Abstract
Background
Septic shock is a highly lethal condition. Early recognition of tissue hypoperfusion and its reversion are key factors for limiting progression to multiple organ dysfunction and death. Lactate-targeted resuscitation is the gold-standard under current guidelines, although it has several pitfalls including that non-hypoxic sources of lactate might predominate in an unknown proportion of patients. Peripheral perfusion-targeted resuscitation might provide a real-time response to increases in flow that could lead to a more timely decision to stop resuscitation, thus avoiding fluid overload and the risks of over-resuscitation. This article reports the rationale, study design and analysis plan of the ANDROMEDA-SHOCK Study.
Methods
ANDROMEDA-SHOCK is a randomized controlled trial which aims to determine if a peripheral perfusion-targeted resuscitation is associated with lower 28-day mortality compared to a lactate-targeted resuscitation in patients with septic shock with less than 4 h of diagnosis. Both groups will be treated with the same sequential approach during the 8-hour study period pursuing normalization of capillary refill time versus normalization or a decrease of more than 20% of lactate every 2 h. The common protocol starts with fluid responsiveness assessment and fluid loading in responders, followed by a vasopressor and an inodilator test if necessary. The primary outcome is 28-day mortality, and the secondary outcomes are: free days of mechanical ventilation, renal replacement therapy and vasopressor support during the first 28 days after randomization; multiple organ dysfunction during the first 72 h after randomization; intensive care unit and hospital lengths of stay; and all-cause mortality at 90-day. A sample size of 422 patients was calculated to detect a 15% absolute reduction in mortality in the peripheral perfusion group with 90% power and two-tailed type I error of 5%. All analysis will follow the intention-to-treat principle.
Conclusions
If peripheral perfusion-targeted resuscitation improves 28-day mortality, this could lead to simplified algorithms, assessing almost in real-time the reperfusion process, and pursuing more physiologically sound objectives. At the end, it might prevent the risk of over-resuscitation and lead to a better utilization of intensive care unit resources.
Trial registration ClinicalTrials.gov Identifier: NCT03078712 (registered retrospectively March 13th, 2017)
Keywords: Septic shock, Resuscitation, Peripheral perfusion, Lactate, Fluid responsiveness
Background
Septic shock is a potentially lethal condition associated with a mortality risk of up to 30–60% [1, 2]. Early recognition of tissue hypoperfusion and its reversion are key factors for limiting progression to multiple organ dysfunction and death [1–6].
Hyperlactatemia has been traditionally considered as a hallmark of ongoing tissue hypoxia and anaerobic metabolism [7, 8]. A recent study targeting a decrease in lactate levels as a resuscitation goal in critically ill patients showed a significant improvement in organ failure and outcomes associated with this endpoint [9]. Therefore, normalization of lactate levels has been recommended as a resuscitation target by current guidelines [10]. However, other non-hypoperfusion-related causes of hyperlactatemia might predominate in an unknown number of patients [11, 12]. In that setting, sustained efforts to increase cardiac output (CO) with fluids or vasoactive drugs could lead to detrimental effects of over-resuscitation [13, 14]. In addition, lactate exhibits a biphasic recovery rate even after a successful resuscitation [15], introducing an important confounder for practitioners.
Monitoring peripheral perfusion is particularly attractive because of its easy clinical accessibility and more importantly, because it could reflect the adequacy of intraabdominal visceral organ perfusion [16, 17]. The skin territory lacks auto-regulatory flow control, and therefore, sympathetic activation impairs skin perfusion during circulatory dysfunction [17], a phenomenon that could be evaluated by peripheral perfusion assessment. A robust body of evidence confirms that abnormal peripheral perfusion after initial resuscitation is associated with increased morbidity and mortality [18–23], whereby it could be used as a potential resuscitation goal [24]. In fact, the presence of a cold clammy skin, mottling or prolonged capillary refill time (CRT) has been suggested as indicators to initiate fluid resuscitation in patients with septic shock [17]. Interestingly, CRT was the first parameter to be normalized in patients surviving from septic shock and predicted lactate normalization at 24 h [18]. A recent pilot study suggests that targeting peripheral perfusion during septic shock resuscitation is safe and associated with less fluid administration and organ dysfunctions [25]. Therefore, the excellent prognosis associated with CRT recovery, its rapid-response time to fluid loading, its relative simplicity, its availability in resource-limited settings and its capacity to change in parallel with perfusion of physiologically relevant territories such as the hepatosplanchnic region [16] constitute strong reasons to evaluate the usefulness of CRT to guide resuscitation in septic shock patients.
Consequently, we decided to conduct a randomized controlled trial (RCT) comparing peripheral perfusion-targeted resuscitation (PPTR) versus lactate-targeted resuscitation (LTR) in patients with septic shock, hypothesizing that resuscitation aimed at peripheral perfusion will be associated with lower mortality rates. We also hypothesize that patients assigned to PPTR will require less volume of fluids with subsequent lower positive fluid balances. Accordingly, PPTR should be associated with less organ dysfunctions, especially at respiratory, renal and gastrointestinal levels.
Methods
Primary objective
To determine if PPTR is associated with lower mortality rates at 28 day than a LTR in patients with septic shock.
Secondary objectives
To determine if a PPTR is associated with less severe multiple organ dysfunction; more mechanical ventilation (MV) free days; and less vasopressor load and renal replacement therapies (RRT) than a LTR strategy in patients with septic shock.
Outcomes
Primary outcome will be all-cause mortality at 28-day.
Secondary outcomes:
Free days of MV, RRT and vasopressor support during the first 28 days after randomization;
Multiple organ dysfunction during the first 72 h after randomization [26];
Intensive care unit (ICU) and hospital lengths of stay;
All-cause mortality at 90-day.
