Abstract
A study was performed of Staphylococcus aureus and methicillin-resistant S. aureus (MRSA) strains isolated from nasal preoperative samples. Of 663 samples assessed, staphylococcus was detected in 143 (21.57%). The disc diffusion method (cefoxitin 30 μg), a screening test (oxacillin 6 μg/mL) and a search for Protein Binding Additional Penicillin 2 (PLP2a) allowed the detection and confirmation of resistance to methicillin for 36 strains, a rate of 5.43% of the total population studied. Eight MRSA carriers received care in the trauma service, 14 in cardiology, five in ear, nose and throat, four in neurosurgery and paediatrics, and one in SCI. Thirty-six methicillin-resistant of the nasal portage strains are in their great majority, 27 of 36, rather limited multi-R character (two to three families namely resistance: tetracyclines, fluoroquinolones, aminoglycosides, macrolides). One of the MRSA strains was found to have intermediate sensitivity to vancomycin.
Keywords: Antibiotic resistance, Healthy volunteers, MRSA, Prevalence, Staphylococcus aureus
Introduction
Staphylococcus aureus is classified as one of the most common pathogens causing nosocomial infections [1]. Because of its virulence and its resistance to the usual antibiotics, this bacterium occupies great importance in human pathology. This saprophytic, ubiquitous species is present in humans in the commensal state. Twenty-five to 50% of individuals are healthy yet carry S. aureus in their nasal cavities, skin flora or mucosa. Along with Escherichia coli and Pseudomonas aeruginosa, S. aureus is the most frequently isolated bacterium in in-hospital sampling [2]. In the general population, the prevalence of permanent nasal carriage is between 20% and 25%, whereas transient colonization by this bacterium affects at least 60% of the remaining population [3]. S. aureus rapidly adapted to the selective pressure of antibiotics, leading to the diffusion of methicillin-resistant Staphylococcus aureus (MRSA) strains, which are responsible for approximately 30% of nosocomial infections [4].
In Algeria, MRSA accounts for 45.6% of strains isolated in hospitals, compared to 27.9% for those of external origin [5]. In Blida, a study published in 2007 on the prevalence of MRSA nasal carriage among 1005 patients indicated that 45 (4.478%) had a MRSA strain [6]. The recommended sampling for MRSA is nasal sampling [7] because the anterior nasal cavity is one of the preferred carrier sites of this bacterium, and the frequency of skin portage depends on nasal carriage [8]. Laboratory screening is based on evidence of Staphylococcus aureus resistance to methicillin. Several different techniques for rapid in vitro detection of the resistance to methicillin of staphylococci have been developed. They included phenotypic techniques using sensitized latex to detect PLP2a [9]; screening tests for oxacillin and cefoxitin; and genotypic techniques searching for the mecA gene by classical PCR [10], [11] as well as by real-time PCR [12], [13].
The objectives of this study were the isolation, identification and prevalence of strains of S. aureus collected from the nasal cavity by swab in different services in Frantz Fanon Hospital, Blida, Algeria; and the determination of the prevalence of MRSA and resistance associated with MRSA. Screening of patients at admission and during hospitalization identified subjects with asymptomatic MRSA. This screening strategy is a major component of any control programme [14]. This study proposes a strategy to prevent infection.
Results
Presentation of data
The present study involved a sample of 663 specimens taken from hospitalized patients and from patients before 48 hours of their admission to the hospital, divided as follows. Of the 663, a total of 389 subjects were male and 274 female. Patients were grouped into four major age groups: 12.52% were aged 0 to 15 years; 24.89% were aged 15 to 40 years; 34.99% were aged 40 to 60 years; and 27.60% were aged 60 years and over. A total of 319 people had already been hospitalized, and 197 people had a history of catheter or other material insertion, or a history of disease. Forty-two people had already received an antibiotic. A total of 222 patients were hospitalized in the trauma service, 278 in cardiology, 41 in neurosurgery, 72 in ear, nose and throat (ENT), 33 in paediatrics and 17 in infant surgery. Of the 663 patients studied, 143 (21.57%) were carriers of S. aureus. Of the 143 strains of S. aureus, 86 strains were found in male subjects and 57 strains in female subjects (sex ratio of 1.51). During the experimental period, the positive cases occurred in patients in different age groups. Subjects aged between 40 and 60 years occurred most often, with 48 cases in this age group of a positive culture for S. aureus, followed by 44 cases in subjects aged 60 years and older.
The percentage of nasal carriage according to hospitalization was 24.45%. The percentage of nasal carriage as a function of antecedents was 47.51%. The prevalence of S. aureus nasal carriage as a function of previous antibiotic therapy was 35.71%. The percentage of nasal carriage according to service was 17.78% in trauma service, 21.94% in cardiology, 19.51% in neurosurgery, 25.00% in ENT, 27.27% in paediatrics and 41.18% in infant surgery.
Statistical analysis by chi-square test revealed the absence of a statistically significant relationship among sex, service and nasal carriage of S. aureus. However, a significant relationship was demonstrated for age, previous antibiotic intake and hospitalization history (p ≤ 0.05) (Table 1).
Table 1.
Relationship between variable risk factors and carriage of Staphylococcus aureus by age, sex and history of patients and hospitalization service
| Variable | Chi-square test | p | Statistically significant |
|---|---|---|---|
| Sex | 0.16 | 0.6891 | No |
| Age | 11.48 | 0.0093 | Yes |
| Previous hospitalization | 3.02 | 0.0822 | No |
| Catheter or other material insertion | 10.94 | 0.0042 | Yes |
| Previous antibiotic therapy | 5.30 | 0.0213 | Yes |
| Service | 7.19 | 0.2068 | No |
Prevalence of MRSA
Research identified 36 MRSA strains. Different techniques can be used to investigate resistance to oxacillin and are summarized in Table 2.
