Abstract
Background
Annual pancreatic cancer incidence rates have been increasing. We examine pancreatic cancer incidence trends by demographics and histologic type.
Methods
Data from the Surveillance, Epidemiology and End Results (SEER) registries were available to assess temporal trends and pancreatic cancer rates from 1974 to 2013.
Results
Pancreatic cancer incidence rates declined between the 1970s and 1990s but increased from 1994 to 2013 among White males. Among non-Hispanic White and Hispanic males, the annual percent change (APC) in incidence between 1992 and 2013 was 0.84% and 0.73%, respectively. Rates also rose among White non-Hispanic, Hispanic and Asian females (APC = 0.81%, 0.56% and 1.23%, respectively) and even more rapidly among females aged 25–34 years (APC > 2.5%). Rates among Black males and females remained unchanged, but higher compared with the other racial/ethnic groups. By histologic type, the increases were greatest for non-secretory endocrine cancers ( > 6%), followed by ductal adenocarcinomas (∼5%) and adenocarcinoma, NOS (∼1.4%)—the largest histologic subgroup of pancreatic cancer. Rates for mucinous adenocarcinomas and poorly specified pancreatic cancer decreased. Overall, incidence rates during 2000–13 were higher among males than females [MF incidence rate ratio (IRR) = 1.28]. The IRR was >1.00 at all ages ≥ 35, but rates among females were higher at younger ages (IRRs 15–24: 0.66, 25–34: 0.81). The MF IRRs for most of the histologic types were elevated among males apart from solid pseudopapillary adenocarcinoma and cystic carcinomas (IRR = 0.22, confidence interval: 0.14–0.34 and 0.52, 0.41–0.65, respectively).
Conclusion
Pancreatic cancer has been increasing overall, but patterns differ by demographic group and histologic type. Many of the trends parallel changing prevalence of lifestyle risk factors such as smoking, overweight and obesity, and diabetes in the USA, particularly for pancreatic adenocarcinoma, and improved diagnosis methods during the past 40 years.
Keywords: Pancreatic, cancer, incidence
Key Messages
Pancreatic cancer is nearly always fatal and overall incidence of the disease has been increasing both within the USA and globally.
Our study found differences in rates of pancreatic cancer by race and sex.
Analyses of pancreatic cancer by histologic type showed rates of non-secretory endocrine, ductal adenocarcinomas and adenocarcinoma NOS were increasing whereas those for mucinous adenocarcinomas and poorly specified decreased.
Compared with women, men were found to have increased rates of the majority of pancreatic cancer histologic types.
Introduction
The recent Cancer Statistics Review (CSR) reported increases in pancreatic cancer incidence during 1992–2013.1 Pancreatic cancer is currently the third leading cause of cancer mortality in the USA, and projections to 2030 estimate that the disease will become the second leading cancer-related cause of death, after lung.1–3 In 2016, an estimated 53 070 new cases of pancreatic cancer were diagnosed within the USA and 41 780 individuals were expected to die of the disease.4,5 The risk of developing pancreatic cancer increases with age—90% of cases are diagnosed after the age of 55 and the median age of diagnosis is 71 years.6,7 Patients with pancreatic cancer have very poor survival, with a relative 5-year survival rate of only 8.2%.1 Pancreatic cancer rates are higher among males than females and among Black compared with White and other racial/ethnic groups. More than 90% of cases diagnosed are exocrine adenocarcinomas but pancreatic neuroendocrine cancer rates have been rising.8
Tobacco use, overweight (including obesity) and diabetes are all modifiable risk factors associated with pancreatic adenocarcinoma.4,9,10 Little is known about modifiable causes of the less common pancreatic cancer histologic types.6 Diabetes mellitus and family history have been associated with sporadic neuroendocrine tumours.11,12 Although cigarette-smoking rates are declining, rates of overweight and obesity and diabetes in the USA are increasing, and there are differences in the prevalence of each of these factors by sex and racial/ethnic group.13 Rare inherited syndromes also play a role in both exocrine and endocrine tumours.6
In our study, we examine pancreatic cancer incidence trends over the past 40 years and patterns by key demographic groupings, namely sex, age, race and tumour histologic type using data from the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) programme and compare them with available data for the major pancreatic risk factors. The SEER population is similar to the US population with respect to measures of poverty and education.14 Only a few studies have carefully assessed the time trends in pancreatic cancer rates8,15 and, to the best of our knowledge, none has examined trends within the main histologic subtypes of pancreatic cancer.
