Abstract
Objective
To examine blood transfusion practices and develop a standardized bundle of interventions to address the high rate of perioperative red blood cell transfusion among open ovarian and endometrial cancer cases..
Methods
This was a retrospective cohort study. Our primary aim was to determine if implemented a bundled intervention was associated with a reduction of perioperative red blood cell transfusions among cases of laparotomy for cancer. Secondary aims included comparing perioperative demographic, surgical, complication, and cost data. Interventions included: blood transfusion practice standardization utilizing American Society of Anesthesiologists guidelines, an intraoperative hemostasis checklist, standardized intraoperative fluid status communication, and evidence-based use of tranexamic acid. Prospective data from women undergoing laparotomy for ovarian or endometrial cancer from September 28, 2015 to May 31, 2016, defined the study cohort and were compared to historical controls (September 1, 2014 to September 25, 2015). Outcomes were compared in the full unadjusted cohorts and in propensity-matched cohorts.
Results
In the intervention and historic cohorts, respectively, 89 and 184 women underwent laparotomy for ovarian cancer (n=74 and 152) or advanced endometrial cancer (n=15 and 32). Tranexamic acid was administered in 54 (60.7%) patients. The perioperative transfusion rate was lower for the intervention group compared to historic controls (18.0% (16/89) versus 41.3% (76/184), p<0.001); a 56.4% reduction. This improvement in the intervention group remained significant after propensity matching (16.2% (13/80) versus 36.2% (29/80), p=0.004). The hospital readmission rate was also lower for the intervention group compared to historic controls (1.1% (1/89) versus 12.5% (23/184), p=0.002); however, this improvement did not attain statistical significance after propensity matching (1.2% (1/80) versus 7.5% (6/80), p=0.12). Cost analysis demonstrated that this intervention was cost-neutral during index hospitalization plus 30-day follow-up.
Conclusion
Application of a standardized bundle of evidence-based interventions was associated with reduced blood utilization in our gynecologic oncology practice.
Introduction
Prior studies have shown increased rates of perioperative complications such as venous thromboembolism and infection in patients who have received perioperative blood transfusion (1-3). Moreover, women with ovarian cancer who receive perioperative blood transfusion after debulking surgery have shorter recurrence free survival (4, 5). Following established guidelines, a more conservative approach to transfusion has emerged, allowing for continued patient safety with less use of allogeneic red blood cells (6-9).
Along with evidence-based blood transfusion guidelines, interventions such as the antifibrinolytic agent, tranexamic acid, may reduce blood loss. Tranexamic acid has been utilized in the perioperative setting across several surgical subspecialties, and in some specialties is used routinely (10-12). Furthermore, a recent randomized, double-blind, placebo-controlled trial demonstrated that preoperative administration of tranexamic acid reduced blood loss and transfusion rates in women with advanced ovarian cancer (13).
There are a limited number of published studies on blood transfusion reduction interventions specific to surgical gynecologic oncology (13-16). When assessing our institution’s National Surgical Quality Improvement Program data, we discovered our perioperative blood transfusion rate was double the national average at 10.5% for all gynecologic surgery cases compared to 5.1% nationally for 2013; this rate was disproportionally affected by the high transfusion rate for patients undergoing hysterectomy for malignancy. Our aim was to examine blood transfusion practices and develop a standardized bundle of interventions to address the high rate of perioperative red blood cell transfusion among open ovarian and endometrial cancer cases at our institution.
Materials and Methods
This was a retrospective cohort study. We implemented a bundled intervention with the primary goal of reducing perioperative red blood cell transfusions among cases of laparotomy for cancer. Secondary aims included comparing intraoperative and postoperative outcomes, and 30-day costs of care, between the intervention cohort and a historical cohort of similar patients. Women aged ≥ 18 years, diagnosed with presumed or biopsy-proven ovarian, fallopian tube, primary peritoneal, or stage III or IV or recurrent endometrial cancer undergoing surgical treatment via laparotomy between September 28, 2015 and May 31, 2016 were included in the intervention cohort. A historical cohort of women meeting the same inclusion criteria who underwent surgical treatment using laparotomy were identified from September 1, 2014 to September 25, 2015. Patients receiving neoadjuvant chemotherapy were excluded from analysis. Surgical site infection reduction and enhanced recovery algorithms were standard of care for all patients who underwent surgery in both the intervention and historical cohorts (17, 18).
Initial quality improvement measures leading to standardization of blood utilization practices were deemed not to be research by the Mayo Clinic Institutional Review Board. Institutional Review Board approval was obtained for retrospective cohort comparison of the post-implementation cohort with the historical cohort. Only the medical records of patients who had previously signed a standard Minnesota Research Authorization form allowing the use of their electronic health record for research were reviewed and included in this study.
