Abstract
Objective
To determine relationships of memory complaints to cognitive function and decline, incident dementia, and neurodegenerative and other neuropathologies, as well as the population-attributable risk for dementia in older black and white persons.
Methods
4015 community-based persons (28% black; 74% women; mean baseline age 78 years), were enrolled in one of four longitudinal cohort studies, and another 2937 in a population-based cohort. Memory scores, assessed using two questions (5-point Likert scales) were categorized as complaints present or absent. Global cognition and five cognitive domains were derived from annual neuropsychological tests. Dementia was assessed from these tests and additional data. Neuropathologic data were available for 1350 deceased with brain autopsies. Regression and mixed effects models were used to examine relationships of memory complaints to cognition and neuropathology.
Results
Baseline memory complaints (n=1310; 33% of 4015) were associated with lower cognition and faster decline in all domains (global score estimate = −0.032 [SE=0.004], p<0.0001), during a mean follow-up of 6 (SD=2) years. Persons with memory complaints had higher dementia risk (HR=1.64; 95%CI: 1.42–1.89) and odds of pathologic Alzheimer’s disease (OR=1.96; 95%CI: 1.51–2.54), neocortical Lewy bodies (OR=2.47, 95%CI: 1.54–3.96), and other neurodegenerative pathologies. Results for dementia risk were similar among blacks and whites. Among 2937 older persons in a population-based cohort with similar data, the population-attributable risk for incident dementia due to memory complaints was 14.0% (95%CI: 2.6–23.0), and did not vary between the black and white groups.
Interpretation
Memory complaints are common in older black and white persons, and relate to cognitive decline, dementia risk, and neurodegenerative pathologies.
Keywords: Memory complaint, dementia, Alzheimer’s disease
Introduction
Dementia is among the most common, disabling, costly, and deadly conditions of aging, and presents a major challenge to public health in high, but also increasingly, in middle- and low-income nations.1 With the development of dementia treatments since the early 1990’s and promise of more effective treatments and preventions, the efficient identification of persons at-risk for dementia is a high priority. Memory complaints are among the most common cognitive complaints in aging, and their assessment is simple, rapid, inexpensive, and safe. Yet, while memory complaints have been associated with higher risk of progression to dementia, other data suggest progression is not certain, and results of studies in the field are challenging to compare and contextualize, in part given differing methods to recruit study participants and to ascertain subjective memory complaints.2–4 Further, associations of memory complaints with cognitive decline, especially in different domains and among different racial groups, but also with underlying neuropathology, remain unclear. Thus, further scientific knowledge about memory complaints and dementia is needed.
Here, we used data from four longitudinal community-based cohort studies of more than 4000 diverse older women and men, to determine the relationship of memory complaints to baseline and incident dementia, as well as cognitive level and decline in five different cognitive domains. We also examined for racial differences in these relationships between blacks and whites. Among a large number of deceased and autopsied participants (n=1350), we examine for underlying mechanisms linking memory complaints to dementia, by assessing the associations of complaints with a range of neurodegenerative and other common neuropathologies of aging. Finally, using data from an independent sample of approximately 3000 older persons participating in a population-based study, we determine for the first time, the population-attributable risk for dementia due to memory complaints.
Participants and Methods
Cohorts
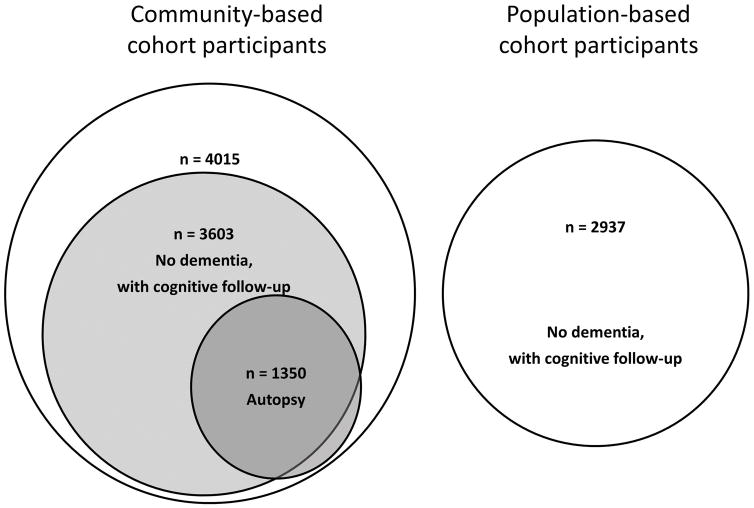
This research was approved by the Institutional Review Board of Rush University Medical Center. To analyze clinical and neuropathologic data, we used data from participants who were enrolled in one of four longitudinal, community-based cohort studies of aging and cognition (Figure 1, left side). Information regarding obtaining data for research use can be found at the Rush Alzheimer’s Disease Center Research Resource Sharing Hub (www.radc.rush.edu). Because the four studies were designed to have essentially identical recruitment techniques and a large overlap of data collection including cognitive testing, combining data from the cohorts increases statistical power and facilitates studies on risk and protective factors in health and aging in a large group of older women and men. Briefly, the Religious Orders Study (ROS)5 began enrolling Catholic clergy men and women in 1994, and of 1412 persons enrolled to-date, 1294 met inclusion criteria (age older than 65 years, with valid data on demographic variables, memory complaints, and cognition) and were included in analyses of cognitive outcomes. Of the 756 participants who died over the course of the study, 700 came to autopsy, of which 672 had neuropathologic data collected to date for inclusion in this study. The Rush Memory and Aging Project (MAP)6 began in 1997, and of 1930 lay-persons in the Chicagoland area enrolled, 1758 were included in analyses. Of 867 who died, 707 came to autopsy, and 655 were included in analyses. The Minority Aging Research Study (MARS)7 has enrolled 732 blacks since 2004, of which 677 were included in this study. Of 134 who have died, 21 came to autopsy, and 19 were included in the analyses. From the Rush Alzheimer’s Disease Core Center Clinical Core,7 286 black participants without dementia were included in this study, of which 4 were also included in analyses with neuropathologic outcomes. In total across the four community-based cohort studies, there were 4015 persons included in cross-sectional analyses and 3603 in longitudinal analyses with cognitive outcomes, and 1350 deceased and autopsied persons included in cross-sectional analyses with neuropathologic outcomes (Figure 1, left side).
Figure 1.
For separate analyses to determine the population-attributable risk for dementia, data from participants in the Chicago Health and Aging Project (CHAP),8 a population-based biracial study of adults older than 65 years and living in four geographically-defined Chicago communities, were also used. The clinical evaluations were identical in all respects to the other four cohort studies described above. The dementia risk was based on the same two memory complaint questions from 2937 participants without baseline dementia (Figure 1, right side). For these participants, the mean age was 78.2 years and education was 13.3 years, and 65.7% were females and 57% black.