Methods
Data
The SEER 9 programme includes data for cancer cases diagnosed during the 40-year period 1974–2013 among residents of eight registries in Connecticut, Hawaii, Iowa, New Mexico, Utah, Detroit (Michigan), San Francisco-Oakland (California), Seattle-Puget Sound (Washington) and Atlanta (Georgia) since 1975.1 The SEER 13 registries include the SEER 9 plus four additional registries (Alaska Natives, Los Angeles, San Jose-Monterey (California) and rural Georgia) for cases diagnosed since 1992. SEER 18 includes the SEER 13 plus an additional five registries (Greater California, Greater Georgia, Kentucky, Louisiana and New Jersey) for cases diagnosed since 2000; these registries now include approximately 30% of the US population.1
Within the SEER 13 and 18, the race/ethnicity of cases diagnosed since 1992 was categorized as White non-Hispanic, White Hispanic, Black, Asian/Pacific Islander and American Indian/Alaskan Native. Among the latter, only those living in Contract Health Services Delivery Areas (CHSDA) were included. Race was categorized as White and Black during the years prior to 1992 (SEER 9). The US White non-Hispanic population has changed little over the past several decades, increasing by 2% in the SEER 9 and decreasing by 2% in the SEER 13 from 1992 to 2013. In contrast, the White Hispanic population rose by 61% in the SEER 13 and 116% in the SEER 9 over the same time period. Thus, the percentage of the White population that was Hispanic grew from 9% to 17% in the SEER 9 and from 20% to 29% in the SEER 13. This underscores the importance of considering the rates among White populations according to ethnic origin and comparing the rates among White residents in earlier years with those among those identified as White non-Hispanic in more recent years. For age-specific analyses, we used the age group 0–24, then 10-year age groups (25–34, 35–44, 45–54, 55–64, 65–74, 75–84, 85+), age-adjusting within each age group.
Within SEER data, the Manual of Tumor Nomenclature and Coding (MOTNAC) was used to code the primary site and histologic type for cases diagnosed until 1976,16 the International Classification of Diseases—Oncology (ICD-0) for those diagnosed until 1991,17 ICD-0–2 through 200018 and ICD-0–3 since 2001.19 The MOTNAC used three digits to classify histologic type, ICD-O used four digits, and ICD-O-2 and 3 each added several more specific codes. Prior to the start of this study, all cases were machine converted to ICD-0–3. We selected all pancreatic cancers using SEER*Stat 20 site recode ICD-0–3/WHO 2008: Pancreas (ICD-O-3 site C25). Our analysis by histologic type included only those cases that were microscopically confirmed. Using the morphological classification of pancreatic cancer set out in the International Classification of Disease for Oncology19 and, on the advice of Dr Michael Goggins, cases were categorized into the following mutually exclusive groups: adenocarcinoma, not otherwise specified (NOS) (8140), ductal adenocarcinoma excluding cystic or mucinous (8255, 8490, 8500, 8507, 8510, 8514, 8521, 8523, 8560, 8570), ductal specified as mucinous adenocarcinoma (8480, 8481), ductal specified as cystic adenocarcinoma (8440, 8470, 8504), ductal specified as arising from an intraductal papillary mucinous neoplasm (IPMN) (8144, 8450, 8453, 8471, 8503), endocrine: non-secretory (8150, 8246), endocrine: secretory (8151, 8152, 8153, 8155, 8156), endocrine: carcinoid (8240, 8241, 8243, 8244, 8245), other specified adenocarcinoma: acinar cell (8550, 8551), other specified solid pseudopapillary (8452), other specified adenocarcinoma: other (8145, 8154, 8260, 8574, 8441, 8460) and specified non-carcinomas (8680–9999); all other cases were categorized as poorly specified type. Only cancers defined as malignant (behaviour = 3) were included.
