Abstract
The current industrial ammonia synthesis relies on Haber–Bosch process that is initiated by the dissociative mechanism, in which the adsorbed N2 dissociates directly, and thus is limited by Brønsted–Evans–Polanyi (BEP) relation. Here we propose a new strategy that an anchored Fe3 cluster on the θ-Al2O3(010) surface as a heterogeneous catalyst for ammonia synthesis from first-principles theoretical study and microkinetic analysis. We have studied the whole catalytic mechanism for conversion of N2 to NH3 on Fe3/θ-Al2O3(010), and find that an associative mechanism, in which the adsorbed N2 is first hydrogenated to NNH, dominates over the dissociative mechanism, which we attribute to the large spin polarization, low oxidation state of iron, and multi-step redox capability of Fe3 cluster. The associative mechanism liberates the turnover frequency (TOF) for ammonia production from the limitation due to the BEP relation, and the calculated TOF on Fe3/θ-Al2O3(010) is comparable to Ru B5 site.
The current industrial ammonia synthesis relies on the Haber-Bosch process that is limited by the Brønsted–Evans–Polanyi relation. Here, the authors propose a new strategy that an anchored Fe3 on θ-Al2O3(010) surface serves as a heterogeneous single cluster catalyst for ammonia synthesis from first-principles calculations and microkinetic analysis.
Introduction
Ammonia synthesis is one of the most important industrial catalytic reactions, which is based on the Haber–Bosch process (N2 + H2 → NH3) since the World War II, and it plays a key role in the growth of human population1,2. Although the process using Fe and Ru metal-based catalysts with promoters has been developed for more than one hundred years, it still requires high pressure (~100 bar) and moderately high temperature (~700 K)3, which is dictated by the compromise between thermodynamic equilibrium and kinetics4. Ammonia synthesis on Fe and Ru metal surfaces is widely regarded as a classical example of correlating the experimentally observable turnover frequency (TOF) with the predicted atomistic mechanism5, where the N2 activation is confirmed to be a direct N≡N dissociation process6–8. The performance of promoted Fe- and Ru-based catalysts clearly shows a site dependence of the N2 dissociation and NHx desorption9. N2 molecules are firstly dissociated on specific active sites, such as the B5 site of Ru(0001) step and the C7 site of Fe(111) or Fe(211) surface5,10,11, then the adsorbed *N is hydrogenated step by step to produce *NH3. This is the well-studied dissociative mechanism.
Previous theoretical studies discussed the possibility of ammonia synthesis at low temperature and low pressure12,13, but the TOF was limited, due to the Brønsted–Evans–Polanyi (BEP) relation14,15. The BEP relation regulates that the dissociation barrier of N2 and the desorption energies of NHx scale linearly with the adsorption energy of N atom12,14,16. Stronger adsorption of N atom implies lower N2 dissociation barrier but higher NHx desorption energies, such as on Re, Mo, Fe metal surfaces; while weaker adsorption of N atom indicates higher N2 dissociation barrier and lower NHx desorption energies, such as on Pd, Co, Ni metal surfaces. Thus a good metal catalyst for ammonia synthesis must have a moderate atomic N adsorption energy, around where the top of volcano plot locates15,16.
Several molecular catalysts and naturally occurring nitrogenase enzymes are capable of reducing N2 under ambient conditions17–21, and the associated mechanisms are likely initiated by the associative adsorption or hydrogenation of N2, rather than the dissociative adsorption that is the key to the Haber–Bosch process. Recently, there were some theoretical indications showing that in the electrochemical ammonia synthesis the N2 molecule did not dissociate upon adsorption, but was hydrogenated to *NNH instead22–29. Even for the thermal catalytic process, Skúlason et al.25 showed that the proportion of associative process (i.e., *N2 + *H→*NNH + *) is underestimated based on Bayesian statistics. For heterogeneous catalysis, O2 and CO can be hydrogenated via an associative mechanism to *OOH and *HCO, respectively, which have been proved to be key intermediates for O2 and CO activation30–36. When the N2 hydrogenation becomes the dominating process, the N–N bond is much weakened, and consequently the dissociation barrier no longer obeys the BEP relation. Thus, designing a catalyst with surface active centers that hydrogenate N2 first can be a new strategy to accomplish ammonia synthesis at low temperature and pressure.
The remarkable recent development of surface single-atom catalyst (SAC) and single-cluster catalyst (SCC) demonstrates the possibility to build homogeneous catalytic active centers on heterogeneous solid surfaces37–41. Inspired by nitrogenase, in which the FeMoco is responsible for N2 activation and ammonia synthesis42–45, Holland and co-workers designed a series of multinuclear iron complexes to mimic the nitrogenase, and showed that the formally Fe(I) and Fe(0) complexes can weaken or even break N2 triple bond at or below room temperature18,19,46. Hosono and co-workers also reported a series of stable electrides as efficient electron donor for loaded Ru metal, which is proved to be more reactive than the bare metal surface47.
In this work, we propose an active center of Fe3 cluster that is anchored on the θ-Al2O3(010) surface, and we predict that the direct dissociation of *N2 on this center is difficult (dissociative mechanism), but the *N2 is easily hydrogenated to form the *NNH species (associative mechanism), which has a much lower N–N bond dissociation barrier than that of *N2. We further reveal that the large spin polarization of Fe3 is responsible for N2 activation, and the low oxidation state iron atom works as an electron reservoir, regulating the charge variation of the whole process. The surface-anchored Fe3 SCC renders a robust multi-step redox capability necessary for ammonia synthesis from dinitrogen.
Results
N2 adsorption on supported Fe3 cluster
The FeMoco site of nitrogenase has been considered responsible for biological nitrogen fixation. Its performance in activating N2 molecule originates from the highly efficient redox cycle between Fe(II) and Fe(III) of the Mo-Fe-S-C cluster of FeMoco. Based on the model of FeMoco, various Fe containing complexes are designed to mimic the N2 activation process on FeMoco19,20,44,48–51. A general implication for designing such complex is to keep the Fe center at a reduced state to facilitate electron donation to the N2 molecule.
In recent years, embedded Fe clusters with low oxidation state, such as three diketiminate-bound Fe(I) ions in Fig. 1a, are shown to synergistically facilitate N2 reduction18,19,46,48,52. Inspired by this finding, we conceive that the small Fe clusters supported by θ-Al2O3(010) surface should be capable of efficient N2 activation (Fig. 1b, c), because the inert support has little electronic interaction with the Fe cluster and thus retains Fe in an even more reduced state for metal-metal bonded Fe clusters3,53. This type of Fe clusters on alumina surface may be experimentally prepared by soft-landing cluster method or thermal treatment of ligated tri-iron cluster (e.g., Fe3(CO)n)54,55. By testing the binding energies for Fen clusters (n = 1–5) (Supplementary Fig. 1 and 2), we have shown that the triangular Fe3 and the pyramidal Fe4 are the most stable clusters on Al2O3 substrate, with the Fe3 cluster kinetically stable against aggregation.
Fig. 1.
