Summary
Besides its essential role in protein synthesis, cysteine plays vital roles in redox homeostasis, being a component of the major antioxidant glutathione and a potent antioxidant by itself. In addition, cysteine undergoes a variety of post-translational modifications which modulate several physiological processes. It is becoming increasingly clear that redox modulated events play important roles not only in peripheral tissues but also in the brain where cysteine disposition is central to these pathways. Dysregulated cysteine metabolism is associated with several neurodegenerative disorders. Accordingly, restoring cysteine balance has therapeutic benefits. This review discusses metabolic signaling pathways pertaining to cysteine disposition in the brain during normal and pathological conditions highlighting recent findings on cysteine metabolism during aging and in neurodegenerative conditions such as Huntington’s disease and molybdenum cofactor deficiency among others.
Keywords: Cysteine, Neurodegeneration, Huntington’s disease, ATF4
Introduction
The brain is one of the most metabolically active organs in the body generating elevated levels of reactive oxygen and nitrogen species and necessitating efficient redox homeostatic controls. Disrupted redox homeostasis plays pivotal roles in disease progression in neurodegenerative disorders including, but not limited to, Huntington’s, Alzheimer’s and Parkinson’s diseases. The major antioxidants glutathione and cysteine are responsible for neutralizing much of the oxidative damage generated. Oxidation of DNA, protein, lipids and carbohydrates has been associated with aging and neurodegenerative conditions, which have been linked to depletion of these versatile sulfur containing molecules [1, 2]. Availability of cysteine is the rate limiting step for glutathione synthesis [3]. Thus, regulated control of cysteine metabolism is central to optimal neuronal function.
Sources of cysteine
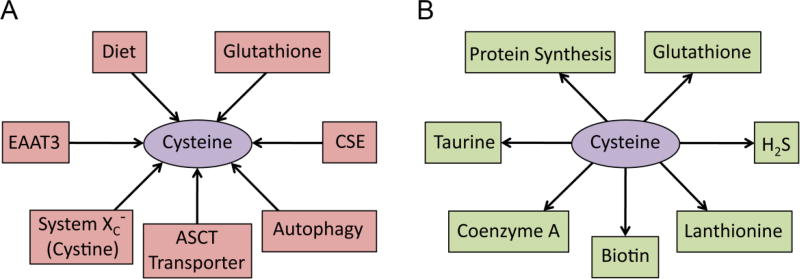
Cells have evolved multiple mechanisms to maintain a constant supply of cysteine, which is utilized for multiple purposes (Figure 1). Cysteine can be obtained from the diet as well as synthesized endogenously by cystathionine γ-lyase (CSE). Cysteine can also be obtained by breakdown of glutathione and proteins by autophagy (Figure 1A). Once generated, cysteine is consumed by various metabolic pathways such as protein synthesis, generation of sulfur containing molecules such as glutathione, taurine, lanthionine, coenzyme A and the gasotransmitter hydrogen sulfide (H2S) (Figure 1B). Interestingly, cysteine is an important part of keratin, the major protein in hair and nails and exoskeleton of several species. Skin and hair contain almost 10–14% cysteine. The cysteine-rich nature of keratin allows the formation of multiple disulfide bonds, which impact the integrity and stability of keratin. Treatment with cysteine donors has been reported to be beneficial in counteracting hair loss. Supplementation of cysteine in the cell line HaCaT enhanced keratin biogenesis [4].
Figure 1. Metabolism of cysteine.
(A) Cysteine is derived from various sources in the brain. It can be obtained from the diet via the transporters system xc−, excitatory amino acid transporter 3 (EAAT3), the alanine, serine, cysteine transporter (ASCT) as well as synthesized endogenous by cystathionine γ-lyase (CSE). Cysteine can also be obtained by breakdown of glutathione and proteins by autophagy. (B) Cysteine is utilized by multiple pathways. Once generated, cysteine is consumed by various metabolic pathways such as protein synthesis, generation of sulfur containing molecules such as glutathione, taurine, lanthionine, coenzyme A and the gasotransmitter hydrogen sulfide (H2S).
Cysteine biosynthesis
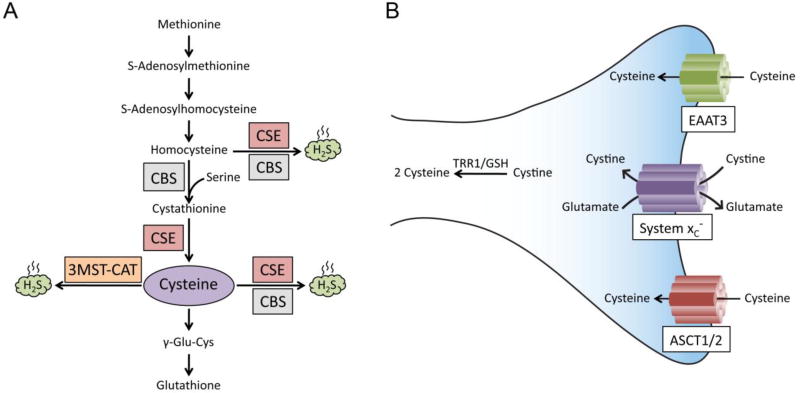
Cysteine, a semi-essential amino acid, can be obtained both from the diet as well as synthesized endogenously via the reverse transsulfuration pathway from methionine [5–8](Figure 2A). Cysteine is generated by the enzyme cystathionase/cystathionine γ-lyase (CSE) from cystathionine, which in turn is generated by the condensation of homocysteine and serine by cystathionine β-synthase (CBS). CSE is the only known biosynthetic enzyme for cysteine in mammals, hence its deficiency leads to a dependence on exogenous cysteine. Mice deleted for CSE lose weight on a cysteine-free diet and die within two weeks [9–11]. In humans, CSE is expressed shortly after birth so that pre-term infants require cysteine supplementation [12, 13]. CSE is highly expressed in the peripheral tissues such as the liver and the gastrointestinal tract. Recent studies have conclusively shown that CSE is also expressed in the brain with major neuroprotective functions [5, 14]. Human CSE exists as a homotetramer with each subunit being 45 kDa and uses pyridoxal 5-phosphate (PLP) as a cofactor bound to each subunit [15]. CSE is a highly inducible enzyme and responds to a wide variety of stimuli ranging from endoplasmic reticulum stress to amino acid deprivation. Depletion of CSE causes oxidative stress, aberrant stress responses, vascular deficits and hyperhomocysteinemia [11, 16, 17].
Figure 2. Cysteine biosynthesis and import.
(A) The reverse transsulfuration pathway. Methionine, derived from the diet, is converted to homocysteine, which is condensed with serine by cystathionine β-synthase (CBS) to generate cystathionine. Cystathionine is acted on by cystathionine γ-lyase (CSE) to generate cysteine. Cysteine is utilized to generate hydrogen sulfide (H2S) by CSE and CBS. H2S can also be generated from homocysteine by CSE and CBS. Cysteine can also be converted to glutathione. In addition, cysteine aminotransferase (CAT), also known as aspartate aminotransferase (AAT) or glutamate oxaloacetate transaminase (GOT) generates 3-mercaptopyruvate from L-cysteine which is utilized by 3-mercaptopyruvate sulfurtransferase (3-MST) to generate H2S in the presence of reducing agents [99] (B) Transport of cyst(e)ine. Cysteine is imported via the excitatory amino acid transporter 3 (EAAT3) and the alanine, serine, cysteine transporter (ASCT). Its oxidized form, cystine, is transported by the transporters system xc−. The EAAT3 and ASCT transporters are Na+-dependent, whereas the system xc− is Na+-independent and Cl−-dependent and functions as an antiporter, exchanging 1 molecule of glutamate for cystine. Once inside the cell, cystine is rapidly reduced to cysteine by either thioredoxin reductase 1 (TRR1) or glutathione (GSH).
