SUMMARY
Bacteriophages rely on their hosts for replication, and many host genes critically determine either viral progeny production or host success via phage resistance. A random insertion transposon library of 240,000 mutants in Salmonella enterica serovar Typhimurium was used to monitor effects of individual bacterial gene disruptions on bacteriophage P22 lytic infection. These experiments revealed candidate host genes that alter the timing of phage P22 propagation. Using a False Discovery Rate of <0.1, mutations in 235 host genes either blocked or delayed progression of P22 lytic infection, including many genes for which this role was previously unknown. Mutations in 77 genes reduced the survival time of host DNA after infection, including mutations in genes for enterobacterial common antigen (ECA) synthesis and osmoregulated periplasmic glucan (OPG). We also screened over 2,000 Salmonella single gene deletion mutants to identify genes that impacted either plaque formation or culture growth rates. The gene encoding the periplasmic membrane protein YajC was newly found to be essential for P22 infection. Targeted mutagenesis of yajC shows that an essentially full-length protein is required for function, and potassium efflux measurements demonstrated that YajC is critical for phage DNA ejection across the cytoplasmic membrane.
Keywords: bacteriophage, yajC, P22, DNA ejection, LPS biosynthesis, ECA pathway
Graphical Abstract
INTRODUCTION
Bacteriophages (or “phages”) are viruses that infect bacteria and are the most abundant biological entities on earth, with an estimated population greater than 1031 (Hatfull & Hendrix, 2011). Pathogenic microbes such as Salmonella interact in complex environments, where genetic exchange between bacteria and their phages can occur readily. One means of exchange is mediated by transducing phages, such as phage P22, which can occasionally package host (i.e. bacterial) DNA (Casjens & Weigele, 2005). Phage transduction can drive the rapid evolution of bacteria, potentially resulting in emerging pathogenicity and antibiotic resistance (Bearson et al., 2014, Schmieger & Schicklmaier, 1999) or serotype conversion (Mavris et al., 1997). Serotype conversion is a process that alters the host lipopolysaccharide (LPS) to protect the host and any integrated prophages from attack by the animal immune response, as well as by other phages. As a result of these evolutionary relationships, cell envelope structures such as LPS are the most variable structures in pathogenic microbes (Reyes et al., 2012), and it is important to study the role phages play in shaping these features. Therefore, it is imperative to have a detailed understanding of the bacterial genes and gene products that modulate interactions between bacteria such as Salmonella and their associated bacteriophages.
The dsDNA tailed phage virions such as bacteriophage P22 adsorb to specific features on the surface of target cells and then release their genomes through the cell membranes into the cytoplasm by a process called “injection” or “ejection”. Host encoded surface proteins or polysaccharides mediate the initial adsorption or attachment, and other host proteins can be required for successful DNA injection. Infectivity of bacteriophages and/or host cell survival have been examined by genetic studies of a wide array of phage-host pairs (e.g. phages λ, T7, T4, P22, and bacteria Escherichia coli, Salmonella enterica, etc.) (Lindberg, 1973, Bertozzi Silva et al., 2016). The majority of these studies utilized cell survival selection schemes to identify host genes whose products are critical for this process and typically identified surface receptors, since phage DNA delivery is completely blocked in such mutant cells. These experiments were transformative and important in the development of modern day molecular genetics (Luria & Delbruck, 1943, Meneely, 2016); however, not all genes that affect phage infection were identified.
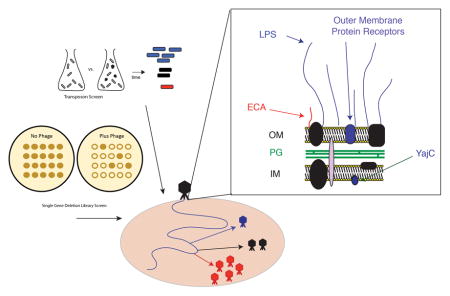
Early studies of phage P22 entry into its host S. enterica serovar Typhimurium showed that LPS is critically important for virion attachment and is the cell’s primary receptor for phage P22 (Susskind & Botstein, 1978, Steinbacher et al., 1997). Consequently, genetic screens to identify mutations that affect P22 entry predominantly revealed Salmonella mutants with defects in O-antigen biosynthesis genes. The predominance of LPS mutants identified in such functional genetic screens is due to the large number of genes involved in LPS biosynthesis (Liu et al., 2014) as well as the severity of the phenotype, since most LPS variants are completely defective for P22 adsorption. P22 virions bind the O-antigen polysaccharide portion of LPS through six tailspike protein trimers, encoded by phage gene 9, that hydrolyze the O-antigen repeats during attachment (Iwashita & Kanegasaki, 1976, Israel et al., 1972, Israel, 1967, Botstein et al., 1973).
The mechanistic steps in DNA delivery by the short-tailed Podoviridae phage family, which includes bacteriophage P22, are currently poorly understood, whereas the process is more well described in other tailed phages (Casjens & Molineux, 2012). Understanding the complexities of infections by lytic phages and the resultant bacterial population response to phage attack is critical, if the prospect of phage therapy is to be put into clinical application. Here, we took advantage of a combination of high-throughput methods to identify a comprehensive set of the bacterial host’s nonessential genes that influence host survival either positively or negatively after P22 infection, including genes important for later steps in the infection cycle. We employed a transposon (Tn) mutant library constructed in S. enterica serovar Typhimurium 14028s (de Moraes et al., 2017) and a systematic screen of a single gene deletion (SGD) library in the same host (Porwollik et al., 2014) to determine genes that affect propagation of phage P22. We comprehensively enumerate, for the first time, which specific genes and parts of the complex LPS pathways affect P22 infection, and also which do not. Furthermore, we identify a previously unexplored role of the enterobacterial common antigen (ECA) in Salmonella susceptibility to P22. We identify many additional genes, most of which do not encode known cell envelope components, that have either a positive or a negative effect on host survival time after phage P22 infection.
Our research expands upon previous genetic screens that identified bacterial genes essential to phage propagation, by also encompassing host genes associated with delayed or accelerated host cell death upon infection. Determination of novel bacterial genes with a role in this process provides a deeper insight into the mechanistic processes by which bacteriophage successfully propagate in and destroy their host cells. Further, we identified a novel gene that is essential to P22 infection, yajC, which we show encodes a protein required for successful delivery of DNA from the virion into the cell.
RESULTS
Global analysis of Salmonella genes that affect phage P22 lytic infection
We used two global approaches to discover Salmonella genes that affect the P22 lytic life cycle. First, a library of random integrations of a transposon (Tn) into bacterial genomes was employed, a technique that can identify genes under selection in various growth conditions (Canals et al., 2012, de Moraes et al., 2017, Sassetti et al., 2001). We used a high complexity Tn5 library of S. enterica serovar Typhimurium strain 14028s to identify either genes whose products enable or promote phage infection (mutation resulted in blocked or delayed infection by P22) or genes that increased bacterial host resistance to P22 infection (mutation resulted in faster loss of host DNA, likely as a result of increased phage propagation). This 14028s Tn5 library contained 240,000 mutants, and insertions were present at an average density of about one every 25 bp and an average of 42.5 per gene (de Moraes et al., 2017). Almost all non-essential genes were represented by multiple independent insertions at different locations. Of the 5,642 annotated 14028s protein encoding genes (Accession No. CP001363), only 514 had four or fewer integrations. Eighty-three of the latter are very small hypothetical <100 bp long genes with no known function and over 300 of the rest are known to be essential or near essential (Canals et al., 2012). Many of the essential genes have DNA replication, RNA synthesis and protein synthesis functions that are also essential for a successful phage P22 life cycle (Susskind & Botstein, 1978).
We infected an actively growing culture of the host Tn5 mutant library in LB with a P22 clear mutant (which is unable to lysogenize the host as it contains the clear plaque repressor mutation c1-7) and used a multiplicity of infection of 7.5, which was experimentally determined to be a condition where >98% of the culture was infected. We compared the genetic profile of mutants with no infection at “time zero” (T0), and after 90 minutes of further growth (T90-control), to the genetic profile of mutants present after 90 minutes of infection (T90-infected). The quantitative pattern of Tn5 insertions was measured by PCR of a region that contains the barcode unique to each Tn insertion, followed by Illumina sequencing of the amplified DNA, as previously described (de Moraes et al., 2017). See Experimental Procedures for details.
After infection, initiation of host genome replication slowed or ceased whereas active replication forks proceeded (Supplementary Figure S1). Because there is only one origin, and the population of infected cells is not synchronized, different individual bacteria will be at different stages of replication. Overall, most hosts are actively replicating and will therefore have two copies of DNA at the origin and one copy at the terminus. When initiation ceases, and the replication fork begins to move, this nearly 2:1 ratio will begin to diminish (Frye et al., 2005, Porwollik et al., 2003). The amplitude observed for this characteristic change in copy number across the genome when replication initiation slows was used to adjust the raw data by position in the genome to reduce the effect of copy number. Next, differences in the relative abundance of transposons within each assayed gene and intergenic region were calculated, along with probabilities, including the False Discovery Rates (FDR). The FDR is an estimate of how often a host gene mutation may be incorrectly attributed as being differentially fit in a P22 infection, and was calculated according to (Love et al., 2014). An FDR of 0.1 indicates a 10% chance that mutations in the gene are not differentially fit in the assay, and a 90% chance they are differentially fit in the assay. We identified many significant changes when comparing Tn profiles of infected cells at T0 and T90. There were no significant changes in the Tn5 profile in uninfected cells at T0 and T90.
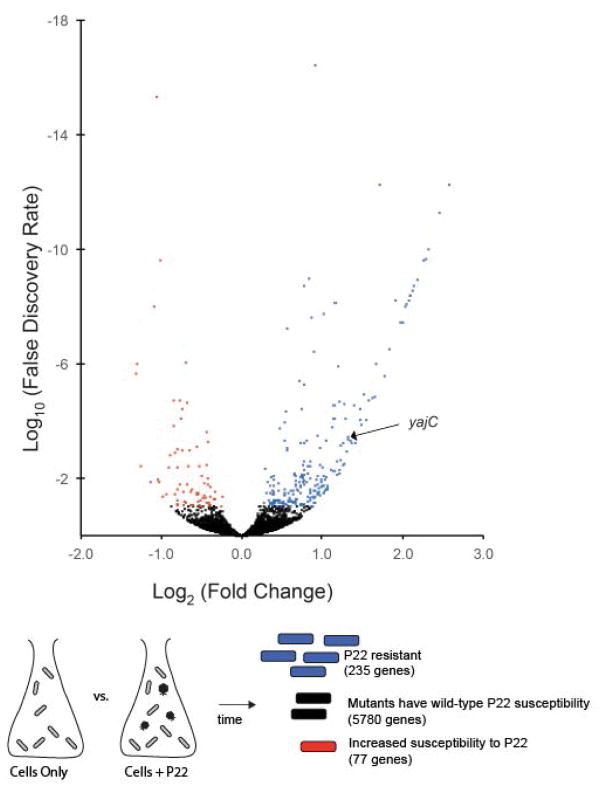
Figure 1 presents a volcano plot of the log10 FDR versus the log2 Fold Change for all 5313 known and putative protein-coding genes assayed in the comparison of Tn profiles of infected cells at T0 and T90. The genes with an FDR of less than 0.1 (less than a 10% chance of being identified in error) are marked in blue for those 235 genes in which mutation blocks or slows the progression of infection, and in red for those 77 genes in which mutation is associated with increased loss of host DNA. Although not formally proven, in each case host DNA loss is very likely to be the result of, and therefore a close surrogate for, successful phage replication and cell lysis. The genes under positive and negative selection during phage infection are listed in Supplementary Tables S1 and S2, respectively. The entire set of data for all genes and intergenic regions is presented in Supplementary Table S3.
Figure 1. Volcano plot of all 5313 mutated host protein coding genes that were screened by transposon analysis.