Quality Improvement Initiative
A multidisciplinary team consisting of gynecologic oncology surgeons, anesthesia providers, blood management specialists, and nursing staff collaborated on the initial quality improvement project to create an evidence-based blood management intervention bundle. Bundled blood transfusion reduction interventions were developed based on quality improvement methods such as intraoperative and postoperative cause-mapping and swim lanes, retrospective data analysis, and evidence-based chart review of patients diagnosed with presumed or biopsy-proven ovarian, fallopian tube, primary peritoneal, or advanced endometrial cancer undergoing surgical treatment through laparotomy.
The final intervention bundle included: standardization of blood transfusion practices according to the American Association of Blood Banks (AABB), American Society of Anesthesiologists (ASA), an intraoperative hemostasis checklist, enhanced intraoperative fluid status communication, and evidence-based use of tranexamic acid (9, 13, 19) (Boxes 1 and 2). Tranexamic acid was dosed according to Lundin, et al, at 15 mg/kg IV within 30 minutes of incision in accordance with the randomized controlled trial of tranexamic acid in ovarian cancer patients (13). A hemostasis checklist was developed to ensure all surgical sites were hemostatic prior to closure (Figure 1). Communication checkpoints, which consisted of nurse-initiated communication to verbalize point in procedure and patient status with each 500 mL of fluid collected in a suction canister, were developed to increase awareness of fluid and patient status for all individuals in the operating room. In hemodynamically stable patients, transfusion was guided by hemoglobin level and one unit of packed red blood cells at a time was the standardized transfusion practice.
Box 1: Intervention Bundle for Blood Transfusion Reduction.
Standardization of blood transfusion practices according to vetted institutional guidelines including those from the AABB and ASA (7,9)
Intraoperative hemostasis checklist performed prior to closure
Standardized intraoperative fluid status communication at every 500 mL of fluid in the suction canister
Evidence-based use of tranexamic acid (15 mg/kg within 30 minutes of incision)(13)
Box 2. Blood Transfusion Guidelines (7, 9).
Active bleeding with cardiovascular instability
Hemoglobin ≤ 7g/dL
Hemoglobin ≤ 8g/dL in a patient who has stable coronary artery disease, evidence of end-organ ischemia, acute brain injury, or symptoms thought to be related to anemia (hypotension unresponsive to fluid resuscitation, unexplained tachycardia unresponsive to fluid resuscitation, cardiac chest pain, congestive heart failure)
Hemoglobin ranging from 8–10 g/dL in a patient who has evidence of acute coronary syndrome.
Figure 1.
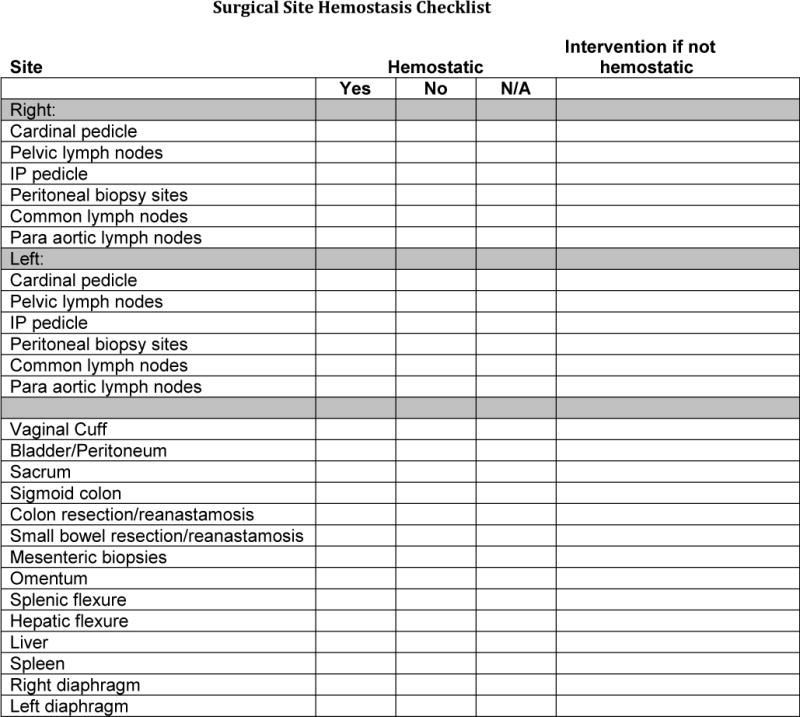
Intraoperative hemostasis checklist included in intervention bundle. IP, infundibulopelvic.