Cysteine uptake
In addition to its biosynthesis via the reverse transsulfuration pathway, cysteine and its oxidized form cystine, can be imported into cells though specific transporters (Figure 2B). Cysteine mainly exists as cystine, its dimeric form, in the extracellular space and in diet because of rapid oxidation. In cells, where the environment is reducing, the amino acid exists as cysteine [18]. In neurons, cysteine is taken up by the Excitatory amino acid transporter 3 (EAAT3/EAAC1). Its oxidized form, cystine is imported by the system xc−.
System x−c
System xc− transports cystine in a Na+-independent but chloride dependent manner. The xc− transporter also transports cystathionine, the precursor for cysteine [19].The xc− transporter is a heterodimer composed of disulfide-linked light (xCT/SLC7A11) and heavy chains (4F2hc/CD98/SLC3A2). The xCT subunit is responsible for substrate specificity and is induced in response to a variety of stimuli such as oxidative stress, lipopolysaccharides (LPS) and electrophilic compounds[20]. The heavy chain is required for trafficking of xCT to the plasma membrane. Cystine transported inside the cell is reduced to cysteine by glutathione or thioredoxin reductase 1 (TRR1)[21]. Mice deleted for xCT, the light chain subunit of the xc− transporter, exhibit elevated redox stress and oxidative damage [22]. Moreover, mice treated with sulfasalazine, an inhibitor of the cystine/glutamate antiporter, exhibit myelin disruption and reduction in levels of myelin, which attests to a role for the xc− transporter in oligodendrocytes[23]. The system xc− plays significant roles in the growth and proliferation of cancer cells. Glioma cells rely primarily on cystine uptake via system xc− for their glutathione synthesis. In certain gliomas, cystine is prooncogenic; thus inhibition of the system xc− reduces tumor growth [24]. The most aggressive gliomas have elevated expression of SLC7A11 (xCT transporter) and as the transport of cystine and glutamate are linked, elevated xCT expression leads to increased extracellular glutamate, excitotoxicity and seizures [25]. Other studies have reported that depleting extracellular cysteine and cystine by administration of cyst(e)inase enzyme causes cell cycle arrest and death in cancer cells due to depletion of intracellular glutathione (GSH) and consequent oxidative stress [26]. Thus, the fine balance between cystine import and glutamate export seems to have been perturbed in these gliomas.
System xc− has also been implicated in memory and behavior. Mice deleted for xCT subunit had decreased extracellular glutamate in the hippocampus and impaired spatial memory, which was more pronounced in younger mice, and susceptibility to limbic seizures [27]. These findings seem to result from long-term adaptive changes to xCT deficiency or the reduction of chronic excitotoxicity due to lower extracellular glutamate levels
System xAG: The Excitatory Amino Acid transporters
Excitatory amino acid transporters (EAATs) are a class of neuronal transporters involved in the transport of glutamate and or aspartate. Of these, EAAT3, also known as EAAC1/SLC1A1, exchanges cysteine for glutamate. Similarly, EAAT2 transports cysteine in exchange for aspartate. Accordingly, glutamate and aspartate inhibit cysteine entry into neurons and are responsible for resulting oxidative damage. Mice lacking EAAC1 have decreased neuronal glutathione and exhibit age-dependent neurodegeneration with enlarged ventricles, cortical thinning and elevated oxidative stress related damage [28]. EAAC1 deleted mice exhibit age-dependent loss of dopaminergic neurons in the substantia nigra and heightened oxidative stress. In Huntington’s disease (HD), decreased cell surface trafficking of EAAC1 occurs leading to decreased cysteine intake [29].
The ASCT transporters
ASCT transporters are stereospecific and carry out Na+-dependent exchange of small neutral amino acids such as L-Ala, L-serine, L-cysteine and L-threonine. Two transporters, ASCT1 (SLC1A4) and ASCT2 (SLC1A5), are also involved in cysteine transport. Of these, ASCT1 transports cysteine and is enriched in glial cells expressing the biosynthetic enzyme for serine, 3-phosphoglycerate dehydrogenase [30, 31]. ASCT2 is not considered to be a major contributor to cysteine transport, but its activity can be modulated by cysteine [32, 33]. ASCT2 is highly expressed in peripheral tissues such as the lung, where it maintains intracellular concentrations of neutral amino acids. In addition to L-alanine, L-serine and L-threonine, ASCT2 also transports L-glutamine and L-asparagine with high affinity, and certain other neutral amino acids with lower affinity, such as methionine, leucine, glycine and valine [34].
Glutathione as a source of cysteine
Glutathione is a tripeptide of glycine, cysteine and glutamate and was proposed to serve as a storage form of cysteine [35]. It is synthesized by the consecutive action of the enzymes γ-glutamylcysteine synthetase and glutathione synthetase in two ATP-dependent reactions. The extracellular enzyme γ-glutamyl transpeptidase (GGT) cleaves the γ-peptide linkage of glutathione to produce cysteinylglycine and glutamate. The cysteinylglycine can be hydrolyzed to generate cysteine and glycine, which can be imported into cells [36, 37].
Autophagy and cysteine
Autophagy is an evolutionarily conserved process involving degradation of cellular components, including soluble and aggregated proteins, organelles and macromolecular complexes. Autophagy (Greek for self-eating), a lysosomal degradation pathway, was first termed by Christian de Duve, who also discovered the lysosome, where intracellular protein degradation occurs [38]. Subsequently, starvation-induced autophagy was described by Ohsumi in yeast [39, 40]. Autophagy plays essential roles in protein turnover and quality control in cells, degrading unwanted proteins and recycling component amino acids during starvation. Starvation, as Ohsumi puts it, is the most frequent and serious threat to the maintenance and preservation of life [41]. Autophagy is an evolutionarily conserved process from yeast to mammals which is under genetic control [42]. The acidic pH of the lysosome facilitates the degradation of proteins via several proteases to release component amino acids. Thus during starvation conditions, when cysteine becomes limiting, degradation of proteins in lysosomes is an alternate source of cysteine for metabolic needs [43]. Cysteine can then be transported out of the lysosomes through the transporter, cystinosin (CTNS)[44]. Mutations in the ctns gene cause cystinosis, an autosomal recessive disease that causes accumulation of cystine in the lysosomes of the kidney, liver, muscles and brain in addition to other tissues as well as a range of symptoms. Treatment of cystinosis includes the use of cysteamine, a decarboxylated derivative of cysteine [45].
