Abstract
Background
The World Health Organization (WHO) has developed a global health strategy to eliminate viral hepatitis. We project the treatment and prevention requirements to achieve the WHO HCV elimination target of reducing HCV incidence by 80% and HCV-related mortality by 65% by 2030 in Pakistan, which has the second largest HCV burden worldwide.
Methods
We developed an HCV transmission model for Pakistan, and calibrated it to epidemiological data from a national survey (2007), surveys among people who inject drugs (PWID), and blood donor data. Current treatment coverage data came from expert opinion and published reports. The model projected the HCV burden, including incidence, prevalence and deaths through 2030, and estimated the impact of varying prevention and direct-acting antiviral (DAA) treatment interventions necessary for achieving the WHO HCV elimination targets.
Results
With no further treatment (currently ∼150 000 treated annually) during 2016–30, chronic HCV prevalence will increase from 3.9% to 5.1%, estimated annual incident infections will increase from 700 000 to 1 100 000, and 1 400 000 HCV-associated deaths will occur. To reach the WHO HCV elimination targets by 2030, 880 000 annual DAA treatments are required if prevention is not scaled up and no treatment prioritization occurs. By targeting treatment toward persons with cirrhosis (80% treated annually) and PWIDs (double the treatment rate of non-PWIDs), the required annual treatment number decreases to 750 000. If prevention activities also halve transmission risk, this treatment number reduces to 525 000 annually.
Conclusions
Substantial HCV prevention and treatment interventions are required to reach the WHO HCV elimination targets in Pakistan, without which Pakistan’s HCV burden will increase markedly.
Keywords: Mathematical model, direct-acting antivirals, prevention, incidence, mortality, LMIC
Key Messages
Hepatitis C virus (HCV) is a global health problem, with 71 million people actively infected worldwide in 2015 and 80% of the global burden being concentrated in lower-middle income countries (LMIC).
Following the advent of new highly effective treatments for HCV, the World Health Organization (WHO) has developed a global health strategy to eliminate viral hepatitis, with aims to reduce HCV incidence by 80% and mortality by 65% by 2030.
Pakistan is an LMIC which harbours 10% of the global HCV burden (7.0 million chronic infections in 2013).
To achieve the WHO HCV elimination targets in Pakistan, model projections suggest substantial scale-up in treatment is required (up to 880 000 treatments per year), although this can be minimized (to 525 000 per year) through scaling up prevention interventions and targeting treatment to people with cirrhosis and people who inject drugs.
This modelling study provides the first full estimation of the treatment and prevention requirements for achieving the WHO HCV elimination targets.
Introduction
Hepatitis C virus (HCV) infection is a major global health problem, with an estimated 71 million chronic infections worldwide and approximately 400 000 annual HCV-related deaths in 2015.1,2 Globally, 80% of the HCV burden is concentrated in low and middle-income countries (LMICs).3 New direct-acting antiviral (DAA) HCV treatments are all oral, well tolerated and achieve cure rates of over 90%.4 The advent of these new treatments has played an important role in the World Health Organization (WHO) recently adopting a global health sector strategy for eliminating viral hepatitis. This strategy commits countries to aggressive targets for eliminating viral hepatitis as a public health threat. For HCV, the targets include an 80% reduction in incident infections and 65% reduction in HCV-associated mortality by 2030.5
Pakistan has the second largest HCV burden in the world,1 with transmission being driven by multiple risk factors including community (barbering, ear/nose piercing) and health care practices (blood transfusion, medical injections) and injecting drug use.6–8 A national survey from 2007 reported that 4.8% of the population, or nearly 8 million individuals, had been exposed to HCV at that time.7 More recent estimates suggest that 7.0 million persons were chronically infected in 2013,1 meaning that Pakistan harbours one-tenth of the global burden of HCV. This emphasizes the crucial importance of tackling the HCV epidemic in Pakistan, for any global effort to eliminate HCV. Since 2005, the Pakistan government has launched national and provincial hepatitis prevention and control programmes, including screening and treatment for HCV-infected individuals.7,9 Further local initiatives have focused on educational interventions for HCV prevention.10 Data are lacking on the effectiveness of these interventions.
To achieve the WHO HCV elimination targets, it is critical to gain an understanding of what is required to control the generalized HCV epidemics in LMICs, something no study has fully undertaken so far. Most previous models considering generalized HCV epidemics in LMICs11–15 have solely evaluated the required level of HCV treatment for achieving the WHO targets for reducing mortality. These models do not incorporate the infectious disease aspect of HCV, and so were unable to project what is needed to reduce HCV incidence. Only two models, both for Egypt,16,17 have evaluated what treatment is needed to reduce incidence, but neither considered impact on mortality. The omissions of these models are important weaknesses, because different treatment strategies and targeting of patient groups will be needed to reduce mortality or reduce incidence. It is therefore critical to consider both outcomes concurrently, and to evaluate how treatment levels can be optimized between targeting people with heightened transmission risk or greater risk of experiencing disease morbidity, which will be especially important in resource-limited settings.
In this study, we develop a dynamic HCV transmission model for the ongoing HCV epidemic in Pakistan, to determine the required level of HCV treatment needed for achieving both WHO elimination targets for incidence and mortality. Importantly, we consider how the required level of treatment can be minimized through targeting treatment to those with HCV-related disease or heightened transmission risk, and by scaling up prevention interventions.