Data represent the relative change in representation of mutations in each gene in bacterial pools collected at T90-infected versus T0. The X axis represents the log2 fold change. The Y axis represents the log10 False Discovery Rate (FDR) plotted in reverse order. About 5200 mutant genes with an FDR above 0.1 (10%), indicating low confidence that relative representation of these mutants changed during infection, are represented by black dots. Mutated genes with an FDR below 0.1 (higher confidence) are shown color-coded according to behavior, and a cartoon schematic is shown below. Red represents genes where insertion mutants became proportionally rarer during infection. Blue represents genes where insertion mutants became proportionally more common during infection. Black represents mutants that did not display a relative change.
Our second approach was to investigate the ability of P22 to propagate in members of a single gene deletion (SGD) panel of S. enterica serovar Typhimurium 14028s. The panel was engineered to contain knockouts of individual genes that are not essential to the host. We tested 2103 SGD mutants, including most genes in which insertions were found to have the strongest effects on P22 infection in the Tn5 experiment (above). These SGD strains were screened by a quantitative plaque assay. Mutants scored as P22-resistant typically exhibited ≥108-fold fewer plaques than the parental wild-type host. In selected cases we also measured host growth rate in 96 well plates with and without P22 infection to determine the timing of the infectious cycle in mutant hosts (see Experimental Procedures).
Table 1 lists a subset of genes of interest for which Tn5 insertion mutations became more abundant at T90-infected due to a failure or delay in P22 killing of the host cell. Table 1 also indicates the ability of P22 to infect members of the corresponding gene deletion in the SGD strain panel. Genes in the SGD panel that exhibited delayed or blocked infection when mutated, displayed a similar phenotype in the Tn5 assay, with the exception of ihfA where P22 infection is delayed in the SGD mutant but which was not assayed in the Tn5 transposon library due to a lack of transposon insertions in this gene.
Table 1. Examples of Salmonella genes that are required for, or assist in, P22 infection.
Genes are ranked according to descending order of selection strength of mutants (see Experimental Procedures). See Supplementary Table S1 for more candidate genes in this class.
| Gene name | STM14 open reading frame locus_tag | Plaques on SGD? | Phenotype in bacterial growth assay? | Pathway | Function |
|---|---|---|---|---|---|
| rfbK | 2577 | No | No lysis | O-antigen | phosphomannomutase |
| rfbF | 2586 | No | nd | O-antigen | glucose-1-phosphate cytidylyltransferase |
| rfaK | 4475 | No | No lysis | LPS core | lipopolysaccharide 1,2-N-acetylglucosaminetransferase |
| rfbM | 2578 | No | No lysis | O-antigen | mannose-1-phosphate guanylyltransferase (GDP) / Mannose-6-phosphate isomerase |
| rfc (wxy) | 1616 | nd | nd | O-antigen | O-antigen polymerase |
| galE | 0901 | No | nd | O-antigen & LPS core | UDP-galactose 4-epimerase |
| rfbD | 2590 | No | No lysis | O-antigen | dTDP-4-dehydrorhamnose reductase |
| rfaJ | 4478 | No | nd | LPS core | UDP-glucose:(glucosyl)lipopolysaccharide alpha-1,2-glucosyltransferase |
| rfbN | 2579 | No | nd | O-antigen | O antigen biosynthesis rhamnosyltransferase |
| rfbI | 2587 | Yes | Delayed lysis | O-antigen | CDP-6-deoxy-delta-3,4-glucoseen reductase |
| rfbP | 2576 | No | nd | O-antigen | undecaprenyl-phosphate galactosephosphotransferase |
| rfbC | 2588 | No | No lysis | O-antigen | dTDP-4-dehydrorhamnose 3,5-epimerase |
| rfaL | 4474 | No | No lysis | O-antigen | O-antigen ligase |
| rfbX (wzx) | 2582 | nd | nd | O-antigen | O-antigen inner membrane flippase |
| rfbU | 2580 | No | No lysis | O-antigen | mannosyl transferase |
| – | 2730 | nd | nd | nd | unknown function; only 34 AA |
| rfaI | 4479.J | nd | nd | LPS core | UDP-glucose:(glucosyl)lipopolysaccharide alpha-1,3-glucosyltransferase |
| rnhA | 0308.J | nd | nd | Nucleic acid metabolism | Ribonuclease HI |
| yidD | 4636 | Yes | Delayed lysis | membrane protein insertion | membrane protein insertion efficiency factor (acts with insertase YidC); small 85 codons |
| corC | 0776 | Yes | Significant delay in lysis in antisense | metal ion efflux | Magnesium and cobalt efflux accessory protein; not a membrane protein? |
| rfaG | 4483 | No | No lysis | LPS core | UDP-glucose:(heptosyl) LPS alpha1,3-glucosyltransferase |
| yibJ | 5097 | Yes | Slight delay in lysis in antisense | nd | unknown function; only 70 AA |
| phoP | 1409 | Yes | Delayed lysis only in antisense | regulatory | response regulator in two-component regulatory system with PhoQ |
| – | 0842 | nd | nd | sugar transport | sugar ABC transporter ATP-binding subunit |
| sufD | 1666 | Yes | Delayed lysis | nd | with SufBC activates cysteine desulfurase SufS |
| dcp | 1826 | Yes | Delayed lysis | protease | Dipeptidyl carboxypeptidase |
| rfaF | 4472 | No | nd | LPS core | ADP-heptose:LPS heptosyltransferase II |
| rfbH | 2584 | nd | nd | O-antigen | CDP-6-deoxy-D-xylo-4-hexulose-3-dehydrase |
| yajC | 0481 | No | No lysis | Sec dependent translocation | preprotein translocase accessory subunit |
| gidA | 4671 | nd | nd | nd | tRNA uridine 5-carboxymethylaminomethyl modification enzyme |
| oafA | 2758 | Yes | Slight delay in lysis in antisense | O-antigen | O-antigen abequose acetylase |
| phoQ | 1408 | Yes | Delayed lysis in antisense | gene regulation | sensor protein in two-component regulatory system with PhoP |
| dam | 4196 | Yes | Delayed lysis | gene regulation, repair | DNA Metabolism_DNA repair, |
| damX | 4197 | Yes | Delayed lysis Delayed lysis |
nd | unknown |
| IhfA** | 1626 | No | No lysis | Recombination, transcription, translation | Integration host factor subunit alpha |
nd = not determined
Assayed with SGD but not Tn5.
Table 2 lists a subset of the genes that associated with delay of host DNA loss during infection. Thus, insertion mutants in these genes become rarer relative to the rest of the host cell population at T90-infected. The plaque assay performed using SGDs of some of these genes showed no noticeable effect on total plaque production, presumably because faster host cell DNA loss, presumably caused by faster progression of infection, did not have a large enough effect on the final titer of phage to be observed in the plaque assay. However, an increase in the rate of death of the host cells was observed in a bacterial growth curve assay which measures the time course of P22 infection (discussed below).
Table 2. Examples of Salmonella genes that delay host DNA degradation after P22 infection.
Genes are ranked according to descending order of selection strength of mutants (see Experimental Procedures). See Supplementary Table S2 for more candidate genes in this class.
| Gene name | STM14 open reading frame locus_tag | Pathway | Function |
|---|---|---|---|
| wzxE | 4724 | ECA | common antigen translocase (flippase) |
| yfhK | 3143 | nd | putative 2-component sensor protein |
| glmU | 4657 | ECA | bifunctional N-acetylglucosamine-1-phosphate uridyltransferase/glucosamine-1-phosphate acetyltransferase |
| mdoG | 1317 | periplasmic glucan synthesis | glucans biosynthesis |
| wecG | 4727 | ECA | Probable UDP-N-acetyl-D-mannosaminuronic acid transferase |
| ygfY | 3679 | nd | unknown function |
| wecD | 4722 | ECA | TDP-fucosamine acetyltransferase |
| fliF | 2390 | flagellar biosynthesis | flagellar M-ring protein |
| wecE | 4723 | ECA | TDP-4-oxo-6-deoxy-D-glucose transaminase |
| – | 4350 | nd | unknown function |
| yebA | 2299 | nd | cell wall endopeptidase, family M23/M37 |
| fliJ | 2394 | flagellar biosynthesis | flagellar biosynthesis chaperone; rod/hook and filament chaperone |
| – | 5018 | nd | unknown function |
| oxyR | 4959 | nd | transcriptional regulator |
| wecB | 4718 | ECA | UDP-N-acetylglucosamine 2-epimerase |
| ybeB | 0750 | nd | unknown function |
| ycfM | 1381 | nd | putative outer membrane lipoprotein |
| – | 2444 | nd | unknown function |
| wecC | 4719 | ECA | UDP-N-acetyl-D-mannosamine dehydrogenase |
| ompA | 1214 | nd | outer membrane protein A precursor; osmoregulation |
| flgC | 1346 | flagellar biosynthesis | flagellar basal body rod protein; part of proximal portion of the flagellar basal body rod |
| fliO | 2399 | flagellar biosynthesis | flagellar biosynthesis; a membrane components of the flagellar export apparatus |
| sdhD | 0852 | nd | succinate dehydrogenase hydrophobic membrane anchor protein |
nd = not determined
Overall, the combined data from these two approaches, which are discussed in more detail in terms of specific genes and pathways below, give a near comprehensive identification of non-essential host genes whose products have significant effects (positive or negative) on the progression of P22 infection.
Salmonella genes essential for successful phage P22 infection: LPS synthesis genes
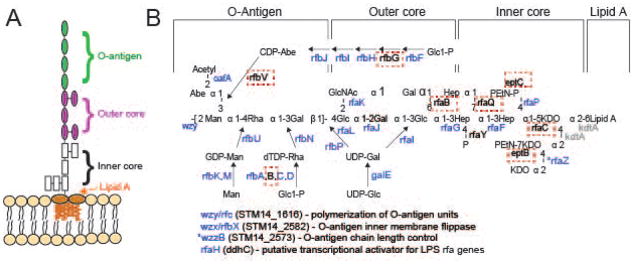
The primary receptor for the adsorption of P22 virions is the cell surface polysaccharide O-antigen (Steinbacher et al., 1997), which is not essential for Salmonella viability in the laboratory. Thus, the most strongly affected genes in the Tn5 screen are genes necessary for O-antigen biosynthesis. Twenty-four of the 36 genes involved in synthesis of the polysaccharide portion of S. enterica serovar Typhimurium LPS were found to be among the top 50 genes in the Tn5 screen that are required for P22 growth and another, wzzB, is in the top 100 (see Supplementary Table S1). These 24 genes are involved in making the O-antigen itself and the outer LPS core on which O-antigen is assembled (Figure 2). Most of the remaining 12 LPS genes that were not among the top genes facilitating P22 infection either encode products that modify the core portion of LPS and/or are essential Salmonella genes that could not be assayed. Each of these 12 genes is discussed in more detail below.
Figure 2. Mutants in the LPS biosynthetic pathway are strongly selected for during P22 infection.
A) The S. enterica serovar Typhimurium O-antigen is shown diagrammatically and in more detail in B), along with pathways of synthesis of the sugars in its trimer repeat unit (shown in square brackets) (reviewed by (Raetz et al., 1996)). Genes, in which mutants slowed P22 infection as measured by the Tn5 experiment, are indicated in blue at the bonds these enzymes catalyze. Genes in which mutation had no significant effects on P22 infection are indicated in black text, and highlighted with a red dashed box. Genes for which mutants were not assayed because the gene is essential or near essential for survival of the host in rich media are indicated in gray. LPS synthesis proceeds generally from right to left in the figure; Wzy/Rfc protein adds tetrasaccharide units to the growing O-antigen chain; RfbP protein adds the first galactose of the trisaccharide repeat to bactoprenol and RfaL transfers the O-antigen chain from there to LPS core-lipid A. Abbreviations are as follows: Glc, glucose; Rha, rhamnose; GlcNAc, N-acetylglucosamine; Man, mannose; OAc, O-acetyl; Hep, heptose; Gal, galactose; KDO, 2-keto-3-deoxyoctulosonic acid; P, phosphate, PEtN, phosphoethanolamine.
