Abstract
Background
In the context of a severe generalized African HIV epidemic, the value of geographically targeted prevention interventions has only recently been given serious consideration. However, to date no study has performed a population-based analysis of the micro-geographical clustering of HIV incident infections, limiting the evidential support for such a strategy.
Methods
We followed 17 984 HIV-uninfected individuals aged 15–54 in a population-based cohort in rural KwaZulu-Natal, South Africa, and observed individual HIV sero-conversions between 2004 and 2014. We geo-located all individuals to an exact homestead of residence (accuracy <2 m). We then employed a two-dimensional Gaussian kernel of radius 3 km to produce robust estimates of HIV incidence which vary across continuous geographical space. We also applied Tango's flexibly shaped spatial scan statistic to identify irregularly shaped clusters of high HIV incidence.
Results
Between 2004 and 2014, we observed a total of 2 311 HIV sero-conversions over 70 534 person-years of observation, at an overall incidence of 3.3 [95% confidence interval (CI), 3.1-3.4] per 100 person-years. Three large irregularly-shaped clusters of new HIV infections (relative risk = 1.6, 1.7 and 2.3) were identified in two adjacent peri-urban communities near the National Road (P = 0.001, 0.015) as well as in a rural node bordering a recent coal mine development (P = 0.020), respectively. Together the clusters had a significantly higher age-sex standardized incidence of 5.1 (95% CI, 4.7-5.6) per 100 person-years compared with a standardized incidence of 3.0 per 100 person-years (95% CI, 2.9-3.2) in the remainder of the study area. Though these clusters comprise just 6.8% of the study area, they account for one out of every four sero-conversions observed over the study period.
Conclusions
Our study has revealed clear ‘corridors of transmission’ in this typical rural, hyper-endemic population. Even in a severely affected rural African population, an approach that seeks to provide preventive interventions to the most vulnerable geographies could be more effective and cost-effective in reducing the overall rate of new HIV infections. There is an urgent need to develop and test such interventions as part of an overall combination prevention approach.
Keywords: HIV incidence, disease clustering, small-area variation, geographical information systems
Introduction
Despite impressive life expectancy gains due to antiretroviral treatment,1,2 the rate of new HIV infections remains high in many communities in sub-Saharan Africa.3,4 Consequently, innovative approaches are needed for prevention strategies that make better use of the limited resources available. Implementing effective HIV prevention programmes requires knowledge of the geographical distribution of HIV incidence and risk factors associated with acquisition of infection.5 However, the value of geographically targeted prevention interventions has only recently been given serious consideration in the context of a severe generalized African epidemic. In this regard, international agencies such as Joint United Nations Programme on HIV/AIDS (UNAIDS)6 and the Global Fund7 have recently recommended that countries move to adopt a geographical prioritization approach to optimize the provision of HIV prevention and treatment services. A particularly prominent adopter of this approach is the United States President's Emergency Plan for AIDS Relief (PEPFAR) which seeks to use a ‘data-driven approach that strategically targets geographic areas and populations where we can achieve the most impact’.8 This approach allocates resources on the basis of a geographical prioritization of districts, focusing on those geographical regions and localities where most transmission is occurring. The strategy recognizes that targeting the populations implicated in these sub-epidemics is vital to achieving large reductions in population-level incidence.
This recent shift in thinking has been driven by high levels of HIV prevalence in some communities,9–11 detection of localized spatial clustering of prevalent cases and HIV-related deaths,12–15 variation in HIV prevalence at a district and clinic level16,17 and geographical variation in HIV incidence among participating women in clinical trials.18 These results have challenged the previous paradigm of a ubiquitous ‘generalized’ epidemic in many hyper-endemic contexts. Furthermore, mathematical models19 and phylogenetic research20,21 have suggested that there may be real prevention gains to be made by harnessing geographical differences in HIV incidence for epidemic control, by intervening aggressively in the most vulnerable, high-risk populations. However, to date there remains limited population-based evidence from a hyper-endemic setting to demonstrate whether incident HIV infections do indeed cluster in space and therefore whether a geographically targeted strategy could potentially pay prevention dividends. Such evidence is difficult and expensive to obtain because of the large sample sizes and long follow-up times required to directly observe a sufficient number of HIV sero-conversions so as to be able to provide robust statistical inference when quantifying geographical variations in HIV incidence. If this type of population-based evidence were forthcoming, it could provide real impetus to more widely adopt a strategy that seeks to deploy specific interventions in high-risk geographical spaces as part of an overall combination prevention approach.
Given this background, we followed up nearly 18 000 HIV-uninfected individuals (observing individual HIV sero-conversions) over a decade, in a typical hyper-endemic, rural South African setting. We precisely geo-locate all participants to an exact homestead of residence and use advanced spatial analytical techniques to identify and characterize micro-geographies with excessive numbers of new HIV infections.
Methods
Setting
The study uses data from one of the most comprehensive demographic surveillance sites in Africa—the Africa Centre (now Africa Health Research Institute) Demographic Information System. 22 The site has collected sociodemographic information on a population of approximately 87 000 individuals within a circumscribed geographical area (438 km2 in area) in rural KwaZulu-Natal, South Africa, for over a decade. One of the notable strengths of the comprehensive demographic platform is its longitudinal integrity and ability to record exact periods of time spent living at multiple locations (including outside the study area) by each individual under surveillance.22 Nested within the demographic information system are the population-based HIV surveillance and sexual behaviour surveys which take place annually. Between 2004 and 2006, all women aged 15–49 years and men aged 15–54 years resident in the surveillance area were eligible for HIV testing. However, starting in 2007, eligibility was extended to cover all resident individuals’ ≥ 15 years of age. The longitudinal dynamics of participation in the HIV survey is described in detail elsewhere.23 Overall, 29% of the adult population aged15 49 are infected with HIV.24 The rate of new HIV infections is high at around 2.7 new infections per 100 person-years in the entire adult population (≥ 15 years of age).4
Ethics approval for all surveillance data collection activities was obtained from the Nelson Mandela Medical School Research Ethics Committee, University of KwaZulu Natal, Durban.
Statistical analysis
Individuals (aged 15–54) were included in the analysis if they tested negative for HIV upon entry into the surveillance cohort and consented to test at least once thereafter (n = 17 984); 80% of participants in the cohort agreed to an HIV test at their first test offer, and 62% observed to be HIV-negative at one point in time were tested on at least one subsequent occasion. Participants seldom test every year, and the median interval of time between last HIV-negative and first HIV-positive test is 2.18 years. The date of HIV seroconversion was assumed to occur according to a uniform random distribution between the date of the last negative and first positive HIV test. This avoided the biases introduced by assuming that the date of HIV sero-conversion occurred at the mid-point of these two dates.25 To investigate the age-sex trends in incidence, we computed HIV incidence [95% confidence interval (CI)] by 5-year age-band. Crude incidence rates per 100 person-years were age-sex standardized against the age and sex structure of the entire cohort for the full period. We also computed incidence by 1-year age band (stratified by gender) and fitted a log-normal function for each group using a maximum likelihood approach.
