Abstract
Objective
Clinical factors contributing to benzodiazepine failure in treating status epilepticus include suboptimal dosing and seizure duration. As many benzodiazepine-refractory episodes of status epilepticus (SE) arise from acute etiologies, we sought to determine whether etiology impacts SE treatment.
Methods
The potency of diazepam to terminate SE induced by lithium-pilocarpine (LiPilo-SE) or kainic acid (KA-SE) in 3-week old rats was studied by video-electroencephalograpy. Synaptic γ-aminobutyric acid type-A receptor (GABAR)-mediated currents were recorded from dentate granule cells using voltage-clamp electrophysiology. Surface expression of γ2 subunit-containing GABARs and Kv4.2 potassium channels in hippocampal slices was determined using a biotinylation assay. Expression of phosphorylated forms of β2/3 and γ2 subunits was determined using phospho-specific antibodies and western blotting.
Results
Diazepam failed to terminate late SE in LiPilo-SE animals but was successful in terminating KA-SE of 1 and 3 hours in duration. One hour after SE onset, GABAR-mediated synaptic inhibition and γ2 subunit-containing GABAR surface expression were reduced in LiPilo-SE animals. These were unchanged in KA-SE animals at 1 and 3 hours. Phosphorylation of γ2 subunit residue S327 was unchanged in both models, though GABAR β3 subunit S408/409 residues were dephosphorylated in the LiPilo-SE animals. Kv4.2 potassium channel surface expression was increased in LiPilo-SE animals but reduced in KA-SE animals.
Interpretation
SE-model-dependent differences support a novel hypothesis that the development of benzodiazepine-pharmacoresistance may be etiologically predetermined. Further studies are required to investigate the mechanisms that underlie such etiological differences during SE and whether etiology-dependent protocols for the treatment of SE need to be developed.
Introduction
Prompt treatment of status epilepticus (SE) is required to reduce the risk of cerebral injury and subsequent morbidity and mortality associated with these self-sustaining, prolonged seizures. Treatment of this neurological emergency frequently commences with a benzodiazepine, a GABAA receptor (GABAR) allosteric modulator, as this class of drugs has proved to be effective in treating SE in the out-of-hospital and in-hospital settings 1–3. Yet, treatment with a benzodiazepine can fail 3. Clinical factors contributing to benzodiazepine pharmacoresistance include suboptimal dosing, seizure duration, and potentially etiology, as many refractory SE cases are the result of acute symptomatic etiologies such as acute head injury, acute stroke, or one of the encephalitides 4–7.
Benzodiazepine pharmacoresistance occurs in animal models in which SE was induced using the muscarinic agonist pilocarpine following a priming dose of lithium 8,9. Subsequent studies using this model demonstrated that the decline in potency of the benzodiazepines as a function of seizure duration may, in part, have resulted from subunit-specific, activity-dependent trafficking of the post-synaptic GABAR population 10–13, which led to a decrease in the surface expression of the benzodiazepine-sensitive γ2 subunit-containing GABARs. This reduced surface expression correlated with a decline in the post-synaptic GABA-mediated chloride current. Once this post-synaptic response to GABA declined, the benzodiazepine dose that effectively terminated early SE failed to effectively increase GABA-mediated inhibition 8,12. As a result, the effective benzodiazepine concentration shifts such that higher doses, often in the anesthetic range, are required to terminate SE.
Several animal models are available to study SE pathogenesis. Prolonged seizures lasting several hours can be induced not only by pilocarpine (Pilo-SE) in isolation or in combination with lithium (LiPilo-SE) but also using organophosphates such as diisopropyl fluorophosphate, a glutamate receptor agonist such as kainic acid (KA-SE), electrical stimulation, and hyperthermia. Preclinical studies demonstrating dynamic changes in the surface expression of the GABARs have been limited to Pilo-SE and LiPilo-SE 10,12,13. In one study 10, GABA-mediated synaptic inhibition was reduced in acute hippocampal slices obtained from animals in which SE was induced by continuous hippocampal stimulation but the GABAR surface expression was not evaluated 10. Therefore, it is not known whether a change in the GABAR surface expression occurs in all episodes of SE or is conditional on etiology.
Not all episodes of SE develop benzodiazepine pharmacoresistance 3. Etiological-dependence of GABAR trafficking during SE could be one potential mechanism to explain why some episodes of SE become refractory to benzodiazepines while other prolonged episodes may continue to respond to a single dose of benzodiazepines. In this study, the etiological influence on benzodiazepine pharmacoresistance, synaptic inhibition, and GABAR subunit expression was compared in the LiPilo and KA models of SE.
Materials and Methods
Animals
Male Sprague-Dawley rats (23–25 day old) were used for according to guidelines approved by the Animal Care and Use Committee of the University of Virginia. Rats were housed in Plexiglas cages with free access to food and water, and kept on regular day/night cycle.
Induction of SE and EEG analysis
SE was induced by administration of lithium (3 mEq) followed 6–18 hours later with pilocarpine (50 mg/kg, ip) or by administration of kainaic acid (8 mg/kg, ip). Animals were monitored for seizures behaviorally and electrographically using video-EEG recordings 11. For treatment studies, continuous spiking greater than 2 Hz and amplitude more than 2 times the background was considered as the start of SE. For biochemical and electrophysiological studies, the time was calculated from the first observed stage V behavioral seizure on the Racine scale 14. Power analysis was performed using house-written software in LabChart7 Pro (ADInstruments; Colorado Springs, CO) as described previously 11,15.
Biochemical studies
Surface expression of γ2 subunits was determined in hippocampi using a previously described biotinylation assay 10,11. The antibodies used in these studies were anti-γ2 subunit antibody (clone 10F10-C1-B8, 2–3 µg/ml) 11, anti-β3 subunit antibody (1:500, Novus), anti-Kv4.2 (1:500, EMD Millipore), and anti-β-actin antibody (1:1000, Sigma-Aldrich).
The expression of phosphorylated γ2 and β3 subunits was determined in hippocampal lysates using anti-phospho S408/409-β3 subunit antibody (1:500, Symansis) and anti-phospho S327-γ2 subunit antibody (1:500, Abcam). The membranes were blocked in 5% BSA in TBST during the Western blotting procedure.
Electrophysiological studies
Action potential-independent synaptic GABAR-mediated currents (mIPSCs) were recorded from dentate granule cells (DGCs) as previously described 10,11. The experiments were conducted within 3 hours of brain slice preparation. All the recordings were performed at 30 °C. The mIPSCs were analyzed using the MiniAnalysis software (Synaptosoft, Decatur, GA) 10,11.
Statistical analysis
Values are expressed as mean ± SEM. Data were analyzed using paired t-test, Wilcoxon matched-pair signed-rank test, or ANOVA with post-hoc Dunnet’s multiple comparison as warranted and a p-value less than 0.05 was considered statistically significant.
RESULTS
Benzodiazepine pharmacoresistance was present during LiPilo-induced SE but not KA-induced SE
Development of benzodiazepine pharmacoresistance during LiPilo-SE in adult animals and animals as young as postnatal day 15 (P15) has been well established 8,11,16,17. We confirmed this finding in P23 to P25 animals in which seizures of 3 minutes (early SE) or 60 minutes (late SE) in duration were treated with diazepam ranging in dose from 3 mg/kg to 30 mg/kg. Seizure termination was operationally defined as the time at which the spiking pattern became non-periodic and fell below 2 Hz 11. Successful treatment was defined as electrographic seizure termination within 30 minutes of diazepam administration without electrographic recurrence for an additional 90 minutes. Each EEG tracing was independently reviewed by two readers (KMH, HPG). A kappa score of 0.90 for seizure termination was achieved. In those cases in which there was not concordance, both reviewers reached a mutual agreement.