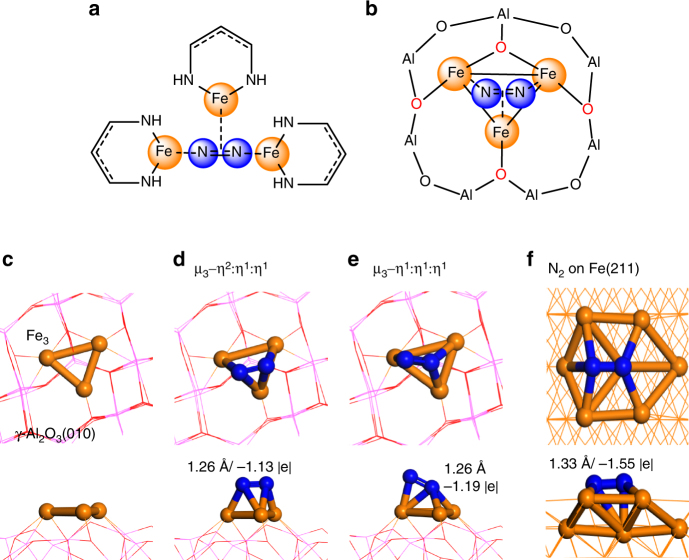
Homogeneous and heterogeneous Fe3 cluster with N2 adsorption. a Schematic representation of N2 coordinated with three Fe(I)-ion homogeneous complexes in the side-on/end-on/end-on (μ3−η2:η1η1) configuration; b schematic representation of N2 coordinated with heterogeneous Fe3/θ-Al2O3(010) in the same configuration; c optimized Fe3 cluster on θ-Al2O3(010); d, e optimized configurations of N2 adsorption on Fe3/θ-Al2O3(010); f N2 adsorption configuration on the C7 site of Fe(211) surface
Here we focus on the catalytic performance of Fe3 on ammonia synthesis, while we have also proved that the pyramid Fe4 cluster, consisting of four triangular planes, exhibits similar catalytic activity to that of Fe3 cluster (Supplementary Fig. 3). Fe3/θ-Al2O3(010) with magnetic moment of 10 μB is the most stable, and the state of 8 μB is only 0.08 eV less stable (Supplementary Fig. 4), which is consistent with the ab initio molecular dynamics (AIMD) simulations in which the magnetic moment oscillates between 10 and 8 μB (Supplementary Fig. 5g). Such large spin polarization on Fe sites is thought to be one of the key factors to activate N2 on FoMoco and Fe complexes19,43,56.
Two of the most stable configurations of N2 adsorption on the Fe3 site are shown in Fig. 1d and e, which are denoted as μ3−η2ːη1ːη1 (side-on/end-on/end-on) and μ3−η1ːη1ːη1 (end-on/end-on/end-on), respectively. These two configurations are also observed in the AIMD simulations (Supplementary Fig. 5). N2 can also adsorb at single or double Fe sites as μ2−η2ːη1, μ1−η1, and μ1−η2 configurations57,58, but these are less stable here (Supplementary Fig. 6). The Bader charge on N2 decreases to −1.13 |e| with its bond length significantly stretched from 1.10 Å in gas phase to 1.26 Å, close to that in N2F2. In comparison, the N–N bond length on C7 site of Fe(221) surface with high surface energy is 1.33 Å with Bader charge of −1.55 |e| (Fig. 1f).
Competition between dissociative and associative hydrogenation
Lots of efforts have been made to investigate the mechanism of ammonia synthesis on metal surfaces. It is found that the N2 molecule dissociates directly at specific sites of the surface, such as the B5 site of Ru(0001) step surface and the C7 site of Fe(111) or Fe(211) surface5,10,59. The barrier of N2 direct dissociation over close-packed Ru(0001) surface is as high as 1.9 eV but lowers to 0.4 eV on the step site6. Different from on the Fe(C7) site, the binding energy of *NHx on Ru surface is lower. According to the BEP relation, the dissociation barrier of N2 (ΔG≠(N2)) and desorption energies of *NHx (ΔG(*NHx)) scale linearly with the adsorption energy of N atom (ΔG(*N)) on the same type of sites16, so the dependence of ΔG≠(N2) and ΔG(*NHx) on ΔG(*N) can be fitted into lines. It is found that the intercepts of such lines for step sites are lower than those for terrace sites, but the slopes are similar for different kinds of sites16. Thus, with the N2 dissociative mechanism on metal surfaces (the slopes of ΔG≠(N2) and |ΔG(*NHx)| against |ΔG(*N)| are of opposite signs), high temperature and high pressure are intrinsically needed to overcome ΔG≠(N2) and ensure the desorption of NHx simultaneously12,14.
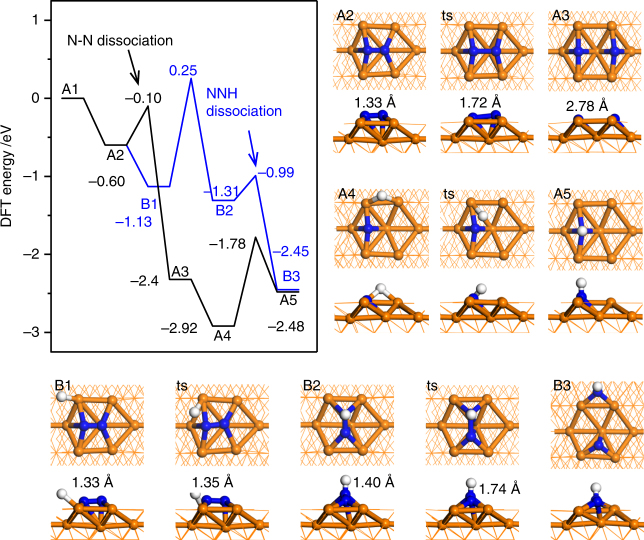
When N2 adsorbs on Fe(211) surface, the anti-bonding π* orbitals of N2 interact strongly with Fe metal surface, and each N is coordinated with four surface Fe, so N2 cannot be hydrogenated without reconstruction. As shown in Fig. 2, the *NNH species (B2) has to rotate from the original *N2 (B1), with the N–N bond length increased to 1.40 Å. Although the dissociation barrier of *NNH (i.e., from B2 to B3) is only 0.32 eV, the hydrogenation barrier of *N2 (from B1 to B2) is as high as 1.38 eV, which is much higher than the *N2 direct dissociation barrier. Thus, it is the high hydrogenation barrier initiating the associative mechanism that makes the dissociative mechanism dominate on Fe(211) surface.
Fig. 2.