Spatial analyses
We geo-located all participants to their respective homesteads of residence which have been comprehensively mapped to an accuracy of < 2 m.14 The population is characterized by a high level of mobility,26 and the demographic information system was set up so as to be able to precisely measure the amount of time each individual was resident at any given location within the study area.22 To ensure unbiased geographical estimates of incidence, it is important that we adequately account for the mobility in the study population and do not impose an overly simplistic static residency assumption on the data. To accomplish this, we included in the incidence denominator only person-days of exposure that accrue to participants while resident in the study area (i.e. person-days spent outside the study area were not included in the incidence rate calculations). Similarly, if the imputed sero-conversion date coincided with a period of time during which the participant lived outside the study area, then the incident event was not included in the numerator. In cases where a participant was resident at multiple homesteads during the period of observation, the exact number of days spent at the location of each homestead was used in the geographical analyses. Incident events were attributed to the location of the homestead at which the participant was resident at the imputed sero-conversion date. This approach ensured that the numerator and denominator in the incidence calculation were not systematically biased in either direction, without the need to super-impose an arbitrary residency criterion or assume that individuals were resident at a single location between survey rounds. We then used two different spatial analytical techniques to measure the spatial distribution of HIV incidence across the study area; these are described in detail below.
First, we used a Gaussian kernel (radius = 3 km) methodology to produce robust estimates of HIV incidence that vary across continuous geographical space.14,27 The size of the kernel was determined on the basis of previous work in this population, which measured the spatial dependence in ward-level HIV prevalence.14 The smaller the radius used in the kernel, the greater the range in estimates obtained and the greater the sensitivity to local variation. The use of a larger kernel will result in smoothing towards the mean, and important variation in HIV incidence may be lost. The Gaussian kernel does not impose any static geographical boundaries on the data, but uses the precise location of each individual to derive the resulting community-level estimates. A median of 463 [interquartile range (IQR): 211-1033] person-years of observation was evaluated in the virtual community surrounding each participant’s homestead in the kernel-based approach. The methodology produces spatially continuous HIV incidence estimates that are sensitive to local variations while at the same time being robust to the effects of random noise.
From a resource allocation perspective, it is important to quantify not only the risk of new HIV infection over person-time but also the density of new HIV infections per unit area. In our previous work, we used the Gaussian kernel approach to estimate the density of existing HIV infections per km2.14 In a similar fashion, to derive the density of HIV incident events, we multiplied the HIV incidence map by the geographical distribution of all HIV-negative residents in 2014 (adjusted for non-consent) to derive the total number of HIV sero-conversions per km2 per year. The latter surface was derived from the 2014 population-based HIV sero-survey of 9508 individuals living in the study area. We applied an edge correction factor within 3 km of the boundary of the study area, to accurately quantify the total number of HIV sero-conversions per km2 across the whole study area in a given year.
Second, for the first time to our knowledge, we applied Tango's flexibly shaped spatial scan statistic28 (implemented in FleXScan software29) to identify micro-geographical clustering of new HIV infections. Spatial scan statistics are designed to detect a local excess of events and to test whether such identified excess could reasonably have occurred by chance.30 A window is imposed on an area of interest by the statistic, and the centre of the windows moves across the study region while also varying in size (diameter). The spatial scan statistic calculates the likelihood of observing the number of events inside and outside each window. The window with maximum likelihood is defined as the most likely cluster, i.e. least likely to have occurred by chance. Most disease or health outcome events are not likely to conform to a predefined geometrical shape (circle or ellipse) as imposed by other commonly used spatial scan statistics, such as the Kulldorff statistic.31 The Tango flexible spatial scan statistic instead allows an irregularly shaped scanning window to be imposed on each location by iteratively adding connected (or adjacent) locations. Therefore, this advanced analytical technique can detect irregularly (arbitrarily) shaped spatial clusters by iteratively (via Monte Carlo replication) combining adjacent locations, and has higher power when the true cluster is non-circular compared with a regularly shaped spatial scan statistic.28,32
We assumed a Poisson model for these analyses, i.e. number of incident events (numerator) scaled by person-years (PY) as the denominator. The Poisson model calculates an expected number of incident events based on the overall incidence rate, and applies this to the observed denominator (PY) within a given scan window (‘cluster’) to calculate the expected number of events. The ratio of the observed vs expected yields the relative risk (RR) for a given cluster. A P-value for the observed cluster (difference) is also calculated, based on the null distribution of likelihood ratio test statistic with a large number of Monte Carlo replications of the data set generated under the null hypothesis.28 We classified any cluster with a P-value of < 0.05 as being statistically significant.
More specifically, consider the scenario where the study area is divided into m nodes (∼800 aggregated homesteads as specified above). The number of cases in a given node i can be represented by the random variable Ni with observed value of incident HIV cases ni, where i = 1, …, m. Under the null hypothesis (H0) of no clustering, the Ni are assumed to be independent Poisson variables whereby:
We employed the likelihood ratio (LLR) with restriction statistic by Tango (2005)28 as implemented in FleXScan, with a default restriction (‘alpha’) parameter setting of 0.2. This approach avoids detecting meaninglessly large clusters (little practical/policy relevance in a relative small geographical area) and also improves calculation time with a large number of locations, as found in our data. Furthermore, the pre-specified maximum length of cluster (or K parameter) for the flexible spatial scan statistic has to be set at a realistic upper bound to avoid computationally infeasible scans. The current practical upper bound as suggested by Tango is around K = 30, which we employed in our analyses.28 Tango has suggested that the execution time of the current algorithm will take more than a week if K > 30 for the number of regions m ∼200–300. However, in our data we had 8590 unique locations before aggregation. With a value of K = 30 it would be statistically impossible to identify significant and meaningful clusters, as the scan statistic would only search through a maximum of 30 adjacent nodes (homesteads), and the number of incident HIV events in such a number of homesteads would be largely 0 across the site. Hence our choice to aggregate the homesteads to a regular grid of n = 760, for the FleXScan analysis to be able to identify meaningful clusters with significance without substantial loss of fine geographical precision/resolution.
Cluster characterization
We employed mixed effects linear and logistic regression models to compare selected characteristics between clusters and non-clusters. The regression models include fixed effects for cluster membership and normally distributed random intercepts that capture the correlation between the samples associated with the same individual. Testing whether there is a relationship between a selected characteristic and the clustering of locations is done by testing the null hypothesis, that the fixed effects for cluster membership are zero, against the alternative hypothesis, that at least one of the fixed effects is different from zero (see Supplementary materials, available as Supplementary data at IJE online).
We examined the relationship between incidence rates and the clustering of locations by fitting separate survival models for men and women. These are Cox proportional hazards models for time to HIV seroconversion, which have cluster membership indicator variables as time-dependent covariates. Assessing whether the clustering of locations is associated with the hazard of HIV acquisition is performed by testing the null hypothesis that the slope coefficients associated with cluster membership are zero. A complete description of the statistical models employed is provided in the Supplementary materials, available at IJE online.