Cysteine and redox homeostasis
Cysteine is readily oxidized to cystine, the predominant form extracellularly. Inside cells, cysteine formation is favored due to the reducing atmosphere of the cell. The cysteine/cystine redox couple has a redox potential of −140 to −160 meV, while in the extracellular milieu, the redox potential is approximately −80 meV. The extracellular concentration of cystine exceeds that of glutathione, about 40–50 µM. GSH is around 2.8 µM and that of its oxidized form GSSG is 0.14 µM. [46]. The redox potential of GSH/GSSG is more reduced than that of cysteine/cystine and has an intracellular value of approximately −230 meV, compared to −140 meV extracellularly. Thus, electron transfer from thiol/disulfide couples such as the GSH/GSSG keeps cysteine in its reduced form in the cytosol. Cysteine participates in a wide variety of redox reactions, due to its sulfur atom, which exists in different oxidation states, ranging from +6 to −2 in oxidizing environments. Accordingly, the −SH group of a cysteine residue can be oxidized to sulfenic, sulfinic or sulfonic groups amongst other modifications.
As cysteine is central to redox balance in cells, perturbations in cysteine levels result in several compensatory or corrective responses. CSE, the biosynthetic enzyme for cysteine, is a highly inducible cytosolic protein and a variety of stress stimuli induce its expression. It is highly induced during cysteine deprivation [11, 47]. Blockade of the xCT transporter can induce its expression. In mouse embryonic fibroblasts deficient for xCT, CSE is upregulated as a compensatory response. Thus, depletion or inhibition of CSE can lead to deleterious effects in both the central nervous system as well as peripheral systems especially under conditions of cysteine limitation. Under certain conditions, CSE translocates to the mitochondria to generate more H2S as in the case of vascular smooth muscle cells subject to ER stress. Interestingly, cysteine levels in the mitochondria are three fold higher than that in the cytosol [48]. Other studies have shown that CSE is sumoylated, which could lead to its nuclear translocation [49]. Thus cysteine metabolism is also controlled in a spatiotemporal manner.
Signaling functions of cysteine residues in proteins
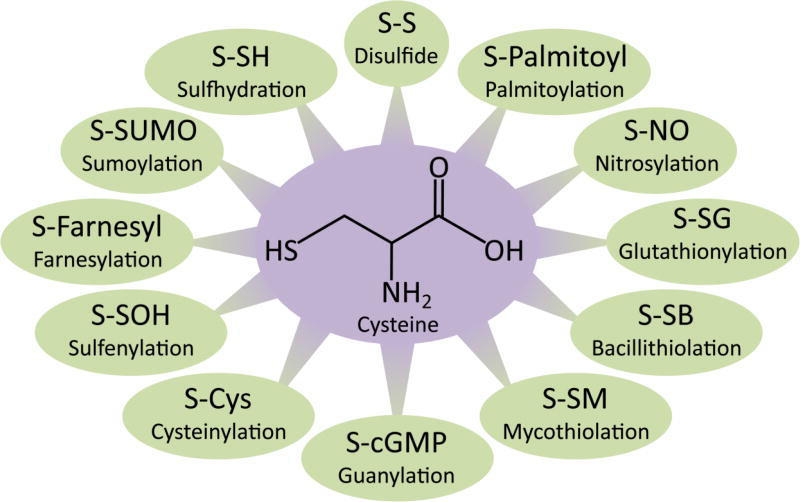
Cysteine constitutes only about 2% of the proteome, but it undergoes the maximum number of posttranslational modifications [50] (Figure 3). This stems from its reactive −SH or thiol group. Cysteine residues on proteins exist as thiolate anions at physiological pH and are targeted by multiple modifications. These modifications include palmitoylation, glutathionylation, guanylation, cysteinylation, sumoylation, farnesylation, palmitoylation, nitrosylation and sulfhydration among several others (Figure 3). Cysteine is also the substrate for the generation of the gasotransmitter, hydrogen sulfide (H2S), which modulates several physiological processes [51]. Recently, sulfhydration, a post-translational modification mediated by hydrogen sulfide (H2S), has garnered attention. Sulfhydration converts the −SH groups of reactive cysteine residues to persulfide or −SSH groups in a manner analogous to nitrosylation, where −SH groups of cysteine residues are converted to −SNO groups. Sulfhydration is a prevalent modification with about 50% of proteins being modified endogenously in the liver [52]. An intriguing aspect of sulfhydration is that it occurs on reactive cysteine residues, and H2S itself is generated by enzymes which utilize cysteine as a substrate [5–7]. In several instances, the same cysteine residue is targeted by different groups and, depending on the modification, the outcome can be very different. For instance, sulfhydration and nitrosylation exert opposite effects on protein function in the case of the glycolytic enzyme glyceraldehyde 3-phosphate dehydrogenase (GAPDH), nuclear factor κB (NF-κB), and the E3-ubiquitin ligase, parkin [52–54].
Figure 3. Posttranslational modifications of cysteine.
Cysteine has a thiol group, where the sulfur atom is nucleophilic and is subject to several post-translational oxidative modifications in cells. Depending on the context, these modifications participate in a diverse array of signaling pathways.
The three enzymes responsible for H2S production, CSE, CBS and 3-mercaptopyruvate sulfurtransferase (3-MST), are also present in the brain. While CBS is present in the astrocytes, CSE is present in neurons and 3-MST is resident in both neurons and astrocytes [55–57]. H2S is involved in several physiological processes in the brain thus precise regulation of its production is vital to optimal functioning of the nervous system. Abnormal H2S signaling has been observed in several neurodegenerative disorders such as AD, PD, ALS and HD.
Dysregulated cysteine homeostasis in neurodegeneration
Altered thiol and redox balance has frequently been linked to neurodegenerative conditions [46].
Huntington’s disease
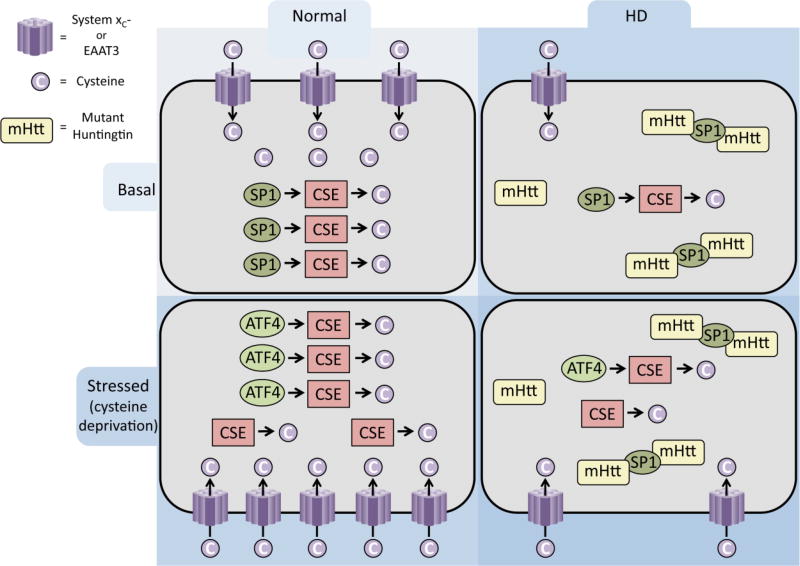
Depletion of cysteine can cause oxidative stress, which has been associated with neurodegenerative diseases such as Huntington’s disease (HD) [47]. The transporters for cysteine and cystine are also dysregulated in HD [29, 58] (Figure 4). HD is an autosomal dominant neurodegenerative disease characterized by expansion of polyglutamine repeats in the protein huntingtin [59]. Mutant huntingtin aggregates and affects multiple cellular processes [60]. We have shown that CSE is depleted in HD, which contributes to disease progression and neurotoxicity. CSE expression and activity was severely depleted in cell culture and mouse models of HD. The depletion was also observed in postmortem human samples with a profound decrease in the striatum, the region most affected in HD. The degree of depletion also correlated with the severity of the disease, with the most advanced stages of the disease exhibiting the greatest depletion. The depletion of CSE was linked to the sequestration of specificity protein 1 (SP1), the transcription factor for CSE, by mHtt. Supplementing the diet of the R6/2 mouse model of HD ameliorates disease symptoms and improves survival. We also demonstrated that not only basal CSE expression, but also its induction in response to cysteine deprivation is affected in HD. Under basal conditions, SP1 is responsible for expression of CSE, while during stress, activating transcription factor 4 (ATF4) regulates induction. In the striatal Q111 cells, a cell culture model of HD, harboring 111 glutamine repeats, response to cysteine deprivation was disrupted [11] (Figure 4). The lack of response was specific to cysteine deprivation in HD, as such as deprivation of other amino acids or other stress signals such as ER stress, induced ATF4 and CSE in HD cells. The elevated oxidative stress generated by lack of CSE was attributed to the failure of corrective responses to cysteine deprivation as supplementation with the antioxidant, sodium ascorbate, restored induction of ATF4 and CSE. Similarly, inducing oxidative stress in the normal cell line, Q7, prevented induction of CSE and ATF4 in response to cysteine starvation. Thus, a feedforward loop involving low CSE and elevated oxidative stress was proposed to be the mechanism underlying dampened response of cysteine biosynthesis in HD (Figure 4). The study is relevant to other neurodegenerative diseases involving oxidative stress and glutathione depletion. It has been shown that agents which can stimulate synthesis of glutathione from cysteine can inhibit apoptosis induced by oxidative stress [61].