Methods
Model formulation
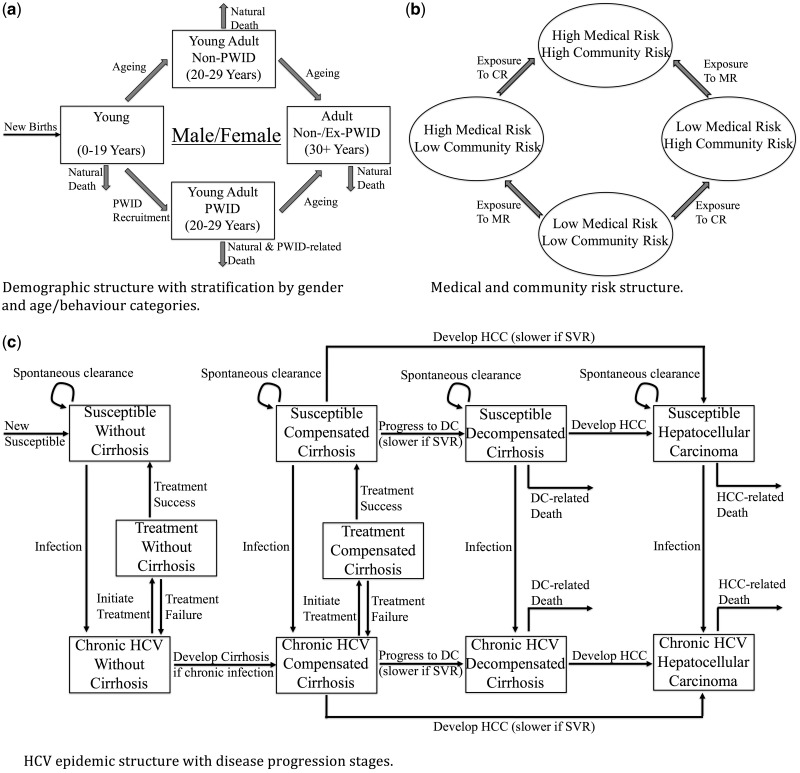
We developed a deterministic HCV transmission model (schematic in Figure 1). The model incorporates population demographics, population growth, HCV transmission in the general population and among people who inject drugs (PWID), natural history of HCV infection, and behavioural risk factors thought to drive HCV transmission. We incorporated three age classes: Young (0–19 years), Young Adult (20–29 years) and Adult (30+ years), with additional stratification among Young Adults to incorporate injecting drug use. These subgroups were divided into four risk strata of low and/or high medical and/or community risk. Individuals can either be susceptible to HCV infection or infected, with the model incorporating HCV disease progression. The Supplementary Materials include a detailed model description (available as Supplementary data at IJE online).
Figure 1.
A schematic illustration showing the structure of the full mathematical model, which incorporates (a) demographic characteristics of the population, including stratification by gender and age, (b) medical and community risk factors that contribute to HCV transmission, and (c) the infection dynamics of the HCV epidemic with disease progression stages. High medical risk is defined as having either over 5 therapeutic injections in the last year, history of blood transfusions, surgery, or haemodialysis, whereas high community risk is defined as ever barbering (males), ear/nose piercings (females), tattoo/acupuncture, or sharing smoking equipment. HCV: hepatitis C virus; PWID: people who inject drugs; DC: decompensated cirrhosis; HCC: hepatocellular carcinoma; CR: community risks; MR: medical risks; SVR: sustained virologic response.
Individuals enter the model in the Young category at a birth rate incorporating population growth, and are initially low risk. Individuals transition through the three age-categories, with a small proportion of Young Adults becoming PWID. Individuals also progress through the different medical and community risk strata that are associated with elevated transmission risk. Individuals experience age-specific mortality, with PWID experiencing additional drug-related mortality. Susceptible individuals become HCV-infected at a per capita rate dependent on gender, age, level of medical and/or community risk and prevalence of infection. PWID have additional infection risk. Some newly infected individuals spontaneously clear infection, whereas the remainder become chronically infected. If untreated, chronic HCV infection persists and gradually progresses to cirrhosis and then end-stage liver disease (ESLD), namely decompensation and hepatocellular carcinoma (HCC), which are associated with heightened mortality.18
Individuals are HCV-treated at a time-varying rate, whereupon they either achieve a sustained virological response (SVR: effective cure), or fail treatment and return to being chronically infected. Following successful treatment, individuals become susceptible to reinfection.18 We assume SVR halts further disease progression for pre-cirrhotic individuals, and slows progression for those with cirrhosis.19
Model parameterisation and calibration
The model was parameterized using demographic, behavioural and HCV epidemiological data from Pakistan and international databases (see Supplementary Materials and Supplementary Tables S2 and S3 for details, available as Supplementary data at IJE online). The model was fit to demographic data for Pakistan from 2015 (population of 189–99 million), and changes in population size and growth rates.20 Age-specific mortality rates were calibrated to give the population age distribution in 2015. The model was calibrated to the estimated proportion of individuals that are PWID (0.24%) in 2013,21 and their estimated HCV chronic prevalence (62.2%).22,23 The overall and age-specific HCV sero-prevalence (4.8%) came from the 2007 national survey,7 adjusted for chronic prevalence. An increasing epidemic was assumed, based on Pakistan blood donor data suggesting HCV sero-prevalence increased by 0.4–2.4% over 1994–2014 (Supplementary Materials). Age- and gender-specific HCV transmission rates were calibrated to fit the model to these data.