Results
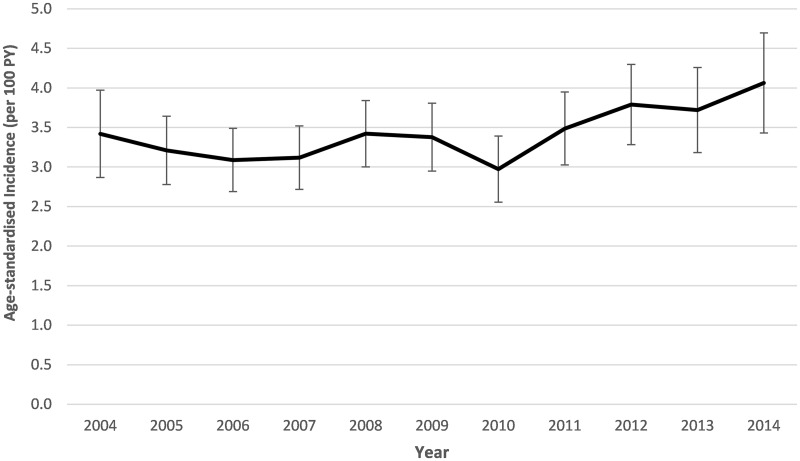
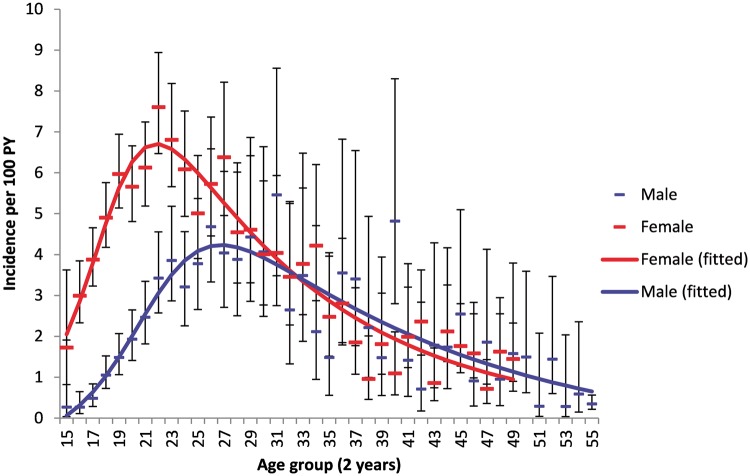
Between 2004 and 2014, we observed a total of 2311 HIV sero-conversions during 70 534 person-years of observation among the cohort, at a crude HIV incidence rate of 3.3 (95% CI, 3.1–3.4) per 100 person-years. The overall HIV incidence rate amongst the male population for the period was 2.0 per 100 person-years (95% CI, 1.8–2.2) compared with 4.1 per 100 person-years (95% CI, 3.9–4.3) among the female population. Overall incidence for the population remained relatively stable when assessed by year (Figure 1). Based on a log normal curve (Figure 2), incidence was highest in females 22 years of age at an incidence of 7.6 cases per 100 person-years (95% CI, 6.5–8.9) and peaked later in males at 27 years of age (4.0 incident cases per 100 person-years, 95% CI, 2.7–6.0). Incidence risk for females was much higher at earlier age but inverted at ∼32 years of age, when males’ risk was subsequently marginally higher. The striking sex differences in HIV incidence are a result of a combination of increased biological susceptibility to infection,33 as well as the unequal cultural, social and economic status of (particularly young) women in society.34,35
Figure 1.
Age-sex standardized HIV incidence by year for individuals aged 15–54 [70 534 person-years (PY), 2311 sero-conversions at crude incidence of 3.3 per 100 PY].
Figure 2.
Female and male age variations in HIV incidence (95% CI) for entire sample of repeat-testers aged 15–54. Superimposed on the graphs are log-normal functions (obtained by maximum likelihood) fitted to 1-year incidence estimates.
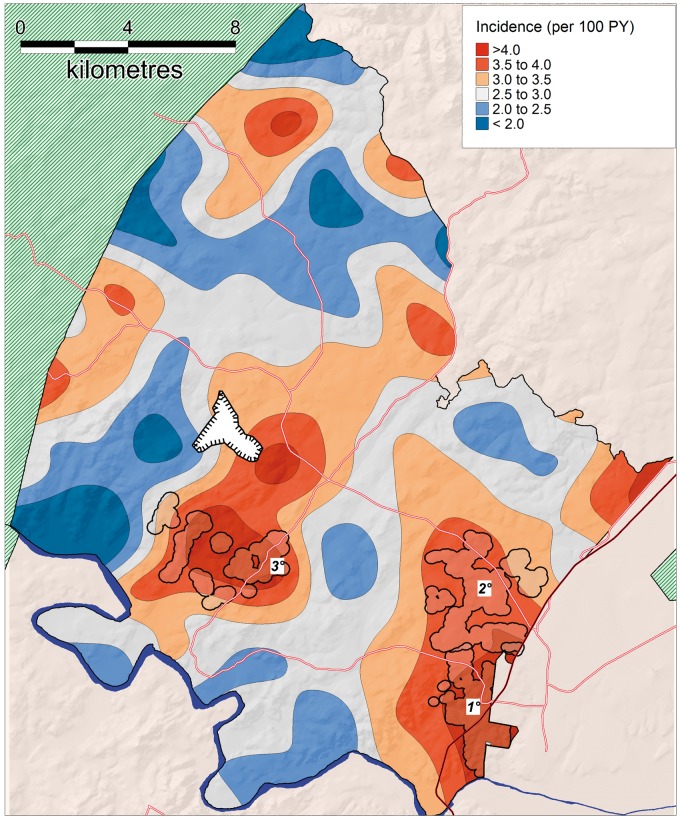
Our analyses revealed considerable spatial variation in HIV incidence (Figure 3). Overall, the kernel-based spatial analytical approach showed that HIV incidence varied between 0.86% and 5.21% per year in the unique virtual community evaluated around each participant’s homestead (a > 6-fold variation). Three clear high-risk spatial clusters (RR = 1.6, 1.7 and 2.3, respectively) were identified using the flexibly shaped spatial scan statistic (Figure 3). Together the clusters had a significantly higher standardized incidence of 5.1 (95% CI, 4.7–5.6) per 100 person-years compared with a standardized incidence of 3.0 per 100 person-years (95% CI, 2.9–3.2) in the remainder of the study area. Clusters 1 and 2 (4.9 and 4.1 km in extent, respectively) were in peri-urban communities in the south-east and eastern portion of the study site, both in close proximity to a National Road (P = 0.001, 0.015, respectively). The third cluster (4.7 km in extent) was situated more internally to the south central of the study area, near a recent coal mine development which first became operational in 2007 P = 0.020). The clusters contained 322, 185 and 64 incident cases, respectively (25% of all incident cases observed over the duration of the study). The clusters had standardized incidence rates of 5.3 (95% CI, 4.7–5.8), 4.6 (95% CI, 3.9–5.3) and 6.7 (95% CI, 4.9–8.4) per 100 person-years, respectively, for the period 2004–14. Whilst the HIV incidence in clusters 1 and 2 (peri-urban communities) remained relatively constant over time, cluster 3 (located near the coal mine) showed a striking increase in standardized HIV incidence (per 100 PY) towards the end of the study period, from 6.1 (2004–08) to 5.6 (2009–12) to 16.2 between 2013 and 2014 (time period 3 vs time period 1 hazard ratio = 3.34, P = 0.001). Analysis of spatial clustering of HIV incidence stratified by gender suggested a similar high-risk cluster respectively for both females and males in the the same location as the primary cluster identified as part of the combined sex analysis, bordering the national highway (Figure 4a, b). Similarly, there was some evidence of a secondary female cluster (P=0.086) in a similar location to the secondary cluster identified as part of the combined sex analysis.
