We show that functional antisporozoite IgM antibodies are induced in Tanzanian volunteers immunized with radiation attenuated, aseptic, purified, cryopreserved Plasmodium falciparum sporozoites (Sanaria PfSPZ Vaccine), arguing for analysis of this class of antibody in future malaria vaccine trials.
Keywords: malaria, PfSPZ vaccine, malaria-preexposed individuals, functional antibodies, IgM
Abstract
Background
The assessment of antibody responses after immunization with radiation-attenuated, aseptic, purified, cryopreserved Plasmodium falciparum sporozoites (Sanaria PfSPZ Vaccine) has focused on IgG isotype antibodies. Here, we aimed to investigate if P. falciparum sporozoite binding and invasion-inhibitory IgM antibodies are induced following immunization of malaria-preexposed volunteers with PfSPZ Vaccine.
Methods
Using serum from volunteers immunized with PfSPZ, we measured vaccine-induced IgG and IgM antibodies to P. falciparum circumsporozoite protein (PfCSP) via ELISA. Function of this serum as well as IgM antibody fractions was measured via in vitro in an inhibition of sporozoite invasion assay. These IgM antibody fractions were also measured for binding to sporozoites by immunofluorescence assay and complement fixation on whole sporozoites.
Results
We found that in addition to anti-PfCSP IgG, malaria-preexposed volunteers developed anti-PfCSP IgM antibodies after immunization with PfSPZ Vaccine and that these IgM antibodies inhibited P. falciparum sporozoite invasion of hepatocytes in vitro. These IgM plasma fractions also fixed complement to whole P. falciparum sporozoites.
Conclusions
This is the first finding that PfCSP and P. falciparum sporozoite-binding IgM antibodies are induced following immunization of PfSPZ Vaccine in malaria-preexposed individuals and that IgM antibodies can inhibit P. falciparum sporozoite invasion into hepatocytes in vitro and fix complement on sporozoites. These findings indicate that the immunological assessment of PfSPZ Vaccine-induced antibody responses could be more sensitive if they include parasite-specific IgM in addition to IgG antibodies.
Clinical Trials Registration
Despite the global reduction in malaria incidence and mortality rates between the years 2000 and 2015, malaria remains a major public health concern. In 2017, the World Health Organization (WHO) estimated 216 million new malaria cases globally, of which 445000 were lethal, mostly due to Plasmodium falciparum, an increase from the previous year [1]. Most of these deaths (92%) occurred in the WHO African region with 70% of all deaths reported in children under 5 years of age [1].
The most advanced malaria vaccine candidate, the P. falciparum circumsporozoite protein (PfCSP)-based subunit vaccine RTS,S/AS01 (Mosquirix), showed limited vaccine efficacy of 36.3% against clinical malaria among children aged 5 to 17 months and of 25.9% in young infants 6 to 12 weeks of age in phase 3 clinical trials in several sub-Saharan countries [2, 3]. This RTS, S/AS01-mediated protection is short-lived and wanes after the first year of vaccination [2], and the vaccine was associated with a significant increase in adverse events [4].
Complete protection against the onset of the disease-causing malaria blood stage infection, known as “sterile protection,” following controlled human malaria infection (CHMI) has been achieved via mosquito bite administration of radiation-attenuated P. falciparum sporozoites [5–7] and by direct venous inoculation (DVI) of aseptic, purified, radiation-attenuated, cryopreserved P. falciparum sporozoites (Sanaria PfSPZ Vaccine) [8–12]. Administration of fully infectious P. falciparum sporozoites either via mosquito bite or DVI to volunteers taking simultaneously the antimalarial drug chloroquine (PfSPZ chemoprophylaxis vaccination) has also been tested recently, with 100% protection at 8–10 weeks after the last immunization [13–15]. P. falciparum attenuation by genetic modification is under investigation clinically, with no CHMI protection data yet reported [16]. However, most of these live-attenuated malaria vaccinations followed by CHMI have been performed in malaria-naive volunteers living in malaria-free countries [5–11, 13–15]. In the first report of immunization of Africans (Malians) with life-long previous exposure to P. falciparum malaria, protection against P. falciparum infection was significant [12]. However, antibody responses to PfCSP by enzyme-linked immunosorbent assay (ELISA), PfSPZ by immunofluorescence assay (IFA), and to PfSPZ by inhibition of sporozoite invasion (ISI) assay were much lower in Malians than in nonimmune American adults who received the exact same PfSPZ Vaccine immunization regimen. This was hypothesized to be a result of immune dysregulation due to lifelong exposure to P. falciparum [12]. It could also be due to anti-P. falciparum sporozoite antibody and anti-liver stage T-cell responses that develop during natural infection [17–20] and/or the fact that prior exposure to blood stage infection might hamper malaria vaccine-induced protection [21]. We propose that better understanding of the type, kinetics, and maintenance of malaria-specific immune responses in malaria-preexposed populations after immunization could improve the development and successful deployment of malaria vaccines [22]. It is thought that the major driver of the powerful protection seen after immunization with PfSPZ-based vaccines is cellular immunity [13]. However, both anti-CSP monoclonal antibodies and IgG from PfSPZ-immunized volunteers can strongly inhibit P. falciparum sporozoite infection of hepatocytes after passive transfer into humanized liver mice, arguing for a role for antibodies in protection [9, 23, 24]. As mentioned above, immunization of previously malaria-exposed volunteers resulted in weaker antisporozoite antibody responses. These studies, however, only focused on IgG responses and recent reports suggest that exposure to blood stage infection results in a significant antiparasite IgM response [21, 25].
Here, we aimed to determine (i) if PfSPZ-specific IgM antibodies were induced by immunization with PfSPZ Vaccine and (ii) if these IgM antibodies had functional activity in inhibiting P. falciparum sporozoite invasion of hepatocytes. To do so, we used sera collected during a clinical trial investigating the safety, immunogenicity, and protective efficacy of DVI- administered PfSPZ Vaccine in Tanzanian adults in Bagamoyo, Tanzania (NCT02132299) (Jongo et al, manuscript submitted).
