Abstract
Background
CD73 is an ecto-enzyme that promotes tumor immune escape through the production of immunosuppressive extracellular adenosine in the tumor microenvironment. Several CD73 inhibitors and adenosine receptor antagonists are being evaluated in phase I clinical trials.
Patients and methods
Full-face sections from formalin-fixed paraffin-embedded primary breast tumors from 122 samples of triple-negative breast cancer (TNBC) from the BIG 02-98 adjuvant phase III clinical trial were included in our analysis. Using multiplex immunofluorescence and image analysis, we assessed CD73 protein expression on tumor cells, tumor-infiltrating leukocytes and stromal cells. We investigated the associations between CD73 protein expression with disease-free survival (DFS), overall survival (OS) and the extent of tumor immune infiltration.
Results
Our results demonstrated that high levels of CD73 expression on epithelial tumor cells were significantly associated with reduced DFS, OS and negatively correlated with tumor immune infiltration (Spearman’s R= −0.50, P < 0.0001). Patients with high levels of CD73 and low levels of tumor-infiltrating leukocytes had the worse clinical outcome.
Conclusions
Taken together, our study provides further support that CD73 expression is associated with a poor prognosis and reduced anti-tumor immunity in human TNBC and that targeting CD73 could be a promising strategy to reprogram the tumor microenvironment in this BC subtype.
Keywords: triple-negative breast cancer, CD73, immunotherapy
Key Message
Our study provides further support that CD73 expression is associated with a poor prognosis and reduced anti-tumor immunity in human triple-negative breast cancer and that targeting CD73 could be a promising strategy to reprogram the tumor microenvironment in this breast cancer subtype.
Introduction
In breast cancer (BC), the extent of lymphocytic infiltration has been associated with a more favorable clinical outcome in numerous studies, particularly in the triple-negative and HER2-positive subtypes [1]. Triple-negative BCs (TNBC) constitute 10%–20% of all BC and are characterized by the lack of hormone receptor and HER2/neu expression [2]. TNBC are also generally more aggressive with poor clinical outcomes and limited treatment options [3]. Due to a higher mutational burden, TNBC appear more immunogenic than other BC subtypes [4]. Yet, in metastatic TNBC, early phase clinical trials have shown modest clinical activity of immune checkpoint inhibitors targeting PD-1 or PD-L1 highlighting the need for new immunotherapeutic approaches [1].
The ecto-nucleotidase CD73 and downstream adenosine receptors are now emerging as attractive therapeutic targets to promote antitumor immune responses [5, 6]. CD73 is expressed on the surface of tumor cells, stromal cells and immune cells [7] where it catalyzes the hydrolysis of AMP into adenosine. Extracellular adenosine is an immunosuppressive metabolite that protects tissues against excessive inflammation [8]. In the tumor microenvironment, adenosine suppresses antitumor immunity, essentially through A2a [9] and A2b [10] adenosine receptors. Independent from its immunosuppressive function, CD73-derived adenosine also promotes tumor cell metastasis and tumor angiogenesis [11].
High levels of CD73 are generally associated with worse clinical outcomes in cancer patients [12]. In BC, however, the prognostic significance of CD73 still remains controversial [13]. In a meta-analysis of over 6000 BC (all subtypes), we recently demonstrated that high levels of CD73 mRNA (microarray data) were significantly associated with worse overall survival (OS) and increased anthracycline resistance in the TNBC subtype alone [14].
Anti-CD73 monoclonal antibodies and A2a antagonists are now entering clinical trials, making it imperative to identify patient populations where the CD73-adenosine pathway contributes to clinical outcome. In this study, we investigated the clinical impact of CD73 protein expression in a large cohort of TNBC from the Breast International Group (BIG) 02-98 adjuvant prospective phase III clinical trial that compared the addition of docetaxel with doxorubicin with doxorubicin-based chemotherapy in node-positive primary BC [15]. Our study demonstrates the prognostic value of CD73 protein expression in TNBC and supports the clinical evaluation of CD73 and/or use of A2a inhibitors in this patient population.
