Abstract
Using various chromatographic methods, a new hexacyclic triterpenoid, 2β, 3β, 24β-trihydroxy-12, 13-cyclo-taraxer-l4-en-28oic acid (1), together with ten known compounds, 2α, 3α, 23-trihydroxyurs-12, 20(30)-dien-28oic acid (2), 6, 7-dehydroroyleanone (3), horminone (4), 7-O-methylhorminone (5), sugiol (6), demethylcryptojaponol (7), 14-deoxycoleon U (8), 5, 6-didehydro-7-hydroxy-taxodone (9), ferruginol (10) and dichroanone (11), were isolated from the roots of Salvia deserta. Their structures were identified on the basis of spectroscopic analysis and comparison with the reported data. The individual compounds (1, 3–8) were screened for cytotoxic activity, using the sulforhodamine B bioassay (SRB) method. As the results, Compounds 3, 5 and 8 showed cytotoxic potency against A549, MDA-MB-231, KB, KB-VIN, and MCF7 cell lines with IC50 values ranging from 6.5 to 10.2 μM.
Keywords: Salvia deserta, Terpenes, Structure elucidation, Cytotoxicity
Graphical abstract

Introduction
Salvia deserta (Xin Jiang Shu Wei Cao) (family Lamiaceae) is a perennial herb widely distributed in northern Xinjiang Uygur Autonomous Region, China. All of the plant parts are used in Chinese folk medicines with antifebrile, detoxifcation, detumescent, lump-dissipation, antitussive and expectorant effects [1]. Salvia species are rich sources of structurally diverse terpenoids, which are likely responsible to a large extent for the therapeutic properties [2]. However, very few studies have been reported on the chemical constituents of the species S. deserta, and only on the isolation of several salvianolic acids. The objective of this current work was the investigation of the roots to identify bioactive terpenoids. The ethanolic extract was fractionated and a new triterpene, salvisertin A (1), was isolated. NOESY correlations allowed us to define the relative configuration of 1. Compounds 1, 2 and 5 were isolated from the genus Salvia for the first time, and 1, 2, 5–11 were firstly isolated from this species. The individual compounds (1, 3–8) were screened for in vitro antiproliferative activity against A549, MDA-MB-231, MCF7, KB, and multidrug-resistant KB subline KB-VIN cell lines. The sulforhodamine B bioassay (SRB) method was used to determine cytotoxic activity.
Results and Discussion
Phytochemical investigation of an ethanol extract of S. deserta using various chromatographic methods led to the isolation of eleven compounds (Figure 1). Compound 1 was obtained as white powder with a molecular formula C30H45O5, deduced from the deprotonated molecular ion peak [M - H]− at m/z 485.3289 (calc. for C30H44O5−: 485.3273), showing eight degrees of unsaturation. The 1H-NMR spectrum (Table 1) of 1 showed the presence of five tertiary methyl groups at δ(H) 0.97 (s, Me(29)), 1.04 (s, Me(25)), 1.08 (s, Me(30)), 1.17 (s, Me(26)), 1.63 (s, Me(23)). In the 1H- and 13C-NMR spectra, H-atoms at δ(H) 5.87 (1H, dd, J = 7.3, 3.7) and carbons at δ(C) 157.0 and δ(C) 118.8 suggested a double bond in the molecule. H-atoms at δ(H) 3.82 (1H, d, J = 10.9), 4.11 (1H, d, J = 10.9) and their corresponding carbons at δ(C) 65.7 indicated the presence of a -CH2OH group. H-atoms at δ(H) 4.45 (1H, dt, J = 11.0, 3.1), 4.57 (1H, d, J = 2.3) and the corresponding carbons at δ(C) 66.8 and δ(C) 74.6 indicated the presence of two -CHOH groups. The 13C-NMR spectrum showed five signals for methyl, ten methylene, seven methine and eight quaternary carbons, including one carboxyl group (δ(C)180.3). The total of 30 carbon resonances indicated a triterpenoid structure. The 1H- and 13C-NMR spectra were completely assigned by detailed 2D-NMR experiments. In the HMBC spectrum (Figure 2), correlations between δ(H) 0.01 (1H, t, J = 4.8) and δ(C) 15.5 (C(12)), 20.1 (C(11)), 24.3 (C(13)), 157.0 (C(14)), δ(H) 1.00 (1H, dd, J = 9.3, 4.8) and δ(C) 20.1 (C(11)), 24.3 (C(13)), 35.4 (C(18)), 157.0 (C(14)) confirmed the presence of a cyclopropane ring.
