Abstract
Importance
Admission medication history (AMH) errors frequently cause medication order errors and patient harm.
Objective
To quantify AMH error reduction achieved when pharmacy staff obtain AMHs before admission medication orders (AMO) are placed.
Design
Three-arm randomized controlled trial.
Population
306 enrolled inpatients.
Interventions
In one intervention arm, pharmacists, and in the second intervention arm, pharmacy technicians obtained initial AMHs prior to admission. They obtained and reconciled medication information from multiple sources. All arms, including the control arm, received usual AMH care, which included variation in several common processes.
Main Outcomes and Measures
The primary outcome was severity-weighted mean AMH error score. To detect AMH errors, all patients received reference standard AMHs, which were compared with intervention and control group AMHs. AMH errors and resultant AMO errors were independently identified and rated by ≥2 investigators as significant, serious or life-threatening. Each error was assigned 1, 4 or 9 points, respectively, to calculate severity-weighted AMH and AMO error scores for each patient.
Results
Patient characteristics were similar across arms (mean±SD age 72±16 years, number of medications 15±7). Analysis was limited to 278 patients (91%) with reference standard AMHs. Mean±SD AMH errors per patient in the usual care, pharmacist and technician arms were 8.0±5.6, 1.4±1.9 and 1.5±2.1, respectively (p<0.0001). Mean±SD severity-weighted AMH error scores were 23.0±16.1, 4.1±6.8 and 4.1±7.0 per patient, respectively (p<0.0001). These AMH errors led to a mean±SD of 3.2±2.9, 0.6±1.1 and 0.6±1.1 AMO errors per patient, and mean severity-weighted AMO error scores of 6.9±7.2, 1.5±2.9 and 1.2±2.5 per patient, respectively (both p<0.0001).
Conclusions
Pharmacists and technicians reduced AMH errors and resultant AMO errors by over 80%. Future research should examine other sites and patient-centered outcomes.
INTRODUCTION
Bates et al defined an adverse drug event (ADE) as an “injury resulting from medical intervention related to a drug”.(1) The Institute of Medicine estimates that hospitalized US patients suffer from 400,000 preventable ADEs annually.(2) Among the most frequent causes of preventable ADEs are errors in admission medication histories (AMHs).(3–5)
Using pharmacists to check AMHs reduces preventable ADEs.(6) Nonetheless, many organizations have encountered difficulties in disseminating pharmacist-led medication reconciliation interventions. The poor uptake of such interventions has been attributed to the complexity of implementing medication reconciliation interventions, which affect multiple interacting workflows, and to the cost of employing pharmacists.(7)
To address both implementation complexity and cost, we modified this intervention by stationing pharmacists in the emergency department to obtain AMHs before admitting physicians place admission medication orders (AMOs). This allows admitting physicians to work from an accurate AMH, which is especially important in an era when electronic health records allow physicians to convert AMHs into AMOs with just a few mouse clicks, and when patients are often admitted by hospitalists unfamiliar with patients’ home medication regimens.
To quantify the reduction in AMH errors achieved by pharmacists and pharmacist-supervised pharmacy technicians obtaining AMHs in the emergency department, we conducted a three-arm randomized controlled trial comparing these providers with usual care processes in a population of medically complex patients. To better understand the effect on more downstream outcomes, including preventable ADEs occurring in the hospital and after discharge, we also compared rates of AMO errors resulting from AMH errors.
METHODS
Trial Design Overview
We conducted a three-arm randomized controlled trial. Intervention arms used pharmacists or pharmacist-supervised pharmacy technicians to obtain AMHs before AMOs were placed. The Cedars-Sinai Institutional Review Board agreed that informed consent of patients should be waived in this randomized allocation of services that had heretofore been allocated via operational convenience.
Setting and Study Population
Cedars-Sinai Medical Center (CSMC) is a large university-affiliated hospital. Providers placing orders for trial patients included community, hospitalist, and resident physicians as well as nurse practitioners and physician assistants. Pharmacists included licensed resident pharmacists.
Eligible participants were medically complex patients admitted to CSMC through the emergency department. Enrollment screening occurred Mondays through Thursdays from approximately 11 AM to 8 PM beginning 1/7/2014 through 2/14/2014. Enrollment ceased at the end of the first day on which the intended sample size was exceeded. Screening was occasionally paused when pharmacy staff were otherwise occupied with clinical or research duties. Inclusion criteria were: ≥10 active chronic prescription medications in the electronic health record (EHR), history of acute myocardial infarction or congestive heart failure in the EHR problem list, admission from a skilled nursing facility (SNF), history of transplant, or active anticoagulant, insulin or narrow therapeutic index medications (Appendix). Patients were excluded if they had previously been enrolled in the study, or if admitted to pediatric or trauma services or transplant services with pharmacists.