Analyses of the 2007 national survey were used to estimate the population proportion and estimated HCV prevalence for the low and/or high medical and community risk categories (defined in Supplementary Materials),24 which were used to estimate the recruitment rate and relative risk of HCV transmission for these categories. HCV disease progression and mortality rates were obtained from the literature, and adjusted for the high proportion of genotype 3 in Pakistan.1,25 The public sector has undertaken treatment with interferon (IFN) and ribavirin (RBV) since 2005,7 with 23 000 treatments between 2005 and 2010, and 45 000–60 000 annual treatments thereafter (unpublished data from the National and State Level Pakistan Hepatitis Control Programs). Equivalent treatment data do not exist for the private sector. However, health care system profile and usage data suggest that for 1994–2014, one-fifth to one-third of health care provision was from the public sector, with no discernable change over time.26–29 To produce a conservative estimate of the total number of historical treatments provided nationally, we assumed a public/private sector split of 40%/60% for HCV treatment between 2005 and 2015, and so estimated the overall treatment rate by scaling the public-sector treatment rate 2.5-fold (Supplementary Table S4, available as Supplementary data at IJE online). For 2015, this estimated 150 000 treatments or a ∼2% treatment rate among infected individuals. The model was calibrated to these treatment rates, evenly distributed across all infected individuals because no disease staging was undertaken. The treatment efficacy for IFN+RBV was estimated (SVR 50–81%) from Pakistan studies.30,31 From 2016, we assumed treatment using new DAAs, with an SVR of 80–95%.4 We assume no treatment for individuals with ESLD.
To account for parameter uncertainty, we calibrated the model within a probabilistic uncertainty analysis framework. Uncertainty was incorporated in most model parameters and calibration data, with each being sampled 1000 times from the distributions in Supplementary Tables S2 and S3 (available as Supplementary data at IJE online). For each sampled parameter set, other model parameters (as discussed above) were varied to fit the model to the calibration data using a non-linear least squares algorithm (Matlab). Overall, 328 model runs fit the data and were used for subsequent analyses.
Model analyses
The calibrated model projected the evolution of the epidemic until 2030, with no further treatment from 2016. We determined the proportion of new HCV infections (2016–30) that could be prevented if the elevated risk among PWID or individuals with high medical and community risk were removed. In comparison with this baseline, we projected the impact of a range of HCV prevention and treatment interventions (described in first column of Table 1) on the epidemic dynamics and burden of HCV-related morbidity over 15 years (2016–30 inclusive). These scenarios assumed no targeting of treatment.
Table 1.
Impact of intervention scenarios over 15-year period from 2016 to 2030 inclusive, compared with baseline scenario of no further treatment from 2016 (also shown)
| Comparator scenario | Number of new HCV infections 2016–30 | Chronic HCV prevalence in 2016 | Number of new HCV-disease 2016–30b | Number of new HCV-related deaths 2016–30 |
|---|---|---|---|---|
| Baseline scenario with no further treatment from 2016 | 13.4 [11.9 to 15.0] million | 3.9% [3.7–4.1%] | 5.4 [4.6 to 6.6] million | 1.4 [1.0 to 2.0] million |
| Intervention scenario | % of new HCV infections prevented 2016–30 | % change in HCV chronic prevalence 2016–30 | % reduction in HCV- disease 2016–30b | % reduction in HCV- related deaths 2016–30 |
| Reducing PWID-related and high medical and community HCV transmission risks by 50% | 21.5 [13.7 to 32.3] | +11.4 [+1.8 to +20.6] | 7.6 [4.6 to 11.5] | 2.4 [1.3 to 4.1] |
| Reducing all HCV transmission risks by 30%a | 38.1 [36.9 to 39.2] | −3.0 [−8.0 to +2.1] | 13.8 [13.1 to 14.4] | 4.4 [3.4 to 6.2] |
| Reducing all HCV transmission risks by 50%a | 59.5 [58.2 to 60.7] | −21.7 [−26.0 to −17.1] | 21.9 [21.0 to 22.9] | 7.1 [5.5 to 10.0] |
| Continuing current treatment rate (2% of infected individuals treated annually) with new DAA treatments from 2016 | 10.4 [9.3 to 11.7] | +2.3 [−3.7 to +8.4] | 12.3 [11.2 to 13.4] | 7.2 [6.3 to 8.3] |
| Scaling up DAA treatment rates from 2016 to 5% of infected individuals treated annually | 23.4 [21.0 to 26.1] | −28.7 [−34.7 to −23.1] | 27.2 [25.0 to 29.3] | 16.3 [14.2 to 18.6] |
| Combined reduction in all transmission risksa (50%) and scaling up DAA treatment rates to 5% of infected individuals treated annually from 2016 | 69.1 [67.3 to 70.7] | −58.8 [−62.4 to −55.0] | 42.1 [40.2 to 44.2] | 21.4 [18.5 to 25.5] |
HCV transmission risk associated with injecting drug use, as well as low and high community and medical risks, is reduced by 30% or 50%.
HCV disease relates to cases of compensated and decompensated cirrhosis and hepatocellular carcinoma.
We then considered what levels of treatment would be needed to reduce the Pakistan HCV epidemic to low levels as advocated by WHO: an 80% reduction in incidence and 65% reduction in HCV-related mortality by 2030, both compared with 2015 levels.5 Different intervention strategies were modelled: (A) No additional prevention, with treatment given equally to all individuals; (B) No additional prevention, with up to 80% of cirrhosis cases treated annually, and the rest treated equally; (C) No additional prevention, with up to 80% of cirrhosis cases treated annually, priority targeting of treatment to PWID (at twice the rate compared with non-cirrhotic non-PWID), and the rest treated equally; (D–F) Treatment scenario (C) with either injecting drug use-related transmission risk, high medical and community risk, or all transmission risks (among PWID and those with low and high medical and community risk) halved.