Several of the LPS synthesis genes found to be under selection were previously known to be required for P22 adsorption; for example, several O-antigen synthesis (rfb) genes and the galE gene (which encodes an enzyme that catalyzes the production of UDP-galactose, a necessary precursor for O-antigen and LPS outer core production) are needed to adsorb P22 (Butela & Lawrence, 2012, Lindberg, 1973, Kong et al., 2011). Furthermore, a plasmid borne Salmonella rfb operon, whose products synthesize the O-antigen portion of LPS, is sufficient to allow P22 to adsorb to E. coli K12 cells (Neal et al., 1993). However, ours is the first systematic analysis that has directly identified all these 24 individual non-essential genes as being important to P22 infection.
In order to understand our results for all of the 36 LPS genes shown in Figure 2, we first examined the LPS core synthesis genes in more detail. We note that rfaF, rfaG, rfaI and rfaJ products are required to build the “main chain” of the core (genes denoted in red in Figure 2), and they stimulate P22 infection. This is expected, because if the main chain is not completed, the O-antigen repeats (which constitute the P22 primary receptor) cannot be added. Second, rfaC and kdtA (green in Figure 2) are nearly essential and essential for the host, respectively (summarized in Canals et al., 2012) so SGD mutants were not available to be assayed. Third, the products of seven genes, rfaB, rfaP, rfaQ, rfaY, rfaZ, eptB and eptC, add side chain sugars to the core or modify them. Of these, rfaQ, rfaY and rfaZ functions are known not to be required for O-antigen addition (Klena et al., 1992), so it is not surprising that their absence does not affect P22 infection in our experiment. The Tn5 experiment also indicates that rfaB and rfaP insertions do not affect P22 infection (Table S1); the products of these genes add galactose and phosphate side groups to the core, respectively. This result is expected from the previous observations that rfaB and rfaP defective point mutants have O-antigen attached to their LPS core (Hoare et al., 2006), and P22 has been shown to successfully infect them (Kadam et al., 1985, Hudson et al., 1978, Yethon et al., 2000, Helander et al., 1989). Somewhat surprisingly, however, rfaB and rfaP SGD mutants are resistant to P22 infection. This is likely due to polar effects on the rfaI and rfaJ genes that are required for O-antigen addition and are immediately transcriptionally downstream from them in the rfa operon. Finally, inactivation of eptB and eptC block addition of phosphoethanolamine at two locations in the Salmonella LPS core (Klein et al., 2013, Reynolds et al., 2005), and insertions in these genes do not affect P22 infection in the Tn5 experiment. It has not been reported whether inactivation of either ept gene blocks O-antigen addition, but the Tn5 experiment suggests that these modifications are not required for O-antigen addition since P22 infects cells with insertions in these genes normally. We also found that our eptC SGD mutant strain (ΔSTM14_4952) allows normal P22 growth, suggesting that the O antigen is present even when EptC is absent.
Of the twenty genes involved in synthesis of the O-antigen portion of LPS, seventeen showed a strong reduction in P22 infection when inactivated by an insertion in the Tn5 library. Again, this is expected because O-antigen is P22’s primary receptor. However, three genes involved in O-antigen synthesis gave unexpected results in the Tn5 experiment; rfbB, G and V (blue text in Figure 2). These should each be required for O-antigen synthesis but did not show decreased P22 infection when Tn5 insertions were present. Among these, rfbB has an 80% identical paralogue, rffG, that acts in the enterobacterial common antigen synthesis pathway (see below). These two dTDP-glucose 4,6-dehydratase enzymes catalyze the same reaction and are able to substitute for one another (Marolda & Valvano, 1995); this is likely the reason that rfbB’s inactivation in the Tn5 experiment did not result in P22 inhibition. Nonetheless, we find that the rfbB SGD mutant (ΔSTM14_2951) is unable to support P22 growth. This failure could be due to either to failure of rffG to fully substitute for rfbB in this strain, or due to polarity from the rfbB SGD insertion on one or more of the immediately downstream genes in the rfb operon, rfbD, rfbA and rfbC, all of which are necessary for synthesis of rhamnose, so their inactivation results in a lack of O-antigen. The oafA and rfbF, G, H, I, J and V genes are required for the synthesis and transfer of the abequose side group to LPS and abequose acetylation (Figure 2). Of these, all but rfbG and rfbV are among the top genes facilitating P22 infection (Tables 1 and S1). The lack of an rfbG phenotype can be explained by this gene having only one Tn5 mutation in the extreme carboxy terminus. The lack of an rfbV phenotype is not yet understood.
In Typhimurium the O-antigen has an acetyl-abequose side group on the main chain mannose, the RfbF, RbfJ and RfbI proteins catalyze steps in the synthesis of the abequose precursor (Wyk & Reeves, 1989, Rubenstein & Strominger, 1974, Lindqvist et al., 1994). Yuasa et al. (Yuasa et al., 1969) and Hong et al. (Hong et al., 2012) have reported that inactivation of rfbJ causes failure to assemble O-antigen chains, and we find that P22 fails to infect rfbF and rfbI SGD mutants (ΔSTM14_2586; ΔSTM14_2587; Tables 1 and S1). Our SGD panel does not include mutants of the other abequose pathway genes so they cannot be tested individually. Thus, the failure to add the abequose moiety to the trimer unit blocks O-antigen polymerization and so blocks P22 adsorption. OafA protein acetylates the abequose after its addition to O-antigen polymer (Slauch et al., 1996). The facts that P22 successfully infects an oafA SGD mutant strain (ΔSTM14_2758) and yet oafA insertions limit P22 infection in the Tn5 experiment (Table S1), indicate that acetylation of the O-antigen abequose enhances but is not essential for P22 adsorption.
Host genes that enhance, but are not required for, P22 infection
Table S1 lists 235 genes that are either required or assist in P22 infection (FDR <0.1). Many of these genes are not found in the major pathways already described such as O antigen or LPS synthesis, and thus may reflect the importance of other cellular functions during P22 infection. The reason that some of these genes were not noted in previous studies is presumably because these genes may enhance, but not be required for, P22 progression.
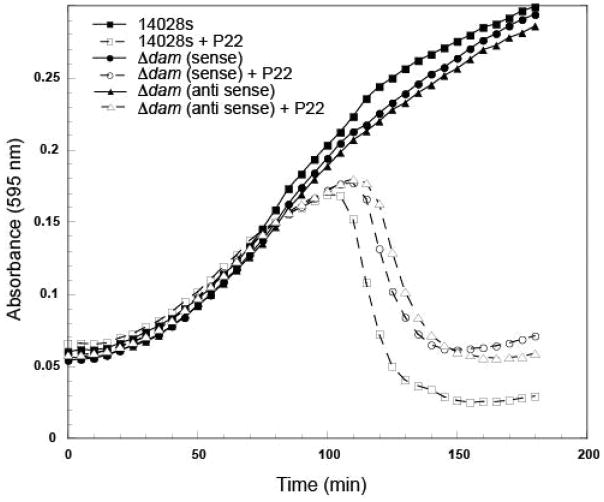
We employed a time course assay in liquid culture to determine if mutants in these genes displayed any delay in host cell lysis after P22 infection compared to the 14028s parental strain. These genes included dam, a DNA methylase involved in DNA repair and gene regulation (Marinus & Casadesus, 2009). The dam gene is involved in phase variation/expression of the P22 gtrABC and the Salmonella STM557-559 chromosomal O-antigen glucosylation genes (Broadbent et al., 2010) as well as in control of the opvAB O-antigen chain length locus (Pirone-Davies et al., 2015) (Table S1). Progression of P22 infection is delayed in dam mutants, regardless of antibiotic resistance promoter orientation both in the SGDs (sense; KanR; antisense CamR) (Figure 3) and in Tn5 (see data for both strands in Table S1). Other genes that enhanced P22 infection include yidD (membrane protein insertion efficiency factor that acts with insertase YidC); sufD (with SufBC activates cysteine desulfurase SufS), and dcp (dipeptidyl carboxypeptidase).
Figure 3. Mutations in dam delay growth of P22.
Representative growth curves for the parental strain 14028s, and ΔSTM14_4196 (Δdam) with antibiotic cassette in the sense and antisense orientation, are shown. Growth curves were performed with and without the addition of P22 phages. Three technical and three biological replicates were performed for each condition, and a representative experiment is shown.
The KanR and CamR SGDs for each gene differ in the orientation of the constitutive resistance gene promoter (Porwollik et al., 2014). Among the mutants that differed in phenotype, depending on the orientation of the antibiotic resistance genes, were phoP and phoQ, which are adjacent in the genome. Mutants in these two genes that carried the resistance gene promoter in the antisense strand appeared to delay P22 progression, whereas mutants with the resistance gene promoter in the sense strand had either no phenotype or a reduced phenotype (data not shown). The same polarity was observed in the Tn5 data, since Tn5 insertion mutants also have a constitutive antibiotic resistance gene and the transposon can integrate in either orientation (see strand specific data in Tables S1 and S3). Encoded directly before phoP/phoQ, in the same strand, is the essential gene purB, suggesting that polar transcriptional interference from phoP/phoQ mutants in the antisense orientation may alter purine metabolism and cause the observed orientation-dependent difference in phenotype. Other genes that showed potentially polar mutant phenotypes delaying P22 progression included yibJ (unknown function); and corC (metal ion efflux, magnesium and cobalt efflux accessory protein).
Host genes that prolong host DNA survival during phage P22 infection
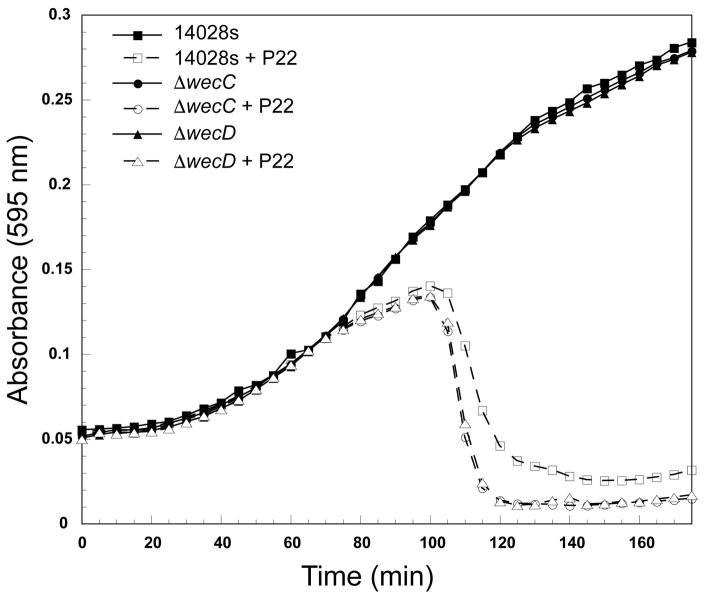
We observed gene mutants that were associated with increased host DNA loss in the Tn5 assay (Tables 2 and S2). When active, the corresponding genes lead to longer host cell survival by an unknown mechanism, though they do not ultimately prevent lysis. This is a generally neglected class of genes that impact phage infection. Interestingly, genes required for the synthesis of two extracellular polysaccharides, enterobacterial common antigen (ECA) and osmoregulated periplasmic glucan (OPG) are in this class (Tables 2 and S2). Bacterial growth assays on wecC and wecD deletion mutants confirm that deletion of these two genes in the ECA pathway increase host cell death after P22 infection (Figure 4).
Figure 4. Mutations in wecC and wecD promote early host cell lysis after P22 infection.
Representative growth curves for the parental strain 14028s, ΔSTM14_4719 (ΔwecC), and ΔSTM14_4722 (ΔwecD) are shown. All growth curves were performed with and without the addition of P22 phages. Three technical replicates were performed for each mutant, and a representative experiment is shown.