Randomization
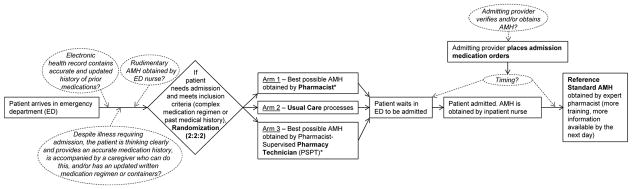
Investigators reviewed the EHR to identify ED patients for whom providers had already placed an admission order. Upon identifying trial candidates, investigators reviewed inclusion/exclusion criteria. After enrolling patients meeting criteria, investigators used RANDI2 randomization software to randomize each patient.(8) Each block of six consecutively enrolled patients was allocated in a 2:2:2 distribution across the three study arms (Figure 1). Patients who left the emergency department (ED) before an AMH could be obtained and patients not ultimately admitted (despite an initial decision to admit) were considered lost to follow-up. Because the number of patients assessed for eligibility on 1/30/14 was lost, we substituted the mean assessed patient count using all other enrollment days.
Figure 1. Workflow Diagram of Admission Medication History (AMH) Processes Occurring During Usual Care and Study Randomization.
Common Usual Care Process Variations Italicized and Circumscribed by Dotted Lines
*Note that both intervention arms also received usual care processes, subject to process variation
Interventions
Patients were randomly allocated to usual care or to one of two intervention arms in which either a pharmacist or pharmacist-supervised pharmacy technician (PSPT) had primary responsibility for obtaining the AMH. Obtaining the initial AMH usually began with reviewing the medication regimen present in the EHR if one was available from a prior encounter. Next, patients, families, and caregivers present in the ED were interviewed. Pill bottles, medication lists, and SNF medication administration records were also reviewed. In cases where sources matched convincingly, no further efforts were undertaken. However, in most cases, other sources including family, pharmacies and/or providers were contacted until questions were resolved. This is consistent with a published protocol for obtaining a “best possible medication history.”(4) Pharmacists and PSPTs attempted to complete all intervention-arm AMHs soon after the ED decision to admit was made and before any AMOs were placed, such that the workflow of admitting physicians would not be affected, and that there would be no need to contact and convince admitting physicians to fix AMHs or AMOs retroactively.
PSPTs presented their AMHs to a supervising pharmacist to allow the pharmacist to decide whether data sources needed further review, or whether the AMH was ready to be entered into the EHR. Requiring pharmacists to enter PSPTs’ AMHs into the EHR ensured that pharmacists reviewed all medications in the AMH, and constituted the pharmacist supervision of PSPTs.
Didactic and Experiential Training of Pharmacists and Pharmacy Technicians
All pharmacists and pharmacy technicians underwent standardized training in obtaining AMHs. Didactic training generally took 8–16 hours and included: review of background publications; review of locally-created general and ED-specific medication reconciliation manuals with detailed guides of AMH workflows, the patient interview, and EHR utilization; and a didactic training evaluation. Experiential training included observing ≥5 AMHs obtained by an expert pharmacist, followed by the trainee obtaining ≥5 AMHs under the proctoring of an expert pharmacist. Training continued until proctors deemed trainees competent.
Usual Care
All arms received usual care for patients admitted from the ED, which commonly involves multiple process variations. EHR-derived medication regimen accuracy is subject to variation in the knowledge and efforts of prior providers, which are often driven by patient acuity and patient care priorities. Patients’ and caregivers’ recall of medication regimens varies over time and across patients. Nurse and physician contributions likely vary in accordance with their pharmacological training and with competing obligations, including patients’ requests for home medications. Finally, physicians may place AMOs before or after patients have had their AMH obtained by an inpatient nurse (dotted lines and italicized text highlight common process variations in Figure 1). To minimize unnecessary overlap, inpatient pharmacists and nurses were advised not to initiate new efforts to improve upon pharmacist-approved AMHs. However, they were able to address any concerning AMH or AMO data that arose during clinical care.
Outcome Measurement
Reference Standard Admission Medication Histories
As per prior studies, we attempted to obtain reference standard AMHs from patients in all arms on the day following admission.(4) When a reference standard AMH was not obtained, patients were considered lost to follow-up. Reference standard AMHs were more comprehensive than initial AMHs in several ways. First, pharmacists obtaining reference standard AMHs started with initial AMH data. As such, study arm could not be masked. Second, reference standard AMHs were only obtained by pharmacists considered to be ‘expert’ in this clinical skill based on their previous experience in obtaining medication histories. These pharmacists were advised to take additional time and to consider additional information (e.g. previous hospital discharge orders) as necessary. Third, these pharmacists often had new information available to them (e.g. medication lists brought in after admission, improved patient mental status). Finally, these pharmacists identified errors that arose during clinical care prior to the reference standard AMH. Some of these pharmacists were study authors. To maximize patient benefit from the study, reference standard AMH findings, including any impact on AMOs, were communicated to the appropriate clinician.