Pertinent data on demographics, past medical history, surgical characteristics and outcomes was abstracted from the medical records by the first author and entered into a Research Electronic Data Capture (REDCap) web-based application designed for this specific study. Current tobacco use was defined as use within 3 months of surgical date, Length of stay was calculated using the day of surgery as day 0, perioperative red blood cell transfusion was defined as intraoperative if administered after surgical incision and prior to discharge from the Post-Anesthesia Care Unit, and postoperative if administered after discharge from the Post-Anesthesia Care Unit through 48 hours postoperative.
With a sample size of 89 patients in the intervention cohort and an estimated 180 patients in the historical cohort, the study had 93% power to detect a 30% decrease in the primary outcome, perioperative red blood cell transfusion rate (i.e. 40% versus 20%), based on a two-sided chi-square test with type I error level of 0.05. A sequential statistical stopping rule was established to ensure the safety of tranexamic acid use in the intervention cohort with respect to venous thromboembolism events within 30 days after surgery. The stopping rule was calculated using the sequential probability ratio test with a type I error of 5% and 85% power, assuming a 30-day VTE rate of 3% and a maximum tolerated rate of 10%. The stopping rule stipulated that use of the bundled intervention would be stopped if 3 patients experienced a venous thromboembolism among the first 18 patients, or 4 among the first 31 patients, or 5 among the first 44 patients, etc.
To account for potential differences in the study groups from observed confounders, propensity score matching was utilized, which enables construction of intervention and control cohorts that are similar in terms of their baseline clinical and other characteristics (20). Logistic regression that models the propensity (probability) of receiving the intervention was used to estimate the propensity scores. Potential confounders included in the logistic modelwere patient residency location (local, regional, national, international), age, tobacco use, clinical diagnosis, cancer stage, insurance status, BMI, and count of Elixhauser comorbidities (21). Intervention and historic controls were matched on the propensity scores using nearest neighbor one-to-one matching without replacement. In both the full unadjusted cohort and the propensity-matched cohorts, comparisons of surgical and postoperative outcomes between the two groups were evaluated using the chi-square or Fisher’s exact test for nominal variables and the two-sample t-test or Wilcoxon rank sum test for continuous variables.
Cost analyses were performed on the propensity-matched cohorts using standardized cost data from the Mayo Clinic Cost Data Warehouse (22). This database applies a standardized costing method using a bottom-up costing approach, which allows costs each of thebilled services. Costs of hospital services are valued by multiplying billed charges by department level cost-to-charge ratios as determined by the Medicare cost reports. Professional services are valued using the Medicare Fee Schedules. All costs were inflated to 2016 US Dollars using the Gross Domestic Product Implicit Price Deflator (23). Cost outcomes included the index hospitalization plus 30-days post discharge. To account for the skewness found in the cost data, we used generalized linear modeling with the gamma distribution to compare costs between the historical and intervention cohorts; the model was also adjusted for confounders with residual imbalance which propensity score matching could not adjust (24). For analyzing the 30-days post discharge follow-up costs, two-part modeling was employed to account for potential patients incurring zero costs in the follow-up period. The first part of this analysis used logistic regression to model the probability of having positive costs, while the second part utilized the generalized linear model described above. Statistical differences in costs of the two study cohorts were determined using 95% CIs of the difference in mean costs. Propensity score matching and all statistical analyses on cost outcomes were performed in Stata 14.0.
Results
We compared 89 women in the intervention cohort (September 28, 2015 to May 31, 2016,) to a historical cohort of 184 women (September 1, 2014 to September 25, 2015). There was no difference in demographic variables among those in the intervention cohort and the historical cohort (Table 1, all p-values >0.05). In the intervention and historic cohorts, respectively, 89 and 184 women underwent laparotomy for ovarian cancer (n=74 and 152) or advanced endometrial cancer (n=15 and 32). Propensity matching resulted in 80 intervention patients being matched to 80 historical controls. Standardized differences indicate that the measured patient and clinical characteristics between the intervention and control cohorts were all well-balanced after propensity matching with standardized differences less than the recommended threshold of 0.10 for all of the characteristics except the Elixhauser comorbidity count and regional residency (Table 1). Tranexamic acid was administered in 54 (60.7%) patients in the intervention group; only 1 patient developed a venous thromboembolism, therefore the statistical stopping rule for venous thromboembolism was not reached.
Table 1.