Figure 3.
Geographical variations in population-level HIV incidence (ages 15–54) as measured by a standard Gaussian kernel (3.0 km radius). Superimposed on the map are the high-risk clusters identified by the Tango's flexibly shaped spatial scan statistic: Cluster 1 [322 sero-conversions, 6233 person-years of observation (PYO), RR = 1.59, P = 0.001, area = 8.3 km2]; Cluster 2 (185 sero-conversions, 3630 PYO, RR = 1.57, P = 0.015, area = 12.7 km2); and Cluster 3 (64 sero-conversions, 891 PYO, RR = 2.27, P = 0.020, area = 9.4 km2). Phase 1 of a recent opencast coal mining development is shown immediately north of cluster 3.
Figure 4.
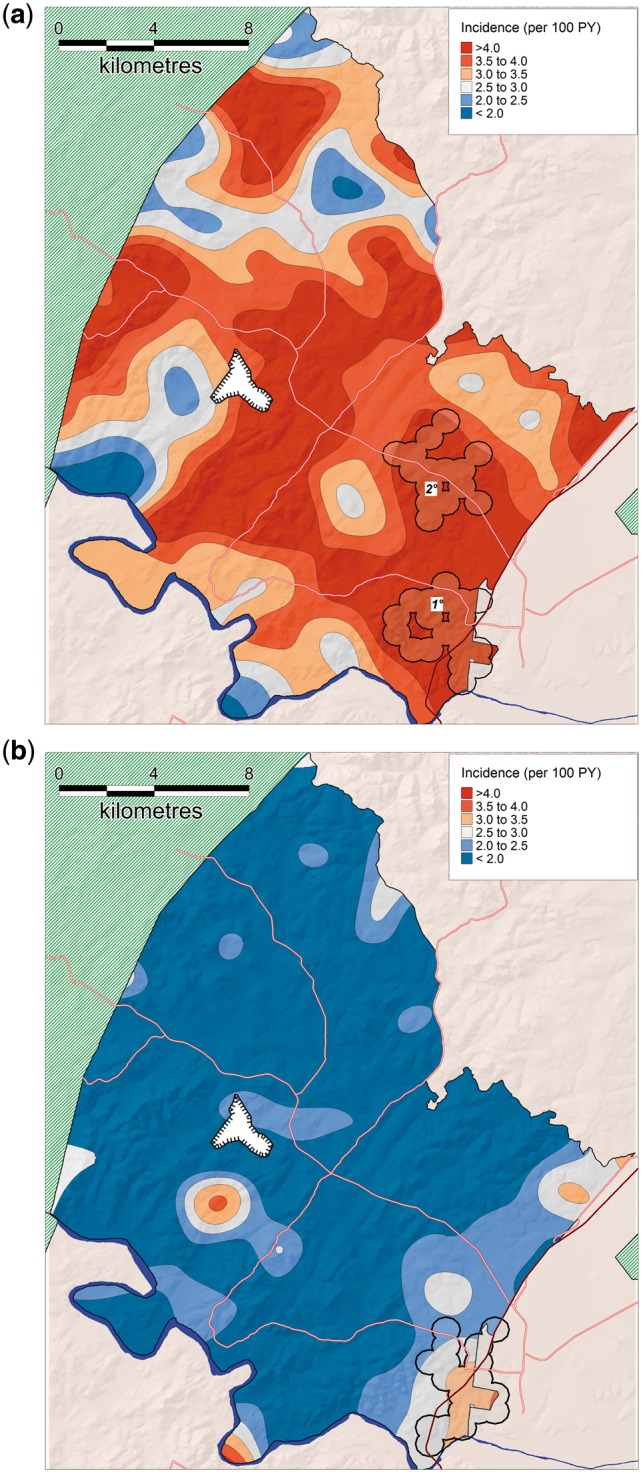
Geographical variations in population-level HIV incidence (ages 15–54) in females (a) and males (b), as measured by a standard Gaussian kernel (3.0 km radius). Superimposed on the map are the high-risk clusters identified by the Tango's flexibly shaped spatial scan statistic: (a) Cluster 1 = 296 sero-conversions, RR = 1.52, P = 0.002; Cluster 2 = 91 sero-conversions, RR = 1.57, P = 0.081; and (b) Cluster = 131 sero-conversions, RR = 1.92, P = 0.001.
Overall, the most prominent differences in the cluster vs non-cluster communities (Figure 3) were the population growth rate between 2004 and 2014 (+6.1% vs -10.9%), migration intensity (160 vs 148 migration events per 100 person-years) and HIV prevalence (38.9% vs 27.5%) (Table 1). The cluster communities also had higher numbers of reported lifetime partners, lower reported number of years of education and lower reported mean age at first sex (females). There was a lower number of births per 1000 females of child-bearing age, and contraceptive use was marginally higher in the cluster communities. Overall, the ratio of female to male incidence was 14% lower (i.e. male incidence was disproportionally higher) in the cluster communities (compared with non-clusters) but this difference was not significant (P = 0.185).
Table 1.
Comparison of key characteristics of three high-incidence clusters identified by the Tango flexible spatial scan-statistic (Figure 3) vs non-clusters
| Characteristic | Non-cluster (n = 15254) | Cluster (n = 2730) | P-valuea |
|---|---|---|---|
| % Female | 58.9 | 58.5 | 0.122 |
| Ratio of female to male incidenceb | 2.2 | 1.9 | 0.185 |
| Mean (SD) age of adults (15–54 years) | 28.0 (11.3) | 28.3 (10.8) | < 0.001 |
| Median (IQR) number of lifetime partners (females) | 1 (1-2) | 2 (1-2) | < 0.001 |
| Mean (SD) years of education of adults (15–54 years)b | 9.2 (3.6) | 8.8 (3.5) | 0.007 |
| Household assets, mean (SD) quintile score | 3.0 (1.4) | 2.9 (1.4) | < 0.001 |
| Fertility rate (births per 1000 females, 15–49 years, PY)b | 62.5 | 62.2 | 0.014 |
| Migration events among ages 15–54 years (per 1000 PY)b | 147.8 | 160.6 | < 0.001 |
| Mean (SD) age first married (females) | 26.1 (7.0) | 27.4 (7.3) | 0.015 |
| Mean (SD) age at first sex (female) | 18.0 (3.0) | 17.7 (3.1) | < 0.001 |
| Current contraception use (females 15–49 years) (%)b | 44.0 | 46.9 | 0.030 |
| Growth over study period (%)c | −10.9 | +6.1% | < 0.001 |
| HIV prevalenced among adults (15–54 years) (%) | 27.5 | 38.9 | < 0.001 |
SD, standard deviation; IQR, interquartile range. Bold indicates a P-value of <0.05.