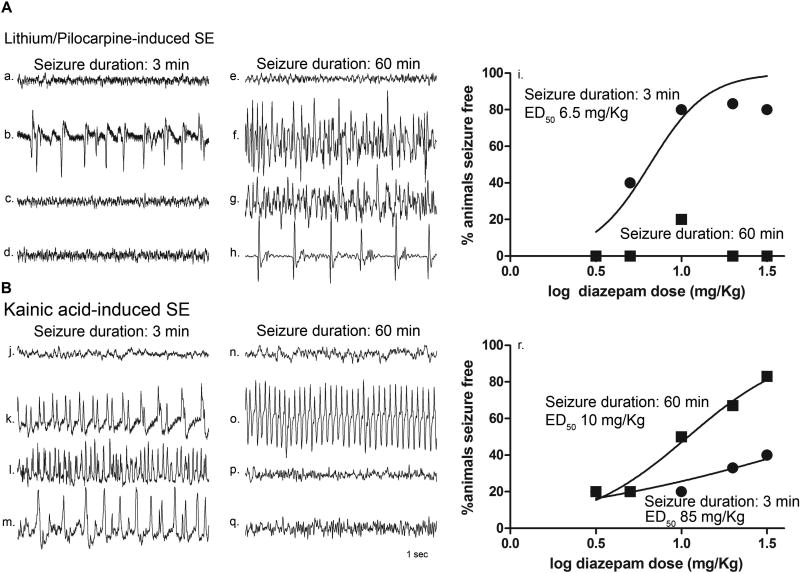
Sample EEG traces obtained from a hippocampal depth electrode from an animal treated with diazepam 10 mg/kg at 3 minutes and at 60 minutes after seizure onset of LiPilo-induced seizures are displayed in the top panel of figure 1. Thirty minutes after diazepam administration, the EEG demonstrates termination of the seizure treated at 3 minutes after onset (figure 1Ac), whereas the seizure treated 60 minutes after onset persisted (figure 1Ag) and a periodic pattern of spikes was still present 2 hours after treatment (figure 1Ah).
Figure 1. Benzodiazepine resistance developed in P23 animals in lithium-pilocarpine-induced SE but not in animals in kainic acid-induced SE.
A, Hippocampal EEG recordings obtained from a P23 rat during seizures induced by a combination of lithium and pilocarpine (LiPilo-SE) and treated with diazepam 10 mg/kg at 3 minutes or 60 minutes after the onset of continuous electrographic seizures. a–d, e–h, 5 minute epochs of baseline prior to pilocarpine injection (a, e), at the time of diazepam administration (b, f), 30 minutes after diazepam administration (c, g), and 2 hours after diazepam administration (d, h). i., Relationship between diazepam dose and percentage (n = 5 animals per dose per seizure duration) of animals seizure free at a time point 30 minutes following diazepam administration without seizure recurrence for an additional 90 minutes. The data were fitted to a sigmoidal dose-response curve with the maximum fitted to 100% and the minimum to 0. The median effective dose (ED50) values were derived from the best-fit equation. B, Hippocampal EEG recordings obtained from a P23 rat during seizures induced by kainic acid (KA-SE) and treated with diazepam 10 mg/kg at 3 minutes or 60 minutes after the onset of continuous electrographic seizures. j–m, n–q, 5 minute epochs of baseline prior to chemoconvulsant injection (j, n), at the time of diazepam administration (k, o), 30 minutes after diazepam administration (l, p), and 2 hours after diazepam administration (m, q). r, Relationship between diazepam dose and percentage (n = 5 animals per dose per seizure duration) of animals seizure free at a time point 30 minutes following diazepam administration without seizure recurrence for an additional 90 minutes. The data were fitted to a sigmoidal dose-response curve with the maximum fixed to 100% and the minimum to 0. The median effective dose (ED50) values were derived from the best-fit equation (• 3 min and ■ 60 min).
The diazepam dose-response of animals seizure free 30 minutes after treatment without recurrence for LiPilo-induced seizures is shown in figure 1Ai. At each dose, 5 animals were treated at each of the two time points. Seizures of 3 minutes in duration were successfully treated with a median effective dose of 6.5 mg/kg. In contrast, the animals treated at the late time point were refractory to the diazepam even at a dose of 30 mg/kg.
Previous studies have demonstrated that a similar pattern of benzodiazepine pharmacoresistance develops for SE induced via electrical stimulation 18 whereas SE induced via the organophosphate paraoxon was responsive to diazepam 30 minutes after seizure onset 19. To determine whether the etiology of SE can affect the response to treatment, the efficacy and potency of diazepam in terminating KA-induced seizures of 3 minutes and 60 minutes in duration was assessed.
Sample EEG traces obtained from a hippocampal depth electrode from an animal treated with diazepam 10 mg/kg at 3 minutes and at 60 minutes after KA-induced seizure onset are displayed in the lower panel of figure 1B. After a period of seizure suppression lasting for 20 minutes after diazepam administratoin, the KA-induced seizure treated at the early time point recurred prior to the 30 minute time point (figure 1Bl) whereas the KA-induced seizure treated 60 minutes after onset stopped prior to the 30 minute time point (figure 1Bp) and did not recur over the next 90 minutes (figure 1Bq).
The diazepam dose-response of animals seizure-free 30 minutes after treatment without recurrence for KA-induced seizures is shown in figure 1Br. At each dose, 5 animals were treated at each of the two time points. Seizures of 3 minutes in duration were difficult to treat with an extrapolated median effective dose of 85 mg/kg. Failures at the lower doses (3 mg/kg and 5 mg/kg) at the early time point resulted from failure to terminate seizure progression. However, at the higher doses (10 mg/kg, 20 mg/kg, and 30 mg/kg), after an initial period of suppression, the seizure recurred within the 90 minute time period. In contrast, diazepam was more effective in terminating the seizures treated 60 minutes after onset without recurrence during the recording period and with better potency (median effective dose of 10 mg/kg).
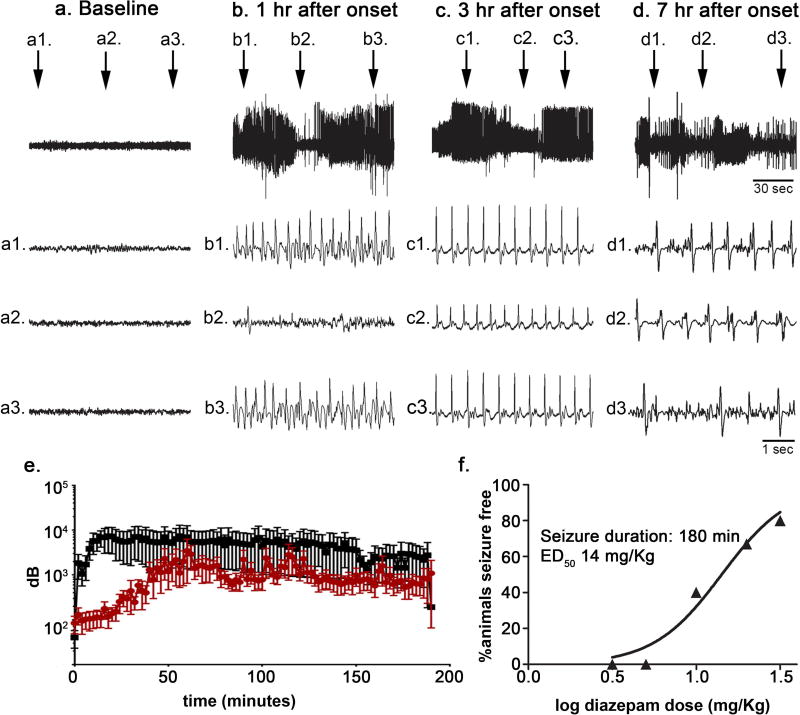
It was previously noted that the effects of diazepam on SE are dependent on clinical and electrographic stage 17,20. In naïve animals (n = 5), the 8 mg/kg dose of KA resulted in seizures that lasted longer than 7 hours in all animals (figure 2Aa–d). The onset of electrographic SE was marked by waxing and waning of electrographic seizures (Treiman stage 2) 21 with short periods of electrographic suppression between the seizures that persisted one hour after SE onset (figure 2Ab). By 3 hours, although the amplitude of the electrographic recording varied (figure 2Ac), periods of suppression were no longer observed, and the seizure had transitioned to a continuous electrographic seizures (Treiman stage 3) with evidence of transitioning to the next stage of continuous ictal activity with flat periods by 7 hours (Treiman stage 4).