METHODS
Detailed experimental methods can be found in the Supplementary Methods.
Ethics Statement
All volunteers gave written informed consent before screening. The clinical trial was performed in accordance with Good Clinical Practices. The protocol was approved by the institutional review boards of the Ifakara Health Institute (ref. no. IHI/IRB/ No: 02-2014), the National Institute for Medical Research Tanzania (NIMR/ HQ/R.8a/Vol.IX/1691), and the Ethikkommission Basel, Basel, Switzerland (ref. no. 261/13). The protocol was approved by the Tanzania Food and Drug Authority (ref. no. TFDA 13/CTR/0003), and the trial was registered at Clinical Trials.gov (NCT02132299) and conducted under US FDA IND 14826.
Clinical Trial Design and Study Population
Details of the trial procedure and volunteers enrolled are given elsewhere (Jongo et al, manuscript submitted). In summary, healthy male volunteers aged 20 to 30 years were randomized to DVI of 5 doses of normal saline, or 1.35 × 105 or 2.7 × 105 of PfSPZ Vaccine in a double-blind clinical trial at the Bagamoyo Clinical Trial Unit of the Ifakara Health Institute in Bagamoyo, Tanzania between 2014 and 2015. Vaccine efficacy was assessed by CHMI by DVI of 3200 PfSPZ of PfSPZ Challenge at 3 weeks after the last PfSPZ immunization, with protected volunteers receiving a second CHMI 24 weeks after the last PfSPZ immunization. The PfSPZ Vaccine proved to be safe and well tolerated in all Tanzanian volunteers. In the low-dose group, 1 volunteer of 18 (6%) was protected against CHMI at 3 weeks and 4 of 20 (20%) volunteers were protected at 3 and 24 weeks in the high-dose group.
Inhibition of Sporozoite Invasion Assay
To determine the capacity of serum antibodies to inhibit sporozoite invasion in vitro, a previously described flow cytometry-based assay was performed [16, 26]. Percent of inhibition of invasion for each volunteer was determined by normalizing to wells containing volunteer-matched preimmune serum or a pooled serum sample from malaria-naive individuals. Normalization of all postimmune ISI values was done as follows: [(%ISI)/(%ISI baseline)] × 100.
CSP Enzyme-Linked Immunosorbent Assay
IgG and IgM antibodies were measured against full-length PfCSP protein, as previously described with minor modifications [16]. All ELISA values were repeated and are referred to as the mean of 2 independent runs. Not all volunteers were tested at all the time points due to sample availability.
Generation of IgM Antibody Fractions
IgM antibody fractions were generated by depletion of IgG and IgA using protein G and Jacalin-sepharose, respectively. Purity of fractions was assessed using total human IgM and IgA ELISAs (Bethyl Laboratories) according to the manufacturer’s protocol.
Sporozoite Immunofluorescence Assays
Sporozoite IFAs were performed as previously described using freshly dissected P. falciparum sporozoites [16]. Sporozoites were visualized using Deltavision microscopy. Images were captured using the same exact exposure conditions in the hIgM/Alexafluor-594 channel to allow comparisons of intensity across samples. Images were modified for clarity with the same exact parameters applied to each sample.
Complement Fixation Assay
The ability of IgM antibody fractions to fix complement was assessed using plate-bound whole P. falciparum sporozoites. C5a was detected in the supernatant following incubation of IgM fractions with human serum complement (Quidel Corp.) by ELISA.
Statistical Analysis
Statistical analyses were carried out using R [27]. The R package ggplot2 was used for data visualization[28] as well as GraphPad Prism 6.
RESULTS
Immunization With PfSPZ Vaccine Induces Sporozoite Inhibitory Antibodies
An overview of the vaccination schedule followed by 2 consecutive homologous CHMIs is given in Supplementary Figure 1 as well as in the publication detailing the clinical trial and protection results (Jongo et al, submitted). Briefly, 2 groups of volunteers (n = 20) were vaccinated 5 times each with a dosage of 1.35 × 105 (low dose) or 2.7 × 105 (high dose) PfSPZ Vaccine and given controlled human malaria infection (CHMI) by direct venous injection of aseptic, purified, cryopreserved wild type sporozoites (PfSPZ challenge) [22]. Volunteers protected from this first CHMI (CHMI1) were then rechallenged 140 days later in a second CHMI (CHMI2), again by PfSPZ challenge.
To verify the inhibitory capacity of serum from PfSPZ vaccinated volunteers reported in Jongo et al (manuscript submitted) in our hands, we used a previously described flow cytometry-based inhibition assay of P. falciparum sporozoite invasion of HC04 cells (ISI assay) [16, 26]. There was variable preexisiting ISI activity in a subset of volunteers as measured using serum from baseline (Supplementary Figure 2). Thus, in order to measure vaccine-induced changes in serum reactivity we normalized each time point to the inhibitory activity of serum taken at baseline.
