Abstract
In typical development, walk onset is accompanied by increased language growth (e.g. Walle & Campos, 2013). The present study explored whether this relation may be disrupted in the infant siblings of children with autism spectrum disorder (ASD; Heightened Risk of receiving an ASD diagnosis; HR), a population exhibiting substantial variability in motor and language development (e.g. Gamliel, Yirmiya & Sigman, 2007; Iverson & Wozniak, 2007). Receptive and expressive language were examined across the transition to walking in three groups of HR infants (no diagnosis, language delay, and ASD; N=91, 8–18 months) and in infants with no family history of ASD (N=25; 9–15 months). Only infants with an eventual ASD diagnosis did not show increased language growth following walk onset.
Keywords: language development, motor development, autism spectrum disorder, infant siblings
The onset of walking appears to be a point of inflection for social and communicative development in typically developing infants (e.g. Karasik, Tamis-Lemonda & Adolph, 2011; Clearfield, 2011). In particular, walk onset is accompanied by an increase in receptive and expressive language, independent of infants’ chronological age (Walle & Campos, 2013; He, Walle & Campos, 2015; Walle & Warlaumont, 2015). These findings support an embodied perspective of early language acquisition, which suggests that the ability to walk affords new opportunities and experiences that may, in turn, bolster infants’ language development. While this phenomenon appears to be robust in typical development, evidence is emerging to suggest that the relation between locomotor status and communicative development may be disrupted in children with autism spectrum disorder (ASD; Srinivasan & Bhat, 2016; Bedford, Pickles & Lord, 2015; Kim, 2008).
A practical constraint to studying walk onset in ASD is that it occurs before the age at which ASD is typically diagnosed. Although reliable diagnosis is possible at 24 months (e.g. Zwaigenbaum et al., 2015), the average age of diagnosis is 40 months (Christensen et al., 2016). To circumvent this constraint, research has focused on the infant siblings of children with ASD, who are at heightened risk (Heightened risk; HR) of developing ASD themselves (18.7% recurrence rate; e.g. Ozonoff et al., 2011). Therefore, prospectively studying HR infants allows identification of early-occurring differences between infants who go on to receive an ASD diagnosis and those who do not. The present study employed this method to examine longitudinal changes in receptive and expressive language across the transition from crawling to walking in four groups of infants: HR infants subsequently diagnosed with ASD (HR-ASD), HR infants with significant language delays (HR-LD), HR infants with no diagnosis (HR-ND), and a comparison group of infants with no family history of ASD (Low Risk; LR).
Walking and Language in Typical Development
Learning to walk is a highly anticipated and striking achievement of infancy, likely because it corresponds to a substantial shift in infants’ interactions with the environment and social partners (e.g. Adolph & Tamis-LeMonda, 2014; Karasik, Adolph & Tamis-LeMonda, 2011). Notably, linkages between walking and growth in both receptive and expressive language have been observed (Oudgenoeg-Paz et al., 2012; Walle & Campos, 2014; He, Walle & Campos, 2015; Walle & Warlaumont, 2015). This phenomenon was first discovered by happenstance by Walle and Campos (2011), who noticed while conducting an unrelated study of infants’ affect understanding that walking infants had significantly larger vocabularies than their age-matched peers who were not yet walking. Subsequently, they conducted an in-depth investigation of this relation by measuring infants’ language growth longitudinally during the transition from crawling to walking (Walle & Campos, 2014). Results revealed that infants’ walking experience significantly predicted both receptive and expressive language growth, and that this growth was above and beyond that accounted for by infants’ chronological age. Furthermore, while chronological age predicted linear growth in language, infants’ walking experience predicted a non-linear trend, with a period of significant acceleration immediately following the onset of walking. Additional studies have replicated these findings, demonstrating that this pattern is stable and is even evident across cultures (Oudgenoeg-Paz et al., 2012; He, Walle & Campos, 2015; Walle & Warlaumont, 2015).
Although the precise mechanism underlying this relation is not yet clear, it is likely that walking acts as an epigenetic phenomenon—a catalyst that drives change across many areas of development (Walle & Campos, 2014). In other words, although the act of walking in and of itself does not directly affect language development, it gives rise to a multitude of changes in infants’ visual input, object interactions, and social interactions, and these in turn are likely to facilitate language acquisition (e.g. Yu & Smith, 2012; Tomasello & Farrar, 1986).
For example, relative to their crawling peers, walking infants are able to locomote farther, more efficiently, and travel with free hands (Adolph, 2008; Adolph et al., 2012; Adolph & Tamis-LeMonda, 2014). Additionally, walking provides a more expansive view of surroundings, allowing infants to view objects and social partners as they locomote (Kretch, Franchak & Adolph, 2014). These changes facilitate visual detection of and access to distal objects, broadening the range of potential communicative referents. Walking infants also no longer rely on their hands for postural support while locomoting and so are better able to carry objects around the environment. Together, these changes afford infants a more active role in selecting objects of interest for object manipulation and social interactions. This may be meaningful for word learning given evidence that infants are more likely to learn labels for objects that are perceptually salient to them (e.g. Pruden, Hirsh-Pasek, Golinkoff & Hennon, 2006; Smith, Jones & Landau, 1996).
Additionally, research suggests that following walk onset, infants perform more frequent communicative gestures with objects (e.g., giving an object to a social partner, or pointing to an object of interest; Clearfield, 2011). Further, these bids are more likely to be “moving” bids—meaning the infant carried the object to share it with a social partner—rather than stationary bids (Karasik, Tamis-Lemonda & Adolph, 2011). This is notable because moving bids may be more salient to caregivers, and therefore more likely to receive a contingent response (see Iverson, 2010, for a discussion). This increase in communicative bids, coupled with infants expanded access to a broader range of objects, is likely to facilitate more frequent bouts of joint attention—i.e. the coordinated attention of infant and adult on an object—given that caregivers frequently provide contingent responses to their infants’ social bids by labeling the referent (e.g. Golinkoff, 1986; Masur, 1982). Classic work on early language acquisition has found that these moments of joint attention are optimal for word learning and are related to language development (e.g. Tomasello & Farrar, 1986; Tomasello, 1988). Thus, based on the available research, it seems likely that the confluence of many co-occurring developmental shifts brought about by walking may underlie the observed growth in language in typical development.
Language Development in ASD
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by deficits in communication and social interactions, as well as the presence of restricted and repetitive behaviors (American Psychiatric Association, 2013). Given that difficulty with communication is a defining feature of ASD, research on HR infants has focused heavily on early language development. This line of study has primarily assessed language development using two instruments: the Mullen Scales of Early Learning, an experimenter-administered assessment that includes subscales for Receptive Language and Expressive Language (MSEL; Mullen, 1995); and the MacArthur-Bates Communicative Development Inventory (CDI; Fenson et al., 1993), a parent report measure assessing infants’ Words Understood (receptive language) and Words Produced (expressive language). Across methodologies, studies have consistently found that between 12–18 months, HR-ASD infants scored significantly lower than HR infants who did not go on to receive an ASD diagnosis (Ozonoff et al., 2010; Estes et al., 2015; Mitchell et al., 2006; Zwaigenbaum et al., 2005; Landa & Garrett-Mayer, 2006). Therefore, there is ample and robust evidence that language delays are characteristic of infant development in ASD.
It is worth noting here that only one of these studies separately examined the subgroup of HR infants with significant language delays but no ASD (Landa & Garrett-Mayer, 2006) despite the fact that HR infants who do not receive an ASD diagnosis also have an elevated prevalence of language and communication delays relative to LR infants (Gamliel, Yirmiya, & Sigman, 2007; Sullivan et al., 2007; Messinger et al., 2013). In this study, no differences between HR-LD and HR-ASD infants were apparent in the first two years. However, by 24 months the HR-LD group had a larger receptive—but not expressive—language vocabulary than their HR-ASD peers (Landa & Garrett-Mayer, 2006). These findings suggest that although both HR-ASD and HR-LD infants demonstrate language delays relative to typically developing infants, their patterns of development may be distinct. Therefore, the inclusion of an HR-LD group is important for two reasons. First, understanding early language development in this subset of infants is informative in its own right because it allows us to probe alternative pathways of language acquisition and how they may go awry. Second, including a HR-LD group allows us to determine whether differences observed in HR-ASD infants are specific to ASD, or whether they are representative of more general communicative delay. To this end, an objective of the present study was to test the possibility that although HR-ASD and HR-LD infants both show delays in language acquisition, the patterns that characterize their development may differ.
