Abstract
Objective
To discuss the state-of-the-art with regards to established or promising bioelectric therapies meant to alter or control neurologic function. We present recent reports on bioelectric technologies that interface with the nervous system at three potential sites – (1) the end organ, (2) the peripheral nervous system, and (3) the central nervous system – while exploring practical and clinical considerations.
Data Sources
Pubmed, IEEE, and Web of Science literature search.
Review Methods
A review of the current literature was conducted to examine functional and histomorphological effects of neuroprosthetic interfaces with a focus on end-organ, peripheral, and central nervous system interfaces.
Conclusions
Innovations in bioelectric technologies are providing increasing selectivity in stimulating distinct nerve fiber populations in order to activate discrete muscles. Significant advances in electrode array design focus on increasing selectivity, stability, and functionality of implantable neuroprosthetics.
Implications for Practice
The application of neuroprosthetics to paretic nerves or even directly stimulating or recording from the central nervous system holds great potential in advancing the field of nerve and tissue bioelectric engineering and contributing to clinical care. Although current physiotherapeutic and surgical treatments seek to restore function, structure, or comfort, they bear significant limitations in enabling cosmetic or functional recovery. Instead, the introduction of bioelectric technology may play a role in the restoration of function in patients with neurologic deficits.
Keywords: Neural Electrode, Neuroprosthetic, Nerve Implant, Prosthetics, Nerve Paresis, Bioelectric Interface
Introduction
The application of bioelectric stimulation to the nervous system has proven itself to be an effective option for restoring or augmenting some degree of function in patients with neurologic dysfunction [55]. In particular, functional stimulation of paretic nerves is a clinically vital and promising area of research that warrants significant investigation. At present, a variety of implantable nerve stimulators have been clinically employed and demonstrated to be efficacious; for example, hypoglossal nerve stimulation in patients with severe obstructive sleep apnea[39,72], chronic spinal cord stimulation in patients with severe neuropathic pain [80, 86], direct electrical stimulation of peripheral nerves in patients with bladder, bowel and sexual dysfunction [14, 20], and even transcutaneous stimulation of the trigeminal nerve in migraine patients [71]have all been successfully used to alleviate clinical pathologies. Deep brain stimulation (DBS), is arguably the most successful example of a bioelectric technology that has made its way into the clinical realm to treat a variety of neurological disorders with an incredible degree of efficacy.
This manuscript reviews the state-of-the-art with regards to established or promising bioelectric therapies meant to alter or control neurologic function. First, we address the three potential sites for bioelectric technologies to interface with the nervous system– both peripheral and central – while exploring practical and clinical considerations. The three sites include, from distalto peripheral, 1) the end organ,2) the peripheral nervous system, or 3) the central nervous system. We also discuss the role of intraneural multichannel microelectrodes in various disease processes. Further, we address emerging technologies that may hold great potential to advance the field of nerve and tissue bioelectric engineering, and how they can be applied in the clinic to address a variety of clinical conditions.Lastly, we discuss the legal, engineering, and biological hurdles limiting the successful translation of bioelectric technologies to the clinical realm.
Materials and Methods
A systematic search was executed on Pubmed, IEEE, and Web of Science databases from database creation to June 2017. PubMed, Ovid, and Cochrane databases were queried using the following keywords: (“bioelectric” or “stimulation” or “technologies” or “recording” or “electrode” or “recording” or “neuroprosthetic” or “implant” or “interface”) and (“paresis” or “paralysis” or “neural” or “muscle” or “end organ” or “peripheral” or “nervous” or “system” or “central” or “brain” or “computer interface” or “nerve”). References of each manuscript were checked for papers that were of potential relevance to our review. Three investigators independently screened each article according to title and abstract, and included any article relating to the application of bioelectric technologies or neuroprosthetics in the peripheral or central nervous system. The full text of each selected article was obtained and analyzed. Because no patient information or animals were involved, Institutional Review Board and Institutional Animal Care and Use Committee approval was not required.
Results and Discussion
Interfacing with the End Organ
End organ stimulation has gained recent prominence within the field of bioelectric technologies and deals with the idea of stimulating or activating organ function (e.g. muscles, visceral organs) through electrodes implanted in the organ itself, or by stimulating the efferent fibers going to the organ. In other words, stimulation modulates the activity of the organ itself. The end organ of an afferent nerve refers to any structure that acts as a receptor, and can be directly stimulated to activate associated nerve fibers (e.g. cochlea of the auditory system, or utricle, saccule, and ampullae of the vestibular system). In contrast, the end or target organ of an efferent neuron is a typically a muscle, in which case a stimulating electrode can be placed near or on the endplate of the neuromuscular junction [Fig. 1]. Using these principles, the end organ can serve as the interface between the electrode and the nerves or muscles to be activated. In fact, recorded bioelectric signals are up to 100 times greater in reinnervated muscles when compared to recordings from peripheral nerve axons, and thus may facilitate signal input acquisition to a greater degree than direct neural recordings [30]. Furthermore, the end organ is often better able to structurally interface with the mechanical composition of an electrode when compared to soft and delicate neural tissue [30].
Fig. 1.

A cartoon example of end organ stimulation within the endplate, exaggerated in size, of a neuromuscular junction (NMJ) is shown. Here the electrode is not in direct contact with the axonal nerve ending, and is depolarized through current spread. Muscle-electrode interfaces have proven more effective with sensory signal clarity due to an increased biocompatibility of the hard metal electrode and the tough mesodermal tissue [30]
Muscle Stimulation
Electrical stimulation of end organs, such as muscles, can be non-invasive, in the case of transcutaneous electrical stimulation, or invasive, as in the case of implantable electrodes. For example, McDonnall et al. used transcutaneous and percutaneous electrodes to activate the orbicularis oculi muscle and restore blink function in patients with facial paralysis, while simultaneously minimizing painful sensations associated with the stimulation [41]. Frigerio and colleagues similarly employed a transcutaneous approach to stimulating the distal facial nerve branches to elicit blinking of the eye [19].Mueller et al. used a laryngeal pacemaker system implanted directly into laryngeal muscles in patients with bilateral vocal fold paralysis to improve breathing and swallowing, without compromising vocalization [52]. In a rodent model, electrodes implanted within the gastrocnemius muscle reduced muscle atrophy without affecting motor reinnervation following tibial nerve transection and repair [85]. Electrodes inserted within muscles may also be able to record movements associated with limb tremors and stimulate the muscles to mitigate the tremors. End organ stimulation offers tremendous potential, but many of the advancements in the field are still within the realm of clinical research and are not yet routinely used in clinical practice. In contrast, end organ bioelectric interfaces are already in routine clinical use within the context of the auditory system.