Materials and methods
Clinical specimens
Primary tumor samples from 122 patients with TNBC included in the BIG 02-98 adjuvant phase III trial were selected for this retrospective analysis (38.2% of all TNBC patients). Additional information on the BIG 02-98 trial is provided in supplementary Materials, available at Annals of Oncology online. The remainder formalin-fixed paraffin-embedded (FFPE) tumor blocks were not retrieved or did not contain enough residual invasive carcinoma after evaluation of a hematoxylin–eosin (H&E)-stained tissue section (supplementary Figure S1, available at Annals of Oncology online). TNBC subtype was defined as estrogen receptor (ER)-negative, progesterone (PR)-negative and HER2-negative based on central immunohistochemistry review that determined ER, PR and HER2 status.
Clinico-pathologic characteristics of the 122 patients included in the analysis are listed in supplementary Table S1, available at Annals of Oncology online. All samples were collected at baseline from the surgical specimen. Patients had consented for ulterior use of their tumor for research purpose. There were no differences in clinicopathologic characteristics (supplementary Table S1, available at Annals of Oncology online) nor any survival differences between tumors included in our study and non-analyzed TNBC tumors.
Multiplexed immunofluorescence staining
CD73 protein expression was revealed with an anti-CD73 mouse monoclonal antibody. Tissues were stained simultaneously with an anti-CK8-18 rabbit monoclonal antibody and an anti-CK5 rabbit polyclonal antibody to reveal the epithelial compartment and an anti-CD45 to reveal immune cells. Slides were stained in two batches to reduce experimental variability and no difference was observed for CD73 expression between the two batch stainings. Briefly, FFPE whole tissue sections were deparaffinized, rehydrated and demasked using a citrate buffer (target retrieval solution; Dako, S1699). Tissues were then blocked with a protein block (Dako, X0909) for 30 min before an overnight incubation at 4 °C with primary antibodies. Secondary antibodies were incubated for 2 h at room temperature. A donkey anti-rabbit IgG AlexaFluor 488 conjugate was used against the anti-cytokeratin. A goat anti-mouse IgG1 AlexaFluor 594 conjugate was used to reveal CD73 and a goat anti-mouse IgG2a AlexaFluor647 conjugate to reveal CD45. All antibodies are listed in supplementary Table S2, available at Annals of Oncology online. Coverslips were mounted on to slides using ProLongGold antifade with DAPI (Thermo Fisher Scientific, P36935) and allowed to dry overnight at 4 °C.
Digital image analysis
Slide images of entire tumor sections were captured at once (in one scene, composed of multiple tiled images) with an Axioscan slide scanner system (Axio Scan.Z1, Carl Zeiss Microscopy) using a 20×/0.75NA objective. All images were captured using the same exposure time, laser power and wavelengths, filter sets, and bit depth. Images were analyzed with Visiomorph DP image analysis software (VIS, Visiopharm). Areas of interest [one to nine areas (median of four per tumor) of 0.2–69 mm2; mean: 6 mm2, median: 4.4 mm2] were determined on each tumor section. Normal breast tissue and ductal carcinoma in situ were excluded of the analysis. All images were visually reviewed to remove staining artifacts and damaged tissue areas. The DAPI signal was used to detect the tissue included in the analysis. Algorithms based on cytokeratin and CD45 positivity determined an epithelial and an immune compartment, respectively. CD45+ tumor-immune infiltration was defined as the CD45+ area relative to the tumor tissue area. To allow a proper comparison across all samples, CD73 expression was quantitatively assessed on a continuous scale as the mean fluorescence intensity (MFI) within each of the three compartments (epithelium, stroma and leukocytes) of each tumor section, relative to the MFI of the whole tumor tissue. The weighted average from all areas of interest for each of the 122 tumors was used to assess CD73 expression in each of the 3 compartments.