Figure 4. Dysregulated cysteine metabolism in Huntington’s disease.
Cysteine and its oxidized form are imported into neurons via the excitatory amino acid transporter 3 (EAAT3) or system xc− respectively under normal conditions. Cysteine is also synthesized endogenously by cystathionine γ-lyase (CSE) whose basal expression is regulated by specificity protein 1 (SP1). In Huntington’s disease (HD), SP1 is sequestered by mutant huntingtin (mHtt) leading to low expression of CSE and cysteine production. In addition, the cystine and cysteine transporters, system xc− and EAAT3/EAAC1 are also dysregulated. During conditions of stress (as in cysteine deprivation), activating transcription factor 4 (ATF4) is induced and regulates CSE expression, leading to increased production of cysteine. System xc−, whose expression is regulated by ATF4, is also induced and transports cystine into neurons. In contrast, HD cells are unable to upregulate ATF4 and induce CSE due to the elevated oxidative stress generated, leading to neuronal cell death.
Spinocerebellar ataxia type 3
Similar to HD, spinocerebellar ataxia type 3 (SCA3) is a polyglutamine repeat disorder where the reverse transsulfuration pathway is disrupted [62]. The disease is characterized by increased oxidative stress and inflammation that contribute to disease progression. Analysis of human patient samples revealed a reduction in CSE expression, possible mediated by aggregation of mutant SCA3. Reduced levels of sulfhydration was also observed in the disease. Overexpression of CSE in a Drosophila model of SCA3 reverses abnormalities.
Amyotrophic lateral sclerosis (ALS)
Amyotrophic Lateral sclerosis is a motor neuron disorder that affects the upper (neurons that project from the cortex to the brainstem and the spinal cord) and lower motor neurons (neurons that project from the brain stem and spinal cord to muscles) and leads to paralysis. The disease can be triggered by both familial as well as sporadic causes. Amongst the familial cases of ALS, mutations in the c9orf72 gene are the most predominant [63]. Amongst the other mutations associated with ALS, the G93A mutation in the antioxidant enzyme superoxide dismutase 1 (SOD1) has been the subject of several studies. The G93A mutation is a gain of function mutant that causes aggregation of SOD1 and degeneration of motor neurons [64, 65]. Cysteine residues on SOD1 have been implicated in the aggregation of SOD1. Human SOD1 has four cysteine residues of which two, Cys57 and Cys146, form an intramolecular disulfide bond [66]. The other cysteine residues, Cys6 and Cys111, do not form disulfide bonds. SOD1 aggregation is increased when the enzyme is in its metal free form and when Cys6 and Cys111 are oxidized. Cys111 is modified by a persulfide group as revealed by mass spectrometry [67]. Modified SOD1 is more resistant to oxidation-induced aggregation. Crystallographic analysis confirms that Cys111 is modified by a covalent polyheptane sulfane sulfur [68]. Sulfane sulfur is elemental sulfur with six valence electrons and no charge, which can bond together to form hydropersulfides (R-S-SH) and polysulfides (-S-Sn-S-). Sulfane sulfur is derived from H2S which in turn, is derived from cysteine and acts as a signaling molecule in vivo [57, 69, 70]. In the G93A mouse model of ALS, very low levels of cysteine were detected as compared to healthy controls [71]. In addition, elevated H2S levels were associated with the condition [72]. Increased levels of H2S are detected in the tissues of the G93A mouse model of ALS and in media of spinal cord cultures of these mice. Increased H2S was also detected in cerebrospinal fluid of ALS patients, suggesting aberrant gasotransmitter signaling. As ALS is associated with elevated oxidative stress, depletion of cysteine could play a role in the process. Supplementation of the diet with a cysteine-rich whey isolate rescues glutathione content in tissues and delays disease onset in the G93A mice [73].
Alzheimer’s disease
Alzheimer’s disease (AD) is the most prevalent neurodegenerative disease reported so far. Symptoms of AD include dementia, impaired spatial memory, deficits in executive functions and cognitive deficits [74, 75]. Causes of AD can be either genetic or sporadic. Aggregation of β-amyloid peptides and tau protein, which form plaques and neurofibrillary tangles, are characteristics of the disease. These insoluble proteins disrupt multiple physiological processes and cause oxidative stress in addition to other abnormalities. Disrupted cysteine and glutathione metabolism has been observed in AD. Some studies reported elevated cysteine levels in the plasma of AD patients [76]. Whether this reflects impaired uptake of cysteine into cells remains to be established. The activity of the neuronal cysteine transporter EAAC1/EAAT3 has been shown to be disrupted in AD. Soluble Aβ can impair cysteine and glutathione metabolism in cells by inhibiting EAAT3 (137). In addition, EAAT3 accumulates in the detergent insoluble fraction of hippocampal neurons instead of its normal localization at the plasma membrane [77]. In addition to these aberrations, decreased levels of H2S have been observed in plasma of AD patients as compared to normal individuals, and there was a negative correlation of H2S levels with the severity of the disease [78]. Thus, multiple aspects of cysteine metabolism are affected in AD.