Cochlear Implants
For the past several decades, end organ stimulating devices have been used to restore hearing in patients with substantial sensorineural hearing loss, which is typically the result of irreversible damage to the cochlear sensory epithelium and auditory nerve. Cochlear implants (CI) consist of a multi-channel electrode array that is surgically inserted deep into the scala tympani within the cochlea, and this array is connected to a receiver-stimulator implanted beneath post-auricular soft tissue [Fig. 2]. Electric current is delivered from select and programmed platinum electrode contacts to the spiral ganglion neurons within Rosenthal’s canal, depolarizing these cells and generating a neural signal at the desired frequency to be propagated along the auditory pathway to the auditory cortex [60]. Approximately half million deaf or hard-of-hearing children and adults have undergone cochlear implantation worldwide, leading to incredible personal, financial, and societal benefits [10, 28, 42, 74].
Fig. 2.

A typical cochlear implant system consists of (1) an external sound processor, which accurately converts pressure changes in the air (soundwaves) into electromagnetic signals, (2) an internal implant, which converts the electromagnetic field into electrical current, and (3) an intracochlear multi-electrode array, with delivers the current and depolarizes the first-order auditory neurons. The current from the electrode bypasses damaged cochlear hair cells and stimulates (4) the cochlear nerve, leading to sound perception and hearing. Images provided by Advanced Bionics, Inc. and modified
Vestibular Prosthetics
Bilateral vestibulopathy, or Dandy’s syndrome, is a debilitating condition characterized by oscillopsia and unsteadiness during locomotion. Unsteadiness, due to a deficient vestibulo-spinal reflex, and oscillopsia, caused by bilaterally impaired vestibulo-ocular reflexes (VOR), both lead to severe impairment of postural control and image stabilization during head and body movement [29]. In recent years, cochlear implants have been modified to be used as vestibular prosthetics to restore inner ear balance function in animal models [16,43, 65]. Many of these experimental devices utilize inertial sensors to detect accelerations of the head, data from which is then used to provide specific electrical signals and patterns to the vestibular system in a compensatory manner.
Pelizzone et al. have recently translated this work to human trials in an effort to restore VOR [58]. Using a modified cochlear implant with a standard intracochlear array and three additional electrode arrays each to be implanted within the ampullae of the three semicircular canals (lateral, superior, and posterior), the authors electrically stimulated the vestibular end organs based on acceleration forces of the head detected by a gyroscope within the device. Electrical stimulation with the prosthesis, at 1Hz, provided a significant VOR gain in three implanted patients with vestibular damage, reaching up to 98% of the average VOR gain in healthy patients. A similar clinical study is underway at Johns Hopkins University [6].
Interfacing with the Peripheral Nervous System
Stimulation of peripheral nerves, or even direct stimulation of the spinal cord, has been routinely implemented in clinical practice. From epidural placement of leads in the dorsal root ganglion (DRG) to treat neuropathic pain [35], to sacral anterior root stimulation to enhance bowel function in spinal cord injury patients [62], direct electrical stimulation of nerves or nerve roots has been demonstrated to be a safe and efficacious clinical intervention. Although long term repercussions of implantable neurostimulators remain to be fully elucidated, the major shortcomings of implantable devices revolve around the fibrotic foreign body response that develops following implantation, particularly following implantation within the peripheral or central nervous system[3]. In order to mitigate the gliotic response to electrode implantation, restricting the neuroprosthetic device to the epineurium can mitigate any ensuing foreign body response. We will now explore several of these epineural interfaces.
Cuff Electrodes
Cuff electrodes are the most basic type of epineural recording or stimulation device. The typical cuff electrode is comprised of a self-coiling, double-layer silicone cuff embedded with 2-3 platinum-foil strips and is wrapped around the outer surface of the nerve, thus providing a direct interface for electrical stimulation [Fig. 3] [22, 38, 54, 67, 74, 77]. The electrode can operate at low stimulus thresholds, thus reducing the likelihood of detrimental nerve damage. Unfortunately, the cuff electrode generally elicits all-or-none neural activity and has a limited ability to target individual fascicles within the nerve fiber [30]. Cuff electrodes have been used safely for years, and although one study in rabbits found long-term cuff implantation will damage myelinated axons, these axons were able to regenerate [31]. Furthermore, impedance and stimulation thresholds of cuff electrodes are stable over time, maintaining functionality for over 12 years in peroneal nerve stimulation experiments in hemiplegic patients [1, 76,81, 83].
Fig. 3.

a) Cuff electrodes and b) Flat interface nerve electrodes (FINE) are the most common epineural nerve-electrode interfaces: FINE compresses the nerve, and thus has a higher resolution to excite specific fascicles. c) Longitudinal intrafascicular electrodes (LIFE) and d) transverse intrafascicular electrodes (TIME) are the most common intraneural nerve-electrode interfaces. e) Multi-channel electrodes have been used by our group to selectively activate fascicles within cranial nerves. For example, if implanted within the facial nerve, specific channels on the electrode array can activate specific fascicles and corresponding facial muscles
The cuff electrode is routinely used in the clinical setting for vagus nerve stimulation (VNS), which has been shown to be effective in the management of epilepsy and treatment resistant depression. The VNS implant is thought to emit diffuse energy centrally towards the brain, disrupting the aberrant signals that contribute to uncontrolled epileptic seizures [19,30,66]. Additionally, the Inspire® hypoglossal nerve stimulator, a Food and Drug Administration (FDA)-approved device used for obstructive sleep apnea, utilizes a cuff electrode wrapped around the hypoglossal nerve [Fig. 4]. This implant is under clinical trials to augment the oropharyngeal airway by stimulating the motor nerve and lowering the tongue in coordination with the breathing cycle [26, 53].
Fig. 4.

