Abstract
Cyclophosphamide (CP) is known to reduce fertility. The protective effects of Spirulina plantesis (SP) against CP-induced testicular toxicity were investigated. Male Wistar rats were categorized into eight groups (n = 7). Four groups of rats were administered CP at a dose of 5 mg in 5 mL distilled water kg-1 per day orally. Two of these groups were received SP (500 and 1000 mg kg-1 per day) orally after CP administration. One of these groups was also received vitamin E (100 mg kg-1 per day) intraperitoneally. A vehicle treated control group, two SP control groups (500 and 1000 mg kg-1 per day) and a vitamin E control group were also included. Body and testes weights, sperm count, serum levels of glutathione peroxidase (GPx), malondialdehyde (MDA), histological and histomorphometric alternations in testes were investigated after four weeks. The CP-treated group exhibited significant decreases in the body and testes weights and spermatogenic activities. Several histological alterations were observed in this group. The CP treatment caused a significant reduction in sperm count, in serum level of GPx, as well increased serum concentration of MDA. The SP co-administration caused an increase in GPx serum level, a decrease in MDA serum level and improvements in histological and histomorphometric alternations. Vitamin E co-treatment showed partial recovery in above-mentioned parameters. These results suggest that SP due to a reduction in oxidative stress has more effective protection against CP-induced reproductive damages in rat than vitamin E.
Key Words: Cyclophosphamide, Male reproductive toxicity, Oxidative stress, Rat, Spirulina plantesis
Introduction
Cyclophosphamide (CP) is the most commonly used immunosuppressant and anticancer drug. The CP is used in chronic and acute leukemia, lymphomas, multiple myeloma, bone marrow transplantation, rheumatic arthritis and some immune-related diseases.1 The CP metabolism, by cytochrome p450 in the hepatocytes, generates active alkylating compounds such as 4-hydroxycyclo-phosphamide, aldophosphamide mustard and acrolein.2 Phosphoramide-mustard alkylates DNA leading to DNA synthesis inhibition, prevents cell division and inhibits tumor cells growth. However, acrolein exerts toxic effects on the body’s healthy cells due to reactive oxygen species (ROS) and nitric oxide productions and leads to peroxynitrite production destroying intracellular lipids, proteins and DNA.3 Many studies have shown that CP causes oxidative stress in the male reproductive system.4,5 More than 50.00% of male patients receiving chemotherapy for various solid tumors suffer severe and irreversible seminiferous epithelia damages.6 Since many of cancer patients are treated with CP before and/or during their reproductive years and cure rates for several types of cancers are high, infertility caused by CP is a very significant concern.7 The toxic effects of CP are associated with increased oxidative stress and its interaction with DNA leading to DNA defects, abnormal cell functions and cell death.8 The CP toxicity is not specific for cancer cells and will affect all dividing cells including those in the immune, reproductive and gastrointestinal systems. The CP interferes with oocytes formation in ovaries and sperm differentiation in testicles and may cause infertilities in males and females.9 Jalali et al. have shown that CP treatment in male rats causes significant increases in oxidative stress and abnormal sperms and decreases in sperm concentration and motility, testosterone level and testicular antioxidant capacity.10 In order to overcome the toxic side effects of anti-cancer drugs, some antioxidant agents were considered useful to decrease oxidative stress.11 Several studies have shown the beneficial effects of antioxidants on CP induced damages.12,13 Accordingly, in a review study, Afkhami-Ardakani et al. have indicated that more than 20 types of plant compounds and antioxidants such as Nigella sativa, Camellia sinensis, American ginseng, Aegle marmelos, Achillea millefolium and Crataegus monogyna have significant improving effects on CP-induced male reproductive toxicities.14 The cyanobacterium Spirulina plantesis (SP) belonging to Oscillatoriaceae family is used traditionally as a source of antioxidants against oxidative stress. The SP naturally grows in high-salt alkaline water in subtropical and tropical areas including America, Mexico, Asia and central Africa.15,16
The SP is the only blue-green alga commercially cultivated for food purpose. In 1996, WHO declared Spirulina as ‘‘the best for tomorrow’’ and it has gained its popularity in recent years as a food supplement.17 The SP has been considered as an influential food rich in proteins, carbohydrates, polyunsaturated fatty acids, sterols and some vital elements such as zinc, manganese, iron, calcium, magnesium and selenium. It is a rich source of vitamin B12, vitamin E, vitamin C, tocopherols, whole spectrum of natural mixed carotene and xanthophyll phytopigments.18
Reportedly, SP has antioxidant, anti-inflammatory and chemoprotective effects.14,19 It has been used for treatment of several diseases including hypercholesterolemia, hyperglycerolemia, cardiovascular and inflammatory diseases, cancers, viral infections, diabetes and hyperlipidemic nephrotic syndrome. It has been shown that SP is able to inhibit oxidative stress.20-22 Yang et al. have shown that SP has phycocyanin pigment with potent antioxidant activity.23 In addition, Chamorro et al. have shown that SP has a protective effect on DNA damage induced by CP.24 Due to its antioxidant and anti-apoptotic properties, SP has also a protective effect on CP-induced renal toxicity.25 Premkumar et al. have also shown that SP has a protective effect against genotoxicity and oxidative stress induced by CP and mitomycin-C.26 Vitamin E (alpha-tocopherol) is one of the fat soluble vitamins regulating oxidation processes in the body.27 It is required for cell membrane and skin integrity maintenance, protects them from harmful oxygen free radicals28 and improves male fertility and semen quality in hypercholesterolemic rats.29 The aim of this study was to investigate the antioxidant and protective effects of SP and vitamin E on CP-induced reproductive toxicity in male rats through examining the sperm quantity, histological and histomorphometric alternations in testis as well as antioxidant indices.