Primary Outcome: Mean Severity-Weighted Admission Medication History (AMH) Error Score
In obtaining reference standard AMHs, expert pharmacists identified AMH errors in the initial AMHs and classified each error according to a previously developed taxonomy as significant, serious or life-threatening.(1) Error severity weights of 12=1, 22=4, and 32=9, respectively, were chosen to reflect the relative capacity of each error type to cause patient harm. A second pharmacist reviewed classifications, and a physician adjudicated disagreements. Because the reference standard pharmacist obtained their AMH while the patient was still hospitalized and used contemporaneous information (e.g., conversations with patients and family members), study arm could not be masked. Because of the vast amount of complex information that might be consulted in determining error severity, we also chose not to mask study arm with case summaries for other reviewers.
For each patient, we calculated a severity-weighted AMH error score. We used this novel error score because it provides a single, severity-weighted measure of error for each AMH. This allowed our power analysis to account for the different potential clinical consequences of different error severities. For each trial arm, we calculated a mean severity-weighted AMH error score.
Secondary Outcome: Mean Severity-Weighted Admission Medication Order (AMO) Error Score
For each AMH error identified, two physicians independently reviewed the relevant medications ordered at hospital admission in the context of the clinical chart. They classified each AMH error as either resulting in no AMO error, or an AMO error of significant, serious, or life-threatening severity. In cases where the admitting physician’s knowledge of an AMH error was unclear and where the resultant orders were clinically reasonable (e.g. the AMH erroneously omitted hydrocodone and it was not ordered at admission, but where it may have been intentionally held for altered mental status, rather than unintentionally omitted), we determined that the AMH error did not clearly lead to any AMO error. A third physician adjudicated disagreements. All adjudicating physicians were study authors. Because all AMO determinations began with a previously identified AMH error, we did not address AMO errors unrelated to AMH errors.
Tertiary Outcomes
Kruskal-Wallis and Fisher’s exact tests were used to compare the three arms in terms of patients’ mean length of stay and the percent of patients readmitted to CSMC within 30 days, respectively. The study was not powered to detect differences in these tertiary outcomes.
Statistical Analysis
Using single factor ANOVA, we determined that a sample of 300 patients would achieve 80% power to detect absolute error score differences of at least 11.2 using the Tukey-Kramer (pairwise) multiple comparison test with an alpha of 0.05.(9, 10) Based on pilot data, we expected patients in the usual care group to have a mean severity-weighted error score of 20.7, with a standard deviation of 16.2. A difference of 11.2 units is clinically significant, representing one life-threatening, almost three severe, or 11 significant AMH errors.
Clinical and demographic variables were summarized using mean or count. Error counts per patient and error scores per patient were summarized by study arm using mean. In accordance with the a priori analysis plan for this randomized trial, we used linear regression models to compare primary outcome and secondary measures across study arms (ANOVA). Because baseline characteristics were balanced across study arms, the linear regression models were not adjusted for any other variables. Post-hoc pair-wise comparisons between study arms used a Tukey-Kramer adjustment for multiple testing. The outcomes were transformed for the models due to outliers in the distributions. To test whether results were robust to the unknown outcomes of patients admitted but lost to follow-up, we conducted a sensitivity analysis whereby all such intervention patients were assumed to have the worst AMH error score measured for any patient, and whereby all such usual care patients were assumed not to have any AMH errors.
To minimize the effect of outliers in the distributions of error counts and scores, a rank transformation was applied to the outcomes in the regression models. The results of hypothesis testing for transformed and non-transformed outcomes were similar, but the residuals in the rank transformed data better fit the model assumptions as the variance of the outcomes in the usual care group was larger than the other two groups. The following variables were compared across study arms with Kruskal-Wallis tests: number of medications, zip code median income, weighted Charlson comorbidity score, and length of stay. Insurance type, race, ethnicity, and readmission rate were analyzed across study arms using Fisher’s Exact test. Analyses used SAS version 9.3 (Cary, NC).
RESULTS
Enrollment and Baseline Characteristics
We enrolled 306 patients. Patient characteristics, including age, sex, race, ethnicity, insurance, number of medications, income and comorbidities were similar across study arms (Table 1). The mean±SD patient age was 72±12 and number of medications present in the EHR prior to obtaining an AMH was 15±7.
Table 1.