Table 2.
Investigation of resistance of Staphylococcus spp. to oxacillin and interpretation of tests (dissemination method).
| Organism | Antibiotic | Interpretation | |
|---|---|---|---|
| Oxacillin (1 μg) | Cefoxitin (30 μg) | ||
| S. aureus | ≥13 mm | ≥22 mm | OXA S strain |
| ≤12 mm | ≤21 mm | OXA R strain | |
Data from République Algérienne Démocratique et Populaire et al. [5].
OXA, oxacillin; R, resistant; S, susceptible.
Antibiogram by diffusion of cefoxitin and oxacillin discs
For S. aureus, the cefoxitin disc test is comparable to that of oxacillin to detect resistance to oxacillin by production of PLP2a (mecA gene); however, the cefoxitin disc is easier to read, and this is therefore the preferred method. In practice, oxacillin (1 μg) and cefoxitin (30 μg) must be tested simultaneously at the level of the S. aureus standard antibiogram for better resistance detection. The principle is as follows: It is an examination which makes it possible to evaluate the sensitivity of the bacterium studied with regard to the antibiotics to which it is brought into contact. It consists of placing the bacterial culture (the subject of the test) in the presence of the antibiotics under study, and observing the development and survival. The effect exerted by the antibiotic on the culture results in an area of inhibition, the diameter measurement of which makes it possible to decide on the strain's sensitivity and resistance to that antibiotic. The antibiogram is performed on Muller-Hinton medium, which allows the homogeneous diffusion of antibiotics.
Search for PLP2a
The search for PLP2a (induced protein) by Slidex MRSA (bioMérieux, Marcy l’Etoile, France) was carried out on colonies taken from the border of the inhibition zone of a cefoxitin disc after 24 hours' incubation. As with any antigen–antibody reaction, the kit reagents should be brought to room temperature before use. After extraction of the protein (according to the manufacturer's recommendations), a rapid slide agglutination test is performed using latex particles sensitized by a monoclonal antibody against PLP2a. These particles will react with the extracted PLP2a, which are optionally present.
A reaction is considered positive if the agglutination is clear with a thinning of the reaction medium. The agglutination appears in 3 to 5 minutes and is improved by depositing the reaction card on a hot plate for a maximum of 1 minute. Agglutination of strains of homogeneous resistance is rapid; that of very heterogeneous strains is finer and appears more slowly [15].
Interpretation of oxacillin resistance testing tests
The interpretation of the tests for resistance to oxacillin is summarized in Table 3. Because of the large sample size, the study focused on the possible relationship between prevalence of nasal carriage of MRSA in the patient population and upstream factors such as patient sex, class, past hospitalization, history of antibiotic therapy and finally the service area in which they were hospitalized. In other words, does the probability of nasal carriage of MRSA strain for a given individual remain homogeneous in the population, or does it vary by sex, age group or previous hospitalization? Of the 663 subjects, 36 were carriers of MRSA strains, i.e. 5.43% of the total sample, and 25.17% of the sample was positive for S. aureus nasal carriage (Table 4). Twenty-three carriers were male (5.91%) and 13 carriers were female (4.74%), with a sex ratio of 1.77.
Table 3.
Interpretation of tests for resistance to oxacillin
| Therapy | Staphylococcus aureus | Staphylococcus lugdunensis | Negative Staphylococcus Coagulase (SCN) | Control strain |
|---|---|---|---|---|
| Cefoxitin 30 μg Oxacillin 1 μg |
≤21 mm ≤12 mm Resistant |
≤21 mm Resistant |
≤24 mm Resistant |
ATCC S. aureus 25923 sensitive |
| Screening test, oxacillin | >1 colony = resistant | >1 colony = resistant | >1 colony = resistant | ATCC S. aureus 29213 sensitive ATCC S. aureus 43300 resistant |
| CMI, oxacillin | ≤2 and ≥ 4 | — | ≤0.25 and ≥ 0.5 | ATCC S. aureus 29213 sensitive 1–4 μg/mL ATCC S. aureus 43300 resistant >4 μg/mL |
| PLP2a | Agglutination: PLP2a+ Absence of agglutination: PLP2a− |
Agglutination: PLP2a+ Absence of agglutination: PLP2a− |
— | — |
Data from République Algérienne Démocratique et Populaire et al. [5].
CMI, chemiluminescent microparticle immunoassay.
Table 4.
Prevalence of MRSA (n = 663)
| MRSA | No. in workforce | % |
|---|---|---|
| Absent | 627 | 94.57 |
| Present | 36 | 5.43 |
| Total | 663 | 100 |
MRSA, methicillin-resistant Staphylococcus aureus.
