Abstract
The poultry red mite (PRM), Dermanyssus gallinae, is a nonburrowing haematophagous nest-dwelling ectoparasite of birds; occasionally it bites humans, inducing dermatitis. The possibility that this parasite may also be involved in transmission of pathogens is an additional concern. We investigated the presence of zoonotic agents in PRMs from bird nests and pets, and related them to urban outbreaks of dermatitis. A total of 98 PRMs from 12 outbreaks of PRM dermatitis that occurred in Italian cities from 2001 to 2017 were molecularly investigated for detection of Coxiella spp. (16S rRNA), Chlamydophila spp. (16S rRNA), Rickettsia spp. (17 kDa protein-encoding gene), Borrelia burgdorferi sensu lato (groEL gene) and Bartonella spp. (16S–23S rRNA intergenic spacer). Of the 12 tested mite pools, one was positive for Coxiella burnetii (100% identity) and two for B. burgdorferi sensu lato (99% with Borrelia afzelii). For the first time, the presence of B. burgdorferi sensu lato and C. burnetii is reported in PRMs from urban areas. Birds, mainly pigeons, can harbour both pathogens. Therefore, birds and their nest-dwelling PRMs may play a role in the epidemiology of these infections.
Keywords: Borrelia afzelii, Coxiella burnetii, Dermanyssus gallinae, Public health, Vectorial role, Zoonotic mites
Introduction
Zoonotic haematophagous mites belonging to the Dermanyssidae and Macronyssidae families (Mesostigmata, Acari) are well known for their capacity to attack and bite humans when their natural primary hosts (birds or rodents) are not available [1], [2]. In particular, the cosmopolitan poultry red mite (PRM) Dermanyssus gallinae (De Geer, 1778) represents relevant veterinary and medical issues [3]. In Europe, most human cases of dermanyssosis are due to exposure to infested poultry in industrial or rural farms. Thus, dermanyssosis is considered an occupational hazard for poultry industry operators [4]. However, an increasing number of PRM dermatitides is being reported from urban dwellers. Urban outbreaks are usually linked to infested bird nests, mainly those of pigeons, located on window ledges or in air conditioners located in close proximity to windows [3], [5], [6], [7]. More occasionally, humans may come in contact with PRM through infested pets such as canaries and gerbils [7], [8].
After the mite bite and the inoculation of salivary fluids, subjects develop erythematous, papular eruptions usually associated with itching. Apart from its potential role in eliciting allergic reactions, the possibility that D. gallinae may act as a vector of infectious diseases should not be overlooked. However, although this vector role remains to be definitively confirmed, a large number of microorganisms have been found associated to PRMs [3], [9], including pathogens such as Salmonella enterica [9], Chlamydia psittaci [10] and avian influenza A virus [11]. However, the available information is scarce, as it is mainly focused on poultry and animal pathogens, and such analyses have not been performed on PRMs causing human urban outbreaks, with the exception of a study that revealed the presence of Bartonella quintana in Dermanyssus sp. mites during an outbreak of trench fever in the Czech Republic [12].
We investigated the presence of zoonotic agents in PRMs collected from urban patients with pruritus and dermatitis.