Statistical analyses
Two end points were analyzed for survival analyses; disease-free survival (DFS) and OS that are defined in supplementary Methods, available at Annals of Oncology online. As there was no significant difference in DFS and OS between anthracycline-only and anthracycline–taxane arms, all treatment arms were pooled for survival analyses [15]. Patients who were alive (for OS) and disease-free (for DFS) were censored at their date of last contact. The associations between CD73 expression (as a continuous variable, per 0.1-unit increment) and CD45+ immune infiltration (as a continuous variable per 10% increment) and prognosis (DFS and OS) were evaluated using Cox regression analyses and likelihood ratio tests in Cox regression models. The added prognostic value of a variable was evaluated using the likelihood ratio statistic test. The SAS macro %findcut was used to determine the optimal cut-off point of the continuous variables CD73 expression and CD45 immune infiltration for DFS [16]. The log-rank test was used to compare groups in terms of DFS or OS. To test the interaction between CD73 expression and treatments arms, the interaction P-value for the interaction term in Cox’s proportional hazards model was calculated. The two-tailed Spearman rank correlation, Mann–Whitney and Kruskal–Wallis tests, Dunn’s multiple comparisons non-parametric tests and the χ2 test were used when appropriate. Statistical analyses were carried out using SAS software for Windows (version 9.4; SAS Institute, Cary, NC). A two-sided P-value of <0.05 was considered significant. The Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK) criteria were followed in this study [17].
Results
CD73 protein expression in TNBC
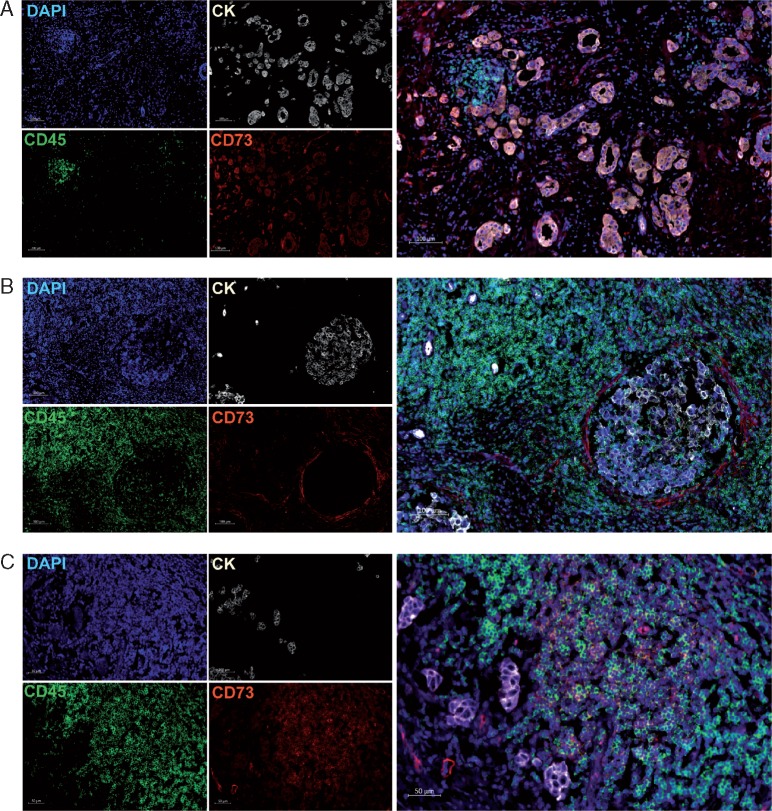
CD73 protein expression was evaluated on full-face tumor sections using immunofluorescence. Consistent with previous studies [18], CD73 was expressed by tumor cells, stromal cells and immune cells (Figure 1). CD73 expression was higher in the tumor epithelium and immune cells compared with stromal cells (supplementary Figure S2 and Table S3, available at Annals of Oncology online). Higher epithelial and immune expressions were primarily observed in lobular tumors (compared with ductal) and in patients with massive lymph node involvement (more than 10 lymph nodes). Higher epithelial expression of CD73 was also detected in histological grade 2 compared with grade 3 tumors. We did not find significant correlations between CD73 and age, menopausal status or tumor size, and stromal CD73 expression was not correlated with any of the characteristics evaluated (supplementary Table S4, available at Annals of Oncology online).
Figure 1.