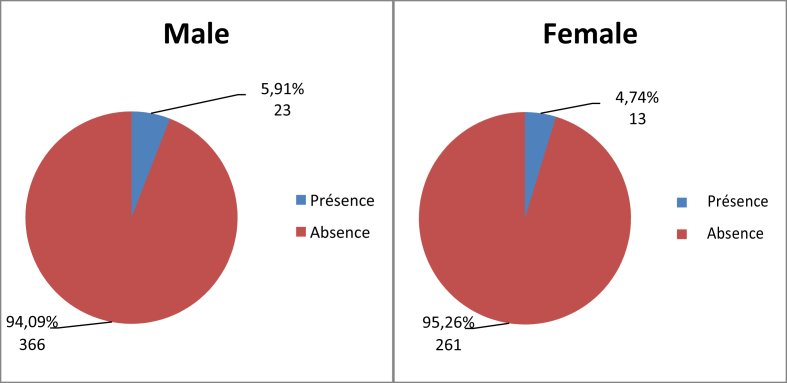
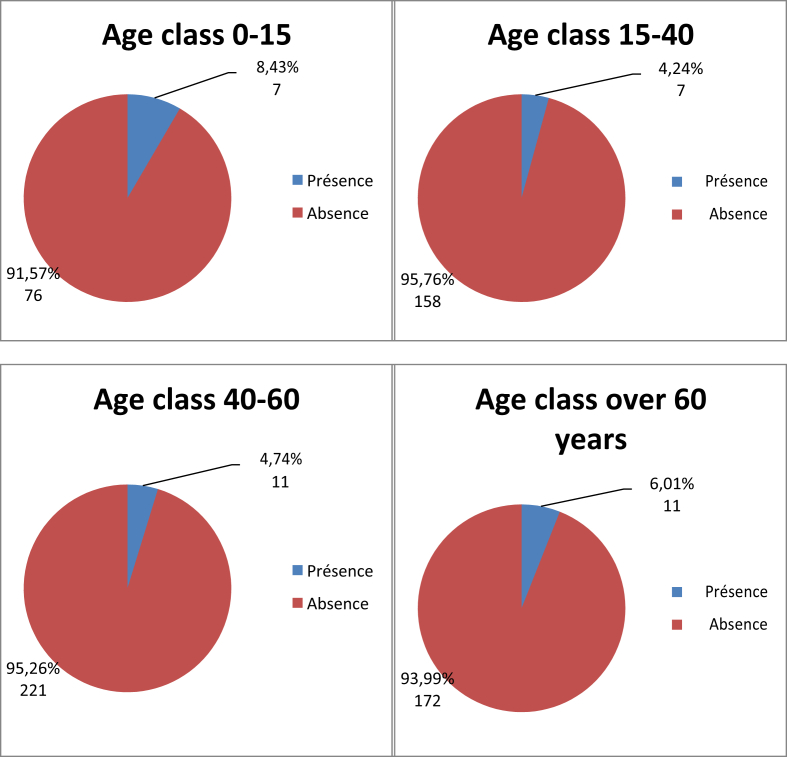
No relationship was found between sex and prevalence of nasal carriage of MRSA (Fig. 1, Table 5). In the population positive for S. aureus (n = 143), 26.74% were male and 22.81% were female (Table 6). Seven carriers (8.43%) were in the age group 0 to 15 years, seven carriers (4.24%) were in the age group 15 to 40 years, 11 carriers (4.74%) were in the age group 40 to 60 years and 11 carriers (6.01%) were in age group 60 years and over (Fig. 2).
Fig. 1.
Distribution of MRSA prevalence by sex (n = 663). MRSA, methicillin-resistant Staphylococcus aureus.
Table 5.
Prevalence of MRSA and MSSA in Staphylococcus aureus population (n = 143)
| Infection | No. of employees | % |
|---|---|---|
| MRSA | 36 | 25.17 |
| MSSA | 107 | 74.83 |
| Total | 143 | 100 |
MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible S. aureus.
Table 6.
Distribution of patients by sex in methicillin-resistant Staphylococcus aureus–positive patients (n = 143)
| Sex | Absence, n (%) | Presence, n (%) |
|---|---|---|
| Male | 63 (73.26%) | 23 (26.74%) |
| Female | 44 (77.19%) | 13 (22.81%) |
| Total | 107 (74.83%) | 36 (25.17%) |
Fig. 2.
Distribution of MRSA prevalence by age group (n = 663). MRSA, methicillin-resistant Staphylococcus aureus.
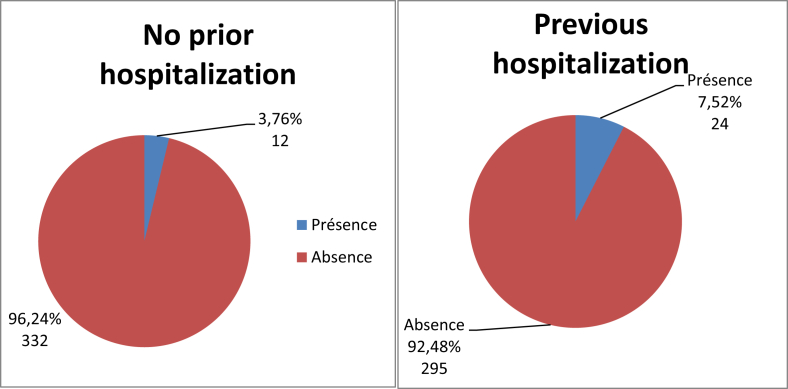
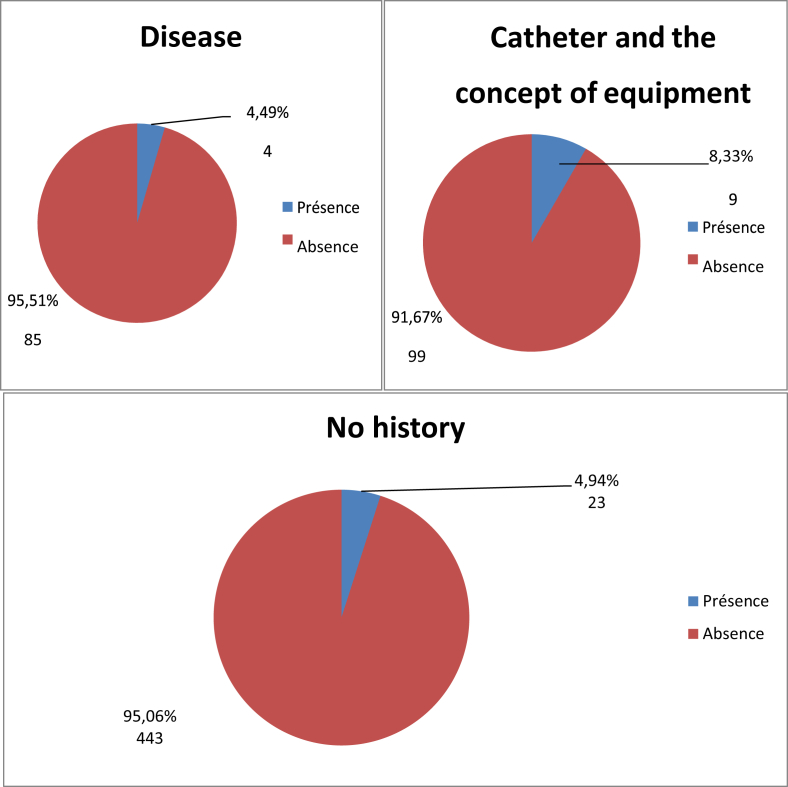
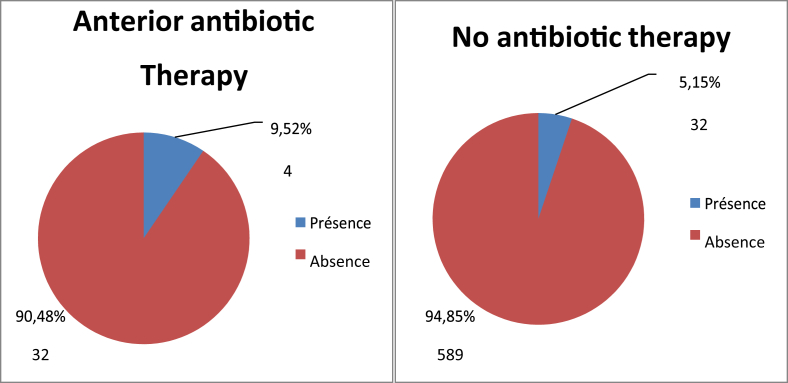
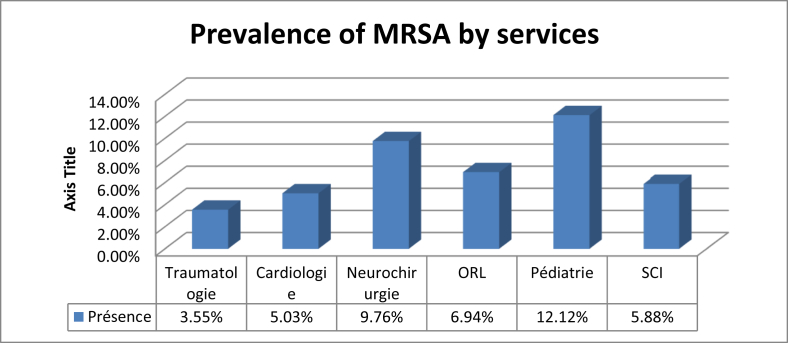
Twenty-four people (7.52%) had been previously hospitalized and were carrying MRSA, compared to only 12 patients (3.76%) for those previously hospitalized (Fig. 3). For the population that screened positive for S. aureus nasal carriage, 30.77% had been previously hospitalized (Table 7). Four patients, or 8.33% of the total population, had a disease such as diabetes, high blood pressure or high cholesterol and had