The energy diagram for the first two steps of NH3 synthesis on Fe(211) C7 site. The black line is for the dissociative mechanism, where *N2 dissociates directly, and the blue line for the associative mechanism, where the N–N bond breaks only after hydrogenation to the *NNH species
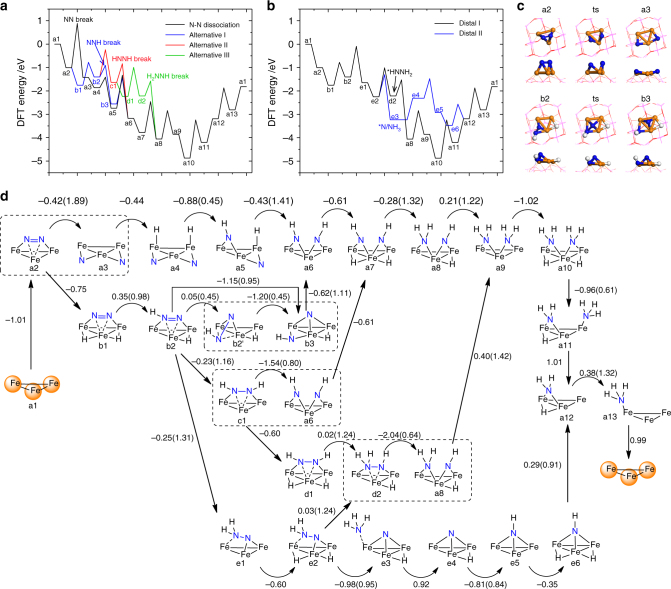
Based on the mechanism on metal catalysts, we investigate the reaction pathways of N2 activation on single-cluster catalyst Fe3/θ-Al2O3(010), as shown in Fig. 3 and Supplementary Fig. 8. The calculated barrier of *N2 direct dissociation is 1.89 eV (Fig. 3c, d), but the associative hydrogenation of *N2 to *NNH only has a barrier of 0.98 eV, which can be driven over with relatively low temperature. Note that the H2 dissociative adsorption barrier is as low as 0.05 eV (Supplementary Fig. 9), which can be neglected comparably. The *N2 dissociation is a structure-sensitive process, which usually needs more than 5 surface metal atoms’ cooperation. The geometry of Fe3 cluster is similar to a close-packed Fe(111) surface, where the dissociation of *N2 is not favored. However, the electronic structure of supported Fe3 cluster is distinct from that of metal surface, which will be discussed in the electronic structures section. On metal surface sites, such as Ru B5 site, the barrier of *N2 hydrogenation is about 0.2 eV higher than that of *N2 dissociation, which leads to around three orders of magnitude difference between the rate constants25. On the contrary, on Fe3/θ-Al2O3(010), the barrier of *N2 hydrogenation is 0.91 eV lower than that of *N2 dissociation, and the calculated rate constants are 9.8 × 105 s−1 and 5.2 × 10−1 s−1 at 700 K and 100 bar, respectively.
Fig. 3.
Energy diagrams for ammonia synthesis. a The dissociative mechanism, and three pathways of associative mechanism with N–N bond dissociation at *NNH, *HNNH, and *HNNH2 intermediates by the alternating hydrogenation route. b Two pathways of associative mechanism by the distal hydrogenation route. c Initial, transition, and final states of *N2 and *NNH dissociation step. d Schematic depiction of the six reaction pathways for conversion of N2 to NH3 catalyzed by Fe3/θ-Al2O3(010). Reaction energies are shown for every step and barriers are enclosed in brackets. The dissociation steps of *N2, *NNH, *HNNH, and *HNNH2 intermediates are enclosed with dashed lines
Reaction mechanisms
As is shown earlier (see also the movie file in the supplementary material), N2 is first activated on Fe3 active center followed by attacking by dissociated H atom, which differs from the procedure in electrochemical condition where proton attacks the adsorbed N2 in solution. With thermochemical condition, after the first N2 hydrogenation step, it is more favorable for *NNH to dissociate into *N and *NH rather than further hydrogenation to *HNNH or *NNH2. Such associative process is believed to occur in the homogeneous catalysis and enzymatic mechanism44,50,60,61. As shown in Fig. 3d, the *NNH can migrate from Fe3 to the Fe3/θ-Al2O3(010) interface (i.e., b2 to b2′) with a barrier of 0.45 eV, where the N-end coordinates with two Fe atoms and the NH-end coordinates with one Fe and one substrate Al ion. The dissociation barrier of *NNH in b2′ is only 0.45 eV with an exothermic reaction energy of −1.20 eV (Fig. 3c, d). Afterwards, the upper *N atom moves to the Fe3 3-fold site, while the lower *NH is anchored at the Fe3/θ-Al2O3(010) interface by coordinating with one surface Al ion and two Fe atoms.
The *NNH can also be hydrogenated further via the alternating or the distal pathway49,51,56. In the alternating pathway, *NNH is hydrogenated to *HNNH43 with a barrier of 1.16 eV (b2 to c1), and the N–N bond length is elongated to 1.42 Å with the N–N stretching frequency of 1012 cm−1, suggesting a single bond between N atoms. The formed *HNNH species can either dissociate into two *NH group or be hydrogenated to *H2NNH that can again dissociate into *NH2 and *NH. The *NHx (x = 1–2) species can be ultimately hydrogenated to *NH3.
In the distal pathway, *NNH is hydrogenated to *NNH2 where both H atoms are on the same end of N–N bond. This process requires a barrier of 1.31 eV, slightly higher than that of the alternating pathway. Next, *NNH2 can be further hydrogenated to *HNNH2 or *N + *NH3. The latter step features spontaneous N–N bond dissociation with a barrier of 0.95 eV, while the former step drives the distal pathway back to the alternating one with a barrier of 1.24 eV.
Overall, we find that the most hydrogenation steps experience barriers ranged from 1.0 to 1.3 eV, which are close to those on metal surfaces5,6, but the indirect N–N bond breaking via *NNH has only a barrier of 0.45 eV, much lower than the direct *N2 dissociation. As a result, the N–N bond breaking step is not the rate-determining step (RDS) of ammonia synthesis on surface-supported Fe3 cluster, distinct from on the traditional metal catalyst surfaces.
The N–N bond dissociation via *NNH bypasses the direct dissociation of *N2 that is one of the severe limitations for the Haber–Bosch process. On the Sabatier volcano curve14, the *N2 dissociation is replaced by the *NNH dissociation, which has a lower barrier that can be driven over with moderate thermodynamic conditions. Therefore, the RDS is no longer the dissociation of N2 but desorption of NHx species. Such an associative mechanism for N2 activation is what nitrogenase does in nature43, and has been reproduced by metal complexes for homogeneous catalysis, where the N2 triple bond is first weakened by single-metal or multiple-metal center, and then hydrogenated by protons and electrons toward NH3 formation42. The surface-anchored Fe3 center with multi-step redox capability, large spin polarization and low oxidation state metal provides a heterogeneous single-cluster catalyst design to mimic the FeMoco in nature.
We further tracked the Bader charges of Fe3 cluster and the N-containing adsorbates along the reaction pathway with associative mechanism, as shown in Fig. 4. The highly reducing Fe3 cluster is strongly oxidized during the whole process. When N2 and H2 are adsorbed on Fe3 cluster, the Bader charge of Fe3 increases from 0.59 to 2.97 |e|, while N2 and H2 are reduced to *N2– and 2*H– adsorbates. The bond order of N2 is reduced to 2.5, with its bond length of 1.26 Å and stretching vibrational frequency of 1384 cm−1. Then, one *H− combines with *N2– to form a *NNH– species, with one electron released back to Fe3 simultaneously. The N–N bond length is then lengthened to 1.36 Å with stretching frequency of 1099 cm−1, and the N–N bond order becomes 2.0 with around two electrons occupying its π* orbitals. Bader charges on Fe3 and *NNH turn to 2.45 and −1.13 |e|, respectively. When *NNH migrates from the Fe3 cluster to the Fe3/θ-Al2O3(010) interface, the N–N bond length is further stretched to 1.40 Å with Bader charge of −1.47 |e|. As shown in Fig. 4, Fe3 is a bifunctional multi-step redox active center for donating electrons at the adsorption steps and accepting electrons at the hydrogenation steps. Thus, the surface-anchored Fe3 single cluster serves as an electron reservoir that regulates the charge variation of the whole process.
Fig. 4.