Materials and methods
Red mite collection
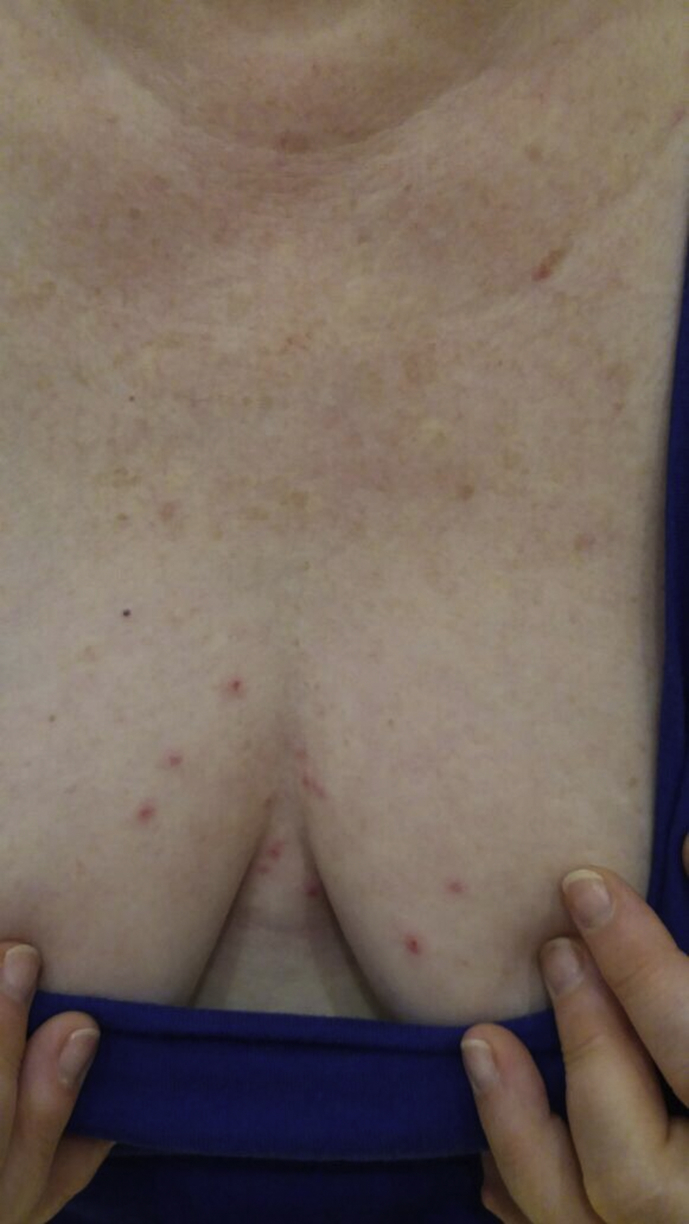
During 2016 and 2017, a total of 98 PRM samples related to 12 outbreaks of itching dermatitis were sent to the Medical Entomology Laboratory of the Istituto Zooprofilattico Sperimentale della Puglia e della Basilicata. Parasites were manually collected in 12 dermanyssosis foci from a hospital, a public office and ten private residences, for a total of 31 subjects. The possible origin of mites were pigeon nests (10/12), sparrow nests (1/12) and pet canaries (1/12). All patients had pruritic, erythematous papules that in some cases were relieved by systemic and/or local antihistaminic and corticosteroid treatment (Fig. 1). Collected mites were identified according to morphologic keys [1], [2], [13] and stored in plastic vials in 70% ethanol.
Fig. 1.
Skin eruptions caused by Dermanyssus gallinae (poultry red mite) bites in 54-year-old woman.
DNA extraction and molecular analyses
Mites from each focus were pooled, for a total of 12 studied pools. The origin and size of each pool are listed in Table 1. Total DNA was extracted from each pool by using the DNeasy Blood and Tissue Kit (Qiagen, Milan, Italy) following the manufacturer's instruction. Before DNA extraction, arthropods were ground in lysis buffer with sterile mortars and pestles. Five distinct PCR assays were performed, using specific primers for Coxiella spp. (16S rRNA) [14], Chlamydia spp. (16S rRNA) [15], Rickettsia spp. (17 kDa protein-coding gene) [16], Borrelia burgdorferi sensu lato (groEL gene) [17] and Bartonella spp. (16S–23S rRNA intergenic spacer) [18]. Amplicons were sequenced, and nucleotide sequences were compared to GenBank using BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastn&PAGE_TYPE=BlastSearch&LINK_LOC=blasthome).
Table 1.
Cases of dermanyssosis and detected pathogens
| Case (date/region) | Collection site | PRM source | No. and sex of affected people | No. of pools (size) | Pool positive | Detected pathogen species | GenBank accession no. |
|---|---|---|---|---|---|---|---|
| 2017/09 Puglia | Bedroom | Pigeon nest | 1F | 1 (5) | – | ||
| 2017/06 Puglia | Bedroom | Pigeon nest | 1F, 1M | 1 (10) | – | ||
| 2015/09 Puglia | Bedroom | Pet canary | 1F | 1 (10) | – | – | – |
| 2011/10 Puglia | Hospital | Pigeon nest | 4 (2M, 2F) | 1 (10) | – | – | |
| 2011/10 Puglia | Bedroom | Pigeon nest | 3 (2M, 1F) | 1 (8) | Borrelia burgdorferi sensu lato | Borrelia afzelii | KY828976 |
| 2009/06 Puglia | Bedroom | Sparrow nest | 1F | 1 (5) | B. burgdorferi sensu lato | B. afzelii | KY828977 |
| 2008/05 Basilicata | Bedroom | Pigeon nest | 3 (1M, 2F) | 1 (10) | Coxiella sp. | Coxiella burnetii | KU215908.1 |
| 2007/05 Puglia | Bedroom | Pigeon nest | 4 (1M, 3) | 1 (10) | – | – | – |
| 2007/03 Campania | Bedroom | Pigeon nest | 1M | 1 (5) | – | _ | |
| 2005/10 Puglia | Bedroom | Pigeon nest | 2 (1M, 1F) | 1 (10) | – | – | – |
| 2005/06 Puglia | Public office | Pigeon nest | 7 (3M, 4F) | 1 (10) | – | – | – |
| 2001/05 Basilicata | Bedroom | Pigeon nest | 2 (1M, 1F) | 1 (5) | – | – | – |
| Total | 31 | 98 | 3 pools |
PRM, poultry red mite.