Walking and Language Development in ASD
To date, no study of HR infants has directly examined language development during the transition from crawling to walking. However, there is some evidence suggesting that the relation between walking experience and communicative development may differ in ASD. One recent study of HR and LR infants examined object sharing behavior (i.e. carrying objects to give or show to a social partner) longitudinally during the transition to walking (Srinivasan & Bhat, 2016). Consistent with previous reports, the LR group demonstrated a sharp increase in object sharing following walk onset. Although the HR infants did show an increasing trend, it was significantly attenuated relative to LR infants. A potential explanation for this finding could be that for the subset of HR infants with ASD, walk onset may not result in a shift in social bids, as is the case in typical development (Karasik, Tamis-Lemonda & Adolph, 2011; Clearfield, 2011). However, this study had a limited sample size and did not include diagnostic outcome data, so it is not clear whether or to what extent this difference may be representative of ASD.
Findings regarding linkages between walking and language in children with ASD are somewhat mixed. Kim (2008) found no relation between parents’ retrospective reports of walk onset and later language in preschool-aged children with ASD. However, a more recent study examined whether parents’ retrospective reports of age at walk onset were related to receptive and expressive language growth longitudinally from 2 to 9 years in a sample of children with ASD (Bedford, Pickles & Lord, 2015). Although findings initially indicated that age at walk onset related both to receptive and expressive language growth, this relation was no longer significant when overall gross motor ability was statistically controlled. In other words, while language growth was related to gross motor ability in general, it was not uniquely predicted by age at walk onset. While these studies provide valuable information regarding general relations between walking and language development, they leave open the question of how language changes during the transition from crawling to walking in ASD.
The Present Study
The present study set out to address this question by examining receptive and expressive language growth longitudinally across a 7-month timeframe, during which infants transitioned from crawling to walking, in 3 groups of HR infants (HR-ND, HR-LD, HR-ASD) and in a comparison group of LR infants. In light of the previously reviewed work demonstrating that language differences are characteristic of ASD in the infant years, and newly emerging work suggesting that the relation between walking and communicative development may differ in ASD, we examined the possibility that HR-ASD infants may show a different pattern of language growth during this transition period. To our knowledge, this is the first study to examine changes in language development at the time of walk onset in HR infants. An understanding of how these processes unfold in ASD is critically important; walk onset represents a period of rapid change in typical development, wherein advances set the stage for future gains, while delays may constrain future language development. Thus, the time of walk onset may represent a point in development when HR-ASD infants begin to lose ground on their peers.
Methods
Participants
Two cohorts of infants participated in this research. The first included 91 HR infants (48 male) who had a full biological sibling with a confirmed diagnosis of Autistic Disorder (AD; DSM-IV-TR; APA, 2000) verified prior to enrollment in the study using DSM-IV-TR criteria and scores above the Autism threshold on the Autism Diagnostic Observation Schedule (ADOS-G; Lord et al., 2000). This cohort was visited at home monthly from 5–14 months of age, and was seen for follow-up visits at 18, 24, and 36 months (i.e. a total of 17 visits were conducted for each HR infant). The second was a comparison group of 25 infants (10 male) with no family history of ASD (i.e. no first or second degree relatives with an ASD; Low Risk; LR) who were seen in their homes biweekly from 2–19 months (i.e. 34 visits were conducted for each LR infant). For both cohorts of infants, visits were conducted by one primary experimenter and several research assistants and lasted approximately 45 minutes to an hour. The present study focused on a window of time defined by the monthly visits that corresponded to infants’ walking experience, regardless of age. This window began with the visit that occurred 4 months prior to infants’ walk onset and ended with the visit that occurred 3 months after walk onset (i.e. 7 data points were examined for each participant). It is important to note that although LR infants were seen at biweekly intervals, only visits made during whole months were included in the present study in order to match the observation schedule of HR infants. For example, if a LR infant’s walk onset occurred at 9.5 months, 10 months was assigned as walk onset. This ensured that any observed differences were not an artifact of a more precise measurement in the LR group. All data were collected between 2002 and 2014 as part of two larger longitudinal studies.
Infants from both cohorts were from English-speaking households and were from uncomplicated pregnancies. One hundred and five infants (81 HR, 24 LR) were Caucasian, 10 (all HR) were Hispanic, and one LR infant was Asian American. Parents in both groups had comparable levels of education, with a majority of parents either holding college degrees or having completed some college. Although family income information was unavailable, Nakao-Treas occupational prestige scores (Nakao & Treas, 1994) were calculated for fathers’ occupation in order to provide an index of social class. HR infants had older mothers, F(1,101)=4.57, p=.035, and fathers, F(1,101)=8.61, p=.004, compared with LR infants. There were no other significant differences between groups. Table 1 displays all demographic information for HR and LR participants in the study.
Table 1.
Demographic Information for Heightened Risk (HR) and Low Risk (LR) groups
| HR (n = 91) | LR (n = 25) | |||
|---|---|---|---|---|
|
|
|
|||
| Gender | ||||
| Female (%) | 43 | (47%) | 10 | (40%) |
| Male (%) | 48 | (53%) | 15 | (60%) |
| Racial or ethnic minority (%) | 10 | (11%) | 1 | (4%) |
| Mean age for Mothers (SD) | 34.19 | (4.01) | 31.92 | (4.85) |
| Mean age for Fathers (SD) | 36.68 | (5.10) | 33.08 | (4.00) |
| Mean Parent Educationa (SD) | 1.22 | (0.50) | 1.38 | (0.50) |
| Mean Paternal Occupational Prestige b (SD) | 53.08 | (18.90) | 48.18 | (22.82) |
Parent education based on averaging education scores for mothers and fathers. 0 = High school, 1 = Some college or college degree; 2 = Graduate or professional school
Nakao–Treas occupational prestige score. Unable to calculate for 4 LR and 5 HR fathers.
Measures
Walk onset
As part of the larger longitudinal studies, parents tracked in a calendar when their infant met the criterion for walk onset (i.e., took 3 consecutive, alternating, and independent steps with no support from furniture or caregivers). Although the onset of walking could have occurred between infants’ monthly anniversaries, we will refer to the first whole-month visit where infants met this criterion as “walk onset”. If the infant did not attain walk onset by the conclusion of the monthly visits, an experimenter called the caregiver at each following month and parent report of this criterion was used to establish infants’ walk onset.
MacArthur-Bates Communicative Development Inventory
Our measure of language development was the MacArthur-Bates Communicative Development Inventory: Words and Gestures form (CDI; Fenson et al, 1993) which was administered at each visit. The CDI is a parent report measure that has been shown to be a valid and reliable measure of infants’ language development. Importantly, it has also been shown to be sensitive to language delay or specific language impairments in many samples, including ASD, Down syndrome, and otherwise typically developing infants (Luyster, Qui, Lopez, & Lord, 2007; Mitchell et al., 2006; Dale, Bates, Reznick, & Morisset, 1989; Fenson et al., 1993, 1994; Heilmann, Weismer, Evans & Hollar, 2005; Miller, Sedey, & Miolo, 1995; Thal, O’Hanlon, Clemmons, & Fralin, 1999). The present study focuses on part 1 of the CDI, which consists of a 396-item vocabulary checklist in which parents are asked to check off items which their infant: a) understands only; or b) both says and understands. The Words Understood variable was calculated by adding together all of the words for which either option was selected in order to measure the infants’ receptive language. The Words Produced variable summed only items marked as both says and understands to measure infants’ expressive language.
Outcome classification
At each follow-up visit (18, 24, and 36 months), parents of HR infants completed the CDI, and HR infants were administered the Mullen Scales of Early Learning (MSEL; Mullen, 1995). At the 36-month visit, HR infants were evaluated and administered the ADOS-G by a research-reliable clinician who was naïve to all previous study data. Infants received a diagnosis of ASD if both of the following criteria were met: an ADOS score that met or exceeded revised algorithm cutoffs for ASD or AD and a clinical best estimate diagnosis of AD or PDD-NOS using DSM-IV-TR criteria (diagnostic evaluations occurred prior to the release of the DSM-V in 2013). Fifteen HR infants (4 female) were diagnosed with ASD (HR-ASD).