Enterobacterial common antigen is a surface glycolipid that is conserved among enteric bacteria and contributes to pathogenicity and virulence in S. enterica (Gilbreath et al., 2012). In Salmonella it is composed of a linear polysaccharide with a tri-saccharide repeat composed of 4-acetamide-4,6-dideoxy-D-galactose -N-acetyl-D-mannosaminuronic acid-N-acetyl-D-glucosamine and can be anchored to the outer membrane by attachment to either the O-antigen core (above) or a phosphoglyceride (Rick et al., 1985). Seven genes in the ECA synthesis pathway, including five in the wec cluster as well as glmU and wzxE, were identified in the Tn5 assay as genes whose products inhibit P22 infection (Table S2, Supplementary Figure S3); these include most of the wec genes that are essential for ECA synthesis but have no role in O-antigen synthesis. The wzxE gene most likely encodes a flippase that moves the bactoprenol-N-acetyl-D-glucosamine ECA intermediate across the inner membrane (Rick et al., 2003), so its detection in the Tn5 assay (Table S1) is not surprising. Disruption of the wzzE gene may alter the ECA chain length (Murray et al., 2003, Marolda et al., 2006, Barr et al., 1999), and, along with wecF and rrfG (see Supplementary Figure S3), it is under significant but mild negative selection in the Tn5 experiment (Table S1). The WecA protein catalyzes the transfer of GlcNAc-1-phosphate moiety from UDP-GlcNAc onto the carrier lipid undecaprenyl phosphate, the first lipid linked intermediate in ECA synthesis, but it was not found to be under selection in our Tn5 experiment. The reason for this is not yet understood.
Osmoregulated periplasmic glucans (OPGs) are branched oligosaccharides that are present in most Proteobacteria (Bontemps-Gallo & Lacroix, 2015). OPG amounts are regulated by the osmolarity of the cell’s environment, and in E. coli under low salt growth conditions they can constitute as much as 5–7% of the dry weight of the cells (Miller et al., 1986). In S. enterica, OPGs are composed of 5–15 glucose units and their presence is linked to virulence and biofilm formation (Bhagwat et al., 2009, Liu et al., 2009). Synthesis of the glucose backbone of OPGs is catalyzed by the products of mboG and mboH (also known as opgG and opgH) (Lacroix et al., 1991), and these genes were detected as having products that reduce the progression of P22 infection in the Tn5 experiment (Table S1). It is not known how OPGs might inhibit or slow the P22 lifecycle, but it is possible that the presence of large amounts of OPG in the periplasm slows P22 DNA delivery. Further work will be required to determine if this hypothesis can be confirmed. To our knowledge, neither ECA nor OPG have been previously shown to play a role in host susceptibility to phage P22 infection, and this highlights the importance of using a systematic genome-wide assay to discover genes that both enhance and delay P22 progression.
Additional genes and gene families are associated with delaying progression after P22 infection. Mutations in these genes lead to faster progression of the infection, as measured by the amount of host DNA present. For example, mutations in genes that affect the synthesis of membrane associated parts of flagella (fliC, F, J, L and O) and mutants in ompA and ycfM that encode outer membrane proteins lead to faster loss of host DNA, presumably because of an increase in the rate of P22 infection progression in these mutants (Tables 2 and S2). We do not know why inactivation of these genes affects progression after P22 infection, but their role in the outer membrane may be relevant to this phenotype, especially concerning phage DNA delivery into cells.
Not all the remaining genes in Tables 2 and S2 can be discussed individually but a few have been observed in other contexts in phage infection. The hflKCX gene cluster encodes proteins known to inhibit bacteriophage lambda CII cleavage and thereby inhibit lysogeny (Kihara et al., 1997). The oxyR gene is known to suppress lambda prophage induction (Glinkowska et al., 2010). Inactivation of the OxyR protein can result in shortening of the O-antigen chains (Cota et al., 2012) and failure to glycosylate the galactose in the O-antigen tri-saccharide (Broadbent et al., 2010, Vander Byl & Kropinski, 2000).
The inner membrane protein YajC is needed for phage P22 DNA entry into the target cell
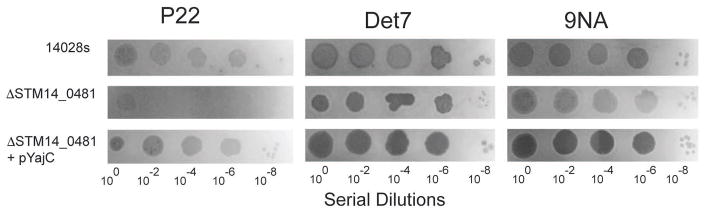
The yajC gene, which encodes an inner membrane protein that is part of the Sec protein translocase complex (Pogliano & Beckwith, 1994b), was found here to be essential for P22 proliferation in several experiments (Table 1). The fraction of bacteria with Tn5 integrations in yajC increased upon challenge with P22 (Table 1), and no plaques were observed in spot tests (Figure 5) when P22 was plated on the yajC deleted strain ΔSTM14_0481 from the SGD panel (Porwollik et al., 2014). Strain ΔSTM14_0481 cell culture growth was unaffected by the presence of P22; there was no cell growth inhibition and no phage-induced cell lysis (Figure 6). Complementation of a plasmid pYajC-borne yajC gene in the ΔSTM14_0481 (ΔyajC) mutant restored P22 infection efficiency as measured by spot tests (Figure 5). Compared with the parental 14028s cell line, complementation of the ΔyajC mutant with a plasmid carrying a wild type yajC gene showed only a slight delay of P22 induced cell lysis (Figure 6). This result could be explained by a decrease in available YajC protein if expression from the pYajC plasmid was not as efficient as in the 14028s strain. Collectively, these results show that in ΔSTM14_0481 it is the yajC defect itself, and not a polar effect on any downstream gene, that is responsible for P22’s failure to propagate, and we conclude that yajC is essential for P22 propagation in Salmonella. Few studies have examined the role of host proteins during P22 entry (Casjens & Molineux, 2012). Therefore, the identification of yajC as important for P22 entry piqued our interest. Here, we focus on further characterization of this novel finding.
Figure 5. YajC is needed for P22 plaque formation.
Plaques are shown after plating of serial dilutions of phages P22, Det7, and 9NA on lawns of the parental strain 14028s or the SGD knockout, ΔSTM14_0482 (ΔyajC) with or without the plasmid expressing YajC.
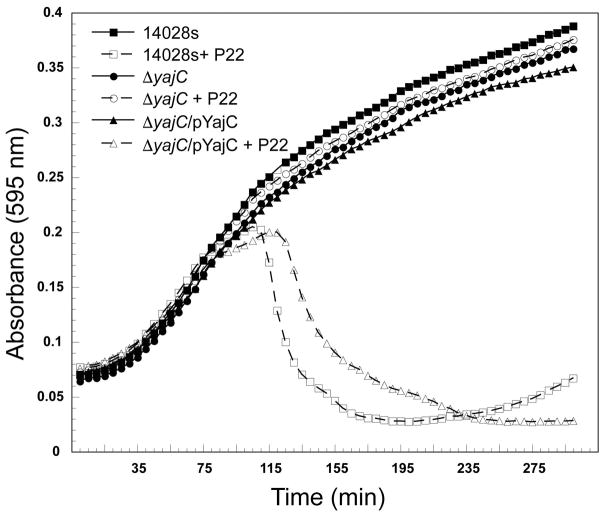
Figure 6. The defect in P22 plaque formation on a ΔSTM14_0481 S. enterica host is repaired by plasmid-borne YajC complementation.
Representative growth curves are shown for the parental strain 14028s and the yajC knockout, ΔSTM14_0481 (ΔyajC) with and without expression of YajC from the plasmid pYajC; all growth curves were performed with and without the addition of P22 phages. Three technical and three biological replicates were performed for each condition, and a representative experiment is shown.
The YajC protein is not essential for Salmonella growth since the yajC SGD strain ΔSTM14_0481 grows apparently normally, but its presence as an accessory factor in the Sec preprotein translocase complex (Pogliano & Beckwith, 1993) suggests that its absence could affect secretion of some Salmonella proteins and therefore affect other features of the bacteria. The most obvious possible effect that could lead to P22 resistance would be a low O-antigen polysaccharide amount or length, since its synthesis requires the secretion of several periplasmic proteins. Thus, in order to determine whether the yajC deletion has an effect on LPS production, we extracted LPS from the parental strain 14028s and the isogenic ΔyajC mutant as previously described and visualized the resulting preparations in silver-stained SDS polyacrylamide electrophoresis gels (Parent et al., 2014, Porcek & Parent, 2015). There was also no effect on LPS functionality as measured by in vitro genome ejection caused by purified LPS nor was there any change in the ability of P22 to adsorb to ΔyajC cells in vivo by phage depletion methods we have previously described (see Supplementary Figure S4) (Jin et al., 2015, Parent et al., 2014). In addition, two other phages, 9NA and Det7, both of which utilize the O-antigen as their primary receptor (Andres et al., 2012, Walter et al., 2008), were tested and neither phage’s growth was affected by the ΔyajC deletion (Figure 5). We conclude from these experiments that LPS and O-antigen production is not affected by the absence of YajC protein. These results also indicate a role for YajC in a narrow range of bacteriophages, as P22 was affected by its absence, but Det7 and 9NA were not.
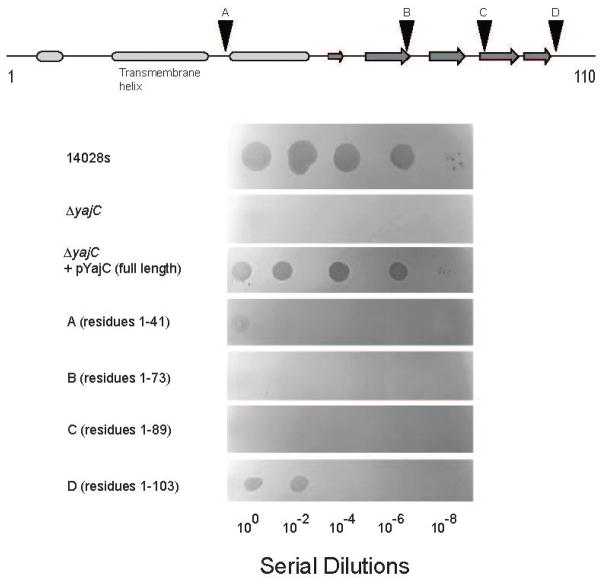
To determine which portions of YajC are critical for its function during P22 infection, we constructed several C-terminally truncated versions of YajC, and tested each for the ability to support P22 growth by spot tests (Figure 7). All truncations showed some effect on P22 entry. A construct containing residues 1-103 (missing only seven C-terminal amino acids) showed some clearing in spot tests (i.e., cell killing at high MOI); however, the plating efficiency was several orders of magnitude lower than on hosts expressing the full-length protein. All other yajC deletions were completely resistant to phage P22 infection, indicating that essentially the full-length protein is required for function.
Figure 7. Regions of YajC critical for P22 infection.
The top schematic shows the YajC secondary structure prediction by PSI-PRED (McGuffin et al., 2000). Ovals represent alpha helices and arrows represent beta strands. Triangles indicate positions where stop codons were engineered into the yajC gene of pYajC. Below, plaque formation after plating of serial dilutions of phage P22 on lawns of bacterial cells, either the parental strain 14028s, ΔSTM14_0482 (ΔyajC), or ΔSTM14_0482 complemented with a plasmid expressing full length (ΔyajC/pYajC) or various truncated YajC versions (panels A–D).