Samples previously tested positive for Bartonella henselae, Coxiella endosymbiont of Rhipicephalus bursa, Rickettsia raoulti, Chlamydia abortus and Borrelia garinii were used as positive controls. For each PCR reaction, two negative control samples were prepared by adding water and DNA from human specific pathogen-free (SPF) blood instead of DNA from mites.
Phylogenetic analysis
Nucleotide sequences from the 16S rRNA and groEL amplicons of Coxiella spp. and B. burgdorferi sensu lato, respectively, were separately aligned with the matching sequences from GenBank (Supplementary Tables S1 and S2, respectively) using ClustalW in MEGA7 [19]. Sequences from Legionella sp. (RefSeq accession no. NR_116014) and Borrelia anserina (CP013704) were used as outgroups, respectively.
For both organisms, phylogenetic inferences were obtained by maximum-likelihood estimation using PhyML software [19], with 1000 nonparametric bootstrap replicates. The best-fitting evolutionary models were determined by the Model test script [20] implemented for the web at Find Model (http://www.hiv.lanl.gov/content/sequence/findmodel/findmodel.html).
Phylogenetic analysis was carried out using the TN+G model (α = 0.24) for the 16S rRNA gene of Coxiella spp., and the HKY+G model (α = 0.16) for the groEL gene of B. burgdorferi sensu lato.
Results
Morphologic identification of mites
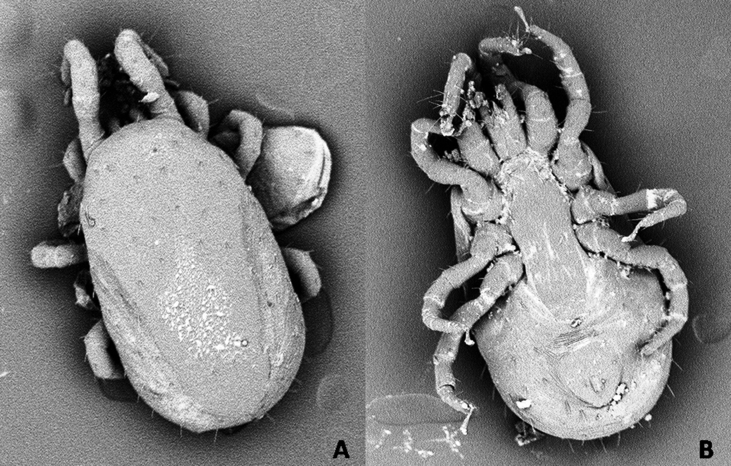
All collected mite specimens were identified as Dermanyssus gallinae (De Geer, 1778) (Fig. 2).
Fig. 2.
Scanning electron microscopy photographs of Dermanyssus gallinae female collected from outbreak. (A) Dorsal view. (B) Ventral view.