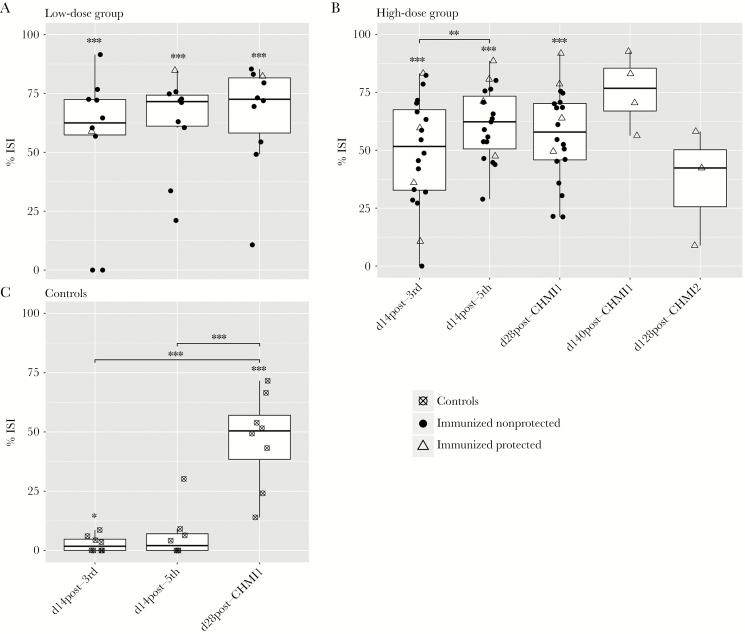
After the third immunization, immunized volunteers in the low-dose group showed an average inhibition of 53.75% ± 10.81% (Figure 1A) while immunized volunteers from the high-dose group showed inhibition by an average of 52.73% ± 5.14% (Figure 1B). Two volunteers from the low-dose group and 1 from the high-dose group showed no ISI activity at this time point. After the fifth immunization, all volunteers in both groups developed functional antibodies with an average inhibition of 62.93% ± 6.36% for the low-dose group and 61.47% ± 3.55% for the high-dose group. ISI activity was unchanged after CHMI1 and was sustained until day 140 after CHMI1 in protected volunteers with an average inhibition of 75.60% ± 7.89%. ISI results of serum samples collected from placebo controls remained undetectable during PfSPZ vaccinations but did increase significantly after CHMI1 (Figure 1C).
Figure 1.
Evaluation of serum from P. falciparum sporozoites (PfSPZ)-immunized malaria pre-exposed Tanzanian volunteers for functional inhibition of sporozoite invasion (ISI) in vitro. Percentage ISI of HC04 cells infected with P. falciparum sporozoites obtained from post-immunization sera from malaria pre-exposed volunteers immunized with (A) 5 × 1.35 × 105 PfSPZ Vaccine (n = 10) and (B) 5 × 2.7 × 105 PfSPZ Vaccine (n = 20). For the high-dose group, 2 subjects had 1 missing data point (at time point d 14 post-fifth and at d 28 post-CHMI2) and were therefore not considered for the statistical analysis. Each data point indicates a volunteer’s mean inhibition as normalized to volunteer-matched preimmune value across 2 independent experiments. The middle bar of each box plot represents the median and the whisker maximum length set to 1.5 IQR (interquartile range). Asterisks above box plots indicate a statistically significant difference of the group mean invasion compared to 0% inhibition of invasion, determined by 1-sample t test. Bars with asterisks show statistically significant changes of inhibition of invasion between visits determined by paired t test. Immunized protected individuals are shown as empty triangles, immunized nonprotected volunteers as solid circles, and control subjects as crossed circles. C, Percentage ISI from sera collected from control volunteers from low- and high-dose group (n = 8), who received normal saline instead of PfSPZ immunization at 14 days after the third and fifth immunization and the same controlled human malaria infection (CHMI) of 3200 nonirradiated PfSPZ of PfSPZ challenge 3 weeks after the final immunization.
Collectively, these results demonstrate that PfSPZ vaccination of malaria-preexposed Tanzanian volunteers induced antibodies that inhibit P. falciparum sporozoite invasion in vitro, in agreement with results of Jongo et al (submitted) using the same samples with an ISI method used across other PfSPZ clinical trials.
PfSPZ Vaccination Induces Anti-PfCSP IgM Antibodies
PfCSP is the major, immunodominant protein on the surface of P. falciparum sporozoites [29]. Thus, we assessed the IgG and IgM antibody titers specific for full-length recombinant PfCSP (amino acids 21–289) [16] using ELISA.
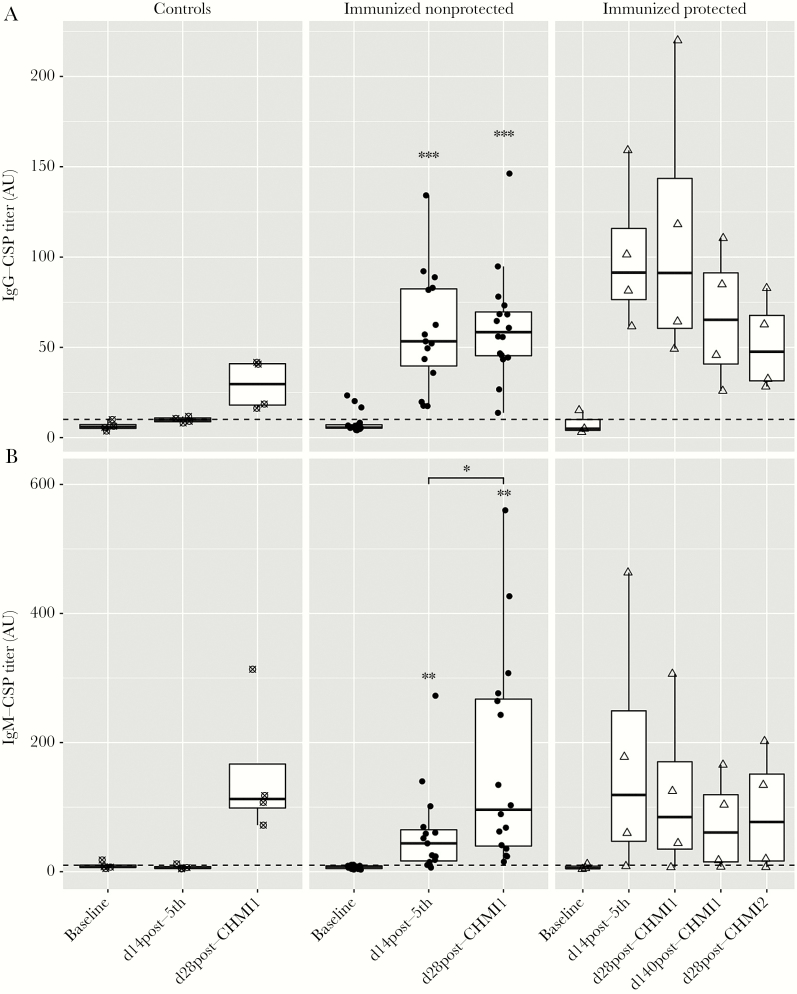
We first verified the presence of anti-PfCSP IgG, as reported in Jongo et al (submitted). We set the positivity cutoff for anti-PfCSP IgG antibody titers at 10.12 arbitrary units (AU) (Figure 2A) based on optimization with malaria-naive volunteers [16]. At baseline, 4 of 23 volunteers showed positive IgG titers (average of 18.89 ± 1.84 AU) with a total group average of 7.94 ± 1.15 AU (Figure 2A). Fourteen days after the fifth immunization, all vaccinees had developed significantly higher anti-PfCSP IgG antibodies with an average of 68.02 ± 8.63 AU (Figure 2A and Supplementary Figure 3). All placebo controls remained negative with an average of 9.89 ± 0.81 AU. Placebo controls and nonprotected vaccinees developed asexual blood stage parasitemia after CHM1, yet, interestingly, the anti-CSP IgG titers after CHMI1 were not boosted in immunized volunteers (average of 71.91 ± 10.23 AU) while the 4 placebo controls became positive after CHMI, albeit with low titers (average of 29.32 ± 6.87 AU) (Figure 2A). On follow-up visits at 140 days post-CHMI1 and on 28 days post-CHMI2, the protected volunteers remained positive for anti-PfCSP IgG.