Diagram of the Inspire Upper Airway Stimulation (UAS0 system. This is an implantable system that stimulates the hypoglossal nerve to treat obstructive sleep apnea (OSA). The components of the system include an implantable pulse generator (IPG), which is normally placed in the chest, and is connected to two leads. The first lead is the sensing lead, and is placed into the fourth intercostal space. This, senses intercostal muscle contraction and activates the stimulating lead, which is interfaced with the hypoglossal nerve. Additionally, external components that include the physician and patient programmer (sleep remote) make up the system. Image provided by Inspire Medical Systems, Inc
For purposes of motor nerve stimulation, a tripolar, or a modified monopolar or bipolar electrode would likely be required. Such designs can facilitate unidirectional action potential propagation while mitigating undesired signals in the opposing direction, though this has been difficult to reliably induce in practice. This contrasts with conventional monopolar or bipolar electrodes, which are impractical for administration of current to a single site [Fig. 5] [51]. Nonetheless, with regards to motor stimulation, bidirectional action potential propagation in stimulated efferent neurons can reliably elicit muscle activation and is less of an issue then with sensory nerve stimulation.
Fig. 5.
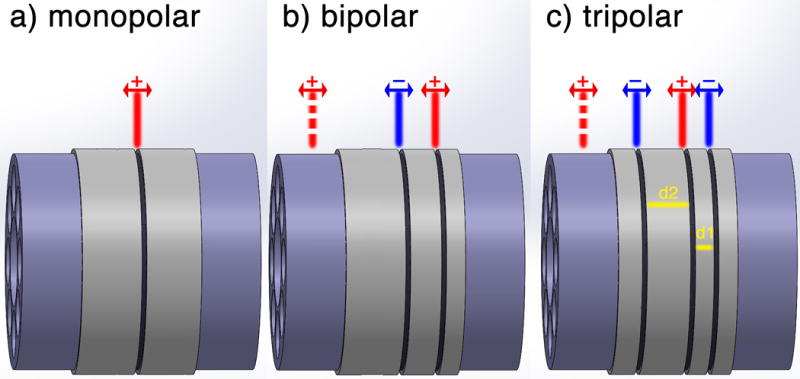
a) Monopolar electrode configuration yields bidirectional action potential propagation due to uncontrolled depolarization of the contact point. b) Bipolar electrode configuration aims to block one direction of action potential propagation by hyperpolarization of the nerve at the anode site. However, such magnitudes of anodic current induce a virtual cathode and negate the cancellation effect, thus yielding a bipolar action potential. c) An important aspect of tripolar electrodes is the platinum placements (d2> d1). Due to a time-shifted hyperpolarization at the further anode (d2) a virtual cathode beyond the closer anode (d1) will not form. However, a virtual cathode will be formed beyond the further anode, which results in a unidirectional action potential
Flat Interface Nerve Electrodes
The flat interface nerve electrode (FINE) is a modified version of the cuff electrode. FINE can be designed in a multi-channel configuration, and moreover, compresses and reshapes the nerve into a flatter conformation, thereby allowing the central fascicles to be closer to the surface and providing more selective axonal population activation [Fig. 3] [30, 33, 57, 79, 87]. Furthermore, intraoperative studies in the human femoral nerve showed that muscles innervated by the femoral nerve could be independently and selectively stimulated with a FINE device [69].Surgical placement of FINE around the femoral trunk lead to selective activation of leg muscles, thereby aiding patients who suffer from lower trunk paralysis to stand from a sitting position [70]. Additionally, the U.S. Department of Defense has investigated the use of FINE in controlling neural prostheses in amputees [48]. Although FINEs have been used in several human studies without any deleterious consequences, FINEs have the potential to compress the nerve, reduce blood flow, or even cause neural damage [61].
Longitudinal and Transverse Intrafascicular Electrode (LIFE and TIME)
In contrast to FINE, intrafascicular electrodes pierce the protective epineurium of the nerve, and can stimulate or record from peripheral nerves with higher sensitivity[25]. Notably, a polymer-based, thin-film longitudinal intrafascicular electrode (tfLIFE/polyLIFE) demonstrated no deleterious effects on nerve fiber count, diameter, or myelin thickness while providing higher recording selectivity than standard metal LIFE following 6 months of implantation in rabbit sciatic nerves [32]. LIFE has also been used to detect neural impulses from the median and ulnar nerves in an amputee to manipulate a robotic hand in a human patient [45, 63]. Transverse intrafascicular multichannel electrodes (TIME) provide superior spatial selectivity than LIFE by preventing distribution of spatial selectivity beyond one fascicle [Fig. 3] [4]. Specifically, TIME was developed to manage phantom limb pain in patients who required simultaneous excitation of multiple parallel fascicles within a nerve. This feat previously required the implantation of multiple LIFEs [4, 25].
Penetrating Multi-Channel Arrays
Penetrating intraneural multi-channel arrays offer researchers vast access to diverse neural pathways [Fig. 3]. Of note, investigators at the University of Utah have developed two types of multi-array electrodes: the Utah Electrode Array (UEA) and the Utah Slanted Electrode Array (USEA) [9, 64]. Both are designed with the capacity to contain up to 100 microneedles which are implanted into neural tissue. Previous experiments with the UEA have reported successful recording of volitional motor commands from the central nervous system, among other accomplishments [56]. However, because this technique is highly invasive, it carries the risk of permanent neural damage[18].Generally, the more invasive the neuroprosthetic interface, the greater the degree of selectivity of neural fiber activation at the sacrifice of potential injury to the nerve. As previously discussed, cuff electrodes, which gently wrap the nerve, generally activate the nerve in an “all-or-none” fashion with limited selectivity, while intraneural multichannel microelectrode arrays violate the perineurium and may induce glial scarring, but offer exquisite selectivity of neural fiber activation.
Using a penetrating multi-electrode array developed at the University of Michigan, Middlebrooks et al. investigated intraneural cochlear nerve stimulation while recording the downstream auditory pathway output at the brainstem inferior colliculus[46–47]. The NeuroNexus (Ann Arbor, MI) stimulating array consists of 16 iridium-plated electrode contacts placed along a silicon shank, and in every critical auditory electrophysiological measurement in anesthetized cats, the penetrating intraneural array consistently out-performed a conventional intracochlear array. Furthermore, our group recently described the role of intraneural stimulation for facial nerve reanimation in the feline model. In short-term experiments, we achieved selective stimulation of facial muscles with the NeuroNexus multi-electrode array implanted into the main trunk of the facial nerve [Fig. 6] [68]. EMG responses of four facial muscles were recorded following stimulation through the 16-channel penetrating array. Electrical pulses from each channel of the array selectively activated discrete neural populations within the facial nerve, resulting in independent activation of specific facial muscles. Increases in stimulation current levels resulted in corresponding increases in EMG voltage response. Moreover, long-term presence of the microelectrode array in the facial nerve confirmed the efficacy and stability of this intraneural stimulation approach (manuscript in revision). Intraneural stimulation of restricted neural populations with one or more multi-channel arrays may play a role in reanimating the face for patients with permanent facial paralysis, and may also help rehabilitate other motor and sensory functions throughout the central and peripheral nervous systems [68].