Comparison of Baseline Characteristics Between the Historical and Intervention Cohorts
| Characteristic | Full unadjusted cohorts
|
Propensity-matched cohorts
|
||||
|---|---|---|---|---|---|---|
| Historical (N=184) |
Intervention (N=89) |
Standardized difference* | Historical (N=80) |
Intervention (N=80) |
Standardized difference* | |
| Age at surgery (years), Mean (SD) | 62.4 (12.4) | 64.0 (11.6) | 0.132 | 64.0 (13.1) | 64.4 (12.0) | 0.030 |
| BMI (kg/m2), Mean (SD) | 28.7 (7.7) | 29.2 (7.9) | 0.064 | 28.8 (8.4) | 29.6 (7.8) | 0.098 |
| Elixhauser comorbidity count, Mean (SD) | 2.3 (1.7) | 2.2 (1.7) | 0.030 | 2.2 (1.8) | 2.4 (1.6) | 0.11 |
| Current tobacco use | 13 (7.1%) | 12 (13.5%) | 0.213 | 9 (11.3%) | 7 (8.8%) | 0.083 |
| Diagnosis | ||||||
| Ovarian cancer | 152 (82.6%) | 74 (83.1%) | 0.014 | 64 (80.0%) | 66 (82.5%) | 0.064 |
| Endometrial cancer | 32 (17.4%) | 15 (16.9%) | 16 (20.0%) | 14 (17.5%) | ||
| Stage | ||||||
| I–II | 31 (16.8%) | 15 (16.9%) | 0.000 | 15 (18.8%) | 14 (17.5%) | 0.032 |
| III–IV | 107 (58.2%) | 46 (51.7%) | 0.130 | 40 (50.0%) | 40 (50.0%) | 0.000 |
| Recurrent | 46 (25.0%) | 28 (31.5%) | 0.144 | 25 (31.3%) | 26 (32.5%) | 0.027 |
| Residency† | ||||||
| Local | 24 (13.2%) | 13 (15.3%) | 0.060 | 10 (12.5%) | 12 (15.0%) | 0.070 |
| Regional | 70 (38.5%) | 35 (41.2%) | 0.060 | 37 (46.3%) | 33 (41.3%) | 0.100 |
| National | 84 (70.6%) | 35 (41.2%) | 0.100 | 31 (38.8%) | 33 (41.3%) | 0.050 |
| International | 4 (2.2%) | 2 (2.4%) | 0.010 | 2 (2.5%) | 2 (2.5%) | 0.000 |
| Insurance status† | ||||||
| Government | 87 (47.8%) | 43 (50.6%) | 0.060 | 45 (56.3%) | 43 (53.8%) | 0.050 |
| Private | 94 (51.7%) | 41 (48.2%) | 0.070 | 34 (42.5%) | 36 (45.0%) | 0.050 |
| Self-pay | 1 (0.6%) | 1 (1.2%) | 0.070 | 1 (1.3%) | 1 (1.3%) | 0.000 |
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index; IQR, interquartile range; SD, standard deviation; VTE, venous thromboembolism.
Covariate imbalance between the historical and intervention groups was assessed by evaluating the standardized difference for each baseline covariate, separately in the full unadjusted cohorts and in the propensity-matched cohorts. The standardized difference for a continuous covariate is defined as the absolute difference in surgical group means divided by an estimate of the pooled standard deviation. The derivation is similar for nominal covariates. A standardized difference less than 0.10 is considered by some authors to denote negligible covariate imbalance between groups.
Data available on 182/184 in the historical cohort and 85/89 in the intervention cohort.
The rate of perioperative blood transfusion was 41.3% (76/184, 95% CI 34.2–48.4%) in the historical cohort, compared to 18.0% (16/89, 95% CI 10.0–26.0%) in the intervention cohort, a 56.4% transfusion reduction (p<0.001, Table 2). This improvement in the intervention cohort remained significant after propensity matching (36.2% (29/80) versus 16.2% (13/80), p=0.004, Table 2). This reduction was driven mostly by the decreased rate of intraoperative blood transfusion of 35% in the historical cohort versus 15% in the intervention cohort after propensity matching (p=0.004, Table 2). There was no difference in postoperative transfusion rates (Table 2).
Table 2.