Figure 2.
Induction of IgG (A) and IgM antibody (B) responses to full-length P. falciparum circumsporozoite protein (CSP) from malaria-preexposed volunteers immunized with 5 × 2.7 × 105P. falciparum sporozoites (PfSPZ) Vaccine, quantified by enzyme-linked immunosorbent assay (ELISA) at the time points: baseline, day 14 post-fifth immunization, day 28 post-controlled human malaria infection 1 (CHMI1), day 140 post-CHMI1, and day 28 post-CHMI2. Antibody titers are specified as arbitrary units (AU) with a positivity cutoff shown by a dashed line. Each data point represents the mean of the duplicate ELISA titers for 1 volunteer. Asterisks indicate statistically significant difference of the mean antibody titer compared to the mean titer measured at the pre-immunization time point as determined by paired t test. Bars with asterisks indicate statistically significant difference of the mean antibody titer between visits determined by paired t test. *P ≤ .05, **P ≤ .01, ***P ≤ .001. The small sample size of control and protected subjects did not allow for statistical testing when grouped by protection status. For whole-group analyses, see Supplementary Figure 3. Control volunteers are shown as crossed circles (n = 4) in the left panel, immunized nonprotected volunteers as solid circles (n = 16) in the middle panel, and immunized protected (n = 4) as empty triangles in the right panel. Serum samples of 2 volunteers were not available, one at baseline (protected subject) and one at visit of day 14 post-fifth (nonprotected subject). The 2 volunteers were not considered for statistical analysis.
Next, we wanted to determine if anti-PfCSP IgM responses were also generated after PfSPZ vaccination. The positivity cutoff for anti-PfCSP IgM antibody titers was set at 9.21 AU based on optimization with malaria-naive serum. At baseline, CSP-binding IgM antibodies (Figure 2B) were detected in 4 of 23 volunteers with an average of 12.78 ± 1.83 AU while the total group average was 7.39 ± 0.71 AU. Fourteen days after the fifth immunization, 18 of 23 volunteers were positive for anti-PfCSP IgM titers with an average of 89.61 ± 27.30 AU. Three of 4 placebo controls remained negative at this time point, as well as 1 vaccinee. Twenty-eight days post CHMI1, a significant increase in anti-PfCSP IgM titers was seen in all volunteers that experienced asexual blood stage infections, including nonprotected vacinees (average: 157.88 ± 34.71 AU) and placebo controls (average: 152.71 ± 54.50 AU) but no increase in anti-CSP IgM was seen in protected volunteers who remained free of parasitemia. In serum samples collected 140 days after CHMI1 and 28 days after CHMI2, these anti-PfCSP IgM responses persisted in 3 of the 4 volunteers sampled at this time point, which was 189 days after the final immunization (Supplementary Figure 1).
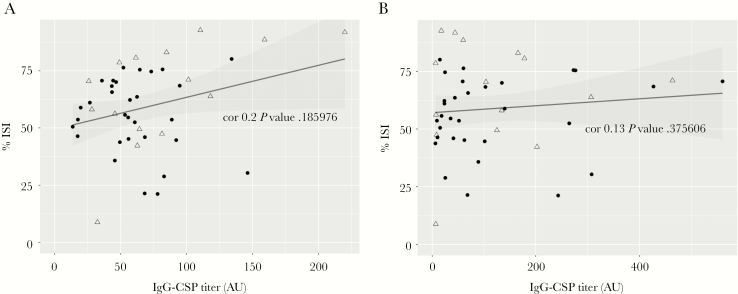
Overall, these results revealed that in addition to IgG, malaria pre-exposed Tanzanian adults developed anti-PfCSP IgM antibody titers that persisted for more than 6 months after PfSPZ immunization and were boosted following exposure to blood stage parasites. No significant correlation became apparent between invasion inhibition and either anti-PfCSP IgG (Figure 3A) or IgM titers (Figure 3B).
Figure 3.
No statistically significant correlation between anti-circumsporozoite protein (CSP) titers and percentage inhibition of sporozoite invasion (ISI) of HC04 cells assessed from malaria-preexposed people immunized with 5 × 2.7 × 105P. falciparum sporozoites (PfSPZ) Vaccine measured at time points of post-immunization and post-controlled human malaria infection. A, Correlation between percentage of ISI and anti-CSP IgG titers and (B) anti-CSP IgM titers determined by Spearman correlation. Immunized nonprotected volunteers are shown as solid circles (n = 31) and immunized protected as empty triangles (n = 15).