Tertiary outcomes:
Amount of resuscitation fluids at 8 and 24-hours;
Total fluid balance at 8, 24, 48 and 72-h;
Occurrence of intraabdominal hypertension (IAH) during the first 72 h after randomization (%);
Use of RRT (%)
In-hospital mortality
Study design
ANDROMEDA-SHOCK is a prospective, multicenter, parallel-group, randomized trial aimed to compare an 8-h protocol of PPTR vs. LTR in patients with septic shock [27].
Patients
Consecutive adult patients (≥ 18 years) with septic shock will be considered eligible. Septic shock is defined as suspected or confirmed infection, plus hyperlactatemia (≥ 2.0 mmol per liter) and vasopressor requirements due to refractory hypotension [27]. This latter is characterized as a systolic blood pressure (SBP) < 90 mmHg or a mean arterial pressure (MAP) < 65 mmHg after an intravenous fluid load of at least 20 ml/kg, administered over the course of 60 min.
Patients will be excluded in case of:
pregnancy;
anticipated surgery or dialysis procedure during the first 8 h after septic shock diagnosis;
Do-Not-Attempt-Resuscitation status;
active bleeding;
acute hematological malignancy;
concomitant severe acute respiratory distress syndrome (ARDS);
more than 4 h after the onset of septic shock criteria.
An active daily screening for potentially eligible patients will be performed at all the participating ICUs.
Randomization
Eligible patients will be randomly allocated to PPTR or LTR groups. PPTR will be aimed to normalize CRT, while LTR will target lactate normalization or a decreasing rate > 20% per 2 h of lactate levels during the 8 h of the study period (Fig. 1).
Fig. 1.
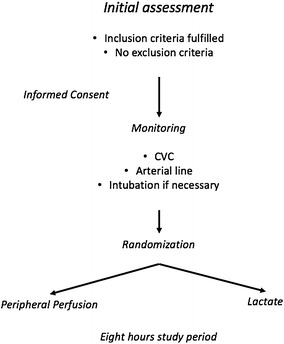
Pre-randomization phase assessments and interventions. CVC central venous catheter
A randomization sequence with an allocation of 1:1 will be generated by a computer program. Study-group assignment will be performed by means of randomized permuted blocks of eight. Allocation concealment will be maintained by means of central randomization. Investigators at the sites will call a representative of the Study Coordinating Center (SCC) available 24 h/7 days through a dedicated phone number. The group to which the patient is allocated will only be disclosed after the information is recorded by the SCC. Such a measure prevents the investigator and the medical team from predicting to which treatment group the patient will be allocated.
Interventions
General management protocol
Both study groups will be treated with a common general management protocol. Sepsis source identification and control, and antimicrobial therapy will be given at the discretion of the treating physician. A central venous catheter (CVC) and an arterial line will be inserted in all cases, while the use of CO monitoring (pulmonary artery catheter or transpulmonary thermodilution techniques) is recommended for patients with a past medical history of heart failure or concomitant ARDS but leaving decision at discretion of the attending physician. Echocardiography will be performed routinely as soon as possible after admission to evaluate basal cardiac function, and to add in assessing fluid responsiveness (FR) [28]. Other dynamic predictors of response to fluids such as pulse pressure variation (PPV), stroke volume variation (SVV) or end-expiratory occlusion test (EEOT) will be used whenever applicable (see below) [28, 29]. MV will be provided (when needed) under light sedation (midazolam, propofol or dexmedetomidine) and analgesia (fentanyl, alfentanil, morphine); tidal volume (Vt) will be limited to 6–8 mL/kg and positive-end-expiratory-pressure (PEEP) will be set according to individual requirements [10]. Glycemic control will be adjusted to maintain glucose levels < 150 mg/dL. Norepinephrine (NE) will be the vasopressor of choice, and its dose will be adjusted to maintain a MAP ≥ 65 mmHg in all patients. Hemoglobin concentrations will be maintained at 8 g/dL or higher to optimize arterial O2 content. The use of other therapies such as epinephrine, vasopressin analogues, steroids or different blood purification techniques like high-volume hemofiltration (HVHF) will be provided according to the usual practice at the involved centers in patients evolving with refractory septic shock. Finally, stress ulcer and venous thrombosis prophylaxis will be managed according to international recommendations [10].
Study protocol
A sequential approach to resuscitation will be followed in both groups as shown in Fig. 2. After fulfilling inclusion criteria and discarding all exclusion conditions, an informed consent will be obtained. Basal measurements including hemodynamics and blood sampling will be performed at Time 0 (T0) representing the starting point just after randomization. The intervention period will be extended for 8 h. All other treatments, during the intervention period and after, will be at the discretion of the treating clinicians according to their local usual clinical practices.
Fig. 2.
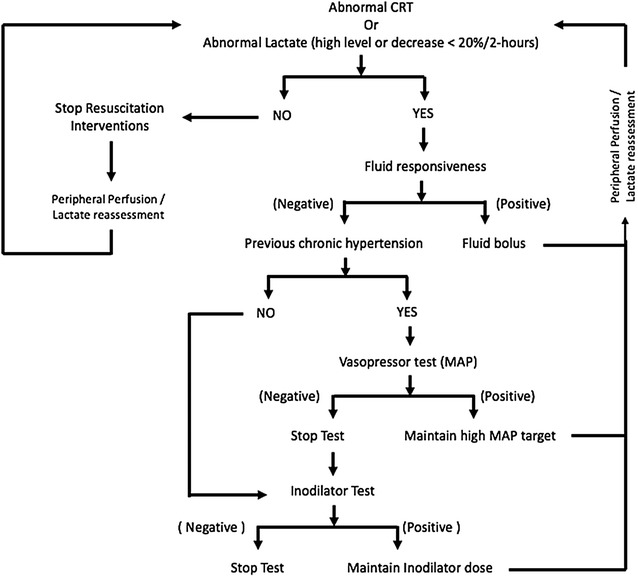
Resuscitation protocol for both groups. The figure describes the sequential approach to resuscitation. The process starts with fluid loading according to the status of fluid responsiveness. If the goal is not obtained, the second step is a vasopressor test, and then an inodilator test. CRT, capillary refill time
Tests and procedures during the study period
Capillary refill time assessment
CRT will be measured by applying firm pressure to the ventral surface of the right index finger distal phalanx with a glass microscope slide. The pressure will be increased until the skin is blank and then maintained for 10 s. The time for return of the normal skin color will be registered with a chronometer. A CRT > 3 s will be considered as abnormal [30].