Materials and Methods
Chemicals. Cyclophosphamide (Baxter Oncology Gmbh, Weiterstadt, Germany) was dissolved in 2 mL sterile distilled water at a dose of 5 mg kg-1, which was in correspondence to the therapeutic dose.10 Spirulina tablets, containing 100% Spirulina platensis microalgae powder, were obtained from OTC Pharma international BV (Gorinchem, The Netherland). The SP tablets were manually crushed, milled and then suspended in distilled water just before administration.
Animals. Fifty-six adult male Wistar rats (200-220 g) were obtained from the Animal Resources Center, Faculty of Veterinary Medicine, Urmia University, Urmia, Iran. The animals were kept in polypropylene cages under standard conditions of temperature (25.00 ± 2.00 °C), relative humidity (50.00 ± 10.00 %) and light (12 hr light/12 hr dark). They were fed commercial rat chow and water ad libitum. All procedures for animal care and use were carried out in accordance with the guidelines approved by the Animal Ethics Committee of Urmia University (AECVU-145-2017).
Study design. After seven days of acclimation to the environment, the rats were randomly divided into eight groups consisting of seven animals each. Control group was administered distilled water vehicle throughout the experiment. The CP group was received cyclophosphamide (5 mg kg-1) dissolved in distilled water by oral gavage.10 The SP(LD) group received low dose of Spirulina (500 mg kg-1) and SP(HD) group received high dose of Spirulina (1000 mg kg-1) dissolved in distilled water orally.30 The CP+SP(LD) and CP+SP(HD) groups were given SP, orally, 4 hr after CP administration with the same doses of CP and SP, respectively.10 The Vit E group received vitamin E (100 mg kg-1) intraperitoneally. The CP+Vit E group was administered vitamin E (100 mg kg-1) 4 hr after CP administration. All treatments were administered daily for four weeks and designed according to previous studies.5,14
Histological parameters. At the end of the treatment period, rats were anesthetized and scarified. The testes and epididymides were rapidly removed and weighed. After fixation of testes in 10% formalin solution, they were directly dehydrated in a graded series of ethanol and embedded in paraffin. Thin sections (4-5 μm) were stained with hematoxylin and eosin and examined using a light microscope. The qualitative changes of testes were recorded and degenerating Leydig cells and abnormal Sertoli cells were considered. One hundred seminiferous tubules were counted and tubule differentiation index (TDI), the percentage of seminiferous tubules containing at least three differentiated germ cells,31 and spermiation index (SPI), the percentage of seminiferous tubules with normal spermiation, were determined.32
Epididymal sperm count. The rats were sacrificed and the epididymis was rapidly removed under sterile conditions and washed two times in phosphate buffered saline to remove blood cells. Then, caudal epididymis was placed in 1 mL of DMEM (Sigma, St. Louis, USA) and 10% fetal bovine serum medium (Sigma). Cauda was cut into 2–3 pieces and incubated at 37 °C for 10 min in CO2 incubator to allow sperms to swim out of the epididymal tubules. Epididymal sperm counts were determined by the method described in the WHO Manual.33 Briefly, 5 μL aliquot of epididymal sperm was diluted with 95 μL of diluent. Then, 10 µL of the diluted sperm suspension was transferred to each counting chamber of hemocytometer and allowed to stand for 5 min in a moist chamber to prevent drying. Heads of sperms were counted with a light microscope at 400×. The sperm count was expressed as a number of spermatozoa per milliliter.