previously carried MRSA, and 9.449% of patients with another antecedent (catheter or other material insertion) had previously carried MRSA (Fig. 4). The study revealed four patients (9.52%) who had previously received antibiotics and who were carriers MRSA, and 32 patients (5.15%) who had not received antibiotics (Fig. 5). There were eight carriers of MRSA (3.55%) who received care in the trauma service, 14 (5.03%) in cardiology, four (9.76%) in neurosurgery, five (94%) in ENT, four (12.12%) in paediatrics and finally one (5.88%) in Infantile Surgery department (ISD) (Fig. 6).
Fig. 3.
Distribution of MRSA prevalence by hospitalization (n = 663). MRSA, methicillin-resistant Staphylococcus aureus.
Table 7.
Patient distribution based on MRSA nasal carriage based on previous hospitalization frequency (n = 143)
| Hospitalization | No MRSA, n (%) | MRSA, n (%) | Total, n (%) |
|---|---|---|---|
| Yes | 54 (75.55) | 24 (30.77) | 78 (100) |
| No | 53 (81.10) | 12 (18.46) | 65 (100) |
| Total | 107 (74.83) | 36 (25.17) | 143 (100) |
MRSA, methicillin-resistant Staphylococcus aureus.
Fig. 4.
Distribution of MRSA prevalence by history (n = 663). MRSA, methicillin-resistant Staphylococcus aureus.
Fig. 5.
Distribution of MRSA prevalence by antibiotic therapy (n = 663). MRSA, methicillin-resistant Staphylococcus aureus.
Fig. 6.
Distribution of the prevalence of MRSA by service. MRSA, methicillin-resistant Staphylococcus aureus.
Statistical analysis
Statistical analysis by chi-square test revealed the absence of a statistically significant relationship among age, sex, previous antibiotic intake, patient history, MRSA nasal carriage and service. However, a significant relationship was demonstrated with respect to previous hospitalization (p ≤ 0.05) (Table 8). The prevalence of nasal carriage of MRSA is thus significantly elevated in any person who has already been hospitalized.
Table 8.
