Abstract
Recent research on the etiology of autism spectrum disorder (ASD) has shifted in part from a singular focus on genetic causes to the involvement of environmental factors and their gene interactions. This shift in focus is a result of the rapidly increasing prevalence of ASD coupled with the incomplete penetrance of this disorder in monozygotic twins. One such area of environmentally focused research is the association of exposures to endocrine disrupting compounds (EDCs) with elevated risk for ASD. EDCs are exogenous chemicals that can alter endogenous hormone activity and homeostasis, thus potentially disrupting the action of sex and other natural hormones at all stages of human development. Inasmuch as sex hormones play a fundamental role in brain development and sexual differentiation, exposure to EDCs in utero during critical stages of development can have lasting neurological and other physiological influences on the developing fetus and, ultimately, the child as well as adult. This review will focus on the possible contributions of EDCs to autism risk and pathogenesis by first discussing the influence of endogenous sex hormones on the autistic phenotype, followed by a review of documented human exposures to EDCs and associations with behaviors relevant to ASD. Mechanistic links between EDC exposures and aberrant neurodevelopment and behaviors are then considered, with emphasis on EDC-induced transcriptional profiles derived from animal and cellular studies. Finally, this review will discuss possible mechanisms through which EDC exposure can lead to persistent changes in gene expression and phenotype, which may in turn contribute to transgenerational inheritance of ASD.
Keywords: Autism, endocrine disrupting compounds, sex hormones, neurodevelopment, gene expression, epigenetics
Introduction
Autism spectrum disorder (ASD) describes a complex set of neurodevelopmental disorders characterized by repetitive behaviors and restricted interests as well as impairments in many areas of social functioning and communication (American Psychiatric Association, 2013). ASD currently affects 1 in 68 children according to the latest estimate from the CDC (Christensen et al., 2016). Studies on the concordance of autism diagnosis between identical twins and among siblings have indicated a strong genetic component contributing to ASD (Bailey et al., 1995). The fact that some genetically-defined disorders such as Fragile X Syndrome, tuberous sclerosis, and Rett Syndrome are also associated with autistic traits further reinforces the notion of ASD as a genetic disorder (Cohen et al., 2005). However, genetic factors alone do not explain all of the pathogenicity and variability in ASD. Studies examining concordance rates between monozygotic twins reveal that although the concordance rate of ASD between monozygotic twins was significantly higher than that of dizygotic twins, the penetrance was still incomplete, suggesting that environmental factors may play a significant role in the etiology and/or pathogenesis of ASD (Hallmayer et al., 2011; Tordjman et al., 2014). Because of the rapidly increasing prevalence of ASD, recent research has focused on potential environmental contributors to the development of ASD (Hu, 2013; LaSalle, 2013). One such area of research is in the effect of prenatal hormone exposure, both endogenous and exogenous, on neurodevelopment and behavior, a subject comprehensively reviewed by Gore et al. (2014). Among the exogenous factors considered, endocrine disrupting compounds (EDCs) include environmental, agricultural, industrial, nutritional, as well as pharmaceutical chemicals that alter hormone activity by either mimicking natural hormones or antagonizing their actions and/or homeostasis in cells and organisms as a whole, since maternal exposure to EDCs could expose developing fetuses by way of the placenta during pregnancy. In addition, persistent EDCs that often accumulate in fatty tissues can be passed postnatally to the newborn through mother’s milk (Grandjean et al., 2004). Table 1 provides examples of ubiquitous EDCs, divided into those with either short (days to <1 year) or long (>1 year) half-lives, and their uses to provide context with respect to possible routes of human exposures.
Table 1.
Examples of ubiquitous endocrine disrupting compounds and their uses
| Short-lived EDCs (half-lives: days to < 1 year) | Found in: |
|---|---|
| Atrazine | Herbicides |
| Bisphenol A | Plastics, thermal receipts, dental sealants |
| Diethylstilbestrol (DES)* | Drug to prevent miscarriage |
| Ethinyl estradiol | Birth control contraceptives |
| Genistein (and other phytoestrogens) | Soy and other plant products |
| Phthalates | Soft toys, cosmetics, air fresheners, flooring material, enteric coatings of pharmaceutical pills, personal care products |
| Triclosan | Antibacterial soaps, toothpaste, detergents, personal care and cleaning products, surgical cleaning solutions |
| Valproic acid (VPA) | Pharmaceutical for epilepsy, bipolar disorder, major depression |
| Vinclozolin | Pesticides |
| Long-lived EDCs (half-lives > 1 year) | Found in: |
| Organochlorines* | Pesticides |
| Perfluorooctanoic acid (PFOA) | Flame retardant, surfactant, nonstick cookware |
| Perfluorooctane sulfonic acid (PFOS) | Flame retardant, surfactant, fabric stain repellents |
| Polychlorinated biphenyls (PCBs)* | Coolants, lubricants |
| Polybrominated diphenyl ethers (PBDEs)* | Flame retardant, textiles |
| Polycyclic aromatic hydrocarbons (PAHs) | Coal, tobacco smoke, automobile emissions, sewage sludge |
| Dichlorodiphenyldichloroethylene (p,p′-DDE) and parent compound Dichlorodiphenyltrichloroethane (p,p′-DDT)* | Pesticides |
Note: Most of the short-lived EDCs are water soluble and measured in urine while most of the long-lived EDCs are fat-soluble and measured in serum.
No longer allowed or manufactured in the US.
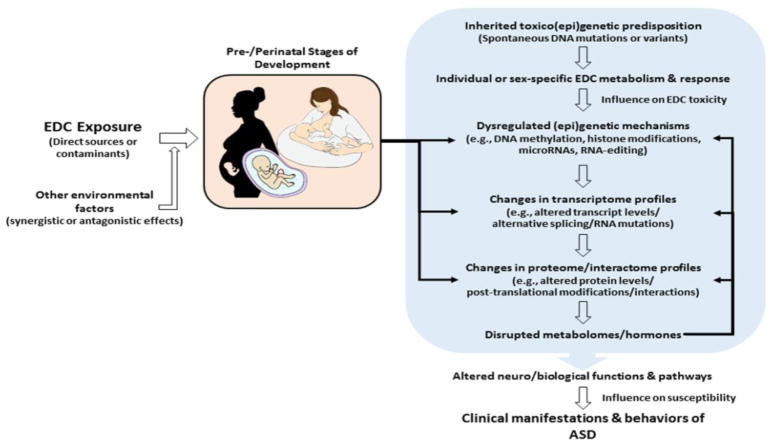
This review will focus on the possible contributions of EDCs to autism risk and pathogenesis by first discussing the impact of EDCs on endogenous hormones and the influence of endogenous hormones on the autistic phenotype, followed by consideration of evidence for human exposures to EDCs that correlate with behaviors relevant to ASD, and a review of evidence for the impact of EDCs on neurodevelopment and behavior derived from animal and cellular studies. Finally, this review will discuss possible mechanisms by which EDC exposure can lead to persistent changes in gene expression and phenotype, which may in turn contribute to transgenerational inheritance of ASD. The schematic in Figure 1 summarizes the ways in which EDCs, in combination with genetic predisposition, can impact various functions and pathways (as well as their cross-talk) to lead to the clinical manifestations and behaviors of ASD.
Figure 1.
Schematic diagram illustrating the various ways in which endocrine disrupting compounds can impact genes and gene regulatory mechanisms (i.e., epigenetic machinery) to cause large-scale changes in gene expression (i.e., the transcriptome) as well as protein and metabolite profiles (i.e., the proteome and metabolome) that may lead to altered neural functions and pathways leading to the clinical manifestations and behaviors associated with ASD. The diagram also suggests feedback interactions between the metabolome and the gene regulatory mechanisms affecting the transcriptome and proteome.
Impact of EDCs on endogenous hormones
During development, the fetal brain is exposed to endogenous hormones from both the fetus’ own developing reproductive system as well as that of its mother. These prenatal hormones play important roles not only in brain development but also in sexual dimorphism in the brain, with changes persisting into adolescence and adulthood during which sexually dimorphic behaviors are manifested (Berenbaum and Beltz, 2016; Cohen-Bendahan et al., 2005; Gore et al., 2014; McCarthy, 2016). Sex-specific manifestations encompass both reproductive and non-reproductive behaviors. It has been known for a long time that injection of testosterone into animal models causes masculinization of behavior (Phoenix et al., 1959). Because the enzyme p450 aromatase converts testosterone to estradiol, injection of estradiol has a similar masculinization effect (McEwen et al., 1977). More recent research has found that androgens directly cause masculinization in nonhuman primates rather than going through an estradiol intermediate as in rodents (Wallen, 2005). However, the precise pathways through which the sex hormones induce masculinization as well as sexually dimorphic brain development and behavior in humans are still not clear. Nevertheless, the condition known as Androgen Insensitivity Syndrome (AIS) in which genetically male individuals exhibit a female phenotype both physically and behaviorally due to mutations in the gene for androgen receptor clearly demonstrates a role for androgens in these developmental processes in humans (Brown et al., 1993; Galani et al., 2008).