Statistical analyses
We used SEER*Stat20 to calculate incidence counts and age-adjusted rates (2000 US Standard Population, 19 age groups), stratified by sex, race/ethnicity, age group and histologic type. The trends analysis used the SEER 13 data with detailed racial/ethnic group categories spanning the 22 years 1992–2013 using grouped years of diagnosis (1992–97, 1998–2003, 2004–08 and 2009–13) and the SEER 9 data for White and Black males and females spanning those years and the additional 18 years 1974–91 (1974–79, 1980–85 and 1986–91).21,22 Temporal trend figures plotted the period-specific rates at their mid-point, e.g. 1974–79: 1977.0 and 2009–13 at 2011.5, and used a semi-logarithmic scale such that a slope of 10 degrees portrayed an annual percent change (APC) of 1%.23 The temporal trend APCs were quantified using annual rates (SEER 13—1992–2013, Table 1) and we include 95% confidence intervals (CIs). To maximize the numbers of cases available to assess recent patterns, we used the SEER 18 data for cases diagnosed during 2000–1324 and incidence rate ratios (IRRs) were calculated by sex and racial/ethnic group. The Tiwari method was used to calculate 95% CIs for the IRRs. These analyses were undertaken using SEER*Stat (version 8.3.4) (National Cancer Institute, Bethesda, MD).20 Rates based on <16 cases are not shown.
Table 1.
Case counts and Annual Percentage Changes (APCs) for pancreatic cancer (total and by histologic type) by racial/ethnic group, gender, and age group, SEER 13 (1992–2013)
| White Non-Hispanic |
White Hispanic |
Black |
Asian/Pacific Islander |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Count | APC1 | LCI | UCI | Count | APC | LCI | UCI | Count | APC | LCI | UCI | Count | APC | LCI | UCI | ||
| Male | 33 206 | 0.84 | 0.67 | 1.01 | 4 000 | 0.73 | 0.04 | 1.43 | 5 104 | −0.31 | −0.82 | 0.20 | 4 208 | 0.16 | −0.52 | 0.84 | |
| Female | 33 331 | 0.81 | 0.59 | 1.03 | 4 364 | 0.56 | 0.07 | 1.05 | 5 899 | −0.22 | −0.70 | 0.26 | 4 389 | 1.23 | 0.74 | 1.72 | |
| Age group | |||||||||||||||||
| Male | |||||||||||||||||
| 0–24 | 22 | ∼ | ∼ | ∼ | 10 | ∼ | ∼ | ∼ | 7 | ∼ | ∼ | ∼ | 3 | ∼ | ∼ | ∼ | |
| 25–34 | 96 | 2.54 | −1.11 | 6.32 | 24 | ∼ | ∼ | ∼ | 33 | ∼ | ∼ | ∼ | 22 | ∼ | ∼ | ∼ | |
| 35–44 | 753 | 0.35 | −0.88 | 1.59 | 174 | 0.63 | −2.22 | 3.58 | 191 | −0.54 | −2.62 | 1.59 | 135 | 3.41 | 0.36 | 6.56 | |
| 45–54 | 3 292 | 0.41 | −0.25 | 1.08 | 566 | 1.19 | −0.24 | 2.64 | 779 | −0.98 | −1.91 | −0.04 | 393 | 1.31 | −0.67 | 3.32 | |
| 55–64 | 7 066 | 0.63 | 0.15 | 1.12 | 945 | 1.20 | 0.23 | 2.18 | 1 436 | 0.27 | −0.69 | 1.24 | 885 | 0.22 | −0.65 | 1.10 | |
| 65–74 | 9 697 | 0.71 | 0.47 | 0.95 | 1 181 | 1.32 | 0.36 | 2.28 | 1 461 | −0.59 | −1.26 | 0.09 | 1 180 | 0.02 | −1.02 | 1.08 | |