Figure 1.
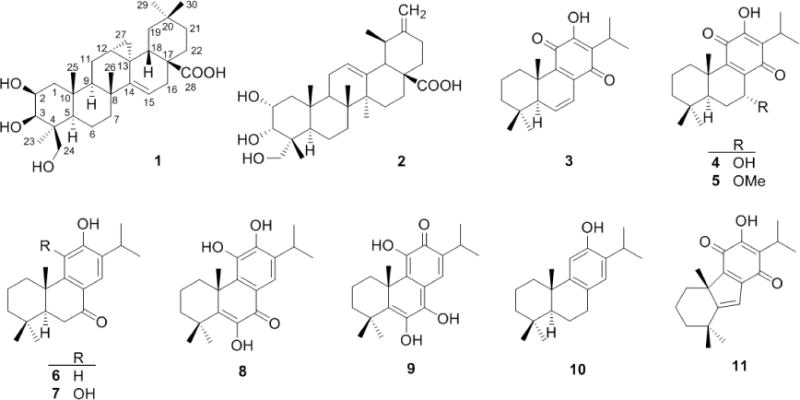
The structures of compounds 1 – 11 isolated from the EtOH extract of S. deserta root.
Table 1.
1H-NMR (500MHz) and 13C-NMR (126 MHz) spectroscopic data in pyridine-d5 (δ in ppm, J in Hz).
Atom numbering as indicated in the text.
| Position | δ (H) | δ (C) | Position | δ (H) | δ (C) |
|---|---|---|---|---|---|
| 1α | 1.74 (m) | 43.6 | 17 | 53.3 | |
| 1β | 2.05 (m) | 18 | 3.12 (dd, J = 13.7, 3.5) | 35.4 | |
| 2 | 4.45 (dt, J = 11.0, 3.1) | 66.8 | 19α | 1.05 (m) | 36.2 |
| 3 | 4.57 (d, J = 2.3) | 74.6 | 19β | 0.79 (dd, J = 12.9, 3.5) | |
| 4 | 45.6 | 20 | 29.5 | ||
| 5 | 1.77 (m) | 50.0 | 21α | 1.32 (dt, J = 13.8, 3.8) | 34.8 |
| 6α | 1.71 (m) | 19.4 | 21β | 1.44 (m) | |
| 6β | 1.48 (m) | 22α | 1.60 (m) | 31.6 | |
| 7α | 1.52 (m) | 39.9 | 22β | 2.08 (m) | |
| 7β | 1.92 (m) | 23 | 1.63 (s) | 24.2 | |
| 8 | 38.0 | 24 | 4.11 (d, J = 10.9) | 65.7 | |
| 9 | 1.13 (dd, J = 13.1, 4.2) | 48.7 | 3.82 (d, J = 10.9) | ||
| 10 | 39.1 | 25 | 1.04 (s) | 18.4 | |
| 11α | 1.97 (dd, J = 13.1, 3.5) | 20.1 | 26 | 1.17 (s) | 23.2 |
| 11β | 1.86 (m) | 27 | 0.01 (t, J = 4.8) | 12.1 | |
| 12 | 1.17 (m) | 15.5 | 1.00 (dd, J = 9.3, 4.8) | ||
| 13 | 24.3 | 28 | 180.3 | ||
| 14 | 157.0 | 29 | 0.97 (s) | 33.0 | |
| 15 | 5.87 (dd, J = 7.3, 3.7) | 118.8 | 30 | 1.08 (s) | 30.0 |
| 16α | 2.12 (m) | 33.5 | |||
| 16β | 2.83 (dd, J = 13.0, 7.3) |
Figure 2.