Clinical evaluations
Participants in four community-based cohorts underwent annual uniform, structured clinical evaluations, including a detailed medical history, physical examination, and neuropsychological testing.5,6 A battery of neuropsychological tests included 19 tests selected to assess a range of cognitive systems, which were grouped to form composite measures of cognition in five cognitive domains, and a measure of overall global cognitive function.9,10 Briefly, there were seven tests of episodic memory (immediate and delayed recall of Story A of the Wechsler Memory Scale-Revised; immediate and delayed recall of the East Boston Story; Word List Memory, Recall and Recognition), three tests of semantic memory (Verbal Fluency; Boston Naming; Reading Test), three of working memory (Digit Span forward and backward; Digit Ordering), four of perceptual speed (Symbol Digit Modalities Test; Number Comparison; two indices from a modified version of the Stroop Test), and two of visuospatial ability (Line Orientation; Progressive Matrices).9 Composite measures of each domain were used in analyses, as well as a global composite of all tests. To create each composite score, individual tests were converted to z scores, using the mean and standard deviation from the combined cohort at baseline, and z scores for the relevant tests were averaged. Further information about the individual tests and five composite scores is published elsewhere.9,10 The Mini-Mental State Examination (MMSE) was also available but not used in the composite. A clinician with dementia expertise reviewed clinical data and classified participants by dementia status, as well as Alzheimer’s disease (AD) and mild cognitive impairment (MCI).5,6 Evaluations also included data on medical conditions, including depression and vascular diseases.5,6
Memory complaints were assessed by two questions on 5-point Likert scales: a) how often memory problems are experienced (coded as 1=never, 2=rarely, 3=sometimes, 4=often, 5=very often); and b) how much worse their memory is compared to 10 years before (1=much better, 2=little better, 3=same, 4=little worse, 5=much worse).11 The sum of the two scores is a memory score, classified as memory complaints if the score is 8 to 10. For deceased, we determined whether memory complaints had ever been reported across the study.
Neuropathology
Structured postmortem neuropathologic evaluations (mean postmortem interval =9.1 (SD=8.5) hours across the four community-based studies) allow for the determination of a pathologic diagnosis of AD,12 as well as measures of individual AD pathologies (plaques and tangles) and other neurodegenerative pathologies including Lewy bodies and others.5,6 Vascular pathologies, including infarcts and cerebral vessel pathologies, are also recorded.5,6
Data analyses
Statistical models included terms to control for age, sex, and education. Primary analyses of associations of baseline cognition with memory complaints employ linear regression analyses of cognitive scores. Additional analyses examined racial differences by repeating primary analyses separately for blacks and whites, as well as by examining if models with terms for black race and for the interaction of memory complaints with black race gave similar results. Further analyses checked for potential confounding effects by adding select clinical factors. Associations with baseline clinical diagnoses were analyzed via logistic regression. Mixed effects regression models employed longitudinal cognitive scores with years from baseline as the time variable. Separate proportional hazards models were employed to examine associations of baseline memory complaints with times to incident dementia, AD, and MCI. Additional analyses examined racial differences. Among persons with neuropathologic data, associations of memory complaints at any time point in the study with pathology findings, employed linear regression models of continuous pathology measures (overall AD pathology; neuritic plaques, diffuse plaques, neurofibrillary tangles) and logistic regression for binary measures (presence of neuropathologic diagnosis of AD, neocortical Lewy bodies, and other neurodegenerative and cerebrovascular pathologies). Finally, a weighted logistic regression model was used to examine the association of memory complaints and incident dementia, and the population-attributable risk was based on the overall incidence of dementia and a nonlinear combination of logistic parameters for relative risk estimate. Additional analyses of race differences were conducted in the total sample (57% black), using an interaction of memory complaints and the indicator for black race. Analyses were programmed in SAS v9.4 (SAS Institute Inc).
Results
Community-based participants and memory complaints
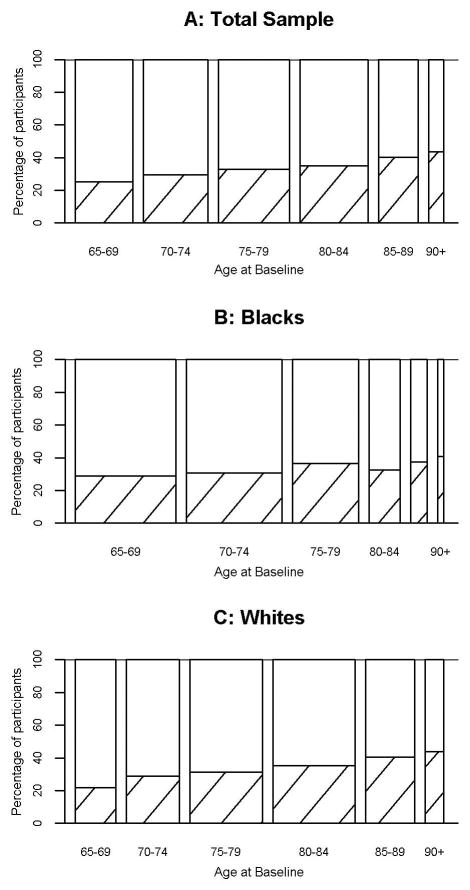
Characteristics of the 4015 participants in the four community-based cohorts are shown in Table 1. At study entry, memory complaints were common, with the mean score on the 10 point scale =7.12 (SD=1.37), and 1310 (32.6%) participants having complaints (score>7). Figure 2 illustrates percentages by age group, and blacks and whites separately. Memory scores tended to be greater among those older at enrollment (Spearman r=0.11). Complaints became more common during follow-up and were present on at least one observation in 1272 (53.0%) participants. Complaints were also fairly consistent within person over time. Overall, about 80% of persons who did not report memory complaints at any one visit, did not report complaints the next year. Among persons who did not have any cognitive impairment either year, 82.5% remained without complaints. Among those with memory complaints at one year, approximately 65% reported complaints the following year.
Table 1.
Baseline clinical characteristics* of 4015 participants in four community-based cohort studies of aging, according to baseline memory complaints**
| Characteristic* | Persons with memory complaints n = 1310 |
Persons without memory complaints n = 2705 |
Total group n = 4015 |
|---|---|---|---|
| Age, years | 78.8 (7.3) | 77.1 (7.2) | 77.7 (7.2) |
| Female sex, n (%) | 949 (72.4%) | 2012 (74.4 %) | 2961 (73.7%) |
| Black, n (%) | 362 (27.7%) | 769 (28.5 %) | 1131 (28.2%) |
| Education, years | 15.7 (3.7) | 15.9 (3.8) | 15.8 (3.8) |
| MMSE score*** | 27.1 (3.3) | 28.0 (2.4) | 27.7 (2.7) |
| Global cognitive score | −0.169 (0.676) | −0.064 (0.596) | −0.012 (0.633) |
| Depressive symptoms score**** | 1.50 (1.82) | 0.97 (1.49) | 1.14 (1.61) |
Values are mean (SD), unless otherwise specified
Persons with memory complaints are those whose memory score was 8 or more on the sum of two questions; see methods for further description
MMSE: Mini-Mental State Examination; maximal (highest) score is 30
Based on the Center for Epidemiologic Studies Depression Scale (CES-D; range: 0–10): number of 10 depressive symptoms endorsed
Figure 2.