Endocrine-disrupting chemicals have been shown to alter endogenous hormone levels in humans. Exposure to multiple types of phthalates was correlated with reduced levels of thyroid hormones and progesterone in pregnant mothers (Johns et al., 2015). Maternal exposure to EDCs during pregnancy has also been linked to hormonal changes in the exposed children. For example, maternal exposure to the flame retardant BDE-153 was associated with a 92.4% increase in their sons’ testosterone levels at age 12 as well as changes in gonadotropic hormones (Eskenazi et al., 2017). Bisphenol A has also been linked with reductions in thyroxine (T4) levels in pregnant mothers in addition to reduced thyroid stimulating hormone (TSH) in their sons but not daughters (Chevrier et al., 2013). Thus, aside from serving as agonists and antagonists of the steroid hormone receptors to interfere with normal hormonal signaling, EDCs can also affect the levels of endogenous hormones and hormonal homeostasis, in part by modulating the activity and expression of key steroid metabolizing enzymes (Alléra et al., 2004; Whitehead and Rice, 2006). These and other mechanisms through which EDCs affect hormone action and homeostasis together with outcomes of EDC exposures have been extensively reviewed in the literature (Diamanti-Kandarakis et al., 2009; Lee and Jacobs, 2015). With respect to developmental disorders, such as ASD, the timing of exposure is also important, with most epidemiological studies focusing on prenatal and early-life exposures during critical periods of development in which hormonal contributions are especially important (Braun et al., 2017a; Braun, 2017; Vrijheid et al., 2016).
The relationship between sex hormones and ASD
Autism spectrum disorder is approximately 4.5 times more prevalent in males than in females according to the latest prevalence estimates reported by the U.S. Centers for Disease Control and Prevention (Christensen et al., 2016). The male dominance in ASD suggests that sex hormones could play a role in the diagnostic gap between males and females. The “extreme male brain theory” of autism in particular postulates that excess prenatal androgen exposure could be a possible cause of this male bias (Baron-Cohen et al., 2005). In support of the extreme male brain theory of autism, elevated levels of testosterone in amniotic fluid have been associated with poor social relationships and the development of restricted interests in the exposed child (Knickmeyer et al., 2005). Additional studies by the Baron-Cohen group associated elevated fetal (but not postnatal (Auyeung et al., 2012)) testosterone levels with a variety of autistic traits, including reduced frequency of eye contact at 12 months of age (Lutchmaya et al., 2002), reduced vocabulary size from 18 to 24 months (Lutchmaya et al., 2001), and reduced scores on the Empathy Quotient (EQ) with increased Systematizing Quotient (SQ) at 96 months (Auyeung et al., 2006; Chapman et al., 2006). Moreover, studies of endocrine disorders such as congenital adrenal hyperplasia (CAH) and polycystic ovary syndrome (PCOS), which are both associated with higher levels of testosterone in females, have found a higher prevalence of autistic traits in female children of the affected women (Knickmeyer et al., 2006; Palomba et al., 2012), further associating higher fetal exposures to testosterone with ASD phenotypes. However, a recent study on androgen levels in umbilical cord blood and ASD phenotypes in infants with an older sibling already diagnosed with ASD did not replicate this direct association (Park et al., 2017). Interestingly, the study revealed a positive association between cord blood testosterone levels and ASD traits in the at-risk younger sibling only when the diagnosed older sibling was a female (regardless of the sex of the infant), suggesting an interaction between the male hormones and a genetic liability factor present in families with females affected by ASD. Without any information on the implied gene-hormone interaction, the mechanistic link connecting testosterone to ASD remains unclear.
A study by Sarachana et al. (2011) offers a plausible explanation for the elevated testosterone levels as well as the male bias in ASD. This study demonstrated the opposing effects of androgen (specifically, dihydrotestosterone) and estrogen (beta-estradiol) on the expression of retinoic acid-related orphan receptor-alpha (RORA), a gene previously found to exhibit reduced expression in both lymphoblastoid cell lines as well as postmortem brain tissues derived from individuals with ASD (Hu et al., 2009; Nguyen et al., 2010). It was noted that dihydrotestosterone reduced the expression of RORA while estradiol increased its expression in SH-SY5Y neuroblastoma cells, a neuronal cell model (Sarachana et al., 2011). Moreover, RORA, a nuclear hormone receptor that functions as a transcriptional modulator, was shown to regulate the expression of aromatase whose protein level was highly correlated (r2 = 0.91) with that of RORA in brain tissues. Collectively, these observations may explain the higher testosterone levels associated with autism inasmuch as lower aromatase levels are expected to lead to a buildup of its substrate testosterone, which can further inhibit RORA expression, thus exacerbating RORA deficiency. Because estradiol has been shown to increase RORA expression, higher estradiol levels in females may protect against conditions that lead to RORA deficiency, thus lowering female susceptibility to ASD. The influence of sex hormones on RORA expression may thus contribute to the sex bias present in autism. Moreover, genome-wide analysis for transcriptional targets of RORA revealed over 2500 other genes potentially regulated by RORA, including more than 400 autism risk genes identified by genetics and functional analyses (Sarachana and Hu, 2013). Based on these findings, we proposed that dysregulation of RORA expression by hormones or hormone-like substances, such as EDCs, may trigger a cascade of transcriptional deregulation of genes relevant to autism pathogenesis (Hu, 2012). The fact that RORA is expressed in the human frontal cortex and cerebellum before birth (from 12–37 weeks post-conception) leaves open the possibility that this gene may be subject to prenatal dysregulation by EDCs as well as by aberrant levels of endogenous hormones (Hu et al., 2015).
Aside from testosterone and estradiol, other steroid hormones, such as progesterone, 17α-hydroxy-progesterone, androstenedione, and cortisol, measured in amniotic fluid of pregnant women, have also been linked to ASD in their children (Baron-Cohen et al., 2014). This finding suggests that an overall elevated fetal steroidogenic activity is associated with autism. Lower thyroid stimulating hormone (TSH) response has also been correlated with autism in boys (Singh et al., 2017; Tareen and Kamboj, 2012). This observation is significant because TSH, like the sex hormones, is also essential for brain development (Howdeshell, 2002; Zoeller and Rovet, 2004), and is a well-known target of EDCs (Miller et al., 2009). In fact, prenatal bisphenol A (BPA) concentrations in maternal serum from late pregnancy has been shown to be associated with reduced TSH in newborn girls, but not boys (Romano et al., 2015).
Human exposure to EDCs
Humans are exposed to low levels of ubiquitous EDCs throughout their lives (Braun, 2017; Colborn and Carroll, 2007; Gore et al., 2014; Heindel et al., 2015; Schug et al., 2016). For example, over 92% of the U.S. population were found to have detectable levels of bisphenol A (BPA), a component of plastics and resins, in their urine (Calafat et al., 2008), and over 57% of the U.S. population had exposure to 4-tertiary-octylphenol (tOP), which is used in rubber tire production as well as in inks for printers. As illustrated in Table 1, many EDCs are organic pollutants, but some can be pharmaceuticals, such as valproic acid (VPA), ethinyl estradiol, and diethylstilbestrol (DES). Interestingly, VPA when taken during pregnancy is a known risk factor for ASD (Christensen et al., 2013), while DES (now discontinued as a medication to prevent miscarriage) has been shown to associate with clear cell adenocarcinoma and other cervical abnormalities in the daughters of women who used DES during pregnancy (Smith et al., 2012). In addition to pharmaceuticals, some foods, such as soy, also have been shown to have hormonal or endocrine disrupting activity. A large part of the exposure to organic pollutants comes from contact with a diverse group of common consumer items, such as the plastic linings inside food and beverage containers, soft toys, thermal receipts, dental sealants, household materials (e.g., flooring, non-stick cookware), flame-retardants in clothing in upholstery as well as air and water pollution from vehicular, agricultural and industrial waste products, with some chemicals (e.g., PFOA, PFOS, PCBs, and PBDEs) persisting in the environment well beyond the initial contamination. These long-lived EDCs, which tend to accumulate in fatty tissues of exposed individuals, can thus pose a threat to human health even years after they are banned from production or use. Humans can be exposed to EDCs via contamination of groundwater by agricultural chemical runoff containing pesticides, such as dichlorodiphenyltrichloroethane (DDT) and herbicides, such as atrazine (Winchester et al., 2009). People living near motorways are also at risk of higher exposure to EDCs (such as PAHs) which are often components of hazardous air pollutants in vehicular exhaust (Windham et al., 2006). Other possible routes of EDC exposure in humans is through personal care products such as cosmetics, soaps, and sunscreens, many of which contain phthalates (Philippat et al., 2015a; Philippat et al., 2015b). Although most human exposures to EDCs are at low dosages which are often below the presumably safe lower limits for exposure established by regulatory agencies, such as the Environmental Protection Agency, they still have the potential to disrupt normal hormonal activity, typically at the levels of endogenous hormones (e.g., in the nanomolar range), that are well below those established as “safe” by toxicological studies (Vandenberg et al., 2012).