The failure of P22 to infect ΔSTM14_0481 (Figures 5–7) indicates that some part of its lytic cycle cannot function properly in this host mutant. To determine whether this defect occurs early or late in the infection cycle, we tested whether infection by P22 results in the formation of lysogens of ΔSTM14_0481, since only early gene functions are required for establishment of lysogeny. S. enterica strains 14028s and ΔSTM14_0481 were infected with a P22 that carries a chloramphenicol resistance gene (see Experimental Procedures), and chloramphenicol resistant lysogen colonies were formed with 14028s about 107 times more frequently than with ΔSTM14_0481. Under the conditions used, about one-third of the infections resulted in lysogen formation in strain 14028s. The failure to establish lysogeny in the absence of YajC protein indicates that some part of P22’s early life cycle is very strongly dependent on YajC. Several P22 early genes are required for stable lysogen formation, for example phage integrase-catalyzed DNA integration and lytic gene repression by the phage-encoded repressor (Susskind & Botstein, 1978). In addition, we tested the ability of strain ΔSTM14_0481 to accept phage P22 transducing DNA as follows: The P22 lysogenic Salmonella strain UB-1988 (described in (Leavitt et al., 2013a)), which carries a chloramphenicol resistance cassette between S. enterica LT2 open reading frames STM764 and STM765 (McClelland et al., 2001), was induced with carbadox. The released P22 virions were used to infect growing cultures of strains 14028s and ΔSTM14_0481 (ΔyajC) at 2 x 108 cells/mL and an MOI of 0.1 for 30 minutes at 37°C. The cultures were then plated for chloramphenicol resistant colonies overnight at 37°C. The 14028s culture contained 6,600 resistant colonies per mL, and the ΔSTM14_0481 (ΔyajC) culture gave <1 resistant transductants per mL (i.e. no transductants were detected). Thus, the yajC defect lowers the frequency of generalized transduction at least 6600-fold. The failure to form lysogens or be transduced by host DNA in P22 virions seems likely to be due to a blockage of DNA delivery into the cytoplasm from the virion. Therefore, we next tested specifically for successful DNA delivery into ΔSTM14_0481.
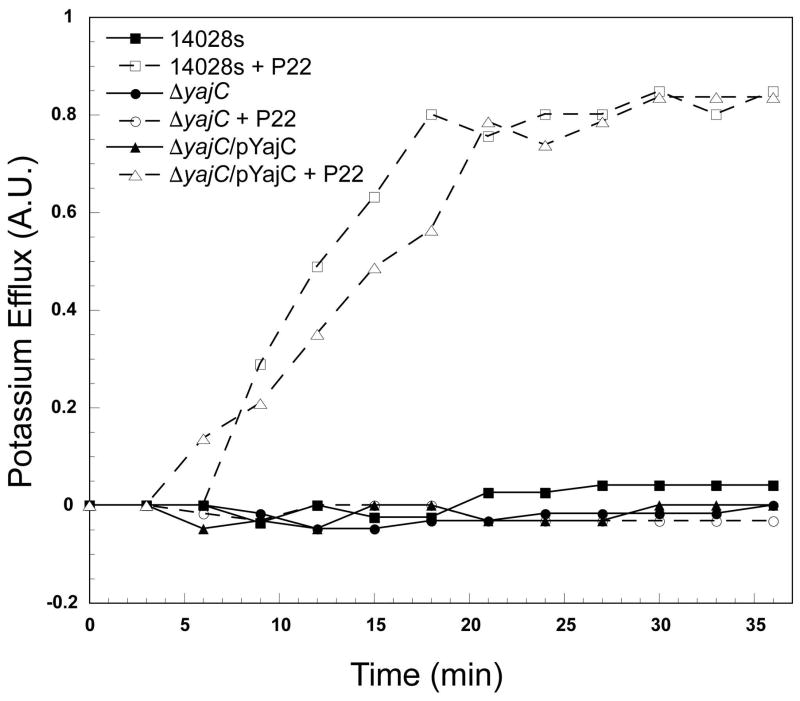
We used a potassium efflux assay (Boulanger & Letellier, 1988, Leavitt et al., 2013b) to test the ability of phage P22 to eject its DNA into the parental 14028s (non deletion strain), ΔSTM14_0481 (ΔyajC), and ΔSTM14_0481 complemented with yajC in trans by plasmid pYajC (above). K+ release correlates with successful P22 delivery of DNA into the cytoplasm of target cells, and can be used to demonstrate phage DNA passage across the host inner membrane (Cumby et al., 2015, Leavitt et al., 2013b). Figure 8 shows that K+ release, and therefore P22 phage DNA ejection into the cytoplasm, is completely blocked in the ΔyajC knockout and is essentially fully restored by the complementing plasmid pYajC. As a control, the ΔSTM14_4474 (ΔrfaL) SGD strain, a mutant that fails to produce O-antigen polysaccharide (above), was also tested and, as previously published (Leavitt et al., 2013a), P22 causes no K+ release. The slight DNA release delay in the plasmid-complemented ΔSTM14_0481 (ΔyajC /pYajC) relative to the parental strain is likely due to different levels of yajC gene expression from the plasmid and the native chromosomal gene. Figure 8 shows the release patterns from strains following P22 infections with an MOI of 5, but even an increased MOI of 15 failed to release K+ from the ΔyajC strain. We conclude that YajC is required for successful DNA delivery into Samonellla cells by phage P22.
Figure 8. P22 DNA entry into hosts requires YajC.
The indicated Salmonella cells were infected by P22 c1-7 phages at an MOI of 5 at 37˚C, and K+ ion release was measured after infection. Hosts that lack a functional YajC (ΔSTM14_0481) with and without the complementing plasmid pYajC were tested, and K+ ion release by the parental strain 14028s is shown for comparison. Potassium ion measurements were performed as described in the Materials and Methods section. Values are the fraction of total K+ released upon cell disruption.
DISCUSSION
Discovery of hundreds of new phenotypes that influence P22 infection
A transposon screen of Salmonella Typhimurium strain 14028s, including mutations in 5313 protein coding genes, revealed selection for 235 mutants during P22 infection and selection against 77 mutants (FDR <0.1) (Figure 1, Tables 1, 2, S1, S2, S3). Many of the Salmonella genes identified in our screens as important or essential for P22 DNA delivery into bacterial host cells are involved in LPS production. This is not surprising, as previous reports have shown that LPS is critical for P22 entry (Liu et al., 2014). LPS has also been shown to contribute to particle ejection in vitro (Andres et al., 2010, Andres et al., 2012); recently reviewed in (Broeker & Barbirz, 2017)). However, LPS causes a slow and inefficient triggering of P22 ejection alone, whereas outer membrane proteins are critical for accelerating this process and for E-protein release which is essential for infection (Jin et al., 2015). Therefore, in this work, we used a transposon assay and SGD screens to identify additional gene products critical for modulating P22 adsorption.
We identified each individual LPS gene that is necessary as well as a few that were not necessary (Figure 2). Essentially all of our results concerning the genes involved in the synthesis of the polysaccharide portion of LPS are in agreement with the known biological facts, or there are reasonable explanations for the small number of apparent exceptions. We take this as a strong indication of the validity of biological conclusions drawn from the Tn5 experiment. Two additional genes, ihfA (Supplemental Figure S2) and yajC (Figures 5–8) were shown to be essential to P22 infection.
We identified many host genes that can modulate P22 infection either by assisting in infection without being essential to infection (Tables 1 and S1) or by delaying host DNA loss (Tables 2 and S2). We demonstrated that some mutants delay but do not stop P22-induced cell lysis (e.g., dam, Figure 3), while other mutants accelerate lysis after infection (e.g., wecC and wecD, Figure 4). Both of these latter gene classes represent novel findings. Genes in the Enterobacterial common antigen (ECA) and OPG pathways are largely in the class of genes that delay progression of infection (Figures 4, S3). The exact biological roles of these polysaccharides are currently unknown. Some evidence suggests that ECA can mask the immunogenicity of surface antigens (Gilbreath et al., 2012, Huang et al., 2016). ECA may also strengthen the outer membrane against harsh conditions such as detergents and bile salts (Ramos-Morales et al., 2003). Thus, P22 might infect ECA or OPG defective Salmonella better than the parent either because its O-antigen or another receptor are masked by the ECA polysaccharide, or because weakening of the outer membrane might allow easier penetration of the outer membrane. Alternatively, a less crowded periplasm may allow easier entry by the P22 DNA delivery machinery.
The inner membrane protein YajC is essential for P22 infection
Previous studies have shown that various inner membrane proteins are essential for DNA delivery by different bacteriophages. For example, the mannose YZ permease complex, which transports mannose across the cytoplasmic membrane, is essential for Siphoviridae E. coli phage λ entry (Erni et al., 1987), and the inner membrane glucose transporter PtsG is essential for Siphoviridae HK97 infection (Cumby et al., 2015). Both ManYZ and PtsG are large proteins that have cellular functions in sugar transport. The large (~85 kDa) inner membrane protein NfrB of unknown cellular function, as well as NfrC (whose gene is now called wecB and is encoded in the same operon as nfrB), were shown to be essential for Podoviridae E. coli phage N4 adsorption (Kiino et al., 1993). The same work showed that the NfrC/WecB protein is directly involved in the ECA synthesis pathway (see Figure S3).
By contrast to the phage receptors listed above, YajC is a small inner membrane protein of only 110 amino acids, and its exact function is currently unknown. It forms a heteromeric complex with SecD and SecF, and this complex in combination with the SecYEG complex increases the affinity and efficiency of the SecA protein translocase (Schulze et al., 2014). Unlike SecD and SecF knockouts that display severe cold sensitive defects (Pogliano & Beckwith, 1994a), YajC is not needed for translocation or for cellular viability (de Keyzer et al., 2003) and is found widely in the Enterobacteriacea but not across other bacterial species (Caufield et al., 2015). Thus, it is not essential for general protein translocation, but may exert a modulatory effect on the Sec translocase. YajC has a single N-terminal transmembrane region as well as a large C-terminal domain that extends into the cytoplasm (Fang & Wei, 2011) (Figure 7). Recently, the location of YajC in Sec holo-transolocon complexes has been determined by cryo-electron microscopy studies (Botte et al., 2016), and it has been suggested that YajC might help to stabilize the translocon complex. When all three proteins are present in the complex (SecD-SecF-YajC), there is a positive effect on translocation efficiency in vivo (Nouwen & Driessen, 2002). We could not directly measure individual effects of SecD or SecF as these proteins are essential to the host and no SGD mutants were available for these genes. Therefore, it is possible that the entire complex, and not just the YajC protein, is essential for P22 entry. To our knowledge, YajC has not been previously been implicated in infection by any phage. Based on the potassium efflux assay (Figure 8), the lack of YajC severely affects P22 DNA entry into the cytoplasm. If the absence of YajC indeed decreases protein translocation efficiency, there could be subtle changes to the outer membrane protein composition in Salmonella that could block P22 entry.
In addition to primary receptors, such as LPS, which mediate initial binding of virions to cells, many phages also must recognize a second cell envelope feature, in order to complete DNA delivery (Casjens & Molineux, 2012). Recently, studies with a close relative of P22 identified multiple outer membrane proteins (Omps) that, in addition to O-antigen, are important for mediating phage Sf6 DNA delivery into Shigella flexneri (Parent et al., 2012, Parent et al., 2014, Porcek & Parent, 2015). OmpA and OmpC were identified as critical for attachment after Sf6 virions conclude their initial interactions with the primary LPS receptor (Verma et al., 1991, Clark et al., 1991, Chua et al., 1999). In vitro ejection experiments suggested that, like Sf6, phage P22 may also require Omps, as these proteins were shown to be necessary for LPS mediated ejection of essential internal phage proteins which are not released when P22 particles are treated with LPS alone (Jin et al., 2015). Previously, individual Salmonella omp gene knockouts have not been found to have an obvious effect on phage P22 ejection into whole cells. Therefore, phage P22 entry may utilize either a greater variety or a different set of proteinaceous surface receptors (Jin et al., 2015).
Single omp knockouts did not show any effect on P22 propagation using SGD mutants, although both ompA and ycfM were identified in the Tn5 assay. Thus, it is difficult to know at this point whether YajC and/or other Sec proteins are a P22 inner membrane receptor or whether YajC drastically affects availability of another surface receptor for P22. P22 escape mutants have yet to be isolated on the ΔyajC knockout cell lines (data not shown). Therefore, we currently do not know if YajC physically interacts with phage P22 structural proteins, such as the phage E-proteins that are released from the virion during DNA delivery and are necessary for successful DNA delivery (Israel, 1977), or if YajC is required for other reasons. The mechanism of YajC action during P22 infection will be explored in future work.
