Abstract
Hepatic encephalopathy (HE) is a major cause of morbidity in cirrhosis. However its severity assessment is often subjective, which needs to be studied systematically.
Aim
To determine how accurately trainee and non-trainee practitioners grade and manage HE patients throughout its severity.
Methods
We performed a survey study using standardized simulated patient videos at 4 US and 3 Canadian centers. Participants were trainees (gastroenterology/hepatology fellows) and non-trainees (faculty, nurse practitioners, physician assistants). We determined the accuracy of HE severity identification and management options between grades<2 or ≥2 HE and trainees/non-trainees
Results
108 respondents (62 trainees, 46 non-trainees) were included. Grades<2 vs.≥2 HE: A higher percentage of respondents were better at correctly diagnosing grades≥2 compared to grades<2 (91 vs 64%, p<0.001). Specialized cognitive testing was checked significantly more often in grades<2, while more aggressive investigation for precipitating factors was ordered in HE grades>2. Serum ammonia levels were ordered in almost a third of ≥2 grade patients. Trainees/non-trainees: HE grades were identified similarly between groups. Trainees were less likely to order serum ammonia and low-protein diets, more likely to order rifaximin, and perform a more thorough work-up for precipitating factors compared to non-trainee respondents.
Conclusions
There was excellent concordance in the classification of grade ≥2 HE between non-trainees vs. trainees but lower grades showed discordance. Important differences were seen regarding blood ammonia, specialized testing and nutritional management between trainees and non-trainees. These results have important implications at the patient level, interpreting multi-center clinical trials and, in the education of practitioners.
Keywords: Cirrhosis, Education, Quality Improvement, West-Haven criteria, Minimal Hepatic Encephalopathy
Introduction
Cirrhosis and hepatic encephalopathy (HE) is a major cause of morbidity and mortality in the United States, and the trend points towards a growing burden over time(1). HE is defined as brain dysfunction caused by liver insufficiency and/or portosystemic shunting in the absence of other brain diseases, which manifests as a wide spectrum of neuropsychiatric abnormalities ranging from subclinical alterations to coma(2). HE accounts for approximately 110,000 hospitalizations yearly (2005-2009) and is the most common cause of readmission in decompensated cirrhotic patient(1,3). Due to the complexity of this condition and its myriad presentations, most hospital and clinic-based specialties such as emergency room, primary care, hospitalist medicine, critical care and gastroenterology/hepatology specialists encounter these patients(2). HE severity is viewed as a continuum but using the West-Haven criteria is divided into grades 1-4 in patients exhibiting clinical signs of the disease(Table 1)(4). The subclinical form, called minimal HE (MHE), cannot be diagnosed using the usual physical examination and requires specialized cognitive testing(3). However, the West-Haven criteria can be subjective and semi-quantitative, and their inter-observer reliability needs to be studied(4). The proper elucidation of HE grades is an important goal for teaching and research since management options vary greatly between different HE grades. In addition, several other questions in the management of HE, including use of blood ammonia levels, restriction of protein, and use of appropriate medications and imaging remain controversial and center-specific, but have gained some clarity in recent guidelines(2). Therefore, an analysis of the accurate diagnosis and subsequent management of HE across its spectrum of severity is a critical clinical, investigational and educational goal.
Table 1.
West-Haven Criteria for Grading the Severity of Hepatic Encephalopathy
| Grade | Descriptors |
|---|---|
| 0 | Normal |
| 1 | Trivial lack of awareness; euphoria or anxiety; shortened attention span; impaired performance of addition or subtraction, altered sleep rhythm |
| 2 | Lethargy or apathy; minimal disorientation for time or place; subtle personality change; inappropriate behavior, |
| 3 | Somnolence to semi-stupor, but responsive to verbal stimuli; confusion; gross disorientation |
| 4 | Coma |
The purpose of this study is to determine how accurately specialist trainee and non-trainee practitioners are able to properly grade and manage HE patients across grades of severity in order to evaluate consistency and management trends in the current practicing population.