| 75–84 | 9 044 | 1.08 | 0.69 | 1.47 | 830 | −0.15 | −1.28 | 0.99 | 926 | −0.73 | −1.80 | 0.35 | 1 116 | −0.04 | −1.26 | 1.19 | |
| 85 + years | 3 236 | 1.15 | 0.56 | 1.75 | 270 | −0.08 | −2.89 | 2.81 | 271 | 0.76 | −1.04 | 2.60 | 474 | −0.81 | −2.29 | 0.68 | |
| Female | |||||||||||||||||
| 0–24 | 33 | ∼ | ∼ | ∼ | 21 | ∼ | ∼ | ∼ | 10 | ∼ | ∼ | ∼ | 12 | ∼ | ∼ | ∼ | |
| 25–34 | 111 | 4.01 | 0.30 | 7.86 | 28 | ∼ | ∼ | ∼ | 32 | ∼ | ∼ | ∼ | 25 | ∼ | ∼ | ∼ | |
| 35–44 | 487 | 2.54 | 1.44 | 3.65 | 127 | 1.71 | −1.49 | 5.01 | 156 | 0.42 | −2.78 | 3.73 | 124 | −1.44 | −4.24 | 1.44 | |
| 45–54 | 2 137 | 0.81 | 0.17 | 1.46 | 423 | 0.34 | −1.43 | 2.14 | 624 | 0.08 | −1.47 | 1.66 | 329 | −0.06 | −2.37 | 2.31 | |
| 55–64 | 4 964 | 1.12 | 0.71 | 1.54 | 846 | 1.47 | 0.22 | 2.74 | 1 179 | −0.42 | −1.35 | 0.52 | 706 | 1.55 | 0.49 | 2.62 | |
| 65–74 | 8 475 | 0.47 | 0.18 | 0.76 | 1 164 | 0.09 | −1.04 | 1.24 | 1 630 | −0.47 | −1.35 | 0.40 | 1 195 | 1.06 | 0.12 | 2.01 | |
| 75–84 | 10 764 | 0.93 | 0.56 | 1.30 | 1 175 | −0.08 | −0.91 | 0.76 | 1 512 | −0.02 | −1.05 | 1.01 | 1 338 | 1.41 | 0.42 | 2.42 | |
| 85 + years | 6 360 | 0.43 | 0.00 | 0.88 | 580 | 1.21 | −0.25 | 2.69 | 756 | −0.45 | −1.73 | 0.84 | 660 | 1.52 | 0.00 | 3.06 | |
| Histologic type* | |||||||||||||||||
| Male | |||||||||||||||||
| Adenocarcinoma, NOS | 18 538 | 1.33 | 1.09 | 1.56 | 2 233 | 2.02 | 1.19 | 2.85 | 2 899 | 0.10 | −0.60 | 0.81 | 2 218 | 0.28 | −0.53 | 1.10 | |
| Ductal Adenocarcinoma | 2 706 | 5.41 | 4.62 | 6.20 | 296 | 5.22 | 2.51 | 8.00 | 384 | 3.01 | 1.27 | 4.78 | 381 | 3.55 | 1.05 | 6.10 | |
| Ductal specified as Mucinous | 1 254 | −3.47 | −4.94 | −1.99 | 143 | −4.89 | −7.75 | −1.95 | 170 | −3.80 | −6.08 | −1.46 | 140 | −2.09 | −4.85 | 0.76 | |
| Endocrine: Non- Secretory | 1 270 | 6.02 | 4.96 | 7.10 | 141 | ∼ | ∼ | ∼ | 163 | ∼ | ∼ | ∼ | 147 | 6.83 | 3.32 | 10.46 | |
| Poorly Specified | 2 601 | −1.92 | −2.64 | −1.20 | 369 | −1.90 | −3.96 | 0.21 | 481 | −1.92 | −4.43 | 0.64 | 356 | 0.17 | −1.87 | 2.24 | |
| Female | |||||||||||||||||
| Adenocarcinoma, NOS | 17 088 | 1.45 | 1.11 | 1.79 | 2 236 | 0.83 | −0.02 | 1.68 | 3 183 | 0.89 | 0.26 | 1.52 | 2 231 | 1.79 | 1.04 | 2.54 | |
| Ductal Adenocarcinoma | 2 546 | 4.94 | 3.91 | 5.99 | 318 | 7.10 | 4.51 | 9.75 | 418 | 3.31 | 1.75 | 4.89 | 437 | 5.47 | 3.58 | 7.40 | |
| Ductal specified as Mucinous | 1 236 | −2.28 | −3.63 | −0.90 | 189 | −3.14 | −5.54 | −0.68 | 198 | −2.99 | −5.31 | −0.61 | 156 | −2.39 | −4.87 | 0.17 | |
| Endocrine: Non- Secretory | 870 | 6.18 | 4.85 | 7.53 | 124 | ∼ | ∼ | ∼ | 160 | ∼ | ∼ | ∼ | 131 | 7.34 | 3.60 | 11.22 | |
| Poorly Specified | 2 360 | −1.21 | −2.18 | −0.22 | 370 | −0.88 | −2.51 | 0.78 | 472 | −3.54 | −5.02 | −2.05 | 322 | −1.42 | −3.11 | 0.31 | |
APC = Annual percent change; LCI, UCI = lower, upper 95% confidence interval.