Uncertainty analysis
We undertook a linear regression analysis of covariance to determine which parameter and calibration data uncertainties contributed most to the variability in the 15-year impact on prevalence, incidence and mortality of a 50% reduction in transmission risk across all groups, and 5% treatment rate per year. The proportion of each model outcome’s sum-of-squares contributed by each parameter was calculated to estimate their importance to the overall uncertainty.
Results
Epidemic projections with no further treatment
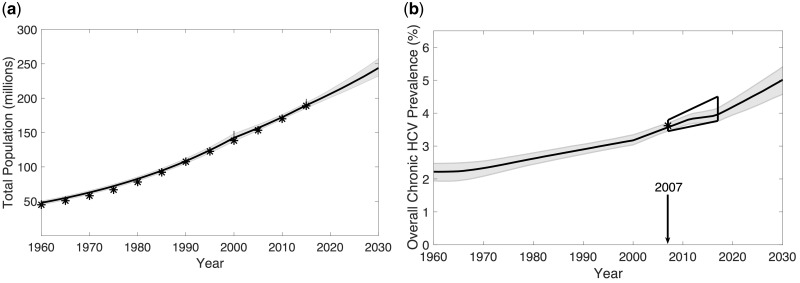
Between 2016 and 2030, the population of Pakistan is projected to increase by a third to approximately 250 million, and chronic HCV prevalence will increase from 3.9% (95% credibility interval 3.7–4.1%) to 5.1% (4.6–5.5%) (Figure 2a, b; Supplementary Table S5, available as Supplementary data at IJE online). Due to these changes, the number of prevalent chronic infections will rise from 7.5 (7.2–7.9) to 12.6 (11.8–13.6) million, and annual incident infections will rise from 700 000 (620 000–780 000) to 1.1 (1.0–1.3) million (from 3.7 to 4.8 per 1000 person-years). Large increases in HCV-related disease will also occur (Figure 3c, d), with 1.4 (1.0–2.0) million HCV-related deaths among those aged over 20 years during this period.
Figure 2.
Model projections for (a) total population size, (b) chronic HCV prevalence in Pakistan from 1960 to 2030. The solid black line and shaded grey areas show the median and 95% credible intervals (95% CrI) for the model projections. For comparison, asterisks indicate available demographic or HCV prevalence data and the wedge-shaped region in Figure 2b indicates the permitted trend in HCV prevalence. Population data for Figure 2a come from the UN Department of Economic and Social Affairs, Population Division, whereas HCV prevalence data for Figure 2b come from the 2007 national survey while reflecting the increasing HCV prevalence trend observed in studies from blood donors for 1994 to 2014.
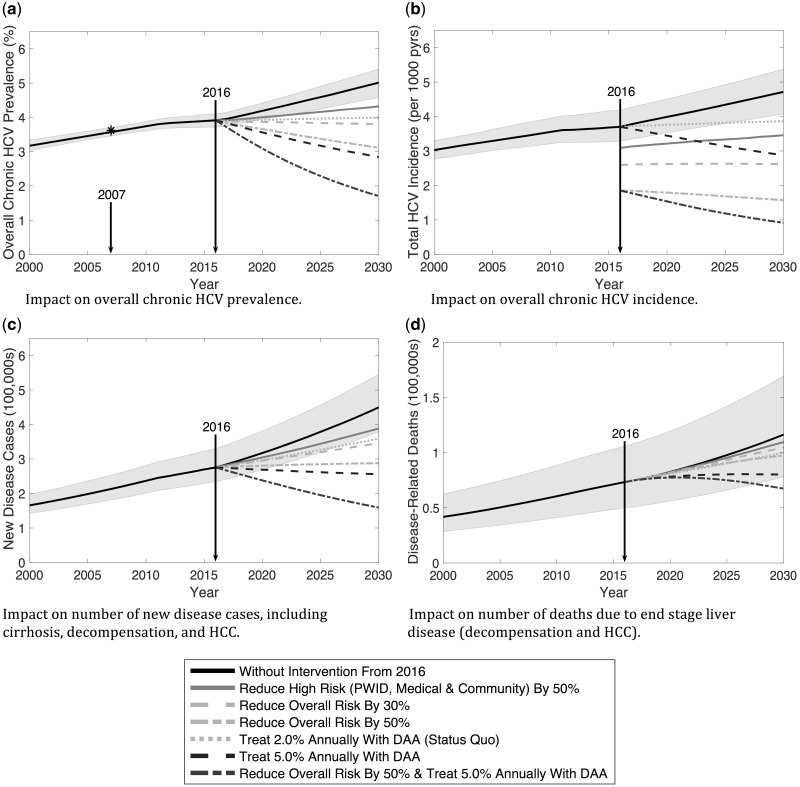
Figure 3.
Projections of the 15-year impact from 2016-2030 of interventions reducing either high or all transmission risks and/or treating a percentage of chronically infected individuals annually. Intervention scenarios are described in Table 1. The solid black line and shaded grey areas show the median and 95% credible intervals for the epidemic projections at baseline without treatment interventions from 2016. Median curves for the various interventions are as indicated.
Heterogeneities in transmission risk
Compared with the overall HCV incidence [3.7 (3.3–4.2) per 1000 person-years (pyrs) in 2016], and HCV incidence among individuals with low community and medical risk [2.8 (2.4–3.2) per 1000 pyrs], PWID have a much higher incidence [366.9 (277.8–490.5) per 1000 pyrs] which, if this risk is removed, would avert 13.9% (9.8–18.2%) of new infections over 2016–30 (Supplementary Table S5, available as Supplementary data at IJE online). Conversely, high community risk was associated with a slightly increased HCV incidence[4.3 (3.7–5.0) per 1000 pyrs by itself and 5.0 (4.1–5.9) per 1000pyrs when combined with high medical risk], whereas high medical risk by itself was not strongly associated [3.3 (2.8–3.7) per 1000 pyrs]. If both high community and medical risks were removed over 2016–30, 30.8% (19.5–46.5%) of new HCV infections would be prevented.