Parkinson’s disease
Dysregulated H2S metabolism has also been observed in Parkinson’s disease (PD). PD is the second most prevalent neurodegenerative disease. Aggregation of α-synuclein to form Lewy bodies is a hallmark of the disease, leading to motor deficits which manifest as shaking and tremors in patients. The substantia nigra of the brain is affected in PD, leading to degeneration of dopaminergic neurons which control motor functions. In PD, the activity of parkin, an E3-ubiquitin ligase, responsible for clearance of toxic protein buildup, is inhibited by nitrosylation, leading to aggregation of its substrate α-synuclein and neurotoxicity [79]. By contrast, sulfhydration activates parkin to degrade misfolded proteins and promote neuroprotection. In postmortem PD brains, sulfhydration of parkin is diminished and nitrosylation increased [54]. Overexpressing cystathionine β-synthase (CBS), which generates H2S, increases parkin sulfhydration and E3 ubiquitin ligase activity. Similarly, administration of H2S donors has proved beneficial in PD. Inhalation of H2S by MPTP injected mice (a chemical model of PD) ameliorates symptoms and reduces the death of dopaminergic neurons [80]. Treating the 6-OHDA-induced PD mouse model with an H2S releasing L-DOPA derivative, ACS84, stimulates the antioxidant defense pathway regulated by Nrf2, preserves dopaminergic neurons, and confers neuroprotection [81]. Protective effects of H2S have also been reported in cell culture models of PD [82].Thus, stimulating H2S-mediated sulfhydration may be beneficial in PD.
Molybdenum cofactor deficiency disorders
Molybdenum cofactor (MoCo) deficiency is a rare inborn error of metabolism caused by defects in the synthesis of molybdenum cofactors [83]. Patients with MoCo deficiency (MoCD) have difficulty feeding, have seizures, exhibit high-pitched crying and severe neurological abnormalities along with a variety of other symptoms in their neonatal period. Molybdenum cofactor containing enzymes play important roles in carbon, sulfur and nitrogen metabolism [84]. Sulfite oxidase (SO), Xanthine oxidase (XO), aldehyde oxidase (AO) and the mitochondrial amidoxime reducing component proteins 1 and 2 (mARC1 and mARC2) are examples. The symptoms of MoCD are recapitulated by a deficiency of SO [85]. Reduction in the activity of SO, which catalyzes the conversion of sulfite derived from cysteine to sulfate, causes the accumulation of sulfite leading to widespread damage and elevated oxidative stress. Cystine, which normally scavenges sulfite by forming S-sulfocysteine (SSC), gets utilized leading to a systemic deficiency of cysteine as well as glutathione[83]. SSC, which is structurally similar to glutamate, is elevated in MoCo deficient patients and causes stimulation of the N-methyl-D-aspartate (NMDA) receptor leading to seizures and brain damage.
Ethylmalonic encephalopathy
The reverse transsulfuration is also dysregulated in Ethylmalonic encephalopathy (EE), a severe mitochondrial disease affecting infants, which leads to progressive encephalopathy, vascular abnormalities, motor dysfunction as well as neurological deficits [86]. Mutations in ethylmalonic encephalopathy 1 (ETHE1) gene cause ethylmalonic encephalopathy. ETHE1 protein, also termed persulfide dioxygenase, is a sulfur dioxygenase localized mainly to the mitochondria which functions in H2S catabolism [87]. As a result, this disease is characterized by elevated levels of H2S, affecting mitochondrial respiration by inhibiting cytochrome c oxidase (COX) [86].
Aging
During aging, the cysteine/cystine redox potential tilts towards a more oxidizing one [88]. A decline in the redox buffering capacity with decreases in glutathione and cysteine levels has been observed in aging [89–91]. Oxidative damage increases as a function of age and has been proposed as a major player in senescence [92]. Thus decreasing oxidative stress or decreasing accumulation of free radicals may slow the aging process. Long-term supplementation of cysteine-based antioxidants delayed muscle loss during aging in mice [93]. Similarly, whey protein, which is highly enriched in cysteine, is used to promote muscle function. In addition, intake of whey protein has shown to increase cellular glutathione content. Consumption of whey protein has been shown to increase the glutathione content and muscle strength in Parkinson’s disease patients and those with AIDS [94, 95]. The reverse transsulfuration pathway plays important roles in longevity. Increased flux through the reverse transsulfuration pathway, which participates in antioxidant defense, is a mediator of longevity [96, 97]. More recently, it was conclusively shown that stimulation of the reverse transsulfuration increases lifespan across evolutionary boundaries and that H2S plays a central role in the process. During dietary restriction, which is known to promote longevity, the H2S produced affords lifespan promoting benefits, although the exact mechanisms underlying the process is yet to be elucidated.
We had proposed previously that H2S mediated sulfhydration/persulfidation can protect proteins from irreversible oxidation on cysteine residues [5]. The CySSO2H (perthiosulfinic) and CySSO3H (perthiosulfonic) oxidation products of persulfides can be recycled back by the reduction of their S–S moieties, which does not occur in the case of the CySO3H oxidation product of unmodified (unsulfhydrated) cysteine residues on proteins [7]. The protection of SH groups of cysteine residues by persulfidation has been verified in the case of phosphatase and tensin homolog deleted on chromosome 10, PTEN [98]. Thus, H2S production and sulfhydration could prevent oxidation of proteins and contribute to longevity, both at the level of protein half-life as well as overall lifespan.
Concluding remarks
The amino acid cysteine is integral to structural and signaling features of the cell and is the precursor of a wide variety of molecules ranging from the gas hydrogen sulfide to coenzyme A. Despite being one of the least abundant amino acids in cells, it undergoes the maximum number of posttranslational modifications, which play significant roles in modulation of protein function. It is also a regulator of cellular redox balance, which is deranged in neurodegenerative disorders. Thus, disruption of cysteine metabolism in neurodegeneration may reflect a form of “cysteine stress” with widespread impact [11]. An analysis of existing evidence shows that restoring cysteine balance in conditions displaying elevated oxidative stress has beneficial effects. Upregulating the reverse transsulfuration pathway can thus be targeted for therapeutic benefits. Our recent studies show that low levels of the Golgi stressor, monensin, can induce ATF4 leading to increased flux via the reverse transsulfuration pathway. Pretreating striatal HD cells with monensin rescues growth in low cysteine medium and preconditions cells to withstand future insults [87]. Thus targeting this pathway to increase cysteine and H2S production can have beneficial effects. This strategy may also be applicable to other pathological conditions involving imbalanced cysteine metabolism. Although substantial progress has been made in the field of cysteine metabolism, several areas of research deserve further investigation (See Outstanding Questions Box). Nonetheless, it is becoming increasingly evident that cysteine homeostasis is linked to a myriad of physiological processes.
Outstanding Questions.
What are conditions under which CSE synthesizes cysteine and H2S? As CSE is the biosynthetic enzyme for cysteine and also utilizes cysteine to generate H2S, the switch between cysteine and H2S production in vivo needs to be elucidated.
What are the relative contributions of the various cysteine generating pathways in different cell types. As cysteine is the rate limiting step for glutathione biosynthesis and the precursor for several sulfur containing molecules, the concentrations of which vary in different cell types and tissues, the source of cysteine may be different in various cell types.
What is the redox state of the Cysteine/Cystine couple during neurodegeneration in different tissues? Cysteine can be readily oxidized to cystine, which alters the redox potential of the cysteine/cystine couple. Several studies calculate the levels of total cysteine, which include the oxidized and reduced forms. It would be more meaningful to measure the Cysteine/cystine ratio.
What is the interplay between various transcription factors that govern the expression of CSE during different developmental stages in different cell types and tissues in response to stress stimuli?
What are the epigenetic changes modulating cysteine and H2S production?
How are various posttranslational modifications occurring on reactive cysteine residues spatially and temporally controlled?
Highlights.
Cysteine is a semi-essential amino acid that is a building block for not only protein synthesis, but also the major antioxidant glutathione.