Baseline clinical diagnosis and cognition
Persons with memory complaints at baseline had an 80% higher odds of cognitive impairment at study entry (n=1184), MCI or dementia (OR=1.78; 95%CI: 1.54–2.07). Separate analyses of blacks (n= 1131) and whites (n= 2849) showed similar results, with OR=1.81 (95%CI: 1.36–2.41) for blacks and OR=1.75 (95%CI: 1.47–2.09) for whites. Complaints were associated with lower scores in global cognition (equivalent to being seven years older at study entry without complaints) and in four cognitive domains (episodic memory, semantic memory, perceptual speed, and visuospatial ability; Table 2). In additional analyses of blacks only, memory complaints were related to global cognition, as well as episodic memory and perceptual speed domains (Table 2). Results of analyses in whites only were similar to those found in the total group (Table 2). In the total group, persons with memory complaints had lower MMSE scores. Findings were consistent across cohorts, including among blacks and whites, and preserved when controlling for number of depressive symptoms or of vascular diseases (not shown).
Table 2.
Relation of memory complaints at baseline, to baseline level of and change in cognitive function, in the total group of community-based participants, and in blacks and whites separately
| Cognitive score | Baseline effect* Estimate (SE), p |
Longitudinal effect** Estimate (SE), p |
|---|---|---|
| Global cognitive score | ||
| Total group | −0.175 (0.019),<0.0001 | −0.032 (0.004), <0.0001 |
| Blacks only | −0.090 (0.032), 0.005 | −0.014 (0.007), 0.046 |
| Whites only | −0.196 (0.022) ),<0.0001 | −0.041 (0.005), <0.0001 |
| Episodic memory | ||
| Total group | −0.267 (0.023), <0.0001 | −0.037 (0.005), <0.0001 |
| Blacks only | −0.154 (0.039),<0.0001 | −0.022 (0.009), 0.013 |
| Whites only | −0.302 (0.028), <0.0001 | −0.045 (0.006),<0.0001 |
| Semantic memory | ||
| Total group | −0.165 (0.026), <0.0001 | −0.038 (0.005), <0.0001 |
| Blacks only | −0.016 (0.049), 0.735 | −0.016 (0.009), 0.081 |
| Whites only | −0.204, (0.029),<0.0001 | −0.049 (0.006),<0.0001 |
| Working memory | ||
| Total group | −0.043 (0.025), 0.088 | −0.014 (0.003), <0.0001 |
| Blacks only | −0.004 (0.046), 0.938 | −0.006 (0.006), 0.331 |
| Whites only | −0.048 (0.030), 0.101 | −0.017 (0.004),<0.0001 |
| Perceptual speed | ||
| Total group | −0.170 (0.028), <0.0001 | −0.018 (0.004), <0.0001 |
| Blacks only | −0.133 (0.051), 0.009 | −0.012 (0.007), 0.096 |
| Whites only | −0.168 (0.032),<0.0001 | −0.022 (0.005),<0.0001 |
| Visuospatial ability | ||
| Total group | −0.053 (0.026), 0.043 | −0.011 (0.004), 0.003 |
| Blacks only | 0.002 (0.051), 0.965 | −0.005 (0.007), 0.478 |
| Whites only | −0.056 (0.029), 0.0503 | −0.013 (0.004), 0.001 |
Separate linear regression analyses, adjusted for age, sex, and education; among 4015 participants (1131 blacks and 2849 whites)
Separate mixed effects analyses, adjusted for age, sex, and education; among 3603 participants (1012 blacks and 2553 whites) with longitudinal data available for analyses
Incident dementia and cognitive decline
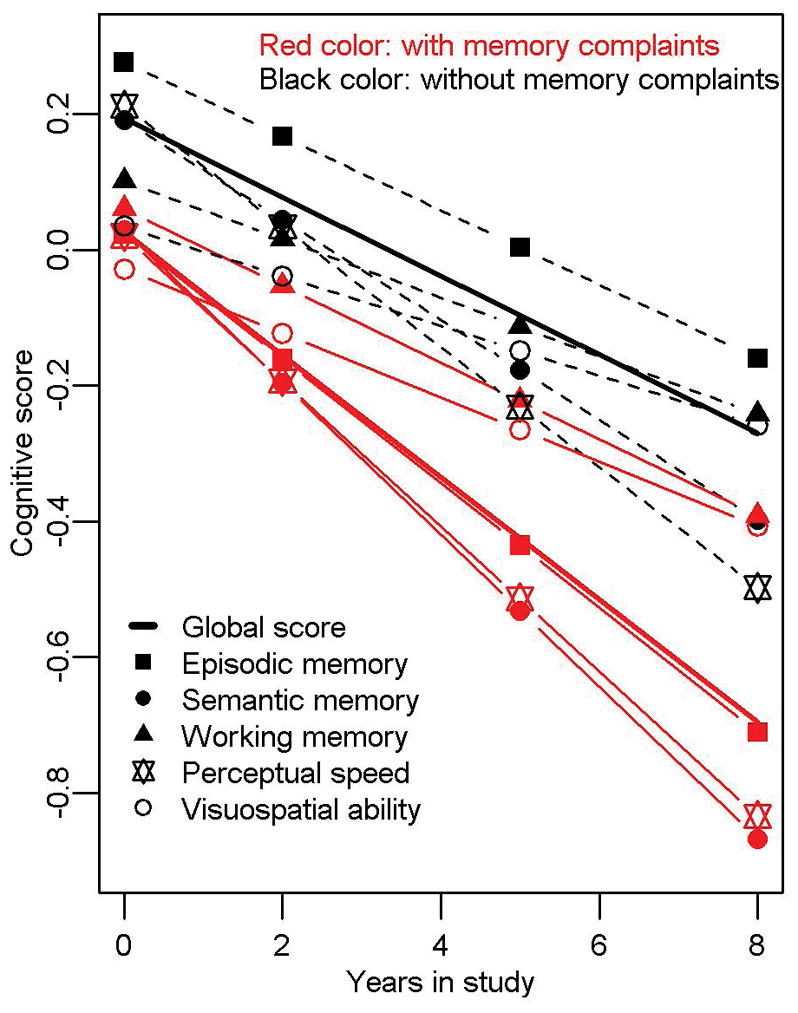
Among 3603 participants with ≥2 cognitive visits available for longitudinal analyses, the mean follow-up was 6 (SD=2) years, and 828 developed dementia. Baseline memory complaints were related to incident dementia (HR=1.64; 95%CI: 1.42–1.89; Figure 3), as well as to dementia-attributable to AD (HR=1.64; 95%CI: 1.42–1.90). Among those without baseline dementia or MCI, memory complaints were related to incident MCI (HR=1.19; 95%CI: 1.05–1.36). Analyses of blacks and whites separately showed significant increased risks in both races. For blacks, the HR=2.31; 95%CI: 1.57–3.41 for incident dementia and HR=2.18; 95%CI: 1.47–3.23 for dementia-attributable to AD. For whites, the HR=1.54; 95%CI: 1.33–1.81 for incident dementia and HR=1.56; 95%CI: 1.33–1.83 for dementia-attributable to AD. In additional analyses in the total group, there was weak evidence for an interaction of memory complaints by race for incident dementia (p for interaction =0.048), but not for incident dementia-attributable to AD (p for interaction >0.1). Persons with memory complaints declined more rapidly in global cognition and in all cognitive domains (Table 2, pFigure 4), as well as in the MMSE. The average decline in global cognition for those with complaints was equivalent to that for persons seven years older without complaints. In additional analyses of blacks only, memory complaints were related to decline in global cognition and in episodic memory specifically, but there was no relation with decline in the other cognitive domains (Table 2). In whites only, results were consistent with those in the total group (Table 2). In an additional analysis in the total group for decline in global cognition, the effect of memory complaints on cognition differed by race ( for interaction =0.0041), with blacks having slower decline than whites.