Potential impact of intervention scenarios
Figure 3 and Table 1 show the potential impact of various intervention scenarios, demonstrating that the HCV epidemic in Pakistan can only be attenuated if transmission risk is reduced or improvements in treatment occur. For instance, a 30% decrease in all transmission risks (PWID and low/high community and medical risks) could stabilize chronic prevalence and would avert 38.1% of new infections over 2016–30, but few HCV-related deaths would be prevented (< 5%). Similarly, continuing current treatment rates (2.0% of infections or ∼150 000 treated annually), while also switching to DAAs, could also stabilize prevalence and would reduce HCV-related mortality by 7.2% over 2016–30, but fewer new infections would be prevented (10.4%). Further impact is achieved through combining interventions, but none of these initial scenarios thus considered achieve the WHO elimination targets.
Requirements for reaching WHO HCV elimination targets
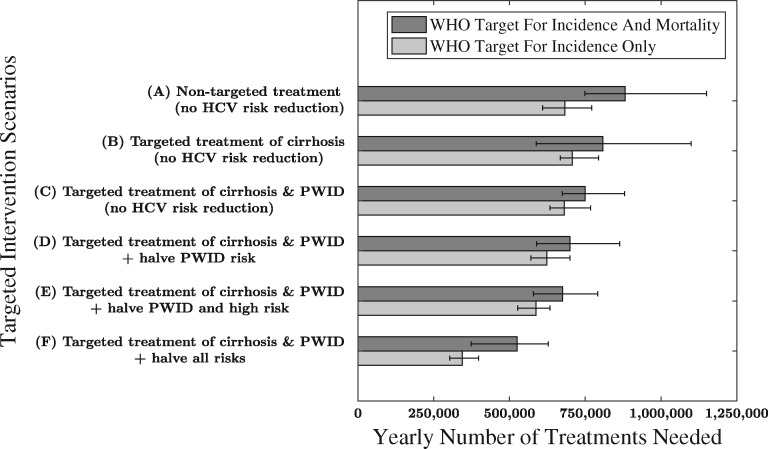
To reach the WHO HCV elimination targets by 2030, the model suggests that substantial treatment scale-up is required (Figure 4). With no targeting of treatment, and no scale-up in prevention, 880 000 (750 000–1 100 000) annual treatments are needed over 2016–30. However, targeting treatment to people with cirrhosis with up to 80% annual coverage results in fewer treatments, requiring 810 000 (590 000–1 100 000) annually to reach the same targets, whereas also targeting PWID (at twice the treatment rate of non-cirrhotic non-PWID) requires 750 000 (670 000–880 000) annual treatments. These annual treatment targets are reduced further if either the HCV transmission risk due to injecting drug use is halved (reduces by 50 000), all high-risk factors are halved (by 100 000) or all transmission risks are halved (by 200 000). For all intervention scenarios, fewer treatments are needed to reach the WHO incidence target compared with the combined mortality and incidence targets (Figure 4).
Figure 4.
Estimated number of annual treatments required to achieve the WHO HCV-elimination target for reducing HCV incidence by 80% and HCV-related mortality by 65% by 2030, for different treatment targeting and prevention interventions. The intervention scenarios consider three treatment intervention scenarios without (Intervention Scenarios A to C) or with HCV risk reduction interventions (Intervention Scenarios D to F). The treatment intervention scenarios considered were: (A) Non-targeted treatment; (B) Targeted treatment towards 80% of chronically infected people with cirrhosis each year; (C to F) Targeted treatment towards 80% of cirrhosis cases and treating PWID at twice the rate of non-PWID. Three HCV risk reduction interventions were considered: (D) Halve HCV transmission risk due to injecting drug use; (E) Halve HCV transmission risk due to injecting drug use and high medical and community risk factors and, lastly, (F) Halve transmission risk amongst PWID as well as amongst those with low and high community and medical risk. Whiskers denote the 95% credibility intervals around all projections.
Uncertainty analysis
For halving all transmission risks and treating 5% of chronic infections annually, analyses of covariance (Supplementary Figure S5, available as Supplementary data at IJE online) indicate that much of the variability in the impact on incidence is due to uncertainty in the chronic HCV prevalence amongst PWID (46.8% of variability), whereas most variability in the impact on mortality is due to uncertainty in the HCC progression rate (65.6%). Conversely, for the impact on prevalence, most variability is due to uncertainty in the DAA SVR rate (47.2%), which also affects incidence (17.5%) and mortality (14.6%). Lastly, the population growth rate affects the impact on prevalence (29.5%) and incidence (17.1%), but not mortality.
Discussion
Best estimates indicate that Pakistan currently harbours one-tenth of the global HCV burden. Our analyses suggest that without further treatment interventions, this HCV burden will increase further by 2030, with the number of prevalent and incident infections increasing by two-thirds to 1.1 million new infections annually and 12.6 million prevalent chronic infections, and close to 1.5 million individuals dying from ESLD. To reverse this increasing burden, and so reach the WHO elimination targets for HCV by 2030, a substantial scale-up in treatment is required, with at least 525 000 treatments being needed per year if treatment is targeted towards PWID and people with cirrhosis, and prevention activities also halve current transmission risk. However, if prevention activities have little impact, then 750 000 individuals will need treatment annually, or 880 000 if treatment is also not targeted to priority groups. These results highlight the urgent need for operationalizing country-wide prevention and treatment interventions to control the HCV epidemic in Pakistan, and the importance of strategies for prioritizing treatment to optimize the impact achieved. The only country currently undertaking a comparable number of treatments is Egypt, where 500 000 patients were treated between January and September 2016.32 For Pakistan to initiate a similar undertaking, a major commitment will be needed from Pakistan federal and provincial governments.