Fig. 6.

Diagram depicting intraneural stimulation of the facial nerve with a multichannel electrode array that can selectively activate discrete nerve fiber populations within the facial nerve and elicit selective stimulation of facial muscle groups. Image credit: Modified from Patrick J. Lynch, medical illustrator; C. Carl Jaffe, MD
Interfacing with the Central Nervous System
Spinal Cord Stimulation
Cortical interfaces
Intracranial brain-computer interfaces (iBMI) are novel brain-machine interfaces that record and decipher neural activity to decode motor intent and elicit a response (e.g. artificial limb movement)[24]. iBMIs utilize microelectrodes implanted into the cortical surface, or electrocorticographic (ECoG) grids to either bypass disrupted neural pathways and directly stimulate nerves or muscles, or directly control artificial limbs. For example, tetraplegic patients have been able to volitionally control robotic arms [13, 23], while other patients have been able to controls their own limbsvia iBMI-based approaches [17, 50].In addition to restoration of motor movement, enhancing communication, as in the case of amyotrophic lateral sclerosis (ALS) patients for example, can also be achieved by iBMI [5, 21, 27]. Future research directed at incorporating sensory feedback in iBMI setupsand improving both recording and stimulation parameters can help improve the efficacy of iBMI approaches in the clinical realm [15, 78].
Auditory Nucleus and Ganglion Stimulation
For patients with trauma or deformations of the cochlea or auditory nerve, the CI, which is designed to be placed in the cochlea and electrically activate the auditory nerve, is not an option for auditory rehabilitation. Most notably, patients with neurofibromatosis type-2 (NF2) suffer from bilateral vestibular schwannomas and as a result, require more central activation of the auditory pathway at the brainstem cochlear nucleus. The auditory brainstem implant (ABI), again a modified cochlear implant with a flat array consisting of 21 platinum electrodes on a Dacron mesh backing, was created for NF2 patients [44]. To date, however, ABIs have proven most beneficial for adults without NF2 and in children with cochlear malformations [8, 11, 75]. In one study involving 48 non-NF2 (non-tumor) patients, a mean score of 59% on open set speech perception tests was achieved [12]. For children suffering from bilateral, ossified cochlea secondary to meningitis, five out of the nine patients experienced a meaningful benefit from ABI, with access to conversational speech and sound field thresholds varying from 25-50 dB (Bayazit Y). Although historically NF2 patients have had exceedingly limited benefit from ABIs due to the detrimental nature of the tumors, more recent studies from Europe have reported considerable success in ABI performance in NF2 patients, including a mean open set speech perception of 41% at 24 months post-ABI activation in 18 NF2 patients (Matthies C). Notably, a penetrating ABI and an auditory midbrain implant inserted into the inferior colliculus did not lead to an increased benefit over the ABI [36–37, 73].
Deep Brain Stimulation
DBS involves the implantation of microelectrodes into specific regions of the brain. An electrical pulse generator is connected to the multiple microelectrodes via microwires, and in the treatment of Parkinson’s disease, will deliver biphasic current pulses (up to 30 microC/cm2) to the subthalamic nucleus or globus pallidus interna [84]. This device has also been successfully used in the management of tremors, depression, obsessive-compulsive disorder, tinnitus, and other conditions, depending on the location of electrode implantation [7, 59, 82].
Brain Computer Interfaces
Furthermore, stimulation and recording of various parts of the brain has been reported to provide tremendous therapeutic potential. Brain computer interfaces (BCI), such as intraparenchymal or subdural cortical electrodes, have proven to be stable and effective implants for the treatment of chronic pain, and long-term monitoring for seizure identification[34].
Cortical Stimulation
Furthermore, motor cortex stimulation (MCS) can successfully treat facial chronic neuropathic painand neuroprosthetic limbs, and with regard to facial reanimation, may hold the potential to enable facial muscle activation [49]. NEED TO EXPAND
Hurdles and Conclusions
In conclusion, the application of neuroprosthetics to paretic nerves holds great potential in advancing the field of nerve and tissue bioelectric engineering and contributing to clinical care. Although current physiotherapeutic and surgical treatments seek to restore function, structure, or comfort, they bear significant limitations in enabling cosmetic or functional recovery. Instead, the introduction of bioelectric technology may play a role in the restoration of volitional function in patients with motor nerve deficits.
Acknowledgments
Funding
This project was supported by grants from the American Neurotology Society, and the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1 TR001414.
Footnotes
Compliance with Ethical Standards
Conflict of Interest
The authors report no conflicts of interest.
Ethical Approval
This research did not involve any animals; therefore, Institutional Animal Care and Use Committee approval was not required.
Informed Consent
This research did not involve human participants orany patient information, thus Institutional Review Board approval was not required.
Contributor Information
Ronald Sahyouni, Division of Neurotology and Skull Base Surgery, Department of Otolaryngology-Head & Neck Surgery, University of California, Irvine, Irvine, CA, USA; Biomedical Engineering, University of California, Irvine, Irvine, CA, USA.
Amin Mahmoodi, Biomedical Engineering, University of California, Irvine, Irvine, CA, USA.
Jefferson W. Chen, Neurological Surgery, University of California, Irvine, Irvine, CA, USA.
David T. Chang, Division of Neurotology and Skull Base Surgery, Department of Otolaryngology-Head & Neck Surgery, University of California, Irvine, Irvine, CA, USA.
Omid Moshtaghi, Division of Neurotology and Skull Base Surgery, Department of Otolaryngology-Head & Neck Surgery, University of California, Irvine, Irvine, CA, USA.
Hamid R. Djalilian, Division of Neurotology and Skull Base Surgery, Department of Otolaryngology-Head & Neck Surgery, University of California, Irvine, Irvine, CA, USA; Biomedical Engineering, University of California, Irvine, Irvine, CA, USA.
Harrison W. Lin, Division of Neurotology and Skull Base Surgery, Department of Otolaryngology-Head & Neck Surgery, University of California, Irvine, Irvine, CA, USA.