Comparison of Intraoperative and Postoperative Outcomes Between the Historical and Intervention Cohorts.
| Outcome | Full unadjusted cohorts
|
Propensity-matched cohorts
|
||||
|---|---|---|---|---|---|---|
| Historical (N=184) |
Intervention (N=89) |
p-value* | Historical (N=80) |
Intervention (N=80) |
p-value* | |
| Intraoperative transfusion rate | 70 (38.0%) | 14 (15.7%) | <0.001 | 28 (35.0%) | 12 (15.0%) | 0.004 |
| Postoperative transfusion rate | 16 (8.7%) | 4 (4.5%) | 0.21 | 3 (3.8%) | 3 (3.8%) | 1.00 |
| Perioperative transfusion rate | 76 (41.3%) | 16 (18.0%) | <0.001 | 29 (36.2%) | 13 (16.2%) | 0.004 |
| Estimated blood loss (mL), Median (IQR) | 500 (250, 800) | 300 (200, 600) | 0.009 | 450 (250, 750) | 300 (200, 500) | 0.021 |
| Operative time (min), Mean (SD) | 279.3 (118.6) | 241.7 (105.6) | 0.01 | 264.6 (116.4) | 234.4 (105.9) | 0.09 |
| Length of stay (days), Median (IQR) | 4 (3, 6) | 4 (3, 6) | 0.87 | 4 (3, 6) | 4 (3, 6) | 0.66 |
| Postoperative complications within 30 days | ||||||
| Venous thromboembolism | 3 (1.6%) | 1 (1.1%) | 1.00 | 1 (1.2%) | 1 (1.2%) | 1.00 |
| Readmission | 23 (12.5%) | 1 (1.1%) | 0.002 | 6 (7.5%) | 1 (1.2%) | 0.12 |
| Reoperation | 8 (4.3%) | 0 | 0.057 | 2 (2.5%) | 0 | 0.50 |
| Infection - Pulmonary | 6 (3.3%) | 2 (2.2%) | 1.00 | 3 (3.8%) | 1 (1.2%) | 0.62 |
| Infection - Sepsis | 8 (4.3%) | 0 | 0.057 | 1 (1.2%) | 0 | 1.00 |
| Infection – Wound or pelvic abscess | 11 (6.0%) | 4 (4.5%) | 0.78 | 2 (2.5%) | 4 (5.0%) | 0.68 |
| Unplanned Intensive Care Unit admit | 6 (3.3%) | 0 | 0.18 | 3 (3.8%) | 0 | 0.25 |
| Other† | 3 (1.6%) | 0 | 0.55 | 1 (1.2%) | 0 | 1.00 |
Abbreviations: IQR, interquartile range; SD, standard deviation.
Comparisons between groups were evaluated using the chi-square or Fisher’s exact test for nominal variables, the two-sample t-t-est for operative time, and the Wilcoxon rank sum test for estimated blood less and length of stay.
Three patients in the historical cohort had a postop anastomosis leak (n=1), postop perforation and anastomosis leak (n=1), and a small bowel obstruction (n=1), respectively.
The transfusion rate among women with ovarian cancer was 40.8% (62/152, 95% CI 33.0–48.6%) in the historical cohort compared to 16.2% (12/74, 95% CI 7.8–24.6%) in the intervention cohort, a 60.3% transfusion reduction (p<0.001). Following propensity score matching, the difference in transfusion rate remained significantly reduced in the intervention cohort (36.5% versus 15.4%, p=0.014). In contrast, the reduction in transfusion rate for the smaller group of women with endometrial cancer, did not reach statistical significance (26.7% (4/15; 95% CI 4.3–49.1%) versus 43.8% (14/32; 95% CI 26.6–60.9%, p=0.26 in the full unadjusted cohorts).
When comparing surgical variables, in addition to the reducedblood transfusion rates, there was a statistically significant reduction in median estimated blood loss from 500 mL to 300 mL (p=0.009), and mean operative time from 279.3 to 241.7 (p=0.01) in the historical and intervention cohorts, respectively (Table 2). This improvement in the intervention cohort remained significant after propensity matching (Table 2).
When comparing postoperative complications between the historical and intervention cohorts, there was a significant reduction in hospital readmission rates in the intervention cohort (12.5% (23/184) versus 1.1% (1/89), p=0.002), however, this reduction did not attain statistical significance after propensity matching (7.5% (6/80) versus 1.2% (1/80), p=0.12). There were no other significant differences in postoperative complication variables between the two groups. (Table 42)
Cost analysis data in the propensity-matched cohorts showed no difference in overall costs, defined as index hospitalization with 30-day follow-up, between the historical and intervention cohorts (Table 3). Total mean cost was $30,168.94 in the historical cohort and $32, 737.39 in the intervention cohort (95% CI for difference in means $-1,361 to $6,498, p=0.2).
Table 3.