Lactate measurements
A lactate value ≥ 2.0 mmol per liter will be considered as abnormal. Arterial lactate levels will be measured at each center, either at point of care or central laboratories (point of care: GEM 4000, Instrumentation Lab, IL, USA; central laboratories: Cobas b221, Roche Diagnostics International; Basel, CH).
Fluid responsiveness (Fig. 3)
Fig. 3.
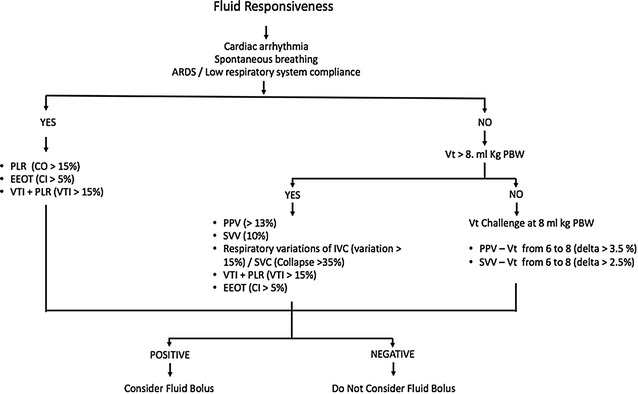
Assessment of fluid responsiveness during the study period. The figure describes an algorithm for assessing fluid responsiveness in different settings depending on the presence or not of mechanical ventilation, arrhythmias or other conditions. Different tests are proposed with the respective cutoff values. ARDS acute respiratory distress syndrome; PLR passive leg rising; CO cardiac output; EEOT end-expiratory occlusion test; CI cardiac index; VTI velocity time integral; Vt tidal volume, PBW predicted body weight; PPV pulse pressure variation; SVV stroke volume variation, IVC inferior vena cava; SVC superior vena cava
FR will be assessed using a structured approach outlined in Fig. 2. Dynamic predictors of FR will be evaluated depending on the individual status, i.e., considering if under MV or spontaneous breathing, Vt, respiratory rate (RR), respiratory system compliance and the presence of arrhythmias. The protocol for patients under MV is shown in Fig. 3 [28, 29].
Fluid challenges
In FR+ patients, the first resuscitation step will be to administer a fluid bolus (FB) of 500 ml of crystalloids every 30 min until normalizing CRT in the PPTR group. In the LTR group, FB will be stopped if at 2 h lactate is normalized or has decreased > 20%, or previously if after any of the fluid boluses, central venous pressure (CVP) has increased ≥ 5 mmHg or the patients have become fluid unresponsive (FR−).
Safety measures during fluid challenges
CVP and FR will be reevaluated after any fluid challenge. If CVP increases < 5 mmHg and FR is still +, another FB will be administered and so on while the perfusion (CRT or lactate) goal are not attained. If CVP increases ≥ 5 mmHg or FR is or become negative, fluids will be stopped and the patient will be moved to the next step.
Vasopressor test
An open-label vasopressor test will be performed increasing MAP up to 80–85 mmHg by using progressive incremental doses of NE in patients with previous history of chronic hypertension and persistently abnormal CRT or unfulfilled lactate goals accompanied by a fluid unresponsive state. Parameters will be reassessed 1 h after in the PPTR and 2 h after in the LTR. If after the vasopressor test, CRT improves, and lactate goals are achieved in PPTR and LTR, respectively, NE will be titrated to maintain this new MAP goal throughout the study period. If goals are not achieved despite increasing MAP, or NE dose surpasses 0.8 mcg/kg/min or adverse effects are observed (heart rate (HR) > 140 ppm, arrhythmias or evident cardiac ischemia), NE dose will be reduced to the level before the vasopressor test, and the protocol will move to the next step.
Inodilator test
An open-label test of dobutamine at fixed 5 mcg/kg/min or milrinone at fixed 0.25 mcg/kg/min doses (at discretion of the attending physician) will be started in patients with persistent abnormal CRT or non-achieved lactate goals, and negative FR status. CRT and lactate goals will be rechecked such as in the vasopressor test. If such resuscitation goals are not reached, drugs will be discontinued and no further action will be taken during the study period, except for rechecking FR every hour and restart fluid challenges if the patient gets again FR + . Dobutamine or milrinone doses will be maintained throughout the study period in those favorably responding to the open-label inodilators test. As a safety measure, inodilators will be stopped if HR increases > 15%, or arrhythmias, ischemia or hypotension develop.
Management of peripheral perfusion-targeted resuscitation
As a safety measure, if signs of inadequate macrohemodynamics persist such as HR > 120 BPM or unstable MAP with increases in vasopressors during the last hour, resuscitation will be continued even if CRT is normal.
After CRT normalization at any step, it will be reassessed hourly during the study period. If at any point it turns abnormal again, the resuscitation sequence will be restarted (Fig. 2)
Management of lactate-targeted resuscitation
Lactate will be assessed every 2 h during the 8-h study period. If after achieving lactate goals, it becomes again abnormal or the decrease rate slow down under 20%/2 h at any of the following controls, the resuscitation sequence will be restarted (Fig. 2).