Impact of EDCs on risk for ASD and neurodevelopment
Due to the importance of sex hormones on brain development, it has been proposed that prenatal and perinatal exposures to EDCs may increase risk for ASD by inducing long-lasting neurological and behavioral effects through the disruption or dysregulation of normal hormonal signaling pathways (Braun, 2012; Rebuli and Patisaul, 2016; Schug et al., 2015). Early epidemiological studies based on geographically or temporally exposed populations have correlated residential distance from agricultural pesticide applications, poundage of pesticide application, and distribution of hazardous air pollutants with an increased risk for ASD (Roberts et al., 2007; Windham et al., 2006). More recent studies based on biomonitoring of EDCs in bodily fluids, such as urine or serum, from pregnant women sought to establish a link between the prenatal body burden of the measured chemicals and risk for ASD in the children of these women. A Finnish case-control study investigating risk associated with exposure to persistent organic pollutants (including a variety of PCB congeners, DDT, and DDE) measured in archived maternal serum and diagnosis of ASD in the child found no significant differences in odds ratios for ASD due to EDC exposures among 75 ASD cases and 75 controls, although the adjusted odds ratio was 1.91 (p = 0.29) for subjects with total PCB levels above the 90th percentile (Cheslack-Postava et al., 2013). A later study on PCBs and organochlorine pesticides which included 545 children with ASD and 418 controls corroborated the findings for at least some of the PCB congeners measured in mid-pregnancy serum, with adjusted odds ratios of 1.79 and 1.82 for PCB138/158 and PCB153, respectively, when comparing the highest and lowest quartiles of exposures (Lyall et al., 2016). Moreover, prenatal exposures to polybrominated diphenyl ether (PBDE) flame retardants that are commonly used in furniture and construction materials, have been found to associate with poorer prefrontal cortex functions, including attention and executive functions (Eskenazi et al., 2017; Sagiv et al., 2015). In contrast to the studies showing associations of PCB and PDBE exposures with ASD-related behaviors, a nested case-control study which focused on exposure to perfluoroalkyl subtances showed no association between prenatal exposure to these long-lived EDCs and risk for ASD (Liew et al., 2015). In fact, another study showed that increasing PFOA exposure was associated with fewer autistic behaviors (Braun et al., 2014). This latter study, which also investigated a wide variety of EDCs including both long-lived (PCBs, PDBEs, and PFOA) and short-lived (phthalates and BPA) compounds for links between maternal exposures and ASD-relevant neurobehaviors revealed a complex pattern of associations (direct, inverse, as well as null) with reciprocal social, repetitive, and stereotypic behaviors in children. With respect to short-lived EDCs that are typically assayed in urine of pregnant women, a series of studies by Braun and colleagues investigating the impact of BPA exposures in utero on neurobehaviors in the exposed children revealed associations with a number of behaviors often impacted by ASD. The affected behaviors included executive function (Braun et al., 2011) and cognition as well as social and repetitive behaviors, some of which were manifested in a sex-dependent manner (Braun et al., 2017a). A recent longitudinal study on the associations between prenatal EDC exposures and neurodevelopmental behaviors further showed that some of the affected behaviors associated with BPA and PBDE exposures persisted in the children for at least 8 years, and were in part sex-dependent (Braun et al., 2017b). Collectively, these studies focusing on prenatal exposures and their outcomes are important because social impairments, repetitive behaviors, and deficits in executive functions are key features of ASD that are generally manifested throughout the individual’s lifetime.
Although the majority of epidemiological studies have focused on the association between exposure to a specific EDC (e.g., BPA) and ASD, most human exposures involve mixtures of EDCs (Braun et al., 2016). These “real-world” exposures complicate the analyses and interpretation of data associating a particular EDC within the mixture with autistic traits. As mentioned above, a recent attempt to correlate gestational exposure to a wide variety of EDCs with autistic behaviors in the children of the exposed mothers revealed a complex set of relationships, with the levels of some EDCs associating with certain ASD behaviors, and other EDCs either having either negligible or opposite associations with the autistic behaviors (Braun et al., 2014). Thus, there is a need to design animal studies that take into consideration exposures to mixtures of EDCs, mimicking the actual exposures at a given location or point in time. One such study which investigated the effects of murine maternal exposures during gestation until weaning to mixtures of four EDCs (atrazine, perfluorooctanoic acid, BPA, and 2,3,7,8-tetrachlorodibenzo-p-dioxin) versus the effects of separate exposures to each of these four chemicals found differences in behavioral responses in the offspring to the mixture of EDCs in comparison to responses induced by the individual EDCs (Sobolewski et al., 2014). Notably, they also reported sex-specific effects where males and females exhibit different behavioral outcomes as a result of the maternal exposures, with males generally displaying more sensitivity to the mixtures as well as to individual chemicals. Identification of the biochemical, physiological, and neurological mechanisms of this differential response to environmental agents may provide clues to the differential susceptibility of males and females towards ASD.
Mechanistic links between EDCs and ASD
Although prenatal exposure to EDCs has been correlated with ASD and autistic traits in humans, the mechanism through which this occurs is largely unknown. One approach towards elucidating this mechanism is to investigate changes in gene expression in both animal and cellular models exposed to EDCs, and to compare the EDC-altered genes to those specifically associated with the etiology and pathogenesis of autism. With respect to animal models, a recent study showed that gestational exposure to BPA induced transgenerational changes in the expression of several estrogen receptors as well as that of the “social” hormones, oxytocin and vasopressin (Wolstenholme et al., 2012). This prenatal exposure to BPA was also found to have transgenerational effects on social recognition and locomotor activity, although no sex differences were noted (Wolstenholme et al., 2013). However, a more recent study reported sexually dimorphic effects of EDC mixtures (administered to dams from gestation day 7 until weaning) on postnatal gene expression in rat brains (Lichtensteiger et al., 2015). Gene expression changes were associated with components of glutamatergic synapses as well as with migration and pathfinding of both glutamateric and GABAergic neurons. These findings may relate to proposed deficits in the excitatory-inhibitory balance in synaptic activity in ASD (Gogolla et al., 2009; Hussman, 2001; Rubenstein and Merzenich, 2003). Fetal exposure to BPA has also been shown to reduce serum estradiol, altering genes involved in X-chromosome inactivation (i.e., Xist and Tsix), and suppressing X-linked ASD candidate genes, including Fmr1 and Nlgn3, as early as 3 weeks postnatally in the cerebrums of prenatally exposed mice (Kumamoto and Oshio, 2013). Aside from in vivo exposures in animals, a recent study showed that in vitro exposures of primary murine neuronal cells to certain pesticides and fungicides, such as rotenone and pyraclostrobin, can induce transcriptional changes associated with ASD (Pearson et al., 2016). Although the majority of animal studies utilize rodents, a recent study using zebrafish as an experimental model has shown that embryonic exposure to BPS (a BPA substitute used in BPA-free plastics), even at a concentration equivalent to 0.1% of the accepted human exposure level of BPA/BPS, can disrupt neurogenesis and cause hyperactivity by mediating androgen receptor activation which leads, in turn, to disrupted aromatase transcription in the brain (Kinch et al., 2015). Because of their rapid maturation, capability of complex behaviors, clear chorion and embryo, as well as the ease of dosing with various chemicals which can be directly added to the holding tanks, zebrafish are rapidly becoming a useful model for environmental studies (Bailey et al., 2013).
With respect to human exposures, a comprehensive survey of the impact of various environmental pollutants on gene expression concludes that many commonly used industrial, agricultural, and pharmaceutical chemicals, including EDCs, can alter gene expression of hundreds of autism susceptibility genes (Carter and Blizard, 2016). A recent review summarizing the effects of estrogenic EDCs, such as BPA, polychlorinated biphenyl compound (PCBs), and phthalates, emphasized alterations in estrogen and nuclear respiratory factor 1 (NRF1) signaling pathways which are impacted in many neurological diseases, including autism (Preciados et al., 2016). Although it is not ethical to deliberately expose humans to EDCs, transcriptional profiling of human peripheral blood mononuclear cells cultured in the presence of PCBs revealed genome-wide deregulation of gene expression, with some genes involved in pathways leading to endocrine system disorders, metabolic diseases, as well as developmental disorders (Ghosh et al., 2015). Collectively, these studies and others which report large-scale changes in gene expression as a result of exposure to EDCs suggest that environmental chemicals may be eliciting epigenetic changes in the genome that may have lasting and widespread changes in gene expression and resultant phenotypes.
Long-lasting influence of EDCs on behavioral phenotypes and the epigenome: implications for ASD
Endocrine disrupting compounds are likely to exert lasting influences on the development and behavior of organisms through the induction of epigenetic modifications of their genome (referred to as the epigenome) which, in turn, can potentially result in heritable changes in gene expression and phenotype without causing changes in DNA sequence. These sometimes reversible epigenetic modifications include DNA methylation, histone modifications, and chromatin remodeling, which together regulate the availability of DNA to transcriptional activators and repressors, as well as microRNA expression which regulates gene expression at post-transcriptional levels (Allis et al., 2006; Kaminsky et al., 2006).
DNA methylation, in particular, has been extensively studied in the context of development and endocrine disruption. Briefly, DNA methylation refers to the process in which a methyl group is reversibly attached to a cytosine base in DNA. Depending on the location of the cytosine in the genome as well as its proximity to specific genes, methylation can either silence or activate gene expression. Importantly, methylation changes may be heritable. For example, if germ cells (e.g., sperm and egg cells) are affected, there is a potential for transgenerational transmission of the altered epigenome, which could influence both gene expression and behavioral phenotypes even in unexposed descendants. Thus, the timing as well as target tissues of environmental chemicals are important, with epigenetic modifications that occur early at critical stages of development likely to be the primary mechanism linking environmental exposures to EDCs with neurodevelopmental disorders (Ladd-Acosta and Fallin, 2016; LaSalle, 2011). Moreover, an individual’s vulnerability to environmental agents can also be influenced by both genetic as well as non-genetic factors, such as diet, metabolic profile, and physical activity.
