Abstract
A 21-year-old male with an SCN1A mutation died of cerebral herniation 3 h after a seizure occurring during physical activity. Cases of fatal cerebral edema in patients with SCN1A mutations after fever and status epilepticus have been recently reported raising the question whether sodium channel dysfunction may contribute to cerebral edema and thereby contribute to the increased premature mortality in Dravet Syndrome. We report on our patient and discuss whether the combination of hyperthermia and ion channel dysfunction may not only trigger seizures but also a fatal pathophysiological cascade of cerebral edema and herniation leading to cardiorespiratory collapse.
Keywords: Dravet Syndrome, SCN1A mutation, Fatality, Cerebral edema, Hyperthermia, SUDEP
1. Introduction
Patients with Dravet Syndrome (DS) are at a significantly increased risk for premature death (16–17 per 1000 patient years), with sudden unexpected death in epilepsy (SUDEP) representing the leading cause of death in childhood followed by status epilepticus [1], [2]. Despite sporadic reports of acute encephalopathy in DS, cerebral edema is not traditionally considered a key pathological mechanism contributing to high mortality in DS. Recently Myers et al. report 5 fatal cases of cerebral edema occurring days after status epilepticus in children with DS. We were recently confronted with a similar case in our emergency department (ED). This was remarkable because our DS patient experienced fatal transtentorial herniation within three hours of a generalized convulsive seizure despite being immediately aborted with buccal midazolam. We therefore find it relevant to report on this case and bring to attention that there may be circumstances under which fatal cerebral edema develops rapidly in patients with DS.
2. Case: 21 year-old male with SCN1A mutation
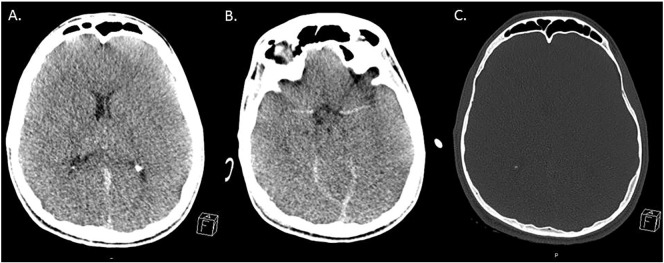
A 21-year-old male patient with an SCN1A mutation and childhood diagnosis of DS was transferred to our ED via helicopter from a hospital nearby requiring cardiopulmonary resuscitation (CPR). According to his father his seizures could be triggered by intense emotion (especially joy) in the past but he had been seizure-free for the past 7 years with a combination of topiramate and potassium bromide. On the day of admission, a warm summer day, he had suffered from a generalized convulsive seizure of unknown duration during a 5 kilometer city run only a few meters from the finish line. Acting upon orders from the patients' father, paramedics standing by quickly applied buccal midazolam, which successfully aborted the seizure. The exact seizure duration was not reported. Upon arrival of an emergency physician the patient was asystolic but was successfully resuscitated in the ambulance. Despite having spontaneous circulation upon admission to the ED of the closest hospital, he quickly became in need of CPR again. He was therefore transferred to our hospital for tertiary care, where CPR was unsuccessfully attempted for 2 h. Arterial blood gas analysis was performed after cumulative 2 h of cardiopulmonary resuscitation. All parameters in the blood gas analysis were within the normal range except potassium (K+ [mmol/l] = 8.7) which is most likely attributable to the failed resuscitation attempts. CT revealed global cerebral edema with completely compressed ventricular cavities and no signs of traumatic brain injury, or lesions of any type. A post-mortem full body CT also did not reveal any intra- or extra-cranial signs of trauma (Fig. 1).
Fig. 1.
Cranial computed tomography after cumulative 3 h of cardiopulmonary resuscitation.
Cranial imaging revealed cerebral herniation due to global cerebral edema without any trauma signs.
(with permission from the Institute of the Diagnostic und Interventional Radiology, Medical Faculty, Heinrich-Heine-University, Düsseldorf).