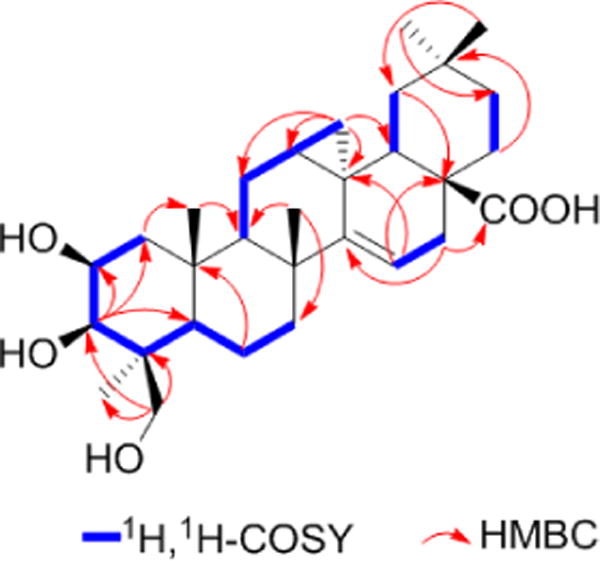
Main 1H,1H-COSY correlations and HMBCs in the spectra of 1.
The 1H- and 13C-NMR data were quite similar to those reported for methyl(12R, 13S)-2α, 3α, 24-trihydroxy-12, 13-cyclo-taraxer-l4-en-28-oate [3] previously isolated from Prunella vulgaris var. lilacina Nakai, except that the 1H and 13C-NMR spectra of 1 lacking a signal for methyl ester group. However, while the structure of 1 resembled that of (12R, 13S)-2α, 3α, 24-trihydroxy-12,13-cyclo-taraxer-14-en-28oic acid [3], which was reported with incomplete spectroscopic data and determination of relative configuration, the A ring proton and carbon signals of 1 did not match completely. Therefore, a 1H,1H-NOESY experiment (Figure 3) was performed and the relative configuration of the stereogenic centers were deduced. The presence of NOE correlations between H-C(27) and H-C(9), H-C(9) and Ha-C(1) confirmed H-C(27) was α-oriented. The configuration of the cyclopropane ring was concluded to be cis to H-C(9). NOESY correlations between H-C(3) and H-C(5), H-C(3) and Ha-C(1), without H-C(3) and Hb-C(1), confirmed H-C(3) was α-oriented and OH-C(3) was β-oriented. The observed coupling constant value for the H-C(2) and H-C(3) (JH-C(2)-H-C(3) = 2.3 Hz), together with H-C(3) proton in an axial configuration, was compatible only with a spatial arrangement in which the H-C(2) proton was equatorial substituent [4][5]. Hence OH-C(2) was β-oriented. A NOESY correlation between H-C(24) and H-C(25), without H-C(23) and H-C(25), confirmed H-C(24) was β-oriented. In addition, if the H-C(24) is α-oriented, the chemical shift of the Me(23) group is generally lower than 18 ppm and C(24) is present at about δ(C) 68 ppm; while if H-C(24) is β-oriented, the chemical shift of the Me(23) group is usually higher than 20 ppm and C(24) is present at around δ(C) 64 ppm. These differences occur because the equatorial hydroxymethylene is less shielded than its axial counterpart [6][7]. The chemical shifts of the C(23) and C(24) in 1 were 24.2 ppm and 65.7 ppm, respectively, indicating that H-C(23) was α-oriented, whereby H-C(24) was β-oriented. Thus, the structure of 1 was characterized as 2β, 3β, 24β-trihydroxy-12, 13-cyclo-taraxer-l4-en-28oic acid. To our knowledge, it is a rare triterpenoid type in nature.
Figure 3.