Cysteine is the precursor for several sulfur containing molecules such as the gaseous signaling molecule hydrogen sulfide, lanthionine, taurine, coenzyme A and biotin.
Although relatively scarce, cysteine undergoes the maximum number of posttranslational modifications.
Sulfhydration, the most recently discovered physiological modification, plays diverse roles in physiology ranging from response to inflammation to neuroprotection.
Dysregulated cysteine and hydrogen sulfide metabolism is frequently encountered in several neurodegenerative disorders.
Upregulating the reverse transsulfuration pathway, via which cysteine and hydrogen sulfide are produced, affords therapeutic benefits.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Droge W. Oxidative stress and ageing: is ageing a cysteine deficiency syndrome? Philos Trans R Soc Lond B Biol Sci. 2005;360(1464):2355–72. doi: 10.1098/rstb.2005.1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Droge W, Kinscherf R. Aberrant insulin receptor signaling and amino acid homeostasis as a major cause of oxidative stress in aging. Antioxid Redox Signal. 2008;10(4):661–78. doi: 10.1089/ars.2007.1953. [DOI] [PubMed] [Google Scholar]
- 3.Griffith OW. Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic Biol Med. 1999;27(9–10):922–35. doi: 10.1016/s0891-5849(99)00176-8. [DOI] [PubMed] [Google Scholar]
- 4.Miniaci MC, et al. Cysteine Prevents the Reduction in Keratin Synthesis Induced by Iron Deficiency in Human Keratinocytes. J Cell Biochem. 2016;117(2):402–12. doi: 10.1002/jcb.25286. [DOI] [PubMed] [Google Scholar]
- 5.Paul BD, Snyder SH. H(2)S signalling through protein sulfhydration and beyond. Nat Rev Mol Cell Biol. 2012;13(8):499–507. doi: 10.1038/nrm3391. [DOI] [PubMed] [Google Scholar]
- 6.Paul BD, Snyder SH. Modes of physiologic H2S signaling in the brain and peripheral tissues. Antioxid Redox Signal. 2015;22(5):411–23. doi: 10.1089/ars.2014.5917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paul BD, Snyder SH. Protein sulfhydration. Methods Enzymol. 2015;555:79–90. doi: 10.1016/bs.mie.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 8.Paul BD, Snyder SH. H2S: A Novel Gasotransmitter that Signals by Sulfhydration. Trends Biochem Sci. 2015;40(11):687–700. doi: 10.1016/j.tibs.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishii I, et al. Cystathionine gamma-Lyase-deficient mice require dietary cysteine to protect against acute lethal myopathy and oxidative injury. J Biol Chem. 2010;285(34):26358–68. doi: 10.1074/jbc.M110.147439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mani S, et al. A critical life-supporting role for cystathionine gamma-lyase in the absence of dietary cysteine supply. Free Radic Biol Med. 2011;50(10):1280–7. doi: 10.1016/j.freeradbiomed.2011.01.038. [DOI] [PubMed] [Google Scholar]
- 11.Sbodio JI, et al. Transcriptional control of amino acid homeostasis is disrupted in Huntington's disease. Proc Natl Acad Sci U S A. 2016;113(31):8843–8. doi: 10.1073/pnas.1608264113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sturman JA, et al. Absence of cystathionase in human fetal liver: is cystine essential? Science. 1970;169(3940):74–6. doi: 10.1126/science.169.3940.74. [DOI] [PubMed] [Google Scholar]
- 13.Gaull G, et al. Development of mammalian sulfur metabolism: absence of cystathionase in human fetal tissues. Pediatr Res. 1972;6(6):538–47. doi: 10.1203/00006450-197206000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Paul BD, Snyder SH. Gasotransmitter hydrogen sulfide signaling in neuronal health and disease. Biochem Pharmacol. 2017 doi: 10.1016/j.bcp.2017.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun Q, et al. Structural basis for the inhibition mechanism of human cystathionine gamma-lyase, an enzyme responsible for the production of H(2)S. J Biol Chem. 2009;284(5):3076–85. doi: 10.1074/jbc.M805459200. [DOI] [PubMed] [Google Scholar]
- 16.Sbodio JI, et al. Golgi stress response reprograms cysteine metabolism to confer cytoprotection in Huntington's disease. Proc Natl Acad Sci U S A. 2018;115(4):780–785. doi: 10.1073/pnas.1717877115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang G, et al. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322(5901):587–90. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conrad M, Sato H. The oxidative stress-inducible cystine/glutamate antiporter, system x (c) (−) : cystine supplier and beyond. Amino Acids. 2012;42(1):231–46. doi: 10.1007/s00726-011-0867-5. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi S, et al. Cystathionine is a novel substrate of cystine/glutamate transporter: implications for immune function. J Biol Chem. 2015;290(14):8778–88. doi: 10.1074/jbc.M114.625053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewerenz J, et al. The cystine/glutamate antiporter system x(c)(−) in health and disease: from molecular mechanisms to novel therapeutic opportunities. Antioxid Redox Signal. 2013;18(5):522–55. doi: 10.1089/ars.2011.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mandal PK, et al. System x(c)− and thioredoxin reductase 1 cooperatively rescue glutathione deficiency. J Biol Chem. 2010;285(29):22244–53. doi: 10.1074/jbc.M110.121327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sato H, et al. Redox imbalance in cystine/glutamate transporter-deficient mice. J Biol Chem. 2005;280(45):37423–9. doi: 10.1074/jbc.M506439200. [DOI] [PubMed] [Google Scholar]
- 23.Soria FN, et al. Cystine/glutamate antiporter blockage induces myelin degeneration. Glia. 2016;64(8):1381–95. doi: 10.1002/glia.23011. [DOI] [PubMed] [Google Scholar]
- 24.Chung WJ, et al. Inhibition of cystine uptake disrupts the growth of primary brain tumors. J Neurosci. 2005;25(31):7101–10. doi: 10.1523/JNEUROSCI.5258-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robert SM, et al. SLC7A11 expression is associated with seizures and predicts poor survival in patients with malignant glioma. Sci Transl Med. 2015;7(289):289ra86. doi: 10.1126/scitranslmed.aaa8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cramer SL, et al. Systemic depletion of L-cyst(e)ine with cyst(e)inase increases reactive oxygen species and suppresses tumor growth. Nat Med. 2017;23(1):120–127. doi: 10.1038/nm.4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Bundel D, et al. Loss of system x(c)− does not induce oxidative stress but decreases extracellular glutamate in hippocampus and influences spatial working memory and limbic seizure susceptibility. J Neurosci. 2011;31(15):5792–803. doi: 10.1523/JNEUROSCI.5465-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aoyama K, et al. Neuronal glutathione deficiency and age-dependent neurodegeneration in the EAAC1 deficient mouse. Nat Neurosci. 2006;9(1):119–26. doi: 10.1038/nn1609. [DOI] [PubMed] [Google Scholar]