Baseline Characteristics of Patients
| Admission Medication History Obtained via: | ||||||
|---|---|---|---|---|---|---|
| Characteristic | Usual Care(n = 101) | Usual Care plus Pharmacist (n = 103) | Usual Care plus Pharmacist-Supervised Pharmacy Technician (n = 102) | |||
| Age, mean, (SD), y | 71 | (18) | 72 | (16) | 71 | (16) |
| Female (n, %) | 48 | (48%) | 54 | (52%) | 55 | (54%) |
| Latino (n, %) | 7 | (7%) | 5 | (5%) | 5 | (5%) |
| Race (n, %) | ||||||
| White | 66 | (65%) | 75 | (73%) | 65 | (64%) |
| Black | 22 | (22%) | 28 | (28%) | 25 | (26%) |
| Asian | 5 | (5%) | 6 | (6%) | 6 | (6%) |
| Other | 0 | (0%) | 0 | (0%) | 1 | (1%) |
| Insurance (n, %) | ||||||
| Commercial | 14 | (14%) | 14 | (14%) | 17 | (17%) |
| Medicaid only | 7 | (7%) | 12 | (12%) | 9 | (9%) |
| Medicare | 78 | (77%) | 76 | (74%) | 75 | (74%) |
| Other | 2 | (2%) | 1 | (1%) | 1 | (1%) |
| Inclusion Criteria, accessed via EHR (n, %)* | ||||||
| ≥10 active chronic prescription medications | 65 | (64%) | 71 | (69%) | 71 | (70%) |
| History of acute myocardial infarction or congestive heart failure | 42 | (42%) | 34 | (33%) | 38 | (37%) |
| Admission from skilled nursing facility | 16 | (16%) | 12 | (12%) | 17 | (17%) |
| History of transplant | 2 | (2%) | 4 | (4%) | 3 | (3%) |
| Anticoagulant, insulin, or other narrow therapeutic index medication | 81 | (80%) | 97 | (94%) | 91 | (89%) |
| Other | ||||||
| Number of active medications in EHR at randomization (mean, SD) | 15 | (7) | 15 | (7) | 15 | (6) |
| Neighborhood household income, median (IQR), annual US$† | 66 063 | (42 615, 71 132) | 66 063 | (43 202, 77 165) | 66 063 | (42 615, 79 233) |
| Weighted Charlson comorbidity score, mean (SD) | 3.1 | (2.4) | 3.5 | (2.8) | 3.6 | (2.6) |
| Inpatient stay within 3 months prior to admission (n, %) | 40 | (40%) | 42 | (41%) | 40 | (40%) |
| >2 encounters w/ PCP or internal medicine consultants within 3 months prior to admission (n, %) | 49 | (49%) | 41 | (40%) | 51 | (50%) |
Many patients qualified for multiple inclusion criteria, such that the percentages sum to more than 100%
Neighborhood household income was estimated by linking patients’ zip codes to 2010 US Census median household income data
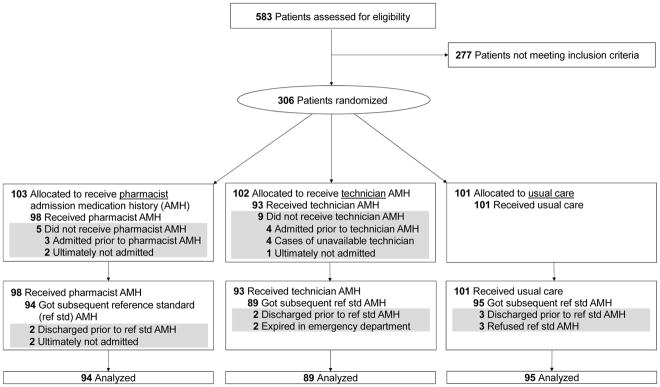
Of 103 and 102 patients randomized to the pharmacist and PSPT arms, only 5 (5%) and 9 (9%) did not receive the intervention, respectively. These patients and 14 others for whom a reference standard AMH was not obtained were classified as dropouts (Figure 2). The primary outcome was not measurable for these 28 (9.2%) patients lacking a reference standard AMH. Therefore, except for the sensitivity analyses, further results are based on the 278 remaining patients.
Figure 2.
CONSORT Flow Diagram
Identification and Adjudication of Admission Medication History (AMH) Errors and Resultant Admission Medication Order (AMO) Errors
Pharmacist raters found that 192 (69%) of 278 patients had 1016 AMH errors. They determined that 399 (39%) AMH errors were significant, 605 (60%) were serious, and 12 (1%) were life-threatening errors. These errors occurred in the AMHs of 138, 164 and 11 patients, respectively.