3. Discussion
SUDEP is the most frequent cause of death directly related to epilepsy, and patients with Dravet Syndrome (DS) display a significantly increased SUDEP risk compared to the general epilepsy population [3]. The majority of observed SUDEP cases occur shortly after a seizure [4], [5], [6], [7], [8], however a previous seizure is not a prerequisite for SUDEP [9]. While the exact pathological mechanism contributing to SUDEP is still unresolved, the 11 cases of monitored SUDEP offer crucial insight into the events leading to SUDEP [10]: following a generalized tonic–clonic seizure (GTCS), heart rate and respiration are transiently increased before a combination of central apnoea, severe bradycardia, and most often transient asystole occurs together with postictal generalized EEG suppression, typically peaking between 1 and 3 min postictally. These observations lead to the hypothesis that SUDEP is an early post-ictal neurovegetative breakdown and potentially a consequence of years of autonomic dysfunction, a common phenomenon in epilepsy —especially in DS patients [11], [12], [13], [14]. The observation of impaired heart-rate variability in patients with DS compared to healthy controls and other epilepsy populations is of interest considering that a voltage-gated sodium channel (VGSC) such as Nav 1.1 is not expressed in cardiac tissue suggesting that cardiac dysfunction in patients with SCN1A mutations is mainly centrally mediated [15].
Edema and increased intracranial pressure (ICP) are not considered a mechanism contributing to SUDEP, however if the post-ictal events occurring in this patient had been unwitnessed (e.g., at night) his death would have likely been attributed to SUDEP. Our patient displayed a similar pattern to SUDEP: fatal cardiac arrest occurring shortly after a generalized seizure. Post-ictal cerebral edema developed rapidly after an aborted seizure probably triggered by a state of prolonged hyperthermia due to physical activity on a warm day. The combination of hyperthermia, ion channel dysfunction and possibly emotional stress may trigger seizures in DS. Hyperthermia is well known to induce seizures and to influence neuronal activity in patients with SCN1A mutations and in experimental conditions involving Nav1.1 dysfunction [16], [17], [18]. Under experimental conditions, exposure to a stressor such as emotional stress increases seizure susceptibility in both SCN1A mutant animals and non-genetically altered controls [19].
According to his father, our patient suffered from drug-resistant seizures in childhood but could ultimately be controlled with anti-seizure drug polypharmacy in adolescence and adulthood which is not uncommon in DS. At his death the patient was taking potassium bromide and topiramate, which may be of interest, as topiramate can decrease sweating and increase body temperature, leading to life-threatening dehydration especially during warm weather. In combination with the warm weather and physical activity, topiramate could have therefore contributed to hyperthermia promoting the seizure.
In neurosurgical practice, edema and raised ICP can be observed rapidly after seizures and edema as a consequence of prolonged seizures is known to represent a predictor of poor outcome [20], [21], [22]. Additionally, the combination of hyperthermia, ion channel dysfunction and possibly emotional stress may lead to fatal cerebral edema and subsequently to increased ICP with cerebral transtentorial herniation. Recently, Myers et al. reported a series of fatal cerebral edema in children with DS. Interestingly, all DS patients with fatal cerebral edema suffered from fever of ≥ 40 °C and hyperthermia-related seizures [23]. Acute encephalopathy referring to non-inflammatory cerebral edema as a complication of febrile illness has been sporadically reported for children with DS [24], [25], [26], [27]. However, pathophysiological mechanisms connecting hyperthermia-induced seizures and fatal cerebral edema have yet to be described: Three distinct possible mechanisms or a combination of thereof are considerations:
-
(1)
Hyperthermia-induced seizures may lead to an early post-ictal compromise, resulting in respiratory failure and cardiac arrest as described in the monitored SUDEP cases in the MORTEMUS study [10]. In addition to autonomic dysfunction, this may further impede CPR during cardiac arrest which promotes cerebral hypoxia. Cerebral hypoxia leads to a disturbance of ion hemostasis and in particular to a neuronal sodium ion influx [28], [29], [30]. Several studies suggested that VGSCs are important in the pathophysiology of hypoxia [28], [29], [30], [31]. It has however yet to be investigated whether SCN1A mutations in DS alter susceptibility for hypoxia and promote hypoxia-related cerebral edema by increasing neuronal sodium ion influx. Altered susceptibility to hypoxia and increased ICP from cerebral edema may impede CPR.
-
(2)
Hyperthermia-related seizures themselves may promote cerebral edema. It is widely accepted that post-ictal cerebral edema begins with excessive influx of sodium and calcium ions through their respective voltage-gated channels into neurons during the repetitive hypersynchronous glutamatergic firing due to seizure activity. Post-ictal hypoxia, energy depletion and lack of sufficient ATP ultimately cause failure of both the sodium-calcium exchange and sodium-potassium exchange further promoting sodium and calcium accumulation with hyperosmolar effects affecting the integrity of the blood-brain barrier [32]. Additionally, neuronal sodium and calcium influx will likely influence the membrane potential facilitating further ion influx through voltage-gated ion channels. Sodium influx through VGSCs during seizure activity may therefore be considered as the crucial mechanism initiating the cascade leading to cerebral edema. It is therefore likely that this cascade may be impacted by both availability of sodium ions and/or changes in the sodium ion gradient as well as altered functioning or expression of VGSCs. Increased excitability due to loss of function from mutations of SCN1A may reflect compensatory upregulation of other VGSC and voltage-gated calcium channels [33] or lack of signaling through GABA-ergic [inhibitory] interneurons in the hippocampus [34]. Increased excitability due to gain of function mutations of SCN1A also reported in DS, may reflect a lowered activation threshold of the channel or prolong channel opening increasing intracellular sodium influx and accumulation [35], [36], [37].