In summary, these studies have revealed many genes that have new phenotypes associated with accelerating or delaying P22 infection. As part of these studies, a new gene involved in the early stages of DNA ejection has been uncovered.
EXPERIMENTAL PROCEDURES
Strains/media
The strains used included Salmonella enterica serovar Typhimurium 14028s and single gene deletion library (SGD) derivatives; SGD mutants were KanR (50 μg/mL), and CamR (20 μg/mL) (Porwollik et al., 2014). The P22 phage used for the Tn5 assay and all SGD screens contains a clear plaque repressor mutant c1-7 that makes it unable to form lysogens (Levine & Curtiss, 1961). Phages Det7 (Casjens et al., 2015) and 9NA (Casjens et al., 2014) have been described. Purified phage stocks were stored in a 10 mM Tris (pH 7.6) and 10 mM MgCl2 buffer. LB Miller broth (Invitrogen) and LB agar (Invitrogen) were used for all experiments.
Transposon library construction, barcoding, and assay for P22 infection
EZ-Tn5™ <KAN-2> (http://www.lucigen.com) was modified to contain an N18 barcode directly adjacent to an Illumina Read 1 sequence. A library of over 240,000 separate Salmonella Typhimurium insertion mutants was constructed by mixing the transposase with the barcoded construct and electroporation in strain 14028s. The barcode associated with each unique Tn5 insertion at each position in the genome was determined using a previously described method (de Moraes et al., 2017). This library was grown to mid-log phase (O.D. 600nm = 0.4, or ~2x108 cells/mL) in LB with Kanamycin at 50 μg/mL. The culture was then split into two parts; one was infected and one not infected by phage. Cells in one portion were infected with phage P22 at a multiplicity of infection (MOI) of 7.5 phage per cell, to ensure that ~98% of the cells were infected. Infection was confirmed by plating the culture for surviving colonies before and after phage infection. One mL aliquots were collected, glycerol was added to a 25% final concentration, the samples were spun for 30 seconds at 13,200 rpm in a microcentrifuge, and the resulting pellets were kept frozen at −80 ˚C. Samples were taken immediately after infection (T0) and at 90 minutes after infection (T90-infected) and uninfected (T90-control). The experiment was performed in triplicate.
Half of each pellet (equivalent to 5 x 107 bacteria in the input) were processed from the input and output library cultures using three washes in water followed by proteinase K digestion as described previously (de Moraes et al., 2017). After inactivation of the protease, a nested PCR regimen was performed to amplify the N18 barcode region and to add sample- and experiment-specific N8 indices, exactly as described before (de Moraes et al., 2017). Different samples, with different indexes, were pooled and subjected to QIAquick PCR product purification (Qiagen) according to the manufacturer’s recommendation. Illumina sequencing proceeded with standard Illumina primers, as described (de Moraes et al., 2017). For identification of barcoded mutants, the first 18 bases, which represented the unique N18 tag for each Tn5 mutant, were extracted, and the abundance of all unique 18-mers was calculated using custom perl scripts. The abundance of all N18 barcodes mapped within each annotated genome feature were summed in a strand specific manner. This represented the aggregated abundance for each feature in the coding strand and the non-coding strand. A plot of the ratios of input and output across the genome indicated that replication initiation had ceased or slowed after infection. The raw ratios were adjusted, accordingly, to account for this change in relative copy number. The aggregated abundances for the input and output libraries were statistically analyzed using DESeq2 and the log2 fold changes and false discovery rates (FDRs) were estimated as described (Love et al., 2014).
Initial screening of the SGD library for resistance against P22 infection
A high throughput assay was developed to screen an SGD S. enterica strain 14028s panel (Porwollik et al., 2014). Using a large format LB agar plate, and a 96-well replicator pin tool, colonies from the SGD library were transferred onto two plates. The first plate contained LB only, and the second plate had a top agar overlay containing P22 phage at a final concentration of 1 x 109 per mL. Plates were grown overnight at 30oC. Colonies that grew equally well on the plates with and without phage were considered potentially phage resistant and were further screened. Additional screening included quantitative measurements of plaque forming units (PFUs) produced on the single gene knockout compared with the wild type strain 14028s, and in some cases, time course assays (see below).
Bacterial culture growth rate (time course) assays
A Molecular Devices FilterMax F5 plate reader using 96-well plates was used to monitor growth rates of cultures with and without phage as described, and as reported previously, subtle differences between strains of 5 minutes are reliably measured (Dover et al., 2016). An overnight culture of each cell type was diluted 1:10 in broth and added to each well. Cells were either uninfected or infected with phage P22 at an MOI of ~0.1. The plates were incubated at 37 °C for 5 h with absorbance measurements taken at 595 nm every 5 min with vigorous shaking before each read.
Construction of pYajC and truncation mutants
The yajC gene was cloned by PCR amplification from S. enterica serovar Typhimurium 14028s genomic DNA with a 5′ primer that introduced a BamHI site and a 3′ primer that introduced a HindIII site. The amplified gene was digested with BamHI and HindIII and ligated into pSE380 (Invitrogen), digested with the same enzymes to create the final plasmid “pYajC”. This plasmid was transformed into ΔSTM14_0481 (ΔyajC) by electroporation and selected for with 100 μg/mL ampicillin. Truncated versions of pYajC were constructed by site-directed mutagenesis using Quikchange (Agilent) to individually replace codons for residues 42, 74, 90, and 104 with a “TAA” stop codon. The RSTF Genomics Core at Michigan State University performed sequencing on all pYajC constructs.
Complementation spot assay for YajC activity
Top agar was seeded with Salmonella cells (either 14028s, ΔSTM14_0481, or ΔSTM14_0481 with pYajC) and spread on LB agar plates. YajC was expressed from pYajC by induction with 2 mM Isopropyl β-D-1-thiogalactopyranoside (IPTG) in the presence of 100 μg/mL ampicillin. Serial dilutions of phages (P22, 9NA, or Det7) were spotted in 2 μL aliquots on top of the cells, and the plates were incubated at 37˚C overnight.
P22 lysogeny establishment assay
Logarithmically growing ΔSTM_0481 and the parental strain 14028s were grown to mid-log stage at a concentration of 2x108 cells/mL in LB at 37°C and were infected with P22 orf25::CamR-EG1, 13−amH101, sieA−Δ1 at an MOI=5. Infected cultures were grown with shaking at 37°C for 90 min, and plated for single colonies on chloramphenicol selective media. The three mutant alleles in this phage do not greatly affect the frequency of lysogeny; the latter two have been previously described (Leavitt et al., 2013b), and orf25::CamR-EG1 is a chloramphenicol resistance cassette inserted into the P22 genome between lysis and head genes (details to be described elsewhere). There is no effect on either the lytic or lysogenic life cycle.
Potassium ion efflux measurement
Potassium ion concentrations were measured with an Orion Ionplus potassium electrode (Thermo Scientific) and a Corning model 430 pH meter as described (Leavitt et al., 2013a).
Supplementary Material
Acknowledgments
Thank you to Dr. Sarah Doore for advice on a cloning strategy for pYajC, and to Dr. Karen Maxwell for helpful discussion and careful reading of the manuscript. This material is based upon work supported by grant NIH R01GM110185 to KNP, NIH R01 GM114817 to SRC, and NIAID Contract No. HHSN272200900040C to MM.
Abbreviations
- SGD
single gene deletion
- PFU
plaque-forming units
- MOI
multiplicity of infection
- Tn
transposon
- LPS
lipopolysaccharide
- Omps
outer membrane proteins
- KanR
kanamycin resistant
- CmR
chloramphenicol resistant
- FDR
false discovery rate
- ECA
enterobacterial common antigen
Footnotes
The authors have no conflicts of interest to declare.
References
- Andres D, Hanke C, Baxa U, Seul A, Barbirz S, Seckler R. Tailspike interactions with lipopolysaccharide effect DNA ejection from phage P22 particles in vitro. J Biol Chem. 2010;285:36768–36775. doi: 10.1074/jbc.M110.169003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres D, Roske Y, Doering C, Heinemann U, Seckler R, Barbirz S. Tail morphology controls DNA release in two Salmonella phages with one lipopolysaccharide receptor recognition system. Mol Micro. 2012;83:1244–1253. doi: 10.1111/j.1365-2958.2012.08006.x. [DOI] [PubMed] [Google Scholar]
- Barr K, Klena J, Rick PD. The modality of enterobacterial common antigen polysaccharide chain lengths is regulated by o349 of the wec gene cluster of Escherichia coli K-12. J Bacteriol. 1999;181:6564–6568. doi: 10.1128/jb.181.20.6564-6568.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearson BL, Allen HK, Brunelle BW, Lee IS, Casjens SR, Stanton TB. The agricultural antibiotic carbadox induces phage-mediated gene transfer in Salmonella. Front in Micro. 2014;5:52. doi: 10.3389/fmicb.2014.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertozzi Silva J, Storms Z, Sauvageau D. Host receptors for bacteriophage adsorption. FEMS Micro Lett. 2016:363. doi: 10.1093/femsle/fnw002. [DOI] [PubMed] [Google Scholar]
- Bhagwat AA, Jun W, Liu L, Kannan P, Dharne M, Pheh B, Tall BD, Kothary MH, Gross KC, Angle S, Meng J, Smith A. Osmoregulated periplasmic glucans of Salmonella enterica serovar Typhimurium are required for optimal virulence in mice. Micro. 2009;155:229–237. doi: 10.1099/mic.0.023747-0. [DOI] [PubMed] [Google Scholar]
- Bontemps-Gallo S, Lacroix JM. New insights into the biological role of the osmoregulated periplasmic glucans in pathogenic and symbiotic bacteria. Environ Micro Rep. 2015;7:690–697. doi: 10.1111/1758-2229.12325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botstein D, Waddell CH, King J. Mechanism of head assembly and DNA encapsulation in Salmonella phage P22. I. Genes, proteins, structures and DNA maturation. J Mol Biol. 1973;80:669–695. doi: 10.1016/0022-2836(73)90204-0. [DOI] [PubMed] [Google Scholar]
- Botte M, Zaccai NR, Nijeholt JL, Martin R, Knoops K, Papai G, Zou J, Deniaud A, Karuppasamy M, Jiang Q, Roy AS, Schulten K, Schultz P, Rappsilber J, Zaccai G, Berger I, Collinson I, Schaffitzel C. A central cavity within the holo-translocon suggests a mechanism for membrane protein insertion. Sci Rep. 2016;6:38399. doi: 10.1038/srep38399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger P, Letellier L. Characterization of ion channels involved in the penetration of phage T4 DNA into Escherichia coli cells. J Biol Chem. 1988;263:9767–9775. [PubMed] [Google Scholar]
- Broadbent SE, Davies MR, van der Woude MW. Phase variation controls expression of Salmonella lipopolysaccharide modification genes by a DNA methylation-dependent mechanism. Mol Micro. 2010;77:337–353. doi: 10.1111/j.1365-2958.2010.07203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broeker NK, Barbirz S. Not a barrier but a key: How bacteriophages exploit host’s O-antigen as an essential receptor to initiate infection. Mol Micro. 2017;105:353–357. doi: 10.1111/mmi.13729. [DOI] [PubMed] [Google Scholar]