Antisporozoite IgM Antibodies Contribute to Inhibition of Sporozoite Invasion and Fix Complement
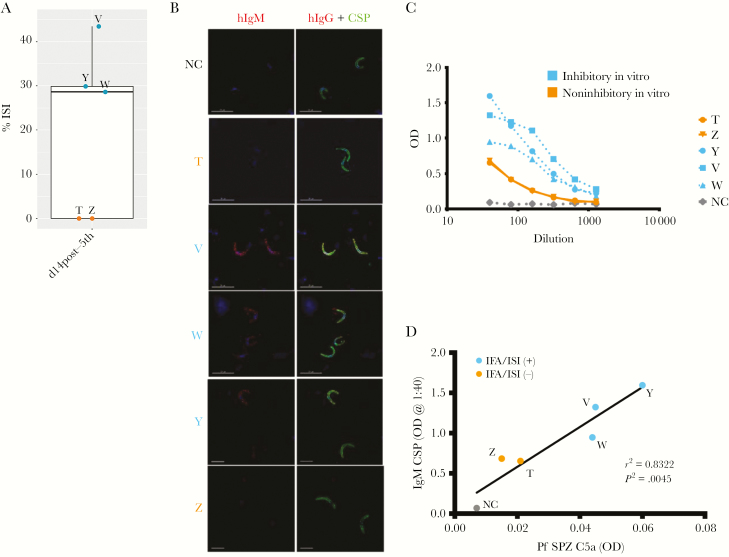
Next, we wanted to understand better the potential contribution of vaccine-induced anti-PfCSP IgM antibodies to in vitro sporozoite invasion inhibition. Here, we selected plasma samples collected from 5 volunteers in the high-dose group whose serum was inhibitory in vitro and had positive anti-PfCSP IgM titers and low anti-PfCSP IgG 14 days after the fifth immunization. Plasma samples were depleted of IgG and IgA antibodies and the IgM-enriched fractions were used to test for function in ISI assay, whole P. falciparum sporozoites in IFA, and binding against full-length PfCSP in ELISA (Figure 4A–C). The resulting fractions after IgG and IgA depletion contained IgM antibodies with a median of 96.6 ± 7.7% pure IgM as measured by total IgM and IgA ELISA (Supplementary Table 1). Given the wide variation in total IgM concentrations found in human serum [30], we chose to test the IgM fractions at a volume-based dilution of 1:20 calculated using the original starting volume of plasma, which was the same dilution used for serum in Figure 1. At a 1:20 dilution the IgM-enriched preparation inhibited P. falciparum sporozoite invasion in 3 out of 5 volunteers, ranging between 28% and 43% (Figure 4A). The 3 ISI-positive volunteers had the highest pre-enrichment serum anti-PfCSP IgM titers (average of 304 AU) while the 2 ISI-negative volunteers had an average of 104 AU. IgM-enriched preparations of these 3 positive volunteers also were positive in sporozoite IFA and had the strongest binding to PfCSP by ELISA (Figure 4B and C). IgM fractions from plasma samples of the 4 high-dose protected volunteers collected 140 days after the first CHMI were tested as well. One volunteer with high anti-PfCSP IgM titers at this time point showed strong binding to purified sporozoites by IFA and showed invasion inhibition at 31% (data not shown).
Figure 4.
Vaccine-induced anti-circumsporozoite protein (CSP) IgM antibodies bind to whole sporozoites and inhibit sporozoite invasion in vitro. Plasma from a subset of volunteers (n = 5) collected at 14 days post-fifth immunization was depleted of IgG and IgA antibodies to generate IgM antibody fractions. Each individual volunteer is indicated with a different letter (T, V, W, Y, Z). “NC” is “negative control,” which is the IgM fraction of the pooled preimmune plasma from respective volunteers. IgM fraction of volunteers that were inhibitory in inhibition of sporozoite invasion assays (ISI) are indicated in blue while those that were not are in orange. A, IgM fractions were tested in inhibition of sporozoite invasion (ISI) at approximately 1:20 dilution from original plasma. Percent ISI as normalized to preimmune pooled IgM fractions are displayed at the 14 days post-fifth immunization time point. Each data point and letter represents a volunteer’s mean value across 2 independent experiments. IgM fractions from volunteers W, Y, and V showed ISI activity between 28% and 43%. B, IgM fractions were applied to fixed, permeabilized whole sporozites and IgM binding detected in immunofluorescence assay (IFA) by staining with anti-human IgM AlexaFluor594 (red) and anti-CSP monoclonal antibody 2A10 conjugated to AlexaFluor488 (green). Each panel represents an individual volunteer indicated by different letters (n = 5), with each image captured under the same exposure conditions with identical modifications. C, IgM fractions were used in enzyme-linked immunosorbent assay (ELISA) with full-length PfCSP at indicated dilutions. Anti-human IgM secondary was used to detect anti-CSP IgM with indicated optical density (OD) at 405 nm. D, IgM fractions at a 1:10 dilution were incubated with whole, air-dried P. falciparum sporozoites (Pf SPZ) in the presence of human complement. Complement fixation was assessed by C5a release in the supernatant by ELISA as compared to wells containing no IgM. OD for each sample is plotted with a correlation to anti-CSP IgM titers for each volunteer with IgM fractions positive for IFA and ISI indicated by color. Correlation between anti-CSP IgM titer and C5a release was performed by Pearson correlation test with resulting r2 and P value shown.
As IgM antibodies are extremely efficient at fixing complement [31], we also wanted to determine if IgM induced after vaccination could fix complement after binding to P. falciparum sporozoites. Indeed, following binding to plate-bound whole P. falciparum sporozoites, we were able to detect complement fixation by C5a release in supernatants in a manner that correlated with both anti-PfCSP IgM titer and IFA/ISI positivity (Figure 4D).