HR infants were identified as language delayed (LD) if they did not receive an ASD diagnosis and either of the following criteria were met (Parlade & Iverson, 2015):
Standardized scores on the CDI-II and or CDI-III at or below the 10th percentile at more than one time point between 18 and 36 month.
Standardized scores on the CDI-III at or below the 10th percentile and standardized scores on the Receptive and or Expressive subscales of the MSEL equal to or greater than 1.5 standard deviations below the mean at 36 months.
These criteria have been used previously to identify language delay in both HR and community samples (e.g., Gershkoff-Stowe, Thal, Smith, & Namy, 1997; Robertson & Weismer, 1999; Weismer & Evans, 2002; Heilmann, Weismer, Evans, & Hollar, 2005; Ozonoff et al., 2010; Parlade & Iverson, 2015). Using these criteria, 26 infants were classified as having Language Delay (HR-LD; 11 female). It should be noted that the designation of HR-LD here is intended to identify infants with a pattern of delayed language development—not a clinically diagnosed language disorder.
Fifty remaining HR infants were classified as No Diagnosis (HR-ND; 28 female). Although the cohort of LR infants did not receive follow-ups at 24 and 36 months, there was no indication of atypical development or parent concerns reported at the 19-month visits.
Data Analytic Approach
The objective of the present study was to examine changes in both receptive and expressive language longitudinally across the transition from crawling to walking in LR infants and in three groups of HR infants: HR-ND, HR-LD, and HR-ASD. To this end, two piecewise Hierarchical Linear Models (HLM) were utilized: one in which receptive language was the dependent variable, and the other with expressive language was the dependent variable. Models were identical with the exception of the dependent variable. HLM is appropriate for these data because it allows us to estimate changes in language at two levels: the first level assesses variation in language scores across time-points, nested within infants (level 1); the second level assesses variation between infants, comparing across outcome classifications (level 2). Additionally, HLM can accommodate unequally-spaced or missing data at level 1, allowing us to include data from infants with incomplete observations (e.g. Huttenlocher et al., 1991; Singer, 1998). Complete data were available for 143 of the 175 observations (81.7%) for LR infants, and 458 of the 637 observations (80.8%) for HR infants.
Fifteen HR infants (6 ND, 4 LD, 5 ASD) did not achieve walk onset until after 14 months—the completion of the HR monthly visits—and so these infants only contributed pre-walking data to the analyses (e.g. if a parent reported infants’ walk onset at 15 months via a monthly phone questionnaire, the infant contributed data for his or her pre-walking time points accordingly, but had missing data for walk onset and beyond). Because this missing data did not occur at random, additional models were estimated with these 15 infants removed. Significant findings were unchanged by excluding these infants, and so results reported include data from the full sample.
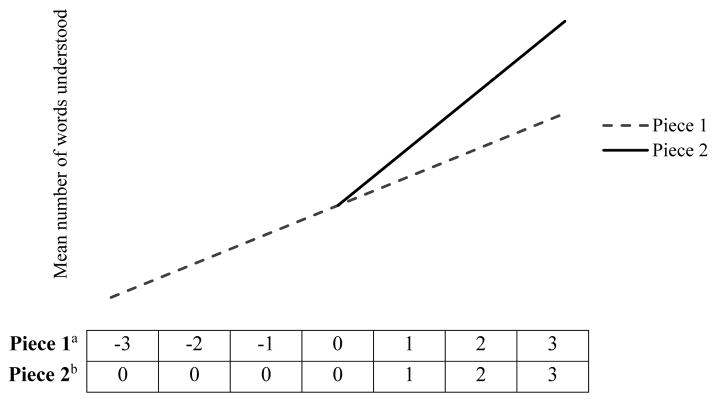
A piecewise model was chosen because it allowed us to estimate and compare growth over time as two pieces, rather than as a single continuous variable. The first piece estimated baseline growth in language (piece 1; all time points), and the second piece estimated additional incremental linear growth from the visit prior to walk onset to the final observation (piece 2; See Figure 1 for further illustration of how these pieces were coded). Therefore, if piece 2 is significantly greater than zero, this indicates a significant increase in linear growth following walk onset, and we can conclude that walk onset is in fact a point of inflection for language growth.
Figure 1.
Schematic illustration of the Piece 1 and Piece 2 time variables
aThe top row illustrates how Piece 1 was coded. Each cell represents walking experience measured in months, where 0 represents the final crawling-only visit, and 1 represents walk onset (a 1 unit increase is equivalent to an increase of 1 month of walking experience). This variable represents a baseline linear growth.
bThe bottom row illustrates how Piece 2 was coded. Unlike Piece 1, only months with positive walking experience are included. Thus, this variable represents additional incremental linear growth during walking months only.
For both models, only two predictors were included at the time-variant level (level 1): the piece 1 and piece 2 variables:
| (1) |
For both models, piece 1 and piece 2 were centered at the visit prior to walk onset—the midpoint of the overall trajectory—for each infant. This point was chosen as the intercept because it marks the very start of the transition to walking, and therefore differences are meaningful in that they tell us how groups differ immediately prior to this transition. Thus, the intercept (π0i) represented an infant i’s language status at the visit prior to walk onset. Here, the term π1i represents the estimated baseline linear growth rate for infant i, and the term π2i represents the estimated additional incremental growth from the visit prior to walk onset forward for infant i.
At level 2, dummy variables for outcome classification were included as predictors on the intercept and both slopes for HR-ND, HR-LD, and HR-ASD infants, using the LR infants as a reference group. This allows us to examine group differences between the HR outcome classification groups and the LR control infants. Additionally, in order to control for differences in chronological age, age at walk onset was included as a predictor on the intercept and on both slope terms. The final level 2 equations for the prediction model were as follows:
| (2) |
| (3) |
| (4) |
In this level of the model, coefficients (the β terms) represent each HR group’s deviation from the LR comparison group in the intercept, piece 1 slope, and piece 2 slope. To illustrate, β00 represents the LR group’s language at the intercept (the visit prior to walk onset), and β02 represents the deviation of the HR-ND group from the LR infants at the intercept. Therefore, the intercept for HR-ND infants can be calculated by adding β00 and β02.
This final prediction model allowed us to assess: a) whether there is an inflection in language growth following walk onset in LR infants; and b) whether each HR outcome group differed from the LR reference group. To further specify whether walk onset reflects a significant shift in each HR group, we rotated the reference group, allowing us to examine language growth separately for each HR outcome group.
Results
The aim of this study was twofold: a) to replicate previous findings showing that walk onset corresponds to a transition in language development (e.g. Walle & Campos, 2013); and b) to examine whether this pattern differs for three outcome groups of HR infants, in particular the HR-ASD group. Given ample evidence of language differences in HR-ASD infants compared to their HR peers without ASD (e.g., Ozonoff et al., 2010; Estes et al., 2015; Mitchell et al., 2006), as well as emerging evidence that the relation between motor and communicative development may differ in ASD (Srinivasan & Bhat, 2016; Bedford, Pickles & Lord, 2015; Kim, 2008), we investigated the possibility that HR-ASD infants would demonstrate a different trajectory of language growth over this developmental transition. Following preliminary analyses, each of these objectives is addressed in turn for receptive and expressive language.
Preliminary Analysis
Two sets of preliminary analyses were carried out before proceeding with final HLM models. First, group differences in age of milestone attainment and gender were examined. We then assessed the fit of our piecewise models relative to other HLM growth models.
Group differences in walk onset age
Before completing analyses, we examined outcome group differences in age at walk onset. Descriptive statistics for each outcome group can be found in Table 2. A one-way ANOVA revealed a significant effect of outcome group on age of walk onset, F(3, 108)=3.23, p=.025, with planned contrasts revealing that the HR-LD group was significantly older at walk onset relative to their LR peers, p = .033. There were no other differences between outcome groups, p’s = ns. In order to ensure that any differences between outcome groups were not an artifact of age differences, we controlled for infants’ age at the time of walk onset by including it as a predictor in our final models.
Table 2.