Figure 2. Kainic acid-induced SE of 180 min duration was also successfully treated with diazepam.
A: Hippocampal EEG recording obtained from a single animal prior to (a) and at various time points (b, c, d) following the onset of kainic acid-induced SE. Top trace, 150-sec epoch. Second trace (a1, b1, c1, d1), third trace (a2, b2, c2, d2), and fourth trace (a3, b3, c3, d3), 5-sec epoch at time indicated in the top trace. B, total power spectra during SE induced using lithium-pilocarpine (black) or kainic acid (red). C, Relationship between diazepam dose and percentage (n = 5 animals per dose per seizure duration) of animals seizure free at a time point 30 minutes following diazepam administration without seizure recurrence for an additional 90 minutes when diazepam was administered 180 minutes after kainic acid-induced seizure onset. The data were fitted to a sigmoidal dose-response curve with the maximum fixed to 100% and the minimum to 0. The median effective dose (ED50) value was derived from the best-fit equation.
To further define the differences in the progression of SE induced by LiPilo compared to KA, total power spectrum analysis was performed. For the LiPilo-SE (n = 4), there was a rapid increase in total power that reached a maximum during the first 30 minutes and then remained essentially stable with only a minimal decline over the displayed 3-hour duration (figure 2B). In contrast, total power for the KA-SE (n = 9) did not reach a maximum as quickly and total power remained lower than that observed for LiPilo-SE (p<0.001, Wilcoxon Matched-pairs Signed Rank test).
Given this slower progression to a continuous electrographic seizure, the efficacy and potency of diazepam to terminate KA-SE of 180 minutes in duration was assessed. Sample EEG tracings obtained from a hippocampal depth electrode from a single animal successfully treated with diazepam 10 mg/kg at 180 minutes after seizure onset are shown in figure 2C. The diazepam dose-response of animals seizure-free 30 minutes after treatment without recurrence for KA-SE of 180 minutes in duration is shown in figure 2C. At each dose, 5 animals were treated. Diazepam efficacy was similar to that observed when treatment occurred after 60 minutes of KA-SE onset and there was only minimal shift in potency from 10 mg/kg at 60 minutes after onset to 14 mg/kg for the KA-SE treated at the 180 minute time point (figure 2C).
The surface expression of the GABAR γ2 subunit was reduced after LiPilo-induced SE but not KA-induced SE
The development of benzodiazepine pharmacoresistance during LiPilo-SE has been associated with a reduced surface expression of the benzodiazepine-sensitive γ2 subunit-containing synaptic GABARs 10,12,13. If this modification in the postsynaptic GABAR pool contributes to benzodiazepine pharmacoresistance then the surface expression of the γ2 subunit would be expected to be preserved during KA-SE.
The surface expression of the γ2 subunit was assessed using a biotinylation assay in whole hippocampal slices acutely obtained after 60 minutes of LiPilo-SE, 60 minutes of KA-SE, and 180 minutes of KA-SE. Control animals and SE-treated animals were run in parallel. All hippocampal slices from an individual animal were pooled.
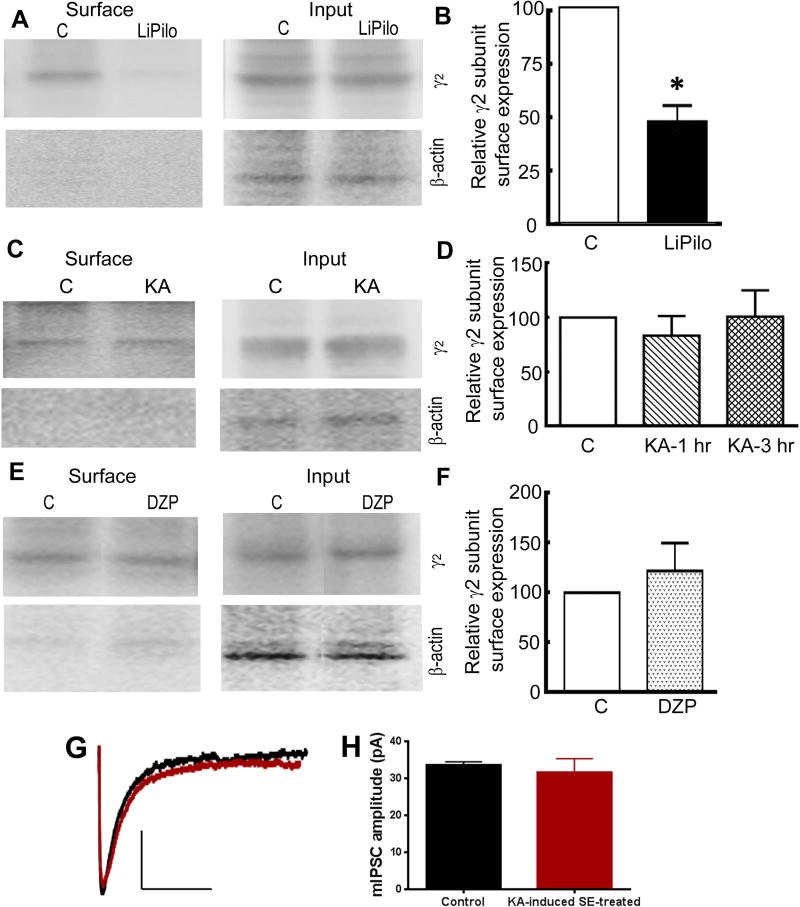
A representative Western blot demonstrating the decrease in the γ2 subunit surface expression following LiPilo-SE compared to the control is displayed in figure 3A. The total γ2 subunit expression in the LiPilo-SE samples was comparable to control. The expression of the γ2 subunit was quantified using scanning densitometry. For the total population of control (n = 6) and LiPilo-SE animals (n = 6), the surface-to-total ratio were 0.64 ± 0.07 and 0.30 ± 0.06, respectively (p<0.005). Therefore, the normalized surface expression of the γ2 subunit in the LiPilo-SE animals was 47 ± 7% of that in controls (Figure 3B).
Figure 3. The hippocampal γ2 subunit surface expression was reduced during LiPilo-induced SE but not during KA-induced SE.