Epigenetic modifications in response to endogenous hormones play a fundamental role in sexual development and differentiation (McCarthy and Nugent, 2013). For example, both DNA methylation and histone modifications have been associated with epigenetic control of puberty (Lomniczi et al., 2013). With respect to sexual development, a recent study found that feminization of the rodent brain requires active methylation to suppress masculinization (Nugent et al., 2015). The discovery of epigenetic contributions to sexual development and differentiation, pathways that are generally thought to be hormone-mediated, has led to the notion that EDCs may contribute to neurodevelopmental disorders by disrupting the epigenome in addition to the endocrine system.
There is increasing evidence from animal studies that a wide range of EDCs can induce nonlethal phenotypic changes in behavior, ranging from male sexual and maternal nurturing behaviors to learning impairments, increased anxiety, and altered stress response (Kajta and Wjtowicz, 2013; Ottinger et al., 2008; Patisaul and Adewale, 2009). Furthermore, studies have shown that some of these behavioral phenotypes may be manifested in F3 individuals (i.e., third generation removed from the EDC-exposed animal), thus raising questions as to the mechanisms of transgenerational transmission of behavioral and even neurological phenotypes (Anway and Skinner, 2008; Crews et al., 2012; Skinner, 2011; Skinner et al., 2011). In mice, BPA exposure during gestation caused impairments in social behaviors down to the F3 generation (Wolstenholme et al., 2013). Social dysfunction is a central feature in autism, but the transgenerational effects of EDCs in humans are unclear. Such transgenerational effects, if mediated through epigenetic modifications, would provide an additional mechanism through which ASD traits may be inherited.
Indeed, EDC-induced transgenerational phenotypic changes have been linked to epigenetic changes in specific genes in rat brain, resulting in altered bionetworks that are revealed by transcriptomic analyses (Skinner et al., 2014). More recently, Skinner and colleagues reported that transient exposure of gestating female rats to atrazine (a common herbicide) induced epigenetic transgenerational inheritance of testis disease, early onset of puberty in females, behavioral changes, and a lean phenotype in both males and females, which were manifested through the F3 generation (McBirney et al., 2017). These investigators further showed that atrazine exposure was associated with DNA methylation changes (called epimutations) in sperm in the F1, F2, and F3 generations, thus providing a mechanism for the transgenerational transmission of disease as well as behavioral and metabolic phenotypes. In mice, in utero EDC exposure has also been shown to induce epigenetic changes in fetal brains which correlated with significant behavioral changes in the offspring (Kundakovic et al., 2013). Briefly, this study showed that environmentally relevant low-doses of BPA induced sex-specific, dose-dependent, and brain region-specific expression changes in genes encoding estrogen receptor 1 (Esr1), estrogen receptor 2 (Esr2), estrogen-related receptor gamma (Esrrg), DNA methyltransferse 1 (Dnmt1), and DNA methyltransferase 3a (Dnmt3a), with the latter two enzymes directly responsible for epigenetic modifications (i.e., DNA methylation). The dose-response trends were often curvilinear, which may reflect the non-monotonic effect of EDCs, and male and female mice were oppositely affected. Kundakovic et al. (2013) also determined that in utero BPA exposure had sexually dimorphic and lasting effects on pup behaviors, specifically social and anxiety-like behaviors in male and female pups. In particular, they found a reduction in play behaviors (e.g., chasing other pups) as a result of in utero BPA exposure of males, but not female pups. Although this study correlated epigenetic changes in the brain of mice with changes in social and anxiety-like behaviors, animal studies do not always directly translate to humans. However, animal studies have provided strong evidence that low-doses of EDCs can significantly induce epigenetic modifications as well as produce behavioral phenotypes relevant to ASD. In fact, a recent study found that the prevalence of traits associated with ASD is higher in children whose grandmothers smoked during pregnancy (Golding et al., 2017). Since PAHs in tobacco smoke are considered EDC pollutants, this study further supports the concept that environmental EDCs can mediate transgenerational effects in humans leading to ASD, although the mechanism for this specific association is unknown.
Evidence for epigenetic changes in ASD
There is already significant evidence for large-scale epigenetic changes in ASD (Ciernia and LaSalle, 2016; Hu, 2013; Keil and Lein, 2016; Siu and Weksberg, 2017). An early study examining global DNA methylation profiles in lymphoblastoid cell lines derived from diagnostically discordant monozygotic twins and sibling pairs (with only one of each twin or sib pair diagnosed with ASD) revealed a number of differentially methylated genes (including RORA) affecting neurodevelopment and neurological functions (Nguyen et al., 2010). Additional large-scale methylation studies on primary lymphocytes (Wong et al., 2014), buccal cells (Berko et al., 2014), as well as postmortem brain tissues (Ginsberg et al., 2012; Ladd-Acosta et al., 2014) derived from individuals with ASD and unaffected controls confirmed the link between changes in the epigenome and ASD diagnosis, although the causes for the epigenomic changes are unknown. In this respect, a study of levels of PCB and PBDE congeners in postmortem brain tissues from individuals with genetically defined and idiopathic ASD in comparison to non-ASD controls showed elevated levels of PCB 95 only in the brain tissues of individuals with maternal 15q11-q13 duplication or paternal deletion in this region, but not in the brain tissues of individuals with idiopathic autism, suggesting an interaction between environmental exposures and genetic predisposition resulting in ASD (Mitchell et al., 2012). This report also noted that methylation of repetitive DNA (specifically LINE-1 elements) was reduced in the PCB 95-exposed brain, which is consistent with the concept of epigenetic changes contributing to the manifestation of ASD. A recent study by the same group demonstrated the interaction of PCB 95 exposure and the ASD-associated 15q11.2-q13.3 maternal duplication on DNA methylation and gene expression in a neuronal cell culture model, offering additional support for the hypothesis of environment by epigenome interactions (Dunaway et al., 2016). Importantly, some of the differentially methylated genes identified in the cellular model overlapped with those found in differentially methylated regions in postmortem brain tissues from individuals with the 15q11.2-q13.3 duplication, but not in brain tissues from individuals with idiopathic ASD or other related syndromic forms of ASD, thus mirroring the earlier findings on the postmortem brain tissues which suggested a specific genetic vulnerability to PCB 95 (Mitchell et al., 2012).
Aside from DNA methylation, additional gene regulatory epigenetic mechanisms, such as histone modifications (Shulha et al., 2012; Sun et al., 2016) and altered microRNA expression (Abu-Elneel et al., 2008; Ander et al., 2015; Ghahramani Seno et al., 2011; Hicks et al., 2016; Kichukova et al., 2017; Mundalil Vasu et al., 2014; Sarachana et al., 2010; Schumann et al., 2017; Talebizadeh et al., 2008; Wu et al., 2016) were also found to be associated with ASD. However, transgenerational inheritance of these ASD-associated epigenetic changes has not been demonstrated so far. Because epigenetic modification is the likely mechanism through which gene by environment interactions occur, further understanding of the impact of EDCs on the epigenome may provide more insight into the role of EDC exposure in the pathogenesis of ASD.
Summary and future research
Although recent studies have found associations between EDC exposure and ASD traits, behaviors, and diagnoses, there is limited research on the exact mechanisms through which EDCs affect neurodevelopment and behavior. Emerging areas of research on EDCs in both animal and cellular models have revealed large-scale changes in gene expression, particularly in pathways relevant to the pathogenesis and pathobiology of ASD. Such genome-wide changes in transcriptional profiles can be mediated by specific master regulator or “ASD-driver” genes, such as RORA, which can have significant downstream consequences through dysregulated transcription of many neurologically relevant target genes, or by epigenetic mechanisms that can coordinately affect large batteries of genes. Additionally, much research is still needed on the identification of pathways (both biochemical and neurological) through which EDC-sensitive genes actually lead to the behaviors and phenotypes associated with autism, with particular attention to sexually dimorphic patterns in susceptibility to EDCs. There is also a paucity of research regarding epigenetic changes as a result of low-dose exposures to EDCs in humans, as most studies are done with rodents. Human studies can involve studying the epigenetic effects of low-dose EDCs on human primary cells and cell lines in culture, or determining epigenetic changes in the DNA of human populations that are chronically and environmentally exposed to specific types of EDCs which are measurable in blood or urine. Furthermore, as most epigenetic analyses to date involved identifying differential DNA methylation, there is a need to understand the impact of EDCs on other epigenetic mechanisms, such as miRNA expression and histone modifications as well as to determine the mechanisms of transgenerational inheritance of such changes, if they occur. Finally, as our understanding of the epigenetic effects of environmental EDCs grows, future studies must also describe the mechanisms by which inherent genetic susceptibilities interact with environmental factors to produce the phenotypic variations observed in ASD.
Finally, aside from the physiological and social impacts of EDCs on individuals, it is important to realize that the potential for increased risk for ASD as a result of exposure to EDCs has significant economic implications. The disease burden of ASD attributed to EDC exposures in the United States is about 1500 cases per year, with an estimated annual cost of over $1.9 billion in U.S. dollars (Attina et al., 2016). In the European Union, approximately 316 cases of ASD per year are attributable to EDC exposure with an estimated annual cost of $265 million (in U.S. dollars). Because the diseases and conditions (such as ASD) associated with EDC exposures are chronic and associated with life-long disability, ubiquitously dispersed EDCs can potentially affect large numbers of individuals worldwide, and exert a significant impact on both the affected individual and their families as well as on society as a whole.