Safety measures
The protocol can be stopped at any moment for safety considerations during the 8-h study period if the attending intensivist considers that the patient has developed unexpected and severe complications or evolves into refractory shock, conditions that under his judgment require liberalization of management. This action has to be reported on the case report form (CRF), and the patient will be followed up with major outcomes, and included in the intention-to-treat (ITT) analysis. Specific safety measures for fluid administration, vasopressor test and inodilator use are specified above.
Suspected unexpected serious adverse reactions (SUSAR)
Any adverse event that occurs in a clinical trial subject, which is assessed by the study investigator as being unexpected, serious and as having a reasonable possibility of a causal relationship with the study procedure will be reported. Reports of these reactions are subject to expedited submission to health authorities. SUSAR’s will be analyzed by both the SCC and the data safety monitoring committee (DSMC).
Blinding
Since the intervention will be administered to critically ill patients (mostly sedated), blinding of these patients is not necessary. Because this is a non-pharmacological intervention, blinding of the medical team is not feasible.
Data collection and management
Study follow-up and the variables that will be collected are described below.
Baseline
Demographics, comorbidities, acute physiology and chronic health evaluation (APACHE) II [31], sepsis source and treatment
pre-ICU resuscitation and fluid balance
Sequential Organ Failure Assessment (SOFA) [26] + and Acute Kidney Injury Network (AKI) criteria [32]
Hemodynamics: HR, SBP, diastolic blood pressure (DBP), MAP, CVP, FR status, intraabdominal pressure (IAP), NE levels, diuresis.
Perfusion: lactate, central venous O2 saturation (ScvO2), central venous arterial pCO2 gradient (P(cv-a)CO2), Hb, central venous and arterial blood gases, CRT, mottling score.
Evolution
SOFA and AKI criteria at 8, 24, 48 and 72 h
Hemodynamics hourly up to 8 h
Fluid administration and balance at 8, 24, 48 and 72 h
Complete perfusion assessment when the targeted parameter is normalized and then at 8, 24, 48 and 72 h
Register of vasoactive drugs and dobutamine/milrinone use
Register of MV and RRT
Source control re-analysis at 4 h
Rescue therapies: HVHF, vasopressin, epinephrine, steroids, others.
Echocardiography at least once during the study period
Follow-up till 28 days for use of MV, RRT and vasopressors
All-cause mortality at hospital discharge, 28 and 90 days
Cause of death.
Quality control
Several procedures will assure data quality, including (1) all investigators will attend a training session before the start of the study to standardize procedures, including data collection (2) the investigators may contact the SCC to solve issues or problems that may arise; (3) CRFs provided by the centers will be subjected to various checks by members of the SCC for missing data, plausible, possible or non-permitted value ranges, and logic checks on a weekly basis. (4) centers will be notified of the inconsistencies or missing data as queries and asked to correct them; (5) the SCC will review detailed reports on screening, enrollment, follow-up, inconsistencies and completeness of data. Immediate actions will follow to solve problems that arise; (6) only after the CRFs are cleared by the SCC, data will be entered in the final electronic database by the data digitizer.
Sample size
Mortality in patients with increased lactate levels in circulatory dysfunction has been shown to exceed 40% [9]. In addition, several studies have shown that abnormal peripheral perfusion is associated with a mortality exceeding 40% as well, whereas a normal CRT in the early phase of septic shock has been associated with a less than 10% mortality [19, 30].
A total sample size of 420 patients (210 per group), analyzing the data using the ITT principle, is expected to provide approximately 90% power to detect a reduction in 28-day mortality from 45 to 30%, considering logistic regression, with a two-sided alpha level of 5%. We consider a decrease of 15% in mortality to have direct clinical implementation effect. Similar effects on mortality have been shown in early resuscitation studies. In addition, limiting fluid administration in patients with septic shock and normal peripheral perfusion has been shown to decrease organ failure, which is the leading cause of death in these patients [25].
However, if a smaller decrease in mortality (such as 10%) is observed at interim analyses, our initial calculated sample size would have only a 57% power to detect benefit. Therefore, we will use an adaptive approach [33] that will allow for a sample-size re-estimation at a preplanned interim analysis after 75% of the sample has been recruited. The sample-size re-estimation will be conducted by the DSMC only if the size effect observed in the interim analysis is between 10 and 15% absolute reduction in mortality [32].
Statistical analysis plan
We will report a detailed statistical analysis plan in a separate document.
Briefly, all analysis will follow the intention-to-treat principle.
Primary outcome
We will assess the effect of PPTR compared to LTR on the primary outcome using time-to-event analysis. Results of our main analysis will be calculated with Cox proportional hazards models, with adjustment for five pre-specified baseline covariates. APACHE II score, SOFA score, lactate level, CRT and source of infection, as fixed (individual-level) effects. Results will be reported as hazard ratios with 95% confidence intervals (CI) and p values. We will also present Kaplan–Meier curves.
Secondary outcomes
We identified several secondary outcomes. First, binary outcomes will be compared through Chi-squared tests, and we will present the results risk ratios (RR), with 95% CI and p values.
Continuous outcomes with normal distribution will be analyzed with t test and reported as mean difference 95% CI and p value. Continuous outcomes with asymmetrical distribution will be analyzed using bootstrapping techniques and reported as absolute difference between medians, 95% CI and p values.
Subgroup analyses
We will analyze the effects of resuscitation strategies on the primary outcome in the following subgroups:
Patients with lactate > 4.0 mmol/L as set by SSC [10]
Patients without a confirmed source of infection (as this could increase the translation of the study to other critically ill).
Patients with low APACHE II/SOFA scores
Patients with a more than 10% difference in lactate levels between the very first one measured and the baseline when starting the study.
Ethical aspects
Each investigator center will submit the study protocol to its Institutional Review Board (IRB). The study will start only after being approved by the IRB. Written informed consent will be obtained from a legal representative of all participants. This study is in compliance with local and international declarations.