In summary, PfSPZ Vaccine-induced anti-PfCSP IgM antibodies in malaria-preexposed volunteers, which persist up to 140 days after CHMI, are capable of binding P. falciparum sporozoites, inhibiting in vitro P. falciparum sporozoite invasion, and fixing complement after P. falciparum sporozoite binding.
DISCUSSION
PfCSP is the most immunodominant antigen expressed on the P. falciparum sporozoite surface [29] and previous studies of PfSPZ Vaccine, including those in Africa, have focused on IgG antibodies to PfCSP [8–12]. In addition to anti-PfCSP IgG responses, we assessed IgM antibodies following PfSPZ vaccination. All immunized volunteers mounted anti-PfCSP IgG antibodies, consistent with previous reports (Jongo et al, manuscript submitted), while 17 of 19 immunized volunteers made anti-PfCSP IgM antibodies (Figure 2A, B). As we saw ISI activity in serum samples from volunteers with high IgM and low IgG titers against PfCSP, we hypothesized that the IgM fraction could be contributing to antibody function in vitro. Indeed, we demonstrated that in 3 volunteers, these anti-PfCSP IgM antibodies mediated ISI activity in plasma fractions after depletion of IgG and IgA. To our knowledge, this is the first demonstration of P. falciparum sporozoite inhibition mediated by enriched IgM preparations. We did not find correlations between anti-PfCSP IgG or IgM titers and ISI activity of whole serum (Figure 3A, B). This is not surprising given that we show that PfSPZ vaccination elicits a complex antibody response composed of both IgG and IgM, and that immunization with irradiated sporozoites elicits responses to a number of antigens [32]. Thus, it is unlikely that a single component will correlate with antibody function in a cohort of vaccinees.
Vaccine-induced IgM antibodies have been regarded in the past as short-lived and less affinity-matured compared to IgG antibodies [33], and early studies of naturally acquired antibodies to PfCSP in Indonesia [34] and Kenya [35] showed that IgM responses were of lower prevalence and magnitude than IgG responses. Therefore, investigation of vaccine-induced humoral immunity has focused on IgG isotypes in malaria-exposed and malaria-naive populations [2, 8–12, 24, 36–38]. Indeed, PfCSP or P. falciparum sporozoite-binding IgG antibodies have correlated with protection in some studies of PfSPZ Vaccine [8, 9, 12] and to some extent in the PfCSP subunit vaccine RTS,S [38].
However, in one study using radiation attenuation sporozoites (RAS)-immunized mice, IgM-dominated antibodies were described as binding to live P. yoelii sporozoites and limiting parasite liver infection by 74% in passive transfer experiments [39]. IgM antibodies cross-reacting with glycoproteins on the P. yoelii sporozoite surface have also been shown to inhibit parasite infection of hepatocytes in vivo and in vitro [40]. In the latter example, these antibodies appeared to function largely through FC-dependent effectors and complement fixation with recruitment of polymorphonuclear cells [40]. Outside of malaria, plasma cells secreting IgM antibodies that are somatically hypermutated and that reside in the spleen have been detected during vaccination and in influenza virus and lymphocytic choriomeningitis virus infections [41].
Blood stage infection can result in IgM memory B cells that are long-lived and somatically hypermutated in humans, and the resulting IgM antibodies can contribute to protection from asexual blood stage infection in mice [25]. Additionally, blood stage infection can also alter germinal center formation and acquisition of antibodies to the pre-erythrocytic stages [21]. However, the development of functional anti-sporozoite IgM responses as a result of blood stage infection has not been investigated. Interestingly, in our study we observed a boost in anti-PfCSP IgM and not IgG after CHMI only in those volunteers that experienced blood stage infection (Figure 2). Thus, it is possible that in our study, prior exposure to blood stage malaria could explain the predisposition of these volunteers to developing anti-CSP IgM in this population as blood stage infection appears to skew the immune response to IgM antibodies. However, this will require similar studies in malaria-naive populations.
Importantly, our data suggest that P. falciparum sporozoite-binding and invasion-inhibitory IgM could contribute to protection against malaria infection. IgM antibodies are excellent in fixing complement, which could either eliminate circulating P. falciparum sporozoites by formation of the membrane attack complex leading to parasite lysis, or by formation of C5a, C3a, and other byproducts, which can subsequently recruit mononuclear cells and stimulate phagocytosis. While complement-mediated lysis has been demonstrated for asexual blood stage merozoites in rodents and using human serum [42, 43], definitive evidence of complement fixation at the sporozoite stage is sparse. Interestingly, we demonstrate here that vaccine-induced IgM antibodies were able to fix complement following binding to whole P. falciparum sporozoites (Figure 4D). To our knowledge, this is the first demonstration of complement fixation with P. falciparum sporozoites and the first to show this using antibodies from humans. Two reports have demonstrated apparent complement-dependent disruption of avian and rodent sporozoite morphology [44, 45], but this was not directly assessed for complement fixation by observing complement byproducts. However, one study in rodents did demonstrate complement C3b deposition on sporozoites after passive transfer of anti-alpha-gal antibodies and a dependence on complement for the protective efficacy of these antibodies [40]. Thus, it is possible that these IgM antibodies could be functioning in prevention of infection in humans as it is conceivable that sporozoites could encounter IgM and complement during their journey from the skin to the liver parenchyma. Directly assessing the role of IgM binding and complement fixation in the prevention of P. falciparum sporozoite infection in vivo will be challenging as laboratory mice have relatively low levels of complement activity [46], particularly in the classical pathway with human antibodies [47]. Nevertheless, determining whether this can be a relevant effector mechanism in protection against P. falciparum sporozoite infection will be critical for better understanding antibody-mediated protection after whole sporozoite immunization, especially in previously malaria-infected individuals. This can be accomplished by carefully designed murine in vivo studies as well as future in vitro studies to determine if the inclusion of complement reveals previously unobserved antisporozoite antibody activity such as has been demonstrated for blood stages [43].