- Butela K, Lawrence JG. Genetic manipulation of pathogenicity loci in non-Typhimurium Salmonella. J Micro Methods. 2012;91:477–482. doi: 10.1016/j.mimet.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canals R, Xia XQ, Fronick C, Clifton SW, Ahmer BM, Andrews-Polymenis HL, Porwollik S, McClelland M. High-throughput comparison of gene fitness among related bacteria. BMC Genomics. 2012;13:212. doi: 10.1186/1471-2164-13-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casjens S, Molineux I. Short Noncontractile Tail Machines: Adsorption and DNA delivery by Podoviruses. In: Rossmann M, Rao V, editors. Viral Molecular Machines. New York: Springer; 2012. [DOI] [PubMed] [Google Scholar]
- Casjens S, Weigele P. Headful DNA packaging by bacteriophage P22. In: Catalano C, editor. Viral Genome Packaging Machines. Georgetown, TX: Landes Bioscience; 2005. pp. 80–88. [Google Scholar]
- Casjens SR, Jacobs-Sera D, Hatfull GF, Hendrix RW. Genome Sequence of Salmonella enterica Phage Det7. Genome Announ. 2015:3. doi: 10.1128/genomeA.00279-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casjens SR, Leavitt JC, Hatfull GF, Hendrix RW. Genome Sequence of Salmonella Phage 9NA. Genome Announ. 2014:2. doi: 10.1128/genomeA.00531-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caufield JH, Abreu M, Wimble C, Uetz P. Protein complexes in bacteria. PLoS Comp Biol. 2015;11:e1004107. doi: 10.1371/journal.pcbi.1004107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua JE, Manning PA, Morona R. The Shigella flexneri bacteriophage Sf6 tailspike protein (TSP)/endorhamnosidase is related to the bacteriophage P22 TSP and has a motif common to exo- and endoglycanases, and C-5 epimerases. Microbiology. 1999;145(Pt 7):1649–1659. doi: 10.1099/13500872-145-7-1649. [DOI] [PubMed] [Google Scholar]
- Clark CA, Beltrame J, Manning PA. The oac gene encoding a lipopolysaccharide O-antigen acetylase maps adjacent to the integrase-encoding gene on the genome of Shigella flexneri bacteriophage Sf6. Gene. 1991;107:43–52. doi: 10.1016/0378-1119(91)90295-m. [DOI] [PubMed] [Google Scholar]
- Cota I, Blanc-Potard AB, Casadesus J. STM2209-STM2208 (opvAB): a phase variation locus of Salmonella enterica involved in control of O-antigen chain length. PloS one. 2012;7:e36863. doi: 10.1371/journal.pone.0036863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumby N, Reimer K, Mengin-Lecreulx D, Davidson AR, Maxwell KL. The phage tail tape measure protein, an inner membrane protein and a periplasmic chaperone play connected roles in the genome injection process of E. coli phage HK97. Mol Micro. 2015;96:437–447. doi: 10.1111/mmi.12918. [DOI] [PubMed] [Google Scholar]
- de Keyzer J, van der Does C, Driessen AJ. The bacterial translocase: a dynamic protein channel complex. Cell & Mol Life Sci. 2003;60:2034–2052. doi: 10.1007/s00018-003-3006-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Moraes MH, Desai P, Porwollik S, Canals R, Perez DR, Chu W, McClelland M, Teplitski M. Salmonella Persistence in Tomatoes Requires a Distinct Set of Metabolic Functions Identified by Transposon Insertion Sequencing. Applied and Environ Micro. 2017:83. doi: 10.1128/AEM.03028-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dover JA, Burmeister AR, Molineux IJ, Parent KN. Evolved Populations of Shigella flexneri Phage Sf6 Acquire Large Deletions, Altered Genomic Architecture, and Faster Life Cycles. Genome Biol & Evo. 2016;8:2827–2840. doi: 10.1093/gbe/evw177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erni B, Zanolari B, Kocher HP. The mannose permease of Escherichia coli consists of three different proteins. Amino acid sequence and function in sugar transport, sugar phosphorylation, and penetration of phage lambda DNA. J Biol Chem. 1987;262:5238–5247. [PubMed] [Google Scholar]
- Fang J, Wei Y. Expression, purification and characterization of the Escherichia coli integral membrane protein YajC. Protein & Pept Lett. 2011;18:601–608. doi: 10.2174/092986611795222713. [DOI] [PubMed] [Google Scholar]
- Frye JG, Porwollik S, Blackmer F, Cheng P, McClelland M. Host gene expression changes and DNA amplification during temperate phage induction. J Bacteriol. 2005;187:1485–1492. doi: 10.1128/JB.187.4.1485-1492.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbreath JJ, Colvocoresses Dodds J, Rick PD, Soloski MJ, Merrell DS, Metcalf ES. Enterobacterial common antigen mutants of Salmonella enterica serovar Typhimurium establish a persistent infection and provide protection against subsequent lethal challenge. Infection & Immunity. 2012;80:441–450. doi: 10.1128/IAI.05559-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinkowska M, Los JM, Szambowska A, Czyz A, Calkiewicz J, Herman-Antosiewicz A, Wrobel B, Wegrzyn G, Wegrzyn A, Los M. Influence of the Escherichia coli oxyR gene function on lambda prophage maintenance. Archives of Microbiology. 2010;192:673–683. doi: 10.1007/s00203-010-0596-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfull GF, Hendrix RW. Bacteriophages and their genomes. Curr Opin in Virol. 2011;1:298–303. doi: 10.1016/j.coviro.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helander IM, Vaara M, Sukupolvi S, Rhen M, Saarela S, Zahringer U, Makela PH. rfaP mutants of Salmonella typhimurium. European J Biochem / FEBS. 1989;185:541–546. doi: 10.1111/j.1432-1033.1989.tb15147.x. [DOI] [PubMed] [Google Scholar]
- Hoare A, Bittner M, Carter J, Alvarez S, Zaldivar M, Bravo D, Valvano MA, Contreras I. The outer core lipopolysaccharide of Salmonella enterica serovar Typhi is required for bacterial entry into epithelial cells. Infection & Immunity. 2006;74:1555–1564. doi: 10.1128/IAI.74.3.1555-1564.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y, Cunneen MM, Reeves PR. The Wzx translocases for Salmonella enterica O-antigen processing have unexpected serotype specificity. Mol Micro. 2012;84:620–630. doi: 10.1111/j.1365-2958.2012.08048.x. [DOI] [PubMed] [Google Scholar]
- Huang C, Liu Q, Luo Y, Li P, Liu Q, Kong Q. Regulated delayed synthesis of lipopolysaccharide and enterobacterial common antigen of Salmonella Typhimurium enhances immunogenicity and cross-protective efficacy against heterologous Salmonella challenge. Vaccine. 2016;34:4285–4292. doi: 10.1016/j.vaccine.2016.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson HP, Lindberg AA, Stocker BA. Lipopolysaccharide core defects in Salmonella typhimurium mutants which are resistant to Felix O phage but retain smooth character. J Gen Micro. 1978;109:97–112. doi: 10.1099/00221287-109-1-97. [DOI] [PubMed] [Google Scholar]
- Israel V. The production of inactive phage P22 particles following infection. Virology. 1967;33:317–322. doi: 10.1016/0042-6822(67)90150-x. [DOI] [PubMed] [Google Scholar]
- Israel V. E proteins of bacteriophage P22. I. Identification and ejection from Wild-Type and defective particles. J Virol. 1977;23:91–97. doi: 10.1128/jvi.23.1.91-97.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel V, Rosen H, Levine M. Binding of bacteriophage P22 tail parts to cells. Journal of virology. 1972;10:1152–1158. doi: 10.1128/jvi.10.6.1152-1158.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwashita S, Kanegasaki S. Enzymic and molecular properties of base-plate parts of bacteriophage P22. European J Bioch / FEBS. 1976;65:87–94. doi: 10.1111/j.1432-1033.1976.tb10392.x. [DOI] [PubMed] [Google Scholar]
- Jin Y, Sdao SM, Dover JA, Porcek NB, Knobler CM, Gelbart WM, Parent KN. Bacteriophage P22 ejects all of its internal proteins before its genome. Virology. 2015;485:128–134. doi: 10.1016/j.virol.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadam SK, Peppler MS, Sanderson KE. Temperature-sensitive mutants in rfaI and rfaJ, genes for galactosyltransferase I and glucosyltransferase II, for synthesis of lipopolysaccharide in Salmonella typhimurium. Can J Micro. 1985;31:861–869. doi: 10.1139/m85-160. [DOI] [PubMed] [Google Scholar]
- Kihara A, Akiyama Y, Ito K. Host regulation of lysogenic decision in bacteriophage lambda: transmembrane modulation of FtsH (HflB), the cII degrading protease, by HflKC (HflA) Proc Natl Acad Sci USA. 1997;94:5544–5549. doi: 10.1073/pnas.94.11.5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiino DR, Singer MS, Rothman-Denes LB. Two overlapping genes encoding membrane proteins required for bacteriophage N4 adsorption. J Bacteriol. 1993;175:7081–7085. doi: 10.1128/jb.175.21.7081-7085.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein G, Muller-Loennies S, Lindner B, Kobylak N, Brade H, Raina S. Molecular and structural basis of inner core lipopolysaccharide alterations in Escherichia coli: incorporation of glucuronic acid and phosphoethanolamine in the heptose region. J Biol Chem. 2013;288:8111–8127. doi: 10.1074/jbc.M112.445981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klena JD, Ashford RS, 2nd, Schnaitman CA. Role of Escherichia coli K-12 rfa genes and the rfp gene of Shigella dysenteriae 1 in generation of lipopolysaccharide core heterogeneity and attachment of O antigen. J Bacteriol. 1992;174:7297–7307. doi: 10.1128/jb.174.22.7297-7307.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Q, Yang J, Liu Q, Alamuri P, Roland KL, Curtiss R., 3rd Effect of deletion of genes involved in lipopolysaccharide core and O-antigen synthesis on virulence and immunogenicity of Salmonella enterica serovar typhimurium. Infection & Immunity. 2011;79:4227–4239. doi: 10.1128/IAI.05398-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix JM, Loubens I, Tempete M, Menichi B, Bohin JP. The mdoA locus of Escherichia coli consists of an operon under osmotic control. Mol Micro. 1991;5:1745–1753. doi: 10.1111/j.1365-2958.1991.tb01924.x. [DOI] [PubMed] [Google Scholar]
- Leavitt JC, Gilcrease EB, Wilson K, Casjens SR. Function and horizontal transfer of the small terminase subunit of the tailed bacteriophage Sf6 DNA packaging nanomotor. Virology. 2013a;440:117–133. doi: 10.1016/j.virol.2013.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavitt JC, Gogokhia L, Gilcrease EB, Bhardwaj A, Cingolani G, Casjens SR. The tip of the tail needle affects the rate of DNA delivery by bacteriophage P22. PloS one. 2013b;8:e70936. doi: 10.1371/journal.pone.0070936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M, Curtiss R. Genetic fine structure of the C region and the linkage map of phage P22. Genetics. 1961;46:1573–1580. doi: 10.1093/genetics/46.12.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg AA. Bacteriophage receptors. Annual Rev Micro. 1973;27:205–241. doi: 10.1146/annurev.mi.27.100173.001225. [DOI] [PubMed] [Google Scholar]
- Lindqvist L, Schweda KH, Reeves PR, Lindberg AA. In vitro synthesis of CDP-d-abequose using Salmonella enzymes of cloned rfb genes. Production of CDP-6-deoxy-D-xylo-4-hexulose, CDP-3,6-dideoxy-D-xylo-4-hexulose and CDP-3,6-dideoxy-D-galactose, and isolation by HPLC. European J Biochem / FEBS. 1994;225:863–872. doi: 10.1111/j.1432-1033.1994.0863b.x. [DOI] [PubMed] [Google Scholar]
- Liu B, Knirel YA, Feng L, Perepelov AV, Senchenkova SN, Reeves PR, Wang L. Structural diversity in Salmonella O antigens and its genetic basis. FEMS Micro Rev. 2014;38:56–89. doi: 10.1111/1574-6976.12034. [DOI] [PubMed] [Google Scholar]