Highlights.
The rapidly increasing prevalence of autism spectrum disorder (ASD) coupled with incomplete penetrance of the disorder in monozygotic twins has stimulated research on environmental factors contributing to autism.
Human exposures to a broad group of ubiquitously dispersed endocrine disrupting compounds (EDCs), a class of chemicals that can interfere with normal hormonal processes in vivo, are steadily increasing.
Because aberrant levels of sex hormones have been implicated in the male bias in ASD, exposures to EDCs are being studied in relation to association with autistic traits.
Prenatal EDC exposures have been shown to associate with ASD-traits as well as neurobehaviors in both humans and animals, with some animal behaviors mimicking the autistic phenotypes.
Large-scale gene expression studies on animal models and cultured human cells exposed to EDCs reveal genome-wide changes in the transcriptome which, in many cases, reflect the transcriptomic profiles associated with ASD.
The long-lasting and transgenerational effects of some EDCs on complex behaviors suggest a role for epigenetic mechanisms that may contribute to the heritability of ASD.
Acknowledgments
Funding: Preparation of this review and studies reported within from Dr. Hu’s laboratory were in part supported by the National Institute of Environmental Health Sciences [grant number R21 ES023061]
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abu-Elneel K, Liu T, Gazzaniga FS, Nishimura Y, Wall DP, Geschwind DH, Lao KQ, Kosik KS. Heterogeneous dysregulation of microRNAs across the autism spectrum. Neurogenetics. 2008;9:153–161. doi: 10.1007/s10048-008-0133-5. [DOI] [PubMed] [Google Scholar]
- Alléra A, Lo S, King I, Steglich F, Klingmüller D. Impact of androgenic/antiandrogenic compounds (AAC) on human sex steroid metabolizing key enzymes. Toxicology. 2004;205:75–85. doi: 10.1016/j.tox.2004.06.039. [DOI] [PubMed] [Google Scholar]
- Allis CD, Jenuwein T, Reinberg D, editors. Epigenetics. Cold Spring Harbor Press; Cold Spring Harbor, NY: 2006. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. American Psychiatric Publishing; Arlington, VA: 2013. [Google Scholar]
- Ander BP, Barger N, Stamova B, Sharp FR, Schumann CM. Atypical miRNA expression in temporal cortex associated with dysregulation of immune, cell cycle, and other pathways in autism spectrum disorders. Molecular Autism. 2015;6:1–13. doi: 10.1186/s13229-015-0029-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anway MD, Skinner MK. Epigenetic programming of the germ line: effects of endocrine disruptors on the development of transgenerational disease. Reprod Biomed Online. 2008;16:23–25. doi: 10.1016/s1472-6483(10)60553-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attina TM, Hauser R, Sathyanarayana S, Hunt PA, Bourguignon J, Myers JP, DiGangi J, Zoeller RT, Trasande L. Exposure to endocrine-disrupting chemicals in the USA: a population-based disease burden and cost analysis. Lancet Diabetes Endocrinol. 2016;4:996–1003. doi: 10.1016/S2213-8587(16)30275-3. [DOI] [PubMed] [Google Scholar]
- Auyeung B, Baron-Cohen S, Chapman E, Knickmeyer R, Taylor K, Hackett G. Foetal testosterone and the child systemizing quotient. European Journal of Endocrinology. 2006;155:123–130. [Google Scholar]
- Auyeung B, Ahluwalia J, Thomson L, Taylor K, Hackett G, O’Donnell KJ, Baron-Cohen S. Prenatal versus postnatal sex steroid hormone effects on autistic traits in children at 18 to 24 months of age. Molecular Autism. 2012:3. doi: 10.1186/2040-2392-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey A, Le Couteur A, Gottesman I, Bolton P. Autism as a strongly genetic disorder: Evidence from a British twin study. Psychol Med. 1995;25:63–77. doi: 10.1017/s0033291700028099. [DOI] [PubMed] [Google Scholar]
- Bailey J, Oliveri A, Levin ED. Zebrafish model systems for developmental neurobehavioral toxicology. Birth Defects Research Part C- Embryo Today Reviews. 2013;99:14–23. doi: 10.1002/bdrc.21027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Auyeung B, Nørgaard-Pedersen B, Hougaard DM, Abdallah MW, Melgaard L, Cohen AS, Chakrabarti B, Ruta L, Lombardo MV. Elevated fetal steroidogenic activity in autism. Mol Psychiatry. 2014:1–8. doi: 10.1038/mp.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Knickmeyer RC, Belmonte MK. Sex Differences in the Brain: Implications for Explaining Autism. Science. 2005;310:819–823. doi: 10.1126/science.1115455. [DOI] [PubMed] [Google Scholar]
- Berenbaum SA, Beltz AM. How early hormones shape gender development. Curr Opin Behav Sci. 2016;7:53–60. doi: 10.1016/j.cobeha.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berko ER, Suzuki M, Beren F, Lemetre C, Alaimo CM, Calder RB, Ballaban-Gil K, Gounder B, Kampf K, Kirschen J, Maqbool SB, Momin Z, Reynolds DM, Russo N, Shulman L, Stasiek E, Tozour J, Valicenti-McDermott M, Wang S, Abrahams BS. Mosaic Epigenetic Dysregulation of Ectodermal Cells in Autism Spectrum Disorder. PLoS Genetics. 2014;10:e1004402. doi: 10.1371/journal.pgen.1004402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM. Endocrine disrupting compounds, gonadal hormones, and autism. Dev Med Child Neurol. 2012;54:1068. doi: 10.1111/j.1469-8749.2012.04372.x. [DOI] [PubMed] [Google Scholar]
- Braun JM. Early-life exposure to EDCs: Role in childhood obesity and neurodevelopment. Nat Rev Endocrionol. 2017;13:161–173. doi: 10.1038/nrendo.2016.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Gennings C, Hauser R, Webster TF. What can epidemiological studies tell us about the impact of chemical mixtures on human health? Environ Health Perspect. 2016;124:A6–A9. doi: 10.1289/ehp.1510569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kalkbrenner AE, Calafat AM, Yolton K, Ye X, Dietrich KN, Lanphear BP. Impact of early-life bisphenol A exposure on behavior and executive function in children. Pediatrics. 2011;128:873–882. doi: 10.1542/peds.2011-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Muckle G, Arbuckle T, Bouchard MF, Fraser WD, Ouellet E, Seguin JR, Oulhote Y, Webster GM, Lanphear BP. Associations of prenatal urinary bisphenol a concentrations with child behaviors and cognitive abilities. Environ Health Perspect. 2017a;125:067008. doi: 10.1289/EHP984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Yolton K, Stacy SL, Erar B, Papandonatos GD, Bellinger DC, Lanphear BP. Prenatal environmental chemical exposures and longitudinal patterns of child neurobehavior. Neurotoxicology. 2017b;192:192–199. doi: 10.1016/j.neuro.2017.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kalkbrenner AE, Just AC, Yolton K, Calafat AM, Sjdin A, Hauser R, Webster GM, Chen A, Lanphear BP. Gestational Exposure to Endocrine-Disrupting Chemicals and Reciprocal Social, Repetitive, and Stereotypic Behaviors in 4- and 5-Year-Old Children: The HOME Study. Environ Health Perspect. 2014;122:513–520. doi: 10.1289/ehp.1307261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TR, Scherer PA, Chang Y, Migeon CJ, Ghirri P, Murono K, Zhou Z. Molecular genetics of human androgen insensitivity. Eur J Pediatr. 1993;152:S62–S69. doi: 10.1007/BF02125442. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Xiaoyun Yea, Lee-Yang Wong a, Reidy JA, Needham LL. Exposure of the U.S. Population to Bisphenol A and 4-tertiary-Octylphenol: 2003–2004. Environ Health Perspect. 2008;116:39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CJC, Blizard RA. Autism genes are selectively targeted by environmental pollutants including pesticides, heavy metals, bisphenol A, phthalates and many others in food, cosmetics or household products. Neurochem Int. 2016;101:83–109. doi: 10.1016/j.neuint.2016.10.011. [DOI] [PubMed] [Google Scholar]
- Chapman E, Baron-Cohen S, Auyeung B, Knickmeyer R, Taylor K, Hackett G. Fetal testosterone and empathy: evidence from the empathy quotient (EQ) and the “reading the mind in the eyes” test. Soc Neurosci. 2006;1:135–148. doi: 10.1080/17470910600992239. [DOI] [PubMed] [Google Scholar]