Trial organization and management
Study Coordinating Center
A team based on the Departmento de Medicina Intensiva, Facultad de Medicina of Pontificia Universidad Católica, Chile, will manage the trial on a day-to-day basis. The SCC is comprised by the chief and co-chair investigators, four project managers, a statistician and a data digitizer. The statistician is based on the Research Institute HCor, São Paulo, Brazil.
The responsibilities of the SCC include:
Planning and conducting the study designing the protocol; designing the CRF; designing the operation guide; managing and controlling data quality; designing, testing and maintaining the electronic database; data quality control; assisting the steering committee;
Managing the research centers selecting and training the research centers; helping the centers prepare a regulatory report to be submitted to the IRBs and assisting the centers with the submission; monitoring recruitment rates and the actions to increase recruitment; monitoring follow-up and implementing actions to prevent follow-up losses; auditing; sending study materials to the research centers; producing a monthly study newsletter; developing supporting material for the study;
Statistical analysis and research reporting complete statistical analysis; helping to write the final manuscript.
Trial Steering Committee
The Trial Steering Committee (TSC) is responsible for the overall study supervision, assisting in developing the study protocol and preparing the final manuscript. All other study committees report to the TSC. The TSC members are investigators trained in designing and conducting randomized clinical trials in critically ill patients.
Study centers
The study centers for ANDROMEDA-SHOCK were selected through a rigorous process. This started with a survey of professional and technical resources as well as processes of care. Centers were contacted trying to make this process representative across public, private and university hospitals, different countries and cultures, and hospital size.
At the end, 34 centers were selected and all applied for IRB approval, leaving finally 26 active centers to start on March 1, 2017, in 5 countries. Brazil is still pending
Details of the centers which accepted to participating in the trial at the time of this manuscript submission are given in the Appendix.
Publication policy
The ANDROMEDA-SHOCK study success depends on all its collaborators. Therefore, the primary results of the trial will be published under the name of ANDROMEDA-SHOCK Investigators. The contributions of all collaborators, their names and respective institutions, will be acknowledged in the manuscript. To safeguard the scientific integrity of the study, data from this study will be submitted to publication only after the final approval from the TSC.
Data Safety Monitoring Committee
The DSMC is set up with independent epidemiologists and intensivists. The DSMC is in charge of providing recommendations for the SCC of continuing the study as planned or discontinuing the recruitment based on evidence that the intervention causes increased mortality in the experimental group (PPTR) as compared to the control group (LTR). Interim analyses will be conducted after recruitment of the first 100 patients and at 75% of the sample.
In addition, the DSMC will discuss and potentially recommend a re-estimation of the sample size according to the interim analysis after recruitment of 75% of the patients. A sample-size re-estimation design is a flexible, adaptive design with the primary purpose of allowing sample size of a study to be reassessed in the mid-course of the study to ensure adequate power.
Discussion
ANDROMEDA-SHOCK is a relevant study in septic shock for several reasons: (1) it determines the value of a simple, bedside, universally available parameter to be used as a resuscitation goal in early septic shock; (2) it proposes an early goal-directed resuscitation strategy based on a holistic physiological view of the reperfusion process; (3) it challenges the gold-standard parameter of lactate since this latter is not universally available and has many interpretation difficulties.
If our hypothesis proves to be correct, resuscitation algorithms might be simplified, assessing almost in real-time the reperfusion process, and in pursuing more physiologically sound objectives through a peripheral perfusion-based strategy, it could prevent the risk of over-resuscitation and lead to a better utilization of ICU resources.
Study status
ANDROMEDA-SHOCK study started recruiting on March 1 in 26 centers from five countries. At the submission of this manuscript, already 388 patients have been recruited.
Authors’ contributions
JB and GH are guarantors of the entire manuscript; JB, JLT, GH, GOT, AD, GF, MC, JH, AC, RC, LA designed the study; All the authors will help in the data interpretation and the final manuscript draft. All authors read and approved this final manuscript.
Acknowledgements
We acknowledge the support from the Departamento de Medicina Intensiva, Facultad de Medicina, Pontificia Universidad Católica de Chile.
Competing interests
The authors declare that they have no competing interests.
Funding
The study is financed in part by an internal grant from the Departamento de Medicina Intensiva, Facultad de Medicina, Pontificia Universidad Católica de Chile.
Ethics approval and consent to participate
Each investigator center will submit the study protocol to its Institutional Review Board (IRB). The study will start only after being approved by the IRB. Written informed consent will be obtained from a legal representative of all participants. This study is in compliance with local and international declarations.