Methods
The study was carried out between February and April 2017 as a quality improvement project. Participants in the study included United States and Canadian trainee and non-trainee gastroenterology practitioners. Trainees included gastroenterology fellows along with internal medicine residents. Non-trainees included gastroenterology faculty members, nurse practitioners, and physician assistants. Each site was given freedom to plan how they performed the survey based on convenience and ability to get maximal participation. The study was described in detail and any participant who attended the sessions was allowed to leave if they did not wish to be involved. All GI faculty and GI fellows were invited to participate in the study at each site and the study was announced at the start of conference. All participants were shown a series of standardized simulated patient videos during the same sitting as published in a prior study(7). The videos demonstrated cirrhotic patients with no HE (normal), grades 1, 2, 3 and 4 HE using the West-Haven criteria. Grades 2-4 occur in a stimulated inpatient setting with a physician, the patient, and the patient’s significant other. For the other two videos normal and grade 1 the videos took place in the outpatient setting in a physician’s office with the physician, the patient, and his mother. The survey was developed under neuro-psychological guidance (JBW, Supplementary Information). The survey was paper based and after completion it was manually collected and mailed or faxed to VCU for analysis. Section one of the survey tested grade identification. Section two of the survey assessed management and was divided into 5 domains (history-taking, initial management, course of action, investigations and lastly therapies). Diagnostic and treatment appropriateness were based upon the recommendations outlined in the 2014 AASLD/EASL Hepatic encephalopathy guidelines(2)(Supplementary table 1).The videos were shown in the same order (grade 4, grade 2, grade 3, normal, and finally grade 1) at all sites and all respondents were asked to complete all questions in the survey after the completion of the video of one grade before moving to the next. Comparisons were performed in the identification and management of the grades, between grades 2 or higher compared to the lower ones and also between trainees and non-trainee practitioners. Data analysis was performed via chi-square and Fisher exact testing using p <0.05.
Sample size
Given the stability of the diagnosis of grade 2 or higher of HE, we assumed that 90% of respondents would be able to evaluate it compared to 60% of respondents in the lower HE grades. With a power of 90% and α of 0.05, we would require at least 42 respondents. Since we wanted to study trainees and non-trainees separately, our aim was to at least enroll 42 subjects in each group.
This project was considered an educational, quality improvement project and was exempt from IRB approval at all institutions.
Results
Demographics
There were slight variations from center to center in how the study was performed, but overall it was consistent. Selected GI faculty and GI fellows at each site, who were trained by JSB and BR, ran the survey sessions. All sessions were combined and included both trainees and non-trainees. The people who invited the participants were the same ones to play the videos and administer the survey within the same session. The study was performed during a regularly scheduled conference time (grand rounds, noon conferences, and didactics sessions.) The response rate was 100% for all conference attendees (trainee and non-trainee) at all sites. In total there were 108 respondents (62 trainees, 46 non-trainees) from 7 centers (77 from 4 US and 31 from 3 Canadian centers) were included. Trainees included 18 1st year, 16 2nd year, 12 3rd year, 14 4th year hepatology fellows & 2 internal medicine residents. Non-trainees included 41 gastroenterology consultants & 5 mid-level practitioners.
Grade Identification
A higher percentage of total respondents correctly diagnosed grades ≥2 compared to grades <2 (Figure 1). HE grades were identified similarly between trainees and non-trainees (Table 2). The breakdown between respondents across groups is shown in supplementary table 2.
Figure 1. Correct characterization of Hepatic Encephalopathy Grades.