Comparison of Total Costs During Index hospitalization Plus 30-Day Follow-Up Between Propensity-Matched Historical and Intervention Cohorts.
| Mean | 95% CI | p-value | |
|---|---|---|---|
| Historical | $30,168.94 | $27,693.93 to $32,643.95 | |
| Intervention | $32,737.39 | $29,473.72 to $36,001.07 | |
| Difference | $2,568.45 | −$1,361.27 to $6,498.17 | 0.200 |
Discussion
In this investigation, we demonstrate that implementation of standardized blood transfusion practices, an intraoperative hemostasis checklist, enhancedintraoperative fluid status communication, and evidence-based use of tranexamic acid was associated with reduced blood loss and red blood cell transfusion rates for patients undergoing laparotomy for ovarian or advanced endometrial cancer. This reduction is clinically important, given that perioperative blood transfusion carries well-described risks and negative outcomes (16, 25). While a bundled approach focused on reducing blood transfusion in gynecologic oncology patients has not been previously reported, our findings are consistent with those published in previous studies showing the efficacy of tranexamic acid and standardized blood transfusion guidelines (7, 13).
Prior studies have shown increased rates of perioperative complications and shorter recurrence free survival for patients with ovarian cancer who received perioperative blood transfusion, while decreasing rates of red blood cell transfusion had a positive effect on perioperative outcomes (1, 2, 4, 26). Similarly, our reduction in blood transfusion was associated with a significant decrease in postoperative hospital readmission rates and a trend toward decreased reoperation rates and sepsis in the intervention group. (1, 2, 27). Additionally, the intervention had no effect on overall costs and was associated with a reduction in readmission rates.
One particular element of the bundle, tranexamic acid, deserves additional discussion. Tranexamic acid has been well studied, and is currently used to aid in the reduction of blood loss and transfusion in orthopedic, urologic, trauma, and other surgical specialties (10-12). Prior studies in the gynecologic oncology patient population have shown similar success in transfusion practices with tranexamic acid. In 2006, Celebi et al compared tranexamic acid to colloid, crystalloid, and epsilon-aminocaproic acid in patients undergoing laparotomy for cervical cancer in a prospective, double-blind randomized trial. Women who received 10 mg/kg of tranexamic acid had statistically significant reductions in blood loss as high as 33.3%(15). More recently, Lundin et al published the results of a randomized, double-blinded, placebo-controlled trial which demonstrated that a single dose of preoperative tranexamic acid at 15 mg/kg IV, significantly reduced both blood loss and blood transfusion rates in women undergoing surgery for advanced stage ovarian cancer (13). Preoperative administration of tranexamic acid did not result in an increase in adverse events, including venous thromboembolism, in our study. Prior studies support the safety of tranexamic acid among women appropriately triaged and screened for contraindications to the medication (28).
Limitations include the retrospective nature of historical data collection with the usual biases of observational, single institution research. Of note, however, our bundled intervention cohort variables were prospectively collected which aids in reducing overall bias and adds consistency to post-intervention data collection; propensity score matching was performed to reduce confounding. Another potential limitation is that a separate quality improvement effort to reduce anastomotic leaks was underway at our institution between 7/2013–1/2016. As this interval overlaps with our intervention bundle for approximately 3 months, potential exists for a confounding effect and this could have contributed to the reduced complication rates (29). In contrast, enhanced recovery and surgical site infection reduction initiatives had already been standardized in our division for both the historical and intervention time frames, making these initiatives an unlikely source of confounding (17, 18). Although bundled interventions are clinically effective, it is not possible to discern if one measure of the bundle is more efficacious, as all measures were implemented simultaneously. Similarly, our findings include data from both ovarian and endometrial cancer cases, and our study was not powered to provide results on these diagnoses individually. Although, all surgeons at our institution agreed with the use of tranexamic acid, in certain scenarios its use was deemed unnecessary such as low likelihood of proceeding with debulking, therefore, tranexamic acid was not administered to all patients due to provider’s preference or contraindication. Finally, as this study was found to be cost neutral, it is possible that other factors in the 30-day post discharge time frame negated any cost savings in the intervention group.
We found that application of a standardized bundle of evidence-based interventions was associated with reduced blood utilization and estimated blood loss in patients undergoing laparotomy for ovarian cancer and advanced or recurrent endometrial cancer. This is clinically important, as reducing blood loss and blood transfusions should translate to reduced risks of the short- and long-term untoward outcomes associated with transfusion. The transfusion reduction bundle can be used by other institutions to standardize blood transfusion practices and reduce blood loss and transfusion rates.
Précis.
Application of a standardized bundle of evidence-based interventions is associated with a reduction in blood utilization in patients with gynecologic cancer.