Biochemical parameters. The blood was collected from left ventricle of heart of anesthetized rats in a tube and centrifuged at 3000 rpm for 15 min. The separated serum was deposited in −20 °C freezer for the biochemical tests. Glutathione peroxidase (GPx) level was measured by the GPx kit as described in the instructions provided by manufacturer (ZellBio Gmbh, Ulm, Germany). Malondialdehyde (MDA) was measured in serum using spectrophotometer at 535 nm wave length. 34
Statistical analysis. Database was set up with SPSS (version 24; SPSS Inc., Chicago, USA). Differences among groups were analyzed by one-way ANOVA model followed by post-hoc Tukey’s test. Data were presented as mean ± SD and resulting p values less than 0.05 were regarded as statistically significant.
Results
Effect of cyclophosphamide and SP on sperm count and body and testis weights. The effects of CP and co-administration of SP on body and testis weights and epididymal sperm count are presented in Table 1. Insignificant (p > 0.05) increases in body and testis weights and epididymal sperm count were observed in SP (500 and 1000 mg kg-1) and Vit E groups compared to control group. On the other hand, CP group showed significant decreases (p < 0.05) in body and testis weights and epididymal sperm count in comparison with control group. Co-administration of SP (500 and 1000 mg kg-1) resulted in significant increases (p < 0.05) in body and testis weights and epididymal sperm count compared to CP group. Co-administration of Vit E resulted in insignificant increases in body and testis weights compared to CP group. Compared to CP group, Vit E co-treatment only could increase sperm count significantly (p < 0.05).
Table 1.
Effects of cyclophosphamide and Spirulina plantesis on body and testis weights (g) and sperm count (106 mL-1). The values are expressed as mean ± SD (n = 7).
| Groups | Control | CP | SP(LD) | SP(HD) | CP+SP(LD) | CP+SP(HD) | Vit E | CP+Vit E |
|---|---|---|---|---|---|---|---|---|
| Initial BW | 206.64 ± 13.53 | 208.04 ± 13.64 | 204.22 ± 14.25 | 207.95 ± 16.92 | 209.45 ± 19.28 | 203.48 ± 15.98 | 211.68 ± 18.26 | 207.65 ± 25.64 |
| Final BW | 230.52 ± 7.62 | 171.14 ± 14.50* | 248.18 ± 10.17† | 277.32 ± 21.90*† | 202.31 ± 9.35† | 203.91 ± 17.13† | 254.94 ± 34.90† | 194.89 ± 7.87* |
| Testis weight | 1.86 ± 0.03 | 1.29 ± 0.04* | 2.01 ± 0.10# | 2.12 ± 0.20*† | 1.67 ± 0.07*† | 1.72 ± 0.05† | 2.02 ± 0.05† | 1.45 ± 0.05* |
| Sperm count | 69.84 ± 1.44 | 5.31 ± 0.61* | 77.32 ± 1.90*† | 79.30 ± 1.23*† | 58.70 ± 2.12*† | 57.38 ± 1.74*† | 75.21 ± 2.07*† | 33.58 ± 3.10*† |
BW: Body weight; CP: Cyclophosphamide; SP: Spirulina plantesis; LD: Low dose; HD: High dose; Vit E: Vitamin E.
Significant difference as compared with control group at p < 0.05, and
Significant difference as compared with cyclophosphamide group at p < 0.05.
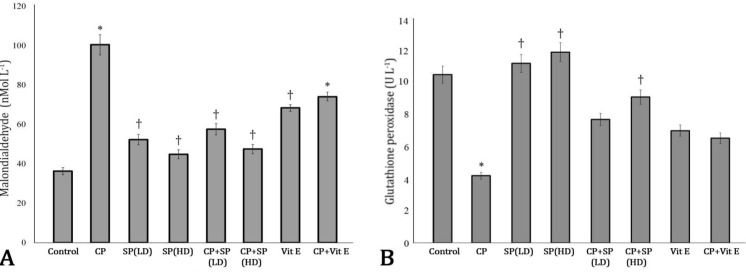
Effect of SP on serum levels of MDA and GPx. As shown in Figure 1A, CP significantly increased serum level of MDA in comparison with control group. Co-administration of SP (500 and 1000 mg kg-1) resulted in significant decrease (p < 0.05) in serum level of MDA in comparison with CP group. Co-administration of Vit E resulted in insignificant decreases in serum level of MDA in comparison with CP group. Serum level of GPx significantly (p < 0.05) decreased in CP group in comparison with control group. Co-administration of SP (1000 mg kg-1) resulted in significant increase (p < 0.05) in serum level of GPx in comparison with CP group. There was no significant increase in serum level of GPx in CP+Vit E group in comparison with CP group (Fig. 1B).
Fig. 1.