Mite-borne pathogens detected in examined red mite pools
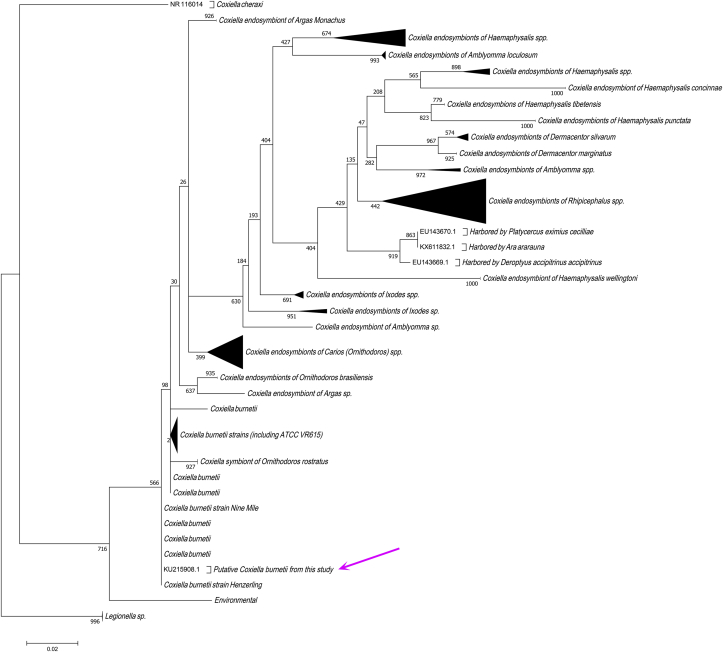
Pathogen detection revealed that one pool out of 12 was positive for Coxiella sp., as were two pools for B. burgdorferi sensu lato (Table 1). The Coxiella sp. amplicon exhibited 100% identity with Coxiella burnetii. The sequence was submitted to GenBank under accession number KU215908. The phylogenetic analysis confirmed the identification of C. burnetii, as shown in Fig. 3.
Fig. 3.
Maximum-likelihood phylogenetic analysis of 16S rRNA sequence from Coxiella sp. detected by PCR and sequencing. Bootstrap values are shown at nodes. Details about sequences included in analysis are provided in Supplementary Table S2. Arrow indicates position of Dermanyssus gallinae. Coxiella burnetii strain was detected in this study.
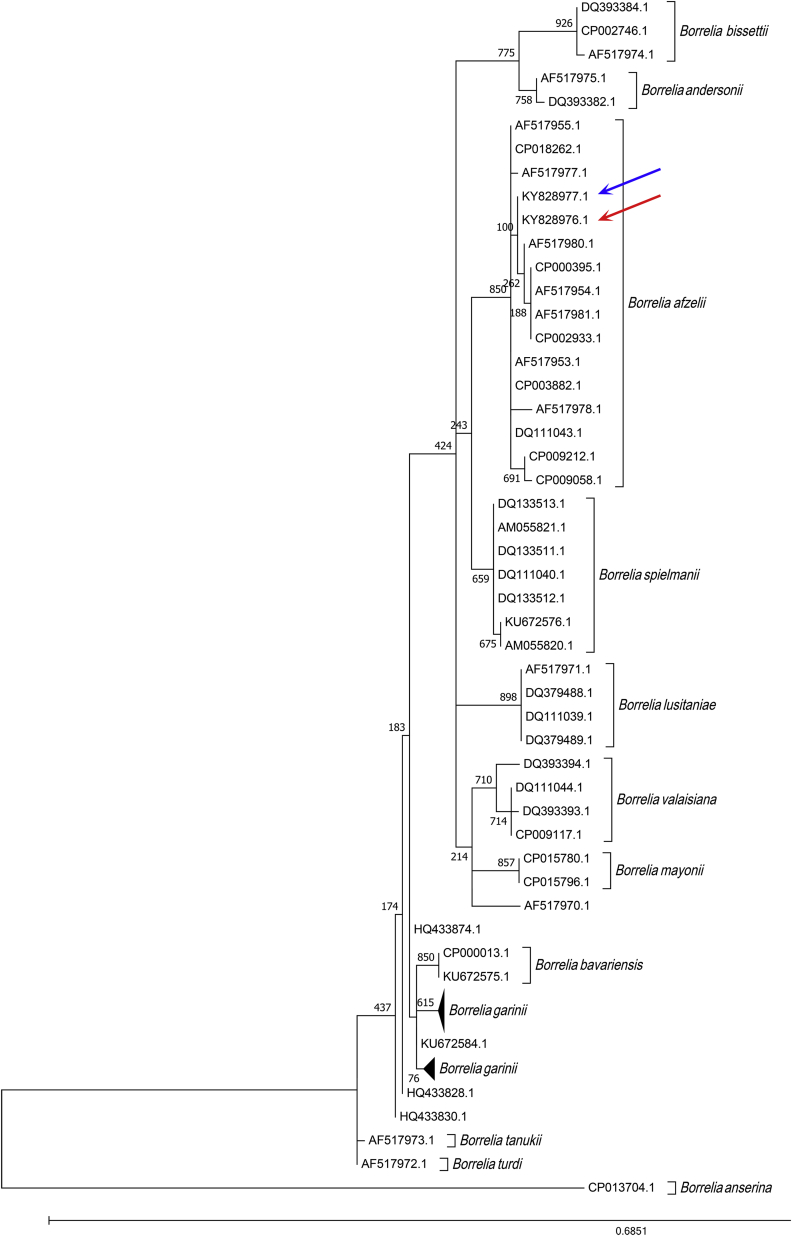
The two positive pools were made of mites collected in a pigeon nest and a sparrow nest, respectively. The nucleotide sequences from the two B. burgdorferi sensu lato amplicons submitted to GenBank under accession numbers KY828976 and KY828977, respectively, were identical but exhibited a 99% identity with Borrelia afzelii. The phylogenetic analysis also confirmed the identification, as demonstrated in Fig. 4.