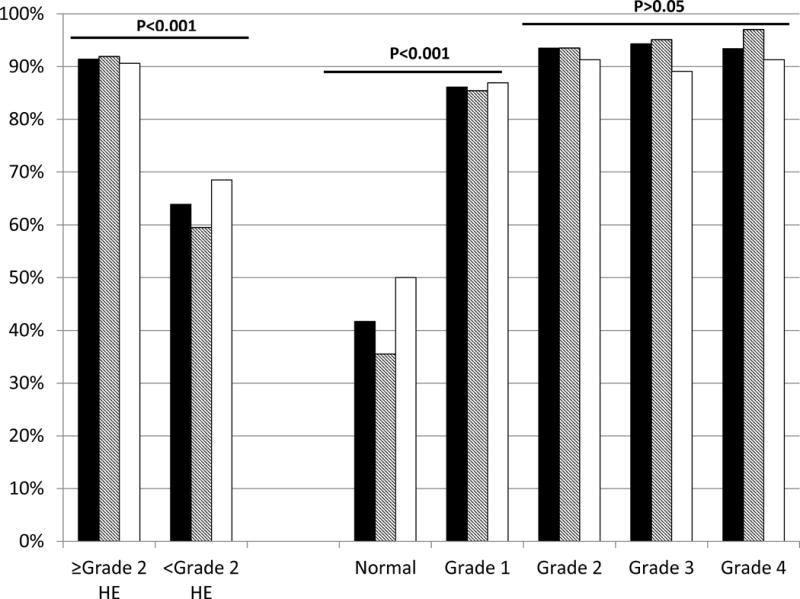
Black bars: All respondents, Diagonally shaded bars: Trainees and White Bars: Non-trainees. The left side of the graph demonstrates concordance between the actual HE grades divided into <Grade 2 vs ≥grade 2. The right side of the graph breaks this down by individual grades. There was a significant discordance between defining normal and grade 1 compared to grades 2 and higher in all respondents.
Table 2.
Identification of HE Grades of Standardized Patients: Percent correct between participant groups. P value using Chi-square test.
| Correct Identification | Trainees (n=62) | Non-Trainees (n=46) | P value |
|---|---|---|---|
| ≥ Grade 2 | 91.9% | 90.6% | 0.23 |
| < Grade 2 HE | 59.5% | 68.5% | 0.67 |
| Subdivisions | |||
| Normal | 35.5% | 50% | 0.13 |
| Grade 1 | 85.4% | 86.9% | 0.83 |
| Grade 2 | 93.5% | 91.3% | 0.66 |
| Grade 3 | 95.1% | 89.1% | 0.24 |
| Grade 4 | 87.0% | 91.3% | 0.49 |
Comparison between grades ≥2 and <grade 2 in all respondents
As shown in table 3, most respondents would inquire about patient stool number and medication history for all grades, while most participants would perform a neurological exam for focal deficits only in grades ≥2. On the other hand, specialized MHE testing was checked significantly more often in HE grades <2. In the work-up, blood tests to define potential precipitating factors and other causes of altered mental status were ordered in the majority of grade ≥2 patients. Serum ammonia levels would be ordered in almost a third of ≥2 grade patients, higher than the lower grades. HE-specific therapies (lactulose and rifaximin), and intravenous albumin and antibiotics, and brain imaging were more commonly ordered for grade ≥2 patients. Patients with more advanced HE were also more likely to receive nutritional consults. Low-protein diets were ordered at a similar low rate across all HE grades.
Table 3.
Clinical Comparison between Management of Standardized Patients with HE < Grade 2 vs HE Grade ≥2.
| <Grade 2 % | Appropriateness | ≥2 Grade % | Appropriateness | p value | |
|---|---|---|---|---|---|
| History | |||||
| Stool Frequency | 82 | Yes | 91 | Yes | 0.002 |
| Medication History | 88 | Yes | 97 | Yes | <0.001 |
| Physical Examination | |||||
| Neurological examination for focal deficits | 23 | Yes | 82 | Yes | <0.001 |
| Neurological examination for herniation | 0 | No | 32 | Yes | <0.001 |
| Investigations | <0.001 | ||||
| MHE Testing | 70 | Yes | 20 | No | <0.001 |
| Brain Scan | 4 | No | 47 | Varies | <0.001 |
| Comprehensive metabolic panel | 62 | Yes | 98 | Yes | <0.001 |
| Complete blood count | 55 | Varies | 99 | Yes | <0.001 |
| Blood cultures | 3 | Varies | 89 | Yes | <0.001 |
| Urinalysis | 11 | Varies | 92 | Yes | <0.001 |
| Blood ammonia | 11 | No | 36 | No | <0.001 |
| Urine drug screen | 27 | Varies | 80 | Varies | <0.001 |
| Blood alcohol level | 24 | Varies | 74 | Varies | <0.001 |
| INR | 38 | Yes | 89 | Yes | <0.001 |
| Hemoccult | 5 | No | 20 | No | <0.001 |
| Management(non-specific) | <0.001 | ||||
| Hospital Admission | 2 | No | 94 | Yes | <0.001 |
| ICU Admission | 0 | No | 33 | Varies | <0.001 |
| Nasogastric intubation | 0 | No | 24 | Varies | <0.001 |
| Endotracheal intubation | 0 | No | 19 | Varies | <0.001 |
| Specific treatments | |||||
| Lactulose | 58 | Case by case basis | 93 | Yes | <0.001 |