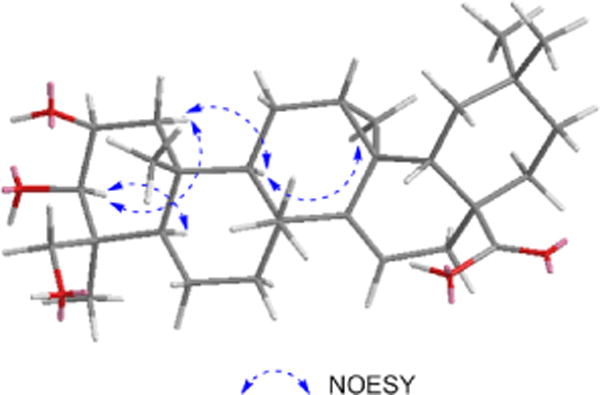
Key NOE correlations of 1 in the NOESY spectrum.
Other 10 known compounds were identified as 2α, 3α, 24-trihydroxyurs-12, 20(30)-dien-28oic acid (2) [8], 6, 7-dehydroroyleanone (3) [9], horminone (4) [10], 7-O-methylhorminone (5) [10], sugiol (6) [11], demethylcryptojaponol (7) [12], 14-deoxycoleon U (8) [13], 5, 6-didehydro-7-hydroxy-taxodone (9) [14], ferruginol (10) [15], and dichroanone (11) [16], by comparing their NMR data with those reported in the literature. Inside, compounds 1, 2 and 5 were isolated from the genus Salvia for the first time, and 1, 2, 5–11 were firstly isolated from S. deserta.
The individual compounds (1, 3–8) were evaluated for their cytotoxicity against A549, MDA-MB-231, KB, KB-VIN, and MCF7 cell lines, using the sulforhodamine B bioassay (SRB) method, and paclitaxel was uesd as an experimental control (Table 2). As a result, compound 3 showed significant antiproliferative potency against A549, KB, KB-VIN cells, with IC50 values of 6.568, 8.916, 8.020 μM, respectively. Compound 5 showed potent cytotoxicity against A549, MDA-MB-231, KB, KB-VIN cells, with IC50 values of 7.194, 8.450, 10.281, 7.303 μM, respectively. Compound 8 also showed significant cytotoxicity against KB, KB-VIN, and MCF7 cells, with IC50 values of 7.689, 7.707, 8.695 μM, respectively. In addition, compounds 4 and 7 exhibited moderate cytotoxic activity against A549, MDA-MB-231, KB, KB-VIN, and MCF7 cells, while the remaining two compounds showed weak activity. It is also worth noticing that, compounds 3 and 5 showed higher cytotoxicity against above cell lines than 4, among which the difference between their structures was C ring, as also found in comparison compounds 7 with 8. It is logical to assume that the activity of these molecules is due to the substituents associated with the C ring [10].
Table 2.
Cytotoxicity data of compounds isolated from the roots of S. deserta.
| Compound | Cytotoxic activitya, IC50 (μM)
|
||||
|---|---|---|---|---|---|
| A549 | MDA-MB-231 | KB | KB-VIN | MCF-7 | |
| 1 2β, 3β, 24β - trihydroxy - 12, 13 - cyclo - taraxer-l4-en-28oic acid | >40 | >40 | >40 | >40 | >40 |
| 3 6, 7-dehydroroyleanone | 6.568±0.176 | 13.320±1.473 | 8.916±1.186 | 8.020±0.258 | 20.779±1.257 |
| 4 horminone | 19.354±0.342 | 23.624±1.812 | 29.581±1.117 | 24.455±0.459 | 34.833±0.922 |
| 5 7-O-methylhorminone | 7.194±0.260 | 8.450±0.174 | 10.281±0.648 | 7.303±0.285 | 13.716±0.654 |
| 6 sugiol | >40 | >40 | >40 | >40 | >40 |
| 7 demethylcryptojaponol | 23.187±0.996 | 26.243±0.140 | 23.501±0.960 | 21.804±0.525 | 28.590±0.336 |
| 8 14-deoxycoleon U | 12.249±0.719 | 18.062±0.008 | 7.689±0.416 | 7.707±0.187 | 8.695±0.975 |
| Paclitaxel (nM)b | 4.191±0.493 | 5.497±0.240 | 3.529±0.398 | 2345.602±35.469 | 11.941±1.305 |
IC50 values based on triplicate five points, presented as the mean ± S.D.