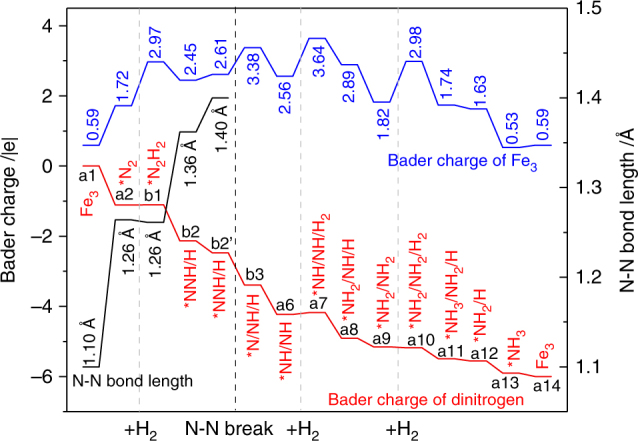
Changes of N–N bond length and charges of Fe3 and the adsorbates. Black, blue, and red curves represent N–N bond lengths, Bader charges of Fe3 cluster, and Bader charges of dinitrogen at every step of the catalytic cycle with the associative mechanism
Electronic structures
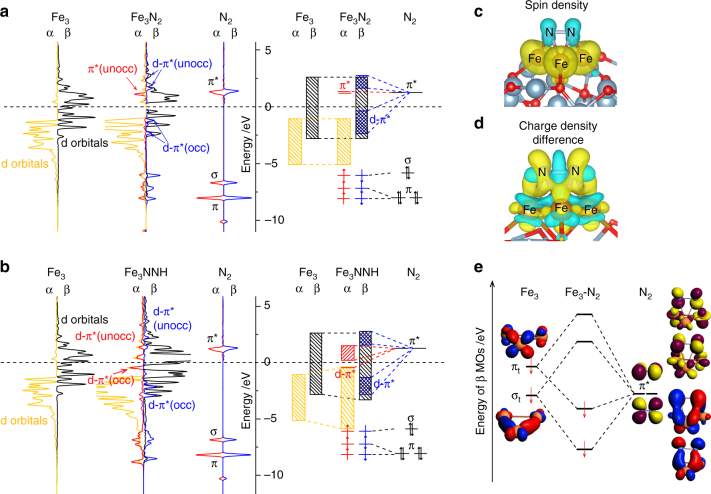
To elucidate the bonding nature of the species involved in the associative mechanism, we investigate the densities of states (DOS) of N2 adsorption on Fe3/θ-Al2O3(010) (with the most stable configuration μ3−η2ːη1ːη1) and C7 site of Fe(211) (Fig. 5a and Supplementary Fig. 10) for comparison. The energy levels of Fe3 minority β-spin d orbitals and N2 π* orbitals are well matched, leading to partial occupation of the formed d-π* orbitals. While the strong spin polarization provides large exchange stabilization energy for the majority α-spin orbitals, leading to that the energy levels of Fe3’s α-spin d orbitals are about 2.5 eV lower than the π* orbitals of N2, and thus no obvious interaction of α orbitals is observed. This indicates that only the β π* orbitals of *N2 are partially occupied, and thus forcing *N2 to be of radical nature (with unpaired electron, Fig. 5c) that is active for hydrogenation. Therefore, the large spin polarization on Fe3 cluster is responsible for the activation of N2. When *N2 is hydrogenated to *NNH, one electron transfers from hydrogen to the α π* orbitals of *N2, and now both α and β DOS of Fe3’s d orbitals overlap with NNH’s π* orbitals, which further weakens the N–N bond. Therefore, the more interaction and thus larger occupation of the π* orbitals in NNH leads to a lower N–N bond order, and is thus responsible for the lower dissociation barrier than that of *N2.
Fig. 5.
Electronic structure analysis. a Projected electronic densities of states (pDOS) and schematic illustrations of 3d orbitals of Fe3 cluster on θ-Al2O3(010), 2p-orbitals of the N2 gas molecule, and their interaction within Fe3N2/θ-Al2O3(010) of μ3−η2:η1η1 configuration. b pDOS and schematic illustrations for the Fe3NNH/θ-Al2O3(010) case. c Spin density of Fe3N2/θ-Al2O3(010). (yellow stands for spin up and cyan for spin down) d Charge density differences (δρ = ρA+B−ρA−ρB) of N2 adsorption on Fe3/θ-Al2O3(010) (cyan stands for holes and yellow for electrons). e The major interactions and energy levels of the scalar relativistic Kohn–Sham β-spin MOs of isolated Fe3N2 with correlation to the orbitals from Fe3 and N2 fragments
Fragment orbital analysis is further performed to provide more details of such interaction between isolated Fe3 and N2 (Fig. 5e, Supplementary Fig. 11 and Supplementary Table 1). The main contribution to the interaction is from the tangential σt, πt molecular orbitals of Fe3 and N2 π* orbitals, which lead to two bonding orbitals and two anti-bonding orbitals. This bonding model shows that the electrons from metal d orbitals partially transfer to the empty π* orbitals of N2, which is consistent with the electron transfer shown in the charge density difference (Fig. 5d) and the DOS analysis. Remarkably, in homogeneous catalysis, the N2 activation process is also initiated with the electron transfer from the electron-rich metal center to the empty π* orbitals of N2, which lowers the bond order in N2 and facilitates the hydrogenation process.
Microkinetic simulations
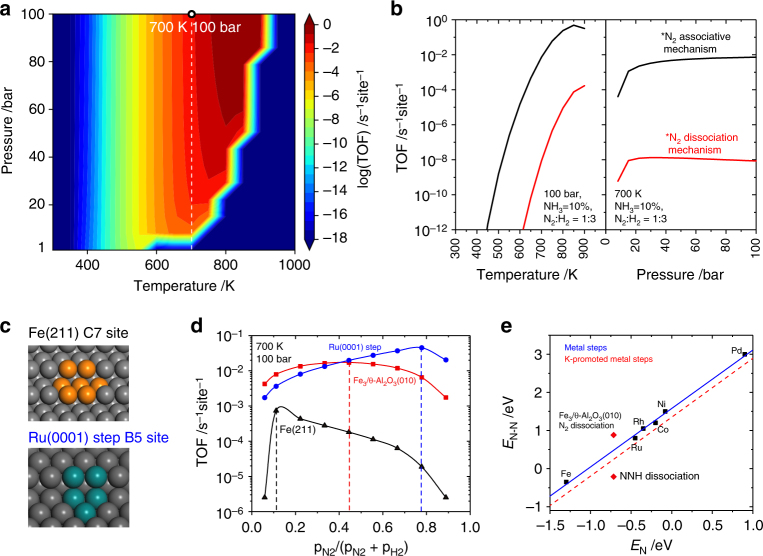
To explore the reactive performance of ammonia synthesis under realistic conditions, we performed comparative kinetic analysis of Fe3/Al2O3, Fe surface, and Ru surface based on the free energy calculations. (Supplementary Fig. 12 and 13) The TOF map (Fig. 6a) is calculated under the pressure range of 1~100 bar and the temperature range of 300–1000 K. The TOF of ammonia production on Fe3/Al2O3 is less than 10−10 s−1 site−1 below 400 K because of too stable adsorption of NHx species (Supplementary Fig. 14a). At high temperature and low pressure, the conversion of NH3 is lower than 10%, and the decomposition of ammonia occurs. With the increase of temperature, the concentrations of NHx decrease and that of bare site increases, since the entropy of free gas molecules becomes dominant. The calculated TOF of ammonia synthesis on Fe3/Al2O3 is 1.4 × 10−2 s−1 site−1 at 100 bar and 700 K, which is comparable to that on the Ru B5 site12. As shown in Fig. 6b, the contribution from the associative mechanism is six orders of magnitude larger than from the dissociative mechanism. While on the Ru step surface, the TOF of the associative mechanism is calculated to be three orders of magnitude lower than that of the dissociative mechanism25.