Adjusted for within-subject correlation (multiple or repeated measurements). See supplementary materials for more details of the modelling approaches used, available at IJE online.
Age-standardized.
Based on growth of entire population under surveillance.
Based on population-based HIV testing survey of 2014.
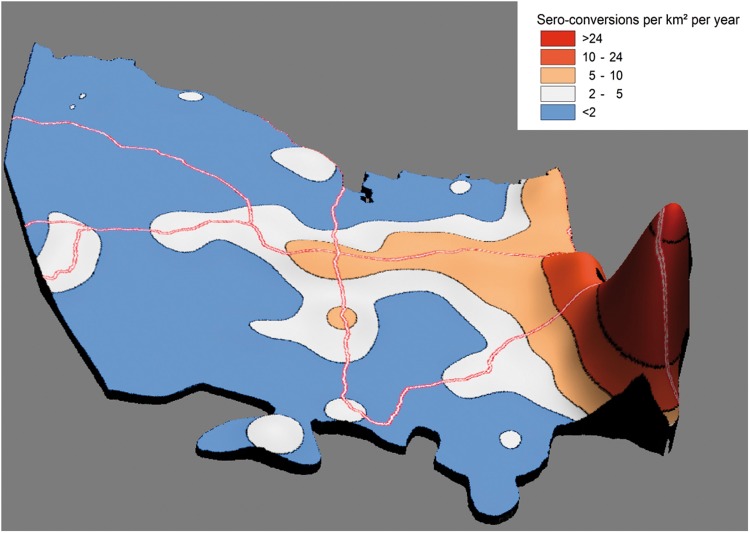
The map showing the estimated number of HIV sero-conversions per km2 per year is presented in Figure 5. Despite the clusters comprising just 6.8% of the study area, they account for one out of every four HIV sero-conversions observed over the study period. Of course, the ‘Achilles heel’ of such a geographically focused strategy is the detailed ‘granular’ data needed to be able to pin-point areas most in need of intervention. In most cases, such data are not available nor indeed feasible to collect. Given this reality, we quantified the implications of a more typical situation in which programme managers did not have knowledge of the exact location of the high-risk clusters, and were instead to target communities within 2 km of the National Road, which are characterized by both overall high incidence as well as a high population density (Table 2). Given this scenario, by focusing efforts on the 8% of the study area that lies within 2 km of the National Road, ∼37% of HIV sero-conversions observed could theoretically be targeted at an ‘effectiveness ratio’ (ratio of the proportion of sero-conversions targeted to proportion of the area covered) of 4.6.
Figure 5.
Estimated HIV sero-conversions per km2 per year for population aged 15–54, obtained using the Gaussian kernel (radius = 3.0 km). The Z axis is proportional to the total HIV sero-conversions per km2 per annum for any geographical location.
Table 2.
Hypothetical targeting strategies to evaluate the number of HIV sero-conversions relative to the size of a given geographical area
| Area km2 (% total) | Person-years (% total) | HIV sero-conversions (% total) | Effectiveness ratioa | |
|---|---|---|---|---|
| High-risk clustersb | 29.9 (6.8%) | 10763 (15.2%) | 550 (23.8%) | 3.5 |
| Communities within 2 km of National Road | 35.8 (8.2%) | 22641 (32.1%) | 866 (37.5%) | 4.6 |
| Study area | 438.1 (100%) | 70534 (100%) | 2311 (100%) | 1.0 |
The ratio of % sero-conversions targeted to % area covered
Only high-risk clusters identified by the Tango flexible scan-statistic (Figure 3) targeted.
Discussion
We have used one of Africa's largest ongoing population-based cohorts to analyse the micro-geographical clustering of HIV incident infections in a hyper-endemic, rural sub-Saharan African context. We use the precise location of each of the nearly 18 000 HIV-uninfected individuals (and exact time spent at each location) followed prospectively over a decade to reveal >6-fold geographical variations in HIV incidence. In addition to the ‘expected’ high-risk clusters in high-density populations near the National Road, the most surprising aspect of the analysis has been the rapid emergence of a rural high-risk cluster with unusually high numbers of new HIV infections near a recent mining development. Overall, the results provide clear empirical evidence for localized ‘corridors of transmission’, and imply that targeting evidence-based interventions to the most vulnerable populations in areas of greatest HIV incidence could be powerful and cost-effective in this typical rural African population, as part of an overall combination prevention approach. Our results add to an increasing body of work from multiple sub-Saharan African environments that have challenged the previous paradigm of a ubiquitous generalized epidemic.9–11,14,15,18,36 Rather, the findings demonstrate the existence of multiple geographically-defined sub-epidemics which make up the composite epidemic in a given population. Taken together, this body of work suggests that there may be a remarkable opportunity to exploit these geographical heterogeneities for epidemic control by intervening in the most vulnerable populations.
Significant clustering of new HIV infections in particular communities can decrease the efficacy of existing population-based intervention measures, but also implies that targeted interventions could be highly effective.19,37 Identification of the localized clustering of HIV incident infections, or so-called ‘hot spots’, also has important local policy relevance, because it has immediate implications for where to focus new prevention programmes or to intensify existing programmes.38 A recent review of research conducted in the 10 highest HIV prevalence countries in the world, concluded that ‘recent empirical findings combined with evidence from phylogenetic studies and supported by mathematical models provide a rationale for testing the feasibility, acceptability, and effectiveness of targeted HIV prevention approaches in hyper-endemic populations to supplement measures aimed at the general population’.39 Although some critical questions remain around the reach and acceptability of such interventions, the results of the review suggested that maximum reductions could be achieved by employing an approach that specifically seeks to target the most vulnerable geographies, in addition to a broad set of interventions targeting the larger population.
Our results raise a fundamental and a related practical question: what is the underlying reason for the clustering of these new HIV infections? High levels of mobility, high rates of sexual partner turnover, younger age at first sex and a high population growth rate are some of the key factors that have been implicated in this research. In recent work, based on the underlying theory of social disequilibrium,40,41 we investigated the individual, social and community challenges to HIV acquisition risk in this study population.42 We found that particularly among men, the effect of migration intensity in predicting HIV acquisition risk was more pronounced and localized in the high-incidence communities near the National Road. This finding was over and above the risk conferred on the basis of individual mobility levels, highlighting the multiple impacts of mobility on HIV acquisition risk and also attesting to the importance of the social and geographical context in predicting risk of HIV acquisition. These risk factors are particularly pertinent in South Africa, given the labour migration history in the apartheid era as well as extremely high levels of socioeconomic inequality.43,44 In addition, high concentrations of sex workers around mines and other industrial developments have been documented,45,46 and this constitutes one possible contributory factor in explaining the high incidence in the high-risk cluster adjacent to the coal mine. Ultimately however, these clusters arise from a complex web of structural, biological and sociodemographic factors which act together synergistically at the level of the individual, the network and the community to produce incidence rates of this magnitude. These factors have been the subject of research in our team and many others over the past 20 years.26,47–49 In the long term, if we more fully understand the underlying causal processes, we will be better placed to design and implement robust interventions across different contexts. Nevertheless, whereas a better understanding this web of causality remains of fundamental importance, basic characterization of the communities in a way that will allow them to be readily identified in other settings is of immense immediate value.