ANDROMEDA–SHOCK Investigators consists of: Writing and Steering Committee
Glenn Hernandez (chair), Gustavo Ospina-Tascón, Alexandre Cavalcanti, Arnaldo Dubin, Javier Hurtado, Gilberto Friedman, Ricardo Castro, Leyla Alegría, Jean-Louis Teboul, Maurizio Cecconi, Fernando Zampieri, Lucas Petri Damiani, Jan Bakker (co-chair). Study Coordinating Center: Glenn Hernandez, Leyla Alegría, Giorgio Ferri, Nicolás Rodriguez, Patricia Holger, Natalia Soto, Mario Pozo, Lucas Petri Damiani, Jan Bakker. Data Safety Monitoring Committee: Deborah Cook, Jean-Louis Vincent, Andrew, Rhodes, Bryan Kavanagh, Phil Dellinger, Wim Rietdijk. Study Centers: Chile: Pontificia Universidad Católica de Chile, Santiago: David Carpio, Nicolás Pavéz, Elizabeth Henriquez, Sebastian Bravo, Emilio Daniel Valenzuela; Hospital Barros-Luco Trudeau, Santiago: Maria Alicia Cid, Ronald Pairumani, Macarena Larroulet, Edward Petruska, Claudio Sarabia; Hospital San Juan de Dios, Santiago: David Gallardo, Juan Eduardo Sanchez, Hugo González, José Miguel Arancibia, Alex Muñoz, Germán Ramirez, Florencia Aravena; Hospital Dr. Sótero del Río, Santiago: Andrés Aquevedo, Fabián Zambrano; Hospital Del Salvador, Santiago: Milan Bozinovic, Felipe Valle, Manuel Ramirez, Victor Rossel, Pilar Muñoz, Carolina Ceballos; Hospital Herminda Martinez, Chillán: Christian Esveile, Cristian Carmona, Eva Candia, Daniela Mendoza; Hospital San Juan de Dios, Curicó: Aída Sanchez; Hospital Guillermo Grant Benavente, Concepción: Paula Fernández, Daniela Ponce, Jaime Lastra, Bárbara Nahuelpán, Fabrizio Fasce; Hospital Clínico Universidad de Chile, Santiago: Cecilia Luengo, Nicolas Medel, Cesar Cortés. Argentina: Clínica La Pequeña Familia, Junín: Luz Campassi; Sanatorio Otamendi y Miroli, Buenos Aires: Arnaldo Dubin, Paolo Rubatto, Brenda Nahime Horna, Mariano Furche; Hospital Provincial del Centenario, Rosario: Juan Carlos Pendino, Lisandro Bettini; Sanatorio Parque, Rosario: Carlos Lovesio, María Cecilia González, Jésica Rodruguez; Hospital Interzonal San Martín, La Plata: Elisa Estenssoro, Héctor Canales, Francisco Caminos; Sanatorio Allende Nueva Córdoba: Cayetano Galletti, Estefanía Minoldo, María José Aramburu, Daniela Olmos; Uruguay: Hospital Español Juan J Crottogini, Montevideo: Javier Hurtado, Nicolás Nin, Jordán Tenzi, Carlos Quiroga, Pablo Lacuesta, Agustín Gaudín, Richard Pais, Ana Silvestre, Germán Olivera; Hospital de Clínicas, Montevideo: Gloria Rieppi, Dolores Berrutti. Ecuador: Hospital Universitario del Rio, Cuenca: Marcelo Ochoa, Paul Cobos, Fernando Vintimilla; Hospital Eugenio Espejo, Quito: Vanessa Ramirez, Milton Tobar, Manuel Jibaja, Fernanda García, Fabricio Picoita, Nelson Remache; Hospital San Francisco de Quito, Quito: Vladimir Granda, Fernando Paredes, Eduardo Barzallo, Paul Garcés; Hospital Carlos Andrade Marín, Quito: Fausto Guerrero, Santiago Salazar, German Torres, Cristian Tana, José Calahorrano, Freddy Solis; Hospital IESS Ibarra, Ibarra: Pedro Torres, Luís Herrera, Antonio Ornes, Verónica Peréz, Glenda Delgado, Alexei Carbonell, Eliana Espinosa, José Moreira; Hospital General Docente Calderón, Quito: Diego Barahona, Blanca Salcedo, Ivonne Villacres, Jhonny Suing, Marco Lopez, Luis Gomez, Guillermo Toctaquiza, Mario Cadena Zapata, Milton Alonso Orazabal, Ruben Pardo Espejo, Jorge Jimenez, Alexander Calderón. Hospital Enrique Garcés, Quito: Gustavo Paredes, José Luis Barberán, Tatiana Moya. Colombia: Hospital San Vicente de Paul, Medellín: Horacio Atehortua, Rodolfo Sabogal; Hospital de Santa Clara, Bogotá: Guillermo Ortiz, Antonio Lara; Hospital Universitario de Ñarino E.S.E, Pasto: Fabio Sanchez, Alvaro Hernán Portilla, Humberto Dávila, Jorge Antonio Mora; Fundación Valle del Lili, Cali: Gustavo-Ospina Tascón, Luis Eduardo Calderón, Ingrid Alvarez, Elena Escobar, Alejandro Bejarano, Luis Alfonso Bustamante.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- CO
cardiac output
- CRT
capillary refill time
- RCT
randomized controlled trial
- PPTR
peripheral perfusion-targeted resuscitation
- LTR
lactate-targeted resuscitation
- MV
mechanical ventilation
- RRT
renal replacement therapy
- ICU
intensive care unit
- IAH
intraabdominal hypertension
- SBP
systolic blood pressure
- MAP
mean arterial pressure
- SCC
Study Coordinating Center
- CVC
central venous catheter
- FR
fluid responsiveness
- PPV
pulse pressure variation
- SVV
stroke volume variation
- EEOT
end-expiratory occlusion test
- Vt
tidal volume
- PEEP
positive-end-expiratory-pressure
- NE
norepinephrine
- HVHF
high-volume hemofiltration
- RR
respiratory rate
- VTI
aortic velocity time integral
- PLR
passive leg raising
- HR
heart rate
- CRF
case report form
- ITT
intention-to-treat
- DSMC
data safety monitoring committee
- APACHE
acute physiology and chronic health evaluation
- AKI
acute kidney injury network
- DBP
diastolic blood pressure
- SUSAR
suspected unexpected serious adverse reactions
- IAP
intraabdominal pressure
- ScvO2
central venous oxygen saturation
- P(cv‐a) CO2
central venous‐arterial PCO2 gradient
- IRB
Institutional Review Board
- TSC
Trial Steering Committee
Contributor Information
Glenn Hernández, Email: glennguru@gmail.com.
Alexandre Biasi Cavalcanti, Email: alexandrebiasi@hotmail.com.
Gustavo Ospina-Tascón, Email: gusospin@gmail.com.
Fernando Godinho Zampieri, Email: fzampieri@hcor.com.br.
Arnaldo Dubin, Email: arnaldodubin@gmail.com.
F. Javier Hurtado, Email: javierhurtado2005@gmail.com.
Ricardo Castro, Email: rcastro.med@gmail.com.
Leyla Alegría, Email: lmalegri@gmail.com.
Maurizio Cecconi, Email: mcecconi@sgul.ac.uk.