CD73 expression in TNBC. Representative images of the immunofluorescence staining with DAPI (blue online), cytokeratins (white online), CD73 (red online) and CD45 (green online) on TNBC tissues. Scans were imaged at 200× magnification using Zen lite software (Carl Zeiss). (A) CD73 is expressed on tumor cells and in the peri-tumoral stroma. (B) CD73 is expressed on few CD45+ leukocytes and stromal cells. (C) CD73 is expressed mainly on CD45+ leukocytes.
CD73 expression and prognosis
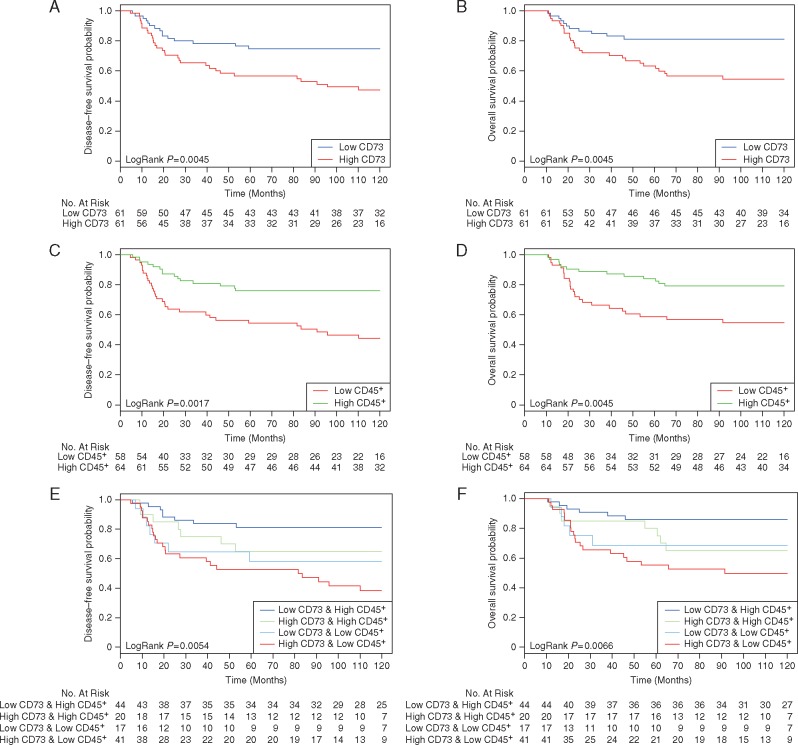
The 10-year DFS and OS of our node-positive TNBC cohort were 62.3% and 68.9%, respectively. We found an association between CD73 expression on epithelial tumor cells—as a continuous variable—and prognosis (Table 1). Increased CD73 expression was correlated with worse survival in both the univariate and multivariate Cox regression models for DFS. The correlation was close to significance for OS in the multivariate model (P = 0.08). Ten-year DFS for CD73-Low TNBC (below median) was 75% versus 49% for CD73-High TNBC (above median) (HR 2.38; 95% CI 1.29–4.42, P = 0.006) and 10-year OS was 82% for CD73-Low versus 55.7% for CD73-High (HR 2.51; 95% CI 1.27–4.96, P = 0.008) (Figure 2A and B). No clear associations between stromal and immune CD73 expression with clinical outcome were found. There was no statistical evidence for interactions between CD73 expression and response to chemotherapy (anthracycline arms or anthracycline–taxane arms) (supplementary Table S5, available at Annals of Oncology online).
Table 1.
Associations between CD73 epithelial expression, CD45+ immune infiltration with prognosis in univariate and multivariate analyses
| Cox regression analyses | DFS |
OS |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N = 122 | Univariate |
Multivariate |
Univariate |
Multivariate |
||||||||
| HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | |
| CD73 epithelial expression (per 0.1 ratio increment) | 1.31 | 1.08–1.60 | 0.007 | 1.26 | 1.00–1.60 | 0.05 | 1.26 | 1.01–1.57 | 0.037 | 1.27 | 0.98–1.66 | 0.08 |
| CD45+ immune infiltration (per 10% increment) | 0.58 | 0.41–0.81 | 0.002 | 0.63 | 0.45–0.89 | 0.008 | 0.64 | 0.45–0.90 | 0.01 | 0.68 | 0.48–0.97 | 0.03 |
The multivariate model contains number of positive lymph nodes, tumor size and histological grade.