Figure 3.
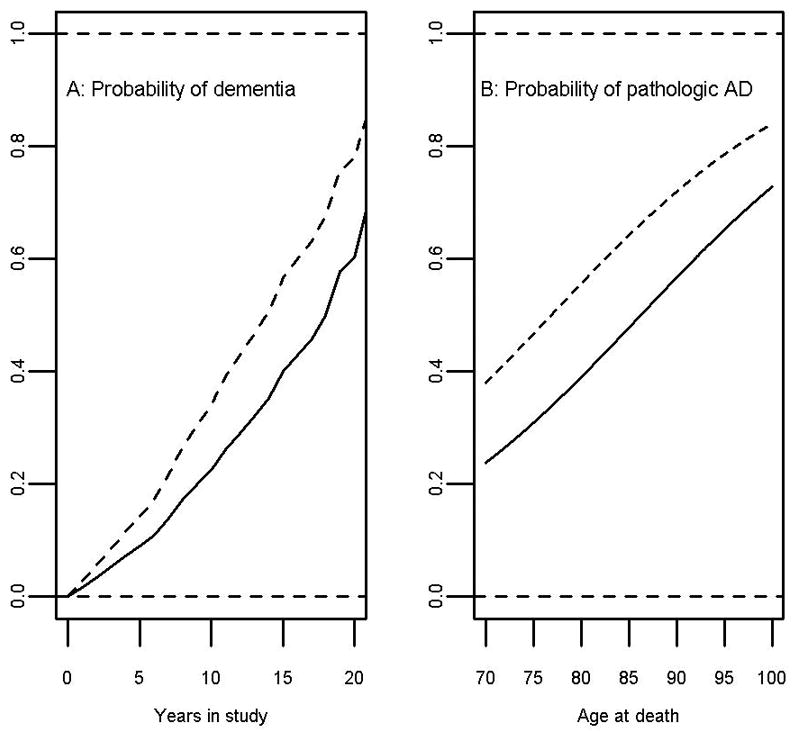
Figure 4.
We conducted additional analyses among the 3471 participants with ≥2 cognitive visits available for longitudinal analyses and without baseline dementia. The frequency of memory complaints at baseline by incident dementia is shown in the Table 3. The sensitivity of baseline memory complaints for predicting incident dementia was low, at 42% (351/1828), and the specificity was 72% (1894/2643). The crude positive predictive value of memory complaints for developing dementia was 32 % ([351/1100] ×100) and the crude negative predictive value was 80 % ([1894/2371] ×100).
Table 3.
Frequency of memory complaints at baseline, by incident dementia
| Incident dementia | |||
|---|---|---|---|
| Yes | No | TOTAL | |
| Memory complaints | |||
| Yes | 351 | 749 | 1100 |
| No | 477 | 1894 | 2371 |
| TOTAL | 828 | 2643 | 3471 |
Neuropathology
Among 1350 deceased participants with neuropathologic data available, the odds of a pathologic diagnosis of AD was almost doubled for those with memory complaints at any time in the study, compared to those without (OR=1.96; 95%CI: 1.51–2.54; Figure 3). Furthermore, complaints were associated with overall AD pathology, as well as individual measures of neuritic plaques, diffuse plaques, and neurofibrillary tangles (all p <0.01). Complaints were also associated with neocortical Lewy bodies (OR=2.47; 95%CI: 1.54–3.96), TDP-43 (OR=1.33; 95%CI: 1.02–1.73), hippocampal sclerosis (OR=2.90; 95%CI: 1.48–5.67), and amyloid angiopathy (OR=1.46; 95%CI: 1.15–1.84), but not with gross infarcts, microinfarcts (p >0.9), or cerebral vessel pathologies including atherosclerosis or arteriolosclerosis (not shown). In additional analyses separately for the 54 blacks with neuropathologic data available, no associations of memory complaints with AD or other pathologies were found (data not shown).
Among the 1350 deceased participants with neuropathologic data available, there were 427 with no cognitive impairment (meaning, no mild cognitive impairment and no dementia) as the summary diagnosis proximate to death. Of these 427 participants, 254 (59.5%) had memory complaints at some time over the course of the study. The postmortem neuropathologic evaluation of the brain of these 427 participants did exhibit a range of neuropathologies: most commonly, AD (181/427, 42.4%). Six other neuropathologies were seen in around 20% to 30%: moderate/severe arteriolosclerosis in 118/423 (27.9%), microinfarcts in 111/426 (26.1%), moderate/severe atherosclerosis in 109/424 (25.7%), gross infarcts in 106/426 (24.9%), moderate/severe cerebral amyloid angiopathy in 103/413 (24.9%), and limbic/cortical TDP43 in 66/346 (19.1%). Two pathologies were rarer: cortical Lewy bodies (24/427, 5.6%) and hippocampal sclerosis (6/424, 1.4%). We conducted analyses restricted to this subgroup of 427 participants with no cognitive impairment, adjusted for age, sex, and education, to examine whether memory complaints were associated with neuropathology. Results showed that there were no associations of memory complaints with any of the measures of neuropathology, whether for the pathologic diagnosis of AD (p =0.25), or for the other AD measures including overall AD pathology (p =0.12), and individual measures of neuritic plaques (p =0.09), diffuse plaques (p =0.26), and neurofibrillary tangles (p =0.48), or measures of neocortical Lewy bodies (p =0.65), TDP-43 (p =0.93), hippocampal sclerosis (p =0.30), or for gross infarcts (p =0.58), microinfarcts (p =0.81), atherosclerosis (p =0.96), arteriolosclerosis (p =0.68), and amyloid angiopathy (p =0.31).