- Cheslack-Postava K, Rantakokko PV, Hinkka-Yli-Salomäki S, Surcel H, McKeague IW, Kiviranta HA, Sourander A, Brown AS. Maternal serum persistent organic pollutants in the Finnish Prenatal Study of Autism: A pilot study. Neurotoxicol Teratol. 2013;38:1–5. doi: 10.1016/j.ntt.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevrier J, Gunier RB, Bradman A, Holland NT, Calafat AM, Eskenazi B, Harley KG. Maternal urinary bisphenol a during pregnancy and maternal and neonatal thyroid function in the CHAMACOS study. Environ Health Perspect. 2013;121:138–144. doi: 10.1289/ehp.1205092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen DL, Baio J, Van NB, Bilder D, Charles J, Constantino JN, Daniels J, Durkin MS, Fitzgerald RT, Kurzius-Spencer M, Lee L, Pettygrove S, Robinson C, Schulz E, Wells C, Wingate MS, Zahorodny W, Yeargin-Allsopp M. Prevalence and characteristics of autism spectrum disorder among children aged 8 Years--autism and developmental disabilities monitoring network, 11 Sites, United States, 2012. MMWR Surveill Summ. 2016;65:1–23. doi: 10.15585/mmwr.ss6503a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen J, Grønborg TK, Sørensen MJ, Schendel D, Parner ET, Pedersen LH, Vestergaard M. Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. Obstet Gynecol Surv. 2013;68:613–614. doi: 10.1001/jama.2013.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciernia AV, LaSalle J. The landscape of DNA methylation amid a perfect storm of autism aetiologies. Nat Rev Neurosci. 2016;17:411–423. doi: 10.1038/nrn.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Bendahan CCC, Van De Beek C, Berenbaum SA. Prenatal sex hormone effects on child and adult sex-typed behavior: Methods and findings. Neurosci Biobehav Rev. 2005;29:353–384. doi: 10.1016/j.neubiorev.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Cohen D, Pichard N, Tordjman S, Baumann C, Burglen L, Excoffier E, Lazar G, Mazet P, Pinquier C, Verloes A, Hron D. Specific genetic disorders and autism: clinical contribution towards their identification. J Autism Dev Disord. 2005;35:103–116. doi: 10.1007/s10803-004-1038-2. [DOI] [PubMed] [Google Scholar]
- Colborn T, Carroll LE. Pesticides, sexual development, reproduction, and fertility: current perspective and future direction. Human and Ecological Risk Assessment: An International Journal. 2007;13:1078–1110. [Google Scholar]
- Crews D, Gillette R, Scarpino SV, Manikkam M, Savenkova MI, Skinner MK. Epigenetic transgenerational inheritance of altered stress responses. Proc Natl Acad Sci U S A. 2012;109:9143–9148. doi: 10.1073/pnas.1118514109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Bourguignon J, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr Rev. 2009;30:293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunaway KW, Islam MS, Coulson RL, Lopez SJ, Vogel Ciernia A, Chu RG, Yasui DH, Pessah IN, Lott P, Mordaunt C, Meguro-Horike M, Horike S, Korf I, LaSalle JM. Cumulative impact of polychlorinated biphenyl and large chromosomal duplications on DNA methylation, chromatin, and expression of autism candidate genes. Cell Rep. 2016;17:3035–3048. doi: 10.1016/j.celrep.2016.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Rauch SA, Tenerelli R, Huen K, Holland NT, Lustig RH, Kogut K, Bradman A, Sjödin A, Harley KG. In utero and childhood DDT, DDE, PBDE and PCBs exposure and sex hormones in adolescent boys: The CHAMACOS study. Int J Hyg Environ Health. 2017;220:364–372. doi: 10.1016/j.ijheh.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galani A, Kitsiou-Tzeli S, Sofokleous C, Kanavakis E, Kalpini-Mavrou A. Androgen insensitivity syndrome: Clinical features and molecular defects. Horm. 2008;7:217–229. doi: 10.14310/horm.2002.1201. [DOI] [PubMed] [Google Scholar]
- Ghahramani Seno MM, Hu P, Gwadry FG, Pinto D, Marshall CR, Casallo G, Scherer SW. Gene and miRNA expression profiles in autism spectrum disorders. Brain Res. 2011;1380:85–97. doi: 10.1016/j.brainres.2010.09.046. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Mitra PS, Loffredo CA, Trnovec T, Murinova L, Sovcikova E, Ghimbovschi S, Zang S, Hoffman EP, Dutta SK. Transcriptional profiling and biological pathway analysis of human equivalence PCB exposure in vitro: Indicator of disease and disorder development in humans. Environ Res. 2015;138:202–216. doi: 10.1016/j.envres.2014.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg MR, Rubin RA, Falcone T, Ting AH, Natowicz MR. Brain transcriptional and epigenetic associations with autism. PLOS ONE. 2012;7:e44736. doi: 10.1371/journal.pone.0044736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogolla N, LeBlanc JJ, Quast KB, Sudhof TC, Fagiolini M, Hensch TK. Common circuit defect of excitatory-inhibitory balance in mouse models of autism. Journal of Neurodevelopmental Disorders. 2009;1:172–181. doi: 10.1007/s11689-009-9023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding J, Ellis G, Gregory S, Birmingham K, Iles-Caven Y, Rai D, Pembrey M. Grand-maternal smoking in pregnancy and grandchild’s autistic traits and diagnosed autism. Sci Rep. 2017;7:46179. doi: 10.1038/srep46179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore AC, Martien KM, Gagnidze K, Pfaff D. Implications of prenatal steroid perturbations for neurodevelopment, behavior, and autism. Endocr Rev. 2014;35:961–991. doi: 10.1210/er.2013-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Jensen AA, Wolf JH. Breastfeeding and the weanling’s dilemma. Am J Public Health. 2004;94:1075–1076. doi: 10.2105/ajph.94.7.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, Miller J, Fedele A, Collins J, Smith K, Lotspeich L, Croen LA, Ozonoff S, Lajonchere C, Grether JK, Risch N. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry. 2011;68:1095–1102. doi: 10.1001/archgenpsychiatry.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindel JJ, Balbus J, Birnbaum L, Brune-Drisse MN, Grandjean P, Gray K, Landrigan PJ, Sly PD, Suk W, Slechta DC, Thompson C, Hanson M. Developmental origins of health and disease: Integrating environmental influences. Endocrinology. 2015;156:3416–3421. doi: 10.1210/EN.2015-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks SD, Ignacio C, Gentile K, Middleton FA. Salivary miRNA profiles identify children with autism spectrum disorder, correlate with adaptive behavior, and implicate ASD candidate genes involved in neurodevelopment. BMC Pediatr. 2016;16:1–11. doi: 10.1186/s12887-016-0586-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howdeshell KL. A model of the development of the brain as a construct of the thyroid system. Environ Health Perspect. 2002;110:337–348. doi: 10.1289/ehp.02110s3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu VW, Sarachana T, Kim KS, Nguyen A, Kulkarni S, Steinberg ME, Luu T, Lai Y, Lee NH. Gene expression profiling differentiates autism case-controls and phenotypic variants of autism spectrum disorders: Evidence for circadian rhythm dysfunction in severe autism. Autism Res. 2009;2:78–97. doi: 10.1002/aur.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu VW. Is retinoic acid-related orphan receptor-alpha (RORA) a target for gene-environment interactions contributing to autism? Neurotoxicology. 2012;33:1434–1435. doi: 10.1016/j.neuro.2012.07.009. [DOI] [PubMed] [Google Scholar]
- Hu VW. From Genes to Environment: Using integrative genomics to build a “systems level” understanding of autism spectrum disorders. Child Development. 2013;84:89–103. doi: 10.1111/j.1467-8624.2012.01759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu VW, Sarachana T, Sherrard RM, Kocher KM. Investigation of sex differences in the expression of RORA and its transcriptional targets in the brain as a potential contributor to the sex bias in autism. Molecular Autism. 2015;6:7. doi: 10.1186/2040-2392-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussman JP. Suppressed gabaergic inhibition as a common factor in suspected etiologies of autism. J Autism Dev Disord. 2001;31:247–248. doi: 10.1023/a:1010715619091. [DOI] [PubMed] [Google Scholar]
- Johns LE, Ferguson KK, Soldin OP, Cantonwine DE, Rivera-González LO, Del Toro LVA, Calafat AM, Ye X, Alshawabkeh AN, Cordero JF, Meeker JD. Urinary phthalate metabolites in relation to maternal serum thyroid and sex hormone levels during pregnancy: A longitudinal analysis. Reprod Biol Endocrinol. 2015:13. doi: 10.1186/1477-7827-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajta M, Wjtowicz AK. Impact of endocrine-disrupting chemicals on neural development and the onset of neurological disorders. Pharmacol Rep. 2013;65:1632–1639. doi: 10.1016/s1734-1140(13)71524-x. [DOI] [PubMed] [Google Scholar]
- Kaminsky Z, Wang SC, Petronis A. Complex disease, gender and epigenetics. Ann Med. 2006;38:530–44. doi: 10.1080/07853890600989211. [DOI] [PubMed] [Google Scholar]
- Keil KP, Lein PJ. DNA methylation: a mechanism linking environmental chemical exposures to risk of autism spectrum disorders? Environmental epigenetics. 2016;2:dvv012. doi: 10.1093/eep/dvv012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kichukova TM, Popov NT, Ivanov IS, Vachev TI. Profiling of circulating serum microRNAs in children with autism spectrum disorder using stem-loop qRT-PCR assay. Folia Medica. 2017;59:43–52. doi: 10.1515/folmed-2017-0009. [DOI] [PubMed] [Google Scholar]
- Kinch CD, Ibhazehiebo K, Jeong J, Habibi HR, Kurrasch DM. Low-dose exposure to bisphenol A and replacement bisphenol S induces precocious hypothalamic neurogenesis in embryonic zebrafish. Proc Natl Acad Sci U S A. 2015;112:1475–1480. doi: 10.1073/pnas.1417731112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knickmeyer R, Baron-Cohen S, Fane BA, Wheelwright S, Mathews GA, Conway GS, Brook CGD, Hines M. Androgens and autistic traits: A study of individuals with congenital adrenal hyperplasia. Hormones and Behavior. 2006;50:148–153. doi: 10.1016/j.yhbeh.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Knickmeyer R, Baron-Cohen S, Raggatt P, Taylor K. Foetal testosterone, social relationships, and restricted interests in children. J Child Psychol Psychiatry. 2005;46:198–210. doi: 10.1111/j.1469-7610.2004.00349.x. [DOI] [PubMed] [Google Scholar]