Many nations worldwide are developing elimination strategies, with 79 countries in the process of or having developed national action plans by early 2017.2,33,34 In April 2015, Georgia began a countrywide programme to eliminate HCV by 2020, with nearly 8500 individuals initiating treatment by April 2016.35 Similarly, other LMICs are working towards achieving HCV elimination within the next decade.32,34
In Pakistan, first-line treatment for chronic HCV infection is changing to the new DAAs. Extensive negotiations with pharmaceutical companies, along with competition from generic DAAs, have resulted in substantial price reductions for generic sofosbuvir, recently reaching US$15 for a 28-day supply (one treatment unit) in the public sector.32 This has set the stage for a scale-up in treatment by the public sector. Although drug costs are higher in the private sector (US$300 for a 28-day supply of sofosbuvir), rapid increases in sales suggest treatment is scaling up in the private sector, with ∼1.1 million treatment units procured between January 2016 and August 2017; data were accessed from IQVIA, formerly Intercontinental Marketing Statistics Health and Quintiles [https://www.iqvia.com]. However, the quality of treatment, including adherence and retention to therapy, remains an issue in Pakistan;30 this could become more acute as generic DAAs become readily available across Pakistan.
A major obstacle confronting all HCV elimination programmes is the identification of infected individuals, which will entail considerable screening. The implementation of nationwide screening programmes will be essential, as few (< 5%) HCV-infected individuals in LMICs know their status.32 In Pakistan, this will require screening of the general population, due to the diffuse nature of the country’s HCV epidemic; this could be optimized through targeting subpopulations with higher prevalence and improving the linkage to treatment following diagnosis. Although beneficial for reducing HCV-related mortality, prioritization of HCV treatment for cirrhosis cases could present further challenges, requiring effective follow-up of diagnosed pre-cirrhotic individuals who may have to wait for treatment. This will require improvements in the Pakistan health care infrastructure to enable them to achieve the WHO HCV elimination targets.
Strengths and limitations
The main strength of our analysis is that we present the first full dynamic transmission and disease progression model of an LMIC HCV epidemic calibrated to detailed context-specific data. However, limitations still exist. First, the national HCV survey7 used to parameterize the model only asked about ever exposure for most HCV risk factors, and used HCV antibody status to measure HCV exposure, which may have occurred long before the survey. Reassuringly, similar risk factors were reported among younger and older individuals, suggesting the same risk factors may be driving HCV transmission at the time of the survey as in the past. Other data limitations include insufficient Pakistan-specific HCV-related progression rates, which may differ due to variations in such things as alcohol consumption or obesity.18
Second, although this analysis used a relatively complex model, it still did not incorporate complexities such as geographical heterogeneity in the Pakistan HCV epidemic or intra-familial transmission.24 Despite this, our model is still useful for projecting the impact of different national treatment and prevention strategies.
Third, our projections considered the impact of reducing specific aspects of transmission risk without defining how this could be achieved. Unfortunately, other than for PWID-targeted HCV prevention interventions,36,37 little evidence exists on the effectiveness of HCV prevention interventions, except for blood safety. Despite this limitation, our analyses do reveal potential targets for prevention interventions, including everyday practices such as barbering, and health care-related risks due to unsafe medical injections. It is now important to design, undertake, and evaluate potential interventions to target these risk modalities.
Fourth, we did not have data on HCV treatment numbers for the private sector, and so derived estimates before 2017 based on assuming a stable split between public/private health care provision in Pakistan.26–29 Although there is uncertainty in these estimates, the small impact achieved by existing treatment rates suggests it is unlikely to affect our model projections. Going forward, our model considered the overall treatment rate needed for achieving elimination, but did not consider how this will be achieved. Despite evidence suggesting recent treatment scale-up in the private sector (see earlier in Discussion), it is possible that, over time, the public sector will provide a larger share as treatment is expanded to those with little access to health care. From a policy perspective, it will be important to determine the contribution of different health care providers for expanding treatment.
Comparison with other studies
Our study is unique in providing the first full estimation of the treatment requirements for achieving the WHO HCV elimination targets for both incidence and mortality in a generalized epidemic setting. Other HCV modelling studies have considered specific high-risk groups,38–40 or the evolving burden of HCV for specific countries without considering the transmission dynamics of HCV.12,14,15 These latter studies cannot estimate the impact of prevention and treatment interventions on HCV incidence, and so could not assess what was needed for reaching the WHO incidence target. Additionally, the only previous model for Pakistan dramatically underestimated the yearly incidence of new HCV infections (230 000 instead of 500 000 in 2014) due to it not incorporating the expanding nature of the Pakistan population and HCV epidemic.15 Conversely, only two studies, both considering Egypt, have modelled the transmission dynamics of HCV in a generalized HCV epidemic setting,16,17 evaluating the treatment requirements for reducing incidence to low levels. Our study adds to these analyses by being the first to estimate the requirements for achieving both incidence and mortality elimination targets, and by evaluating the additional benefits of targeting treatment and scaling up prevention interventions – something that is crucial for reducing the large treatment burden. We also consider an expanding epidemic setting, contrasting with Egypt, with our results suggesting more treatments will be needed in Pakistan than in Egypt to achieve the WHO incidence target.41
Conclusion and implications
This study directly addresses the feasibility of eliminating hepatitis at a national level, a pre-requisite to achieving global elimination as set out in the World Health Assembly resolution. It highlights the considerable effort needed to reach the WHO HCV elimination targets in high-burden settings such as Pakistan. Any global effort to eliminate HCV will have to give special attention to tackling the HCV epidemics in such settings, due to their large contribution to the global epidemic. To succeed in reducing both mortality and incidence, and to minimize the required treatment capacity, these efforts should target testing and treatment interventions to those with disease and those with transmission potential. This should also involve the scale-up of prevention interventions which can dramatically decrease the treatment requirements for reducing incidence.