Population-attributable risk
The population-based study collected the same information on memory complaints and conducted similar clinical evaluations as the other four cohorts. In this study of 2937 persons, 22.4% had baseline memory complaints and 9.8% developed dementia. Memory complaints increased incident dementia risk (HR=1.73; 95%CI: 1.12–2.33). The population-attributable risk for incident dementia based on memory complaints was 14.0% (95%CI: 2.6–23.0) after adjusting for age, sex, education, and race. Inclusion of the terms for race and the interaction of memory complaints by race, showed that the population-attributable risk for incident dementia did not vary between the black and white groups (11.0% vs. 13.7%, p difference=0.68).
Discussion
Of more than 4000 diverse community-dwelling older persons followed annually for an average of 6 years, memory complaints were present in a third of individuals at baseline and associated cross-sectionally with cognitive impairment and lower cognitive function. Further, memory complaints were associated with a more than 60% increased dementia risk and a faster rate of cognitive decline across a range of domains, including memory and perceptual speed. These associations were similar among blacks and whites. Among 1350 deceased persons with neuropathologic data, memory complaints at any point during follow-up were associated with a two-fold higher odds of pathologically-diagnosed AD, and with Lewy bodies and other neurodegenerative pathologies, but not cerebrovascular disease. In separate analyses using data from about 3000 persons from a population-based cohort, the population-attributable risk for incident dementia based on memory complaints was 14%, and similar among blacks and whites.
Literature on memory complaints in aging is difficult to interpret due to differing methods in assessment (e.g., with or without accompanying objective impairment), terminologies employed, populations studied, study design used, and consideration of other factors (e.g., education, depression).13,14 Of particular note, how the memory question(s) are phrased and what groups are studied (e.g., clinic-based vs otherwise) influence responses and therefore results. These and other factors contribute to the controversy regarding the significance of memory complaints, including whether these complaints are simply part of “normal aging”, whether they are the earliest manifestation of AD, and/or represent some other pathologic or experiential process (e.g., personal factors, social constructs, or external factors, including as related to race or environment). In the present study, 33% of older community-dwelling persons and 22% of a population-based cohort had memory complaints (mean age=78), based on two questions about current frequency and comparison to 10 years prior. This percentage is in keeping with smaller studies of community-dwelling persons suggesting a relatively-high frequency of these complaints in older persons,15 but higher than in other reports.16,17 Moreover, consistent with the literature,13 we found that memory complaints increase with increasing age, further supporting the need to understand the relevance of these complaints. And, while there is variability over time in the self-report of memory complaints (consistent with other self-report measures), our measure showed reasonable reliability over time. Nevertheless, interpretation of results on the relation of memory complaints to cognition should be considered at the group level rather than individual level.
Data on the clinical relevance of memory complaints has been mixed. Recent cross-sectional studies show that persons with early dementia or MCI may indeed have memory complaints,18 and complaints may be associated with lower cognition,19 negative well-being,20,21 and mortality.22 Further, many but not all longitudinal studies17,23,24 and recent meta-analyses2,3 suggest that memory complaints predict incident dementia and MCI, and a more precipitous cognitive decline. But other data suggest complaints do not always herald cognitive impairment,4,25 that informant- rather than participant-reported complaints are more predictive,26 or that associations between complaints and demonstrable impairment vary over time.27 Among the largest longitudinal studies, including 2415 persons ≥75 years in a primary care medical registry, memory complaints increased dementia risk at three years of follow-up.28
Our study expands the scientific knowledge about memory complaints in aging in several important ways. Combining four community-based cohort studies with similar data collection, in a large diverse sample of both older women and men, memory complaints were found to be cross-sectionally associated with dementia and lower cognition, including lower MMSE score, one of the most widely-used neuropsychological tools in the assessment of dementia. This suggests that the two memory questions used may be a simple tool to use in the initial assessment for the presence of cognitive impairment. Furthermore, over an average of 6 years of annual data collection, memory complaints increased dementia risk by 64%, including AD and MCI separately, and were related to faster rates of cognitive decline. Interestingly, while decline in memory would be expected given the complaint, we found decline in all five cognitive domains assessed. These results shed some light on possible underlying brain networks involved in linking memory complaints to dementia.
Our data further advances knowledge about race, memory complaints, and cognition. To date, the relation of subjective to objective memory in blacks is unclear29 and there are few data available on memory complaints and cognition in a large group of both blacks and whites. One study of nearly 1700 blacks and 1400 whites, showed that memory complaints were not related to cognitive decline in a single measure of global cognition, but analyses examining specifically for race effects were not done.30 Similarly, a more recent study reported that subjective cognitive concerns were associated with memory performance in whites but not blacks; however they had a small sample of blacks and may have been limited in power.31 Interestingly, they concluded that cognitive concerns may differ as a function of race and that blacks may endorse cognitive concerns differently than whites. In our study, we found that memory complaints were cross-sectionally associated with increased odds of having cognitive impairment, and with lower levels in cognitive function, in blacks and whites separately, suggesting that memory complaints are operating the same across race and there are no significant racial differences in these relations. Further, in longitudinal analyses of blacks and whites separately, memory complaints were related to higher risk of dementia and of dementia-attributable to AD, as well as to faster rates of decline in cognitive function, in each group of blacks and in whites. And, while blacks seem to have higher risk for incident dementia than whites (HR =2.31 in blacks, and HR =1.54 in whites), the relation of memory complaints to risk of incident dementia did not clearly differ across race. We did, however, find that the relation of memory complaints to decline in different cognitive domains was significant only in episodic memory in blacks, but in all domains in whites. So although we found that the patterns of memory complaints and cognitive performance were similar between blacks and whites, the associations of complaints with cognition appears to be weaker among blacks compared to whites. Reasons for these different patterns across race are not clear but could be due to the fact that we had a larger proportion of blacks at younger ages and memory complaints are related to age. Also, we had fewer blacks overall to compare to whites, and power may have been inadequate to detect some relations in the smaller sample of blacks (1012 blacks, compared to 2553 whites). But our results also suggest no clear evidence for effect modification by race in the relation of memory complaints to dementia, as shown by the population-attributable risk for incident dementia not being significantly different for blacks compared to whites in our population-based cohort. Thus, future work is needed to characterize relationships of memory complaints to cognition by race.