Despite the accuracy and strengths of the micro-geographical approach used, our work has some limitations. The length of time between last HIV-negative and first HIV-positive test in this cohort (≈2 years), combined with high levels of mobility in this population,26 mean that any geographical clustering analysis would be biased towards the null hypothesis of spatial randomness. Although these effects cannot explain the false detection of a spatial cluster of new infections, it is nevertheless conceivable that we may have missed detection of some clusters as a result. In addition, the analytical approach used in comparing clusters with non-clusters, in terms of the sociodemographic and behavioural characteristics, is ecological in nature and as such cannot be used to establish causal relationships. Work is ongoing to establish what drives spatial differences in risk, using individual-level spatial models to provide a stronger basis for causal inference.
In this typical rural setting, nearly 40% of all HIV sero-conversions take place in communities occupying only 8% of the study area. The combination of highest individual-level risk of infection as well as high population densities in these communities give rise to this finding. Thus, there will likely be efficiency gains in the deployment of prevention interventions to such communities, driven by economies of scale. Such gains would be further amplified if individuals in these communities play a disproportionate role in re-seeding epidemics in other populations or act as short-term ‘nodes of attraction’ for high-risk, HIV-infected individuals from more rural communities.15,16 In this regard, our results suggest that high-density peri-urban communities with rapid population growth, located along National Roads, should be prioritized for intervention in this and other similar settings. Given the high levels of mobility in these populations,26 it is likely that the effect of such an intervention would extend well beyond the intervention community. Similarly, populations surrounding recent mining and other industrial developments should be given special prevention consideration over and above the clear need to focus prevention efforts on the employees of these developments themselves. This may be particularly pertinent given the scale of the mining industry in South Africa and Southern African in general.50
The cornerstone of any combination prevention approach must be treatment as prevention.51 One obvious component of the prevention strategy, would therefore be to rapidly increase antiretroviral therapy (ART) coverage in these high-risk communities, particularly in light of attaining the UNAIDS 90-90-90 treatment targets.52 Remarkably, in some of the high-incidence communities near the National Road, 65% of HIV-infected individuals had unsuppressed viral loads and > 20% of the entire adult population—i.e. irrespective of HIV status—were viraemic for HIV in 2011 (7 years after roll-out of ART).53 We have previously demonstrated, in this real-world setting, that individual HIV acquisition risk declines significantly with increasing ART coverage in the local community,4 the household54 and the sexual partnership;55 and in forthcoming work, we show a significant population-level decrease in HIV incidence in men (consistent with a higher uptake of ART in women,53 as well as increase in the prevalence of circumcision).56 However, uptake of ART is impeded by geographical and transport-related barriers which can also produce negative HIV treatment outcomes in those individuals who have been initiated on ART.57,58 In this vein, we have shown empirically that there is a steep and immediate fall-off in uptake of ART with increasing distance from a service delivery point.59 For example, holding other factors constant, at approximately 4.8 km from a clinic providing ART, the odds of an HIV-positive individual being on ART are half those of someone living immediately next to a clinic. It follows that by locating ART services in these populous areas of highest transmission intensity, the most vulnerable populations could be intensively targeted to achieve maximum reductions in HIV incidence. Other components of such a strategy could include intensive messaging campaigns,60 the use of micro-financial incentives to increase rates of HIV testing and linkage to care particularly among men,61–63 improved access to voluntary medical male circumcision64 and the use of pre-exposure prophylaxis65 among vulnerable groups such as young women and sex workers66 (who may be over-represented in communities characterized by intense transmission).
Implementing a geographically orientated intervention approach is not without its drawbacks and programmatic challenges even when the epidemiological rationale is clear.67 First, the data required to implement the strategy can be numerous and the burden on health care workers to collect this data could be substantial. However, as we outlined earlier in the paper, even targeting ‘obvious’ high-risk populations, such as peri-urban communities living within 2 km of major transport routes, could achieve substantial prevention dividends. Second, there could be a danger that populations outside the high-risk communities could become marginalized in terms of treatment and prevention efforts. After all, 75% of HIV sero-conversions in this population still occur outside the identified incidence clusters, and individuals in these other communities are also vulnerable to HIV infection. It is therefore essential that good services continue to be provided to these populations as well, if the UNAIDS 90-90-90 targets are to be reached and the tide of the epidemic is to be turned through a sustained combination prevention approach. Third, scaling up services in high-risk communities implies a level of budget flexibility that may not exist in some HIV hyper-endemic contexts. However, notwithstanding these difficulties, the potential rewards of such an approach may be substantial. For example, a recent modelling exercise undertaken in Kenya estimated that, with no additional cost, a geographically tailored approach could result in a 33% drop in the rate of new infections towards the end of a 15-year period.19 A subsequent study estimated the potential impact of a localized, integrated approach to HIV prevention funding that prioritized populations on the basis of both geographical and individual risk factors, across the continent of Africa. The results suggested that for a US$20 billion representative expenditure over a 15-year period, scale-up of prevention along present funding channels could avert 5.3 million new infections, relative to no scale-up.68
Our study has revealed remarkable geographical variation in HIV incidence in this hyper-endemic population, with the existence of clear ‘corridors of transmission’ where the rate of new HIV infections was 70% higher than in surrounding communities. Targeting efforts at settings where HIV transmission is most intense is crucial. A considerable body of evidence now supports the contention that even in a severely affected rural African setting, interventions that strategically target geographically defined high-risk communities, as part of a combination prevention approach, could be more effective in reducing the overall rate of new infections. Most recently, this type of geographical prioritization approach has been adopted by PEPFAR in order to maximize the impact of their investment.8 Despite programmatic and other challenges, our empirical results, combined with insights from recent mathematical modelling studies as well as observations from other generalized epidemic settings, strongly suggest that, given finite (and likely decreasing) resources, targeted HIV prevention strategies could be effective even in a population with very high overall HIV incidence. There is therefore an urgent need to develop and test such interventions as part of an overall combination prevention approach.
Supplementary Data
Supplementary data are available at IJE online.
Funding
This work was supported by National Institute of Health (R01HD084233 and R01AI124389) and South African Medical Research Council Flagship (MRC-RFA-UFSP-01–2013/UKZN HIVEPI) grants as well as a UK Academy of Medical Sciences Newton Advanced Fellowship (NA150161). AD was supported in part by the National Science Foundation Grant DMS/MPS-1737746 to University of Washington. TB was supported by the Alexander von Humboldt Foundation through the Alexander von Humboldt Professorship endowed by the German Federal Ministry of Education and Research.
Conflict of interest: None declared.
Key Messages
Our study has revealed remarkable geographical variation in HIV incidence in this hyper-endemic population, with the existence of clear ‘corridors of transmission’ where the rate of new HIV infections was 70% higher than surrounding communities.