References
- 1.Andreasen LN, Struijk JJ, Lawrence S. Measurement of the performance of nerve cuff electrodes for recording. Med Biol Eng Comput. 2000;38:447–453. doi: 10.1007/BF02345015. [DOI] [PubMed] [Google Scholar]
- 2.Bayazit Y, Kosaner J, Celenk F, Somdas M, Yilmaz I, Altin G, Cevizci R, Yavuz H, Ozluoglu L. Auditory brainstem implant in postlingual postmeningitic patients. Laryngoscope. 2016;126:1889–1892. doi: 10.1002/lary.25731. [DOI] [PubMed] [Google Scholar]
- 3.Benfield J, Maknojia A, Epstein F. Progressive Paraplegia from Spinal Cord Stimulator Lead Fibrotic Encapsulation: A Case Report. Am J Phys Med Rehabil. 2016;95:e30–33. doi: 10.1097/PHM.0000000000000411. [DOI] [PubMed] [Google Scholar]
- 4.Boretius T, Badia J, Pascual-Font A, Schuettler M, Navarro X, Yoshida K, Stieglitz T. A transverse intrafascicular multichannel electrode (TIME) to interface with the peripheral nerve. Biosens Bioelectron. 2010;26:62–69. doi: 10.1016/j.bios.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Bouchard KE, Mesgarani N, Johnson K, Chang EF. Functional organization of human sensorimotor cortex for speech articulation. Nature. 2013;495:327–332. doi: 10.1038/nature11911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carey J. Clinical Trials. Available via Johns Hopkins University; 2016. Multichannel Vestibular Implant Early Feasibility Study. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02725463. Accessed 11 Jul 2017. [Google Scholar]
- 7.Chen XL, Xiong YY, Xu GL, Liu XF. Deep brain stimulation. Interv Neurol. 2013;1:200–212. doi: 10.1159/000353121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi JY, Song MH, Jeon JH, Lee WS, Chang JW. Early surgical results of auditory brainstem implantation in nontumor patients. Laryngoscope. 2011;121:2610–2618. doi: 10.1002/lary.22137. [DOI] [PubMed] [Google Scholar]
- 9.Clark GA, Ledbetter NM, Warren DJ, Harrison RR. Recording sensory and motor information from peripheral nerves with Utah Slanted Electrode Arrays. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:4641–4644. doi: 10.1109/IEMBS.2011.6091149. [DOI] [PubMed] [Google Scholar]
- 10.Clinkard D, Barbic S, Amoodi H, Shipp D, Lin V. The economic and societal benefits of adult cochlear implant implantation: A pilot exploratory study. Cochlear Implants Int. 2015;16:181–185. doi: 10.1179/1754762814Y.0000000096. [DOI] [PubMed] [Google Scholar]
- 11.Colletti V, Shannon RV. Open set speech perception with auditory brainstem implant? Laryngoscope. 2005;115:1974–1978. doi: 10.1097/01.mlg.0000178327.42926.ec. [DOI] [PubMed] [Google Scholar]
- 12.Colletti V, Shannon R, Carner M, Veronese S, Colletti L. Outcomes in nontumor adults fitted with the auditory brainstem implant: 10 years’ experience. Otol Neurotol. 2009;30:614–618. doi: 10.1097/MAO.0b013e3181a864f2. [DOI] [PubMed] [Google Scholar]
- 13.Collinger JL, Wodlinger B, Downey JE, Wang W, Tyler-Kabara EC, Weber DJ, McMorland AJ, Velliste M, Boninger ML, Schwartz AB. High-performance neuroprosthetic control by an individual with tetraplegia. Lancet. 2013;381:557–564. doi: 10.1016/S0140-6736(12)61816-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Creasey GH, Craggs MD. Functional electrical stimulation for bladder, bowel, and sexual function. Handb Clin Neurol. 2012;109:247–257. doi: 10.1016/B978-0-444-52137-8.00015-2. [DOI] [PubMed] [Google Scholar]
- 15.Cronin JA, Wu J, Collins KL, Sarma D, Rao RP, Ojemann JG, Olson JD. Task-Specific Somatosensory Feedback via Cortical Stimulation in Humans. IEEE Trans Haptics. 2016;9:515–522. doi: 10.1109/TOH.2016.2591952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Della Santina CC, Migliaccio AA, Patel AH. A multichannel semicircular canal neural prosthesis using electrical stimulation to restore 3-d vestibular sensation. IEEE Trans Biomed Eng. 2007;54:1016–1030. doi: 10.1109/TBME.2007.894629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ethier C, Oby ER, Bauman MJ, Miller LE. Restoration of grasp following paralysis through brain-controlled stimulation of muscles. Nature. 2012;485:368–371. doi: 10.1038/nature10987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernandez E, Greger B, House PA, Aranda I, Botella C, Albisua J, Soto-Sanchez C, Alfaro A, Normann RA. Acute human brain responses to intracortical microelectrode arrays: challenges and future prospects. Front Neuroeng. 2014;7:24. doi: 10.3389/fneng.2014.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frigerio A, Heaton JT, Cavallari P, Knox C, Hohman MH, Hadlock TA. Electrical Stimulation of Eye Blink in Individuals with Acute Facial Palsy: Progress toward a Bionic Blink. Plast Reconstr Surg. 2015;136:515e–523e. doi: 10.1097/PRS.0000000000001639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.George AT, Dudding TC, Gurmany S, Kamm MA, Nicholls RJ, Vaizey CJ. Pudendal nerve stimulation for bowel dysfunction in complete cauda equina syndrome. Ann Surg. 2014;259:502–507. doi: 10.1097/SLA.0b013e31828e7602. [DOI] [PubMed] [Google Scholar]
- 21.Gilja V, Pandarinath C, Blabe CH, Nuyujukian P, Simeral JD, Sarma AA, Sorice BL, Perge JA, Jarosiewicz B, Hochberg LR, Shenoy KV, Henderson JM. Clinical translation of a high-performance neural prosthesis. Nat Med. 2015;21:1142–1145. doi: 10.1038/nm.3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grill WM, Mortimer JT. Stability of the input-output properties of chronically implanted multiple contact nerve cuff stimulating electrodes. IEEE Trans Rehabil Eng. 1998;6:364–373. doi: 10.1109/86.736150. [DOI] [PubMed] [Google Scholar]