Physician raters agreed that 419 (41%) of these AMH errors clearly led to an AMO error. The 419 AMO errors occurred among 142 (74%) of the 192 patients who had an AMH error. Raters found that 261 (62%) AMO errors occurring among 117 patients were significant, 155 (37%) among 84 patients were serious, and 3 (1%) among 3 patients were life-threatening errors. Examples of AMH and AMO errors identified are detailed in Supplementary Table 1.
Outcome Comparisons Across Arms
There was a mean±SD of 8.0±5.6 AMH errors per patient in the usual care arm vs 1.4±1.9 and 1.5±2.1 AMH errors per patient in the pharmacist and PSPT arms, respectively (pair-wise t tests p< 0.0001) (Table 2). When we accounted for error severity via the primary outcome of severity-weighted AMH error score, patients in the usual care arm had a mean±SD severity-weighted AMH error score of 23.0±16.1 vs scores of 4.1±6.8 and 4.1±7.0 in the pharmacist and PSPT arms, respectively (p< 0.0001).
Table 2.
Outcomes of 278 Patients with Reference Standard Admission Medication Histories (AMH)
| Result | Usual Care (n = 95) | Usual Care plus Pharmacist (n = 94) | Usual Care plus Pharmacist-Supervised Pharmacy Technician (n = 89) | p-value* | |||
|---|---|---|---|---|---|---|---|
| Mean AMH error outcomes (95% CI) | |||||||
| AMH errors per patient | 8.0 | (6.8, 9.1) | 1.4 | (1.0, 1.8) | 1.5 | (1.0, 1.9) | < 0.0001 |
| AMH errors per patient, severe or life-threatening only | 4.6 | (3.8, 5.3) | 0.8 | (0.49, 1.1) | 0.7 | (0.45, 1.1) | < 0.0001 |
| AMH error score per patient† | 23.0 | (19.7, 26.2) | 4.1 | (2.7, 5.5) | 4.1 | (2.6, 5.6) | < 0.0001 |
| Mean Admission Medication Order (AMO) error outcomes (95% CI) | |||||||
| AMO errors per patient | 3.2 | (2.6, 3.8) | 0.6 | (0.42, 0.85) | 0.6 | (0.41, 0.97) | < 0.0001 |
| AMO errors per patient, severe or life-threatening only | 1.2 | (0.85, 1.5) | 0.2 | (0.12, 0.36) | 0.1 | (0.06, 0.24) | < 0.0001 |
| AMO error score per patient | 6.9 | (5.5, 8.4) | 1.5 | (0.89, 2.1) | 1.2 | (0.67, 1.7) | < 0.0001 |
| Mean utilization outcomes | |||||||
| Length of stay (95% CI) | 5.2 | (4.3, 6.1) | 6.5 | (5.1, 7.9) | 6.2 | (5.0, 7.3) | 0.13 |
| Readmission within 30 days (%) | 27 | (27%) | 17 | (17%) | 19 | (19%) | 0.16 |
Rank transformed ANOVA F-test
Primary outcome
Our sensitivity analysis, which assumed that all intervention patients lost to follow-up had the worst measured AMH severity score (100), but that usual care patients lost to follow-up had no AMH errors, resulted in the usual care arm having a mean±SD severity-weighted AMH error score of 22.0±16.4 vs scores of 9.0±22.1 and 13.8±29.8 in the pharmacist and PSPT arms, respectively (p< 0.0001).
Patients in the usual care arm had a mean±SD of 3.2±2.9 AMO errors per patient vs 0.6±1.1 and 0.6±1.1 AMO errors per patient in the pharmacist and PSPT arms, respectively (p<0.0001). Accounting for error severity showed that patients in the usual care arm had a mean±SD severity-weighted AMO error score of 6.9±7.2 vs 1.5±2.9 and 1.2±2.5 in the pharmacist and PSPT arms, respectively (p<0.0001).
Using Cohen’s D to standardize the magnitude of the measured effect revealed that for the primary outcome of AHM error score, the effect size for each intervention was 1.5 (Table 3). For the more downstream outcome of severe or life-threatening AMO errors, the effect size for each intervention was approximately 0.8. These measurements are accepted to represent very large and large effect sizes, respectively.(11) Although this trial was not designed to test for non-inferiority, we found no differences in any outcomes between pharmacists and PSPTs.
Table 3.