- Kumamoto T, Oshio S. Effect of fetal exposure to bisphenol A on brain mediated by X-chromosome inactivation. J Toxicol Sci. 2013;38:485–494. doi: 10.2131/jts.38.485. [DOI] [PubMed] [Google Scholar]
- Kundakovic M, Gudsnuk K, Franks B, Madrid J, Miller RL, Perera FP, Champagne FA. Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol A exposure. Proc Natl Acad Sci U S A. 2013;110:9956–9961. doi: 10.1073/pnas.1214056110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladd-Acosta C, Hansen KD, Briem E, Fallin MD, Kaufmann WE, Feinberg AP. Common DNA methylation alterations in multiple brain regions in autism. Mol Psychiatry. 2014;19:862–871. doi: 10.1038/mp.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladd-Acosta C, Fallin MD. The role of epigenetics in genetic and environmental epidemiology. Epigenomics. 2016;8:271–283. doi: 10.2217/epi.15.102. [DOI] [PubMed] [Google Scholar]
- LaSalle JM. A genomic point-of-view on environmental factors influencing the human brain methylome. Epigenetics. 2011;6:862–869. doi: 10.4161/epi.6.7.16353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaSalle JM. Epigenomic strategies at the interface of genetic and environmental risk factors for autism. J Hum Genet. 2013;58:396–401. doi: 10.1038/jhg.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D-H, Jacobs DR. Methodological issues in human studies of endocrine disrupting chemicals. Rev Endocr Metab Disord. 2015;16:289–297. doi: 10.1007/s11154-016-9340-9. [DOI] [PubMed] [Google Scholar]
- Lichtensteiger W, Bassetti-Gaille C, Faass O, Axelstad M, Boberg J, Christiansen S, Rehrauer H, Georgijevic JKh, Hass U, Kortenkamp A, Schlumpf M. Differential gene expression patterns in developing sexually dimorphic rat brain regions exposed to antiandrogenic, estrogenic, or complex endocrine disruptor mixtures: glutamatergic synapses as target. Endocrinology. 2015;156:1477–1493. doi: 10.1210/en.2014-1504. [DOI] [PubMed] [Google Scholar]
- Liew Z, Ritz B, von Ehrenstein OS, Bech BH, Nohr EA, Fei C, Bossi R, Henriksen TB, Bonefeld-Jørgensen EC, Olsen J. Attention deficit/hyperactivity disorder and childhood autism in association with prenatal exposure to perfluoroalkyl substances: A nested case–control study in the Danish National Birth Cohort. Environ Health Perspect. 2015;123:367–373. doi: 10.1289/ehp.1408412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomniczi A, Loche A, Castellano JM, Ronnekleiv OK, Bosch M, Kaidar G, Knoll JG, Wright H, Pfeifer GP, Ojeda SR. Epigenetic control of female puberty. Nat Neurosci. 2013;16:281–289. doi: 10.1038/nn.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutchmaya S, Baron-Cohen S, Raggatt P. Foetal testosterone and eye contact in 12-month-old human infants. Infant Behavior & Development. 2002;25:327–335. [Google Scholar]
- Lutchmaya S, Baron-Cohen S, Raggatt P. Foetal testosterone and vocabulary size in 18- and 24-month-old infants. Infant Behavior and Development. 2001;24:418–424. [Google Scholar]
- Lyall K, Croen LA, Sjodin A, Yoshida CK, Zerbo O, Kharrazi M, Windham GC. Polychlorinated biphenyl and organochlorine pesticide concentrations in maternal mid-pregnancy serum samples: Association with autism spectrum disorder and intellectual disability. Environ Health Perspect. 2016;125:474–480. doi: 10.1289/EHP277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBirney M, King SE, Pappalardo M, Houser E, Unkefer M, Nilsson E, Sadler-Riggleman I, Beck D, Winchester P, Skinner MK. Atrazine induced epigenetic transgenerational inheritance of disease, lean phenotype and sperm epimutation pathology biomarkers. PLoS ONE. 2017;12:e0184306. doi: 10.1371/journal.pone.0184306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Nugent BM. Epigenetic contributions to hormonally-mediated sexual differentiation of the brain. J Neuroendocrinol. 2013;25:1133–1140. doi: 10.1111/jne.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM. Multifaceted origins of sex differences in the brain. Philos Trans R Soc Lond B Biol Sci. 2016;371:20150106. doi: 10.1098/rstb.2015.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Lieberburg I, Chaptal C, Krey LC. Aromatization: important for sexual differentiation of the neonatal rat brain. Horm Behav. 1977;9:249–263. doi: 10.1016/0018-506x(77)90060-5. [DOI] [PubMed] [Google Scholar]
- Miller MD, Crofton KM, Rice DC, Zoeller RT. Thyroid-disrupting chemicals: Interpreting upstream biomarkers of adverse outcomes. Environ Health Perspect. 2009;117:1033–1041. doi: 10.1289/ehp.0800247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell MM, Woods R, Chi L-H, Schmidt RJ, Pessah IN, Kostyniak PJ, LaSalle JM. Levels of select PCB and PBDE congeners in human postmortem brain reveal possible environmental involvement in 15q11-q13 duplication autism spectrum disorder. Environ Mol Mutagen. 2012;53:589–598. doi: 10.1002/em.21722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundalil Vasu M, Anitha A, Thanseem I, Suzuki K, Yamada K, Takahashi T, Wakuda T, Iwata K, Tsujii M, Sugiyama T, Mori N. Serum microRNA profiles in children with autism. Molecular Autism. 2014;5:40–49. doi: 10.1186/2040-2392-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen A, Rauch TA, Pfeifer GP, Hu VW. Global methylation profiling of lymphoblastoid cell lines reveals epigenetic contributions to autism spectrum disorders and a novel autism candidate gene, RORA, whose protein product is reduced in autistic brain. FASEB Journal. 2010;24:3036–3051. doi: 10.1096/fj.10-154484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent BM, Wright CL, Shetty AC, Hodes GE, Lenz KM, Mahurkar A, Russo SJ, Devine SE, McCarthy MM. Brain feminization requires active repression of masculinization via DNA methylation. Nat Neurosci. 2015;18:690–697. doi: 10.1038/nn.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottinger MA, Lavoie E, Thompson N, Barton A, Whitehouse K, Barton M, Abdelnabi M, Quinn M, Panzica G, Jr, Viglietti-Panzica C. Neuroendocrine and behavioral effects of embryonic exposure to endocrine disrupting chemicals in birds. Brain Res Rev. 2008;57:376–385. doi: 10.1016/j.brainresrev.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Palomba S, Marotta R, Di Cello A, Russo T, Falbo A, Orio F, Tolino A, Zullo F, Esposito R, La Sala GB. Pervasive developmental disorders in children of hyperandrogenic women with polycystic ovary syndrome: a longitudinal case-control study. Clin Endocrinol (Oxf) 2012;77:898–904. doi: 10.1111/j.1365-2265.2012.04443.x. [DOI] [PubMed] [Google Scholar]
- Park BY, Lee BK, Burstyn I, Tabb LP, Keelan JA, Whitehouse AJO, Croen LA, Fallin MD, Hertz-Picciotto I, Montgomery O, Newschaffer CJ. Umbilical cord blood androgen levels and ASD-related phenotypes at 12 and 36 months in an enriched risk cohort study. Molecular Autism. 2017;8:1–12. doi: 10.1186/s13229-017-0118-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patisaul HB, Adewale HB. Long-term effects of environmental endocrine disruptors on reproductive physiology and behavior. Front Behav Neurosci. 2009;3:1–18. doi: 10.3389/neuro.08.010.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson BL, Simon JM, McCoy ES, Salazar G, Fragola G, Zylka MJ. Identification of chemicals that mimic transcriptional changes associated with autism, brain aging and neurodegeneration. Nat Commun. 2016;7:11173. doi: 10.1038/ncomms11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippat C, Bennett DH, Krakowiak P, Rose M, Hwang Hyun-Min, Hertz-Picciotto I. Phthalate concentrations in house dust in relation to autism spectrum disorder and developmental delay in the CHildhood Autism Risks from Genetics and the Environment (CHARGE) study. Environmental Health: A Global Access Science Source. 2015a;14:1–10. doi: 10.1186/s12940-015-0024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippat C, Bennett D, Calafat AM, Picciotto IH. Exposure to select phthalates and phenols through use of personal care products among Californian adults and their children. Environ Res. 2015b;140:369–376. doi: 10.1016/j.envres.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Preciados M, Yoo C, Roy D. Estrogenic Endocrine Disrupting Chemicals Influencing NRF1 Regulated Gene Networks in the Development of Complex Human Brain Diseases. International Journal of Molecular Sciences. 2016;17:1–62. doi: 10.3390/ijms17122086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebuli ME, Patisaul HB. Assessment of sex specific endocrine disrupting effects in the prenatal and pre-pubertal rodent brain. Journal of Steroid Biochemistry & Molecular Biology. 