CONCLUSIONS
Immunization of malaria-preexposed volunteers with PfSPZ Vaccine elicits functional, sporozoite invasion-inhibitory antibodies, including anti-PfCSP IgM antibodies capable of fixing complement in vitro. Immunological assessment of the mode of action of PfSPZ vaccine-induced humoral effector mechanisms should therefore include monitoring of parasite-specific IgG as well as IgM antibodies. Future studies need to expand on our findings and address if (1) parasite-inhibitory IgM production is related to PfSPZ vaccine administration route and dosing; (2) PfSPZ vaccine-induced IgM responses are unique to malaria-preexposed individuals; and (3) the sporozoite-binding IgM antibody repertoire undergoes affinity maturation resulting in improved effector function over repeated vaccinations.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We would like to extend our utmost appreciation to all those who made the BSPZV1 clinical trial possible, especially the volunteers.
Financial support. This work was supported by National Institutes of Health (grant number F32 AI 114113) and the Swiss Vaccine Research Institute, Switzerland. The development, manufacturing, and quality control release and stability studies of PfSPZ Vaccine and PfSPZ Challenge were supported in part by National Institute of Allergy and Infectious Diseases Small Business Innovation Research program (grant number 5R44AI055229).
Potential conflicts of interest. Sanaria Inc. manufactured PfSPZ Vaccine and PfSPZ Challenge. Thus, all authors associated with Sanaria have potential conflicts of interest. There are no other conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part. Gordon Research Conference: Malaria; Les Diablerets, Switzerland, 2017. Annual Meeting of the American Society for Tropical Medicine and Hygiene; Baltimore, Maryland, 2017.
References
- World Health Organization. World malaria report 2017. Geneva: World Health Organization,2017. [Google Scholar]
- 2. Olotu A, Fegan G, Wambua J et al. Seven-year efficacy of RTS,S/AS01 malaria vaccine among young African children. N Engl J Med 2016; 374:2519–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Agnandji ST, Fernandes JF, Bache EB, Ramharter M. Clinical development of RTS,S/AS malaria vaccine: a systematic review of clinical phase I-III trials. Future Microbiol 2015; 10:1553–78. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization. Malaria vaccine: WHO position paper-January 2016. Wkly Epidemiol Rec 2016; 91:33–51. [PubMed] [Google Scholar]
- 5. Clyde DF, McCarthy VC, Miller RM, Hornick RB. Specificity of protection of man immunized against sporozoite-induced falciparum malaria. Am J Med Sci 1973; 266:398–403. [DOI] [PubMed] [Google Scholar]
- 6. Hoffman SL, Goh LM, Luke TC et al. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis 2002; 185:1155–64. [DOI] [PubMed] [Google Scholar]
- 7. Rieckmann KH, Carson PE, Beaudoin RL, Cassells JS, Sell KW. Letter: Sporozoite induced immunity in man against an Ethiopian strain of Plasmodium falciparum. Trans R Soc Trop Med Hyg 1974; 68:258–9. [DOI] [PubMed] [Google Scholar]
- 8. Seder RA, Chang LJ, Enama ME et al. ; VRC 312 Study Team Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science 2013; 341:1359–65. [DOI] [PubMed] [Google Scholar]
- 9. Ishizuka AS, Lyke KE, DeZure A et al. Protection against malaria at 1 year and immune correlates following PfSPZ vaccination. Nat Med 2016; 22:614–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Epstein JE, Paolino KM, Richie TL et al. Protection against Plasmodium falciparum malaria by PfSPZ Vaccine. JCI Insight 2017; 2:e89154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lyke KE, Ishizuka AS, Berry AA et al. Attenuated PfSPZ Vaccine induces strain-transcending T cells and durable protection against heterologous controlled human malaria infection. Proc Natl Acad Sci U S A 2017; 114:2711–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sissoko MS, Healy SA, Katile A et al. Safety and efficacy of PfSPZ vaccine against Plasmodium falciparum via direct venous inoculation in healthy malaria-exposed adults in Mali: a randomised, double-blind phase 1 trial. Lancet Infect Dis 2017; 17:498–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roestenberg M, McCall M, Hopman J et al. Protection against a malaria challenge by sporozoite inoculation. N Engl J Med 2009; 361:468–77. [DOI] [PubMed] [Google Scholar]
- 14. Roestenberg M, Teirlinck AC, McCall MB et al. Long-term protection against malaria after experimental sporozoite inoculation: an open-label follow-up study. Lancet 2011; 377:1770–6. [DOI] [PubMed] [Google Scholar]
- 15. Mordmüller B, Surat G, Lagler H et al. Sterile protection against human malaria by chemoattenuated PfSPZ vaccine. Nature 2017; 542:445–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kublin JG, Mikolajczak SA, Sack BK et al. Complete attenuation of genetically engineered Plasmodium falciparum sporozoites in human subjects. Sci Transl Med 2017; 9: eaad9099. [DOI] [PubMed] [Google Scholar]
- 17. Ryg-Cornejo V, Ly A, Hansen DS. Immunological processes underlying the slow acquisition of humoral immunity to malaria. Parasitology 2016; 143:199–207. [DOI] [PubMed] [Google Scholar]
- 18. Portugal S, Pierce SK, Crompton PD. Young lives lost as B cells falter: what we are learning about antibody responses in malaria. J Immunol 2013; 190:3039–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Egan JE, Weber JL, Ballou WR et al. Efficacy of murine malaria sporozoite vaccines: implications for human vaccine development. Science 1987; 236:453–6. [DOI] [PubMed] [Google Scholar]
- 20. Doolan DL, Hoffman SL, Southwood S et al. Degenerate cytotoxic T cell epitopes from P. falciparum restricted by multiple HLA-A and HLA-B supertype alleles. Immunity 1997; 7:97–112. [DOI] [PubMed] [Google Scholar]