Relationship between variable risk factors and methicillin-resistant Staphylococcus aureus carriage by age, sex, patient history and hospitalization service
| Variable | Chi-square test | p | Statistically significant |
|---|---|---|---|
| Sex | 0.43 | 0.5119 | No |
| Age | 3.57 | 0.3117 | No |
| Previous hospitalization | 5.25 | 0.0219 | Yes |
| Catheter or other material insertion | 2.15 | 0.3412 | No |
| Previous antibiotic therapy | 1.46 | 0.2269 | No |
| Service | 6.13 | 0.2937 | No |
Resistance associated with MRSA profile
The results of the antibiogram of 36 strains of MRSA isolated by nasal swab are listed in Table 9. Isolated MRSA strains have a very high resistance to ofloxacin and kanamycin, and to a lesser extent to erythromycin and tetracycline. The present study found a rare strain with intermediate sensitivity to vancomycin.
Table 9.
Distribution of 36 methicillin-resistant Staphylococcus aureus strains according to antibiotic susceptibility
| Antibiotic | Family | Resistance rate | Sensitivity rate | Intermediate rate | Inductive resistance rate |
|---|---|---|---|---|---|
| TET30 | Tetracyclines | 6/36 | 30/36 | 0/36 | 0/36 |
| OFX5 | Fluoroquinolones | 21/36 | 15/36 | 0/36 | 0/36 |
| KMN30 | Aminosides | 24/36 | 12/36 | 0/36 | 0/36 |
| TEC30 | Glycopeptides | 0/36 | 36/36 | 0/36 | 0/36 |
| ERY15 | Macrolides | 9/36 | 13/36 | 14/36 | 0/36 |
| CD2 | Lincosamides | 0/36 | 31/36 | 0/36 | 5/36 |
| VAN | Glycopeptides | 0/36 | 35/36 | 1/36 | 0/36 |
TET 30: TetracyclineOFX 5: OfloxacinKMN 30: KanamycinTEC 30: TeicoplaninERY 15: ErythromycinCD 2: ClindamycinVAN: Vancomycin.
Discussion
S. aureus is one of the agents responsible for nosocomial infections; the consequences are severe because of the organism's increasing resistance to antibiotics. It is essentially the nasal carriage of this bacterium that is responsible for Nosocomial infections. Staphylococcal infections are ubiquitous and can occur in the form of community-acquired or nosocomial infections.
An epidemiologic study of more than 10 000 patients in 1417 resuscitation units in Europe showed that S. aureus was responsible for 30.1% of infections, or 60% of MRSA [16]. All together, nearly half of the nosocomial infections are linked to this germ. The epidemiology of staphylococcal infections evolves over the life course and almost necessarily involves an essentially nasal port. It is estimated that nasal carriage of S. aureus in adults is present in 20% to 40% of the population, depending on local seasonal and epidemiologic factors. Medical and paramedical hospital staff have a higher rate of nasal carriage than the general population [17].
Some patients have a higher risk of staphylococcal nasal colonization, such as those with insulin-dependent diabetes, chronic dialysis patients, drug addicts, HIV/AIDS patients [8] and elderly patients in nursing homes [18], [19].
To our knowledge, few national studies have been interested in screening for nasal carriage of S. aureus. In the present study, of the 663 patients, 58.67% were male and 41.33% female. Patients were admitted to one of six services, with a distribution of 33.48% in the trauma service, 41.93% in cardiology, 6.18% in neurosurgery, 10.86% in ENT, 4.98% in paediatrics and 2.56% in infant surgery.
Studies on the prevalence of S. aureus nasal carriage have shown that this species colonizes the skin and mucous membranes of living beings of many species [20]. The nasal fossae represent the main portal site. Other reservoirs exist, including skin, perineum, axillary hollows and pharynx [18]. A study conducted in the United States found 28.6% of nasal carriage to be Methicillin-sensitive Staphylococcus aureus (SASM) and 1.5% MRSA [21]. In the present study, the prevalence of nasal carriage of S. aureus was 21.57%. Wertheim et al. [22] reported the importance of nasal carriage of S. aureus by indicating the frequency of porting in different parts of the human body: nose (27%), pharynx (10-20%), neck (10%), skin of the thorax (15%), skin of the abdomen (15%), armpit (8%), forearm (5%) and ankle (10%). In other literature, 15% to 40% of the general population is nasally colonized by this bacterium [23]. In a similar study conducted in several countries, the authors were able to obtain rates of nasal carriage by site: France (Paris) 18%, Mali (Bamako) 22%, Algeria (Tlemcen) 27%, Moldova (Chisinau) 25% and Cambodia (Phnom Penh) 12%.
The nasal carriage of S. aureus according to sex did not show a statistically significant relationship. According to Fleurette [24], portage is independent of sex, although several studies found a significant relationship between this parameter and sex, with male subjects more frequently affected than female subjects.
Age is a risk factor for colonization of S. aureus [25], [26]. In the present study, the prevalence of nasal colonization by S. aureus in children was 32.53%, and for seven strains of MRSA was 8.43%.
A study in Brazil of children attending day nurseries found a colonization rate with strains of MRSA of 1.2% [27]. Hussain et al. [28] showed that 122 (24.4%) of 500 children with S. aureus were carriers of MRSA (both nasal and perineal) in Chicago; three of these strains (0.6%) were MRSA. Another study reported a 1.7% MRSA nasal colonization rate among 300 healthy children [29]. A similar study found that among 500 healthy subjects aged between 2 weeks and 21 years recruited during routine health maintenance visits, 29% were carriers of S. aureus, and four patients (0.8%) had MRSA [30]. The majority of these authors recruited their population at hospital admission during the first 24 hours (or sometimes the first 48 hours), or during routine visits for vaccinations.