A) Effects of cyclophosphamide and Spirulina plantesis on serum level of malondialdehyde (MDA). The values are expressed as mean ± SD (n = 7). * indicates significant difference as compared with control group at p < 0.05 and † indicates significant difference as compared with cyclophosphamide group at p < 0.05. B) Effects of cyclophosphamide and Spirulina plantesis on serum level of glutathione peroxidase (GPx). The values are expressed as mean ± SD (n = 7). * indicates significant difference as compared with control group at p < 0.05 and † indicates significant difference as compared with cyclophosphamide group at p < 0.05. CP: Cyclophosphamide, SP: Spirulina plantesis, LD: Low dose, HD: High dose, and Vit E: Vitamin E
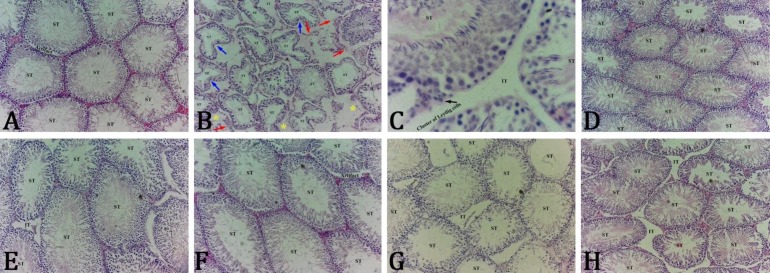
Effect of SP on histological and histomorphometric parameters of testicular tissue. Histopathological studies of the testes showed that CP causes seminiferous collapses and spermatogenesis reduction with central debris in tubules lacking sperms. The CP increased the number of degenerating tubules. Moreover, testicular tissue investigations showed Leydig cells hypoplasia and dispersion with pyknotic nuclei as well as interstitial edema and increased interstitial spaces. The junction of Sertoli cells with germ cells was lost and their nuclei were seemed irregular and smaller than control rats (Figs. 2A and 2B). The SP-co-treated animals showed minimal histological aberrations and were recovered from CP-induced injuries (Figs. 2E and 2F). As shown in Fig. 2G, Vit E-co-treated animals showed nearly normal histoarchitecture in comparison with CP group, but this improvement in SP-co-treated animals was more distinctive. As seen in Table 2, TDI and SPI percentages reduced in CP-treated animals and co-treatment with SP at two doses increased both indices significantly. Likewise, Vit E could increase TDI and SPI percentages insignificantly.
Fig. 2.
Hematoxylin and eosin stained photomicrographs of testis in experimental groups. Rats of control (A; 200×) and vitamin E (H; 200×) groups as well as Spirulina-treated rats (C; 400× and D; 200×) present normal seminiferous tubules (ST) and interstitial tissue (IT) histoarchitectures with active spermatogenesis. The cyclophosphamide group (B; 200×) exhibits collapsed seminiferous tubules (blue arrows), impaired spermatogenesis, edematous fluid accumulation (yellow stars), scattered and degenerated Leydig cells with pyknotic nuclei (red arrows) and germ cells detachment. Spirulina plantesis-co-treated animals show normal testicular histoarchitecture (E and F; 200×). Vitamin E co-treated rat show nearly normal testicular histoarchitecture (G; 200×).
Table 2.
Effects of cyclophosphamide and Spirulina plantesis on tubule differentiation index (TDI) and spermiation index (SPI). The values are expressed as mean ± SD (n = 7).
| Indices | Control | CP | SP(LD) | SP(HD) | CP+SP(LD) | CP+SP(HD) | Vit E | CP+Vit E |
|---|---|---|---|---|---|---|---|---|
| TDI (%) | 95.00 ± 1.00 | 12.00 ± 4.00* | 96.00 ± 1.00† | 97.33 ± 2.08† | 66.33 ± 18.03† | 72.00 ± 19.00† | 96.00 ± 2.00† | 43.00 ± 18.00* |
| SPI (%) | 95.85 ± 1.95 | 10.00 ± 2.16* | 96.00 ± 1.73† | 97.71 ± 0.95† | 69.42 ± 16.65*† | 73.28 ± 11.27*† | 96.85 ± 1.06† | 22.00 ± 1.15* |
CP: Cyclophosphamide SP: Spirulina plantesis; LD: Low dose HD: High dose Vit E: Vitamin E.
Significant difference as compared with control group at p < 0.05, and
Significant difference as compared with cyclophosphamide group at p < 0.05.