Experimental control.
Conclusions
In conclusion, two triterpenoids (one oleanane- and one ursane-type), together with nine abietane-type diterpenoids were isolated from the roots of S. deserta. The investigation enriched the library of compounds isolated from this plant. Notably, the discovery of compound 1 and the determination of its relative configuration contributed to the chemical diversity of the oleanane-type triterpenoid family. These results might be helpful for explaining the use of S. deserta in traditional medicine.
Experimental Section
General
The HR-ESI-MS data were obtained on an Agilent 6520B Q-TOF spectrometer. The optical rotation was determined on a JASCO P-1020 polarimeter. Analytical HPLC measurements were conducted on a Shimadzu LC-20AT pump with a Shimadzu SPD-20A UV-Vis detector, using a ZORBAX SB-C18 column (250×4.6 mm, 5 μm). Semi-preparative HPLC separation was performed on a Shimadzu LC-6AD pump with a Shimadzu SPD-20A UV-Vis detector, using a ZORBAX SB-C18 column (250×9.4 mm, 5 μm). NMR spectra were recorded on a Bruker ACF (300 and 500 MHz) spectrometer with deuterated solvent signals used as internal standards. Column chromatography was conducted on MCI gel (75–150 μm; Mitsubishi Chemical Corp., Tokyo, Japan), ODS (PrePAK-500/C18, YMC) and Sephadex LH-20 (20–100 μm, Pharmacia, U.S.A.) columns.
Plant materials
The dried roots of S. deserta were collected in 2015 from Urumqi County, Xinjiang Uygur Autonomous Region, China. The identity of the plant material was verified by Dr. Wei Li of Tasly R&D Institute, Tasly Pharmaceutical Co., Ltd., Tianjin, P. R. China and a voucher specimen (No. 20150916) was deposited at the Department of Natural Medicinal Chemistry, China Pharmaceutical University, Nanjing, China.
Extraction and isolation
The air-dried roots of Salvia deserta (4.0kg) were extracted four times with 75% ethanol under reflux (85°C). After filtration, the solvent was evaporated in vacuum to yield a crude ethanol extract (170.0g). All of the extract were suspended in water and successively partitioned with petroleum ether, EtOAc and BuOH to obtain PE fraction (10.0g), EtOAc fraction (25.0g), and BuOH fraction (20.0g), respectively. The EtOAc extract (25.0g) was separated by MCI gel column chromatography and eluted with a gadient of CH3OH/H2O (10:90, 30:70, 50:50, 70:30, 90:10, 100:0, v/v), to yield 10 fractions (Fr.1—Fr.10) based on TLC analysis.
Fr.6 (1000.0mg) was applied to ODS column with CH3OH/H2O (30:70, 50:50, 70:30, 100:0, v/v) as the eluent to give eight subfractions Fr.6.1—Fr.6.5. Fr.6.1 was further subjected to semi-preparative HPLC eluting with CH3CN/H2O/CF3COOH (60:40:0.02, v/v/v) to give compounds 5 (9.6mg) and 6 (4.1mg), Fr.6.2 eluting with CH3CN/H2O/CF3COOH (65:35:0.02, v/v/v) to give compound 4 (8.6mg), Fr.6.4 eluting with CH3CN/H2O/CF3COOH (50:50:0.02, v/v/v) to give compounds 7 (32.4mg) and 8 (69.6mg).
Fr.7 (1270.0mg) was subjected to silica gel column chromatography and gradiently eluted with the solvent of CH2Cl2/MeOH(50:1, 20:1, 8:1,5:1, v/v/v) to give twelve subfractions Fr.7.1—Fr.7.12. Fr.7.6 was subjected to Sephadex LH-20 column eluting with CH2Cl2/CH3OH (1:1, v/v) to produce compound 2 (5.9mg). Fr.7.10 was recrystallized with methanol to afford compound 1 (10.3mg).