In the present study, previous hospitalization, history of disease, history of catheter or other material insertion and previous antibiotic therapy were risk factors for S. aureus carriage. Several studies have found that these factors remain the most important in the carriage of S. aureus in community settings or during admission to hospital [29].
Regarding sensitivity to antibiotics, S. aureus has developed various types of resistance to antistaphylococcal agents. More than 80% of the strains produce penicillinase. Oxacillin remains active against these strains, but hospital-acquired and more recently community-acquired staphylococci have developed cross-resistance between oxacillin and other β-lactams by production of protein-binding penicillins (PLP) of low affinity. This latter resistance is more easily detected by cefoxitin [31]. According to the present study, the latter is active in 5.42% of the strains, for a MRSA carriage rate of 5.42%—a frequency that remains normal compared to several countries. However, according to Toualbia et al. [6] in a 2007 study conducted in the same hospital in Blida, the prevalence of MRSA was 4.48% higher than the prevalence found in the present study.
The resistance to kanamycin is 66.67%, tetracycline 16.67% and ofloxacin 58.33%. Macrolide sensitivity and erythromycin resistance are intermediate in respectively 25% and 38.89% of the strains. On the other hand, there is an inducible resistance of the strains to clindamycin; it is estimated at 13.89%. The present study found a sensitivity of the isolated strains to teicoplanin as well as a strain with intermediate sensitivity to vancomycin. Indeed, vancomycin remains the most effective antibiotic treatment for this type of infection. Disturbingly, however, strains of MRSA with intermediate sensitivity to vancomycin have been isolated in Japan, the United States and Europe (France, England, Spain, France, Greece).
Some studies have suggested that the problem of endemic MRSA does not require a particular preventive measure [32]; others consider that knowledge of MRSA dissemination can pose more problems than it solves. Most specialists recognize the need to develop a policy to combat the spread of MRSA [33]. Prevention programmes include screening patients at admission and taking barrier precautions. In a study conducted in a resuscitation unit in the Netherlands, the frequency of transmission was estimated to be 38 times higher in carriers without an identified transmission source [34]. A mathematical model demonstrated that a 12% increase in hand hygiene compliance could offset the influence on MRSA transmission, taking into account work overload due to a decrease in staffing in a resuscitation service [35]; the authors further discuss wearing gloves, which is part of the recommended standard precautions to protect personnel from blood and other body fluids; wearing masks when in the presence of cases of respiratory infection with potentially contaminating secretions; maintaining the environment; and hospitalizing patients singly (i.e. geographic isolation). One study reported a reduction in the nosocomial transmission of MRSA with a prevention policy based on individual chamber isolation and wearing gloves without the introduction of an intake screening strategy [36]. Some studies have revealed frequent contamination of the surfaces in patients' rooms. One study of the contamination of door handles in 196 patient rooms found that MRSA was isolated from 11.3% of samples, with an amount ranging from 103 to 106 bacteria per handle. The contamination of the door handles of the patients' rooms was 2.5 times greater than that of noncarrier patients [37]. However, contamination of the doors of noncarrier patients underlines the extent of their dissemination in the hospital environment.
Conclusion
The present study focused on MRSA strains, isolated from nasal specimens, carried out on random external volunteers. The samples were taken from male and female children and adults who had never been infected with MRSA and who sought care at the university hospital.
Of the 663 samples taken, 143 golden staphylococci (21.57%) were detected. Of these S. aureus, 36 MRSA were isolated. A prevalence of 5.43% is thus deduced from the total population studied.
The results of the present study emphasize the high rate of resistance to methicillin found in screened patients, which can increase the incidence of nosocomial infections and also aggravate prognosis. Screening MRSA carriers at admission is considered a critical determinant in the prevention of cross-transmission. Most studies emphasize that the mastering programmes put in place show a certain efficiency by reducing the level of cross-transmission and the occurrence of infections.
With high frequency, potential severity and significant increase, MRSA presents all the characteristics that could lead to a major public health emergency. Faced with this problem, the search for improvement (feasibility, cost, efficiency, acceptability) in master's programmes is particularly important. Among the most commonly used methods of prevention are antibiotic prescribing, screening patients at admission, decolonizing patients who undergo surgical procedures, taking contact precautions and isolating patients individually.
In order to limit the spread of MRSA, it is necessary to set up an information policy for healthcare providers in the city (nurses, physiotherapists and doctors) when one of their patients carries MRSA. This information is not routinely obtained in hospitals during intra- or interhospital transfers, although this has been long recommended [38], [39], [40]. It will therefore probably be even more difficult to generalize this recommendation from the hospital to the larger city. In addition, details regarding carriage should be obtained, including telephone contacts and hospitalization reports. A policy for training urban healthcare providers on controlling the transmission of MRSA, in particular hand disinfection methods, should also be implemented.
Thus, the results of the present study suggest that preventive measures ought to be based mainly on better compliance with hand hygiene, sterilization and disinfection of equipment, and better continuity of personnel in the service of patients.
On the one hand, the recommendation of the wide use of hydroalcoholic solutions, which have proved their effectiveness and which improve the observance of hand disinfection protocols, should make it possible to simplify barrier measures. On the other hand, family contact transmissions (not often described) are likely to be more difficult to limit. There are currently no specific recommendations for the management of antibiotic-resistant bacteria at our facility.
To learn the dispersion of MRSA clones in the general population, molecular characterization (e.g. pvl, mecA, agr, cassettes) is indispensable. It was not carried out in this study because of lack of resources.
Conflict of interest
None declared.