DFS, disease-free survival; OS, overall survival.
Figure 2.
Association of CD73 epithelial expression and tumor-infiltrating leukocytes with clinical outcome. Kaplan–Meier analyses of DFS and OS of 122 patients with TNBC stratified according to CD73 epithelial expression (A and B), CD45+ tumor-infiltrating immune cells (C and D) and to the combination of both markers (E and F). Log-rank tests were used to derive P-values for comparisons between groups.
CD73 expression and tumor-infiltrating immune cells
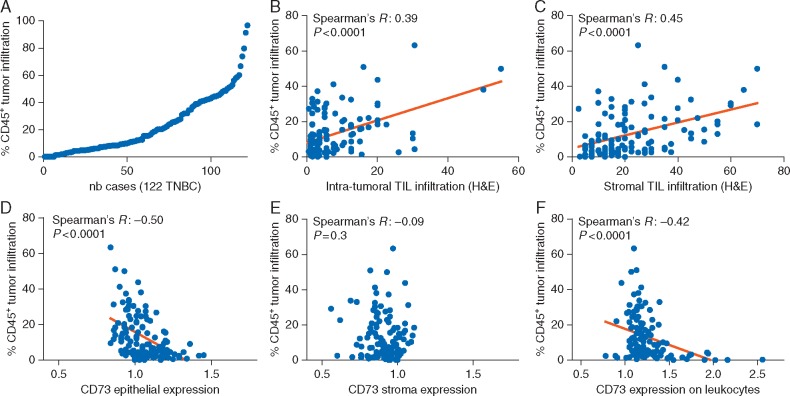
The extent of tumor-infiltrating leukocytes was determined by measuring the CD45+ area relative to the total tumor tissue area. As shown in our previous study [19], tumor immune infiltration forms a continuum with a subset of extensively infiltrated tumors (Figure 3A; supplementary Table S6, available at Annals of Oncology online). Our measurement of the CD45+ infiltration was correlated with intra-tumoral (Spearman’s R = 0.39, P < 0.0001) and stromal (Spearman’s R = 0.46, P < 0.0001) TIL previously scored by pathologists on H&E-stained sections from the same tumors (Figure 3B and C). Consistent with the results of Loi et al., the extent of immune infiltration assessed as the CD45+ area in our analysis was associated with better DFS and OS (Table 1; supplementary Table S7, available at Annals of Oncology online). In the multivariate analysis, adding CD45 to H&E TIL scores appeared to improve the prognostic model, but not the reverse (supplementary Table S8, available at Annals of Oncology online). Kaplan–Meier curves for high and low CD45+ areas are shown in Figure 2B and D (for DFS: HR 0.36; 95% CI 0.19–0.65, P = 0.001; and for OS: HR 0.39; 95% CI 0.20–0.76, P = 0.006).
Figure 3.
CD45+ tumor-immune infiltration. Distribution of CD45+ tumor-infiltrating leukocytes area relative to the tumor area, determined by immunofluorescence and image analysis (A). Correlations between CD45+ tumor-infiltrating leukocytes with intra-tumoral (B) and stromal (C) tumor-infiltrating lymphocytes (TIL) assessed by pathologists on hematoxylin and eosin (H&E)-stained sections. Correlations between CD45+ tumor-immune infiltration and CD73 expression in the epithelium (D), stroma (E) and on leukocytes (F).
Assessment of the association between CD73 expression and CD45+ area revealed a significant negative correlation between epithelial CD73 expression and immune infiltration (Figure 3D). There was no correlation between immune infiltration and CD73 stromal expression (Figure 3E). Interestingly, CD73 expression on leukocytes was also found to negatively correlate with immune infiltration (Figure 3F).