Discussion
The CP as an alkylating agent is the main cause of spermatogenesis dysfunction in cancer patients.35 The CP metabolization by hepatic microsomal cytochrome P450, causes alkylating agents production such as 4-hydroxy-cyclophosphamide, aldophosphamide-mustard and acrolein.2 Acrolein is a toxic compound causing oxidative stress through free radicals production.36 It is able to react with certain cell macromolecules including proteins, membrane lipids and DNA.11 A previous study has indicated that CP upsurges apoptosis speed due to increasing levels of ROS and inducing oxidative stress.37 Lipid peroxidation is one of the main indicators of oxidative damage initiated by ROS and it has been linked to altered membrane structure and enzyme inactivation. Increased lipid peroxidation could be detected by MDA production, the most frequently used biomarker.38 Using compounds with protective and potent antioxidant properties can facilitate the clinical use of CP to improve the quality of life among patients under chemotherapy through reducing its toxicity.14 Our aim was to investigate the antioxidant effects of SP, as a potent antioxidant agent, against CP-induced toxicity in rats testicular tissue via seminiferous tubules histopathological alternations, serum levels of oxidative stress indicators changes and sperm quantity examinations. In the current study, co-administration of SP provided significant protection against CP-induced reductions in body and testis weights. Dysfunction and malnutrition induced by decreased feed consumption after CP treatment may attribute to CP-induced reduction in body weight, while the decreases in testes weight may be due to marked parenchymal atrophy, histopathological changes (including degeneration and necrosis( and reductions in sperm and testosterone productions observed in animals treated with CP.32,39 However, administration of SP (500 and 1000 mg kg-1) significantly inhibited the CP-induced reductions in body and testes weights. This protective effect may be attributed to the antioxidant effect of SP under conditions of oxidative stress. Compared to SP, Vit E at a dose of 100 mg kg-1 did not significantly ameliorate the reductions in body and testes weights induced by CP. It was also observed that CP reduces epididymal sperm count significantly. The decreased sperm count may be elucidated by the increased oxidative stress, as spermatozoa are particularly susceptible to oxidative stress.40 According to a previous report, SP protects sperm against oxidative stress induced by doxorubicin.41 In this study, administration of SP (500 and 1000 mg kg-1) CP-treated rats prevented the CP-induced deteriorations in sperm count. Besides, it was found that compared to control rats, CP treatment also induce marked histological alterations in testis including reduction in the numbers of germ cell, vacuolization of seminiferous epithelium, spermatogenesis arrest and degeneration of Leydig cells, confirming previous reports.42 The germ cells reduction, seminiferous epithelia vacuolization and spermatogenesis arrest are in agreement with decreased sperm count observed in current study and can be the results of oxidative stress induced by CP. In this study, oxidative stress was confirmed by decreased antioxidant enzyme GPx as well as increased MDA level. Administration of SP (500 and 1000 mg kg-1) significantly attenuated the severity of CP-induced histological changes and effects on spermato-genesis, which can be attributed to its antioxidant activities. The Vit E-co-treated animals showed nearly improved parameters compared to CP group, but this improvement was stronger in SP-co-treated animals. These findings indicate that SP antioxidant activities due to having several antioxidant compounds such as various vitamins, phycocyanin, selenium, polyunsaturated fatty acids are much more potent than those of Vit E. Abd el-Baky et al. have demonstrated that SP is able to increase its antioxidant activity during oxidative stress elevation and has a self-regulating antioxidant activity against the intensity of oxidative stress.43 Therefore, self-regulating antioxidant activity of SP is more likely the reason of non-significant difference between low and high doses of SP seen in this study. Consistently, many studies have reported similar findings. Jalali et al. have reported that CP administration increase oxidative stress significantly and cause sperm concentration and motility reductions.44 It was also revealed that a single dose of CP is able to increase MDA level in testis and epididymis significantly.11 Selvakumar et al. have demonstrated that CP increase testicular levels of MDA and hydrogen peroxide and change superoxide dismutase and GPx activities in testicular tissue.45 The SP prevents oxidative stress and DNA damages through scavenging free radicals and increasing antioxidant enzymes levels.46 A previous study has revealed that SP-derived phycocyanin and selenium can exert potent antioxidant activity in chemo-prevention.47 In addition, SP was reported to exert protective effects against genotoxicity,48 hemopoietic system toxicity,49 mutagenicity,24 ovarian toxicity,50 nephrotoxicity and urotoxicity25 induced by CP administration.
The results of our study confirmed the antioxidant effects of SP against CP-induced adverse effects in testicular tissue and spermatogenic cells. It is therefore recommended to conduct clinical trials to investigate the use of SP and CP combination in chemotherapy and immunosuppressive therapy to develop complementary medications to decrease anticancer drugs side effects.
Acknowledgements
This study was a part of a PhD thesis No. D2-127 and supported by Urmia University, Urmia, Iran.