-
(3)
Post-ictal bradycardia and asystole as a consequence of ictal activity spreading to the cardiorespiratory nuclei of the brainstem is a proposed mechanism of SUDEP. Bradycardia is also a well-documented reaction to increased ICP (Cushing Reflex) and may further contribute to post-ictal cardio–respiratory dysfunction. Furthermore it is also possible autonomic dysfunction observed in DS patients may alter physiological compensatory mechanisms to increased ICP mediated given the complexity of brainstem-subcortical networks.
This case report is limited by the inherent nature of an isolated patient report but also by the limited documentation due to the emergency situation. However, disease-directed treatment strategies aimed at attenuating autonomic dysfunction and preventing cardiac arrest in DS patients require further investigation.
4. Conclusion
Cytotoxic brain edema, neuronal swelling and subsequently Ca2 +-independent neuronal death are mediated by a sodium ion influx into neurons, which peaks during the repetitive firing of neurons underlying seizures. It is therefore possible that sodium channel dysfunction can promote fatal cerebral edema under certain circumstances including the post-ictal period. The combination of hyperthermia, ion channel dysfunction and possibly emotional stress may therefore not only trigger seizures but also facilitate a fatal pathophysiological cascade of cerebral edema leading to cerebral herniation causing cardiorespiratory collapse. Furthermore, SCN1A mutations may alter susceptibility for neuronal hypoxia. Further analyses are required to elucidate underlying pathophysiological mechanisms of SUDEP and post-ictal edema in DS patients.
Acknowledgments
Acknowledgements
None.
Disclosure of potential conflicts of interest
Maxine Dibué-Adjei is an employee of LivaNova PLC, manufacturer of vagus nerve stimulators. All other authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers' bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Grant support
The present study was not funded.
References
- 1.Cooper M.S., McIntosh A., Crompton D.E., McMahon J.M., Schneider A., Farrell K. Mortality in Dravet syndrome. Epilepsy Res. 2016;128:43–47. doi: 10.1016/j.eplepsyres.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Skluzacek J.V., Watts K.P., Parsy O., Wical B., Camfield P. Dravet syndrome and parent associations: the IDEA League experience with comorbid conditions, mortality, management, adaptation, and grief. Epilepsia. 2011;52(Suppl. 2):95–101. doi: 10.1111/j.1528-1167.2011.03012.x. [DOI] [PubMed] [Google Scholar]
- 3.Surges R., Thijs R.D., Tan H.L., Sander J.W. Sudden unexpected death in epilepsy: risk factors and potential pathomechanisms. Nat Rev Neurol. 2009;5:492–504. doi: 10.1038/nrneurol.2009.118. [DOI] [PubMed] [Google Scholar]
- 4.Ermolyuk Y.S., Alder F.G., Surges R., Pavlov I.Y., Timofeeva Y., Kullmann D.M. Differential triggering of spontaneous glutamate release by P/Q-, N- and R-type Ca2 + channels. Nat Neurosci. 2013;16:1754–1763. doi: 10.1038/nn.3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langan Y. Sudden unexpected death in epilepsy (SUDEP): risk factors and case control studies. Seizure. 2000;9:179–183. doi: 10.1053/seiz.2000.0388. [DOI] [PubMed] [Google Scholar]
- 6.Langan Y., Nashef L., Sander J.W. Sudden unexpected death in epilepsy: a series of witnessed deaths. J Neurol Neurosurg Psychiatry. 2000;68:211–213. doi: 10.1136/jnnp.68.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Langan Y., Nashef L., Sander J.W. Case-control study of SUDEP. Neurology. 2005;64:1131–1133. doi: 10.1212/01.WNL.0000156352.61328.CB. [DOI] [PubMed] [Google Scholar]