Fig. 4.
Maximum-likelihood phylogenetic analysis of groEL gene from Borrelia burgdorferi sensu lato detected by PCR and sequencing. Bootstrap values are shown at nodes. Details about sequences included in analysis are provided in Supplementary Table S1. Blue and red arrows show position of Borrelia afzelii strains detected in this study.
Discussion
Dermanyssus gallinae is well known for being associated with a number of microorganisms, but to date little attention has been paid to its potential role as a vector or reservoir of human pathogens. Our findings demonstrate that zoonotic pathogens might be associated to D. gallinae. In particular, the detection of C. burnetii in mites causing human dermatitis may represent a major concern, as the pathogen is the agent of the Q fever, a worldwide zoonosis [20]. Previous studies noted that birds living in urban environments, mostly pigeons, can harbour C. burnetii [21], [22] and may be the source of Q fever outbreaks that atypically occurred in people not in contact with ruminants, the classical source of infection [20]. In 1955, Zemskaia and Pchelkina [23] showed that D. gallinae could acquire infection while feeding on infected animals and that C. burnetii survived about 6 months in live PRMs and about 1 year in dead mites. As a nidicolous mite, D. gallinae may also come in contact with C. burnetii through contaminated nesting materials such as bird faeces. Observations in poultry farms showed that chickens usually remove mites by picking, and other avian species, including pigeons, also adopt the same strategy to remove parasites. Therefore, ingestion of infected D. gallinae may represent a further route for the transmission of C. burnetii in avian species, as well as in some mammals [24].
By considering those factors, one may speculate that the almost ubiquitous distribution of D. gallinae might contribute to the almost global diffusion of C. burnetii. Confirming such a hypothesis would require accurate and comprehensive analysis of urban mites to promptly detect the possible diffusion of this pathogen.
Similar conclusions may be drawn after the detection of B. afzelii in naturally infected PRMs infesting humans. Borrelia afzelii is a genospecies of B. burgdorferi sensu lato, and it is one of the agents of Lyme borreliosis in humans [25]. This disease is a multisystemic infection caused by spirochetes belonging to the B. burgdorferi sensu lato complex. In Europe, most genospecies of that group are specifically host associated [26]. For example, rodents and insectivores are usually infected by B. afzelii or B. garinii [27], while birds are frequently associated with B. garinii or B. valasiana [28]. However, some authors suspect that birds may also play a role in the transmission of B. afzelii.
Although Lyme disease is considered essentially to be a tick-borne disease [29], other species of blood-sucking parasites are able to spread the spirochetes [30], [31], [32]. This study extends the group of potential Lyme borreliosis vectors by also including D. gallinae. Overall, our study demonstrates the association between D. gallinae and pathogens such as C. burnetii and B. afzelii that are potentially dangerous for animals and humans. These findings should focus attention on the frequent infestation of synanthropic birds, mainly pigeons, by D. gallinae, including in urban areas [22].
To our knowledge, no study has yet been carried out to ascertain the ability of D. gallinae to transmit disease fever related to C. burnetii or Lyme borreliosis to humans, but this possibility should be no longer be neglected. Unfortunately, no data were available about the serologic response of patients bitten by infected C. burnetii and B. afzelii, respectively, and no blood or biosamples were collected from these patients, which prevented further analysis from being performed. Therefore, we suggest that physicians, dermatologists and clinicians in general consider carrying out more in-depth investigations in cases of human dermanyssosis.