- 29.Li X, et al. Aberrant Rab11-dependent trafficking of the neuronal glutamate transporter EAAC1 causes oxidative stress and cell death in Huntington's disease. J Neurosci. 2010;30(13):4552–61. doi: 10.1523/JNEUROSCI.5865-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakai K, et al. Neutral amino acid transporter ASCT1 is preferentially expressed in L-Ser-synthetic/storing glial cells in the mouse brain with transient expression in developing capillaries. J Neurosci. 2003;23(2):550–60. doi: 10.1523/JNEUROSCI.23-02-00550.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arriza JL, et al. Cloning and expression of a human neutral amino acid transporter with structural similarity to the glutamate transporter gene family. J Biol Chem. 1993;268(21):15329–32. [PubMed] [Google Scholar]
- 32.Pingitore P, et al. Large scale production of the active human ASCT2 (SLC1A5) transporter in Pichia pastoris--functional and kinetic asymmetry revealed in proteoliposomes. Biochim Biophys Acta. 2013;1828(9):2238–46. doi: 10.1016/j.bbamem.2013.05.034. [DOI] [PubMed] [Google Scholar]
- 33.Scalise M, et al. Cysteine is not a substrate but a specific modulator of human ASCT2 (SLC1A5) transporter. FEBS Lett. 2015;589(23):3617–23. doi: 10.1016/j.febslet.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 34.Utsunomiya-Tate N, et al. Cloning and functional characterization of a system ASC-like Na+-dependent neutral amino acid transporter. J Biol Chem. 1996;271(25):14883–90. doi: 10.1074/jbc.271.25.14883. [DOI] [PubMed] [Google Scholar]
- 35.Meister A, Anderson ME. Glutathione. Annu Rev Biochem. 1983;52:711–60. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 36.Meister A. Glutathione, ascorbate, and cellular protection. Cancer Res. 1994;54(7 Suppl):1969s–1975s. [PubMed] [Google Scholar]
- 37.Hanigan MH, Ricketts WA. Extracellular glutathione is a source of cysteine for cells that express gamma-glutamyl transpeptidase. Biochemistry. 1993;32(24):6302–6. doi: 10.1021/bi00075a026. [DOI] [PubMed] [Google Scholar]
- 38.de Duve C. The lysosome turns fifty. Nat Cell Biol. 2005;7(9):847–9. doi: 10.1038/ncb0905-847. [DOI] [PubMed] [Google Scholar]
- 39.Takeshige K, et al. Autophagy in yeast demonstrated with proteinase-deficient mutants and conditions for its induction. J Cell Biol. 1992;119(2):301–11. doi: 10.1083/jcb.119.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baba M, et al. Ultrastructural analysis of the autophagic process in yeast: detection of autophagosomes and their characterization. J Cell Biol. 1994;124(6):903–13. doi: 10.1083/jcb.124.6.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohsumi Y. Historical landmarks of autophagy research. Cell Res. 2014;24(1):9–23. doi: 10.1038/cr.2013.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakatogawa H, et al. Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat Rev Mol Cell Biol. 2009;10(7):458–67. doi: 10.1038/nrm2708. [DOI] [PubMed] [Google Scholar]
- 43.Thoene JG, et al. Cystinotic fibroblasts accumulate cystine from intracellular protein degradation. Proc Natl Acad Sci U S A. 1977;74(10):4505–7. doi: 10.1073/pnas.74.10.4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Town M, et al. A novel gene encoding an integral membrane protein is mutated in nephropathic cystinosis. Nat Genet. 1998;18(4):319–24. doi: 10.1038/ng0498-319. [DOI] [PubMed] [Google Scholar]
- 45.Gahl WA, et al. Cysteamine therapy for children with nephropathic cystinosis. N Engl J Med. 1987;316(16):971–7. doi: 10.1056/NEJM198704163161602. [DOI] [PubMed] [Google Scholar]
- 46.McBean GJ, et al. Thiol redox homeostasis in neurodegenerative disease. Redox Biol. 2015;5:186–94. doi: 10.1016/j.redox.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paul BD, et al. Cystathionine gamma-lyase deficiency mediates neurodegeneration in Huntington's disease. Nature. 2014;509(7498):96–100. doi: 10.1038/nature13136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fu M, et al. Hydrogen sulfide (H2S) metabolism in mitochondria and its regulatory role in energy production. Proc Natl Acad Sci U S A. 2012;109(8):2943–8. doi: 10.1073/pnas.1115634109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Agrawal N, Banerjee R. Human polycomb 2 protein is a SUMO E3 ligase and alleviates substrate-induced inhibition of cystathionine beta-synthase sumoylation. PLoS One. 2008;3(12):e4032. doi: 10.1371/journal.pone.0004032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miseta A, Csutora P. Relationship between the occurrence of cysteine in proteins and the complexity of organisms. Mol Biol Evol. 2000;17(8):1232–9. doi: 10.1093/oxfordjournals.molbev.a026406. [DOI] [PubMed] [Google Scholar]
- 51.Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol Rev. 2012;92(2):791–896. doi: 10.1152/physrev.00017.2011. [DOI] [PubMed] [Google Scholar]
- 52.Mustafa AK, et al. H2S signals through protein S-sulfhydration. Sci Signal. 2009;2(96):ra72. doi: 10.1126/scisignal.2000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sen N, et al. Hydrogen sulfide-linked sulfhydration of NF-kappaB mediates its antiapoptotic actions. Mol Cell. 2012;45(1):13–24. doi: 10.1016/j.molcel.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vandiver MS, et al. Sulfhydration mediates neuroprotective actions of parkin. Nat Commun. 2013;4:1626. doi: 10.1038/ncomms2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Enokido Y, et al. Cystathionine beta-synthase, a key enzyme for homocysteine metabolism, is preferentially expressed in the radial glia/astrocyte lineage of developing mouse CNS. FASEB J. 2005;19(13):1854–6. doi: 10.1096/fj.05-3724fje. [DOI] [PubMed] [Google Scholar]
- 56.Morikawa T, et al. Hypoxic regulation of the cerebral microcirculation is mediated by a carbon monoxide-sensitive hydrogen sulfide pathway. Proc Natl Acad Sci U S A. 2012;109(4):1293–8. doi: 10.1073/pnas.1119658109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shibuya N, et al. 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid Redox Signal. 2009;11(4):703–14. doi: 10.1089/ars.2008.2253. [DOI] [PubMed] [Google Scholar]
- 58.Frederick NM, et al. Dysregulation of system xc(−) expression induced by mutant huntingtin in a striatal neuronal cell line and in R6/2 mice. Neurochem Int. 2014;76:59–69. doi: 10.1016/j.neuint.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. The Huntington's Disease Collaborative Research Group. Cell. 1993;72(6):971–83. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- 60.Bates GP, et al. Huntington disease. Nat Rev Dis Primers. 2015;1:15005. doi: 10.1038/nrdp.2015.5. [DOI] [PubMed] [Google Scholar]
- 61.Ratan RR, et al. Macromolecular synthesis inhibitors prevent oxidative stress-induced apoptosis in embryonic cortical neurons by shunting cysteine from protein synthesis to glutathione. J Neurosci. 1994;14(7):4385–92. doi: 10.1523/JNEUROSCI.14-07-04385.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Snijder PM, et al. Overexpression of cystathionine gamma-lyase suppresses detrimental effects of spinocerebellar ataxia type 3. Mol Med. 2015 doi: 10.2119/molmed.2015.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.DeJesus-Hernandez M, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72(2):245–56. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rosen DR, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362(6415):59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 65.Gurney ME, et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264(5166):1772–5. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 66.Tainer JA, et al. Determination and analysis of the 2 A-structure of copper, zinc superoxide dismutase. J Mol Biol. 1982;160(2):181–217. doi: 10.1016/0022-2836(82)90174-7. [DOI] [PubMed] [Google Scholar]