The basis for the relation of memory complaints to dementia is uncertain. One possibility is that another factor associated with both complaints and dementia may account for their relation. While depression and vascular diseases (e.g., stroke) may be confounders, we did not find evidence for this. Another possibility is that memory complaints are an early manifestation of underlying neuropathology which may lead to the clinical expression of dementia. Indeed, several studies have found that memory complaints are associated with brain abnormalities, including in cerebrospinal fluid32 and on neuroimaging.33 However, only neuropathologic evaluation provides direct visualization of AD and allows for simultaneous consideration of other common causes of dementia such as Lewy bodies. We are aware of only three previously published studies that used human brain tissue to examine the relation of memory complaints to neuropathology, and all showed a relation to AD but included a much smaller number of participants and did not examine a wide range of neuropathologies. In the first study of 237 men, complaints were associated with pathologic AD using two sets of criteria, as well as with Lewy bodies and hippocampal sclerosis.34 The second, by our group, in a small subset of 90 autopsied participants in the current study, showed that complaints were associated with both amyloid and tangles.11 In the most recent study of 243 persons, those with complaints had higher levels of AD-type pathology (plaques), even when cognitive impairment was not present.35 The current study supports these findings of a relation with AD pathology, and expands on earlier data, with a larger number of persons with neuropathologic data (n=1350), inclusion of both women and men, and a wider range of pathologic conditions considered, including neurodegenerative and cerebrovascular. We found that memory complaints were associated with a pathologic diagnosis of AD, as well as higher levels of neuritic plaques, diffuse plaques, and neurofibrillary tangles. Furthermore, complaints were associated with several other common neurodegenerative pathologies of dementia, including neocortical Lewy bodies,36 TDP-43, hippocampal sclerosis, and amyloid angiopathy. While clinical data suggest that memory complaints increase stroke risk,37 and we found an association of complaints with perceptual speed (commonly associated with vascular processes), we did not find evidence for associations with infarcts or cerebral vessel pathologies. The reason for this finding is unclear and more research is needed to examine potential associations between memory complaints and vascular pathologies. In addition, several clinical and other factors have now been identified as predicting cognitive decline and dementia-attributable to AD, and yet are not found to be associated with any of the key defining characteristics of AD neuropathology such as plaques or tangles.38–40 This underscores that much needs to be learnt about neuropathology of cognitive decline in aging beyond the known neuropathologies of dementia.
Importantly, a novel contribution of this study is the examination of the population-attributable risk for incident dementia from memory complaints. In this case, the attributable risk does not likely imply causality. Indeed, memory complaints are more likely to be an early symptom of pre-clinical AD and other neurodegeneration, occurring on the pathway to cognitive decline and dementia, before objective impairment is demonstrable. Our findings suggest that 14 out of 100 older persons who develop dementia could be identified by the two memory questions used in our study, and that these questions may be informative for both groups of blacks and whites.
There is a need to develop standardized approaches to study and care for persons with memory complaints, including determining which complaints to solicit and not missing potential opportunities to manage cognitive impairment.41,42 US federal laws stipulate that Medicare recipients be covered for annual cognitive assessment and planning of dementia care (S.857, H.R.1559). However, there is no widely-accepted approach to screen for cognitive impairment in primary care43 and a recent systematic review concluded that the evidence for screening is lacking in the absence of a complaint.44 These conflicting messages create a quandary for primary care clinicians. For clinicians who conduct annual cognitive assessments in the current environment of uncertainty, two questions about memory may be a pragmatic way to identify persons requiring monitoring for dementia. If a person in the health care system is identified as having memory complaints, for instance on review of systems, then a cognitive screen via the MMSE or otherwise, could be the next step in a clinical evaluation, along with a medical history, examination, and testing as indicated (e.g., blood work, neuroimaging, neuropsychological testing). Indeed, our study along with others,11,45,46 suggest that asking about memory complaints in older persons may offer some, albeit incomplete, prognostic value regarding cognitive decline, dementia risk, and dementia etiology, that could be useful in shared decision making regarding care and management approaches to cognitive concerns. Given challenges with establishing dementia biomarkers, the assessment of memory complaints may be a useful tool for identifying persons at-risk for dementia, as well as for clinical trials enrichment, particularly as it is simple, rapid, inexpensive, safe, and practical for implementation in large and underserved populations.47 Also, memory complaints are more easily assessed compared to commonly-used bed-side dementia screens.48 While we found a 14% population-attributable risk for incident dementia due to memory complaints, further research into the properties and utility of memory complaints as a potential option for identifying who might benefit from screening for cognitive impairment in primary care settings is needed.
This study has several limitations. First, memory complaints were assessed in a limited fashion, using two simple questions, and data (on a 10 point scale) were used to dichotomize participants into having complaints (complaints present) vs. not having complaints. Given that subjective memory complaints have been studied using a variety of approaches across other studies, though in smaller samples, the fundamental issues about external validity of our measure remain. Given the low sensitivity and moderate specificity, the memory complaints measure is not appropriate to determine risk of dementia at the individual level. However, in our study and across several large cohorts, the measure appears to be informative at the group level. Further, the intermediate group of persons with memory score of 7 did not differ importantly from the reference group without complaints (score <7; data not shown) and our previous study of 90 persons using this memory score showed consistent results with higher scores being associated with AD pathology.11 A second limitation is that we did not consider incident memory complaints or worsening complaints over time, and further studies are needed to examine effects of these on risk of dementia. Third, we did not fully disentangle specific pathophysiologic pathways linking memory complaints to dementia. Fourth, power to detect race differences using postmortem autopsy data is limited in the current study, with only 54 blacks among 1350 deceased participants with neuropathologic data available. These limitations notwithstanding, the study has strengths. First, data were derived from a large number of about 4000 community-dwelling women and men who underwent systematic, annual clinical evaluations allowing for the relation of memory complaints to both a) baseline and change in cognitive function across different domains assessing a range of cognitive systems, and b) baseline and incident dementia, including AD and MCI. Annual assessments enhanced the ability to more accurately model change in cognition and identify incident conditions, over an average of 6 years, and up to 22 years of follow-up. Second, detailed neuropathologic data, collected blinded to clinical data, were available in the largest study to date, and we were able to examine associations of memory complaints with AD and other common neurodegenerative and cerebrovascular pathologies not detectable without autopsy. Finally, in another cohort of about 3000 persons with similar data collection, we determined for the first-time, the population-attributable risk of dementia for persons with memory complaints, further supporting the frequency and potential clinical relevance of ascertaining for these complaints.
Acknowledgments
We are grateful for the altruism of the thousands of participants in the Religious Orders Study, the Rush Memory and Aging Project, the Minority Aging Research Study, the Rush Alzheimer’s Disease Core Center Clinical Core, and the Chicago Health and Aging Project. We thank study coordinators, data and analytic programmers, and staff and faculty of the Rush Alzheimer’s Disease Center and the Rush Institute for Healthy Aging.
Funding: This work was supported by the Illinois Department of Public Health and the National Institutes of Health grants: P30 AG010161, RF1 AG015819, R01 AG17917, RF1 AG22018, R01 AG11101, R01 AG051635, R01 AG40039, R01 NS084965.
Supported by the Illinois Department of Public Health and the National Institutes of Health grants: P30 AG010161, RF1 AG015819, R01 AG17917, RF1 AG22018, R01 AG11101, R01 AG051635, R01 AG40039, R01 NS084965
Footnotes
Author Contributions
Study concept and design: Z.A., S.E.L., and D.A.B. Data acquisition and analysis: Z.A., S.E.L., D.A.F., J.A.S., K.B.R., D.A.E., L.L.B., and D.A.B. Manuscript and figure drafting and preparation: Z.A., S.E.L., D.A.F., J.A.S., K.B.R., J.J.P., R.C.S., D.A.E., L.L.B., and D.A.B.