Supplementary Data
Supplementary data are available at IJE online.
Funding
This work was supported by the U.S. Centers for Disease Control and Prevention (CDC) and UNITAID. Additionally, we would like to acknowledge the National Institute of Health Research (NIHR) Health Protection Research Unit (HPRU) in Evaluation of Interventions. N.K.M., H.F., P.V. and M.H. were additionally supported by the National Institute for Drug Abuse (grant number R01 DA037773-01A1), and N.K.M. was partially funded by the University of California San Diego Center for AIDS Research (CFAR), a National Institute of Health (NIH)-funded programme (grant number P30 AI036214). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Supplementary Material
Acknowledgements
The authors would like to thank two anonymous reviewers, whose constructive feedback improved the clarity and exposition of the manuscript. All modelling has been done in collaboration with the Pakistan HCV Task Force and the US Centers for Disease Control, Division of Viral Hepatitis (CDC DVH).
Author Contributions
P.V., F.A. and N.G. initiated the study with the Pakistan HCV Technical Advisory Group (H.Q., H.M., S.H. and Q.S.). P.V. provided overall leadership for the study design, analysis and interpretation of the findings. F.A., N.G. and the Pakistan TAG guided the analysis plan developed by P.V. and A.G.L. A.G.L. developed the final model, with preliminary models developed by H.F. and C.M. A.G.L. performed all model analyses. A.T., M.M. and C.F.D. undertook analyses of the National Survey dataset and blood donor data for parameterizing the model. H.Q., H.M. and S.H. provided data for the model. A.G.L. wrote the first draft of the manuscript with P.V. All authors have contributed to the overall collaboration through guiding the analysis plan, interpreting the results and writing subsequent versions of the manuscript.
Conflict of interest: N.K.M. and P.V. have received unrestricted research grants from Gilead unrelated to this work, and N.K.M. has received honoraria from Merck, AbbVie and Janssen. M.H. has received honoraria unrelated to this work from Merck, AbbVie, Janssen, MSD and Gilead. H.F. has received an honorarium from MSD unrelated to this work.
References
- 1. Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H.. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol 2014;61(Suppl 1):S45–57. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization. Global Hepatitis Report, 2017. Geneva: WHO, 2017. [Google Scholar]
- 3. Graham CS, Swan T.. A path to eradication of hepatitis C in low- and middle-income countries. Antiviral Res 2015;119:89–96. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization. Guidelines for the Screening Care and Treatment of Persons with Chronic Hepatitis C Infection: Updated Version. Geneva: World Health Organization, 2016. [PubMed] [Google Scholar]
- 5. World Health Organization. Global Health Sector Strategy on Viral Hepatitis 2016-2021. Towards Ending Viral Hepatitis. Geneva: WHO, 2016. [Google Scholar]
- 6. Janjua NZ, Hamza HB, Islam M. et al. Health care risk factors among women and personal behaviours among men explain the high prevalence of hepatitis C virus infection in Karachi, Pakistan. J Viral Hepat 2010;17:317–26. [DOI] [PubMed] [Google Scholar]
- 7. Qureshi H, Bile KM, Jooma R, Alam SE, Afridi HUR.. Prevalence of hepatitis B and C viral infections in Pakistan: findings of a national survey appealing for effective prevention and control measures. East Mediterr Health J 2010;16(Suppl):S15–23. [PubMed] [Google Scholar]
- 8. Waheed Y, Shafi T, Safi SZ, Qadri I.. Hepatitis C virus in Pakistan: a systematic review of prevalence, genotypes and risk factors. World J Gastroenterol 2009;15:5647–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Centers for Disease Control and Prevention (CDC). Establishment of a viral hepatitis surveillance system, Pakistan, 2009-2011. MMWR Morb Mortal Wkly Rep 2011;60(40):1385–90. [PubMed] [Google Scholar]
- 10. Krishanani MK, Qidwai W, Ali BS, Khuwaja AK.. Educational intervention among barbers about liver cancer-inducing viruses: a pilot study from a developing country. J Cancer Educ 2010;25:632–36. [DOI] [PubMed] [Google Scholar]
- 11. European Union HCV Collaborators. Hepatitis C virus prevalence and level of intervention required to achieve the WHO targets for elimination in the European Union by 2030: a modelling study. Lancet Gastroentero Hepatol 2017;2:325–36. [DOI] [PubMed] [Google Scholar]
- 12. Hatzakis A, Chulanov V, Gadano AC. et al . The present and future disease burden of hepatitis C virus (HCV) infections with today's treatment paradigm. Vol. 2. J Viral Hepat 2015;22(Suppl 1):26–45. [DOI] [PubMed] [Google Scholar]
- 13. Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol 2017;2:161–76. [DOI] [PubMed] [Google Scholar]
- 14. Razavi H, Waked I, Sarrazin C. et al. The present and future disease burden of hepatitis C virus (HCV) infection with today's treatment paradigm. J Viral Hepat 2014;21(Suppl 1):34–59. [DOI] [PubMed] [Google Scholar]
- 15. Sibley A, Han KH, Abourached A. et al. The present and future disease burden of hepatitis C virus infections with today's treatment paradigm. Vol. 3. J Viral Hepat 2015;22(Suppl 4):21–41. [DOI] [PubMed] [Google Scholar]
- 16. Ayoub HH, Abu-Raddad LJ.. Impact of treatment on hepatitis C virus transmission and incidence in Egypt: A case for treatment as prevention. J Viral Hepat 2016;24:486–95. [DOI] [PubMed] [Google Scholar]
- 17. Breban R, Arafa N, Leroy S. et al. Effect of preventive and curative interventions on hepatitis C virus transmission in Egypt (ANRS 1211): a modelling study. Lancet Global Health 2014;2:e541–49. [DOI] [PubMed] [Google Scholar]
- 18. Westbrook RH, Dusheiko G.. Natural history of hepatitis C. J Hepatol 2014;61(Suppl 1):S58–68. [DOI] [PubMed] [Google Scholar]
- 19. Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y.. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med 2013;158(Pt 1):329–37. [DOI] [PubMed] [Google Scholar]
- 20. United Nations, Department of Economic and Social Affairs, Population Division. United Nations World Population Prospects: The 2015 Revision https://esa.un.org/unpd/wpp/ (30 June 2016, date last accessed).