- 67.de Beus MD, et al. Modification of cysteine 111 in Cu/Zn superoxide dismutase results in altered spectroscopic and biophysical properties. Protein Sci. 2004;13(5):1347–55. doi: 10.1110/ps.03576904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.You Z, et al. Characterization of a covalent polysulfane bridge in copper-zinc superoxide dismutase. Biochemistry. 2010;49(6):1191–8. doi: 10.1021/bi901844d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kimura H. Signaling molecules: hydrogen sulfide and polysulfide. Antioxid Redox Signal. 2015;22(5):362–76. doi: 10.1089/ars.2014.5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abiko Y, et al. Polysulfide Na2S4 regulates the activation of PTEN/Akt/CREB signaling and cytotoxicity mediated by 1,4-naphthoquinone through formation of sulfur adducts. Sci Rep. 2017;7(1):4814. doi: 10.1038/s41598-017-04590-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bame M, et al. Amino acids as biomarkers in the SOD1(G93A) mouse model of ALS. Biochim Biophys Acta. 2014;1842(1):79–87. doi: 10.1016/j.bbadis.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 72.Davoli A, et al. Evidence of hydrogen sulfide involvement in amyotrophic lateral sclerosis. Ann Neurol. 2015;77(4):697–709. doi: 10.1002/ana.24372. [DOI] [PubMed] [Google Scholar]
- 73.Ross EK, et al. A Cystine-Rich Whey Supplement (Immunocal((R))) Delays Disease Onset and Prevents Spinal Cord Glutathione Depletion in the hSOD1(G93A) Mouse Model of Amyotrophic Lateral Sclerosis. Antioxidants (Basel) 2014;3(4):843–65. doi: 10.3390/antiox3040843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Scheltens P, et al. Alzheimer's disease. Lancet. 2016;388(10043):505–17. doi: 10.1016/S0140-6736(15)01124-1. [DOI] [PubMed] [Google Scholar]
- 75.Lane CA, et al. Alzheimer's disease. Eur J Neurol. 2017 doi: 10.1111/ene.13439. [DOI] [PubMed] [Google Scholar]
- 76.Heafield MT, et al. Plasma cysteine and sulphate levels in patients with motor neurone, Parkinson's and Alzheimer's disease. Neurosci Lett. 1990;110(1–2):216–20. doi: 10.1016/0304-3940(90)90814-p. [DOI] [PubMed] [Google Scholar]
- 77.Duerson K, et al. Detergent-insoluble EAAC1/EAAT3 aberrantly accumulates in hippocampal neurons of Alzheimer's disease patients. Brain Pathol. 2009;19(2):267–78. doi: 10.1111/j.1750-3639.2008.00186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu XQ, et al. Plasma levels of endogenous hydrogen sulfide and homocysteine in patients with Alzheimer's disease and vascular dementia and the significance thereof. Zhonghua Yi Xue Za Zhi. 2008;88(32):2246–9. [PubMed] [Google Scholar]
- 79.Chung KK, et al. S-nitrosylation of parkin regulates ubiquitination and compromises parkin's protective function. Science. 2004;304(5675):1328–31. doi: 10.1126/science.1093891. [DOI] [PubMed] [Google Scholar]
- 80.Kida K, et al. Inhaled hydrogen sulfide prevents neurodegeneration and movement disorder in a mouse model of Parkinson's disease. Antioxid Redox Signal. 2011;15(2):343–52. doi: 10.1089/ars.2010.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xie L, et al. Therapeutic effect of hydrogen sulfide-releasing L-Dopa derivative ACS84 on 6-OHDA-induced Parkinson's disease rat model. PLoS One. 2013;8(4):e60200. doi: 10.1371/journal.pone.0060200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tiong CX, et al. Protective effect of hydrogen sulphide against 6-OHDA-induced cell injury in SH-SY5Y cells involves PKC/PI3K/Akt pathway. Br J Pharmacol. 2010;161(2):467–80. doi: 10.1111/j.1476-5381.2010.00887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schwarz G. Molybdenum cofactor and human disease. Curr Opin Chem Biol. 2016;31:179–87. doi: 10.1016/j.cbpa.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 84.Hille R, et al. The mononuclear molybdenum enzymes. Chem Rev. 2014;114(7):3963–4038. doi: 10.1021/cr400443z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Relinque B, et al. Isolated sulfite oxidase deficiency. J Neonatal Perinatal Med. 2015 doi: 10.3233/NPM-15814029. [DOI] [PubMed] [Google Scholar]
- 86.Tiranti V, Zeviani M. Altered sulfide (H(2)S) metabolism in ethylmalonic encephalopathy. Cold Spring Harb Perspect Biol. 2013;5(1):a011437. doi: 10.1101/cshperspect.a011437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kabil O, Banerjee R. Characterization of patient mutations in human persulfide dioxygenase (ETHE1) involved in H2S catabolism. J Biol Chem. 2012;287(53):44561–7. doi: 10.1074/jbc.M112.407411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Roede JR, et al. Characterization of plasma thiol redox potential in a common marmoset model of aging. Redox Biol. 2013;1:387–93. doi: 10.1016/j.redox.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ravindranath V, et al. Low glutathione levels in brain regions of aged rats. Neurosci Lett. 1989;101(2):187–90. doi: 10.1016/0304-3940(89)90528-4. [DOI] [PubMed] [Google Scholar]
- 90.Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273(5271):59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Emir UE, et al. Noninvasive quantification of ascorbate and glutathione concentration in the elderly human brain. NMR Biomed. 2011;24(7):888–94. doi: 10.1002/nbm.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Balaban RS, et al. Mitochondria, oxidants, and aging. Cell. 2005;120(4):483–95. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 93.Sinha-Hikim I, et al. Long-term supplementation with a cystine-based antioxidant delays loss of muscle mass in aging. J Gerontol A Biol Sci Med Sci. 2013;68(7):749–59. doi: 10.1093/gerona/gls334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tosukhowong P, et al. Biochemical and clinical effects of Whey protein supplementation in Parkinson's disease: A pilot study. J Neurol Sci. 2016;367:162–70. doi: 10.1016/j.jns.2016.05.056. [DOI] [PubMed] [Google Scholar]
- 95.Micke P, et al. Oral supplementation with whey proteins increases plasma glutathione levels of HIV-infected patients. Eur J Clin Invest. 2001;31(2):171–8. doi: 10.1046/j.1365-2362.2001.00781.x. [DOI] [PubMed] [Google Scholar]
- 96.Kabil H, et al. Increased transsulfuration mediates longevity and dietary restriction in Drosophila. Proc Natl Acad Sci U S A. 2011;108(40):16831–6. doi: 10.1073/pnas.1102008108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Uthus EO, Brown-Borg HM. Methionine flux to transsulfuration is enhanced in the long living Ames dwarf mouse. Mech Ageing Dev. 2006;127(5):444–50. doi: 10.1016/j.mad.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ohno K, et al. Endogenous S-sulfhydration of PTEN helps protect against modification by nitric oxide. Biochem Biophys Res Commun. 2015;456(1):245–9. doi: 10.1016/j.bbrc.2014.11.066. [DOI] [PubMed] [Google Scholar]
- 99.Kimura H. Hydrogen sulfide: its production, release and functions. Amino Acids. 2011;41(1):113–21. doi: 10.1007/s00726-010-0510-x. [DOI] [PubMed] [Google Scholar]