2016;160:148–159. doi: 10.1016/j.jsbmb.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts EM, English PB, Grether JK, Windham GC, Somberg L, Wolff C. Maternal residence near agricultural pesticide applications and autism spectrum disorders among children in the California central valley. Environ Health Perspect. 2007;115:1482–1489. doi: 10.1289/ehp.10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano ME, Webster GM, Vuong AM, Thomas Zoeller R, Chen A, Hoofnagle AN, Calafat AM, Karagas MR, Yolton K, Lanphear BP, Braun JM. Gestational urinary bisphenol A and maternal and newborn thyroid hormone concentrations: The HOME Study. Environ Res. 2015;138:453–460. doi: 10.1016/j.envres.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JLR, Merzenich MM. Model of autism: Increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv SK, Kogut K, Gaspar FW, Gunier RB, Harley KG, Parra K, Villaseñor D, Bradman A, Holland N, Eskenazi B. Prenatal and childhood polybrominated diphenyl ether (PBDE) exposure and attention and executive function at 9–12years of age. Neurotoxicol Teratol. 2015;52:151–161. doi: 10.1016/j.ntt.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarachana T, Hu VW. Genome-wide identification of transcriptional targets of RORA reveals direct regulation of multiple genes associated with autism spectrum disorder. Molecular Autism. 2013;4:1–19. doi: 10.1186/2040-2392-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarachana T, Xu M, Wu RC, Hu VW. Sex hormones in autism: androgens and estrogens differentially and reciprocally regulate RORA, a novel candidate gene for autism. PLoS ONE. 2011;6:e17116. doi: 10.1371/journal.pone.0017116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarachana T, Zhou R, Chen G, Manji HK, Hu VW. Investigation of post-transcriptional gene regulatory networks associated with autism spectrum disorders by microRNA expression profiling of lymphoblastoid cell lines. Genome Medicine. 2010;2:1–18. doi: 10.1186/gm144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schug TT, Blawas AM, Gray K, Heindel JJ, Lawler CP. Elucidating the links between endocrine disruptors and neurodevelopment. Endocrinology. 2015;156:1941–1951. doi: 10.1210/en.2014-1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schug TT, Johnson AF, Birnbaum LS, Colborn T, Guillette LJ, Crews DP, Jr, Collins T, Soto AM, Vom Saal FS, McLachlan JA, Sonnenschein C, Heindel JJ. Minireview: Endocrine Disruptors: Past Lessons and Future Directions. Mol Endocrinol. 2016;30:833–847. doi: 10.1210/me.2016-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann CM, Sharp FR, Ander BP, Stamova B. Possible sexually dimorphic role of miRNA and other sncRNA in ASD brain. Molecular Autism. 2017;8:1–10. doi: 10.1186/s13229-017-0117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulha HP, Cheung I, Whittle C, Wang J, Virgil D, Lin CL, Guo Y, Lessard A, Akbarian S, Weng Z. Epigenetic signatures of autism: Trimethylated H3K4 landscapes in prefrontal neurons. Arch Gen Psychiatry. 2012;69:314–324. doi: 10.1001/archgenpsychiatry.2011.151. [DOI] [PubMed] [Google Scholar]
- Singh S, Yazdani U, Gadad B, Zaman S, Hynan LS, Roatch N, Schutte C, Marti CN, Hewitson L, German DC. Serum thyroid-stimulating hormone and interleukin-8 levels in boys with autism spectrum disorder. J Neuroinflammation. 2017;14:1–7. doi: 10.1186/s12974-017-0888-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu MT, Weksberg R. Epigenetics of Autism Spectrum Disorder. In: Delgado-Morales R, editor. Neuroepigenomics in Aging and Disease. Springer International Publishing; Cham: 2017. pp. 63–90. [DOI] [PubMed] [Google Scholar]
- Skinner MK. Role of epigenetics in developmental biology and transgenerational inheritance. Birth Defects Research Part C: Embryo Today: Reviews. 2011;93:51–55. doi: 10.1002/bdrc.20199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner MK, Manikkam M, Guerrero-Bosagna C. Epigenetic transgenerational actions of endocrine disruptors. Reproductive Toxicology. 2011;31:337–343. doi: 10.1016/j.reprotox.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner MK, Savenkova MI, Zhang B, Gore AC, Crews D. Gene bionetworks involved in the epigenetic transgenerational inheritance of altered mate preference: environmental epigenetics and evolutionary biology. BMC Genomics. 2014;15:1–38. doi: 10.1186/1471-2164-15-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EK, White MC, Weir HK, Peipins LA, Thompson TD. Higher incidence of clear cell adenocarcinoma of the cervix and vagina among women born between 1947 and 1971 in the United States. Cancer Causes Control. 2012;23:207–211. doi: 10.1007/s10552-011-9855-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobolewski M, Conrad K, Allen JL, Weston H, Martin K, Lawrence BP, Cory-Slechta DA. Sex-specific enhanced behavioral toxicity induced by maternal exposure to a mixture of low dose endocrine-disrupting chemicals. Neurotoxicology. 2014;45:121–130. doi: 10.1016/j.neuro.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Poschmann J, Cruz-Herrera DR, Parikshak NN, Hajan HS, Kumar V, Ramasamy R, Belgard TG, Elanggovan B, Wong CCY, Mill J, Geschwind DH, Prabhakar S. Histone Acetylome-wide Association Study of Autism Spectrum Disorder. Cell. 2016;167:1385–1397. doi: 10.1016/j.cell.2016.10.031. [DOI] [PubMed] [Google Scholar]
- Talebizadeh Z, Butler MG, Theodoro MF. Feasibility and Relevance of Examining Lymphoblastoid Cell Lines to Study Role of microRNAs in Autism. Autism Research. 2008;1:240–250. doi: 10.1002/aur.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tareen RS, Kamboj MK. Role of Endocrine Factors in Autistic Spectrum Disorders. The Pediatric Clinics of North America. 2012;59:75–88. doi: 10.1016/j.pcl.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Tordjman S, Somogyi e, Coulon N, Kermarrec S, Cohen D, Bronsard G, eWeismann-Arcache Catherine, Botbol M, Lauth B, Barburoth M, Guinchat V, Roubertoux P, Kovess V, eGeoffray Marie-Maude, Xavier J. Gene X environment interactions in autism spectrum disorders: role of epigenetic mechanisms. Frontiers in Psychiatry. 2014;5:1–17. doi: 10.3389/fpsyt.2014.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs David R, Jr , Lee D, Shioda T, Soto AM, vom Saal FS, Welshons WV, Zoeller RT, Myers JP. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev. 2012;33:378–455. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrijheid M, Casas M, Gascon M, Valvi D, Nieuwenhuijsen M. Environmental pollutants and child health-A review of recent concerns. Int J Hyg Environ Health. 2016;219:331–342. doi: 10.1016/j.ijheh.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Wallen KK. Hormonal influences on sexually differentiated behavior in nonhuman primates. Front Neuroendocrinol. 2005;26:7–26. doi: 10.1016/j.yfrne.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Whitehead SA, Rice S. Endocrine-disrupting chemicals as modulators of sex steroid synthesis. Best Pract Res Clin Endocrinol Metab. 2006;20:45–61. doi: 10.1016/j.beem.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Winchester PD, Huskins J, Ying J. Agrichemicals in surface water and birth defects in the United States. Acta Paediatr. 2009;98:664–669. doi: 10.1111/j.1651-2227.2008.01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windham GC, Lixia Zhang a, Robert Gunier a, Croen LA, Grether JK. Autism spectrum disorders in relation to distribution of hazardous air pollutants in the san francisco bay area. Environ Health Perspect. 2006;114:1438–1444. doi: 10.1289/ehp.9120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme JT, Edwards M, Shetty S, Gatewood JD, Taylor JA, Rissman EF, Connelly JJ. Gestational exposure to bisphenol A produces transgenerational changes in behaviors and gene expression. Endocrinology. 2012;153:3828–3838. doi: 10.1210/en.2012-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme JJ, Goldsby JA, Rissman EF. Transgenerational effects of prenatal bisphenol A on social recognition. Horm Behav. 2013;64:833–839. doi: 10.1016/j.yhbeh.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CCY, Meaburn EL, Ronald A, Price TS, Jeffries AR, Schalkwyk LC, Plomin R, Mill J. Methylomic analysis of monozygotic twins discordant for autism spectrum disorder and related behavioural traits. Mol Psychiatry. 2014;19:495–503. doi: 10.1038/mp.2013.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YE, Parikshak NN, Belgard TG, Geschwind DH. Genome-wide, integrative analysis implicates microRNA dysregulation in autism spectrum disorder. Nat Neurosci. 2016;19:1463–1476. doi: 10.1038/nn.4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoeller RT, Rovet J. Timing of thyroid hormone action in the developing brain: Clinical observations and experimental findings. J Neuroendocrinol. 2004;16:809–818. doi: 10.1111/j.1365-2826.2004.01243.x. [DOI] [PubMed] [Google Scholar]