1APCs were calculated using weighted least squares method, based on rates per 100 000 person-years, age-adjusted to the 2000 US Standard Population (19 age groups - Census P25–1130).
^Excludes 74 male and 89 female American Indian/Alaskan Native cases who live outside a CHSDA area.
∼Statistic could not be calculated because at least one year had zero cases.
*Includes microscopically confirmed cases only.
The numbers of cases were too few for temporal trends analysis by histologic type groups: cystic (n = 325), papillary (361), endocrine: secretory (n = 160) and carcinoid (n = 457) as well for the other specified adenocarcinoma: acinar cell (196), solid pseudopapillary (77), serous (n = 9) and other (84).
We also conducted analyses using the Joinpoint software for the SEER 9 and 13 data sets.25,26 The Joinpoint method did not detect any changes in trends within the SEER 13 annual age-adjusted rates by gender or racial/ethnic group and estimated the same APCs as the SEER*Stat method. However, some changes in slope were detected in the longer-term SEER 9 data, and we note those findings in the text.
Results
Temporal trends
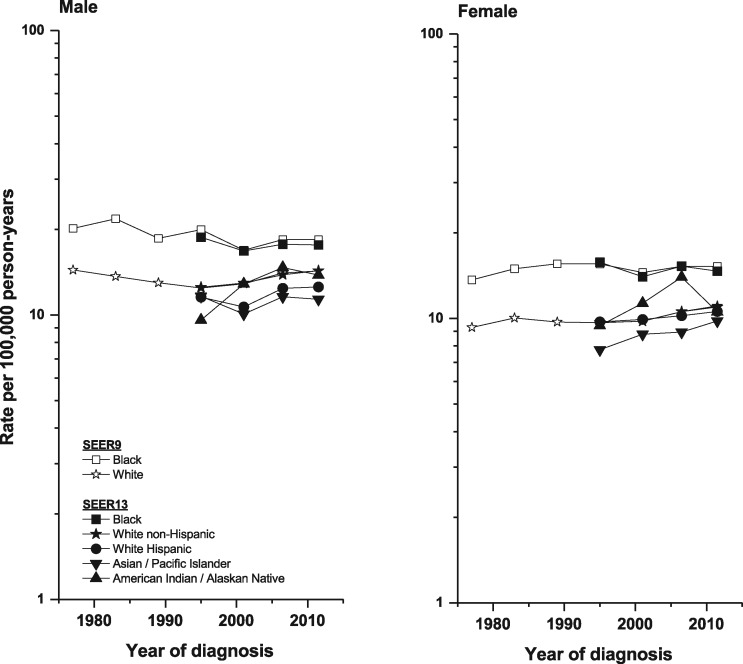
Using data from SEER 9 allowed an exploration of long-term trends in pancreatic cancer. Incidence rates among White males declined until 1994 (1975–94, APC = –0.98%) and subsequently increased during 1994–2013 (APC = 0.95%). For White females, rates increased during 1975–84 (APC = 1.18%), were stable during 1984–99 (APC = –0.34%) and increased during 1999–2013 (APC = 1.29%). In contrast, the rates among Black males decreased during 1975–2013 (APC = –0.45%) and remained unchanged (APC = 0.17%) among Black females in the same time period.
Utilizing the more detailed racial and ethnic categories available within SEER 13 (1992–2013), we further explored the above trends (Figure 1 and Table 1). Among White non-Hispanic and Hispanic men, rates of pancreatic cancer increased during 1992–2013 (APC = 0.84% and 0.73%, respectively), whereas rates for Black and Asian/Pacific Islander males remained unchanged. Incidence rose among White non-Hispanic, White Hispanic and Asian/Pacific Islander females (APC = 0.81%, 0.56% and 1.23%, respectively), but not Black females.