Means and standard deviations for age of walk onset (in months) by outcome group
| LR | HR-ND | HR-LD | HR-ASD | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | range | M | SD | range | M | SD | range | M | SD | range | |
|
|
|
|
|
|||||||||
| Agea | 11.76 | 1.56 | 9–15 | 12.51 | 1.82 | 8–17 | 13.15 | 1.791 | 10–17 | 13.14 | 1.88 | 11–16 |
Infants’ chronological age in months when the walk onset milestone was attained
Group differences in gender
Given discrepant ratios of males to females across outcome groups, we also examined the relation between infant gender and language prior to conducting analyses. Language ability has been shown to relate to gender in typical development, with female infants demonstrating higher language scores (e.g. Huttenlocker, Haight, Bryk, Seltzer & Lyons, 1991). A multivariate ANOVA with two independent variables (Gender and Outcome group) and two dependent variables (mean Words Understood and mean Words Produced) revealed no significant effect of Gender, F(1,108)=0.02, p=.889; and no significant interaction between Gender and Outcome group, F(3,108)=.068, p=.977; for Words Understood. There was also no significant effect for Gender, F(1,108)=2.097, p=.151; and no significant Gender by Outcome group interaction F(3,108)=0.787, p=.504 for Words Produced. Descriptive statistics for these data are presented in Table 3.
Table 3.
Mean Words Produced and Words Understood separated by Gender and Outcome group
| LR | HR-ND | HR-LD | HR-ASD | |||||
|---|---|---|---|---|---|---|---|---|
| M | F | M | F | M | F | M | F | |
|
|
|
|
|
|||||
| N | 10 | 15 | 23 | 27 | 15 | 11 | 10 | 5 |
| Words produced | M=4.1 (3.2) | M=3.6 (3.5) | M=4.3 (4.4) | M=8.5 (11.5) | M=1.8 (1.8) | M=3.2 (5.8) | M=1.5 (2.7) | M=4.4 (5.2) |
| Words understood | M=44.0 (27.3) | M=51.2 (38.7) | M=55.7 (41.6) | M=55.8 (47.6) | M=30.8 (20.8) | M=31.4 (39.0) | M=29.3 (23.3) | M=26.0 (25.9) |
Means (standard deviations) collapsed across the 7 time-points for total Words Produced and Words Understood from the MacArthur-Bated Communicative Development Inventory by Gender and Outcome (LR= lower risk; HR-ND=high risk with no diagnosis; HR-LD= heightened risk with language delay; HR-ASD=heightened risk with a confirmed ASD diagnosis; M= male, F=female).
Assessment of model fit
A piecewise model was selected because it allowed us to test the specific hypothesis that walk onset marks a point of inflection in language development. However, there is also evidence demonstrating that both receptive and expressive language growth follow a quadratic trend across the first few years of life (e.g. Fenson et. al., 1994). For this reason, it was necessary to assess whether piecewise models were a better fit for these data than either linear or quadratic models. For both receptive and expressive language, two chi-square deviance tests were calculated in order to measure how much the actual observed data varied from the predicted values of each model and to compare this variance between models. A lower deviance signifies that the data more closely fit the model predictions. The first chi-square test compared a piecewise and a linear model, and the second test compared the piecewise model with a quadratic model. Results for the receptive language data revealed that deviance was significantly lower for the piecewise model compared to both the linear model; χ2 = 642.35, p<.001 and the quadratic model, χ2 = 414.71, p<.001. These analyses confirmed that a piecewise model more precisely accounted for variance in receptive language growth. This process was also carried out with the expressive language data. Again, chi-square deviance tests indicated that data deviated from the model significantly less for a piecewise model than for a linear, χ2 = 345.37, p<.001, and for a quadratic model; χ2 = 389.82 p<.001. Therefore, we proceeded with the final prediction piecewise models.
Walk Onset as a Point of Inflection in LR Infants
Receptive language
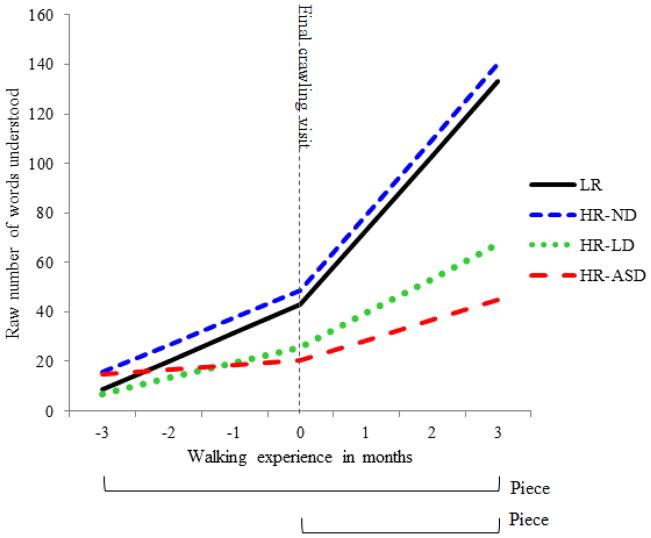
The numbers of Words Understood on the CDI were totaled for each infant at seven monthly time-points across the transition from crawling to walking. Table 4 displays the coefficients for our final conditional piecewise model; the model is displayed graphically in Figure 2. As can be seen in the figure, it appears that there was substantial additional incremental growth in receptive language for the LR reference group following the final crawl-only visit. This pattern was confirmed by the conditional model. LR infants’ piece 1 slope (i.e. the baseline linear slope) was significant, with an increase of 11.35 words understood per month (β10 = 11.35, SE = 2.0, t = 5.69, p<.001). Importantly, LR infants’ piece 2 slope was also significant, showing an additional incremental increase of 18.78 words understood per month after the transition to walking (β20 = 18.78, SE = 3.18, t = 5.90, p<.001). Thus, following walk onset, LR infants’ receptive language increased from 11.35 words per month to 30.13 (11.35 + 18.78) words per month.
Table 4.
Receptive Language
| FIXED EFFECTS | Coefficient | s.e. |
|---|---|---|
| For INTRCPT1, π0 | ||
| Mean LR intercept, β00 | 42.87*** | 7.71 |
| Age at walk onset, β01 | 11.71*** | 2.16 |
| HR-ND, β02 | 5.66 | 9.44 |
| HR-LD, β03 | −17.22 | 10.86 |
| HR-ASD, β04 | −22.43~ | 12.99 |
| For Piece 1 slope, π1 | ||
| Mean LR slope, β10 | 11.35*** | 1.99 |
| Age at walk onset, β11 | 1.84*** | 0.64 |
| HRND, β12 | −0.44 | 2.60 |
| LD, β13 | −5.15~ | 2.82 |
| ASD, β14 | −9.50** | 3.44 |
| For Piece 2 slope, π2 | ||
| Mean LR slope, β20 | 18.78*** | 3.18 |
| Age at walk onset, β21 | 0.67 | 1.24 |
| HRND, β22 | 0.84 | 3.72 |
| LD, β23 | −11.13* | 4.52 |
| ASD, β24 | −13.48* | 5.43 |
|
| ||
| RANDOM EFFECTS | Variance Component | s.d. |
|
| ||
| INTRCPT1, r0 | 1544.97*** | 39.31 |
| Piece 1 slope, r1 | 60.76*** | 7.79 |
| Piece 2 slope, r2 | 93.46** | 9.67 |
Note: df = 108 for fixed effects and 88 for random effects;
p<.10,
p<.05,
p<.01,
p<.001
Figure 2.
Final conditional model projections for the number of words understood over time across outcome classifications.
Expressive Language
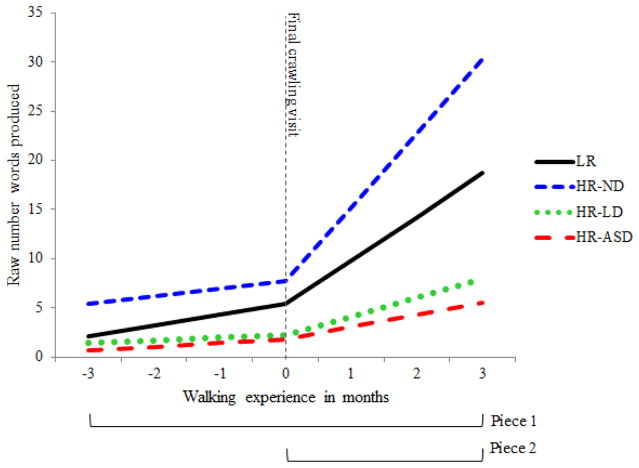
Because the transition to walking generally coincides with a time when many infants are just beginning to produce their first few words, many infants had scores of “0” words produced at early time-points (as can be inferred from Figure 3). Thus, data for expressive language were characterized by significant positive skew. Here we present data with robust standard errors—a more conservative estimate—in order to mitigate the potential effect of violating the assumption of normality.