Despite the overall high incidence of HIV in many rural African populations, these findings, and observations from similar settings, support an approach that seeks to provide preventive interventions to the most vulnerable geographies as part of an overall combination prevention approach.
Supplementary Material
References
- 1. Bor J, Herbst AJ, Newell M-L, Bärnighausen T.. Increases in adult life expectancy in rural South Africa: valuing the scale-up of HIV treatment. Science 2013;339:961–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jahn A, Floyd S, Crampin AC. et al. Population-level effect of HIV on adult mortality and early evidence of reversal after introduction of antiretroviral therapy in Malawi. Lancet 2008;371:1603–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Piot P, Karim SSA, Hecht R. et al. Defeating AIDS—advancing global health. Lancet 2015;386:171–218. [DOI] [PubMed] [Google Scholar]
- 4. Tanser F, Bärnighausen T, Grapsa E, Zaidi J, Newell ML.. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science 2013;339:966–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wilson D, Halperin DT. ‘ Know your epidemic, know your response’: a useful approach, if we get it right. Lancet 2008;372:423–26. [DOI] [PubMed] [Google Scholar]
- 6. Joint United Nations Programme on HIV/AIDS (UNAIDS). Location, Location: Connecting People Faster to HIV Services. Geneva: UNAIDS, 2013. [Google Scholar]
- 7. The Global Fund. The Global Fund’s New Funding Model: Fourth Replenishment (2014-2016). Geneva: The Global Fund, 2013. [Google Scholar]
- 8. President's Emergency Plan for AIDS Relief (PEPFAR). PEPFAR 3.0:Controlling the Epidemic: Delivering on the Promise of an AIDS-free Generation .Washington, DC: PEPFAR, 2014. [Google Scholar]
- 9. Madise N, Ziraba AK, Inungu J. et al. Are slum dwellers at heightened risk of HIV infection than other urban residents? Evidence from population-based HIV prevalence surveys in Kenya. Health Place 2012;18:1144–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nel A, Mabude Z, Smit J. et al. HIV incidence remains high in KwaZulu-Natal, South Africa: evidence from three districts. PLoS One 2012;7:e35278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Opio A, Muyonga M, Mulumba N.. HIV infection in fishing communities of Lake Victoria Basin of Uganda - a cross-sectional sero-behavioral survey. PLoS One 2013;8:e70770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mee P, Collinson MA, Madhavan S. et al. Evidence for localised HIV related micro–epidemics associated with the decentralised provision of antiretroviral treatment in rural South Africa: a spatio–temporal analysis of changing mortality patterns (2007–2010). J Glob Health 2014;4:010403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Namosha E, Sartorius B, Tanser F.. Spatial clustering of all-cause and HIV-related mortality in a rural South African population (2000-2006). PLoS One 2013;8:e69279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tanser F, Bärnighausen T, Cooke GS, Newell ML.. Localized spatial clustering of HIV infections in a widely disseminated rural South African epidemic. Int J Epidemiol 2009;38:1008–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cuadros DF, Awad SF, Abu-Raddad LJ.. Mapping HIV clustering: a strategy for identifying populations at high risk of HIV infection in sub-Saharan Africa. Int J Health Geogr 2013;12:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Coburn BJ, Okano JT, Blower S.. Current drivers and geographic patterns of HIV in Lesotho: implications for treatment and prevention in Sub-Saharan Africa. BMC Med 2013;11:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abdool Karim Q, Abdool Karim SS, Singh B, Short R, Ngxongo S.. Seroprevalence of HIV infection in rural South Africa. AIDS 1992;6:1535–39. [DOI] [PubMed] [Google Scholar]
- 18. Wand H, Ramjee G.. Targeting the hotspots: investigating spatial and demographic variations in HIV infection in small communities in South Africa. J Int AIDS Soc 2010;13:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Anderson S-J, Cherutich P, Kilonzo N. et al. Maximising the effect of combination HIV prevention through prioritisation of the people and places in greatest need: a modelling study. Lancet 2014;384:249–56. [DOI] [PubMed] [Google Scholar]
- 20. Hué S, Hassan AS, Nabwera H. et al. HIV type 1 in a rural coastal town in Kenya shows multiple introductions with many subtypes and much recombination. AIDS Res Hum Retroviruses 2012;28:220–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nazziwa J, Njai HF, Ndembi N. et al. Short communication: HIV type 1 transmitted drug resistance and evidence of transmission clusters among recently infected antiretroviral-naive individuals from Ugandan fishing communities of Lake Victoria. AIDS Res Hum Retroviruses 2013;29:788–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tanser F, Hosegood V, Bärnighausen T. et al. Cohort Profile: Africa Centre Demographic Information System (ACDIS) and population-based HIV survey. Int J Epidemiol 2008;37:956–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Larmarange J, Mossong J, Bärnighausen T, Newell ML.. Participation dynamics in population-based longitudinal HIV surveillance in rural South Africa. PLoS One 2015;10:e0123345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zaidi J, Grapsa E, Tanser F, Newell ML, Bärnighausen T.. Dramatic increase in HIV prevalence after scale-up of antiretroviral treatment. AIDS 2013;27:2301–05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vandormael A, Dobra A, Bärnighausen T, de Oliveira T, Tanser F.. Incidence rate estimation, periodic testing, and the limitations of the mid-point imputation approach. Int J Epidemiol 2018;47:236–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dobra A, Bärnighausen T, Vandormael A, Tanser F.. Space-time migration patterns and risk of HIV acquisition in rural South Africa. AIDS 2017;31:137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Waller LA, Gotway CA.. Applied Spatial Statistics for Public Health Data. Hoboken, NJ: Wiley, 2004. [Google Scholar]
- 28. Tango T, Takahashi K.. A flexibly shaped spatial scan statistic for detecting clusters. Int J Health Geogr 2005;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Takahashi K, Yokoyama T, Tango T.. FleXScan 3.1:Software for the Flexible Spatial Scan Statistic. 2010. https://sites.google.com/site/flexscansoftware/download_e (30 June 2014, date last accessed).