A, Sample Western blots of the surface protein fraction and the total protein fraction (input) of the GABAR γ2 subunit in hippocampal slices acutely obtained from P23 – 25 controls animals (C) and animals in status epilepticus of 1 hour in duration induced by a combination of lithium and pilocarpine (LiPilo). The purity of surface proteins was checked in each assay by confirming the absence of β-actin expression in the surface protein fraction. B, The surface expression of the γ2 subunit presented as mean ± SEM of the ratio of the surface-to-total expression in control slices and LiPilo-induced SE-treated slices acutely obtained one hour after seizure onset. n=6, * p < 0.05. C, Sample Western blots of the surface protein fraction and the total protein fraction of the GABAR γ2 subunit in hippocampal slices acutely obtained from P23 – 25 control animals (C) and animals in status epilepticus of 1 hour in duration induced by kainic acid (KA). The purity of surface proteins was checked in each assay by confirming the absence of β-actin expression in the surface protein fraction. D, The surface expression of the γ2 subunit presented as mean ± SEM of the ratio of the surface-to-total expression in control slices and KA-induced SE-treated slices obtained either 1 hour or 3 hours after seizure onset. n=5 at 1 hour post-KA-induced SE and n=6 at 3 hours post-KA-induced SE. E, Sample Western blots of the surface protein fraction and total protein fraction (input) of the GABAR γ2 subunit in hippocampal slices acutely obtained from P23 – 25 control animals (C) or lithium-pretreated animals that received diazepam (10 mg/kg) 10 minutes prior to the injection of pilocarpine (DZP). The slices were obtained 90 minutes after the administration of the pilocarpine. The purity of surface proteins was checked in each assay by confirming the absence of β-actin expression in the surface protein fraction. F, The surface expression of the γ2 subunit presented as mean ± SEM of the ratio of the surface-to-total expression in control slices and DZP slices, n=5. G, Averaged mIPSC traces from a control (black) DGC and a KA-induced SE-treated (red) DGC voltage clamped to a holding potential of −60 mV. The median amplitude for the mIPSCs recorded from the control neuron displayed here was 34 pA (black tracing) and that for the KA-induced SE-treated neurons displayed here was 31 pA (red tracing). H, A bar graph displaying the mean of the median mIPSC amplitude for the population of control and KA-induced SE-treated DGCs. N=8 neurons from 4 control animals and N=8 neurons from 5 SE animals.
In contrast to the LiPilo-SE animals, the surface expression of the γ2 subunit in the acutely obtained hippocampal slices from KA-SE animals was not altered. For the total population of control (n = 5) and KA-SE animals (n = 5), the surface-to-total ratio were 0.52 ± 0.05 and 0.41 ± 0.08, respectively (p>0.05). Thus, the normalized surface expression of the γ2 subunit in the KA-SE animals was 83 ± 18% of that in controls (figure 3D). The surface and total expression of the γ2 subunit was also measured in hippocampal slices acutely obtained after KA-SE of 3 hours in duration. For the total population of 5 KA-SE animals, the surface-to-total ratio was 0.48 ± 0.07 (100 ± 24% of controls, n=6, p > 0.05; figure 3D). Thus, even 3 hours after onset, the surface expression of the GABAR γ2 subunit was not decreased.
The change in the γ2 subunit surface expression was previously correlated with a decrease in the post-synaptic response to the presynaptic release of GABA 10,12,13. Receptor internalization requires lateral movement of the receptor out of the synapse prior to internalization 22. The finding that γ2 subunit surface expression was not reduced during KA-SE does not exclude the possibility that receptors had moved out of the synapse to an extrasynaptic location but not been internalized. To investigate this possibility, mIPSCs were recorded from dentate granule cells (DGCs) in hippocampal slices acutely obtained after 3 hours of KA-SE and the mIPSC amplitude was compared to that recorded from DGCs in hippocampal slices obtained from control animals. Representative “averaged” mIPSCs for a control neuron and KA-SE-treated neuron are shown in figure 3G. For a population of 8 control neurons (4 animals) and 8 KA-SE-treated neurons (5 animals), the mIPSC amplitude were similar, 34.2 ± 2.4 pA and 31.5 ± 3.8 pA, respectively (p > 0.05; figure 3H). For the control and KA-SE-treated neuron populations, the charge transfer was also similar (940.2 ± 203.6 pC vs. 828.4 ± 143.9 pC, p > 0.05). This complementary experiment demonstrates that the GABAR population present at the synapse was preserved during KA-SE.
These findings raised the possibility that the altered GABAR surface expression may be a direct, seizure-independent effect of lithium and pilocarpine. To address this possibility, the γ2 subunit surface expression was compared in control animals and LiPilo-treated animals that received a single dose of diazepam (10 mg/kg) 10 minutes before the injection of pilocarpine. These animals did not develop seizures (data not shown). The hippocampal slices were acutely obtained 90 minutes after pilocarpine injection, the approximate duration after pilocarpine injection for the experiments using the LiPilo-SE animals. A representative Western blot displaying the surface and total expression for a control animal and LiPilo-treated animal in which the seizure was prevented by diazepam (DZP) are displayed in figure 3E. For the total population of control (n = 5) animals and diazepam-treated animals (n = 5), the surface-to-total expression was 0.54 ± 0.09 and 0.72 ± 0.2, respectively. Therefore, as shown in figure 3F, the normalized γ2 subunit surface expression was similar to that of the control animals (p > 0.05). Therefore, the change in the γ2 subunit surface expression was most likely in response to the seizure and not a direct effect of the lithium and pilocarpine.
Changes in the surface expression of the Kv4.2 potassium channel during SE are also model-dependent
The findings above demonstrate that activity-dependent GABAR trafficking during SE is, in part, dependent on etiology. A prior study demonstrated a decrease in the surface expression of Kv4.2 after KA-SE 23. To determine if there was a similar etiological influence on the surface expression of these channels during SE, Kv4.2 surface expression was measured in hippocampal slices acutely obtained from animals after 1 hour of LiPilo-SE and after 3 hours of KA-SE and compared to the surface expression of these channels in hippocampal slices obtained from controls.
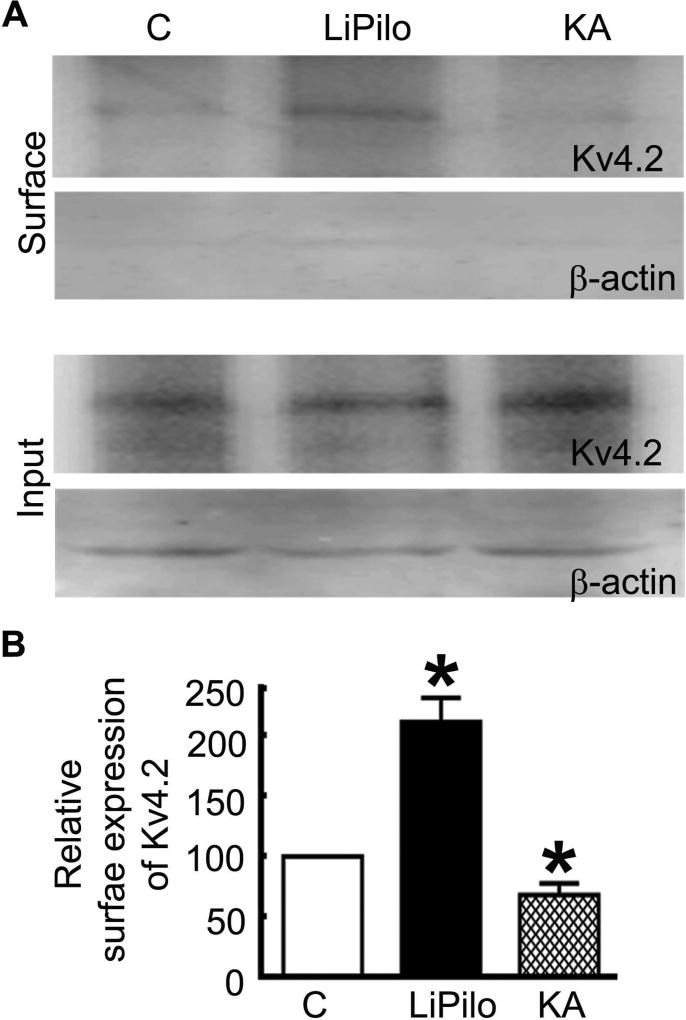
A representative Western blot for the surface and total expression of Kv4.2 demonstrating an increase in the surface expression of Kv4.2 after LiPilo-SE and a decrease after KA-SE compared to a control is displayed in figure 4A. The total expression of Kv4.2 was similar for the control and SE-treated animals. Kv4.2 expression was quantified using scanning densitometry. For the total population of control (n = 6), LiPilo-SE (n =6), and KA-SE (n = 6), the surface-to-total Kv4.2 expression ratios were 0.42 ± 0.06, 0.60 ± 0.08, and 0.27 ± 0.04, respectively. The normalized Kv4.2 expression after LiPilo-SE was 143 ± 20% of controls (p <0.05; figure 4B) and the normalized Kv4.2 expression after KA-SE was 67 ± 10% that of controls (p<0.05; figure 4B). Thus, the surface expression of Kv4.2 was also differentially regulated in LiPilo- and KA-SE.