Figure 3.
Final conditional model projections for the number of words produced over time across outcome classifications.
At each time point, expressive language was calculated as the sum of Words Produced for each infant. Model estimates are presented in Table 5 and graphed in Figure 3. It is clear from the figure that LR infants’ expressive language follows a similar trajectory as in receptive language: there was again a sharp increase in expressive language following the final crawling visit. The LR reference group showed significant linear growth in piece 1 of the model—i.e. the baseline growth—with an increase of 1.1 words per month (β10 = 1.1, SE = 0.23, t = 4.72, p<.001. Additionally, as was observed in receptive language, the slope for piece 2 was also significant for LR infants, indicating that there was significant additional linear growth of 3.35 words per month following the final crawling visit (β20 = 3.35, SE = 0.7, t = 4.76, p<.001). That is, following walk onset, the LR group demonstrated a new linear slope of 4.45 new words produced per month (1.1 + 3.35).
Table 5.
Expressive Language
| FIXED EFFECTS | Coefficient | s.e. |
|---|---|---|
| For INTRCPT1, π0 | ||
| Mean LR intercept, β00 | 5.35*** | 0.75 |
| Age at walk onset, β01 | 1.99*** | 0.37 |
| HR-ND, β02 | 2.32~ | 1.26 |
| HR-LD, β03 | −3.17*** | 0.94 |
| HR-ASD, β04 | −3.57*** | 1.07 |
| For Piece 1 slope, π1 | ||
| Mean LR slope, β10 | 1.10*** | 0.23 |
| Age at walk onset, β11 | 0.025 | 0.14 |
| HRND, β12 | −0.35 | 0.37 |
| LD, β13 | −0.86** | 0.31 |
| ASD, β14 | −0.71~ | 0.38 |
| For Piece 2 slope, π2 | ||
| Mean LR slope, β20 | 3.35*** | 0.70 |
| Age at walk onset, β21 | 1.41** | 0.44 |
| HRND, β22 | 3.46** | 1.65 |
| LD, β23 | −1.67* | 0.83 |
| ASD, β24 | −2.48* | 0.99 |
|
| ||
| RANDOM EFFECTS | Variance component | s.d. |
|
| ||
| INTRCPT1, r0 | 29.83*** | 5.46 |
| Piece 1 slope, r1 | 0.18 | 0.42 |
| Piece 2 slope, r2 | 45.56*** | 6.75 |
Note: df = 108 for fixed effects, and df = 88 for random effects;
p<.10,
p<.05,
p<.01,
p<.001
In light of the significant positive skew in the data noted above, these results should be interpreted cautiously. Nevertheless, we believe that the pattern of growth described here accurately represents expressive language growth across the transition to walking for two reasons. First, it is consistent with the pattern of growth we observed for receptive language—in which data are normally distributed. This is encouraging because receptive and expressive language scores on the CDI correlate significantly with one another (r = .53–.65 in a sample of 1,803 children; Fenson et al., 1994). Second, findings reported here for typically developing infants are consistent with previously observed patterns of expressive language growth during the transition to walking (e.g. Walle & Campos, 2014).
Outcome Group Differences in Receptive Language
In this section, we report results on outcome group differences on the Words Understood subscale of the CDI (receptive language). As described in the analytic approach, dummy variables for each HR outcome group were added onto the intercept and slopes, allowing for comparison of each outcome group to the LR reference group (i.e. the coefficients represent deviations from the LR group). Below we describe group differences in the intercept and in both slope terms.
Group differences in the receptive language intercept
We first examined group differences in the intercept term, i.e. in infants’ receptive language status at the visit prior to walk onset. It is important to note that age at walk onset was included as a predictor on the intercept to account for potential age differences between groups. For the LR reference group, the mean number of words understood was 42.87 (β00 = 42.87, SE = 7.71, t = 5.55, p < .001). Coefficients representing outcome group comparisons revealed a tendency for HR-ND infants to understand more words than LR infants at this time point—on average 48.53 words—but this was not significant (β02 = 5.66, SE = 9.44, t = 0.6, p > .05). Not surprisingly, there was a trend for the HR-LD group to understand fewer words at the final crawling visit than their LR peers, with 25.65 words reported on average; however, this difference also did not achieve significance (β03 = −17.22, SE = 10.86, t = −1.59, p > .05). Only the HR-ASD infants differed marginally from the LR comparison group, with an average of 20.44 words understood at the final crawling visit (β04 = −22.43, SE = 13.0, t = −1.73, p = .087). Although this difference was marginal, it may be a meaningful difference, as it suggests that even before walk onset, HR-ASD infants were already behind their LR peers in receptive language development.
Group differences in the receptive language baseline slope
Next, we examined group differences in the piece 1 slope, that is, the baseline slope for the entire trajectory. Significant coefficients here represent differences in the HR groups’ baseline rates of receptive language growth relative to the LR reference group. As previously discussed, LR infants demonstrated an average linear increase of 11.35 words understood per month (β10 = 11.35, SE = 2.0, t = 5.69, p <.001). The model revealed that the HR-ND infants did not differ significantly from the LR group, with an average baseline increase of 10.91 words understood per month (β12 = −0.44, SE = 0.64, t = 2.87, p > .05). The HR-LD group had a marginally attenuated baseline growth rate of receptive language relative to the LR group: a 1 month interval was associated with a 6.2 word increase on average (β13 = −5.16, SE = 2.83, t = −1.82, p = 0.071). Compared to LR infants, HR-ASD infants had a significantly reduced baseline rate of receptive language growth, with a mean increase of only 1.85 words per month (β14 = −9.5, SE = 3.44, t = −2.76, p = 0.007).
Group differences in receptive language after the transition to walking
Finally, we examined differences in the piece 2 slope. Significant coefficients here denote that an outcome group differs from the LR reference group in additional incremental growth in receptive language following the transition from crawling to walking. Because variability between groups overall is already accounted for by the piece 1 coefficients, significant coefficients here convey variability in incremental growth specifically from the final crawl-only visit through the remainder of the observation period as infants gained increased walking experience.
As noted above, the LR group displayed an incremental increase of 18.78 words per month for the piece 2 slope (β20 = 18.78; SE = 3.18; t = 5.903; p<.001); recall that this is in addition to gains from baseline growth. The HR-ND group did not differ significantly from the LR group and showed similar incremental growth in receptive language (19.62 words per month; β22= 0.84; SE = 3.72; t = 0.23; p>.05). Both the HR-LD and the HR-ASD groups showed significantly attenuated growth in receptive language relative to LR infants, with the HR-LD group showing an incremental increase of 7.65 additional words understood per month beyond baseline (β23 = −11.13; SE = 4.52; t = −2.46; p = 0.015), and HR-ASD increasing by only 5.3 words per month (β24 = −13.48; SE = 5.43; t = −2.48; p = 0.015).
Although these findings indicate that the HR-LD and HR-ASD infants exhibit slower growth in receptive language relative to their LR peers, they do not tell us whether the additional growth is significant for each HR outcome group. Thus, post hoc analyses were performed by rotating the reference group. Both the HR-ND and HR-LD groups had significant additional incremental growth in receptive language following walk onset (p < .001, p = .024, respectively). However, the HR-ASD infants did not show significant additional growth, p = 0.25
Outcome Group Differences in Expressive Language
Results on outcome group differences on the Words Produced subscale of the CDI (expressive language) are reported below. As in the previous analysis, dummy variables were included for each HR outcome group, such that these coefficients on the intercept and slope terms represent deviations from the LR reference group.
Group differences in the expressive language intercept
We first examined group differences in the intercept term, which represents infants’ expressive language at the visit that occurred immediately prior to walk onset. As in the previous analysis, age at walk onset was also included as a predictor in order to control for any differences explained by chronological age. The LR reference group had a mean of 5.35 words produced at the intercept (β00 = 5.35, SE = 0.75, t = 7.13, p<.001), and the HR-ND infants had an average of about 7.67 words at this time (β02 = 2.32, SE = 1.26, t =1.85, p=.068). Both the HR-LD and HR-ASD produced significantly fewer words at the intercept, with the HR-LD infants producing on average 2.18 words (β03= −3.17, SE = 0.94, t = −3.36, p<.001), and HR-ASD infants producing 1.78 words on average at the final crawling visit (β04= −3.57, SE = 1.07, t = −3.34, p<.001). These findings suggest that even before the onset of walking, HR-LD and HR-ASD infants show delays in expressive language ability compared to their typically developing peers.