- 8.Terrence C.F., Jr., Wisotzkey H.M., Perper J.A. Unexpected, unexplained death in epileptic patients. Neurology. 1975;25:594–598. doi: 10.1212/wnl.25.6.594. [DOI] [PubMed] [Google Scholar]
- 9.Lhatoo S.D., Nei M., Raghavan M., Sperling M., Zonjy B., Lacuey N. Nonseizure SUDEP: sudden unexpected death in epilepsy without preceding epileptic seizures. Epilepsia. 2016;57:1161–1168. doi: 10.1111/epi.13419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryvlin P., Nashef L., Lhatoo S.D., Bateman L.M., Bird J., Bleasel A. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol. 2013;12:966–977. doi: 10.1016/S1474-4422(13)70214-X. [DOI] [PubMed] [Google Scholar]
- 11.Nei M., Ho R.T., Abou-Khalil B.W., Drislane F.W., Liporace J., Romeo A. EEG and ECG in sudden unexplained death in epilepsy. Epilepsia. 2004;45:338–345. doi: 10.1111/j.0013-9580.2004.05503.x. [DOI] [PubMed] [Google Scholar]
- 12.DeGiorgio C.M., DeGiorgio A.C. SUDEP and heart rate variability. Epilepsy Res. 2010;90:309–310. doi: 10.1016/j.eplepsyres.2010.03.013. [author reply 311-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delogu A.B., Spinelli A., Battaglia D., Dravet C., De Nisco A., Saracino A. Electrical and autonomic cardiac function in patients with Dravet syndrome. Epilepsia. 2011;52(Suppl. 2):55–58. doi: 10.1111/j.1528-1167.2011.03003.x. [DOI] [PubMed] [Google Scholar]
- 14.Ergul Y., Ekici B., Tatli B., Nisli K., Ozmen M. QT and P wave dispersion and heart rate variability in patients with Dravet syndrome. Acta Neurol Belg. 2013;113:161–166. doi: 10.1007/s13760-012-0140-z. [DOI] [PubMed] [Google Scholar]
- 15.Zimmer T., Haufe V., Blechschmidt S. Voltage-gated sodium channels in the mammalian heart. Glob Cardiol Sci Pract. 2014;2014:449–463. doi: 10.5339/gcsp.2014.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dravet C., Bureau M., Oguni H., Fukuyama Y., Cokar O. Severe myoclonic epilepsy in infancy: Dravet syndrome. Adv Neurol. 2005;95:71–102. [PubMed] [Google Scholar]
- 17.Oakley J.C., Kalume F., Yu F.H., Scheuer T., Catterall W.A. Temperature- and age-dependent seizures in a mouse model of severe myoclonic epilepsy in infancy. Proc Natl Acad Sci U S A. 2009;106:3994–3999. doi: 10.1073/pnas.0813330106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liautard C., Scalmani P., Carriero G., de Curtis M., Franceschetti S., Mantegazza M. Hippocampal hyperexcitability and specific epileptiform activity in a mouse model of Dravet syndrome. Epilepsia. 2013;54:1251–1261. doi: 10.1111/epi.12213. [DOI] [PubMed] [Google Scholar]
- 19.Sawyer N.T., Helvig A.W., Makinson C.D., Decker M.J., Neigh G.N., Escayg A. Scn1a dysfunction alters behavior but not the effect of stress on seizure response. Genes Brain Behav. 2016;15:335–347. doi: 10.1111/gbb.12281. [DOI] [PubMed] [Google Scholar]
- 20.Canas N., Breia P., Soares P., Saraiva P., Calado S., Jordao C. The electroclinical-imagiological spectrum and long-term outcome of transient periictal MRI abnormalities. Epilepsy Res. 2010;91:240–252. doi: 10.1016/j.eplepsyres.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 21.Lassmann H., Petsche U., Kitz K., Baran H., Sperk G., Seitelberger F. The role of brain edema in epileptic brain damage induced by systemic kainic acid injection. Neuroscience. 1984;13:691–704. doi: 10.1016/0306-4522(84)90089-7. [DOI] [PubMed] [Google Scholar]
- 22.Gao Q., Ou-Yang T.P., Sun X.L., Yang F., Wu C., Kang T. Prediction of functional outcome in patients with convulsive status epilepticus: the END-IT score. Crit Care. 2016;20:46. doi: 10.1186/s13054-016-1221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Myers K.A., McMahon J.M., Mandelstam S.A., Mackay M.T., Kalnins R.M., Leventer R.J. Fatal cerebral edema with status epilepticus in children with Dravet syndrome: report of 5 cases. Pediatrics. 2017;139 doi: 10.1542/peds.2016-1933. [DOI] [PubMed] [Google Scholar]