Low CD73 and high immune infiltrates characterize good outcome in TNBC
We finally investigated whether the combination of CD73 epithelial expression and CD45+ leukocytes identified subgroups of patients with a distinct prognosis. Our analysis indicates that CD73 mitigated the favorable prognosis associated with the presence CD45+ leukocytes and patients with low CD73 epithelial expression and high CD45+ immune infiltration (36% of tumors; Figure 2D and E) had the best outcome with a 10-year DFS of 82% and a 10-year OS of 86.5%. In contrast, patients with high CD73 expression and low CD45+ immune infiltration (35% of tumors) had the worst outcome with a 10-year DFS of 41.5% and a 10-year OS of 51.2% (for DFS: HR 4.17, 95% CI 1.87–9.3, P < 0.001; for OS: HR 4.38 95% CI 1.75–10.93, P = 0.0015). In the multivariate analysis, the addition of CD45+ immune infiltration to CD73 add further prognostic information, but adding CD73 to CD45+ did not (supplementary Tables S8 and S9, available at Annals of Oncology online).
Discussion
This study describes an extensive analysis of CD73 protein expression in 122 patients with early-stage TNBC from the BIG 02-98 phase III clinical trial. Specimens from patients associated with long-term follow-up data from clinical trials, such as those evaluated here, are ideal for measuring the prognostic value of a given biomarker [20]. Survival outcomes of our series of TNBC patients were in the range of previously reported TNBC survival data [3]. Our study found that high levels of CD73 expression in breast epithelial tumor cells are significantly associated with poor DFS and OS in TNBC. These results support our previous findings based on gene-expression data [14]. Furthermore, we show that the extent of immune infiltration in TNBC, measured as the CD45+ area, is negatively correlated with CD73 expression on tumor cells. Our findings are, therefore, consistent with a role for CD73 and downstream adenosine-signaling in TNBC immune suppression [7].
The TNBCs evaluated in this study were previously shown to support a prognostic value for lymphocytic infiltration after scoring TIL on H&E sections [21]. Our evaluation of immune infiltration using automated quantification of total CD45+ area, confirmed the good prognosis conferred by immune infiltration in TNBC and appeared to be more robust in predicting DFS and OS than H&E TIL scores evaluated by pathologists. Furthermore, even if CD73 expression and CD45+ infiltration were negatively correlated, the combination of the two markers, allowed us to retrospectively differentiate patients with either an excellent prognosis (high immune infiltration and low CD73 expression) or a poor prognosis (low immune infiltration and high CD73 expression).
Our results are particularly interesting in this era of immunotherapy as data accumulates showing that an increased presence of lymphocytes at the tumor site is linked with the clinical efficacy of immune checkpoint blockade [22]. Accordingly, patients with low tumor immune infiltration are generally more resistant to immune checkpoint blockade but quantification of TIL alone is not sufficient to clearly distinguish responders from non-responders. Tumor CD73 expression levels might additionally (or alternatively) identify patients most likely to derive benefit from CD73- or adenosine-targeting agents or a synergistic immunotherapeutic combination with an immune checkpoint inhibitor. For instance, our data suggest that patients with high levels of TIL and high levels of CD73 epithelial expression may most likely to benefit from a combination of a checkpoint inhibitor with a CD73- or adenosine-targeting agents to decrease immunosuppression of the pre-existing immune response. Conversely, patients with low or no detectable TIL and high CD73 expression may most likely benefit from these CD73- or adenosine receptors inhibitors in combination with drugs able to increase tumor immune infiltration as vaccines or adoptive cell transfer. Interrogating multiple immune biomarkers as those investigated in this study might help to stratify patients and guide for the best combination strategy.
Several inhibitors targeting CD73 or adenosine A2a receptor are currently being evaluated in phase I clinical trials, including in TNBC. Interim clinical safety data revealed that targeting adenosine A2a receptor is well tolerated and can increase frequencies of activated immune cells and tumor immune cell infiltration (AACR 2017, Abstract #5593). In TNBC, 7 out of the 17 (41%) assessable patients treated with an A2a antagonist alone (i.e. CPI-444; Corvus Pharmaceuticals) experienced disease control at interim reporting. Since pre-clinical studies demonstrated that combining adenosine-targeting agents with a PD-1, PD-L1 or CTLA-4 inhibitor significantly improves therapeutic responses [23, 24], on-going phase I trials are also evaluating combining adenosine-targeting agents with a PD-1 or PD-L1 inhibitor.