Fig. 6.
Microkinetic simulations. a TOF per site of ammonia synthesis on Fe3/θ-Al2O3(010) mapped with pressure (1–100 bar) and temperature (300–1000 K). H2:N2 ratio is fixed at 3, and the conversion ratio of NH3 is 10%. b TOF contributions from the associative mechanism (black curve) and the dissociative mechanism (red curve) at constant pressure of 100 bar and constant temperature of 700 K, respectively. c Structures of B5 site on Ru(0001) step surface and C7 site on Fe(211) surface. d TOFs per site of ammonia synthesis over the three catalysts as a function of N2 partial pressure at 700 K and 100 bar. e Calculated transition state energies (EN–N) for N2 dissociation or NNH dissociation as a function of the nitrogen adsorption energy (EN) over step sites on Fe, Ru, Rh, Co, Ni, and Pd surfaces. The solid blue line represents a least-squared interpolation between the points, the red line depicts the cases with addition of alkali (potassium) promotion. Note that the energy data for the metal surfaces in e are taken from ref. [12]
Based on the above microkinetic analysis of the ammonia synthesis on Fe3/θ-Al2O3(010), we further compare it with the widely reported cases on Fe C7 site and Ru B5 site (Fig. 6c)5,6,12,62. The coverages and free energy diagrams for surface species are shown in Supplementary Fig. 13 and 14. For Fe C7 site, although the transition state energy of *N2 dissociation is only −0.10 eV with respect to the gas phase N2 and clean surface, the adsorption energy of two *N is as high as −2.4 eV (Fig. 2), which results in dominant coverage of *N on the active center (Supplementary Fig. 14f). Thus, the RDS on Fe C7 site is the desorption of *NHx species. For Ru B5 site, the transition state energy of *N2 dissociation is 0.4 eV, with two *N adsorption energy of −0.8 eV. At low (<450 K) and high (>450 K) temperature, the Ru B5 site is covered by *NH2 and *H, respectively. Thus, Ru B5 site is the closest to the top of volcano curve, which balances the *N2 dissociation and *NHx desorption processes.
For our single-cluster catalyst Fe3/θ-Al2O3(010), the Fe3 active site is covered by *NH2 and *NH3 at low temperature, and at constant temperature or pressure (Supplementary Fig. 14), the TOF on Fe3/θ-Al2O3(010) is comparable to that on Ru B5 site, and is about two orders of magnitude larger than on Fe C7 site. (Supplementary Fig. 15) By plotting the TOF against the partial pressure of N2 as shown in Fig. 6d, the maxima for Fe C7, Fe3/θ-Al2O3(010), and Ru B5 site are 0.06, 0.44, and 0.78, respectively. As the Fe C7 site is mainly covered by *N, lowering the N2 partial pressure from 25% to 6% increases the TOF. On the contrary, for Ru B5 site, the main surface species is *H at 700 K and 100 bar, and thus increasing the proportion of N2 accelerates the reaction. For Fe3/θ-Al2O3(010), to reach a high TOF, one should balance the partial pressure of N2:H2, because the associative mechanism requires the co-adsorption of N2 and H2 to form the *NNH species, which is the key intermediate as discussed above.
As shown in Fig. 6e, for most active sites on metal surfaces, there is a linear relation (i.e., BEP relation) between the adsorption energies of N atom (EN) and the transition state energies (EN–N, which is equivalent to the apparent activation barrier) for N2 dissociation. On Fe3/θ-Al2O3(010), although the EN–N is above the line for metal cases, the transition state energy of NNH dissociation is even much lower than the line for potassium-promoted metal cases. It is thus possible to bypass the BEP relation with the associative mechanism, and the limitation underlying one side of the volcano curve is then removed. As a counterpart, very recently Chen et al. showed63,64 very interesting cases that the other scaling relation between EN and desorption energies of NHx can also be broken by nitrogen transfer from metal to LiH, which separates the N2 dissociation site from the hydrogenation and desorption site. With these strategies of breaking the BEP relation, ammonia synthesis with ambient conditions does not seem to be impossible. While this study focus on the Fe3/Al2O3 system, it is conceivable that change of support can influence the charge state of the metal cluster and alteration of the transition metal can also affect the N–NH bond breaking barrier. Especially, the procedure of N2 activation and hydrogenation can be affected by moisture or solvents if existing. Further study of the optimal surface metal clusters and support for N2-to-NH3 conversion under different chemical conditions will be interesting. The nature of the surface SCCs will likely offer high selectivity like single-atom catalysts.
Discussion
We propose a surface-anchored Fe3 single-cluster active center for ammonia synthesis by first-principle calculations and microkinetic analysis. This Fe3 cluster can be stably anchored on the θ-Al2O3(010) surface, and its multi-step redox capacity, large spin polarization and low oxidation state metal enable efficient N2 activation, due to the spin-polarized charge transfer from Fe’s 3d orbitals to N2 π* orbitals. The partial occupation of N2’s β-spin π* orbitals both lowers the N–N bond order and also grants *N2 a novel radical nature that leads to an associative mechanism for N2 activation, which mimics the initiation process in the nitrogenase as well as artificial metal complexes for homogeneous catalysis involving nitrogen fixation.
We predict the whole catalytic mechanism for ammonia synthesis on Fe3/θ-Al2O3(010) that is distinctly different from on the industrially used Fe and Ru metal surfaces. The dissociative mechanism dominates the ammonia synthesis on metal surfaces, where *N2 dissociates directly. Thus, the TOF of ammonia synthesis on metal surfaces obeys the BEP relation that demands the balance between N2 dissociation and NHx desorption. However, we find that in our associative mechanism at the single-cluster catalyst the first hydrogenation of N2 to *NNH is much faster than the dissociative mechanism on Fe3/θ-Al2O3(010), and the following dissociation of *NNH only has a barrier of 0.45 eV. Remarkably, the associative mechanism bypasses the BEP relation and thus the limitation underlying one side of the volcano curve. Such surface-anchored Fe3 center represents a class of new catalyst—single-cluster catalyst, which features identical yet isolated active centers on support and thus bridges the gap between heterogeneous and homogeneous catalysis, serving as a heterogeneous catalyst design that enables the associative mechanism for ammonia synthesis from dinitrogen.
The calculated TOF of ammonia synthesis on Fe3/θ-Al2O3(010) is comparable to that on Ru B5 site, which is known as the most active metal catalyst, and is two orders of magnitude faster than Fe C7 site. Thus, the anchored Fe3 center is a promising candidate heterogeneous catalyst for highly selective ammonia synthesis via the associative mechanism. In the future work, we will conduct extensive investigation of various surface-anchored metal trimer and polynuclear clusters, in order to find the optimum of such design for ammonia synthesis toward high TOF at low temperature and low pressure. Highly stable single-cluster catalysts with well-accommodating support hold promises for rational design of highly selective and active catalysts for complicated catalytic reactions such as N2-to-NH3 conversion.