Comparing Admission Medication History (AMH) Error and Admission Medication Order (AMO) Error Rates Across Study Arms
| Result | Usual Care minus Pharmacist (n = 95, 94) | Pharmacist minus Pharmacist-Supervised Pharmacy Technician (n = 94, 89) | Usual Care minus Pharmacist-Supervised Pharmacy Technician (n = 95, 89) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean AMH error outcomes | Δ* | pSD† | C‡ | Δ | pSD | C | Δ | pSD | C |
| AMH errors per patient | 6.6§ | 4.2 | 1.6 | −0.08 | 2.0 | −0.04 | 6.5§ | 4.2 | 1.5 |
| AMH errors per patient, severe or life-threatening only | 3.8§ | 2.9 | 1.4 | 0.04 | 1.5 | 0.03 | 3.8§ | 2.8 | 1.4 |
| AMH error score per patient** | 18.8§ | 12.4 | 1.5 | 0.05 | 6.8 | 0.01 | 18.9§ | 12.4 | 1.5 |
| Mean AMO error outcomes | |||||||||
| AMO errors per patient | 2.5§ | 2.2 | 1.2 | −0.002 | 1.1 | −0.002 | 2.5§ | 2.2 | 1.2 |
| AMO errors per patient, severe or life-threatening only | 0.92§ | 1.2 | 0.76 | 0.10 | 0.55 | 0.17 | 1.0§ | 1.2 | 0.85 |
| AMO error score per patient | 5.4§ | 5.5 | 0.99 | 0.29 | 2.7 | 0.11 | 5.7§ | 5.4 | 1.1 |
Δ: Difference in means
pSD: pooled standard deviation
C: Cohen’s D calculated as difference in means divided by pooled standard deviation of the two groups
p < 0.0001 (pairwise comparison with Tukey-Kramer adjustment for multiple testing)
Primary outcome
Of 183 patients randomized to either intervention, 29 (16%) had a serious or life-threatening AMO. Compared to 56 (59%) of 95 control patients with such errors, this represents a number needed to treat (NNT) of three (point estimate 2.3, 95%CI 1.8–3.2). This number underestimates the intervention’s impact because many patients had multiple serious AMO errors. Although there were no statistically significant differences in utilization outcomes across arms, point estimates for length of stay were approximately one day longer in the intervention arms (p=0.13), and point estimates for 30-day readmission rates were approximately 10% lower in the intervention arms (p=0.16).
DISCUSSION
In this three-arm randomized controlled trial, adding AMH interviews by pharmacists or PSPTs to usual care processes reduced AMH errors by over 80%. The most downstream and clinically meaningful result was reducing the severe and life-threatening AMO error rate from 1.2 per patient in the usual care arm to 0.2 per patient in the intervention arms. Preventing AMOs should allow patients to avoid ADEs, which are known to increase length of stay, cost, morbidity, and mortality.(2, 12)
We found a much larger benefit than prior research. Many prior studies checked AMHs after AMOs were placed, thus resembling our usual care arm. For example, one systematic review found that the median study only identified (and in some cases addressed) 0.35 clinically significant unintentional medication discrepancies per patient.(13) In contrast, our usual care arm reference standard AMHs identified a mean of 1.2 severe or life-threatening AMO errors per patient, which translated to a much greater opportunity for reductions.
We attribute the high baseline error rate to the medically complex patient population we studied, which resulted from our inclusion criteria. Two prior systematic reviews had conflicting findings regarding targeting interventions at high-risk patients. One review found such targeting in 13 of 26 studies, and deemed it to be a “key aspect of successful interventions.”(14) The other review found such targeting in seven of 20 interventions, and determined that “commonly used criteria for selecting high-risk patients do not consistently improve the effect of medication reconciliation.”(13) Our study patients had a mean of 15 medications present at enrollment, versus prior study population means ranging from seven to eleven medications.(15) The strong effect of our intervention suggests that targeting may be helpful if it is used to identify these patients at extremely high risk for ADEs. Such patients are already prevalent at CSMC, and this cohort is growing quickly throughout the developed world due to population aging and increasing prescription drug use.(16)
The second factor likely contributing to the strong effect, and likely related to the high-risk patient population, is the substantial time spent by the pharmacist and PSPTs who conducted the intervention. In a time and motion study reported elsewhere, they spent 58.5 and 79.4 minutes per patient, respectively (p=0.14). (17) Although one other study reported similar results,(18) this represents substantially more time than the 20 to 40 minutes reported in several prior studies conducted on younger, healthier patients.(19, 20) Beyond these substantial time requirements, these interventions also require pharmacy personnel to be stationed in the ED and able to attend to AMHs as soon as a determination to admit a patient has been made – before AMOs are placed. As such, these interventions may be best suited to large hospitals with sufficient ED patient volume to justify stationing pharmacy personnel in the ED.