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
- 1.Sakr SA, Mahran HA, Abo-El-Yazid SM. Effect of fenugreek seeds extract on cyclophosphamide-induced histomorphometrical, ultrastructural and biochemical changes in testes of albino mice. Toxicol Ind Health. 2012;28(3):276–288. doi: 10.1177/0748233711412427. [DOI] [PubMed] [Google Scholar]
- 2.Kehrer JP, Biswal SS. The molecular effects of acrolein. Toxicol Sci. 2000;57(1):6–15. doi: 10.1093/toxsci/57.1.6. [DOI] [PubMed] [Google Scholar]
- 3.Korkmaz A, Topal T, Oter S. Pathophysiological aspects of cyclophosphamide and ifosfamide induced hemorrhagic cystitis; implication of reactive oxygen and nitrogen species as well as PARP activation. Cell Biol Toxicol. 2007;23(5):303–312. doi: 10.1007/s10565-006-0078-0. [DOI] [PubMed] [Google Scholar]
- 4.Singh S, Lata S, Tiwari KN. Antioxidant potential of Phyllanthus fraternusWebster on cyclophosphamide induced changes in sperm characteristics and testicular oxidative damage in mice. Indian J Exp Biol. 2015;53(10):647–656. [PubMed] [Google Scholar]
- 5.Jalali AS, Hasanzadeh S, Malekinejad H. Achillea millefolium inflorescence aqueous extract ameliorates cyclophosphamide-induced toxicity in rat testis: Stereological evidences. Chin J Nat Med. 2012;10(4):247–254. [PubMed] [Google Scholar]
- 6.Howell SJ, Shalet SM. Testicular function following chemotherapy. Hum Reprod Update. 2001;7(4):363–369. doi: 10.1093/humupd/7.4.363. [DOI] [PubMed] [Google Scholar]
- 7.Meistrich ML. Hormonal stimulation of the recovery of spermatogenesis following chemo- or radiotherapy. Acta Pathol Microbiol Immunol Scand. 1998;106(1):37–45. doi: 10.1111/j.1699-0463.1998.tb01317.x. [DOI] [PubMed] [Google Scholar]
- 8.Lee S, Schmitt CA. Chemotherapy response and resistance. Curr Opin Genet Dev. 2003;13(1):90–96. doi: 10.1016/s0959-437x(02)00014-x. [DOI] [PubMed] [Google Scholar]
- 9.Pendse S, Ginsburg E, Singh AK. Strategies for preservation of ovarian and testicular function after immunosuppression. Am J Kidney Dis. 2004;43(5):772–781. doi: 10.1053/j.ajkd.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Shalizar Jalali A, Hasanzadeh S, Malekinejad H. Chemo-protective effect of Crataegus monogyna aqueous extract against cyclophosphamide-induced repro-ductive toxicity. Vet Res Forum. 2011;2(4):266–273. [Google Scholar]
- 11.Zanchi MM, Manfredini V, Brum DS, et al. Green tea infusion improves cyclophosphamide-induced damage on male mice reproductive system. Toxicol Rep. 2015;2(2015):252–260. doi: 10.1016/j.toxrep.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tripathi P, Tripathi R, Patel RK, et al. Investigation of antimutagenic potential of Foeniculum vulgare essential oil on cyclophosphamide induced geno-toxicity and oxidative stress in mice. Drug Chem Toxicol. 2013;36(1):35–41. doi: 10.3109/01480545.2011.648328. [DOI] [PubMed] [Google Scholar]
- 13.Patra K, Bose S, Sarkar S, et al. Amelioration of cyclophosphamide induced myelosuppression and oxidative stress by cinnamic acid. Chem Biol Interact. 2012;195(3):231–239. doi: 10.1016/j.cbi.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Afkhami-Ardakani M, Hasanzadeh S, Shahrooz R, et al. Phytotherapy and phytopharmacology for reduction of cyclophosphamide-induced toxicity in the male urinary system. J Renal Inj Prev. 2017;6(3):164–170. [Google Scholar]
- 15.Vonshak A. Spirulina platensis (Arthrospira): Physiology, cell biology and biotechnology. 1st ed. London, UK: Taylor & Francis ; 1997. pp. 150–163. [Google Scholar]
- 16.Gershwin ME, Belay A. Spirulina in human nutrition and health. Florida, USA: CRC Press ; 2008. pp. 132–137. [Google Scholar]
- 17.Simpore J, Kabore F, Zongo F, et al. Nutrition rehabilitation of undernourished children utilizing Spiruline and Misola. Nutr J. 2006;5(3):1–7. doi: 10.1186/1475-2891-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chamorro G, Salazar M, Favila L, et al. Pharmacology and toxicology of Spirulina alga [Spanish] Rev Invest Clin. 1996;48(5):389–399. [PubMed] [Google Scholar]