| Rifaximin | 14 | No | 50 | Varies | <0.001 |
| Antibiotics | 0 | No | 19 | Varies | <0.001 |
| IV Albumin | 0 | No | 11 | No | <0.001 |
| Nutritional management | <0.001 | ||||
| Nutritional consultation | 42 | Yes | 65 | Yes | <0.001 |
| Low Protein diet | 9 | No | 12 | No | 0.19 |
P values were calculated using Chi-Square or Fisher Exact Test as necessary. Appropriateness was based on the EASL/AASLD 2014 HE guidelines. MHE: minimal hepatic encephalopathy, ICU: intensive care unit
Trainee vs Non-Trainee Management
When management was compared between trainee and non-trainee respondents, we found a statistically similar response to most questions except a few important ones (Table 4). Specifically, trainees were more likely to; i) inquire about bowel movement frequency irrelevant of the severity of HE, ii) order rifaximin and iii) perform a more thorough work-up for precipitating factors and less likely to order i) serum ammonia and ii) low-protein diets compared to non-trainee respondents.
Table 4.
Clinical Comparison between Management of Standardized Patients with HE < Grade 2 vs HE Grade ≥2 between specialty trainees vs on-trainees.
| <Grade 2 | ≥2 Grade | |||||
|---|---|---|---|---|---|---|
| Trainees % |
Non-trainees % |
p value | Trainees % |
Non-trainees % |
p value | |
| History | ||||||
| Stool Number | 89 | 73 | <0.001 | 95 | 86 | <0.001 |
| Medication History | 90 | 86 | 0.31 | 96 | 99 | 0.14 |
| Physical Examination | ||||||
| Neurological examination for focal deficits | 26 | 29 | 0.27 | 78 | 86 | 0.07 |
| Neurological examination for herniation | 0 | 1 | 0.43 | 36 | 26 | 0.05 |
| Investigations | ||||||
| MHE Testing | 73 | 66 | 0.32 | 15 | 20 | 0.23 |
| Brain Scan | 3 | 4 | 0.67 | 49 | 45 | 0.48 |
| Comprehensive metabolic panel | 60 | 64 | 0.50 | 98 | 99 | 0.64 |
| Complete blood count | 53 | 58 | 0.52 | 99 | 99 | 0.83 |
| Blood cultures | 2 | 3 | 0.70 | 94 | 83 | <0.001 |
| Urinalysis | 10 | 12 | 0.73 | 95 | 88 | 0.04 |
| Blood ammonia | 7 | 16 | 0.03 | 33 | 41 | 0.15 |
| Urine drug screen | 22 | 34 | 0.05 | 80 | 80 | 0.93 |
| Blood alcohol level | 19 | 29 | 0.08 | 72 | 76 | 0.41 |
| INR | 39 | 36 | 0.67 | 88 | 91 | 0.49 |
| Hemoccult | 0 | 12 | <0.001 | 10 | 31 | <0.001 |
| Management (non-specific) | ||||||
| Hospital Admission | 2 | 1 | 0.64 | 96 | 91 | 0.10 |
| ICU Admission | 1 | 0 | 1.00 | 35 | 29 | 0.22 |
| Nasogastric intubation | 0 | 0 | 1.00 | 25 | 23 | 0.75 |
| Endotracheal intubation | 0 | 0 | 1.00 | 23 | 15 | 0.10 |
| Specific treatments | ||||||
| Lactulose | 60 | 54 | 0.36 | 95 | 91 | 0.16 |
| Rifaximin | 10 | 20 | 0.59 | 68 | 30 | <0.001 |
| Antibiotics | 0 | 0 | 1.00 | 17 | 21 | 0.32 |
| IV Albumin | 0 | 0 | 1.00 | 12 | 11 | 0.79 |
| Nutritional management | ||||||
| Nutritional consultation | 46 | 36 | 0.14 | 69 | 64 | 0.41 |
| Low Protein diet | 6 | 12 | 0.15 | 8 | 18 | 0.006 |
P value using Chi-Square or Fisher Exact Test as appropriate MHE: minimal hepatic encephalopathy, ICU: intensive care unit
Discussion
The current study results show that an accurate and reproducible assessment of lower grades of HE remains problematic even among trainees and practitioners of the subspecialty of gastroenterology. The results also demonstrate that trainees and practitioners had significant differences in the management of HE grades primarily in investigations, nutrition and medical therapies.