Potential Conflicts of Interest
Nothing to report.
References
- 1.Alzheimer’s Disease International. World Alzheimer Report 2015. [Accessed March 15, 2017];The Global Impact of Dementia: An analysis of prevalence, incidence, cost & trends. http://www.worldalzreport2015.org/
- 2.Mitchell AJ, Beaumont H, Ferguson D, et al. Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: meta-analysis. Acta Psychiatr Scand. 2014;130(6):439–451. doi: 10.1111/acps.12336. [DOI] [PubMed] [Google Scholar]
- 3.Mendonça MD, Alves L, Bugalho P. From subjective cognitive complaints to dementia: Who is at risk? A systematic review. Am J Alzheimers Dis Other Demen. 2016;31(2):105–114. doi: 10.1177/1533317515592331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blackburn DJ, Wakefield S, Shanks MF, et al. Memory difficulties are not always a sign of incipient dementia: a review of the possible causes of loss of memory efficiency. Br Med Bull. 2014;112(1):71–81. doi: 10.1093/bmb/ldu029. [DOI] [PubMed] [Google Scholar]
- 5.Bennett DA, Schneider JA, Arvanitakis Z, Wilson RS. Overview and findings from the Religious Orders Study. Curr Alzheimer Res. 2012;9(6):628–645. doi: 10.2174/156720512801322573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennett DA, Schneider JA, Buchman AS, et al. Overview and findings from the Rush Memory and Aging Project. Curr Alzheimer Res. 2012;9(6):646–663. doi: 10.2174/156720512801322663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnes LL, Shah RC, Aggarwal NT, et al. The Minority Aging Research Study: ongoing efforts to obtain brain donation in African Americans without dementia. Curr Alzheimer Res. 2012;9(6):734–745. doi: 10.2174/156720512801322627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajan KB, Wilson RS, Weuve J, et al. Cognitive impairment 18 years prior to clinical diagnosis of Alzheimer’s disease dementia. Neurology. 2015;85(10):898–904. doi: 10.1212/WNL.0000000000001774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson RS, Beckett LA, Barnes LL, et al. Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging. 2002;17(2):179–193. [PubMed] [Google Scholar]
- 10.Barnes LL, Yumoto F, Capuano A, et al. Examination of the factor structure of a global cognitive function battery across race and time. JINS. 2016;22:66–75. doi: 10.1017/S1355617715001113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnes LL, Schneider JA, Boyle PA, et al. Memory complaints are related to Alzheimer disease pathology in older persons. Neurology. 2006;67(9):1581–1585. doi: 10.1212/01.wnl.0000242734.16663.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hyman BT, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 2012;8:1–13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jonker C, Geerlings MI, Schmand B. Are memory complaints predictive for dementia? A review of clinical and population-based studies. Int J Geriatr Psychiatry. 2000;15(11):983–991. doi: 10.1002/1099-1166(200011)15:11<983::aid-gps238>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 14.Alagoa João A, Maroco J, Ginó S, et al. Education modifies the type of subjective memory complaints in older people. Int J Geriatr Psychiatry. 2016;31(2):153–160. doi: 10.1002/gps.4305. [DOI] [PubMed] [Google Scholar]
- 15.Schofield PW, Marder K, Dooneief G, et al. Association of subjective memory complaints with subsequent cognitive decline in community-dwelling elderly individuals with baseline cognitive impairment. Am J Psychiatry. 1997;154(5):609–615. doi: 10.1176/ajp.154.5.609. [DOI] [PubMed] [Google Scholar]
- 16.Adams ML, Deokar AJ, Anderson LA, Edwards VJ. Self-Reported Increased Confusion or Memory Loss and Associated Functional Difficulties Among Adults Aged ≥60 Years: 21 States, 2011. Morbidity & Mortality Weekly Report. 2013;62(18):347–350. © 2013 Centers for Disease Control and Prevention (CDC) [PubMed] [Google Scholar]
- 17.Kaup AR, Nettiksimmons J, LeBlanc ES, Yaffe K. Memory complaints and risk of cognitive impairment after nearly 2 decades among older women. Neurology. 2015;85(21):1852–1858. doi: 10.1212/WNL.0000000000002153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Juncos-Rabadán O, Pereiro AX, Facal D, et al. Prevalence and correlates of mild cognitive impairment in adults aged over 50 years with subjective cognitive complaints in primary care centers. Geriatr Gerontol Int. 2014;14(3):667–673. doi: 10.1111/ggi.12157. [DOI] [PubMed] [Google Scholar]
- 19.Burmester B, Leathem J, Merrick P. Subjective Cognitive Complaints and Objective Cognitive Function in Aging: A Systematic Review and Meta-Analysis of Recent Cross-Sectional Findings. Neuropsychol Rev. 2016;26(4):376–393. doi: 10.1007/s11065-016-9332-2. [DOI] [PubMed] [Google Scholar]
- 20.Grut M, Jorm AF, Fratiglioni L, et al. Memory complaints of elderly people in a population survey: variation according to dementia stage and depression. J Am Geriatr Soc. 1993;41(12):1295–1300. doi: 10.1111/j.1532-5415.1993.tb06478.x. [DOI] [PubMed] [Google Scholar]
- 21.Zuniga KE, Mackenzie MJ, Kramer A, McAuley E. Subjective memory impairment and well-being in community-dwelling older adults. Psychogeriatrics. 2016;16(1):20–26. doi: 10.1111/psyg.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roehr S, Luck T, Bickel H, et al. AgeCoDe Study Group. Mortality in incident dementia - results from the German Study on Aging, Cognition, and Dementia in Primary Care Patients. Acta Psychiatr Scand. 2015;132(4):257–269. doi: 10.1111/acps.12454. [DOI] [PubMed] [Google Scholar]
- 23.Luck T, Luppa M, Matschinger H, et al. Incident subjective memory complaints and the risk of subsequent dementia. Acta Psychiatr Scand. 2015;131(4):290–296. doi: 10.1111/acps.12328. [DOI] [PubMed] [Google Scholar]
- 24.Rönnlund M, Sundström A, Adolfsson R, Nilsson LG. Subjective memory impairment in older adults predicts future dementia independent of baseline memory performance: Evidence from the Betula prospective cohort study. Alzheimers Dement. 2015;11(11):1385–1392. doi: 10.1016/j.jalz.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Silva D, Guerreiro M, Faria C, Maroco J, Schmand BA, Mendonça Ad. Significance of subjective memory complaints in the clinical setting. J Geriatr Psychiatry Neurol. 2014 Dec;27(4):259–265. doi: 10.1177/0891988714532018. [DOI] [PubMed] [Google Scholar]