- 19.Pak W, Takayama F, Mine M, et al. Anti-oxidative and anti-inflammatory effects of spirulina on rat model of non-alcoholic steatohepatitis. J Clin Biochem Nutr. 2012;51(3):227–234. doi: 10.3164/jcbn.12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim MY, Cheong SH, Lee J H, et al. Spirulina improves antioxidant status by reducing oxidative stress in rabbits fed a high-cholesterol diet. J Med Food. 2010;13(2):420–426. doi: 10.1089/jmf.2009.1215. [DOI] [PubMed] [Google Scholar]
- 21.Juarez-Oropeza MA, Mascher D, Torres-Duran PV, et al. Effects of dietary Spiurlina on vascular reactivity. J Med Food. 2009;12(1):15–20. doi: 10.1089/jmf.2007.0713. [DOI] [PubMed] [Google Scholar]
- 22.Deng R, Chow TJ. Hypolipidemic, antioxidant, and anti-inflammatory activities of microalgae Spirulina. Cardiovasc Ther. 2010;28(4):33–45. doi: 10.1111/j.1755-5922.2010.00200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang F, Wong KH, Yang YF, et al. Purification and in vitro antioxidant activities of tellurium-containing phycobiliproteins from tellurium-enriched Spirulina platensis. J Inflamm Res. 2014;8(2014):1789–1800. doi: 10.2147/DDDT.S62530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chamorro-Cevallos G, Garduño-Siciliano L, Barrón BL, et al. Chemoprotective effect of Spirulina (Arthrospira) against cyclophosphamide-induced mutagenicity in mice. Food Chem Toxicol. 2008;46(2):567–574. doi: 10.1016/j.fct.2007.08.039. [DOI] [PubMed] [Google Scholar]
- 25.Sinanoglu O, Yener AN, Ekici S, et al. The protective effects of spirulina in cyclophosphamide induced nephrotoxicity and urotoxicity in rats. Urology. 2012;80(6):1392–1396. doi: 10.1016/j.urology.2012.06.053. [DOI] [PubMed] [Google Scholar]
- 26.Premkumar K, Abraham SK, Santhiya ST, et al. Protective effect of Spirulina fusiformis on chemical-induced genotoxicity in mice. Fitoterapia. 2004;75(1):24–31. doi: 10.1016/j.fitote.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 27.Ishaq GM, Saidu Y, Bilbis LS, et al. Effects of α-tocopherol and ascorbic acid in the severity and management of traumatic brain injury in albino rats. J Neurosci Rural Pract. 2013;4(3):292–297. doi: 10.4103/0976-3147.118784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanoka D, Miesel R, Jedzejczak R, et al. Oxidative stress and male fertility. J Androl. 1997;12(1):2434–2436. [Google Scholar]
- 29.Saki G, Jasemi M, Sarkaki AR, et al. Effect of administration of vitamins C and E on fertilization capacity of rats exposed to noise stress. Noise Health. 2013;15(64):184–198. doi: 10.4103/1463-1741.112374. [DOI] [PubMed] [Google Scholar]
- 30.Abdel-daim MM, Abuzead MM, Halawa SM. Protective role of Spirulina platensis against acute deltamethrin-induced toxicity in rats. PLoS One. 2013;8(9):1–7. doi: 10.1371/journal.pone.0072991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Porter KL, Shetty G, Meistrich ML. Testicular edema is associated with spermatogonial arrest in irradiated rats. Endocrinology. 2006;147(3):1297–1305. doi: 10.1210/en.2005-0890. [DOI] [PubMed] [Google Scholar]
- 32.Rezvanfar M, Sadrkhanlou R, Ahmadi A, et al. Protection of cyclophosphamide-induced toxicity in reproductive tract histology, sperm characteristics, and DNA damage by an herbal source; evidence for role of free-radical toxic stress. Hum Exp Toxicol. 2008;27(12):901–910. doi: 10.1177/0960327108102046. [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization (WHO) WHO laboratory manual for the examination and processing of human semen. 5th ed. Geneva, Switzerland: WHO Press ; 2010. pp. 167–169. [Google Scholar]
- 34.Aghaei S, Nikzad H, Taghizadeh M, et al. Protective effect of pumpkin seed extract on sperm characteristics, biochemical parameters and epididymal histology in adult male rats treated with cyclophosphamide. Andrologia. 2014;46(8):927–935. doi: 10.1111/and.12175. [DOI] [PubMed] [Google Scholar]