Acknowledgments
Supported by CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Footnotes
Presented at Society of Gynecologic Oncology Annual Meeting, March 24–27, 2018.
Financial Disclosure
The authors did not report any potential conflicts of interest.
Each author has indicated that he or she has met the journal’s requirements for authorship.
References
- 1.Xenos ES, Vargas HD, Davenport DL. Association of blood transfusion and venous thromboembolism after colorectal cancer resection. Thrombosis Research. 2012;129(5):568–72. doi: 10.1016/j.thromres.2011.07.047. [DOI] [PubMed] [Google Scholar]
- 2.Kaneko K, Kawai K, Tsuno NH, Ishihara S, Yamaguchi H, Sunami E, et al. Perioperative Allogeneic Blood Transfusion Is Associated With Surgical Site Infection After Abdominoperineal Resection—a Space for the Implementation of Patient Blood Management Strategies. International Surgery. 2015;100(5):797–804. doi: 10.9738/INTSURG-D-14-00174.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakkum-Gamez JN, Dowdy SC, Borah BJ, Haas LR, Mariani A, Martin JR, et al. Predictors and costs of surgical site infections in patients with endometrial cancer. Gynecologic Oncology. 2013;130(1):100–6. doi: 10.1016/j.ygyno.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Oliveira GS, Schink JC, Buoy C, Ahmad S, Fitzgerald PC, McCarthy RJ. The association between allogeneic perioperative blood transfusion on tumour recurrence and survival in patients with advanced ovarian cancer. Transfusion Medicine. 2012;22(2):97–103. doi: 10.1111/j.1365-3148.2011.01122.x. [DOI] [PubMed] [Google Scholar]
- 5.McGehee RP, Dodson MK, Moore JL, Morrison FS, Bass JD, Burrow P, et al. Effect of blood transfusion in patients with gynecologic malignancy. International Journal of Gynecology & Obstetrics. 1994;46(1):45–52. doi: 10.1016/0020-7292(94)90308-5. [DOI] [PubMed] [Google Scholar]
- 6.Mirski MA, Frank SM, Kor DJ, Vincent J-L, Holmes DR. Restrictive and liberal red cell transfusion strategies in adult patients: reconciling clinical data with best practice. Critical Care. 2015;19(1):202. doi: 10.1186/s13054-015-0912-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nuttall G, Brost B, Connis R, Gessner J, Harrison C, Miller R, et al. Practice Guidelines for Perioperative Blood Transfusion and Adjuvant Therapies: An Updated Report by the American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Anesthesiology. 2006;105(1):198–208. doi: 10.1097/00000542-200607000-00030. [DOI] [PubMed] [Google Scholar]
- 8.Shander A, Gross I, Hill S, Javidroozi M, Sledge S. A new perspective on best transfusion practices. Blood Transfusion. 2013;11(2):193–202. doi: 10.2450/2012.0195-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carson JL, Grossman BJ, Kleinman S, et al. Red blood cell transfusion: A clinical practice guideline from the aabb*. Annals of Internal Medicine. 2012;157(1):49–58. doi: 10.7326/0003-4819-157-1-201206190-00429. [DOI] [PubMed] [Google Scholar]
- 10.Crescenti A, Borghi G, Bignami E, Bertarelli G, Landoni G, Casiraghi GM, et al. Intraoperative use of tranexamic acid to reduce transfusion rate in patients undergoing radical retropubic prostatectomy: double blind, randomised, placebo controlled trial. The BMJ. 2011;343:d5701. doi: 10.1136/bmj.d5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henry DA, Carless PA, Moxey AJ, O’Connell D, Stokes BJ, Fergusson DA, et al. Anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion. The Cochrane database of systematic reviews. 2011;(3) doi: 10.1002/14651858.CD001886.pub4. CD001886-CD0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kagoma YK, Crowther MA, Douketis J, Bhandari M, Eikelboom J, Lim W. Use of antifibrinolytic therapy to reduce transfusion in patients undergoing orthopedic surgery: A systematic review of randomized trials. Thrombosis Research. 2009;123(5):687–96. doi: 10.1016/j.thromres.2008.09.015. 2009/03/01/ [DOI] [PubMed] [Google Scholar]
- 13.Lundin ES, Johansson T, Zachrisson H, Leandersson U, Bäckman F, Falknäs L, et al. Single-dose tranexamic acid in advanced ovarian cancer surgery reduces blood loss and transfusions: double-blind placebo-controlled randomized multicenter study. Acta Obstetricia et Gynecologica Scandinavica. 2014;93(4):335–44. doi: 10.1111/aogs.12333. [DOI] [PubMed] [Google Scholar]