References
- 1.National Nosocomial Infections Surveillance System National nosocomial infections surveillance (NNIS) system report, data summary from January 1992 through June 2003. Am J Infect Control. 2003;31:481–498. doi: 10.1016/j.ajic.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Joubert O. Université de Strasbourg; 2005. Identification, stabilisation et inhibition de l’interaction monomère S–monomère F des leucotoxines à deux composés de Staphyloccocus aureus.http://scd-theses.u-strasbg.fr/1055/ Thesis. Available at: [Google Scholar]
- 3.Peacock S.J., de Silva I., Lowy F.D. What determines nasal carriage of Staphylococcus aureus? Trends Microbiol. 2001;9:605–610. doi: 10.1016/s0966-842x(01)02254-5. [DOI] [PubMed] [Google Scholar]
- 4.Tristan A., Durand G., Durupt F., Ferry T., Res M., Reverdy M.E. SARM: Données épidémiologiques récentes et évolution de la résistance. Revue Francophone des laboratoires. 2005;376:4–98. [Google Scholar]
- 5.République Algérienne Démocratique et Populaire; Ministère de la Santé . 2015. de la Population et de la Réforme Hospitalière; Réseau Algérien de Surveillance de la Résistance des Bactéries aux Antibiotiques (AARN)http://www.sante.dz/aarn/documents/pdf/Rapport2015%20.pdf Surveillance de la résistance des bactéries aux antibiotiques. Available at: [Google Scholar]
- 6.Toualbia N., Saidi N., Mostapha S. 2007. Prévalence du portage nasal en MRSA (methicillin-resistant Staphylococcus aureus) dans la population de Blida. Thesis. [Google Scholar]
- 7.Lucet J.C. The importance of detecting methicillin-resistant Staphylococcus aureus in an intensive care setting. Ann Fr Anesth Reanim. 2002;5:384–391. doi: 10.1016/s0750-7658(02)00623-8. [DOI] [PubMed] [Google Scholar]
- 8.Kluytmans J., van Belkum A., Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997;10:505–520. doi: 10.1128/cmr.10.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakatomi Y., Sugiyama J. A rapid latex agglutination assay for the detection of penicillin-binding protein 2. Microbiol Immunol. 1998;42:739–743. doi: 10.1111/j.1348-0421.1998.tb02347.x. [DOI] [PubMed] [Google Scholar]
- 10.Unal S., Hoskins J., Flokowitsch J.E., Wu C.Y., Preston D.A., Skatrud P.L. Detection of methicillin-resistant staphylococci by using the polymerase chain reaction. J Clin Microbiol. 1992;30:1685–1691. doi: 10.1128/jcm.30.7.1685-1691.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murakami K., Minamide W., Wada K., Nakamura E., Teraoka H., Wayanabe S. Identification of methicillin-resistant strains of staphylococci by polymerase chain reaction. J Clin Microbiol. 1991;29:2240–2244. doi: 10.1128/jcm.29.10.2240-2244.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reischl U., Linde H.J., Metz M., Leppmeier B., Lehn N. Rapid identification of methicillin-resistant Staphylococcus aureus and simultaneous species confirmation using real-time fluorescence PCRJ. Clin Microbiol. 2000;38:2429–2433. doi: 10.1128/jcm.38.6.2429-2433.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fang H., Hedin G. Rapid screening and identification of methicillin resistant Staphylococcus aureus from clinical samples by selective-broth and real-time PCR assay. J Clin Microbiol. 2003;41:2894–2899. doi: 10.1128/JCM.41.7.2894-2899.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyce J.M. Strategies for controlling methicillin-resistant Staphylococcus aureus in hospitals. J Chemother. 1995;7(Suppl 3):81–85. [PubMed] [Google Scholar]
- 15.Société Française de Microbiologie; Comité de l’antibiogramme. Communiqué. 2006. http://www.sfm-microbiologie.org/ Available at: [Google Scholar]
- 16.Vincent J.L., Bihari D.J., Suter P.M., Bruining H.A., White J., Nicolas-Chanoin M.H. The prevalence of nosocomial infection in intensive care units in Europe. Results of the European prevalence of infection in intensive care (EPIC) study. JAMA. 1995;274:639–644. [PubMed] [Google Scholar]
- 17.Waldvogel F.A. Staphylococcus aureus. In: Mandell I., Gerald L., Dolin R., editors. Mandell, Douglas and Bennett’s principles and practice of infectious diseases. Churchill Livingstone; New York: 1995. pp. 77–1754. [Google Scholar]
- 18.Terpenning M.S., Ramsey M.A., Zarins L.T., Jorgensen K.A., Sottile W.S., Schaberg D.R. Methicillin-resistant Staphylococcus aureus: colonization and infection in a long-term care facility. Ann Intern Med. 1991;115:417–422. doi: 10.7326/0003-4819-115-6-417. [DOI] [PubMed] [Google Scholar]
- 19.Bradley S.F. Methicillin-resistant Staphylococcus aureus in nursing homes. Epidemiology prevention and management. Drugs Aging. 1997;10:185–198. doi: 10.2165/00002512-199710030-00003. [DOI] [PubMed] [Google Scholar]
- 20.Williams R.E. Healthy carriage of Staphylococcus aureus: its prevalence and importance. Bacteriol Rev. 1963;27:56–71. doi: 10.1128/br.27.1.56-71.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorwitz R.J., Kruszon-Moran D., McAllister S.K., McQuillan G., McDougal L.K., Fosheim G.E. Changes in the prevalence of nasal colonization with Staphylococcus aureus in the United States, 2001–2004. J Infect Dis. 2008;197:1226–1234. doi: 10.1086/533494. [DOI] [PubMed] [Google Scholar]