- 21. Keitany GJ, Kim KS, Krishnamurty AT et al. Blood stage malaria disrupts humoral immunity to the pre-erythrocytic stage circumsporozoite protein. Cell Rep 2016; 17:3193–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Richie TL, Billingsley PF, Sim BK et al. Progress with Plasmodium falciparum sporozoite (PfSPZ)-based malaria vaccines. Vaccine 2015; 33:7452–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Behet MC, Foquet L, van Gemert GJ et al. Sporozoite immunization of human volunteers under chemoprophylaxis induces functional antibodies against pre-erythrocytic stages of Plasmodium falciparum. Malar J 2014; 13:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sack BK, Mikolajczak SA, Fishbaugher M et al. Humoral protection against mosquito bite-transmitted Plasmodium falciparum infection in humanized mice. NPJ Vaccines 2017; 2:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Krishnamurty AT, Thouvenel CD, Portugal S et al. Somatically hypermutated plasmodium-specific IgM(+) memory B cells are rapid, plastic, early responders upon malaria rechallenge. Immunity 2016; 45:402–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kaushansky A, Rezakhani N, Mann H, Kappe SH. Development of a quantitative flow cytometry-based assay to assess infection by Plasmodium falciparum sporozoites. Mol Biochem Parasitol 2012; 183:100–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2011. http://www.R-project.org/. Accessed 15 February 2018. [Google Scholar]
- 28. Wickham H. ggplot2: Elegant graphics for data analysis. New York: Springer Verlag, 2009. [Google Scholar]
- 29. Plassmeyer ML, Reiter K, Shimp RL Jr et al. Structure of the Plasmodium falciparum circumsporozoite protein, a leading malaria vaccine candidate. J Biol Chem 2009; 284:26951–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gonzalez-Quintela A, Alende R, Gude F et al. Serum levels of immunoglobulins (IgG, IgA, IgM) in a general adult population and their relationship with alcohol consumption, smoking and common metabolic abnormalities. Clin Exp Immunol 2008; 151:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Garson JA, Quindlen EA, Kornblith PL. Complement fixation by IgM and IgG autoantibodies on cultured human glial cells. J Neurosurg 1981; 55:19–26. [DOI] [PubMed] [Google Scholar]
- 32. Trieu A, Kayala MA, Burk C et al. Sterile protective immunity to malaria is associated with a panel of novel P. falciparum antigens. Mol Cell Proteomics 2011; 10:M111.007948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Seifert M, Przekopowitz M, Taudien S et al. Functional capacities of human IgM memory B cells in early inflammatory responses and secondary germinal center reactions. Proc Natl Acad Sci U S A 2015; 112:E546–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hoffman SL, Wistar R Jr, Ballou WR et al. Immunity to malaria and naturally acquired antibodies to the circumsporozoite protein of Plasmodium falciparum. N Engl J Med 1986; 315:601–6. [DOI] [PubMed] [Google Scholar]
- 35. Hoffman SL, Oster CN, Plowe CV et al. Naturally acquired antibodies to sporozoites do not prevent malaria: vaccine development implications. Science 1987; 237:639–42. [DOI] [PubMed] [Google Scholar]
- 36. Epstein JE, Tewari K, Lyke KE et al. Live attenuated malaria vaccine designed to protect through hepatic CD8⁺ T cell immunity. Science 2011; 334:475–80. [DOI] [PubMed] [Google Scholar]
- 37. Chaudhury S, Ockenhouse CF, Regules JA et al. The biological function of antibodies induced by the RTS,S/AS01 malaria vaccine candidate is determined by their fine specificity. Malar J 2016; 15:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. White MT, Bejon P, Olotu A et al. The relationship between RTS,S vaccine-induced antibodies, CD4⁺ T cell responses and protection against Plasmodium falciparum infection. PLoS One 2013; 8:e61395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kumar KA, Sano G, Boscardin S et al. The circumsporozoite protein is an immunodominant protective antigen in irradiated sporozoites. Nature 2006; 444:937–40. [DOI] [PubMed] [Google Scholar]
- 40. Yilmaz B, Portugal S, Tran TM et al. Gut microbiota elicits a protective immune response against malaria transmission. Cell 2014; 159:1277–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bohannon C, Powers R, Satyabhama L et al. Long-lived antigen-induced IgM plasma cells demonstrate somatic mutations and contribute to long-term protection. Nat Commun 2016; 7:11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sack BK, Keitany GJ, Vaughan AM, Miller JL, Wang R, Kappe SH. Mechanisms of stage-transcending protection following immunization of mice with late liver stage-arresting genetically attenuated malaria parasites. PLoS Pathog 2015; 11:e1004855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Boyle MJ, Reiling L, Feng G et al. Human antibodies fix complement to inhibit Plasmodium falciparum invasion of erythrocytes and are associated with protection against malaria. Immunity 2015; 42:580–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Touray MG, Seeley DC Jr, Miller LH. Plasmodium gallinaceum: differential lysis of two developmental stages of malaria sporozoites by the alternative pathway of complement. Exp Parasitol 1994; 78:294–301. [DOI] [PubMed] [Google Scholar]
- 45. McCoy ME, Golden HE, Doll TA et al. Mechanisms of protective immune responses induced by the Plasmodium falciparum circumsporozoite protein-based, self-assembling protein nanoparticle vaccine. Malar J 2013; 12: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ong GL, Mattes MJ. Mouse strains with typical mammalian levels of complement activity. J Immunol Methods 1989; 125:147–58. [DOI] [PubMed] [Google Scholar]
- 47. Ratelade J, Verkman AS. Inhibitor(s) of the classical complement pathway in mouse serum limit the utility of mice as experimental models of neuromyelitis optica. Mol Immunol 2014; 62:104–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.