These results have important implications with respect to patient care, resource utilization, conduct of multi-center clinical trials and, most importantly, in the training and continuing education of practitioners who deal with this complex patient population. The lack of reproducibility in the diagnosis of the lower HE stages is not unexpected given the semi-quantitative nature of the “gold-standard,” the West-Haven criteria for HE. Given these uncertainties, regulatory agencies such as the Food and Drug Administration (FDA) have mandated use of other instruments to gauge severity of HE, especially for the earlier grades for future clinical trials(7). Also in response to this difficulty, the recent international guidelines for HE have combined grade 1 HE and minimal HE into covert HE(2,8). This study highlights the utility of this approach in the guidelines since there is a major gap in differentiating between “normal” and grade 1 HE, while there is excellent agreement between grades 2, 3 and 4.
The inability to accurately identify HE grades <2 could potentially result in inappropriate management of this patient population and affect multi-center clinical research. This is particularly important in trials in which grade 1 HE is a major endpoint. This lack of agreement in the specialist community (both trainees and non-trainee practitioners) does not bode well for the adequate resolution of this situation. In most practices, grade 1 HE is diagnosed clinically through the experience of the investigator or in most cases on inquiry with the companions of the patients. However, this is highly dependent on the presence of the companion, familiarity of the patient to the clinician, as well as availability of time that is required for the practitioner to ask these questions. A recent study to better define grade 1 using the simple animal naming test has been published, which requires further validation in other centers to study its ability to define this grade(9). The deficiency in this knowledge was also highlighted by the relatively few respondents who ordered specialized testing for MHE to better define lower grades of HE. These tests, which range from simple App-based tests to sophisticated neuro-psychological tools, can be important in prognosticating OHE development, indicate poor quality of life, and help educate patients and caregivers about HE(2,10,11). In cases such as these where the identification of “normal” and grade 1 is in doubt, MHE testing results could add an important component to the decision-making process and is recommended for every at-risk cirrhotic patient per guidelines(2). Given the stability of the diagnosis beyond grade 2, the definition of all other grades below that as covert HE seems to be an important option until better operative criteria are defined for grade 1 in multi-center studies. Efforts must be made to improve training in this area at every level including during medical school, residency, and fellowship. Online learning tutorials, phone applications, and standardized curricula are all possible supplemental options for improving trainee and non-trainee knowledge in this area. Specific standardized patients to simulate inpatient and outpatient visits may be necessary tools for this complex population as well.
This study also provides evidence of knowledge deficiencies in the proper management of this patient population including improper understanding of MHE testing, precipitant evaluations, diet/nutritional needs, and treatment options. The appropriateness was judged based on the 2014 HE AASLD/EASL guideline recommendations. The burden of HE and chronic liver disease will continue to grow and proper management is key for improving patient survival, reducing hospital cost/readmission rates, and maximizing reimbursement. In 2003, patients hospitalized with HE generated charges of approximately 1 billion US dollars and this cost will continue to rise in coming years(1,12). Under-staging could lead to an incomplete workup of precipitating factors, delay in escalation of care, and ineffective treatment strategies, while the reverse could leads to resource mismanagement with increased costs and a higher patient exposure to medications, expensive imaging studies, and invasive procedures.