Conflict of interest
None declared.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.nmni.2018.01.004.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Varma M.G.R. Ticks and mites (Acari) In: Lane R.P., Crosskey R.W., editors. Medical insects and arachnids. Chapman & Hall; London: 1993. pp. 597–658. [Google Scholar]
- 2.Baker A.S. Natural History Museum, London; Stationery Office; London: 1999. Mites and ticks of domestic animals. An identification guide and information source. [Google Scholar]
- 3.George D.R., Finn R.D., Graham K.M., Mul M.F., Maurer V., Moro C.V. Should the poultry red mite Dermanyssus gallinae be of wider concern for veterinary and medical science? Parasit Vectors. 2015;25:178. doi: 10.1186/s13071-015-0768-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cafiero M.A., Galante D., Camarda A., Giangaspero A., Sparagano O. Why dermanyssosis should be listed as an occupational hazard. Occup Environ Med. 2011;68:628. doi: 10.1136/oemed-2011-100002. [DOI] [PubMed] [Google Scholar]
- 5.Haag-Wackernagel D., Bircher A.J. Ectoparasites from feral pigeons affecting humans. Dermatology. 2010;220:82–92. doi: 10.1159/000266039. [DOI] [PubMed] [Google Scholar]
- 6.Bellanger A.P., Bories C., Foulet F., Bretagne S., Botterel F. Nosocomial dermatitis caused by Dermanyssus gallinae. Infect Control Hosp Epidemiol. 2008;29:282–283. doi: 10.1086/528815. [DOI] [PubMed] [Google Scholar]
- 7.Cafiero M.A., Galante D., Raele D.A., Nardella M.C., Piccirilli E., Lomuto M. Outbreaks of Dermanyssus gallinae (Acari, Mesostigmata) related dermatitis in humans in public and private residences, in Italy (2001–2017): an expanding skin affliction. J Clin Case Rep. 2017;7(10):1035. [Google Scholar]
- 8.Lucky A.W., Sayers C., Argus J.D., Lucky A. Avian mite bites acquired from a new source—pet gerbils: report of 2 cases and review of the literature. Arch Dermatol. 2001;137:167–170. [PubMed] [Google Scholar]
- 9.Valiente Moro C., De Luna C.J., Tod A., Guy J.H., Sparagano A.O.E., Zenner L. The poultry red mite, Dermanyssus gallinae: a potential vector of pathogenic agents. Exp Appl Acarol. 2009;48:93–104. doi: 10.1007/s10493-009-9248-0. [DOI] [PubMed] [Google Scholar]
- 10.Circella E., Pugliese N., Todisco G., Cafiero M.A., Sparagano O.A., Camarda A. Chlamydia psittaci infection in canaries heavily infested by Dermanyssus gallinae. Exp Appl Acarol. 2011;55:329–338. doi: 10.1007/s10493-011-9478-9. [DOI] [PubMed] [Google Scholar]
- 11.Boseret G., Losson B., Mainil J.G., Thiry E., Saegerman C. Zoonoses in pet birds: review and perspectives. Vet Res. 2013;20(44):36. doi: 10.1186/1297-9716-44-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melter O., Arvand M., Votypka J., Hulinska D. Bartonella quintana transmission from mite to family with high socio-economic status. Emerg Infect Dis. 2012;18:163–165. doi: 10.3201/eid1801.110186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Palma A., Giangaspero A., Cafiero M.A., Germinara G.S. A gallery of the key characters to ease identification of Dermanyssus gallinae (Acari: Gamasida: Dermanyssidae) and allow differentiation from Ornithonyssus sylviarum (Acari: Gamasida: Macronyssidae) Parasit Vectors. 2014;5:104. doi: 10.1186/1756-3305-5-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Almeida A.P., Marcili A., Leite R.C., Nieri-Bastosa F.A., Domingues L.N., Martins J.R. Coxiella symbiont in the tick Ornithodoros rostratus (Acari: Argasidae) Ticks Tick Borne Dis. 2012;3:203–206. doi: 10.1016/j.ttbdis.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Thomas V., Casson N., Greub G. Criblamydia sequanensis, a new intracellular Chlamydiales isolated from Seine river water using amoebal co-culture. Environ Microbiol. 2006;8:2125–2135. doi: 10.1111/j.1462-2920.2006.01094.x. [DOI] [PubMed] [Google Scholar]