- 23.Hochberg LR, Bacher D, Jarosiewicz B, Masse NY, Simeral JD, Vogel J, Haddadin S, Liu J, Cash SS, van der Smagt P, Donoghue JP. Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature. 2012;485:372–375. doi: 10.1038/nature11076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu K, Bounni F, Williams Z. Editorial. Advancement in brain-machine interfaces for patients with tetraplegia: neurosurgical perspective. Neurosurg Focus. 2017;43:E5. doi: 10.3171/2017.5.FOCUS17244. [DOI] [PubMed] [Google Scholar]
- 25.IEEE Robotics and Automation Society., IEEE Engineering in Medicine and Biology Society. Proceedings of the First IEEE/RAS-EMBS International Conference on Biomedical Robotics and Biomechatronics BioRob 2006 understanding how biological systems work, to guide the design of novel, high performance bio-inspired machines and to develop novel devices that can better act on, substitute parts of, and assist human beings; Pisa, Italy. February 20–22, 2006.2006. [Google Scholar]
- 26.Inspire Medical Systems, Inc. Clinical Trials. Available via Inspire Medical Systems, Inc; 2016. Stimulation Therapy for Apnea Reduction. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02725463. Accessed 11 Jul 2017. [Google Scholar]
- 27.Jarosiewicz B, Sarma AA, Bacher D, Masse NY, Simeral JD, Sorice B, Oakley EM, Blabe C, Pandarinath C, Gilja V, Cash SS, Eskandar EN, Friehs G, Henderson JM, Shenoy KV, Donoghue JP, Hochberg LR. Virtual typing by people with tetraplegia using a self-calibrating intracortical brain-computer interface. Sci Transl Med. 2015;7:313ra179. doi: 10.1126/scitranslmed.aac7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jung D, Bhattacharyya N. Association of hearing loss with decreased employment and income among adults in the United States. Ann Otol Rhinol Laryngol. 2012;121:771–775. doi: 10.1177/000348941212101201. [DOI] [PubMed] [Google Scholar]
- 29.Kim S, Oh YM, Koo JW, Kim JS. Bilateral vestibulopathy: clinical characteristics and diagnostic criteria. Otol Neurotol. 2011;32:812–817. doi: 10.1097/MAO.0b013e31821a3b7d. [DOI] [PubMed] [Google Scholar]
- 30.Langhals NB, Urbanchek MG, Ray A, Brenner MJ. Update in facial nerve paralysis: tissue engineering and new technologies. Curr Opin Otolaryngol Head Neck Surg. 2014;22:291–299. doi: 10.1097/MOO.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larsen JO, Thomsen M, Haugland M, Sinkjaer T. Degeneration and regeneration in rabbit peripheral nerve with long-term nerve cuff electrode implant: a stereological study of myelinated and unmyelinated axons. Acta Neuropathol. 1998;96:365–378. doi: 10.1007/s004010050907. [DOI] [PubMed] [Google Scholar]
- 32.Lawrence SM, Larsen JO, Horch KW, Riso R, Sinkjaer T. Long-term biocompatibility of implanted polymer-based intrafascicular electrodes. J Biomed Mater Res. 2002;63:501–506. doi: 10.1002/jbm.10303. [DOI] [PubMed] [Google Scholar]
- 33.Lertmanorat Z, Montague FW, Durand DM. A flat interface nerve electrode with integrated multiplexer. IEEE Trans Neural Syst Rehabil Eng. 2009;17:176–182. doi: 10.1109/TNSRE.2008.2009307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leuthardt EC, Schalk G, Moran D, Ojemann JG. The emerging world of motor neuroprosthetics: a neurosurgical perspective. Neurosurgery. 2006;59:1–14. doi: 10.1227/01.NEU.0000221506.06947.AC. discussion 11–14. [DOI] [PubMed] [Google Scholar]
- 35.Liem L, Russo M, Huygen FJ, Van Buyten JP, Smet I, Verrills P, Cousins M, Brooker C, Levy R, Deer T, Kramer J. One-year outcomes of spinal cord stimulation of the dorsal root ganglion in the treatment of chronic neuropathic pain. Neuromodulation. 2015;18:41–48. doi: 10.1111/ner.12228. discussion 48–49. [DOI] [PubMed] [Google Scholar]
- 36.Lim HH, Lenarz M, Lenarz T. Auditory midbrain implant: a review. Trends Amplif. 2009;13:149–180. doi: 10.1177/1084713809348372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lim HH, Lenarz T. Auditory midbrain implant: research and development towards a second clinical trial. Hear Res. 2015;322:212–223. doi: 10.1016/j.heares.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loeb GE, Peck RA. Cuff electrodes for chronic stimulation and recording of peripheral nerve activity. J Neurosci Methods. 1996;64:95–103. doi: 10.1016/0165-0270(95)00123-9. [DOI] [PubMed] [Google Scholar]
- 39.Malhotra A. Hypoglossal-nerve stimulation for obstructive sleep apnea. N Engl J Med. 2014;370:170–171. doi: 10.1056/NEJMe1314084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matthies C, Brill S, Varallyay C, Solymosi L, Gelbrich G, Roosen K, Ernestus RI, Helms J, Hagen R, Mlynski R, Shehata-Dieler W, Muller J. Auditory brainstem implants in neurofibromatosis Type 2: is open speech perception feasible? J Neurosurg. 2014;120:546–558. doi: 10.3171/2013.9.JNS12686. [DOI] [PubMed] [Google Scholar]
- 41.McDonnall D, Guillory KS, Gossman MD. Restoration of blink in facial paralysis patients using FES. I Ieee Embs C Neur E: 76-+ 2009 doi: 10.1109/Ner.2009.5109238. [DOI] [Google Scholar]
- 42.McKinnon BJ. Cost effectiveness of cochlear implants. Curr Opin Otolaryngol Head Neck Surg. 2014;22:344–348. doi: 10.1097/MOO.0000000000000091. [DOI] [PubMed] [Google Scholar]
- 43.Merfeld DM, Haburcakova C, Gong W, Lewis RF. Chronic vestibulo-ocular reflexes evoked by a vestibular prosthesis. IEEE Trans Biomed Eng. 2007;54:1005–1015. doi: 10.1109/TBME.2007.891943. [DOI] [PubMed] [Google Scholar]
- 44.Merkus P, Di Lella F, Di Trapani G, Pasanisi E, Beltrame MA, Zanetti D, Negri M, Sanna M. Indications and contraindications of auditory brainstem implants: systematic review and illustrative cases. Eur Arch Otorhinolaryngol. 2014;271:3–13. doi: 10.1007/s00405-013-2378-3. [DOI] [PubMed] [Google Scholar]