Most HE episodes have a precipitating factor, the identification and correction of which is essential for the improvement of symptoms(2,13,14). For HE grades ≥ 2, there was an adequate workup of these factors for most practitioners, although specific issues were identified. While the latest guidelines do not advise it, non-trainee practitioners had a higher rate of inappropriate ammonia level ordering across all HE grades despite evidence that high blood-ammonia levels alone do not add any diagnostic, staging, or prognostic value(2). There is a strong consensus that low protein diets should be avoided in HE patients(15,16). Unfortunately almost 10% of participants continued to order low protein diets in all HE grades instead of ordering robust nutritional assessments for patients. This highlights nutrition as an area of concern. Another response element group that was concerning was the relatively high proportion of respondents who would perform brain scanning with CT or MRI in patients with advanced HE grades, which although useful to detect intracerebral hemorrhage, which has been shown to be of doubtful value in patients without focal neurological deficits(17,18).
Currently only overt HE is routinely treated. While most practitioners ordered lactulose correctly for overt HE patients they also treated patients with grades<2 over half the time leading to concerns for overtreatment in this patient population. Rifaximin alternatively was ordered less frequently for overt HE patients by non-trainees. This could be logistically challenging in Canada, where rifaximin has only recently become easier to access. However, in patients who are intolerant of lactulose, there is a good evidence basis for the use of rifaximin(19).
When comparing trainees to non-trainees, the general trend emerged that trainees were more aggressive in their workup and management of overt HE patients while non-trainees were more thorough in their workup of HE patients grade <2. Maintaining currency on newer guideline changes is critical in providing standard of care treatment. The responses of non-trainees being more likely to order ammonia levels and low protein diets, while showing hesitancy prescribing rifaximin demonstrates these differences. Therefore there remains a need for continued re-education of the practitioner population regarding guideline changes during continuing medical education.
Although this study identified a major inconsistency in diagnosing HE, it also had limitations including practitioner sample size and the number of included gastrointestinal programs in the US and Canada. Participants were obliged to make staging decisions and management options based on another physician’s interview which may not have included all or some of questions the participant would ask in their clinical practice. Due to the structure of the study some participants may have thought that a “normal” patient wasn’t included as part of the videos even though this was listed as a possible staging selection choice. Ideally it would have beneficial to expand the detail of certain management topics, for example MHE testing could have been split into the many different possible tests available, but due to an overall lack of consensus in this field, relatively finer details of MHE testing were not included. We also determined that anonymity regarding center location would aid in a more transparent educational experience, therefore individual center data could not be compared. In most centers both trainees and non-trainees completed the survey in the same room, which could encourage for possible contamination of responses. We only limited ourselves to GI practitioners and trainees given a higher likelihood of encountering HE patients routinely but further research into the understanding of HE needs to be expanded to other medicine residents, hospitalists, emergency room and critical care specialties.
We conclude that in this multi-center international survey using simulated standardized patients, there was excellent concordance between stage 2 or higher for HE whereas lower stages showed significant discordance. There were also important differences found regarding blood ammonia levels, specialized cognitive testing and nutritional management between trainees and non-trainee practitioners. Further research in this area should be directed at operationalizing the grading of earlier HE grades, and improving education of trainees and non-trainee practitioners regarding the current treatment guidelines to improve patient care. This could have an important impact on resource utilization, clinical research, and education.