- Liu L, Tan S, Jun W, Smith A, Meng J, Bhagwat AA. Osmoregulated periplasmic glucans are needed for competitive growth and biofilm formation by Salmonella enterica serovar Typhimurium in leafy-green vegetable wash waters and colonization in mice. FEMS Micro Lett. 2009;292:13–20. doi: 10.1111/j.1574-6968.2008.01462.x. [DOI] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biology. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luria SE, Delbruck M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943;28:491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinus MG, Casadesus J. Roles of DNA adenine methylation in host-pathogen interactions: mismatch repair, transcriptional regulation, and more. FEMS Micro Rev. 2009;33:488–503. doi: 10.1111/j.1574-6976.2008.00159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marolda CL, Tatar LD, Alaimo C, Aebi M, Valvano MA. Interplay of the Wzx translocase and the corresponding polymerase and chain length regulator proteins in the translocation and periplasmic assembly of lipopolysaccharide o antigen. J Bacteriol. 2006;188:5124–5135. doi: 10.1128/JB.00461-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marolda CL, Valvano MA. Genetic analysis of the dTDP-rhamnose biosynthesis region of the Escherichia coli VW187 (O7:K1) rfb gene cluster: identification of functional homologs of rfbB and rfbA in the rff cluster and correct location of the rffE gene. J Bacteriol. 1995;177:5539–5546. doi: 10.1128/jb.177.19.5539-5546.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavris M, Manning PA, Morona R. Mechanism of bacteriophage SfII-mediated serotype conversion in Shigella flexneri. Mol Micro. 1997;26:939–950. doi: 10.1046/j.1365-2958.1997.6301997.x. [DOI] [PubMed] [Google Scholar]
- McClelland M, Sanderson KE, Spieth J, Clifton SW, Latreille P, Courtney L, Porwollik S, Ali J, Dante M, Du F, Hou S, Layman D, Leonard S, Nguyen C, Scott K, Holmes A, Grewal N, Mulvaney E, Ryan E, Sun H, Florea L, Miller W, Stoneking T, Nhan M, Waterston R, Wilson RK. Complete genome sequence of Salmonella enterica serovar Typhimurium LT2. Nature. 2001;413:852–856. doi: 10.1038/35101614. [DOI] [PubMed] [Google Scholar]
- McGuffin LJ, Bryson K, Jones DT. The PSIPRED protein structure prediction server. Bioinformatics. 2000;16:404–405. doi: 10.1093/bioinformatics/16.4.404. [DOI] [PubMed] [Google Scholar]
- Meneely PM. Pick Your Poisson: An Educational Primer for Luria and Delbruck’s Classic Paper. Genetics. 2016;202:371–375. doi: 10.1534/genetics.115.184564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KJ, Kennedy EP, Reinhold VN. Osmotic adaptation by gram-negative bacteria: possible role for periplasmic oligosaccharides. Science. 1986;231:48–51. doi: 10.1126/science.3941890. [DOI] [PubMed] [Google Scholar]
- Murray GL, Attridge SR, Morona R. Regulation of Salmonella typhimurium lipopolysaccharide O antigen chain length is required for virulence; identification of FepE as a second Wzz. Mol Micro. 2003;47:1395–1406. doi: 10.1046/j.1365-2958.2003.03383.x. [DOI] [PubMed] [Google Scholar]
- Neal BL, Brown PK, Reeves PR. Use of Salmonella phage P22 for transduction in Escherichia coli. J Bacteriol. 1993;175:7115–7118. doi: 10.1128/jb.175.21.7115-7118.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouwen N, Driessen AJ. SecDFyajC forms a teterotetrameric complex with YidC. Mol Micro. 2002;44:1397–1405. doi: 10.1046/j.1365-2958.2002.02972.x. [DOI] [PubMed] [Google Scholar]
- Parent KN, Erb ML, Cardone G, Nguyen K, Gilcrease EB, Porcek NB, Pogliano J, Baker TS, Casjens SR. OmpA and OmpC are critical host factors for bacteriophage Sf6 entry in Shigella. Mol Micro. 2014;92:47–60. doi: 10.1111/mmi.12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent KN, Gilcrease EB, Casjens SR, Baker TS. Structural evolution of the P22-like phages: Comparison of Sf6 and P22 procapsid and virion architectures. Virology. 2012;427:177–188. doi: 10.1016/j.virol.2012.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirone-Davies C, Hoffmann M, Roberts RJ, Muruvanda T, Timme RE, Strain E, Luo Y, Payne J, Luong K, Song Y, Tsai YC, Boitano M, Clark TA, Korlach J, Evans PS, Allard MW. Genome-wide methylation patterns in Salmonella enterica Subsp. enterica Serovars. PloS one. 2015;10:e0123639. doi: 10.1371/journal.pone.0123639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogliano JA, Beckwith J. SecD and SecF facilitate protein export in Escherichia coli. EMBO J. 1994a;13:554–561. doi: 10.1002/j.1460-2075.1994.tb06293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogliano KJ, Beckwith J. The Cs sec mutants of Escherichia coli reflect the cold sensitivity of protein export itself. Genetics. 1993;133:763–773. doi: 10.1093/genetics/133.4.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogliano KJ, Beckwith J. Genetic and molecular characterization of the Escherichia coli secD operon and its products. J Bacteriol. 1994b;176:804–814. doi: 10.1128/jb.176.3.804-814.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcek NB, Parent KN. Key residues of S. flexneri OmpA mediate infection by bacteriophage Sf6. J Mol Biol. 2015;427:1964–1976. doi: 10.1016/j.jmb.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porwollik S, Frye J, Florea LD, Blackmer F, McClelland M. A non-redundant microarray of genes for two related bacteria. Nucl Acids Res. 2003;31:1869–1876. doi: 10.1093/nar/gkg298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porwollik S, Santiviago CA, Cheng P, Long F, Desai P, Fredlund J, Srikumar S, Silva CA, Chu W, Chen X, Canals R, Reynolds MM, Bogomolnaya L, Shields C, Cui P, Guo J, Zheng Y, Endicott-Yazdani T, Yang HJ, Maple A, Ragoza Y, Blondel CJ, Valenzuela C, Andrews-Polymenis H, McClelland M. Defined single-gene and multi-gene deletion mutant collections in Salmonella enterica sv Typhimurium. PloS one. 2014;9:e99820. doi: 10.1371/journal.pone.0099820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz C, Ratetz C, Raetz C. Bacterial lipopolysaccharides: a remarkable family of bioactive macroamphiphiles. In: Neidhardt F, editor. Escherichia coli and Salmonella cellular and molecular biology. 1996. pp. 1035–1063. [Google Scholar]
- Ramos-Morales F, Prieto AI, Beuzon CR, Holden DW, Casadesus J. Role for Salmonella enterica enterobacterial common antigen in bile resistance and virulence. J Bacteriol. 2003;185:5328–5332. doi: 10.1128/JB.185.17.5328-5332.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes RE, Gonzales CR, Jimenez RC, Herrera MO, Andrade AA. Mechanisms of O-Antigen Structural Variation of Bacterial Lipopolysaccharide (LPS) In: Karunaratne DN, editor. The complex world of polysaccharides. 2012. [Google Scholar]
- Reynolds CM, Kalb SR, Cotter RJ, Raetz CR. A phosphoethanolamine transferase specific for the outer 3-deoxy-D-manno-octulosonic acid residue of Escherichia coli lipopolysaccharide. Identification of the eptB gene and Ca2+ hypersensitivity of an eptB deletion mutant. J Biol Chem. 2005;280:21202–21211. doi: 10.1074/jbc.M500964200. [DOI] [PubMed] [Google Scholar]
- Rick PD, Barr K, Sankaran K, Kajimura J, Rush JS, Waechter CJ. Evidence that the wzxE gene of Escherichia coli K-12 encodes a protein involved in the transbilayer movement of a trisaccharide-lipid intermediate in the assembly of enterobacterial common antigen. J Biol Chem. 2003;278:16534–16542. doi: 10.1074/jbc.M301750200. [DOI] [PubMed] [Google Scholar]
- Rick PD, Mayer H, Neumeyer BA, Wolski S, Bitter-Suermann D. Biosynthesis of enterobacterial common antigen. J Bacteriol. 1985;162:494–503. doi: 10.1128/jb.162.2.494-503.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein PA, Strominger JL. Enzymatic synthesis of cytidine diphosphate 3,6-dideoxyhexoses. 8. Mechanistic roles of enzyme E-1 and pyridoxamine 5′-phosphate in the formation of cytidine diphosphate-4-keto-3,6-dideoxy-D-glucose from cytidine diphosphate-4-keto-6-deoxy-D-glucose. J Biol Chem. 1974;249:3776–3781. [PubMed] [Google Scholar]
- Sassetti CM, Boyd DH, Rubin EJ. Comprehensive identification of conditionally essential genes in mycobacteria. Proc Natl Acad Sci USA. 2001;98:12712–12717. doi: 10.1073/pnas.231275498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmieger H, Schicklmaier P. Transduction of multiple drug resistance of Salmonella enterica serovar typhimurium DT104. FEMS Micro Lett. 1999;170:251–256. doi: 10.1111/j.1574-6968.1999.tb13381.x. [DOI] [PubMed] [Google Scholar]
- Schulze RJ, Komar J, Botte M, Allen WJ, Whitehouse S, Gold VA, Lycklama ANJA, Huard K, Berger I, Schaffitzel C, Collinson I. Membrane protein insertion and proton-motive-force-dependent secretion through the bacterial holo-translocon SecYEG-SecDF-YajC-YidC. Proc Natl Acad Sci USA. 2014;111:4844–4849. doi: 10.1073/pnas.1315901111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slauch JM, Lee AA, Mahan MJ, Mekalanos JJ. Molecular characterization of the oafA locus responsible for acetylation of Salmonella typhimurium O-antigen: oafA is a member of a family of integral membrane trans-acylases. J Bacteriol. 1996;178:5904–5909. doi: 10.1128/jb.178.20.5904-5909.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbacher S, Miller S, Baxa U, Budisa N, Weintraub A, Seckler R, Huber R. Phage P22 tailspike protein: crystal structure of the head-binding domain at 2.3A, fully refined strucutre of the endorhamnosidase at 1.56A resolution, and the molecular basis of O-antigen recognition and cleavage. J Mol Biol. 1997;267:865–880. doi: 10.1006/jmbi.1997.0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susskind MM, Botstein D. Molecular genetics of bacteriophage P22. Micro Rev. 1978;42:385–413. doi: 10.1128/mr.42.2.385-413.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Byl C, Kropinski AM. Sequence of the genome of Salmonella bacteriophage P22. J Bacteriol. 2000;182:6472–6481. doi: 10.1128/jb.182.22.6472-6481.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma NK, Brandt JM, Verma DJ, Lindberg AA. Molecular characterization of the O-acetyl transferase gene of converting bacteriophage SF6 that adds group antigen 6 to Shigella flexneri. Mol Micro. 1991;5:71–75. doi: 10.1111/j.1365-2958.1991.tb01827.x. [DOI] [PubMed] [Google Scholar]
- Walter M, Fiedler C, Grassl R, Biebl M, Rachel R, Hermo-Parrado XL, Llamas-Saiz AL, Seckler R, Miller S, van Raaij MJ. Structure of the receptor-binding protein of bacteriophage det7: a podoviral tail spike in a myovirus. J Virol. 2008;82:2265–2273. doi: 10.1128/JVI.01641-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyk P, Reeves P. Identification and sequence of the gene for abequose synthase, which confers antigenic specificity on group B salmonellae: homology with galactose epimerase. J Bacteriol. 1989;171:5687–5693. doi: 10.1128/jb.171.10.5687-5693.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yethon JA, Gunn JS, Ernst RK, Miller SI, Laroche L, Malo D, Whitfield C. Salmonella enterica serovar Typhimurium waaP mutants show increased susceptibility to polymyxin and loss of virulence In vivo. Infection & Immunity. 2000;68:4485–4491. doi: 10.1128/iai.68.8.4485-4491.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuasa R, Levinthal M, Nikaido H. Biosynthesis of cell wall lipopolysaccharide in mutants of Salmonella. V. A mutant of Salmonella typhimurium defective in the synthesis of cytidine diphosphoabequose. J Bact. 1969;100:433–444. doi: 10.1128/jb.100.1.433-444.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.