To better understand the potential impact of the studied interventions, we consulted previous literature showing that 0.9% of AMO errors results in an ADE during hospitalization.(21) Critically, the studied interventions have potential advantages that we did not evaluate. The intervention workflows should be more efficient than using pharmacists to retrospectively check usual care processes and to contact and convince ordering physicians to request changes before errors cause harm. Furthermore, it seems likely that the interventions streamlined physicians’ workflows and saved them time by allowing them to order from accurate AMHs, to minimize downstream pharmacist contacts and to reduce the need for corrections. Finally, and most importantly, prior research has shown the greatest benefit of reducing AMH errors to be a reduction in post-discharge prescription errors and resultant ADEs.(4) Future research should endeavor to evaluate these hypothesized benefits.
Because one sought-after benefit of using PSPTs is to reduce costs, it is notable that we found no difference in the benefit provided by PSPTs versus pharmacists. This is consistent with other similar studies.(22, 23) However, our aforementioned time and motion analysis also did not find intervention costs to be lower in the PSPTs arm, as compared to the pharmacist arm, once the costs of pharmacist supervision were included.(17) Nonetheless, the current study may allay concerns of effectiveness that have hindered PSPT adoption. With effectiveness established, these results point to an opportunity to improve PSPT efficiency, through altered work processes and the use of electronic pharmacy claims data, that could make PSPT both a better and less expensive intervention.
Generalizability is a known gap in medication reconciliation intervention research.(7) Beyond embracing an intervention that we thought would improve efficiency and reduce implementation complexity, we also designed our trial to be pragmatic. In contrast to prior work,(15) we included many patients admitted by community physicians. Because the interventions did not require physician workflow changes, many physicians were unaware of the trial entirely. We included resident pharmacists to ensure that experience was unnecessary. We minimized biases associated with requiring patients to opt-in. All of these factors should contribute to strong external validity.
The findings must be interpreted in the context of limitations. First, the study was powered on intermediate endpoints, rather than on patient-centered outcomes (PCO). Although there is an established linkage between AMH errors and PCO,(1) it would be useful to study PCO directly, especially because systematic reviews have drawn conflicting conclusions about whether previously-studied medication reconciliation interventions affect PCO.(6, 13, 15, 24) Second, we only used one site. Third, not all aspects of randomization were masked from study personnel. Because block size was not masked, selection bias could have occurred. Furthermore, we could not practicably mask arm allocation. Fortunately, we were able to increase objectivity by leveraging accepted methodology, which used agreement of independent raters to identify and rate the severity of AMH and AMO errors.(4) Finally, study providers could not access electronic pharmacy claims data (EPCD). Because EPCD is likely now available in most US hospitals, and because it has good potential to reduce AMH errors and to reduce the time needed to obtain AMHs, it will be important to retest these interventions with EPCD.(25)
CONCLUSIONS
Among medically-complex older adults, pharmacists and PSPTs reduced AMH errors and resultant AMO errors by over 80% by obtaining AMHs in the ED. This effect was robust to severity-weighting, and thus shows promise for reducing patient harm. We attribute the strong effect to a high-risk patient population and an intensive intervention. Future research should test whether these results generalize to other settings and affect patient-centered outcomes, and whether hypothesized efficacy and efficiency benefits are indeed demonstrable.
Supplementary Material
Actual Examples of Admission Medication History (AMH) and Admission Medication Order (AMO) Error Severity Ratings.
References
- 1.Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group. Jama. 1995;274(1):29–34. [PubMed] [Google Scholar]
- 2.Aspden PWJ, Bootman JL, Cronenwett LR. Preventing Medication Errors: Quality Chasm Series. Washington, DC: The National Academic Press; 2007. [Google Scholar]
- 3.Cornish PL, Knowles SR, Marchesano R, Tam V, Shadowitz S, Juurlink DN, et al. Unintended medication discrepancies at the time of hospital admission. Archives of internal medicine. 2005;165(4):424–9. doi: 10.1001/archinte.165.4.424. [DOI] [PubMed] [Google Scholar]