In this study, we used multiplex immunofluorescence to label multiple markers in tumor tissues to specifically identify epithelial tumor cells and leukocytes. Image analysis is a useful tool for quantitatively and objectively measuring meaningful information from a digital image [25]. This approach allowed us to evaluate CD73 expression in distinct compartments of the tumor microenvironment as continuous variables. The stroma was defined as the cytokeratin-negative plus CD45− compartment in this study. Because the evaluation of stromal CD73 was less precise than the measurements for tumor or immune cells, we cannot exclude the possibility that CD73 expression on stromal cell subsets are also associated with prognosis.
Overall, our data validate important correlations in TNBC between CD73 expression and worst outcome and increased immune infiltration with a better prognosis. These associations provide a strong rationale for the development of immune therapeutic strategies and specifically for targeting immunomodulation through CD73 in TNBC patients [26]. Monoclonal antibodies directed against CD73 and adenosine receptors could help to reprograming the tumor microenvironment, particularly as a synergistic immunotherapeutic combination with immune checkpoint blockade.
Supplementary Material
Acknowledgements
We thank Battista Calvieri from the Microscopy Imaging Lab Faculty of Medicine of the University of Toronto, Canada for the scans, Guillaume Chouinard from the CRCHUM Cell Imaging Core Facility, Tina Gruosso, PhD, from the Goodman Cancer Research Centre, McGill University, Montreal, Canada for insight into the analysis, Gert Van den Eyden, MD, PhD, and Roberto Salgado, MD, PhD, from the Department of pathology, GZA Ziekenhuizen, Wilrijk and Anaïs Boisson from the Molecular Immunology Unit for technical help.
We thank all investigators and participants of the BIG 2-98 clinical trial who have permitted the use of their tissue for research. BIG 2-98 was conducted under the umbrella of the Breast international group (BIG), with sponsorship and funding provided by Sanofi Aventis.
Funding
This work was supported by a Project Grant from the Canadian Institutes of Health Research (CIHR, grant number: 364476). JS is supported by the Famille Jean-Guy Sabourin Research Chair in Pharmacology. LB is a fellow of the Belgian Fund for Scientific Research (FRS-FNRS).
Disclosure
JS is a consultant and member of the Scientific Advisory Board for Surface Oncology Inc. All remaining authors have declared no conflicts of interest.
References
- 1. Luen SJ, Savas P, Fox SB. et al. Tumour-infiltrating lymphocytes and the emerging role of immunotherapy in breast cancer. Pathology 2017; 49(2): 141–155. [DOI] [PubMed] [Google Scholar]
- 2. Goldhirsch A, Winer EP, Coates AS. et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 2013; 24(9): 2206–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dent R, Trudeau M, Pritchard KI. et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 2007; 13(15 Pt 1): 4429–4434. [DOI] [PubMed] [Google Scholar]
- 4. García-Teijido P, Pelaez-Fernández I, Fernández-Pérez Y, Luque-Cabal M.. Tumor-infiltrating lymphocytes in triple negative breast cancer: the future of immune targeting. Clin Med Insights Oncol 2016; 10(Suppl 1): 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Antonioli L, Blandizzi C, Malavasi F. et al. Anti-CD73 immunotherapy: a viable way to reprogram the tumor microenvironment. Oncoimmunology 2016; 5(9): e1216292.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Allard D, Turcotte M, Stagg J.. Targeting A2 adenosine receptors in cancer. Immunol Cell Biol 2017; 95(4): 333–339. [DOI] [PubMed] [Google Scholar]