Acknowledgments
Grant and Financial Support—Partly funded using VA merit review grant I0CX001076 and NCATS NIH R21TR002024 to JSB
Abbreviations
- HE
hepatic encephalopathy
- CHE
covert hepatic encephalopathy
- OHE
overt hepatic encephalopathy
Footnotes
Conflicts of interest– Authors have nothing to disclose
References
- 1.Stepanova M, Mishra A, Venkatesan C, et al. In-hospital mortality and economic burden associated with hepatic encephalopathy in the United States from 2005 to 2009. Clin Gastroenterol Hepatol. 2012;10:1034–41 e1. doi: 10.1016/j.cgh.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 2.Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715–35. doi: 10.1002/hep.27210. [DOI] [PubMed] [Google Scholar]
- 3.Bajaj JS, Reddy KR, Tandon P, et al. The 3-month readmission rate remains unacceptably high in a large North American cohort of patients with cirrhosis. Hepatology. 2016;64:200–8. doi: 10.1002/hep.28414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conn HO, Leevy CM, Vlahcevic ZR, et al. Comparison of lactulose and neomycin in the treatment of chronic portal-systemic encephalopathy. A double blind controlled trial Gastroenterology. 1977;72:573–83. [PubMed] [Google Scholar]
- 5.Ferenci P, Lockwood A, Mullen K, et al. Hepatic encephalopathy–definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716–21. doi: 10.1053/jhep.2002.31250. [DOI] [PubMed] [Google Scholar]
- 6.Hassanein TI, Tofteng F, Brown RS, Jr, et al. Randomized controlled study of extracorporeal albumin dialysis for hepatic encephalopathy in advanced cirrhosis. Hepatology. 2007;46:1853–62. doi: 10.1002/hep.21930. [DOI] [PubMed] [Google Scholar]
- 7.Bajaj JS, Frederick RT, Bass NM, et al. Overt hepatic encephalopathy: development of a novel clinician reported outcome tool and electronic caregiver diary. Metab Brain Dis. 2016 doi: 10.1007/s11011-016-9851-9. [DOI] [PubMed] [Google Scholar]
- 8.Bajaj JS, Cordoba J, Mullen KD, et al. Review article: the design of clinical trials in hepatic encephalopathy–an International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN) consensus statement. Aliment Pharmacol Ther. 2011;33:739–47. doi: 10.1111/j.1365-2036.2011.04590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campagna F, Montagnese S, Ridola L, et al. The animal naming test: An easy tool for the assessment of hepatic encephalopathy. Hepatology. 2017;66:198–208. doi: 10.1002/hep.29146. [DOI] [PubMed] [Google Scholar]
- 10.Amodio P, Montagnese S, Gatta A, et al. Characteristics of minimal hepatic encephalopathy. Metab Brain Dis. 2004;19:253–67. doi: 10.1023/b:mebr.0000043975.01841.de. [DOI] [PubMed] [Google Scholar]
- 11.Allampati S, Duarte-Rojo A, Thacker LR, et al. Diagnosis of Minimal Hepatic Encephalopathy Using Stroop EncephalApp: A Multicenter US-Based, Norm-Based Study. Am J Gastroenterol. 2016;111:78–86. doi: 10.1038/ajg.2015.377. [DOI] [PubMed] [Google Scholar]
- 12.Leise MD, Poterucha JJ, Kamath PS, et al. Management of hepatic encephalopathy in the hospital. Mayo Clin Proc. 2014;89:241–53. doi: 10.1016/j.mayocp.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pantham G, Post A, Venkat D, et al. A New Look at Precipitants of Overt Hepatic Encephalopathy in Cirrhosis. Dig Dis Sci. 2017;62:2166–2173. doi: 10.1007/s10620-017-4630-y. [DOI] [PubMed] [Google Scholar]
- 14.Bajaj JS, Sanyal AJ, Bell D, et al. Predictors of the recurrence of hepatic encephalopathy in lactulose-treated patients. Aliment Pharmacol Ther. 2010;31:1012–7. doi: 10.1111/j.1365-2036.2010.04257.x. [DOI] [PubMed] [Google Scholar]
- 15.Amodio P, Bemeur C, Butterworth R, et al. The nutritional management of hepatic encephalopathy in patients with cirrhosis: International Society for Hepatic Encephalopathy and Nitrogen Metabolism Consensus. Hepatology. 2013;58:325–36. doi: 10.1002/hep.26370. [DOI] [PubMed] [Google Scholar]
- 16.Cordoba J, Lopez-Hellin J, Planas M, et al. Normal protein diet for episodic hepatic encephalopathy: results of a randomized study. J Hepatol. 2004;41:38–43. doi: 10.1016/j.jhep.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 17.Donovan LM, Kress WL, Strnad LC, et al. Low likelihood of intracranial hemorrhage in patients with cirrhosis and altered mental status. Clin Gastroenterol Hepatol. 2015;13:165–169. doi: 10.1016/j.cgh.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 18.Rahimi RS, Rockey DC. Overuse of head computed tomography in cirrhosis with altered mental status. Am J Med Sci. 2016;351:459–466. doi: 10.1016/j.amjms.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 19.Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071–81. doi: 10.1056/NEJMoa0907893. [DOI] [PubMed] [Google Scholar]