Group differences in the expressive language baseline slope
Group differences were also examined in the piece 1 slope; that is, the linear growth across all time-points. Again, significant coefficients represent each outcome group’s deviation in the baseline linear growth from the LR reference group. For the LR reference group, the baseline linear growth was 1.1 new words produced per month (β10 = 1.10, SE = 0.23, t = 4.72, p<.001). For HR-ND infants, the piece 1 slope did not significantly differ from the LR reference group, with an increase of 0.75 new words produced per month (β12 = −0.35, SE = 0.37, t = −0.94, p>.05). HR-LD infants showed a significantly attenuated baseline growth in expressive language compared LR infants, adding on average 0.24 new words to their expressive language repertoire per month (β13 = −0.86, SE = 0.31, t = −2.75, p<.01). Compared to LR infants, the HR-ASD group also showed a tendency for a reduced linear baseline growth, with an average of 0.39 new words per month; however, this was only marginally significant (β14 = −0.71, SE = 0.38, t = −1.85, p=0.068).
Group differences in expressive language after the transition to walking
The final set of coefficients examined whether each outcome group differed from the LR reference growth in the piece 2 slope. In other words, it examined differences in additional incremental linear growth specifically following the final crawling visit (i.e. the transition into walking). As was previously reported, the LR reference group demonstrated a significant increase in linear growth following walk onset: on average they produced 4.45 new words per month following walk onset, as opposed to only 1.1 new words prior to walk onset (β20 = 3.35, SE = 0.70, t= 4.76, p<.001). Model estimates revealed that HR-ND infants showed a small but significant difference of 3.46 additional new words produced per month following walk onset compared to the LR group (β22 = 3.46, SE = 1.65, t = 2.10, p = 0.038). Relative to LR infants, the HR-LD group also showed significantly less piece 2 growth, increasing by 1.68 words produced per month following walk onset (β23 = −1.67, SE = 0.83, t = −2.01, p<.05). HR-ASD infants showed the most dramatic attenuation in expressive language growth following walk onset with only 0.87 additional new words produced per month (β24= −2.48, SE = 0.99, t = −2.51, p=0.14).
Again, post hoc analyses were performed by rotating the reference group. The pattern of results revealed in these analyses replicated the pattern found for receptive language: the HR-ND and HR-LD groups had significant additional linear growth in expressive language following the onset of walking (p<.001 and p<.01 respectively). Again, the HR-ASD infants did not significantly increase in expressive language growth after the onset of walking (p=0.30).
Discussion
The present study was designed to assess whether walk onset is a point of inflection for language development in three groups of HR infants who varied in developmental outcomes and in a comparison group of LR infants. We replicated previous research showing that typically developing infants—both LR and HR-ND—demonstrated a significant increase in receptive and expressive vocabulary acquisition at walk onset. Our findings provide further evidence that in typical development, walking supports early language learning. They also revealed striking differences in patterns of language growth following the onset of walking between the LR and HR-ND, HR-LD and HR-ASD groups. Following a discussion of these differences, we highlight a set of potential mechanisms that may account for the observed relation between walking experience and language as candidates for future investigation.
Outcome Group Differences in Language
Examination of overall baseline language growth during the observation period revealed that HR-ND and LR infants did not significantly differ in receptive or expressive language. As expected, HR-LD infants showed an attenuated pattern of overall linear growth compared to LR peers; this difference was significant for expressive language, but did not achieve significance for receptive language. This finding is consistent with studies of infants with language delay—often termed “late talkers”—which find that receptive language ability and gesture use are comparable to typically developing peers (e.g. Thal et al., 1991; Rescorla & Schwartz, 1990).
HR-ASD infants showed significantly reduced growth in receptive language overall, and a marginally significant attenuation in expressive language. This finding aligns with previous research reporting that HR-ASD infants differ from their unaffected HR peers in early language development (Mitchell et al., 2006; Ozonoff et al., 2010; Estes et al., 2015; Zwaigenbaum et al., 2005). Our finding builds on this literature in that it utilized a milestone-based approach, rather than examining change in relation to chronological age. This is important because it demonstrates that even when infants were matched on walking experience—which may occur on a different developmental timescale—differences in language persisted. Thus, this finding indicates that atypical trajectories of language growth in HR-ASD infants are particularly robust, spanning multiple methodologies.
Outcome Group Differences in the Relation between Walking and Language
To our knowledge, this is the first study to examine language development during the transition from crawling to walking in HR infants. Results revealed that for the HR-ND and HR-LD groups, the onset of walking marked a point of inflection in both receptive and expressive language development, with these groups demonstrating significant additional linear growth following milestone attainment. Only the HR-ASD group did not show a significant increase following walk onset. This diverging pattern of language growth during the transition to walking may provide insight for understanding communicative development in ASD. Although ample work has documented differences in communication in the first few years in ASD (see Jones, Gliga, Bedford, Charman & Johnson, 2014, for a review), little is known about the specific developmental pathways by which they may arise. Walk onset may be of particular importance in clarifying these pathways because it is a period of rapid change in typical language development, wherein advances are likely to set the stage for future gains, while delays may constrain future development. Therefore, HR-ASD infants may stand to lose significant ground on their typically developing peers during this transition.
Given the complexity of ASD (e.g. Wozniak et al., 2016) and of the development of language, an atypical relation between walking and language clearly does not fully account for deficits in communication exhibited by young children with ASD. Rather, it seems likely that infants with ASD are vulnerable to deficits in communication prior to walking—as is evidenced by their pattern of language growth leading up to walk onset—and that this gap in language widens as a result of the gains walking affords typically developing infants. Thus, an area for future research is the investigation of real-time processes that account for why this language “spurt” is present in typical development at walk onset, but absent in ASD. Here, we suggest several possible, non-mutually-exclusive explanations that could serve as starting points for future investigations.
One possibility is that infants with ASD do not exhibit the same increase in communicative bids (e.g. gestures) around walk onset as their typically developing peers. Research on gesture use in HR-ASD infants indicates that they produce fewer gestures—and fewer social bids in general—than their LR peers (Cassel et al., 2007; Goldberg et al., 2005; Yirmiya et al., 2007; Parlade & Iverson, 2015). If it is the case that HR-ASD infants are not matching their typically developing peers’ increase in communicative gestures (such as showing and giving objects to caregivers) following walk onset, their opportunities for and the content of their social exchanges may differ substantially.
This hypothesis is consistent with recent work finding that HR infants as a group show attenuated growth in social bids following walk onset relative to LR peers (Srinivasan & Bhat, 2016). It is possible that this attenuation is driven by a subset of HR infants who go on to receive an eventual ASD diagnosis. However, because outcome data were not available in this study, it is unclear whether it was specific to ASD. Future studies should investigate this relation in infants with ASD by measuring both communicative bids and language growth longitudinally during the transition to walking.
A second explanation is that while walking may afford typically developing infants more frequent opportunities for establishing social interactions, this may not be the case for infants with ASD, for whom walking may allow for more opportunities to physically disengage during social interactions. Anecdotally, in this study we observed the HR-ASD infants locomoting just as frequently, but approaching caregivers noticeably less often than did their unaffected peers. While our observation requires formal investigation, this explanation does fit with studies of infants’ visual attention which find that, in general, infants with ASD attend less to social stimuli than typically developing infants (e.g. Moriuchi, Klin & Jones, 2016). They may also be less likely to use walking to seek out social stimuli, and this difference may consequently impact language development.
Finally, differences in postural control or gait in children with ASD may make walking a more effortful task. In a retrospective home video study, Esposito and colleagues (2008, 2011) examined the gait of infants with ASD and found that relative to typically developing infants, the gait of infants with ASD was less fluid and symmetrical. These difficulties are likely to persist, as studies find that older children and adults with ASD exhibit differences in balance, gait, and walking speed compared to neurotypical participants (e.g., Jansiewicz et al., 2006; Minshew et al., 2004). This presents the possibility that differences in postural control may already exist at walk onset in HR-ASD infants, making the task of walking more effortful, and potentially hindering infants’ ability to integrate walking with other communicative behaviors (e.g. carrying objects to share with social partners). Future studies should investigate whether there may be linkages between characteristics of infants’ postural control, gait, and communicative behaviors during the transition from crawling to walking.