Jean-Louis Teboul, Email: jlteboul.bicetre@invivo.edu.
Jan Bakker, Email: jan.bakker@erasmusmc.nl.
The ANDROMEDA-SHOCK Study Investigators:
Glenn Hernandez, Gustavo Ospina-Tascón, Alexandre Cavalcanti, Arnaldo Dubin, Javier Hurtado, Gilberto Friedman, Ricardo Castro, Leyla Alegría, Jean-Louis Teboul, Maurizio Cecconi, Fernando Zampieri, Lucas Petri Damiani, Jan Bakker, Giorgio Ferri, Nicolás Rodriguez, Patricia Holger, Natalia Soto, Mario Pozo, Deborah Cook, Jean-Louis Vincent, Andrew Rhodes, Bryan Kavanagh, Phil Dellinger, Wim Rietdijk, David Carpio, Nicolás Pavéz, Elizabeth Henriquez, Sebastian Bravo, Emilio Daniel Valenzuela, Maria Alicia Cid, Ronald Pairumani, Macarena Larroulet, Edward Petruska, Claudio Sarabia, David Gallardo, Juan Eduardo Sanchez, Hugo González, José Miguel Arancibia, Alex Muñoz, Germán Ramirez, Florencia Aravena, Andrés Aquevedo, Fabián Zambrano, Milan Bozinovic, Felipe Valle, Manuel Ramirez, Victor Rossel, Pilar Muñoz, Carolina Ceballos, Christian Esveile, Cristian Carmona, Eva Candia, Daniela Mendoza, Aída Sanchez, Paula Fernández, Daniela Ponce, Jaime Lastra, Bárbara Nahuelpán, Fabrizio Fasce, Cecilia Luengo, Nicolas Medel, Cesar Cortés, Luz Campassi, Paolo Rubatto, Brenda Nahime Horna, Mariano Furche, Juan Carlos Pendino, Lisandro Bettini, Carlos Lovesio, María Cecilia González, Jésica Rodruguez, Elisa Estenssoro, Héctor Canales, Francisco Caminos, Cayetano Galletti, Estefanía Minoldo, María José Aramburu, Daniela Olmos, Nicolás Nin, Jordán Tenzi, Carlos Quiroga, Pablo Lacuesta, Agustín Gaudín, Richard Pais, Ana Silvestre, Germán Olivera, Gloria Rieppi, Dolores Berrutti, Marcelo Ochoa, Paul Cobos, Fernando Vintimilla, Vanessa Ramirez, Milton Tobar, Manuel Jibaja, Fernanda García, Fabricio Picoita, Nelson Remache, Vladimir Granda, Fernando Paredes, Eduardo Barzallo, Paul Garcés, Fausto Guerrero, Santiago Salazar, German Torres, Cristian Tana, José Calahorrano, Freddy Solis, Pedro Torres, Luís Herrera, Antonio Ornes, Verónica Peréz, Glenda Delgado, Alexei Carbonell, Eliana Espinosa, José Moreira, Diego Barahona, Blanca Salcedo, Ivonne Villacres, Jhonny Suing, Marco Lopez, Luis Gomez, Guillermo Toctaquiza, Mario Cadena Zapata, Milton Alonso Orazabal, Ruben Pardo Espejo, Jorge Jimenez, Alexander Calderón, Gustavo Paredes, José Luis Barberán, Tatiana Moya, Horacio Atehortua, Rodolfo Sabogal, Guillermo Ortiz, Antonio Lara, Fabio Sanchez, Alvaro Hernán Portilla, Humberto Dávila, Jorge Antonio Mora, Gustavo Ospina-Tascón, Luis Eduardo Calderón, Ingrid Alvarez, Elena Escobar, Alejandro Bejarano, and Luis Alfonso Bustamante
References
- 1.Vincent JL, De Backer D. Circulatory shock. N Engl J Med. 2013;369:1726–1734. doi: 10.1056/NEJMra1208943. [DOI] [PubMed] [Google Scholar]
- 2.Cecconi M, De Backer D, Antonelli M, Beale R, Bakker J, Hofer C, et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensiv Care Med. 2014;40:1795–1815. doi: 10.1007/s00134-014-3525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shoemaker WC, Appel PL, Kram HB. Tissue oxygen debt as a determinant of lethal and nonlethal postoperative organ failure. Crit Care Med. 1988;16:1117–1120. doi: 10.1097/00003246-198811000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Vallet B. Vascular reactivity and tissue oxygenation. Intensiv Care Med. 1998;24:3–11. doi: 10.1007/s001340050507. [DOI] [PubMed] [Google Scholar]
- 5.Bellomo R, Reade MC, Warrillow SJ. The pursuit of a high central venous oxygen saturation in sepsis: growing concerns. Crit Care. 2008;12:130. doi: 10.1186/cc6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.PRISM Investigators. Rowan KM, Angus DC, Bailey M, Barnato AE, Bellomo R, et al. Early, goal-directed therapy for septic shock—a patient-level meta-analysis. N Engl J Med. 2017;376:2223–2234. doi: 10.1056/NEJMoa1701380. [DOI] [PubMed] [Google Scholar]
- 7.Bakker J, Gris P, Coffernils M, Kahn R, Vincent J. Serial blood lactate levels can predict the development of multiple organ failure following septic shock. Am J Surg. 1996;171:221–226. doi: 10.1016/S0002-9610(97)89552-9. [DOI] [PubMed] [Google Scholar]
- 8.Friedman G, De Backer D, Shahla M, Vincent JL. Oxygen supply dependency can characterize septic shock. Intensiv Care Med. 1998;24:118–123. doi: 10.1007/s001340050531. [DOI] [PubMed] [Google Scholar]
- 9.Jansen TC, van Bommel J, Schoonderbeek FJ, Sleeswijk Visser SJ, van der Klooster JM, Lima AP, et al. Early lactate-guided therapy in intensive care unit patients: a multicenter, open-label, randomized controlled trial. Am J Respir Crit Care Med. 2010;182:752–761. doi: 10.1164/rccm.200912-1918OC. [DOI] [PubMed] [Google Scholar]
- 10.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensiv Care Med. 2017;43:304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Alvarez M, Marik P, Bellomo R. Sepsis-associated hyperlactatemia. Crit Care. 2014;18:503. doi: 10.1186/s13054-014-0503-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hernandez G, Bruhn A, Castro R, Regueira T. The holistic view on perfusion monitoring in septic shock. Curr Opinion Crit Care. 2012;18:280–286. doi: 10.1097/MCC.0b013e3283532c08. [DOI] [PubMed] [Google Scholar]