Fr.8 (1540.0mg) was subjected to silica gel column chromatography and gradiently eluted with the solvent of CH2Cl2/MeOH(100:1, 70:1, 50:1, 20:1, 5:1, v/v/v) to give seven subfractions Fr.8.1—Fr.8.7. Fr.8.1 was subjected to semi-preparative HPLC using CH3CN/H2O/CF3COOH (67:33:0.02, v/v/v) as the mobile phase to provide compounds 10 (10.2mg) and 11 (6.9mg), Fr.8.4 using CH3CN/H2O/CF3COOH (60:40:0.02, v/v/v) to provide compound 9 (4.9mg). Fr.9 (500.0mg) was recrystallized with methanol to afford compound 3 (153.2mg).
2β, 3β, 24β-trihydroxy-12, 13-cyclo-taraxer-l4-en-28oic acid (1)
White powder. [α]25D = +58.6 (c = 0.10, MeOH). 1H- and 13C-NMR: see Table 1. HR-ESI-MS: 485.3289 ([M - H]−, C30H44O5−; calc. 485.3273).
Bioassays
Human lung carcinoma (A549), originally isolated as epidermoid carcinoma of the nasopharynx (KB), P-glycoprotein-overexpressing multidrug-resistant KB subline (KB-VIN), and triple-negative or hormone-responsible breast cancer (MDA-MB-231 or MCF7, respectively) cell lines were obtained from ATCC or UNC Lineberger Comprehensive Cancer Center. All cell lines were maintained in RPMI-1640 medium containing 25 mM HEPES and 2 mM L-glutamine (Gibco, Invitrogen Corporation, NY) supplemented with 10% fetal bovine serum (Gibco, Invitrogen Corporation, NY), in a humidified environment with 5% CO2 at 37 °C.
Cytotoxic activity was determined by the sulforhodamine B (SRB) colorimetric assay as previously described [17]. In brief, the cells (4-11×103 cells/well) were seeded in 96-well plates filled with culture medium containing various concentrations of samples, and incubated for 72 h. At the end of the exposure period, the attached cells were fixed in cold 10% trichloroacetic acid for 30 min followed by staining with 0.04% SRB (Sigma Chemical Co.) for 30 min. The bound SRB was solubilized in 10 mM Tris-base and the absorbance was measured at 515 nm on a Microplate Reader ELx800 (Bio-Tek Instruments, Winooski, VT) with a Gen5 software. All results were representative of three or more experiments.
The inhibition was calculated with the formula: Inhibition % = (ODc − ODt)/ODc×100%
where ODc is the absorbance of negative control, and ODt is the absorbance of tested drug.
Supplementary Material
Acknowledgments
This work was supported by National Natural Science Foundation of China (Grant No. 81573560) and National Natural Science Foundation of China (Grant No. 81673513). Partial support from US NIH grant CA177584 from the National Cancer Institute awarded to K.H.L. is also acknowledged.
Footnotes
Supplementary Material
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/MS-number.
Author Contribution Statement
L, S. M. Li and X. F. Huang contributed in the collection of plant material. Y. R. Wang, X. F. Huang designed the experiments and wrote the initial draft. Y. R. Wang, Y. Yu, W. Liu and X. F. Huang participated in the acquisition and interpretation of the data. K. H. Lee., M. Goto, and S. L. Morris-Natschke conducted the biological assays and refined the manuscript. All authors approved the final version for publication.