- 21. United Nations Office on Drugs and Crime. Drug Use in Pakistan 2013 http://www.unodc.org (30 June 2016, date last accessed).
- 22. Nelson PK, Mathers BM, Cowie B. et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet 2011;378:571–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Micallef JM, Kaldor JM, Dore GJ.. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J Viral Hepat 2006;13:34–41. [DOI] [PubMed] [Google Scholar]
- 24. Trickey A, May MT, Davies C. et al. Importance and contribution of community, social, and healthcare risk factors for hepatitis C Infection in Pakistan. Am J Trop Med Hyg 2017;97:1920–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thein H-H, Yi Q, Dore GJ, Krahn MD.. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology 2008;48:418–31. [DOI] [PubMed] [Google Scholar]
- 26. Nishtar S. Health Indicators of Pakistan. Gateway Paper II. Islamabad: Federal Bureau of Statistics and Ministry of Health, 2007. [Google Scholar]
- 27. Pakistan Medical Research Council. National Health Survey of Pakistan: Health Profile of the People of Pakistan, 1990-94. Islamabad: Pakistan Medical Research Council, 1998. [Google Scholar]
- 28. Pakistan Medical Research Council, Directorate of Malaria Control, Save The Children. Malaria Indicator Survey in 38 High Risk Districts of Pakistan 2013-2014. Islamabad: PMRC, 2015. [Google Scholar]
- 29. Regional Health Systems Observatory, World Health Organization. Health System Profile 2007 – Pakistan. Geneva: WHO, 2008. [Google Scholar]
- 30. Qureshi H, Mohamud BK, Alam SE, Arif A, Ahmed W.. Treatment of hepatitis B and C through national programme – an audit. J Pak Med Assoc 2013;63:220–24. [PubMed] [Google Scholar]
- 31. Umar M, Bilal M.. Hepatitis C, a mega menace: a Pakistani perspective. J Pioneer Med Sci 2012;2:68. [Google Scholar]
- 32. World Health Organization. Global Report on Access to Hepatitis C Treatment. Geneva: WHO, 2016. [Google Scholar]
- 33. National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on a National Strategy for the Elimination of Hepatitis B and C. A National Strategy for the Elimination of Hepatitis B and C. Washington, DC: National Academies Press, 2017. [PubMed] [Google Scholar]
- 34. World Health Organization. Combating Hepatitis B and C to Reach Elimination by 2030: Advocacy Brief. Geneva: WHO, 2016. [Google Scholar]
- 35. Gvinjilia L, Nasrullah M, Sergeenko D. et al. National progress toward hepatitis C elimination – Georgia, 2015-2016. MMWR Morb Mortal Wkly Rep 2016;65:1132–35. [DOI] [PubMed] [Google Scholar]
- 36. Platt L, Reed J, Minozzi S. et al. Effectiveness of needle/syringe programmes and opiate substitution therapy in preventing HCV transmission among people who inject drugs. Cochrane Database Syst Rev 2016;2016:CD012021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Platt L, Minozzi S, Reed J. et al. Needle syringe programmes and opioid substitution therapy for preventing HCV transmission among people who inject drugs: findings from a Cochrane Review and meta-analysis. Addiction 2017, Sep 11. doi: 10.1111/add.14012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cousien A, Tran VC, Deuffic-Burban S, Jauffret-Roustide M, Dhersin JS, Yazdanpanah Y.. Dynamic modelling of hepatitis C virus transmission among people who inject drugs: a methodological review. J Viral Hepat 2015;22:213–29. [DOI] [PubMed] [Google Scholar]
- 39. Martin NK, Vickerman P, Grebely J. et al. Hepatitis C virus treatment for prevention among people who inject drugs: Modeling treatment scale-up in the age of direct-acting antivirals. Hepatology 2013;58:1598–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Martin NK, Vickerman P, Dore GJ, Hickman M.. The hepatitis C virus epidemics in key populations (including people who inject drugs, prisoners and MSM): the use of direct-acting antivirals as treatment for prevention. Curr Opin HIV AIDS 2015;10:374–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kandeel A, Genedy M, El-Refai S, Funk AL, Fontanet A, Talaat M.. The prevalence of hepatitis C virus infection in Egypt 2015: implications for future policy on prevention and treatment. Liver Int 2017;37:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.