Methods
DFT parameters
Energetics calculations for reaction mechanisms were carried out by using spin-polarized density functional theory (DFT) with Perdew-Burke-Ernzerhof (PBE)65 generalized gradient approximation as implemented in VASP 5.3.566. The cutoff energy of plane-wave basis set is 400 eV and single gamma-point grid sampling was used for Brillouin zone integration. Atomic positions were optimized until the forces were less than 0.02 eV/Å. Transition states were searched by climbing image nudged elastic-band method (CI-NEB) and further confirmed by vibrational frequency analysis67.
Computational model
A θ-Al2O3(010)-p(2 × 4) surface slab was used to model the substrate with cell parameters of a = 11.24 Å, b = 11.79 Å, and c = 25.00 Å. The slab consists of seven O−Al layers, where the bottom two O−Al layer were frozen while the remaining layers were allowed to relax. The slab lattice parameters were fixed to the optimized cell parameters of bulk θ-Al2O3, in order to mimic the support bulk. The trinuclear Fe clusters were anchored by coordinating with surface oxygen as shown in Fig. 1c, and Supplementary Fig. 1. The formation energy of adsorbed iron clusters is defined as Ef = E(Fen/Al2O3)–E(Al2O3)–nE(Fe), where E(Fen/Al2O3) is the total energy of Al2O3 surface with Fen adsorbed, E(Al2O3) is the energy of pristine Al2O3 surface, and E(Fe) is the energy per atom of Fe metal. So the formation energy includes both the formation energy of gas phase Fen cluster and its binding energy on surface. Therefore, we can directly evaluate the stability by Ef. The binding energy of Fe3 cluster is defined as Ebind = E(Fe3/Al2O3)–E(Al2O3)–E(Fe3), where E(Fe3) is the energy of gas phase Fe3 cluster. The adsorption energies of molecules are defined as Eads(X) = E(X/Al2O3)–E(Al2O3)–E(X), where X is H2, N2, and NH3. The adsorption energy of N atom is defined as Eads(N) = 1/2 (E(2 N/Al2O3)–E(Al2O3)–E(N2)), where E(2 N/Al2O3) is the total energy for N2 dissociative adsorption.
Chemical bonding analysis
Electronic structure analyses were performed using spin-unrestricted DFT with PBE exchange-correlation functional and TZ2P Slater basis sets as implemented in the Amsterdam Density Functional (ADF 2016.101) program68. Frozen core approximations were applied to N [1 s2] and Fe[1s2–2p6]. The scalar relativistic (SR) effect was included by the zero-order-regular approximation (ZORA)69. Fe3 and Fe3N2 were optimized under D3h and Cs point-group symmetries, respectively, at all possible spin configurations. Molecular orbitals (MOs) of Fe3N2 and their corresponding contributions from Fe3 and N2 were obtained from fragment MO analyses.
Microkinetic method
Microkinetic modeling was carried out using the CatMAP software package70. The model was constructed by numerically solving the differential equations that describe the coverage of each surface intermediates under the steady-state approximation. The rate constant of each elementary step was calculated by using harmonic transition state theory. The free energies for gas molecules were estimated using the ideal gas approximation considering the vibrational, rotational, and translational contributions to both entropy and enthalpy, while the free energies for surface adsorbates were approximated using the harmonic approximation that treats all degrees of freedom as vibrational modes. The steady-state TOFs were calculated based on the steady-state surface coverages.
Data avalability
All other data supporting the findings of this study are available in the article and its Supplementary Information files and from the corresponding author on request.
Electronic supplementary material
Descriptions of Additional Supplementary Files
Acknowledgements
This work is supported by National Natural Science Foundation of China (NSFC, grant nos. 21590792, 91645203, and 21521091), China Postdoctoral Science Foundation (No. 2017M610863) and Beijing Natural Science Foundation (2184105). The calculations were performed by using supercomputers at Tsinghua National Laboratory for Information Science and Technology, the Supercomputer Center of the Computer Network Information Center at the Chinese Academy of Sciences, the National Supercomputer Centre in Guangzhou (NSCC-GZ, Tianhe II), and the Computational Chemistry Laboratory of Department of Chemistry at Tsinghua University, which is supported by Tsinghua Xuetang Talents Program.
Author contributions
J.L. conceived and directed the research. J.-C.L. performed all the calculations. J.-C.L., X.M. and Y.L. designed the study, analyzed the data. J.-C.L., Y.-G.W., H.X. and J.L. co-wrote the paper. All the authors discussed the results and commented on the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-018-03795-8.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Haber F, van Oordt GZ. Über die Bildung von Ammoniak den Elementen. Anorg. Chem. 1905;44:341–378. doi: 10.1002/zaac.19050440122. [DOI] [Google Scholar]
- 2.Ertl G. Reactions at Surfaces: From Atoms to Complexity (Nobel Lecture) Angew. Chem. Int. Ed. 2008;47:3524–3535. doi: 10.1002/anie.200800480. [DOI] [PubMed] [Google Scholar]
- 3.Cherkasov N, Ibhadon AO, Fitzpatrick P. A review of the existing and alternative methods for greener nitrogen fixation. Chem. Eng. Process Process Intensif. 2015;90:24–33. doi: 10.1016/j.cep.2015.02.004. [DOI] [Google Scholar]
- 4.Hammer B, Nørskov JK. Theoretical surface science and catalysis—calculations and concepts. Adv. Catal. 2000;45:71–129. [Google Scholar]
- 5.Honkala K, et al. Ammonia synthesis from first-principles calculations. Science. 2005;307:555–558. doi: 10.1126/science.1106435. [DOI] [PubMed] [Google Scholar]
- 6.Logadóttir Aacute, Nørskov JK. Ammonia synthesis over a Ru(0001) surface studied by density functional calculations. J. Catal. 2003;220:273–279. doi: 10.1016/S0021-9517(03)00156-8. [DOI] [Google Scholar]
- 7.Ertl G, Lee S, Weiss M. Kinetics of nitrogen adsorption on Fe (111) Surf. Sci. 1982;114:515–526. doi: 10.1016/0039-6028(82)90702-6. [DOI] [Google Scholar]
- 8.Bozso F, Ertl G, Grunze M, Weiss M. Interaction of nitrogen with iron surfaces: I. Fe (100) and Fe (111) J. Catal. 1977;49:18–41. doi: 10.1016/0021-9517(77)90237-8. [DOI] [Google Scholar]
- 9.Egeberg R, et al. N 2 dissociation on Fe (110) and Fe/Ru (0001): what is the role of steps? Surf. Sci. 2001;491:183–194. doi: 10.1016/S0039-6028(01)01397-8. [DOI] [Google Scholar]
- 10.Strongin D, Carrazza J, Bare SR, Somorjai G. The importance of C7 sites and surface roughness in the ammonia synthesis reaction over iron. J. Catal. 1987;103:213–215. doi: 10.1016/0021-9517(87)90109-6. [DOI] [Google Scholar]
- 11.Reuter K, Plaisance CP, Oberhofer H, Andersen M. Perspective: On the active site model in computational catalyst screening. J. Chem. Phys. 2017;146:040901. doi: 10.1063/1.4974931. [DOI] [PubMed] [Google Scholar]
- 12.Vojvodic A, et al. Exploring the limits: A low-pressure, low-temperature Haber–Bosch process. Chem. Phys. Lett. 2014;598:108–112. doi: 10.1016/j.cplett.2014.03.003. [DOI] [Google Scholar]