- 45.Micera S, Citi L, Rigosa J, Carpaneto J, Raspopovic S, Di Pino G, Rossini L, Yoshida K, Denaro L, Dario P, Rossini PM. Decoding information from neural signals recorded using intraneural electrodes: toward the development of a neurocontrolled hand prosthesis. Proc IEEE. 2010;98:407–417. doi: 10.1109/JPROC.2009.2038726. [DOI] [Google Scholar]
- 46.Middlebrooks JC, Snyder RL. Intraneural stimulation for auditory prosthesis: modiolar trunk and intracranial stimulation sites. Hear Res. 2008;242:52–63. doi: 10.1016/j.heares.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 47.Middlebrooks JC, Snyder RL. Selective electrical stimulation of the auditory nerve activates a pathway specialized for high temporal acuity. J Neurosci. 2010;30:1937–1946. doi: 10.1523/JNEUROSCI.4949-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miranda RA, Casebeer WD, Hein AM, Judy JW, Krotkov EP, Laabs TL, Manzo JE, Pankratz KG, Pratt GA, Sanchez JC, Weber DJ, Wheeler TL, Ling GS. DARPA-funded efforts in the development of novel brain-computer interface technologies. J Neurosci Methods. 2015;244:52–67. doi: 10.1016/j.jneumeth.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 49.Monsalve GA. Motor cortex stimulation for facial chronic neuropathic pain: A review of the literature. Surg Neurol Int. 2012;3:S290–311. doi: 10.4103/2152-7806.103023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moritz CT, Perlmutter SI, Fetz EE. Direct control of paralysed muscles by cortical neurons. Nature. 2008;456:639–642. doi: 10.1038/nature07418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mortimer JT, Bhadra N. Neuroprosthetics: theory and practice. Vol. 2. World Scientific Publishing Company; New Jersey: 2004. Peripheral nerve and muscle stimulation; pp. 638–682. [Google Scholar]
- 52.Mueller AH, Hagen R, Foerster G, Grossmann W, Baumbusch K, Pototschnig C. Laryngeal pacing via an implantable stimulator for the rehabilitation of subjects suffering from bilateral vocal fold paralysis: A prospective first-in-human study. Laryngoscope. 2016;126:1810–1816. doi: 10.1002/lary.25792. [DOI] [PubMed] [Google Scholar]
- 53.Mwenge GB, Rombaux P, Lengele B, Rodenstein D. Hypoglossal Nerve Stimulation for Obstructive Sleep Apnea. Prog Neurol Surg. 2015;29:94–105. doi: 10.1159/000434660. [DOI] [PubMed] [Google Scholar]
- 54.Naples GG, Mortimer JT, Scheiner A, Sweeney JD. A spiral nerve cuff electrode for peripheral nerve stimulation. IEEE Trans Biomed Eng. 1988;35:905–916. doi: 10.1109/10.8670. [DOI] [PubMed] [Google Scholar]
- 55.Navarro X, Krueger TB, Lago N, Micera S, Stieglitz T, Dario P. A critical review of interfaces with the peripheral nervous system for the control of neuroprostheses and hybrid bionic systems. J Peripher Nerv Syst. 2005;10:229–258. doi: 10.1111/j.1085-9489.2005.10303.x. [DOI] [PubMed] [Google Scholar]
- 56.Normann RA, Fernandez E. Clinical applications of penetrating neural interfaces and Utah Electrode Array technologies. J Neural Eng. 2016;13:061003. doi: 10.1088/1741-2560/13/6/061003. [DOI] [PubMed] [Google Scholar]
- 57.Park HJ, Durand DM. Motion control of the rabbit ankle joint with a flat interface nerve electrode. Muscle Nerve. 2015;52:1088–1095. doi: 10.1002/mus.24654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pelizzone M, Fornos AP, Guinand N, van de Berg R, Kos I, Stokroos R, Kingma H, Guyot JP. First functional rehabilitation via vestibular implants. Cochlear Implants Int. 2014;15(Suppl 1):S62–64. doi: 10.1179/1467010014Z.000000000165. [DOI] [PubMed] [Google Scholar]
- 59.Perlmutter JS, Mink JW. Deep brain stimulation. Annu Rev Neurosci. 2006;29:229–257. doi: 10.1146/annurev.neuro.29.051605.112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peterson NR, Pisoni DB, Miyamoto RT. Cochlear implants and spoken language processing abilities: review and assessment of the literature. Restor Neurol Neurosci. 2010;28:237–250. doi: 10.3233/RNN-2010-0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Raffe MR. UPenn Veterinery Medicine Computer Aided Learning. CAL UPenn; 1985. Principles of peripheral nerve repair. Available via UPenn. http://cal.vet.upenn.edu/projects/saortho/chapter_65/65mast.htm. Accessed 11 Jul 2017. [Google Scholar]
- 62.Rasmussen MM, Kutzenberger J, Krogh K, Zepke F, Bodin C, Domurath B, Christensen P. Sacral anterior root stimulation improves bowel function in subjects with spinal cord injury. Spinal Cord. 2015;53:297–301. doi: 10.1038/sc.2015.2. [DOI] [PubMed] [Google Scholar]
- 63.Rossini PM, Micera S, Benvenuto A, Carpaneto J, Cavallo G, Citi L, Cipriani C, Denaro L, Denaro V, Di Pino G, Ferreri F, Guglielmelli E, Hoffmann KP, Raspopovic S, Rigosa J, Rossini L, Tombini M, Dario P. Double nerve intraneural interface implant on a human amputee for robotic hand control. Clin Neurophysiol. 2010;121:777–783. doi: 10.1016/j.clinph.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 64.Rousche PJ, Normann RA. Chronic recording capability of the Utah Intracortical Electrode Array in cat sensory cortex. J Neurosci Methods. 1998;82:1–15. doi: 10.1016/s0165-0270(98)00031-4. [DOI] [PubMed] [Google Scholar]
- 65.Rubinstein JT, Bierer S, Kaneko C, Ling L, Nie K, Oxford T, Newlands S, Santos F, Risi F, Abbas PJ, Phillips JO. Implantation of the semicircular canals with preservation of hearing and rotational sensitivity: a vestibular neurostimulator suitable for clinical research. Otol Neurotol. 2012;33:789–796. doi: 10.1097/MAO.0b013e318254ec24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sackeim HA, Rush AJ, George MS, Marangell LB, Husain MM, Nahas Z, Johnson CR, Seidman S, Giller C, Haines S, Simpson RK, Jr, Goodman RR. Vagus nerve stimulation (VNS) for treatment-resistant depression: efficacy, side effects, and predictors of outcome. Neuropsychopharmacology. 2001;25:713–728. doi: 10.1016/S0893-133X(01)00271-8. [DOI] [PubMed] [Google Scholar]