- 26.Slavin MJ, Sachdev PS, Kochan NA, et al. Predicting Cognitive, Functional, and Diagnostic Change over 4 Years Using Baseline Subjective Cognitive Complaints in the Sydney Memory and Ageing Study. Am J Geriatr Psychiatry. 2015;23(9):906–914. doi: 10.1016/j.jagp.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Snitz BE, Small BJ, Wang T, et al. Do Subjective Memory Complaints Lead or Follow Objective Cognitive Change? A Five-Year Population Study of Temporal Influence. J Int Neuropsychol Soc. 2015;21(9):732–742. doi: 10.1017/S1355617715000922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jessen F, Wiese B, Bachmann C, et al. German Study on Aging, Cognition and Dementia in Primary Care Patients Study Group. Prediction of dementia by subjective memory impairment: effects of severity and temporal association with cognitive impairment. Arch Gen Psychiatry. 2010;67(4):414–422. doi: 10.1001/archgenpsychiatry.2010.30. [DOI] [PubMed] [Google Scholar]
- 29.Sims RC, Whitfield KE, Ayotte BJ, Gamaldo AA, Edwards CL, Allaire JC. Subjective memory in older African Americans. Exp Aging Res. 2011;37(2):220–240. doi: 10.1080/0361073X.2011.555640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blazer DG, Hays JC, Fillenbaum GG, Gold DT. Memory complaint as a predictor of cognitive decline: a comparison of African American and White elders. J Aging Health. 1997;9(2):171–184. doi: 10.1177/089826439700900202. [DOI] [PubMed] [Google Scholar]
- 31.Jackson JD, Rentz DM, Aghjayan SL, et al. Subjective cognitive concerns are associated with objective memory performance in Caucasian but not African-American persons. Age and Ageing. 2017;46(6):988–993. doi: 10.1093/ageing/afx077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Harten AC, Smits LL, Teunissen CE, et al. Preclinical AD predicts decline in memory and executive functions in subjective complaints. Neurology. 2013;81(16):1409–1416. doi: 10.1212/WNL.0b013e3182a8418b. [DOI] [PubMed] [Google Scholar]
- 33.Snitz BE, Weissfeld LA, Cohen AD, et al. Subjective Cognitive Complaints, Personality and Brain Amyloid-beta in Cognitively Normal Older Adults. Am J Geriatr Psychiatry. 2015;23(9):985–993. doi: 10.1016/j.jagp.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jorm AF, Masaki KH, Davis DG, et al. Memory complaints in nondemented men predict future pathologic diagnosis of Alzheimer disease. Neurology. 2004;63(10):1960–1961. doi: 10.1212/01.wnl.0000144348.70643.f2. [DOI] [PubMed] [Google Scholar]
- 35.Kryscio RJ, Abner EL, Cooper GE, et al. Self-reported memory complaints: implications from a longitudinal cohort with autopsies. Neurology. 2014;83(15):1359–1365. doi: 10.1212/WNL.0000000000000856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Erro R, Santangelo G, Barone P, et al. Do subjective memory complaints herald the onset of mild cognitive impairment in Parkinson disease? J Geriatr Psychiatry Neurol. 2014;27(4):276–281. doi: 10.1177/0891988714532015. [DOI] [PubMed] [Google Scholar]
- 37.Sajjad A, Mirza SS, Portegies ML, et al. Subjective memory complaints and the risk of stroke. Stroke. 2015;46(1):170–175. doi: 10.1161/STROKEAHA.114.006616. [DOI] [PubMed] [Google Scholar]
- 38.Wilson RS, Arnold SE, Schneider JA, Li Y, Bennett DA. Chronic distress, age-related neuropathology, and late-life dementia. Psychosom Med. 2007;69(1):47–53. doi: 10.1097/01.psy.0000250264.25017.21. [DOI] [PubMed] [Google Scholar]
- 39.Wilson RS, Capuano AW, Boyle PA, Hoganson GM, Hizel LP, Shah RC, Nag S, Schneider JA, Arnold SE, Bennett DA. Clinical-pathologic study of depressive symptoms and cognitive decline in old age. Neurology. 2014;83(8):702–709. doi: 10.1212/WNL.0000000000000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu L, Boyle PA, Segawa E, Leurgans S, Schneider JA, Wilson RS, Bennett DA. Residual decline in cognition after adjustment for common neuropathologic conditions. Neuropsychology. 2015;29(3):335–343. doi: 10.1037/neu0000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Snitz BE, Yu L, Crane PK, et al. Subjective cognitive complaints of older adults at the population level: an item response theory analysis. Alzheimer Dis Assoc Disord. 2012;26(4):344–351. doi: 10.1097/WAD.0b013e3182420bdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adams M. Routine Check-Ups and Other Factors Affecting Discussions With a Health Care Provider About Subjective Memory Complaints, Behavioral Risk Factor Surveillance System, 21 States, 2011. Prev Chronic Dis. 2016;13:E15. doi: 10.5888/pcd13.150471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cordell CB, Borson S, Boustani M, et al. Medicare Detection of Cognitive Impairment Workgroup. Alzheimer’s Association recommendations for operationalizing the detection of cognitive impairment during the Medicare Annual Wellness Visit in a primary care setting. Alzheimers Dement. 2013;9(2):141–150. doi: 10.1016/j.jalz.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 44.Lin JS, O’Connor E, Rossom R, et al. Screening for Cognitive Impairment in Older Adults: An Evidence Update for the U.S. Preventive Services Task Force. Rockville, MD: Agency for Healthcare Research and Quality; 2013. Evidence Report No. 107. AHRQ Publication No. 14-05198-EF-1. [PubMed] [Google Scholar]
- 45.Buckley RF, Ellis KA, Ames D, et al. Australian Imaging Biomarkers and Lifestyle Study of Ageing (AIBL) Research Group. Phenomenological characterization of memory complaints in preclinical and prodromal Alzheimer’s disease. Neuropsychology. 2015;29(4):571–581. doi: 10.1037/neu0000156. [DOI] [PubMed] [Google Scholar]
- 46.Salem LC, Vogel A, Ebstrup J, et al. Subjective cognitive complaints included in diagnostic evaluation of dementia helps accurate diagnosis in a mixed memory clinic cohort. Int J Geriatr Psychiatry. 2015;30(12):1177–1185. doi: 10.1002/gps.4272. [DOI] [PubMed] [Google Scholar]
- 47.Hildreth KL, Church S. Evaluation and management of the elderly patient presenting with cognitive complaints. Med Clin North Am. 2015;99(2):311–335. doi: 10.1016/j.mcna.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramlall S, Chipps J, Bhigjee AI, Pillay BJ. The sensitivity and specificity of subjective memory complaints and the subjective memory rating scale, deterioration cognitive observee, mini-mental state examination, six-item screener and clock drawing test in dementia screening. Dement Geriatr Cogn Disord. 2013;36(1–2):119–135. doi: 10.1159/000350768. [DOI] [PubMed] [Google Scholar]