- 35.Zhao H, Jin B, Zhang X, et al. Yangjing capsule ameliorates spermatogenesis in male mice exposed to cyclophosphamide. Evid Based Complement Alternat Med. 2015;1(2015):1–8. doi: 10.1155/2015/980583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kern JC, Kehrer JP. Acrolein-induced cell death: A caspase-influenced decision between apoptosis and oncosis/necrosis. Chem Biol Interact. 2002;139(1):79–95. doi: 10.1016/s0009-2797(01)00295-2. [DOI] [PubMed] [Google Scholar]
- 37.Asiri YA. Probucol attenuates cyclophosphamide- induced oxidative apoptosis, p53 and Bax signal expression in rat cardiac tissues. Oxid Med Cell Longev. 2010;3(5):308–316. doi: 10.4161/oxim.3.5.13107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oh MS, Chang MS, Park W, et al. Yukmijihwang-tang protects against cyclophosphamide-induced reproductive toxicity. Reprod Toxicol. 2007;24(3):365–370. doi: 10.1016/j.reprotox.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 39.Ceribasi AO, Turk G, Sonmez M, et al. Toxic effect of cyclophosphamide on sperm morphology, testicular histology and blood oxidant-antioxidant balance, and protective roles of lycopene and ellagic acid. Basic Clin Pharmacol Toxicol. 2010;107(3):730–736. doi: 10.1111/j.1742-7843.2010.00571.x. [DOI] [PubMed] [Google Scholar]
- 40.Vernet P, Aitken RJ, Drevet JR. Antioxidant strategies in the epididymis. Mol Cell Endocrinol. 2004;216(2):31–39. doi: 10.1016/j.mce.2003.10.069. [DOI] [PubMed] [Google Scholar]
- 41.Sudha M, Kawimani S. Protective effect of spirulina on doxorubicin induced testicular toxicity. Int J Pharm Bio Sci. 2011;2(3):214–222. [Google Scholar]
- 42.Yuan D, Wang H, He H, et al. Protective effects of total flavonoids from Epimedium on the male mouse reproductive system against cyclophosphamide-induced oxidative injury by up-regulating the expressions of SOD3 and GPX1. Phytother Res. 2014;28:1) 88–97. doi: 10.1002/ptr.4956. [DOI] [PubMed] [Google Scholar]
- 43.Abd El-Baky HH, El-Baz FK, El-Baroty GS. Enhancement of antioxidant production in Spirulina platensis under oxidative stress. Acta Physiol Plant. 2009;31(3):623–631. [Google Scholar]
- 44.Jalali AS, Hasanzadeh S, Malekinejad H. Beneficial effects of Achillea millefolium aqueos extract against cyclophosphamide-induced reproductive toxicity. J Exp Integr Med. 2013;3(2):113–119. [Google Scholar]
- 45.Selvakumar E, Prahalathan C, Mythili Y, et al. Beneficial effects of DL-alpha-lipoic acid on cyclophosphamide-induced oxidative stress in mitochondrial fractions of rat testis. Chem Biol Interact. 2005;152(1):59–66. doi: 10.1016/j.cbi.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 46.Wu Q, Liu L, Miron A, et al. The antioxidant, immuno-modulatory, and anti-inflammatory activities of Spirulina: An overview. Arch Toxicol. 2016;90(8):1–24. doi: 10.1007/s00204-016-1744-5. [DOI] [PubMed] [Google Scholar]
- 47.Chen T, Wong YS. In vitro antioxidant and antiproliferative activities of selenium-containing phycocyanin from selenium-enriched Spirulina platensis. J Agric Food Chem. 2008;56(12):4352–4358. doi: 10.1021/jf073399k. [DOI] [PubMed] [Google Scholar]
- 48.Premkumar K, Pachiappan A, Abraham SK, et al. Effect of Spirulina fusiformis on cyclophosphamide and mitomycin-C induced genotoxicity and oxidative stress in mice. Fitoterapia. 2001;72(8):906–911. doi: 10.1016/s0367-326x(01)00340-9. [DOI] [PubMed] [Google Scholar]
- 49.Zhang HQ, Lin AP, Sun Y, et al. Chemo- and radio-protective effects of polysaccharide of Spirulina platensis on hemopoietic system of mice and dogs. Acta Pharmacol Sin. 2001;22(12):1121–1124. [PubMed] [Google Scholar]
- 50.Yener NA, Sinanoglu O, Ilter E, et al. Effects of spirulina on cyclophosphamide-induced ovarian toxicity in rats: Biochemical and histomorphometric evaluation of the ovary. Biochem Res Int. 2013;1(2013):1–6. doi: 10.1155/2013/764262. [DOI] [PMC free article] [PubMed] [Google Scholar]