Figure 4. The surface expression of Kv4.2 was increased during LiPilo-induced SE but reduced during KA-induced SE.
A, Sample Western blots of the surface protein fraction and the total protein fraction (input) of the potassium channel Kv4.2 in hippocampal slices acutely obtained from P23-25 control animals (C), animals in status epilepticus of 1 hour in duration induced by a combination of lithium and pilocarpine (LiPilo), and animals in status epilepticus of 3 hours in duration induced by kainic acid (KA). The purity of surface proteins was checked in each assay by confirming the absence of β-actin expression in the surface protein fraction. B, The surface expression of the Kv4.2 subunit presented as mean ± SEM of the ratio of the surface-to-total expression in slices obtained from control animals (C), LiPilo-SE-treated animal (LiPilo), and KA-SE-treated animals (KA). n=6 each, * p < 0.05.
Phosphorylation-dependent regulation of GABARs during SE is site-specific
The β3 and γ2 GABAR subunits contain putative AP2 binding sites, which when dephosphorylated can interact with the AP2 domain of clathrin leading to receptor internalization 24. A prior study 13 demonstrated that the β3 site is dephosphorylated during Pilo-induced SE. Other studies have demonstrated that phosphatase inhibition can block activity-dependent GABAR trafficking during SE 11,25. What is not known is whether the dephosphorylation of the β subunit during SE resulted from a promiscuous process that leads to dephosphorylation of all the receptor’s phosphorylation sites or site-directed process that leads to post-translational changes at a limited number of phosphorylation sites. To confirm and extend that prior finding 13, the phosphorylation of the β3 (S408/409) and γ2 (S327) subunits was determined after LiPilo-SE of 1 hour and KA-SE of 3 hours in duration.
A comparison of SE-induced changes in the expression of pS408/409β3 and pS327γ2 in hippocampal slices acutely obtained in animals in LiPilo- and KA-SE is displayed in figure 5. As with a prior study 13, there was a reduction in the phosphorylated fraction of the β3 GABAR subunit in hippocampal proteins obtained from LiPilo-SE animals. For 6 experimental replicates, the pS408/409β3-to-total-β3 expression following LiPilo-SE was only 39 ± 3% of the expression seen in control animals (N=7, p < 0.05; Figure 5A, B).
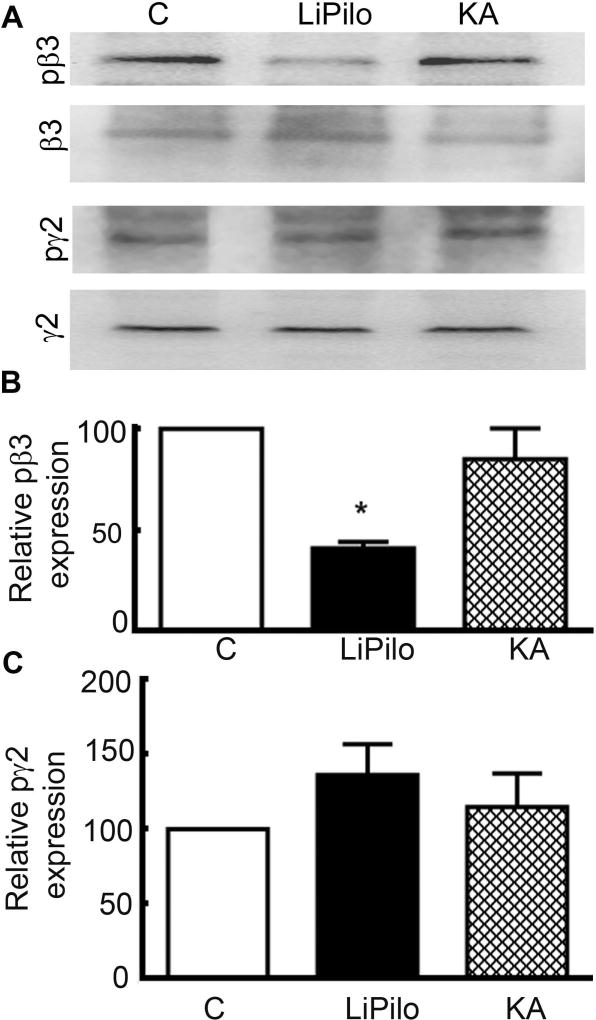
Figure 5. The phosphorylation of β3 subunit was decreased in animals in LiPilo-induced SE, whereas the phosphorylation of β3 was unaltered during KA-induced SE.
A, Sample Western blot of the fraction of phosphorylated GABAR β3 subunit (S408/409), GABAR β3 subunit, phosphorylated GABAR γ2 subunit (S327), and GABAR γ2 subunit in whole hippocampal slices acutely obtained from control animals (C), animals in status epilepticus of 1 hour in duration induced by the combination of lithium and pilocarpine (LiPilo), and animals in status epilepticus of 3 hours in duration induced by kainic acid (KA). In the representative blot shown in this figure, the pS408/409β3 to total β3 expression in the LiPilo-induced SE animals was 0.03 compared to 0.13 for the control (C). B, Phosphorylated GABAA receptor β3 subunit expression normalized to total GABAR β3 subunit expression presented as mean ± SEM in hippocampal slices obtained from control animals (C), LiPilo-induced SE-treated animals (LiPilo), and KA-induced SE-treated animals (KA). n=7 each for pβ3 and n=7 for pγ2 Li-pilo and n=5 for pγ2 KA, * p < 0.05. C, Phosphorylated GABAA receptor γ2 subunit normalized to total GABAR γ2 subunit expression presented as mean ± SEM in whole hippocampal slices obtained from control animals (C), LiPilo-induced SE-treated animals (LiPilo), and KA-induced SE-treated animals (KA). The reactivity of the pS408/409β3 and pS327γ2 antibodies for the phosphorylated forms of the subunits was confirmed using brain tissue lysates treated with λ-phosphatase (1 µg/100 µg protein) for 30 min at 37°C. Treatment with λ-phosphatase reduced the phosphorylated form of the GABAR subunit; the immunoreactivity of pS04/409β3 and pS327γ2 antibodies in the λ-phosphatase-treated lysates was lower than that in the untreated lysates. For 3 experimental replicates, the pS408/409β3 expression in the λ-phosphatase-treated lysate was 28 ± 8.5% of that in the untreated lysate and pS327γ2 expression in the λ-phosphatase-treated lysate was 39 ± 12% of that in the untreated lysates (p < 0.05; data not shown).
To address the question of site-specificity of post-translational modifications during SE, the ratio of the γ2 subunit phosphorylated at serine 327 (S327) to the total γ2 fraction following 1 hour of LiPilo-SE was compared to controls. Across 7 experimental replicates, the ratio of the pS327γ2-to-total-γ2 expression in the LiPilo-SE animals was 131 ± 19% of that in controls (p > 0.05; figure 5A, C). This result suggests that post-translational modifications of the GABAR population is not the result a non-specific process but a site-specific process that targets only a subset of the receptor’s phosphorylation sites.