- 13.Rod TH, Logadottir A, Nørskov JK. Ammonia synthesis at low temperatures. J. Chem. Phys. 2000;112:5343. doi: 10.1063/1.481103. [DOI] [Google Scholar]
- 14.Medford AJ, et al. From the Sabatier principle to a predictive theory of transition-metal heterogeneous catalysis. J. Catal. 2015;328:36–42. doi: 10.1016/j.jcat.2014.12.033. [DOI] [Google Scholar]
- 15.Bligaard T, et al. The Brønsted–Evans–Polanyi relation and the volcano curve in heterogeneous catalysis. J. Catal. 2004;224:206–217. doi: 10.1016/j.jcat.2004.02.034. [DOI] [Google Scholar]
- 16.Dahl S, Logadottir A, Jacobsen CJ, Nørskov JK. Electronic factors in catalysis: the volcano curve and the effect of promotion in catalytic ammonia synthesis. Appl. Catal. A. 2001;222:19–29. doi: 10.1016/S0926-860X(01)00826-2. [DOI] [Google Scholar]
- 17.Sellmann D, Sutter J. In quest of competitive catalysts for nitrogenases and other metal sulfur enzymes. Acc. Chem. Res. 1997;30:460–469. doi: 10.1021/ar960158h. [DOI] [Google Scholar]
- 18.Rodriguez MM, Bill E, Brennessel WW, Holland PL. N2 reduction and hydrogenation to ammonia by a molecular iron-potassium complex. Science. 2011;334:780–783. doi: 10.1126/science.1211906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McWilliams SF, Holland PL. Dinitrogen binding and cleavage by multinuclear iron complexes. Acc. Chem. Res. 2015;48:2059–2065. doi: 10.1021/acs.accounts.5b00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coric I, Mercado BQ, Bill E, Vinyard DJ, Holland PL. Binding of dinitrogen to an iron-sulfur-carbon site. Nature. 2015;526:96–99. doi: 10.1038/nature15246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Falcone M, Chatelain L, Scopelliti R, Zivkovic I, Mazzanti M. Nitrogen reduction and functionalization by a multimetallic uranium nitride complex. Nature. 2017;547:332–335. doi: 10.1038/nature23279. [DOI] [PubMed] [Google Scholar]
- 22.Montoya JH, Tsai C, Vojvodic A, Nørskov JK. The challenge of electrochemical ammonia synthesis: a new perspective on the role of nitrogen scaling relations. ChemSusChem. 2015;8:2180–2186. doi: 10.1002/cssc.201500322. [DOI] [PubMed] [Google Scholar]
- 23.Skúlason E, Jónsson H. Atomic scale simulations of heterogeneous electrocatalysis: recent advances. Adv. Phys. X. 2017;2:481–495. [Google Scholar]
- 24.Skúlason E, et al. A theoretical evaluation of possible transition metal electro-catalysts for N2 reduction. Phys. Chem. Chem. Phys. 2012;14:1235–1245. doi: 10.1039/C1CP22271F. [DOI] [PubMed] [Google Scholar]
- 25.Garden AL, Skúlason E. The mechanism of industrial ammonia synthesis revisited: calculations of the role of the associative mechanism. J. Phys. Chem. C. 2015;119:26554–26559. doi: 10.1021/acs.jpcc.5b08508. [DOI] [Google Scholar]
- 26.Abghoui Y, Skúlason E. Onset potentials for different reaction mechanisms of nitrogen activation to ammonia on transition metal nitride electro-catalysts. Catal. Today. 2017;286:69–77. doi: 10.1016/j.cattod.2016.11.047. [DOI] [Google Scholar]
- 27.Abghoui Y, Garden AL, Howalt JG, Vegge T, Skúlason E. Electroreduction of N2 to Ammonia at Ambient Conditions on Mononitrides of Zr, Nb, Cr, and V: A DFT Guide for Experiments. ACS Catal. 2016;6:635–646. doi: 10.1021/acscatal.5b01918. [DOI] [Google Scholar]
- 28.Abghoui Y, et al. Enabling electrochemical reduction of nitrogen to ammonia at ambient conditions through rational catalyst design. Phys. Chem. Chem. Phys. 2015;17:4909–4918. doi: 10.1039/C4CP04838E. [DOI] [PubMed] [Google Scholar]
- 29.Zhao J, Chen Z. Single Mo atom supported on defective boron nitride monolayer as an efficient electrocatalyst for nitrogen fixation: A Computational Study. J. Am. Chem. Soc. 2017;139:12480–12487. doi: 10.1021/jacs.7b05213. [DOI] [PubMed] [Google Scholar]
- 30.Liu JC, Tang Y, Chang CR, Wang YG, Li J. Mechanistic insights into propene epoxidation with O2–H2O mixture on Au7/α-Al2O3: a hydroproxyl pathway from ab initio molecular dynamics simulations. ACS Catal. 2016;6:2525–2535. doi: 10.1021/acscatal.6b00021. [DOI] [Google Scholar]
- 31.Saavedra J, Doan HA, Pursell CJ, Grabow LC, Chandler BD. The critical role of water at the gold-titania interface in catalytic CO oxidation. Science. 2014;345:1599–1602. doi: 10.1126/science.1256018. [DOI] [PubMed] [Google Scholar]
- 32.Chang CR, Yang XF, Long B, Li J. A water-promoted mechanism of alcohol oxidation on a Au(111) surface: understanding the catalytic behavior of bulk gold. ACS Catal. 2013;3:1693–1699. doi: 10.1021/cs400344r. [DOI] [Google Scholar]
- 33.Fedoseev G, Cuppen HM, Ioppolo S, Lamberts T, Linnartz H. Experimental evidence for glycolaldehyde and ethylene glycol formation by surface hydrogenation of CO molecules under dense molecular cloud conditions. Mon. Not. R. Astron. Soc. 2015;448:1288–1297. doi: 10.1093/mnras/stu2603. [DOI] [Google Scholar]
- 34.Studt F, Abild-Pedersen F, Varley JB, Nørskov JK. CO and CO2 hydrogenation to methanol calculated using the BEEF-vdW functional. Catal. Lett. 2013;143:71–73. doi: 10.1007/s10562-012-0947-5. [DOI] [Google Scholar]
- 35.Behrens M, et al. The active site of methanol synthesis over Cu/ZnO/Al2O3 industrial catalysts. Science. 2012;336:893–897. doi: 10.1126/science.1219831. [DOI] [PubMed] [Google Scholar]
- 36.Chang CR, Wang YG, Li J. Theoretical investigations of the catalytic role of water in propene epoxidation on gold nanoclusters: a hydroperoxyl-mediated pathway. Nano Res. 2011;4:131–142. doi: 10.1007/s12274-010-0083-8. [DOI] [Google Scholar]
- 37.Qiao B, et al. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 2011;3:634–641. doi: 10.1038/nchem.1095. [DOI] [PubMed] [Google Scholar]
- 38.Zhang S, et al. Catalysis on singly dispersed bimetallic sites. Nat. Commun. 2015;6:7938. doi: 10.1038/ncomms8938. [DOI] [PubMed] [Google Scholar]
- 39.Yang XF, et al. Single-atom catalysts: a new frontier in heterogeneous catalysis. Acc. Chem. Res. 2013;46:1740–1748. doi: 10.1021/ar300361m. [DOI] [PubMed] [Google Scholar]
- 40.Zhang B, et al. Homogeneously dispersed multimetal oxygen-evolving catalysts. Science. 2016;352:333–337. doi: 10.1126/science.aaf1525. [DOI] [PubMed] [Google Scholar]
- 41.Ma XL, Liu JC, Xiao H, Li J. Surface Cluster Catalyst for N2-to-NH3 Thermal Conversion. J. Am. Chem. Soc. 2018;140:46–49. doi: 10.1021/jacs.7b10354. [DOI] [PubMed] [Google Scholar]
- 42.van der Ham CJ, Koper MT, Hetterscheid DG. Challenges in reduction of dinitrogen by proton and electron transfer. Chem. Soc. Rev. 2014;43:5183–5191. doi: 10.1039/C4CS00085D. [DOI] [PubMed] [Google Scholar]
- 43.Hoffman BM, Lukoyanov D, Yang ZY, Dean DR, Seefeldt LC. Mechanism of nitrogen fixation by nitrogenase: the next stage. Chem. Rev. 2014;114:4041–4062. doi: 10.1021/cr400641x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anderson JS, Rittle J, Peters JC. Catalytic conversion of nitrogen to ammonia by an iron model complex. Nature. 2013;501:84–87. doi: 10.1038/nature12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Musaev, D. G., Bobadova‐Parvanova, P. & Morokuma, K. in Computational Modeling for Homogenous and Enzymatic Catalysis. (eds Morokuma K. & Musaev D. G.) 83–108 (Wiley‐VCH., Weinheim, Germany, 2008).