References
- 1.Wang XL, Wang XQ, Wang XM, Hu JP, Rena K. Study on the chemical constituents of EtOAc extraction from Salvia deserta. West China J Pharm Sci. 2014;29:257–259. [Google Scholar]
- 2.Peng Q, Liu JX. Advances in chemical constituents and bioactivity of Salvia genus. Chin J Chin Mater med. 2015;40:2096–2105. [PubMed] [Google Scholar]
- 3.Kojima H, Tominaga H, Sato S, Takayanagi H, Ogura H. Two novel hexacyclic triterpenoids from Prunella vulgaris. Phytochemistry. 1988;27:2921–2925. [Google Scholar]
- 4.Fang Q, Huang CS, Chen XH, Liu HX, Zhong ZG. Survey on the Spectral Features of Ursane Triterpense in Actinidia Planties. J Guangxi Teach Edu Univ. 2007;24:53–60. [Google Scholar]
- 5.Ballesta-Acosta MC, Pascual-Villalobos MJ, Rodriguez B. A new 24-nor-oleanane triterpenoid from Salvia carduacea. J Nat Prod. 2002;65:1513–1515. doi: 10.1021/np020178u. [DOI] [PubMed] [Google Scholar]
- 6.Mahato SB, Kundu AP. 13C NMR Spectra of pentacyclic triterpenoids—a compilation and some salient features. Phytochemistry. 1994;37:1517–1575. [Google Scholar]
- 7.Xu YX, Xiang ZB, Jin YS, Xu W, Sun LN, Chen WS, Chen HS. Constituents from the Roots of Actinidia chinensis and Their Cytochrome P450 Enzyme Inhibitory Activities. Chem Biodivers. 2016;13:1454–1459. doi: 10.1002/cbdv.201500518. [DOI] [PubMed] [Google Scholar]
- 8.Zhang XR, Ding LS, Peng SL, Wang MK. Chemical constituents from clematoclethra scandens. Nat Prod Res Dev. 2000;12:38–41. [Google Scholar]
- 9.Kusumoto N, Ashitani T, Hayasaka Y, Murayama T, Ogiyama K, Takahashi K. Antitermitic activities of abietane-type diterpenes from Taxodium distichum cones. J Chem Ecol. 2009;35:635–642. doi: 10.1007/s10886-009-9646-0. [DOI] [PubMed] [Google Scholar]
- 10.Jonathan LT, Che CT, Pezzuto JM, Fong HHS, Farnsworth NR. 7-O-Methylhorminone and Other Cytotoxic Diterpene Quinones from Lepechinia bullata. J Nat Prod. 1989;52:571–575. doi: 10.1021/np50063a016. [DOI] [PubMed] [Google Scholar]
- 11.Ma HY, Wang CH, Yang L, Zhang M, Wang ZT. Chemical Constituents of Senecio cannabifolius var. Integrilifolius. Chin J Nat Med. 2009;1:28–30. [Google Scholar]
- 12.Xu LL, Lu J, Li WJ, Kong LY. Studies on the chemical constituents in root of Coleus forskohlii. Chin J Chin Mater med. 2005;30:1753–1755. [PubMed] [Google Scholar]
- 13.Braulio MF, Carmen ED, Ana G, Azucena GC. Diterpenes from Salvia broussonetii Transformed Roots and Their Insecticidal Activity. J Agr Food Chem. 2005;53:5200–5206. doi: 10.1021/jf058045c. [DOI] [PubMed] [Google Scholar]
- 14.Mothana RA, Al-Said MS, Al-Musayeib NM, Gamel AE, Al-Massarani SM, Al-Rehaily AJ, Abdulkader M, Maes L. In Vitro Antiprotozoal Activity of Abietane Diterpenoids Isolated from Plectranthus barbatus Andr. Int J Mol Sci. 2014;15:8360–8371. doi: 10.3390/ijms15058360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li WH, Chang ST, Chang SC, Chang HT. Isolation of antibacterial diterpenoids from Cryptomeria japonica bark. Nat Prod Res. 2008;22:1085–1093. doi: 10.1080/14786410802267510. [DOI] [PubMed] [Google Scholar]
- 16.Kawazoe K, Yamamoto M, Takaishi Y, Honda G, Fujita T, Sezik E, Yesilada E. Rearranged abietane-type diterpenes from Salvia dichroantha. Phytochemistry. 1999;30:493–497. [Google Scholar]
- 17.Wang XF, Guan F, Ohkoshi E, Guo WJ, Wang LL, Zhu DQ, Wang SB, Wang LT, Hamel E, Yang DX, Li LN, Qian KD, Morris-Natschke SL, Yuan SJ, Lee KH, Xie L. Optimization of 4-(n-cycloamino) phenylquinazolines as a novel class of tubulin-polymerization inhibitors targeting the colchicine site. J Med Chem. 2014;57:1390–1402. doi: 10.1021/jm4016526. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