In contrast to these results obtained following 1 hour of LiPilo-SE, the phosphorylated fraction of both the β3 and γ2 subunit in whole hippocampal slices obtained from KA-SE animals was unchanged. For 7 experimental replicates, the pS408/409β3-to-total-β3 expression was also similar to control (83 ± 13% of control, n=7, p>0.05, ANOVA; figure 5A, B). For 5 experimental replicates, the pS327γ2-to-total-γ2 ratio was 115 ± 17% that of controls (p> 0.05; figure 5A, C). These findings are concordant with the absence of a change in the GABAR surface expression after 3 hours of KA-SE.
Discussion
The novel findings of this study are (1) diazepam remained a potent and effective agent for terminating KA-SE of 1 and 3 hours in duration, (2) the surface expression of the benzodiazepine-sensitive γ2 subunit and GABA-mediated synaptic inhibition were not reduced as a result of KA-SE, and (3) the change in the surface expression of the Kv4.2 potassium channel was differentially regulated during LiPilo- and KA-SE.
This study confirms previous findings of reduced potency of diazepam in terminating late LiPilo-SE with an associated decrease in the surface expression of the benzodiazepine-sensitive γ2 subunit-containing GABARs resulting from a dephosphorylation of a β subunit 8,10,11,13,16. It extends those findings by demonstrating that the receptor’s post-translational modification, which is permissive for the receptor’s internalization, results from a site-directed process and not the indiscriminate removal of all phosphate groups on all subunits.
Preserved GABA-mediated synaptic inhibition during KA-SE
Successful seizure termination in this study was defined as the seizure stopping within 30 minutes without recurrence for an additional 90 minutes. This criterion was based on diazepam’s pharmacokinetic properties that result in this medications redistribution leading to a lowering of its initial high brain concentration such that it has a shorter duration of action and the potential for seizure recurrence 26,27. Based on this criterion, the prolonged 1 and 3 hour KA-seizures were responsive to diazepam and benzodiazepine-pharmacoresistance was not observed. This finding combined with the preserved surface expression of the benzodiazepine-sensitive γ2 subunit-containing GABAA receptors and preserved post-synaptic response to GABA demonstrated that the post-synaptic GABA-mediated synaptic apparatus was maintained during KA-SE.
The potential that the prolonged KA-induced seizures recurred after the EEG recording session was completed cannot be excluded. Recurrence of seizures after successful termination has been reported for both short and long KA-induced seizure durations 27, was the primary reason in our study for failed termination after early treatment of KA-induced seizures, and may be the reason that one study 28 in which recording after diazepam was performed for up to 48 hours concluded that benzodiazepine-pharmacoresistance was not dependent on etiology.
Distinct pattern of seizure evolution between LiPilo- and KA-SE
In comparison to LiPilo-SE, a slower progression of KA-SE through the electrographic SE stages was observed. Further, analysis of EEG power during the LiPilo-SE and KA-SE demonstrated clear differences in seizure progression suggesting etiology may influence neuronal activation patterns, which may have a subsequent effect on which and the frequency with which certain neurotransmitters are released and which second messenger systems are activated. These differences may not only influence the response to treatment observed in this study but could also account for the well-described difference in functional anatomy and histopathological outcome observed following SE in these two animal models 29,30.
To assure that the changes in the surface expression of the γ2 subunit-containing GABARs was not a direct pharmacological effect of the lithium and pilocarpine, the receptor surface expression was measured in hippocampal slices acutely obtained from LiPilo-treated animals in which the seizure was prevented by the administration of diazepam prior to the pilocarpine. In this situation, in the absence of the seizure, a change in the surface expression was not observed. This finding in combination with similar experiments performed by Wasterlain and colleagues 12 provides additional evidence for neuronal activity-dependent modulation of GABAR trafficking during seizures.
The extracellular glutamate concentration and the extent of calcium influx can lead to differential signaling and surface membrane stability of GABARs. For example, brief application of NMDA increases GABAR surface expression in cultured hippocampal neurons, whereas a brief application of glutamate reduced GABAR surface expression 31,32. These differences were attributed to differences in the association of CaMKII with GABARs. Furthermore, a sustained calcium influx through NMDAR activation is proposed to activate calcineurin leading to destabilization of surface GABARs. In contrast, a moderate increase in glutamate, which can activate metabotrophic glutamate receptors, stabilizes surface GABARs 33. Future studies will be required to determine if the model-dependent differences that were observed in the phosphorylation and surface expression of GABARs in these two models are the result of differences in excitatory amino acid release, differences in the excitatory amino acid receptors activated, or differences in calcium influx that occurred during the SE induced by LiPilo vs KA.
Internalization of GABARs and dephosphorylation of specific residues
Post-translational modifications such as phosphorylation and dephosphorylation of serine/threonine residues on α1, β2/3, α4, and γ2 subunits are critical in regulating the pool of functional GABARs present on the cell surface under pathophysiological conditions 34. Phosphorylation of S408/409 residues on the β2/3 subunits by PKC and CaMKII increases the GABAR surface expression whereas dephosphorylation of these residues by protein phosphatases increases the interaction of GABARs with the AP2 domain of clathrin-endocytic machinery leading to receptor internalization 13,24. A dephosphorylation of β3 S408/409 residues in the adult mice in SE was associated with reduced GABAR surface expression 13. In a prior study, blockade of protein phosphatases was efficacious in preventing or recovering the reduced expression of functional GABARs during in vivo or in vitro SE 11. The observed dephosphorylation of the β3 subunit in the hippocampi of LiPilo-SE animals but not in KA-SE animals point to a phosphorylation-dependent mechanism underlying the differential changes in the surface expression of GABARs seen in the two models of SE employed in this study.
In contrast to dephosphorylation of β3 subunits in the hippocampi of LiPilo-SE animals, the phosphorylation of γ2 subunits on S327 remained unaltered in both models of SE. The γ2 subunit S327 is a target of calcineurin, which is activated during SE 35,36. These findings suggest that protein phosphatase activation does not trigger a general dephosphorylation of different proteins. The mechanisms that protect phosphorylated residues on the γ2 subunit are currently unknown. In neurotransmitter receptors assembled from heteromeric subunit combinations, one of the subunits often plays a dominant role in regulating the surface membrane trafficking 37,38; it appears that the dephosphorylation of β3 subunits plays a prominent role in the internalization of GABARs in SE irrespective of post-translational modification of other subunits.
Model-specific changes in the expression of Kv4.2 channels
Rapidly activating K+ current (A-type current) mediated by voltage-gated potassium channel such as Kv4.2 regulates neuronal activity. The surface expression of Kv4.2 channels was also differentially regulated in LiPilo-SE and KA-SE animals; it was reduced in KA-SE animals as has been reported before 23 but increased in LiPilo-SE animals. Whether this upregulation follows a similar time course as that of the reduction in GABAR-mediated inhibition, which occurs as early as 15 min after the onset of SE 39, is not known. But it could compensate for the reduced GABAR-mediated inhibition. Phosphorylation of Kv4.2 channels by EKR1/2 MAP kinases, which are activated during SE 40,41, reduces channel surface expression 23. The activation of protein phosphatases during SE may also play a role in dephosphorylation and increased surface expression of Kv4.2 channels during LiPilo-SE. Taken together these findings show etiology-dependent differences in the regulation of GABARs and KV4.2 channel.