- 67.Sahin M, Haxhiu MA, Durand DM, Dreshaj IA. Spiral nerve cuff electrode for recordings of respiratory output. J Appl Physiol (1985) 1997;83:317–322. doi: 10.1152/jappl.1997.83.1.317. [DOI] [PubMed] [Google Scholar]
- 68.Sahyouni R, Bhatt J, Djalilian HR, Tang WC, Middlebrooks JC, Lin HW. Selective stimulation of facial muscles with a penetrating electrode array in the feline model. Laryngoscope. 2017;127:460–465. doi: 10.1002/lary.26078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schiefer MA, Polasek KH, Triolo RJ, Pinault GC, Tyler DJ. Selective stimulation of the human femoral nerve with a flat interface nerve electrode. J Neural Eng. 2010;7:26006. doi: 10.1088/1741-2560/7/2/026006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schiefer MA, Triolo RJ, Tyler DJ. A model of selective activation of the femoral nerve with a flat interface nerve electrode for a lower extremity neuroprosthesis. IEEE Trans Neural Syst Rehabil Eng. 2008;16:195–204. doi: 10.1109/TNSRE.2008.918425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schoenen J, Vandersmissen B, Jeangette S, Herroelen L, Vandenheede M, Gerard P, Magis D. Migraine prevention with a supraorbital transcutaneous stimulator: a randomized controlled trial. Neurology. 2013;80:697–704. doi: 10.1212/WNL.0b013e318282505. [DOI] [PubMed] [Google Scholar]
- 72.Schwartz AR, Bennett ML, Smith PL, De Backer W, Hedner J, Boudewyns A, Van de Heyning P, Ejnell H, Hochban W, Knaack L, Podszus T, Penzel T, Peter JH, Goding GS, Erickson DJ, Testerman R, Ottenhoff F, Eisele DW. Therapeutic electrical stimulation of the hypoglossal nerve in obstructive sleep apnea. Arch Otolaryngol Head Neck Surg. 2001;127:1216–1223. doi: 10.1001/archotol.127.10.1216. [DOI] [PubMed] [Google Scholar]
- 73.Schwartz MS, Otto SR, Shannon RV, Hitselberger WE, Brackmann DE. Auditory brainstem implants. Neurotherapeutics. 2008;5:128–136. doi: 10.1016/j.nurt.2007.10.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Semenov YR, Yeh ST, Seshamani M, Wang NY, Tobey EA, Eisenberg LS, Quittner AL, Frick KD, Niparko JK, Team CDI Age-dependent cost-utility of pediatric cochlear implantation. Ear Hear. 2013;34:402–412. doi: 10.1097/AUD.0b013e3182772c66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sennaroglu L, Ziyal I, Atas A, Sennaroglu G, Yucel E, Sevinc S, Ekin MC, Sarac S, Atay G, Ozgen B, Ozcan OE, Belgin E, Colletti V, Turan E. Preliminary results of auditory brainstem implantation in prelingually deaf children with inner ear malformations including severe stenosis of the cochlear aperture and aplasia of the cochlear nerve. Otol Neurotol. 2009;30:708–715. doi: 10.1097/MAO.0b013e3181b07d41. [DOI] [PubMed] [Google Scholar]
- 76.Struijk JJ, Thomsen M. Tripolar nerve cuff recording: stimulus artifact, EMG and the recorded nerve signal. IEEE Eng Med Biol Soc. 1995:1105–1106. doi: 10.1109/IEMBS.1995.579534. [DOI] [Google Scholar]
- 77.Struijk JJ, Thomsen M, Larsen JO, Sinkjaer T. Cuff electrodes for long-term recording of natural sensory information. IEEE Eng Med Biol Mag. 1999;18:91–98. doi: 10.1109/51.765194. doi: 10.1109/51.765194. [DOI] [PubMed] [Google Scholar]
- 78.Thrasher A, Graham GM, Popovic MR. Reducing muscle fatigue due to functional electrical stimulation using random modulation of stimulation parameters. Artif Organs. 2005;29:453–458. doi: 10.1111/j.1525-1594.2005.29076.x. [DOI] [PubMed] [Google Scholar]
- 79.Tyler DJ, Durand DM. Functionally selective peripheral nerve stimulation with a flat interface nerve electrode. IEEE Trans Neural Syst Rehabil Eng. 2002;10:294–303. doi: 10.1109/TNSRE.2002.806840. [DOI] [PubMed] [Google Scholar]
- 80.Van Buyten JP, Al-Kaisy A, Smet I, Palmisani S, Smith T. High-frequency spinal cord stimulation for the treatment of chronic back pain patients: results of a prospective multicenter European clinical study. Neuromodulation. 2013;16:59–65. doi: 10.1111/ner.12006. discussion 65–56. [DOI] [PubMed] [Google Scholar]
- 81.Veraart C, Grill WM, Mortimer JT. Selective control of muscle activation with a multipolar nerve cuff electrode. IEEE Trans Biomed Eng. 1993;40:640–653. doi: 10.1109/10.237694. [DOI] [PubMed] [Google Scholar]
- 82.Volkmann J, Herzog J, Kopper F, Deuschl G. Introduction to the programming of deep brain stimulators. Mov Disord. 2002;17(Suppl 3):S181–187. doi: 10.1002/mds.10162. [DOI] [PubMed] [Google Scholar]
- 83.Waters RL, McNeal DR, Faloon W, Clifford B. Functional electrical stimulation of the peroneal nerve for hemiplegia. Long-term clinical follow-up. J Bone Joint Surg Am. 1985;67:792–793. [PubMed] [Google Scholar]
- 84.Wei XF. Analysis and design of electrodes for deep brain stimulation. Duke University; North Carolina: 2009. [Google Scholar]
- 85.Willand MP, Holmes M, Bain JR, Fahnestock M, De Bruin H. Electrical muscle stimulation after immediate nerve repair reduces muscle atrophy without affecting reinnervation. Muscle Nerve. 2013;48:219–225. doi: 10.1002/mus.23726. [DOI] [PubMed] [Google Scholar]
- 86.Wolter T. Spinal cord stimulation for neuropathic pain: current perspectives. J Pain Res. 2014;7:651–663. doi: 10.2147/JPR.S37589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yoo PB, Durand DM. Selective recording of the canine hypoglossal nerve using a multicontact flat interface nerve electrode. IEEE Trans Biomed Eng. 2005;52:1461–1469. doi: 10.1109/TBME.2005.851482. [DOI] [PubMed] [Google Scholar]