- 24.Okumura A., Uematsu M., Imataka G., Tanaka M., Okanishi T., Kubota T. Acute encephalopathy in children with Dravet syndrome. Epilepsia. 2012;53:79–86. doi: 10.1111/j.1528-1167.2011.03311.x. [DOI] [PubMed] [Google Scholar]
- 25.Takayanagi M., Haginoya K., Umehara N., Kitamura T., Numata Y., Wakusawa K. Acute encephalopathy with a truncation mutation in the SCN1A gene: a case report. Epilepsia. 2010;51:1886–1888. doi: 10.1111/j.1528-1167.2010.02600.x. [DOI] [PubMed] [Google Scholar]
- 26.Berkovic S.F., Harkin L., McMahon J.M., Pelekanos J.T., Zuberi S.M., Wirrell E.C. De-novo mutations of the sodium channel gene SCN1A in alleged vaccine encephalopathy: a retrospective study. Lancet Neurol. 2006;5:488–492. doi: 10.1016/S1474-4422(06)70446-X. [DOI] [PubMed] [Google Scholar]
- 27.Chipaux M., Villeneuve N., Sabouraud P., Desguerre I., Boddaert N., Depienne C. Unusual consequences of status epilepticus in Dravet syndrome. Seizure. 2010;19:190–194. doi: 10.1016/j.seizure.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 28.Plant L.D., Marks J.D., Goldstein S.A. SUMOylation of NaV1.2 channels mediates the early response to acute hypoxia in central neurons. Elife. 2016:5. doi: 10.7554/eLife.20054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fung M.L., Croning M.D., Haddad G.G. Sodium homeostasis in rat hippocampal slices during oxygen and glucose deprivation: role of voltage-sensitive sodium channels. Neurosci Lett. 1999;275:41–44. doi: 10.1016/s0304-3940(99)00728-4. [DOI] [PubMed] [Google Scholar]
- 30.Fung M.L. Role of voltage-gated Na + channels in hypoxia-induced neuronal injuries. Clin Exp Pharmacol Physiol. 2000;27:569–574. doi: 10.1046/j.1440-1681.2000.03309.x. [DOI] [PubMed] [Google Scholar]
- 31.Stys P.K., Lopachin R.M. Mechanisms of calcium and sodium fluxes in anoxic myelinated central nervous system axons. Neuroscience. 1998;82:21–32. doi: 10.1016/s0306-4522(97)00230-3. [DOI] [PubMed] [Google Scholar]
- 32.Rosenberg G.A. Brain edema and disorders of cerebrospinal fluid circulation. In: Bradley WGD R.B., Ferichel G.M., Marsden C.D., editors. Neurology in clinical practice. Butterworth Heinmann; Boston: 2000. pp. 1545–1559. [Google Scholar]
- 33.Yu F.H., Mantegazza M., Westenbroek R.E., Robbins C.A., Kalume F., Burton K.A. Reduced sodium current in GABAergic interneurons in a mouse model of severe myoclonic epilepsy in infancy. Nat Neurosci. 2006;9:1142–1149. doi: 10.1038/nn1754. [DOI] [PubMed] [Google Scholar]
- 34.Ogiwara I., Miyamoto H., Morita N., Atapour N., Mazaki E., Inoue I. Nav1.1 localizes to axons of parvalbumin-positive inhibitory interneurons: a circuit basis for epileptic seizures in mice carrying an Scn1a gene mutation. J Neurosci. 2007;27:5903–5914. doi: 10.1523/JNEUROSCI.5270-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Volkers L., Kahlig K.M., Verbeek N.E., Das J.H., van Kempen M.J., Stroink H. Nav 1.1 dysfunction in genetic epilepsy with febrile seizures-plus or Dravet syndrome. Eur J Neurosci. 2011;34:1268–1275. doi: 10.1111/j.1460-9568.2011.07826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rhodes T.H., Lossin C., Vanoye C.G., Wang D.W., George A.L., Jr. Noninactivating voltage-gated sodium channels in severe myoclonic epilepsy of infancy. Proc Natl Acad Sci U S A. 2004;101:11147–11152. doi: 10.1073/pnas.0402482101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cossette P., Loukas A., Lafreniere R.G., Rochefort D., Harvey-Girard E., Ragsdale D.S. Functional characterization of the D188V mutation in neuronal voltage-gated sodium channel causing generalized epilepsy with febrile seizures plus (GEFS) Epilepsy Res. 2003;53:107–117. doi: 10.1016/s0920-1211(02)00259-0. [DOI] [PubMed] [Google Scholar]