Limitations and Conclusions
The present study has several strengths, including a prospective, longitudinal design with frequent observations, and the inclusion of a language-delayed comparison group. However, there are also limitations. First, as is frequently the case with studies of HR infants, the sample of infants who went on to receive an ASD diagnosis is relatively small, and replication with a larger cohort of infants with ASD is necessary. Additionally, the research should be replicated with other measures of language. Although the CDI does have high concurrent validity with standardized assessments of language (r = .72; Bates, Bretherton & Snyder, 1988; Fenson et al, 1994), it may capture something different about infants’ language use than data from behavioral coding of observations. For instance, on the Words Produced portion of the CDI parents identify words they have heard their infant use, but information about the frequency or context of word use is not collected.
It is also important to acknowledge a limitation of the CDI for assessing receptive language in particular. Because the CDI is a parent-report measure, it is unclear precisely what information caregivers utilize when they estimate whether a word is understood or not. This makes it difficult to disentangle receptive language from infants’ social engagement more generally; and it is therefore possible that parents underreport receptive language for infants who are less socially responsive. Difficulty separating these two distinct constructs—infants’ understanding of language and their outward communicative behavior—pervades studies of receptive language, and may confound receptive language scores for infants with ASD. Even frequently utilized standardized behavioral assessments of receptive language (e.g. the MSEL) are embedded within a social exchange between the infant and the experimenter, and therefore successful performance hinges on socially responsive behavior and gestural replies to prompts (Tager-Flusberg, 1999). Future studies should address this limitation to understand receptive language development in ASD. In particular, measures utilizing visual fixation (e.g. the “looking while listening” paradigm; Fernald, Zangl, Portillo, & Marchman, 2008) may provide a useful means of measuring language comprehension because they are independent of social engagement.
The present findings suggest that walk onset may play a different role in language acquisition in typical development and in ASD. It is likely that the onset of walking offers all infants more autonomy in shaping their experiences with objects and social partners—and that this autonomy may lead infants with ASD to different experiences than their typically developing peers. This underscores the importance of studying typical and atypical communicative development within the context of the developing body. During the first few years, infants’ bodies—and consequently their abilities for action—are changing rapidly, and these changes have a dramatic influence on their social, verbal, and perceptual experiences (Needham & Libertus, 2011; Iverson, 2010). Thus, in addition to examining developmental change in relation to infants’ chronological age, comparing patterns of change at points of motor transition may provide an additional means for understanding development.
Acknowledgments
Funding: This study was funded by Autism Speaks and the National Institutes of Health (R01 HD41607 and R01 HD54979), with additional support from HD35469 and HD055748.
This research was supported by grants from Autism Speaks and the National Institutes of Health (R01 HD41607 and R01 HD54979) to JMI. Additional support was provided by HD35469 and HD055748 to N.J. Minshew. Special thanks to the members of the Infant Communication Lab at the University of Pittsburgh for help with data collection, and to the families and infants who participated in the research. Portions of these data were presented at the 2016 Annual International Meeting for Autism Research.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- Adolph KE, Tamis-LeMonda CS. The costs and benefits of development: The transition from crawling to walking. Child development perspectives. 2014;8(4):187–192. doi: 10.1111/cdep.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolph KE. Learning to move. Current Directions in Psychological Science. 2008;17(3):213–218. doi: 10.1111/j.1467-8721.2008.00577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolph KE, Cole WG, Komati M, Garciaguirre JS, Badaly D, Lingeman JM, … Sotsky RB. How do you learn to walk? Thousands of steps and dozens of falls per day. Psychological science. 2012;23(11):1387–1394. doi: 10.1177/0956797612446346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders, text revision (DSM-IV-TR) 4. Washington DC: APA; 2000. text revision. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5®) American Psychiatric Pub; 2013. [Google Scholar]
- Bedford R, Pickles A, Lord C. Early gross motor skills predict the subsequent development of language in children with autism spectrum disorder. Autism Research. 2015 doi: 10.1002/aur.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel TD, Messinger DS, Ibanez LV, Haltigan JD, Acosta SI, Buchman AC. Early social and emotional communication in the infant siblings of children with autism spectrum disorders: An examination of the broad phenotype. Journal of autism and developmental disorders. 2007;37(1):122–132. doi: 10.1007/s10803-006-0337-1. [DOI] [PubMed] [Google Scholar]
- Clearfield MW. Learning to walk changes infants’ social interactions. Infant Behavior and Development. 2011;34(1):15–25. doi: 10.1016/j.infbeh.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Dale PS, Bates E, Reznick JS, Morisset C. The validity of a parent report instrument of child language at twenty months. Journal of child language. 1989;16(02):239–249. doi: 10.1017/s0305000900010394. [DOI] [PubMed] [Google Scholar]
- Estes A, Zwaigenbaum L, Gu H, St John T, Paterson S … IBIS Network. Behavioral, cognitive, and adaptive development in infants with autism spectrum disorder in the first 2 years of life. Journal of Neurodevelopmental Disorders. 2015;7:1. doi: 10.1186/s11689-015-9117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenson L, Dale P, Reznick JS, Thal D, Bates E, Hartung J, Pethick SJ, Reilly J. The MacArthur Communicative Development Inventories: User’s guide and technical manual. San Diego, CA: Singular Publishing Group; 1993. [Google Scholar]
- Fenson L, Dale PS, Reznick JS, Bates E, Thal DJ, Pethick SJ. Variability in early communicative development. Monographs of the Society for Research in Child Development. 1994;59(5 Serial No. 242) doi: 10.2307/1166093. [DOI] [PubMed] [Google Scholar]
- Fernald A, Zangl R, Portillo AL, Marchman VA. Looking while listening: Using eye movements to monitor spoken language. Developmental psycholinguistics: On-line methods in children’s language processing. 2008:113–132.
- Gamliel I, Yirmiya N, Sigman M. The development of young siblings of children with autism from 4 to 54 months. Journal of autism and developmental disorders. 2007;37(1):171–183. doi: 10.1007/s10803-006-0341-5. [DOI] [PubMed] [Google Scholar]
- Gershkoff-Stowe L, Thal DJ, Smith LB, Namy LL. Categorization and its developmental relation to early language. Child Development. 1997;68(5):843–859. doi: 10.1111/j.1467-8624.1997.tb01966.x. [DOI] [PubMed] [Google Scholar]
- Goldberg WA, Jarvis KL, Osann K, Laulhere TM, Straub C, Thomas E, … Spence MA. Brief report: Early social communication behaviors in the younger siblings of children with autism. Journal of Autism and Developmental Disorders. 2005;35(5):657–664. doi: 10.1007/s10803-005-0009-6. [DOI] [PubMed] [Google Scholar]
- Golinkoff RM. ‘I beg your pardon?’: the preverbal negotiation of failed messages. Journal of child language. 1986;13(03):455–476. doi: 10.1017/S0305000900006826. [DOI] [PubMed] [Google Scholar]
- He M, Walle EA, Campos JJ. A Cross-National Investigation of the Relationship Between Infant Walking and Language Development. Infancy. 2015;20(3):283–305. doi: 10.1111/infa.12071. [DOI] [Google Scholar]
- Heilmann J, Weismer SE, Evans J, Hollar C. Utility of MacArthur-Bates Communicative Development Inventory in identifying language abilities of late-talking and typically developing toddlers. American Journal of Speech-Language Pathology. 2005;14:40–51. doi: 10.1044/1058-0360. [DOI] [PubMed] [Google Scholar]
- Huttenlocher J, Haight W, Bryk A, Seltzer M, Lyons T. Early vocabulary growth: Relation to language input and gender. Developmental psychology. 1991;27(2):236. doi: 10.1037/0012-1649.27.2.236. [DOI] [Google Scholar]