- 30. Kulldorff M, Heffernan R, Hartman J, Assuncao R, Mostashari F.. A space-time permutation scan statistic for disease outbreak detection. PLoS Med 2005;2:e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kulldorff M. A spatial scan statistic. Commun Stat Theory Methods 1997;26:1481–96. [Google Scholar]
- 32. Takahashi K, Kulldorff M, Tango T, Yih K.. A flexibly shaped space-time scan statistic for disease outbreak detection and monitoring. Int J Health Geogr 2008;7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Quinn TC, Overbaugh J.. HIV/AIDS in women: an expanding epidemic. Science 2005;308:1582–83. [DOI] [PubMed] [Google Scholar]
- 34. Joint United Nations Programme on HIV/AIDS (UNAIDS). The Gap Report. Geneva: UNAIDS, 2014. [Google Scholar]
- 35. Jewkes RK, Dunkle K, Nduna M, Shai N.. Intimate partner violence, relationship power inequity, and incidence of HIV infection in young women in South Africa: a cohort study. Lancet 2010;376:41–48. [DOI] [PubMed] [Google Scholar]
- 36. Wand H, Whitaker C, Ramjee G.. Geoadditive models to assess spatial variation of HIV infections among women in local communities of Durban, South Africa. Int J Health Geogr 2011;10:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sumartojo E, Carey JW, Doll LS, Gayle H.. Targeted and general population interventions for HIV prevention:towards a comprehensive approach. AIDS 1997;11:1201–09. [DOI] [PubMed] [Google Scholar]
- 38. Aral SO, Torrone E, Bernstein K.. Geographical targeting to improve progression through the sexually transmitted infection/HIV treatment continua in different populations. Curr Opin HIV AIDS 2015;10:477–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tanser F, de Oliveira T, Maheu-Giroux M, Bärnighausen T.. Concentrated HIV subepidemics in generalized epidemic settings. Curr Opin HIV AIDS 2014;9:115–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sampson RJ, Groves WB.. Community structure and crime: Testing social-disorganization theory. Am J Sociol 1989;94:774–802. [Google Scholar]
- 41. Shaw CR, McKay HD.. Juvenile Delinquency and Urban Areas. Chicago. IL: University of Chicago Press, 1942. [Google Scholar]
- 42. Tomita A, Vandormael AM, Bärnighausen T, de Oliveira T, Tanser F.. Social disequilibrium and the risk of HIV acquisition: A multilevel study in rural KwaZulu-Natal Province, South Africa. J Acquir Immune Defic Syndr 2017;75:164–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wabiri N, Taffa N.. Socio-economic inequality and HIV in South Africa. BMC Public Health 2013;13:1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Coovadia H, Jewkes R, Barron P, Sanders D, McIntyre D.. The health and health system of South Africa: historical roots of current public health challenges. Lancet 2009;374:817–34. [DOI] [PubMed] [Google Scholar]
- 45. Williams BG, Taljaard D, Campbell CM. et al. Changing patterns of knowledge, reported behaviour and sexually transmitted infections in a South African gold mining community. AIDS 2003;17:2099–107. [DOI] [PubMed] [Google Scholar]
- 46. Shahmanesh M, Patel V, Mabey D, Cowan F.. Effectiveness of interventions for the prevention of HIV and other sexually transmitted infections in female sex workers in resource poor setting: a systematic review. Trop Med Int Health 2008;13:659–79. [DOI] [PubMed] [Google Scholar]
- 47. Auvert B, Buve A, Ferry B. et al. Ecological and individual level analysis of risk factors for HIV infection in four urban populations in sub-Saharan Africa with different levels of HIV infection. AIDS 2001;15(Suppl 4):S15–30. [DOI] [PubMed] [Google Scholar]
- 48. Tanser F, Bärnighausen T, Hund L, Garnett GP, McGrath N, Newell ML.. Effect of concurrent sexual partnerships on rate of new HIV infections in a high-prevalence, rural South African population: a cohort study. Lancet 2011;378:247–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bärnighausen T, Hosegood V, Timaeus IM, Newell ML.. The socioeconomic determinants of HIV incidence: evidence from a longitudinal, population-based study in rural South Africa. AIDS 2007;21(Suppl 7):S29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Williams B, Campbell C.. Creating alliances for disease management in industrial settings: a case study of HIV/AIDS in workers in South African gold mines. Int J Occup Environ Health 1998;4:257–64. [DOI] [PubMed] [Google Scholar]
- 51. Mayer K, Gazzard B, Zuniga JJM. et al. Controlling the HIV epidemic with antiretrovirals: IAPAC consensus statement on treatment as prevention and preexposure prophylaxis. J Int Assoc Provid AIDS Care 2013;12:208–16. [DOI] [PubMed] [Google Scholar]
- 52. Joint United Nations Programme on HIV/AIDS UNAIDS. 90–90–90 - An Ambitious Treatment Target to Help End the AIDS Epidemic. Geneva: UNAIDS, 2014. [Google Scholar]
- 53. Tanser F, Vandormael A, Cuadros D. et al. Effect of population viral load on prospective HIV incidence in a hyper-endemic rural African community. Sci Transl Med 2017;9:eaam8012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Vandormael A, Newell M-L, Bärnighausen T, Tanser F.. Use of antiretroviral therapy in households and risk of HIV acquisition in rural KwaZulu-Natal, South Africa, 2004–12: a prospective cohort study. Lancet Glob Health 2014;2:e209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Oldenburg CE, Bärnighausen T, Tanser F. et al. Antiretroviral therapy to prevent HIV acquisition in serodiscordant couples in a hyperendemic community in rural South Africa. Clin Infect Dis 2016;63:548–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Vandormael A, Akullian AN, Dobra A et al. Sharp decline in male HIV incidence in a rural South African population (2004–2015). Conference on Retroviruses and Opportunistic Infections; 2018 Mar 4–7. Boston, MA.
- 57. Lankowski AJ, Siedner MJ, Bangsberg DR, Tsai AC.. Impact of geographic and transportation-related barriers on HIV outcomes in sub-Saharan Africa: a systematic review. AIDS Behav 2014;18:1199–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Siedner MJ, Lankowski A, Tsai AC. et al. GPS-measured distance to clinic, but not self-reported transportation factors, are associated with missed HIV clinic visits in rural Uganda. AIDS 2013;27:1503–08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cooke G, Tanser F, Bärnighausen T, Newell M.. Uptake of antiretroviral treatment through decentralized primary healthcare in rural South Africa BMC Public Health 2010;10:585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Muessig KE, Nekkanti M, Bauermeister J, Bull S, Hightow-Weidman LB.. A systematic review of recent smartphone, Internet and Web 2.0 interventions to address the HIV continuum of care. Curr HIV/AIDS Rep 2015;12:173–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bassett IV, Wilson D, Taaffe J et al. Financial incentives to improve progression through the HIV treatment cascade. Curr Opin HIV AIDS 2015;10:451–63. [DOI] [PMC free article] [PubMed]
- 62. van Rooyen H. Advice dilemmas: Managing advice against the competing public health and counselling imperatives that shape VCT in South Africa. Afr J AIDS Res 2013;12:211–20. [DOI] [PubMed] [Google Scholar]
- 63. Hensen B, Lewis JJ, Schaap A. et al. Factors associated with HIV-testing and acceptance of an offer of home-based testing by men in rural Zambia. AIDS Behav 2015;19:492–504. [DOI] [PubMed] [Google Scholar]
- 64. Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou J, Sitta R, Puren A.. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med 2005;2:e298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Baeten JM, Donnell D, Ndase P. et al. Antiretroviral Prophylaxis for HIV-1 Prevention among Heterosexual Men and Women. N Engl J Med 2012;367:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Baral S, Beyrer C, Muessig K. et al. Burden of HIV among female sex workers in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Infect Dis 2012;12:538–49. [DOI] [PubMed] [Google Scholar]
- 67. Hallett T, Anderson S-J, Asante CA. et al. Evaluation of geospatial methods to generate subnational HIV prevalence estimates for local level planning. AIDS 2016;30:1467–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. McGillen JB, Anderson S-J, Dybul MR, Hallett TB.. Optimum resource allocation to reduce HIV incidence across sub-Saharan Africa: a mathematical modelling study. Lancet HIV 2016;3:e441–48. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.