- 13.Marik PE, Linde-Zwirble WT, Bittner EA, Sahatjian J, Hansell D. Fluid administration in severe sepsis and septic shock, patterns and outcomes: an analysis of a large national database. Intensiv Care Med. 2017;43:625–632. doi: 10.1007/s00134-016-4675-y. [DOI] [PubMed] [Google Scholar]
- 14.Hjortrup PB, Haase N, Bundgaard H, Thomsen SL, Winding R, Pettilä V, et al. Restricting volumes of resuscitation fluid in adults with septic shock after initial management: the CLASSIC randomised, parallel-group, multicentre feasibility trial. Intensiv Care Med. 2016;42:1695–1705. doi: 10.1007/s00134-016-4500-7. [DOI] [PubMed] [Google Scholar]
- 15.Hernandez G, Luengo C, Bruhn A, Kattan E, Friedman G, Ospina-Tascon GA, et al. When to stop septic shock resuscitation: clues from a dynamic perfusion monitoring. Ann Intensive Care. 2014;4:30. doi: 10.1186/s13613-014-0030-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brunauer A, Koköfer A, Bataar O, Gradwohl-Matis I, Dankl D, Bakker J, et al. Changes in peripheral perfusion relate to visceral organ perfusion in early septic shock: a pilot study. J Crit Care. 2016;35:105–109. doi: 10.1016/j.jcrc.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Lima A, Bakker J. Clinical assessment of peripheral circulation. Curr Opin Crit Care. 2015;21:226–231. doi: 10.1097/MCC.0000000000000194. [DOI] [PubMed] [Google Scholar]
- 18.Hernandez G, Pedreros C, Veas E, Bruhn A, Romero C, Rovegno M, et al. Evolution of peripheral vs metabolic perfusion parameters during septic shock resuscitation. J Crit Care. 2012;27:283–288. doi: 10.1016/j.jcrc.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 19.Ait-Oufella H, Lemoinne S, Boelle PY, Galbois A, Baudel JL, Lemant J, et al. Mottling score predicts survival in septic shock. Intensive Care Med. 2011;37:801–807. doi: 10.1007/s00134-011-2163-y. [DOI] [PubMed] [Google Scholar]
- 20.Lima A, Jansen TC, Van Bommel J, Ince C, Bakker J. The prognostic value of the subjective assessment of peripheral perfusion in critically ill patients. Crit Care Med. 2009;37:934–938. doi: 10.1097/CCM.0b013e31819869db. [DOI] [PubMed] [Google Scholar]
- 21.Coudroy R, Jamet A, Frat JP, Veinstein A, Chatellier D, Goudet V, et al. Incidence and impact of skin mottling over the knee and its duration on outcome in critically ill patients. Intensive Care Med. 2015;41:452–459. doi: 10.1007/s00134-014-3600-5. [DOI] [PubMed] [Google Scholar]
- 22.Ait-Oufella H, Bige N, Boelle PY, Pichereau C, Alves M, Bertinchamp R, et al. Capillary refill time exploration during septic shock. Intensive Care Med. 2014;40:958–964. doi: 10.1007/s00134-014-3326-4. [DOI] [PubMed] [Google Scholar]
- 23.van Genderen ME, Paauwe J, de Jonge J, van der Valk RJ, Lima A, Bakker J, et al. Clinical assessment of peripheral perfusion to predict postoperative complications after major abdominal surgery early: a prospective observational study in adults. Crit Care. 2014;18:R114. doi: 10.1186/cc13905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dünser MW, Takala J, Brunauer A, Bakker J. Re-thinking resuscitation: leaving blood pressure cosmetics behind and moving forward to permissive hypotension and a tissue perfusion-based approach. Crit Care. 2013;17:326. doi: 10.1186/cc12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Genderen ME, Engels N, van der Valk RJP, Lima A, Klijn E, Bakker J, et al. Early peripheral perfusion-guided fluid therapy in patients with septic shock. Am J Respir Crit Care Med. 2015;191:477–480. doi: 10.1164/rccm.201408-1575LE. [DOI] [PubMed] [Google Scholar]
- 26.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the work-ing group on sepsis-related problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 27.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller A, Mandeville J. Predicting and measuring fluid responsiveness with echocardiography. Echo Res Pract. 2016;3:G1–G12. doi: 10.1530/ERP-16-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monnet X, Teboul JL. Assessment of volume responsiveness during mechanical ventilation: recent advances. Crit Care. 2013;17:217. doi: 10.1186/cc12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lara B, Enberg L, Ortega M, Leon P, Kripper C, Aguilera P, et al. Capillary refill time during fluid resuscitation in patients with sepsis-related hyperlactatemia at the emergency department is related to mortality. PLoS ONE. 2017;12:e0188548. doi: 10.1371/journal.pone.0188548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Kellum JA, Lameire N. KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1) Crit Care. 2013;17:204. doi: 10.1186/cc11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhatt DL, Mehta C. Adaptive designs for clinical trials. N Engl J Med. 2016;375:65–74. doi: 10.1056/NEJMra1510061. [DOI] [PubMed] [Google Scholar]