- 7. Allard B, Longhi MS, Robson SC, Stagg J.. The ectonucleotidases CD39 and CD73: Novel checkpoint inhibitor targets. Immunol Rev 2017; 276(1): 121–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eltzschig HK. Extracellular adenosine signaling in molecular medicine. J Mol Med 2013; 91(2): 141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sitkovsky MV, Hatfield S, Abbott R. et al. Hostile, hypoxia-A2-adenosinergic tumor biology as the next barrier to overcome for tumor immunologists. Cancer Immunol Res 2014; 2(7): 598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mittal D, Sinha D, Barkauskas D. et al. Adenosine 2B receptor expression on cancer cells promotes metastasis. Cancer Res 2016; 76(15): 4372–4382. [DOI] [PubMed] [Google Scholar]
- 11. Allard B, Turcotte M, Spring K. et al. Anti-CD73 therapy impairs tumor angiogenesis. Int J Cancer 2014; 134(6): 1466–1473. [DOI] [PubMed] [Google Scholar]
- 12. Gao Z, Dong K, Zhang H.. The roles of CD73 in cancer. Biomed Res Int 2014; 2014: 460654.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Supernat A, Markiewicz A, Welnicka-Jaskiewicz M. et al. CD73 expression as a potential marker of good prognosis in breast carcinoma. Appl Immunohistochem Mol Morphol 2012; 20(2): 103–107. [DOI] [PubMed] [Google Scholar]
- 14. Loi S, Pommey S, Haibe-Kains B. et al. CD73 promotes anthracycline resistance and poor prognosis in triple negative breast cancer. Proc Natl Acad Sci USA 2013; 110(27): 11091–11096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sonnenblick A, Francis PA, Azim HA. et al. Final 10-year results of the Breast International Group 2-98 phase III trial and the role of Ki67 in predicting benefit of adjuvant docetaxel in patients with oestrogen receptor positive breast cancer. Eur J Cancer 2015; 51(12): 1481–1489. [DOI] [PubMed] [Google Scholar]
- 16. Williams BA, Mandrekar JN, Mandrekar SJ. et al. Finding Optimal Cutpoints for Continuous Covariates with Binary and Time-to-Event Outcomes. Technical Report Series #79, 2006. citeseerx.ist.psu.edu/viewdoc/versions?doi=10.1.1.123.8124.
- 17. McShane LM, Altman DG, Sauerbrei W. et al. REporting recommendations for tumour MARKer prognostic studies (REMARK). Br J Cancer 2005; 93(4): 387–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Allard B, Turcotte M, Stagg J.. CD73-generated adenosine: orchestrating the tumor-stroma interplay to promote cancer growth. J Biomed Biotechnol 2012; 2012: 485156.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Buisseret L, Garaud S, de Wind A. et al. Tumor-infiltrating lymphocyte composition, organization and PD-1/PD-L1 expression are linked in breast cancer. Oncoimmunology 2017; 6(1): e1257452.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Simon RM, Paik S, Hayes DF.. Use of archived specimens in evaluation of prognostic and predictive biomarkers. J Natl Cancer Inst 2009; 101(21): 1446–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Loi S, Sirtaine N, Piette F. et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol 2013; 31(7): 860–867. [DOI] [PubMed] [Google Scholar]
- 22. Tumeh PC, Harview CL, Yearley JH. et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014; 515(7528): 568–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Allard B, Pommey S, Smyth MJ, Stagg J.. Targeting CD73 enhances the antitumor activity of anti-PD-1 and anti-CTLA-4 mAbs. Clin Cancer Res 2013; 19(20): 5626–5635. [DOI] [PubMed] [Google Scholar]
- 24. Mittal D, Young A, Stannard K. et al. Antimetastatic effects of blocking PD-1 and the adenosine A2A receptor. Cancer Res 2014; 74(14): 3652–3658. [DOI] [PubMed] [Google Scholar]
- 25. Lloyd MC, Johnson JO, Kasprzak A, Bui MM.. Image analysis of the tumor microenvironment. Adv Exp Med Biol 2016; 936: 1–10. [DOI] [PubMed] [Google Scholar]
- 26. Allard B, Turcotte M, Stagg J.. Targeting CD73 and downstream adenosine receptor signaling in triple-negative breast cancer. Expert Opin Ther Targets 2014; 18(8): 863–881. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.