Clinical Implications
Although triggered by chemoconvulsants, LiPilo-SE and KA-SE share commonalities with SE in the human including changes in cerebral blood flow, breakdown of the blood brain barrier, cerebral edema, neuronal injury, and the later development of recurrent, unprovoked seizures and cognitive deficits 42–48. Therefore, studies of SE in these models allows for an approximation of the molecular and cellular mechanisms that may occur during SE in the human. Certainly, studies that serve to confirm and extend these findings will seek additional SE models in which SE is triggered by etiologies that more commonly occur in the human.
Independent of etiology, all prolonged seizures of SE are self-sustaining and share a low probability of spontaneous seizure termination 49. Our findings of model-dependent differences in the response to diazepam, activity-dependent trafficking, and synaptic inhibition provide additional support for the hypothesis that a modification in the post-synaptic GABAR population contributes to the development of benzodiazepine pharmacoresistance. These findings also support a novel hypothesis that the molecular changes that occur during SE, changes that may impact response to treatment, are also dependent on etiology. A mechanism that depends on both etiology and duration may explain why some seizures remain responsive to benzodiazepines and other agents -- even at the most prolonged durations such as in the Veterans Affairs Status Epilepticus Cooperative Study in which 10 to 30% of patients in the subtle SE group responded to treatment -- and why some etiologies are more likely to result in SE episodes that are resistant to treatment 3. Until the role of etiology in determining response is further defined with the potential future development of etiology-dependent protocols 50 these results support current SE treatment protocols in which a 1,4-benzodiazepine is recommended as the first line-treatment independent of seizure duration and etiology.
Acknowledgments
This work was supported by NS067439 from the National Institute of Neurological Disorders and Stroke (NINDS).
Footnotes
Author Contributions:
SJ, KR, KMH, SJC, HPG contributed to the study concept and design; SJ, KR, KMH, SJC, HPG performed data acquisition and analysis; and SJ, KR, HPG contributed to the drafting of the manuscript and preparing the figures.
Potential Conflicts of Interest: Nothing to report
References
- 1.Goodkin HP, Kapur J. The impact of diazepam's discovery on the treatment and understanding of status epilepticus. Epilepsia. 2009;50:2011–2018. doi: 10.1111/j.1528-1167.2009.02257.x. [DOI] [PubMed] [Google Scholar]
- 2.Silbergleit R, Lowenstein D, Durkalski V, et al. RAMPART (Rapid Anticonvulsant Medication Prior to Arrival Trial): A double-blind randomized clinical trial of the efficacy of intramuscular midazolam versus intravenous lorazepam in the prehospital treatment of status epilepticus by paramedics. Epilepsia. 2011;52:45–47. doi: 10.1111/j.1528-1167.2011.03235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Treiman DM, Meyers PD, Walton NY, et al. A comparison of four treatments for generalized convulsive status epilepticus. Veterans Affairs Status Epilepticus Cooperative Study Group. N Engl J Med. 1998;339:792–798. doi: 10.1056/NEJM199809173391202. [DOI] [PubMed] [Google Scholar]
- 4.Chateauneuf AL, Moyer JD, Jacq G, et al. Super-refractory status epilepticus: epidemiology, early predictors, and outcomes. Intensive Care Medicine. 2017 doi: 10.1007/s00134-017-4837-6. [DOI] [PubMed] [Google Scholar]
- 5.Delaj L, Novy J, Ryvlin P, et al. Refractory and super-refractory status epilepticus in adults: a 9-year cohort study. Acta Neurologica Scandinavica. 2017;135:92–99. doi: 10.1111/ane.12605. [DOI] [PubMed] [Google Scholar]
- 6.Holtkamp M, Othman J, Buchheim K, et al. Predictors and prognosis of refractory status epilepticus treated in a neurological intensive care unit. Journal of Neurology, Neurosurgery &amp; Psychiatry. 2005;76:534. doi: 10.1136/jnnp.2004.041947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Novy J, Logroscino G, Rossetti AO. Refractory status epilepticus: A prospective observational study. Epilepsia. 2010;51:251–256. doi: 10.1111/j.1528-1167.2009.02323.x. [DOI] [PubMed] [Google Scholar]
- 8.Kapur J, Macdonald RL. Rapid seizure-induced reduction of benzodiazepine and Zn2+ sensitivity of hippocampal dentate granule cell GABAA receptors. J Neurosci. 1997;17:7532–7540. doi: 10.1523/JNEUROSCI.17-19-07532.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walton NY, Treiman DM. Response of status epilepticus induced by lithium and pilocarpine to treatment with diazepam. Exp Neurol. 1988;101:267–275. doi: 10.1016/0014-4886(88)90010-6. [DOI] [PubMed] [Google Scholar]
- 10.Goodkin HP, Joshi S, Mtchedlishvili Z, et al. Subunit-specific trafficking of GABAA receptors during status epilepticus. J Neurosci. 2008;28:2527–2538. doi: 10.1523/JNEUROSCI.3426-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joshi S, Rajasekaran K, Hawk KM, et al. Phosphatase inhibition prevents the activity-dependent trafficking of GABAA receptors during status epilepticus in the young animal. Epilepsia. 2015;56:1355–1365. doi: 10.1111/epi.13098. [DOI] [PubMed] [Google Scholar]
- 12.Naylor DE, Liu H, Wasterlain CG. Trafficking of GABAA receptors, loss of inhibition, and a mechanism for pharmacoresistance in status epilepticus. J Neurosci. 2005;25:7724–7733. doi: 10.1523/JNEUROSCI.4944-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terunuma M, Xu J, Vithlani M, et al. Deficits in phosphorylation of GABAA receptors by intimately associated protein kinase C activity underlie compromised synaptic inhibition during status epilepticus. J Neurosci. 2008;28:376–384. doi: 10.1523/JNEUROSCI.4346-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 15.Raol YH, Lapides DA, Keating JG, et al. A KCNQ channel opener for experimental neonatal seizures and status epilepticus. Ann Neurol. 2009;65:326–336. doi: 10.1002/ana.21593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodkin HP, Liu X, Holmes GL. Diazepam terminates brief but not prolonged seizures in young, naive rats. Epilepsia. 2003;44:1109–1112. doi: 10.1046/j.1528-1157.2003.62402.x. [DOI] [PubMed] [Google Scholar]
- 17.Jones DM, Esmaeil N, Maren S, et al. Characterization of pharmacoresistance to benzodiazepines in the rat Li-pilocarpine model of status epilepticus. Epilepsy Res. 2002;50:301–312. doi: 10.1016/s0920-1211(02)00085-2. [DOI] [PubMed] [Google Scholar]
- 18.Mazarati AM, Baldwin RA, Sankar R, et al. Time-dependent decrease in the effectiveness of antiepileptic drugs during the course of self-sustaining status epilepticus. Brain Res. 1998;814:179–185. doi: 10.1016/s0006-8993(98)01080-4. [DOI] [PubMed] [Google Scholar]
- 19.Todorovic MS, Cowan ML, Balint CA, et al. Characterization of status epilepticus induced by two organophosphates in rats. Epilepsy Research. 2012;101:268–276. doi: 10.1016/j.eplepsyres.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang NC, Good LB, Marsh ST, et al. EEG stages predict treatment response in experimental status epilepticus. Epilepsia. 2009;50:949–952. doi: 10.1111/j.1528-1167.2008.01911.x. [DOI] [PubMed] [Google Scholar]