- 22.Wertheim H.F.L., Melles D.C., Vos M.C., van Leeuwen W., van Belkum A., Verbrugh H.A. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis. 2005;5:751–762. doi: 10.1016/S1473-3099(05)70295-4. [DOI] [PubMed] [Google Scholar]
- 23.Kluytmans W., Van Leeuwen W., Goessens R., Hollis S., Messer L., Herwaldt H. Food-initiated outbreak of methicillinresistant Staphylococcus aureus analyzed by pheno- and genotyping. J Clin Microbiol. 1995;33:1121. doi: 10.1128/jcm.33.5.1121-1128.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fleurette J. Taxonomie et écologie des staphylocoques à coagulase négatives. Med Mal Infect. 1990:6–15. HS3. [Google Scholar]
- 25.Nouwen J.L., Ott A., Kluytmans-Vandenbergh M.F., Boelens H.A., Hofman A., van Belkum A. Predicting the Staphylococcus aureus nasal Carrier state: derivation and validation of a ‘culture rule. Clin Infect Dis. 2004;39:806–811. doi: 10.1086/423376. [DOI] [PubMed] [Google Scholar]
- 26.Bishoff W., Wallis M., Tucker K. Staphylococcus aureus nasal carriage in a student community: prevalence, clonal relationships,and risk factors. Infect Control Hosp Epidemiol. 2004;25:485–491. doi: 10.1086/502427. [DOI] [PubMed] [Google Scholar]
- 27.Lamaro-Cardoso J., de Lancastre H., Kipnis A., de Lencastre H., Kipnis A., Pimenta F.C. Molecular epidemiology and risk factors for nasal carriage of Staphylococcus aureus and methicillin-resistant S. aureus in infants attending daycare centers in Brazil. J Clin Microbiol. 2009;47:3991–3997. doi: 10.1128/JCM.01322-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hussain F.M., Boyle-Vavra S., Daum R.S. Community acquired methicillin-resistant Staphylococcus aureus colonization in healthy children attending an outpatient pediatric clinic. Pediatr Infect Dis J. 2001;20:763–767. doi: 10.1097/00006454-200108000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Cheng Immergluck L., Kanungo S., Schwartz A., McIntyre A., Schreckenberger P.C., Diaz P.S. Prevalence of Streptococcus pneumoniae and Staphylococcus aureus nasopharyngeal colonization in healthy children in the United States. Epidemiol Infect. 2004;132:159–166. doi: 10.1017/s0950268803001791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakamura M.M., Rohling K.L., Shashaty M., Lu H., Tang Y.W., Edwards K.M. Prevalence of methicillin-resistant Staphylococcus aureus nasal carriage in the community pediatric population. Pediatr Infect Dis J. 2002;21:917–922. doi: 10.1097/00006454-200210000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Daurel C., Leclercq R. L’antibiogramme de Staphylococcus aureus. Revue Francophone des laboratoires. 2008;407:83. [Google Scholar]
- 32.Teare E.L., Cookson B., French G.L., Jenner E.A., Scott G., Pallett A. Hand washing initiative. J Hosp Infect. 1999;43:1–3. doi: 10.1053/jhin.1999.0251. [DOI] [PubMed] [Google Scholar]
- 33.Hidron A.I., Kourbatova E.V., Halvosa J.S., Terrell B.J., McDougal L.K., Tenover F.C. Risk factors for colonization with methicillin-resistant Staphylococcus aureus (MRSA) in patients admitted to an urban hospital: emergence of community-associated MRSA nasal carriage. Clin Infect Dis. 2005;41:159–166. doi: 10.1086/430910. [DOI] [PubMed] [Google Scholar]
- 34.Walker E.S., Vasquez J.E., Dula R., Bullock H., Sarubbi F.A. Mupirocin-resistant, methicillin-resistant Staphylococcus aureus: does mupirocin remain effective? Infect Control Hosp Epidemiol. 2003;24:342–346. doi: 10.1086/502218. [DOI] [PubMed] [Google Scholar]
- 35.Grundmann H., Tami A., Hori S., Halwani M., Slack R. Nottingham Staphylococcus aureus population study: prevalence of MRSA among elderly people in the community. BMJ. 2002;324:1365–1366. doi: 10.1136/bmj.324.7350.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hecker M.T., Aron D.C., Patel N.P., Lehmann M.K., Donskey C.J. Unnecessary use of antimicrobials in hospitalized patients. Arch Intern Med. 2003;163:972–978. doi: 10.1001/archinte.163.8.972. [DOI] [PubMed] [Google Scholar]
- 37.Oliveira D.C., Tomasz A., de Lencastre H. Secrets of success of a human pathogen: molecular evolution of pandemic clones of methicillin-resistant Staphylococcus aureus. Lancet Infect Dis. 2002;2:180–189. doi: 10.1016/s1473-3099(02)00227-x. [DOI] [PubMed] [Google Scholar]
- 38.Hospital Infection Control Advisory Committee Guidelines for isolation precautions in hospitals. Am J Infect Control. 1996;24:24–52. doi: 10.1016/s0196-6553(96)90050-4. [DOI] [PubMed] [Google Scholar]
- 39.Shlaes D.M., Gerdin D.N., John J.F., Craig W.A., Bornstein D.L., Duncan R.A. Society for Healthcare Epidemiology of America and Infectious Diseases Society of America Joint Committee on the prevention of antimicrobial resistance: guidelines for the prevention of antimicrobial resistance in hospitals. Infect Control Hosp Epidemiol. 1997;18:275–291. doi: 10.1086/647610. [DOI] [PubMed] [Google Scholar]
- 40.Comité technique national des infections nosocomiales (CTIN) Ministère de l’Emploi et de la Solidarité; Paris: 1999. Maîtrise de la diffusion des bactéries multirésistantes aux antibiotiques—recommandations pour les établissements de santé. [Google Scholar]