- 16.Webb L., Mitchell C., Malloy D.C., Dasch G.A., Azad A.F. Detection of murine typhus infection in fleas by using the polymerase chain reaction. J Clin Microbiol. 1990;28:530–534. doi: 10.1128/jcm.28.3.530-534.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gil H., Barral M., Escudero R., García-Perez A.L., Anda P. Identification of a new Borrelia species among small mammals in areas of Northern Spain where Lyme disease is endemic. Appl Environ Microbiol. 2005;71:1336–1345. doi: 10.1128/AEM.71.3.1336-1345.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roux V., Raoult D. The 16S-23S rRNA intergenic spacer region of Bartonella (Rochalimaea) species is longer than usually described in other bacteria. Gene. 1995;156:107–111. doi: 10.1016/0378-1119(94)00919-j. [DOI] [PubMed] [Google Scholar]
- 19.Kumar S., Stecher G., Tamura K. MEGA7: Molecular evolutionary Genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Filippitzi M.E., Goumperis T., Robinson T., Saegerman C. Microbiological zoonotic emerging risks, transmitted between livestock animals and humans. Transbound Emerg Dis. 2007–2015;2017(64):1059–1070. doi: 10.1111/tbed.12484. [DOI] [PubMed] [Google Scholar]
- 21.Stein A., Raoult D. Pigeon pneumonia in provence: a bird-borne Q fever outbreak. Clin Infect Dis. 1999;29:617–620. doi: 10.1086/598643. [DOI] [PubMed] [Google Scholar]
- 22.Haag-Wackernagel D., Moch H. Health hazards posed by feral pigeons. J Infect. 2004;48:307–313. doi: 10.1016/j.jinf.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Zemskaia A.A., Pchelkina A.A. Experimental infection of ticks Dermanyssus gallinae Redi and Bdelonyssus bacoti Hirst with Q fever. Dokl Akad Nauk SSSR. 1955;101:391–392. [PubMed] [Google Scholar]
- 24.Eldin C., Mélenotte C., Mediannikov O., Ghigo E., Million M., Edouard S. From Q fever to Coxiella burnetii infection: a paradigm change. Clin Microbiol Rev. 2017;30:115–190. doi: 10.1128/CMR.00045-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pritt B.S., Mead P.S., Hoang Johnson D.K., Neitzel D.F., Respicio-Kingry L.B., Davis J.P. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: a descriptive study. Lancet Infect Dis. 2016;16:556–564. doi: 10.1016/S1473-3099(15)00464-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurtenbach K., Hanincová K., Tsao J.I., Margos G., Fish D., Ogden N.H. Fundamental processes in the evolutionary ecology of Lyme borreliosis. Nat Rev Microbiol. 2006;4:660–669. doi: 10.1038/nrmicro1475. [DOI] [PubMed] [Google Scholar]
- 27.Hanincová K., Schäfer S.M., Etti S., Sewell H.S., Taragelová V., Ziak D. Association of Borrelia afzelii with rodents in Europe. Parasitology. 2003;126:11–20. doi: 10.1017/s0031182002002548. [DOI] [PubMed] [Google Scholar]
- 28.Hanincová K., Taragelová V., Koci J., Schäfer S.M., Hails R., Ullmann A.J. Association of Borrelia garinii and B. valaisiana with songbirds in Slovakia. Appl Environ Microbiol. 2003;69:2825–2830. doi: 10.1128/AEM.69.5.2825-2830.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson J.F. Epizootiology of Lyme borreliosis. Scand J Infect Dis Suppl. 1991;77:23–34. [PubMed] [Google Scholar]
- 30.Melaun C., Zotzmann S., Santaella V.G., Werblow A., Zumkowski-Xylander H., Kraiczy P. Occurrence of Borrelia burgdorferi s.l. in different genera of mosquitoes (Culicidae) in Central Europe. Ticks Tick Borne Dis. 2016;7:256–263. doi: 10.1016/j.ttbdis.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 31.Netušil J., Žákovská A., Vostal K., Norek A., Stanko M. The occurrence of Borrelia burgdorferi sensu lato in certain ectoparasites (Mesostigmata, Siphonaptera) of Apodemus flavicollis and Myodes glareolus in chosen localities in the Czech Republic. Acta Parasitol. 2013;58:337–341. doi: 10.2478/s11686-013-0147-5. [DOI] [PubMed] [Google Scholar]
- 32.Magnarelli L.A., Anderson J.F. Ticks and biting insects infected with the etiologic agent of Lyme disease, Borrelia burgdorferi. J Clin Microbiol. 1988;26:1482–1486. doi: 10.1128/jcm.26.8.1482-1486.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.