- 21.Treiman DM, Walton NY, Kendrick C. A progressive sequence of electroencephalographic changes during generalized convulsive status epilepticus. Epilepsy Res. 1990;5:49–60. doi: 10.1016/0920-1211(90)90065-4. [DOI] [PubMed] [Google Scholar]
- 22.Jacob TC, Moss S, Jurd R. GABAA receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat Rev Neurosci. 2008;9:331–343. doi: 10.1038/nrn2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lugo JN, Barnwell LF, Ren Y, et al. Altered phosphorylation and localization of the A-type channel, Kv4.2 in status epilepticus. J Neurochem. 2008;106:1929–1940. doi: 10.1111/j.1471-4159.2008.05508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kittler JT, Chen G, Honing S, et al. Phospho-dependent binding of the clathrin AP2 adaptor complex to GABAA receptors regulates the efficacy of inhibitory synaptic transmission. Proc Nat Aca Sci. 2005;102:14871–14876. doi: 10.1073/pnas.0506653102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eckel R, Szulc B, Walker MC, et al. Activation of calcineurin underlies altered trafficking of α2 subunit containing GABAA receptors during prolonged epileptiform activity. Neuropharmacology. 2015;88:82–90. doi: 10.1016/j.neuropharm.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friedman H, Abernethy DR, Greenblatt DJ, et al. The pharmacokinetics of diazepam and desmethyldiazepam in rat brain and plasma. Psychopharmacology (Berl) 1986;88:267–270. doi: 10.1007/BF00180822. [DOI] [PubMed] [Google Scholar]
- 27.Fritsch B, Stott JJ, Joelle Donofrio J, et al. Treatment of early and late kainic acid-induced status epilepticus with the noncompetitive AMPA receptor antagonist GYKI 52466. Epilepsia. 2010;51:108–117. doi: 10.1111/j.1528-1167.2009.02205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hasson H, Kim M, Moshé SL. Effective treatments of prolonged status epilepticus in developing rats. Epilepsy & Behavior. 2008;13:62–69. doi: 10.1016/j.yebeh.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Covolan L, Mello LEAM. Temporal profile of neuronal injury following pilocarpine or kainic acid-induced status epilepticus. Epilepsy Res. 2000;39:133–152. doi: 10.1016/s0920-1211(99)00119-9. [DOI] [PubMed] [Google Scholar]
- 30.Rajasekaran K, Zanelli SA, Goodkin HP. Lessons from the laboratory: the pathophysiology, and consequences of status epilepticus. Semin Pediatr Neurol. 2010;17:136–143. doi: 10.1016/j.spen.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marsden KC, Beattie JB, Friedenthal J, et al. NMDA receptor activation potentiates inhibitory transmission through GABA receptor-associated protein-dependent exocytosis of GABAA receptors. J Neurosci. 2007;27:14326–14337. doi: 10.1523/JNEUROSCI.4433-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marsden KC, Shemesh A, Bayer KU, et al. Selective translocation of Ca2+/calmodulin protein kinase IIα (CaMKIIα) to inhibitory synapses. Proc Nat Aca Sci. 2010;107:20559–20564. doi: 10.1073/pnas.1010346107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bannai H, Lqvi S, Schweizer C, et al. Activity-dependent tuning of inhibitory neurotransmission based on GABAA receptor diffusion dynamics. Neuron. 2009;62:670–682. doi: 10.1016/j.neuron.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 34.Vithlani M, Terunuma M, Moss SJ. The dynamic modulation of GABAA receptor trafficking and its role in regulating the plasticity of inhibitory synapses. Physiol Rev. 2011;91:1009–1022. doi: 10.1152/physrev.00015.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kurz JE, Sheets D, Parsons JT, et al. A significant increase in both basal and maximal calcineurin activity in the rat pilocarpine model of status epilepticus. J Neurochem. 2001;78:304–315. doi: 10.1046/j.1471-4159.2001.00426.x. [DOI] [PubMed] [Google Scholar]
- 36.Wang A, Chi Z, Wang S, et al. Calcineurin-mediated GABAA receptor dephosphorylation in rats after kainic acid-induced status epilepticus. Seizure. 2009;18:519–523. doi: 10.1016/j.seizure.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 37.Sheng M, Lee SH. AMPA receptor trafficking and the control of synaptic transmission. Cell. 2001;105:825–828. doi: 10.1016/s0092-8674(01)00406-8. [DOI] [PubMed] [Google Scholar]
- 38.Joshi S, Keith KJ, Ilyas A, et al. GABAA receptor membrane insertion rates are specified by their subunit composition. Mol Cell Neurosci. 2013;56:201–211. doi: 10.1016/j.mcn.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun HY, Goodkin HP. The pervasive reduction of GABA-mediated synaptic inhibition of principal neurons in the hippocampus during status epilepticus. Epilepsy Res. 2016;119:30–33. doi: 10.1016/j.eplepsyres.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berkeley JL, Decker MJ, Levey AI. The role of muscarinic acetylcholine receptor-mediated activation of extracellular signal-regulated kinase 1/2 in pilocarpine-induced seizures. J Neurochem. 2002;82:192–201. doi: 10.1046/j.1471-4159.2002.00977.x. [DOI] [PubMed] [Google Scholar]
- 41.Kim YS, Hong KS, Seong YS, et al. Phosphorylation and activation of mitogen-activated protein kinase by kainic acid-induced seizure in rat hippocampus. Biochem Biophys Res Commun. 1994;202:1163–1168. doi: 10.1006/bbrc.1994.2050. [DOI] [PubMed] [Google Scholar]
- 42.Engelhorn T, Weise J, Hammen T, et al. Early diffusion-weighted MRI predicts regional neuronal damage in generalized status epilepticus in rats treated with diazepam. Neurosci Let. 2007;417:275–280. doi: 10.1016/j.neulet.2007.02.072. [DOI] [PubMed] [Google Scholar]
- 43.Sperk G, Lassmann H, Baran H, et al. Kainic acid induced seizures: neurochemical and histopathological changes. Neuroscience. 1983;10:1301–1315. doi: 10.1016/0306-4522(83)90113-6. [DOI] [PubMed] [Google Scholar]
- 44.Zucker DK, Wooten GF, Lothman EW. Blood-brain barrier changes with kainic acid-induced limbic seizures. Expet Neurol. 1983;79:422–433. doi: 10.1016/0014-4886(83)90223-6. [DOI] [PubMed] [Google Scholar]
- 45.Lassmann H, Petsche U, Kitz K, et al. The role of brain edema in epileptic brain damage induced by systemic kainic acid injection. Neuroscience. 1984;13:691–704. doi: 10.1016/0306-4522(84)90089-7. [DOI] [PubMed] [Google Scholar]
- 46.Nakasu Y, Nakasu S, Kizuki H, et al. Changes in water diffusion of rat limbic system during status epilepticus elicited by kainate. Psychiatry and Clinical Neurosciences. 1995;49:S228–S230. doi: 10.1111/j.1440-1819.1995.tb02184.x. [DOI] [PubMed] [Google Scholar]
- 47.Hsu YH, Lee WT, Chang C. Multiparametric MRI evaluation of kainic acid-induced neuronal activation in rat hippocampus. Brain. 2007 doi: 10.1093/brain/awm207. awm207. [DOI] [PubMed] [Google Scholar]
- 48.Gorter JA, van Vliet EA, Aronica E. Status epilepticus, blood-brain barrier disruption, inflammation, and epileptogenesis. Epilepsy Behav. 2015;49:13–16. doi: 10.1016/j.yebeh.2015.04.047. [DOI] [PubMed] [Google Scholar]
- 49.Trinka E, Cock H, Hesdorffer D, et al. A definition and classification of status epilepticus GÇô Report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia. 2015;56:1515–1523. doi: 10.1111/epi.13121. [DOI] [PubMed] [Google Scholar]
- 50.Riviello JJ, Jr, Holmes GL. The treatment of status epilepticus. Semin Pediatr Neurol. 2004;11:129–138. doi: 10.1016/j.spen.2004.03.011. [DOI] [PubMed] [Google Scholar]