- 46.Grubel K, Brennessel WW, Mercado BQ, Holland PL. Alkali metal control over N–N cleavage in iron complexes. J. Am. Chem. Soc. 2014;136:16807–16816. doi: 10.1021/ja507442b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kitano M, et al. Ammonia synthesis using a stable electride as an electron donor and reversible hydrogen store. Nat. Chem. 2012;4:934–940. doi: 10.1038/nchem.1476. [DOI] [PubMed] [Google Scholar]
- 48.Figg TM, Holland PL, Cundari TR. Cooperativity between low-valent iron and potassium promoters in dinitrogen fixation. Inorg. Chem. 2012;51:7546–7550. doi: 10.1021/ic300150u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rittle J, Peters JC. An Fe-N2 complex that generates hydrazine and ammonia via Fe·NNH2: demonstrating a hybrid distal-to-alternating pathway for N2 reduction. J. Am. Chem. Soc. 2016;138:4243–4248. doi: 10.1021/jacs.6b01230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Creutz SE, Peters JC. Catalytic reduction of N2 to NH3 by an Fe-N2 complex featuring a C-atom anchor. J. Am. Chem. Soc. 2014;136:1105–1115. doi: 10.1021/ja4114962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anderson JS, et al. Characterization of an Fe identical with N-NH2 Intermediate Relevant to Catalytic N2 Reduction to NH3. J. Am. Chem. Soc. 2015;137:7803–7809. doi: 10.1021/jacs.5b03432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wucherer EJ, Vahrenkamp H. Azoalkane‐nitrene cleavage on an Fe3‐cluster. Angew. Chem. Int. Ed. 1987;26:355–356. doi: 10.1002/anie.198703551. [DOI] [Google Scholar]
- 53.Rayment T, Schlögl R, Thomas J, Ertl G. Structure of the ammonia synthesis catalyst. Nature. 1985;315:311–313. doi: 10.1038/315311a0. [DOI] [Google Scholar]
- 54.Ji S, et al. Confined pyrolysis within metal-organic frameworks to form uniform Ru3 clusters for efficient oxidation of alcohols. J. Am. Chem. Soc. 2017;139:9795–9798. doi: 10.1021/jacs.7b05018. [DOI] [PubMed] [Google Scholar]
- 55.Lei Y, et al. Increased silver activity for direct propylene epoxidation via subnanometer size effects. Science. 2010;328:224–228. doi: 10.1126/science.1185200. [DOI] [PubMed] [Google Scholar]
- 56.Li XF, et al. Conversion of dinitrogen to ammonia by FeN3-embedded graphene. J. Am. Chem. Soc. 2016;138:8706–8709. doi: 10.1021/jacs.6b04778. [DOI] [PubMed] [Google Scholar]
- 57.Holland PL. Metal-dioxygen and metal-dinitrogen complexes: where are the electrons? Dalton. Trans. 2010;39:5415–5425. doi: 10.1039/c001397h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.MacLachlan EA, Fryzuk MD. Synthesis and reactivity of side-on-bound dinitrogen metal complexes. Organometallics. 2006;25:1530–1543. doi: 10.1021/om051055i. [DOI] [Google Scholar]
- 59.Somorjai G, Materer N. Surface structures in ammonia synthesis. Top. Catal. 1994;1:215–231. doi: 10.1007/BF01492277. [DOI] [Google Scholar]
- 60.Coric I, Holland PL. Insight into the iron-molybdenum cofactor of nitrogenase from synthetic iron complexes with sulfur, carbon, and hydride ligands. J. Am. Chem. Soc. 2016;138:7200–7211. doi: 10.1021/jacs.6b00747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rao L, Xu X, Adamo C. Theoretical Investigation on the Role of the Central Carbon Atom and Close Protein Environment on the Nitrogen Reduction in Mo Nitrogenase. ACS Catal. 2016;6:1567–1577. doi: 10.1021/acscatal.5b02577. [DOI] [Google Scholar]
- 62.Medford AJ, et al. Assessing the reliability of calculated catalytic ammonia synthesis rates. Science. 2014;345:197–200. doi: 10.1126/science.1253486. [DOI] [PubMed] [Google Scholar]
- 63.Wang P, et al. Breaking scaling relations to achieve low-temperature ammonia synthesis through LiH-mediated nitrogen transfer and hydrogenation. Nat. Chem. 2017;9:64–70. doi: 10.1038/nchem.2595. [DOI] [PubMed] [Google Scholar]
- 64.Wang P, et al. The formation of surface lithium-iron ternary hydride and its function on catalytic ammonia synthesis at low temperatures. Angew. Chem. Int. Ed. Engl. 2017;56:8716–8720. doi: 10.1002/anie.201703695. [DOI] [PubMed] [Google Scholar]
- 65.Perdew JP, Burke K, Ernzerhof M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996;77:3865. doi: 10.1103/PhysRevLett.77.3865. [DOI] [PubMed] [Google Scholar]
- 66.Kresse G, Furthmüller J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B: Condens. Matter Mater. Phys. 1996;54:11169. doi: 10.1103/PhysRevB.54.11169. [DOI] [PubMed] [Google Scholar]
- 67.Henkelman G, Uberuaga BP, Jónsson H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000;113:9901–9904. doi: 10.1063/1.1329672. [DOI] [Google Scholar]
- 68.te Velde G, et al. Chemistry with ADF. J. Comput. Chem. 2001;22:931–967. doi: 10.1002/jcc.1056. [DOI] [Google Scholar]
- 69.Lenthe Ev, Baerends EJ, Snijders JG. Relativistic regular two‐component Hamiltonians. J. Chem. Phys. 1993;99:4597–4610. doi: 10.1063/1.466059. [DOI] [Google Scholar]
- 70.Medford AJ, et al. CatMAP: a software package for descriptor-based microkinetic mapping of catalytic trends. Catal. Lett. 2015;145:794–807. doi: 10.1007/s10562-015-1495-6. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Descriptions of Additional Supplementary Files