- Iverson JM, Wozniak RH. Variation in vocal-motor development in infant siblings of children with autism. Journal of autism and developmental disorders. 2007;37(1):158–170. doi: 10.1007/s10803-006-0339-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson JM. Developing language in a developing body: The relationship between motor development and language development. Journal of child language. 2010;37(02):229–261. doi: 10.1017/S0305000909990432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansiewicz EM, Goldberg MC, Newschaffer CJ, Denckla MB, Landa R, Mostofsky SH. Motor signs distinguish children with high functioning autism and Asperger’s syndrome from controls. Journal of autism and developmental disorders. 2006;36(5):613–621. doi: 10.1007/s10803-006-0109-y. [DOI] [PubMed] [Google Scholar]
- Jones EJ, Gliga T, Bedford R, Charman T, Johnson MH. Developmental pathways to autism: a review of prospective studies of infants at risk. Neuroscience & Biobehavioral Reviews. 2014;39:1–33. doi: 10.1016/j.neubiorev.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasik LB, Tamis-LeMonda CS, Adolph KE. Transition from crawling to walking and infants’ actions with objects and people. Child development. 2011;82(4):1199–1209. doi: 10.1111/j.1467-8624.2011.01595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HU. Development of early language and motor skills in preschool children with autism. Perceptual and motor skills. 2008;107(2):403–406. doi: 10.2466/pms.107.2.403-406. [DOI] [PubMed] [Google Scholar]
- Kretch KS, Franchak JM, Adolph KE. Crawling and walking infants see the world differently. Child development. 2014;85(4):1503–1518. doi: 10.1111/cdev.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landa R, Garrett-Mayer E. Development in infants with autism spectrum disorders: A prospective study. Journal of Child Psychology and Psychiatry. 2006;47:629–638. doi: 10.1111/j.1469-7610.2006.01531.x. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, … Rutter M. The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. doi: 10.1023/A:1005592401947. [DOI] [PubMed] [Google Scholar]
- Luyster RJ, Qui S, Lopez K, Lord C. Predicting outcomes of children referred for autism using the MacArthur-Bates Communicative Development Inventory. Journal of Speech, Language, and Hearing Research. 2007;50:667–81. doi: 10.1044/1092-4388. [DOI] [PubMed] [Google Scholar]
- Masur EF. Mothers’ responses to infants’ object-related gestures: Influences on lexical development. Journal of child language. 1982;9(01):23–30. doi: 10.1017/S0305000900003585. [DOI] [PubMed] [Google Scholar]
- Messinger DM, Young GS, Ozonoff S, Dobkins K, Carter AS, Zwaigenbaum L, … Sigman M. Beyond Autism: A Baby Siblings Research Consortium study of high-risk children at three years of age. Journal of the American Academy of Child and Adolescent Psychiatry. 2013;52(3):300–308. doi: 10.1016/j.jaac.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JF, Sedey AL, Miolo G. Validity of parent report measures of vocabulary development for children with Down syndrome. Journal of Speech, Language, and Hearing Research. 1995;38(5):1037–1044. doi: 10.1044/jshr.3805.1037. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Sung K, Jones BL, Furman JM. Underdevelopment of the postural control system in autism. Neurology. 2004;63(11):2056–2061. doi: 10.1212/01.WNL.0000145771.98657.62. [DOI] [PubMed] [Google Scholar]
- Mitchell S, Brian J, Zwaigenbaum L, Roberts W, Szatmari P, Smith I, Bryson S. Early language and communication development of infants later diagnosed with autism spectrum disorder. Developmental and Behavioral Pediatrics. 2006;27:69–78. doi: 10.1097/00004703-200604002-00004. 0196-206X/06/2702-0069. [DOI] [PubMed] [Google Scholar]
- Mullen EM. Mullen: Scales of Early Learning (AGS edn.) Circle Pines, MN: American Guideline Service, Inc; 1995. [Google Scholar]
- Nakao K, Treas J. Updating occupational prestige and socioeconomic scores: How the new measures measure up. Sociological Methodology. 1994;24:1–72. doi: 10.2307/270978. [DOI] [Google Scholar]
- Needham A, Libertus K. Embodiment in early development. Wiley Interdisciplinary Reviews: Cognitive Science. 2011;2(1):117–123. doi: 10.1002/wcs.109. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Iosif A, Baguio R, Cook IC, Moore Hill M, Hutman T, … Young GS. A prospective study of the emergence of early behavioral signs of autism. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(3):256–266. doi: 10.1016/j.jaac.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L, … Hutman T. Recurrence risk for autism spectrum disorders: a Baby Siblings Research Consortium study. Pediatrics. 2011;128(3):e488–e495. doi: 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parladé MV, Iverson JM. The development of coordinated communication in infants at heightened risk for autism spectrum disorder. Journal of autism and developmental disorders. 2015;45(7):2218–2234. doi: 10.1007/s10803-015-2391-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruden SM, Hirsh-Pasek K, Golinkoff RM, Hennon EA. The Birth of Words: Ten-Month-Olds Learn Words Through Perceptual Salience. Child development. 2006;77(2):266–280. doi: 10.1111/j.1467-8624.2006.00869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla L, Schwartz E. Outcome of toddlers with specific expressive language delay. Applied Psycholinguistics. 1990;11(04):393–407. doi: 10.1017/S0142716400009644. [DOI] [Google Scholar]
- Robertson SB, Weismer SE. Effects of treatment on linguistic and social skills in toddlers with delayed language development. Journal of Speech, Language, and Hearing Research. 1999;42(5):1234–1248. doi: 10.1044/jslhr.4205.1234. [DOI] [PubMed] [Google Scholar]
- Singer JD. Using SAS PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. Journal of educational and behavioral statistics. 1998;23(4):323–355. [Google Scholar]
- Smith LB, Jones SS, Landau B. Naming in young children: A dumb attentional mechanism? Cognition. 1996;60(2):143–171. doi: 10.1016/0010-0277(96)00709-3. [DOI] [PubMed] [Google Scholar]
- Srinivasan SM, Bhat AN. Differences in object sharing between infants at risk for autism and typically developing infants from 9 to 15 months of age. Infant Behavior and Development. 2016;42:128–141. doi: 10.1016/j.infbeh.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan M, Finelli J, Marvin A, Garrett-Mayer E, Bauman M, Landa R. Response to joint attention in toddlers at risk for autism spectrum disorder: A prospective study. Journal of Autism and Developmental Disorders. 2007;37(1):37–48. doi: 10.1007/s10803-006-0335-3. [DOI] [PubMed] [Google Scholar]
- Tager-Flusberg H. A psychological approach to understanding the social and language impairments in autism. International review of psychiatry. 1999;11(4):325–334. doi: 10.1080/09540269974203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal DJ, O’Hanlon L, Clemmons M, Fralin L. Validity of a parent report measure of vocabulary and syntax for preschool children with language impairment. Journal of Speech, Language, and Hearing Research. 1999;42:482–496. doi: 10.1044/jslhr.4202.482. [DOI] [PubMed] [Google Scholar]
- Thal D, Tobias S, Morrison D. Language and Gesture in Late Talkers: A 1-Year Follow-up. Journal of Speech, Language, and Hearing Research. 1991;34(3):604–612. doi: 10.1044/jshr.3403.604. [DOI] [PubMed] [Google Scholar]
- Tomasello M, Farrar MJ. Joint attention and early language. Child development. 1986:1454–1463. doi: 10.2307/1130423. [DOI] [PubMed] [Google Scholar]
- Walle EA, Campos JJ. Infant language development is related to the acquisition of walking. Developmental psychology. 2014;50(2):336. doi: 10.1037/a0033238. [DOI] [PubMed] [Google Scholar]
- Walle E, Warlaumont AS. Infant Locomotion, the Language Environment, and Language Development: A Home Observation Study. CogSci 2015 [Google Scholar]
- Weismer SE, Evans JL. The role of processing limitations in early identification of specific language impairment. Topics in Language Disorders. 2002;22(3):15–29. [Google Scholar]
- Wozniak RH, Leezenbaum NL, Northrup JB, West KL, Iverson JM. The development of Autism Spectrum Disorders: Variability and causal complexity. WIRED Cognitive Science. 2016 doi: 10.1002/wcs.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya N, Ozonoff S. The very early phenotype of autism. Journal of Autism and Developmental Disorders. 2007;37(1):1–11. doi: 10.1007/s10803-006-0329-1. [DOI] [Google Scholar]
- Yu C, Smith LB. Embodied attention and word learning by toddlers. Cognition. 2012;125(2):244–262. doi: 10.1016/j.cognition.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson SE, Brian J, Smith IM, Roberts W, Szatmari P, … Vaillancourt T. Stability of diagnostic assessment for autism spectrum disorder between 18 and 36 months in a high-risk cohort. Autism Research. 2015;9(7):790–800. doi: 10.1002/aur.1585. [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. International Journal of Developmental Neuroscience. 2005;23:143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]