- 4.Pippins JR, Gandhi TK, Hamann C, Ndumele CD, Labonville SA, Diedrichsen EK, et al. Classifying and predicting errors of inpatient medication reconciliation. Journal of general internal medicine. 2008;23(9):1414–22. doi: 10.1007/s11606-008-0687-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gleason KM, McDaniel MR, Feinglass J, Baker DW, Lindquist L, Liss D, et al. Results of the Medications at Transitions and Clinical Handoffs (MATCH) study: an analysis of medication reconciliation errors and risk factors at hospital admission. Journal of general internal medicine. 2010;25(5):441–7. doi: 10.1007/s11606-010-1256-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mueller SK, Sponsler KC, Kripalani S, Schnipper JL. Hospital-based medication reconciliation practices: a systematic review. Archives of internal medicine. 2012;172(14):1057–69. doi: 10.1001/archinternmed.2012.2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pevnick JM, Shane R, Schnipper JL. The problem with medication reconciliation. BMJ quality & safety. 2016;25(9):726–30. doi: 10.1136/bmjqs-2015-004734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schrimpf DLP, Pilz LR. Web-based open source application for the randomization process in clinical trials: RANDI2. International journal of clinical pharmacology and therapeutics. 2010;48(July):465–7. doi: 10.5414/cpp48465. [DOI] [PubMed] [Google Scholar]
- 9.Hsu JC. Multiple Comparisons Theory and Methods. Computational Statistics & Data Analysis. 1996;25(2):241–3. [Google Scholar]
- 10.Hintze J. PASS 11. Kaysville, Utah, USA: NCSS, LLC; 2011. [Google Scholar]
- 11.Sawilowsky SS. New effect size rules of thumb. 2009. [Google Scholar]
- 12.Hug BL, Keohane C, Seger DL, Yoon C, Bates DW. The costs of adverse drug events in community hospitals. Jt Comm J Qual Patient Saf. 2012;38(3):120–6. doi: 10.1016/s1553-7250(12)38016-1. [DOI] [PubMed] [Google Scholar]
- 13.Kwan JL, Lo L, Sampson M, Shojania KG. Medication reconciliation during transitions of care as a patient safety strategy: a systematic review. Annals of internal medicine. 2013;158(5_Part_2):397–403. doi: 10.7326/0003-4819-158-5-201303051-00006. [DOI] [PubMed] [Google Scholar]
- 14.Mueller SK, Cunningham SK, Kripalani S, Schnipper JL. Hospital-based medication reconciliation practices: a systematic review. Arch Intern Med. 2012:172. doi: 10.1001/archinternmed.2012.2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christensen M, Lundh A. Medication review in hospitalised patients to reduce morbidity and mortality. The Cochrane database of systematic reviews. 2016;2:Cd008986. doi: 10.1002/14651858.CD008986.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maust DT, Gerlach LB, Gibson A, Kales HC, Blow FC, Olfson M. Trends in Central Nervous System-Active Polypharmacy Among Older Adults Seen in Outpatient Care in the United States. JAMA internal medicine. 2017 doi: 10.1001/jamainternmed.2016.9225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen CB, Shane R, Bell DS, Cook-Wiens G, Pevnick JM. A Time and Motion Study of Pharmacists and Pharmacy Technicians Obtaining Admission Medication Histories. Journal of hospital medicine. 2017;12(3):180–3. doi: 10.12788/jhm.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meguerditchian AN, Krotneva S, Reidel K, Huang A, Tamblyn R. Medication reconciliation at admission and discharge: a time and motion study. BMC health services research. 2013;13:485. doi: 10.1186/1472-6963-13-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kent AJ, Harrington L, Skinner J. Medication reconciliation by a pharmacist in the emergency department: a pilot project. The Canadian journal of hospital pharmacy. 2009;62(3):238–42. doi: 10.4212/cjhp.v62i3.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.ASHP-APhA Medication Management in Care Transitions Best Practices American Society of Health-System Pharmacists and the American Pharmacists Association.
- 21.Bates DW, Boyle DL, Vander Vliet MB, Schneider J, Leape L. Relationship between medication errors and adverse drug events. Journal of general internal medicine. 1995;10(4):199–205. doi: 10.1007/BF02600255. [DOI] [PubMed] [Google Scholar]
- 22.Johnston R, Saulnier L, Gould O. Best Possible Medication History in the Emergency Department: Comparing Pharmacy Technicians and Pharmacists. The Canadian Journal of Hospital Pharmacy. 2010;63(5):359–65. doi: 10.4212/cjhp.v63i5.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Irwin AN, Ham Y, Gerrity TM. Expanded Roles for Pharmacy Technicians in the Medication Reconciliation Process: A Qualitative Review. Hospital Pharmacy. 2017;52(1):44–53. doi: 10.1310/hpj5201-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mekonnen AB, McLachlan AJ, Brien JA. Effectiveness of pharmacist-led medication reconciliation programmes on clinical outcomes at hospital transitions: a systematic review and meta-analysis. BMJ open. 2016;6(2):e010003. doi: 10.1136/bmjopen-2015-010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pevnick JM, Palmer KA, Shane R, Wu CN, Bell DS, Diaz F, et al. Potential benefit of electronic pharmacy claims data to prevent medication history errors and resultant inpatient order errors. Journal of the American Medical Informatics Association : JAMIA. 2016;23(5):942–50. doi: 10.1093/jamia/ocv171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Actual Examples of Admission Medication History (AMH) and Admission Medication Order (AMO) Error Severity Ratings.