- 14.Eisenkop SM, Spirtos NM, Lin W-CM, Gross GM. Reduction of Blood Loss during Extensive Pelvic Procedures by Aortic Clamping—A Preliminary Report. Gynecologic Oncology. 2003;88(1):80–4. doi: 10.1006/gyno.2002.6862. 2003/01/01. [DOI] [PubMed] [Google Scholar]
- 15.Celebi N, Celebioglu B, Selcuk M, Canbay O, Karagoz A, Aypar U. The role of antifibrinolytic agents in gynecologic cancer surgery. Saude Med J. 2006;27(5):637–41. [PubMed] [Google Scholar]
- 16.Boone JD, Kim KH, Marques M, Straughn JM. Compliance rates and outcomes associated with a restrictive transfusion policy in gynecologic oncology patients. Gynecologic Oncology. 2014;132(1):227–30. doi: 10.1016/j.ygyno.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 17.Kalogera E, Bakkum-Gamez JN, Jankowski CJ, Trabuco E, Lovely JK, Dhanorker S, et al. Enhanced Recovery in Gynecologic Surgery. Obstetrics & Gynecology. 2013;122(2, PART 1):319–28. doi: 10.1097/AOG.0b013e31829aa780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson MP, Kim SJ, Langstraat CL, Jain S, Habermann EB, Wentink JE, et al. Using Bundled Interventions to Reduce Surgical Site Infection After Major Gynecologic Cancer Surgery. Obstetrics & Gynecology. 2016;127(6):1135–44. doi: 10.1097/AOG.0000000000001449. [DOI] [PubMed] [Google Scholar]
- 19.Practice Guidelines for Perioperative Blood Transfusion and Adjuvant TherapiesAn Updated Report by the American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Anesthesiology. 2006;105(1):198–208. doi: 10.1097/00000542-200607000-00030. [DOI] [PubMed] [Google Scholar]
- 20.D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998 Oct 15;17(19):2265–81. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 21.Elixhauser A, Steiner C, Harris D, Coffey R. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Visscher SL, Naessens JM, Yawn BP, Reinalda MS, Anderson SS, Borah BJ. Developing a standardized healthcare cost data warehouse. BMC Health Services Research. 2017;17:396. doi: 10.1186/s12913-017-2327-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Medical Expenditure Panel Survey: Using Appropriate Price Indices for Analyses of Health Care Expenditures or Income Across Multiple Years. 2013 [cited 2017 May]; Available from: [Google Scholar]
- 24.Manning WG, Basu A, Mullahy J. Generalized modeling approaches to risk adjustment of skewed outcomes data. J Health Econ. 2005 May;24(3):465–88. doi: 10.1016/j.jhealeco.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 25.Altman AD, Liu X-Q, Nelson G, Chu P, Nation J, Ghatage P. The Effects of Anemia and Blood Transfusion on Patients With Stage III-IV Ovarian Cancer. International Journal of Gynecological Cancer. 2013;23(9):1569–76. doi: 10.1097/IGC.0b013e3182a57ff6. [DOI] [PubMed] [Google Scholar]
- 26.Molena D, Mungo B, Stem M, Feinberg RL, Lidor AO. Prevalence, Impact, and Risk Factors for Hospital-Acquired Conditions after Major Surgical Resection for Cancer: A NSQIP Analysis. Journal of Gastrointestinal Surgery. 2015;19(1):142–51. doi: 10.1007/s11605-014-2642-x. [DOI] [PubMed] [Google Scholar]
- 27.Politsmakher A, Doddapaneni V, Seeratan R, Dosik H. Effective Reduction of Blood Product Use in a Community Teaching Hospital: When Less Is More. The American Journal of Medicine. 2013;126(10):894–902. doi: 10.1016/j.amjmed.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 28.Xie J, Ma J, Kang P, Zhou Z, Shen B, Yang J, et al. Does tranexamic acid alter the risk of thromboembolism following primary total knee arthroplasty with sequential earlier anticoagulation? A large, single center, prospective cohort study of consecutive cases. Thrombosis Research. 2015;136(2):234–8. doi: 10.1016/j.thromres.2015.05.014. 2015/08/01/ [DOI] [PubMed] [Google Scholar]
- 29.Kalogera E, Nitschmann CC, Dowdy SC, Cliby WA, Langstraat CL. A prospective algorithm to reduce anastomotic leaks after rectosigmoid resection for gynecologic malignancies. Gynecologic Oncology. 2017;144(2):343–7. doi: 10.1016/j.ygyno.2016.11.032. [DOI] [PubMed] [Google Scholar]