Figure 1.
Pancreatic cancer incidence trends by sex and racial/ethnic group—SEER 9 (1974–2013) for Black and White populations and SEER 13 (1992–2013) for detailed racial/ethnic categories.
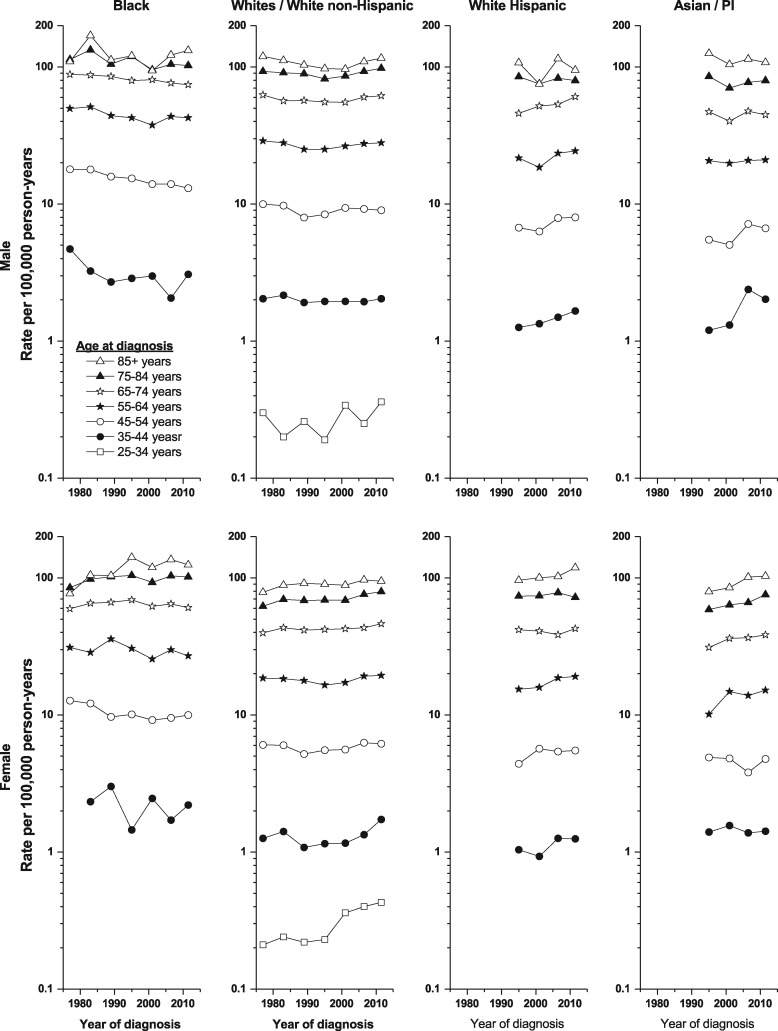
The rising rates among White non-Hispanic males were due to increases among those aged ≥55 years, with APCs of 0.63%, 0.71%, 1.08% and 1.15%, respectively, for the 10-year age groups 55–64 up to 85+ (Table 1 and Figure 2). Among White Hispanic males, rates rose in the two middle age categories—aged 55–64 and 65–74 (APC = 1.20% and 1.32%). There was also an increase in incidence among Asian/Pacific Islander males aged 35–44 (APC = 3.41%) (Table 1).
Figure 2.
Pancreatic cancer incidence trends by sex, racial/ethnic group and age at diagnosis, SEER 9 (1974–91) and 13 (1992–2013).
Within SEER 13, among White non-Hispanic females, the rate rose most rapidly among those aged 25–34 and 35–44 (APC = 4.01% and 2.54%, respectively) and more modestly among those aged 45–84 years (APC = 0.47–1.12%) (Table 1). Age-specific rates also rose among White Hispanic females aged 55–64 years (APC = 1.47%) and among Asian females in the three age groups 55–84 years (APCs = 1.55%, 1.06% and 1.41%, respectively). It was not possible to calculate APCs among White Hispanic, Black and Asian/Pacific Island cases aged 25–34, as there was at least 1 year with zero cases. Further analyses were performed to explore potential cohort effects, but no substantive results were found for either males or females (results not shown).
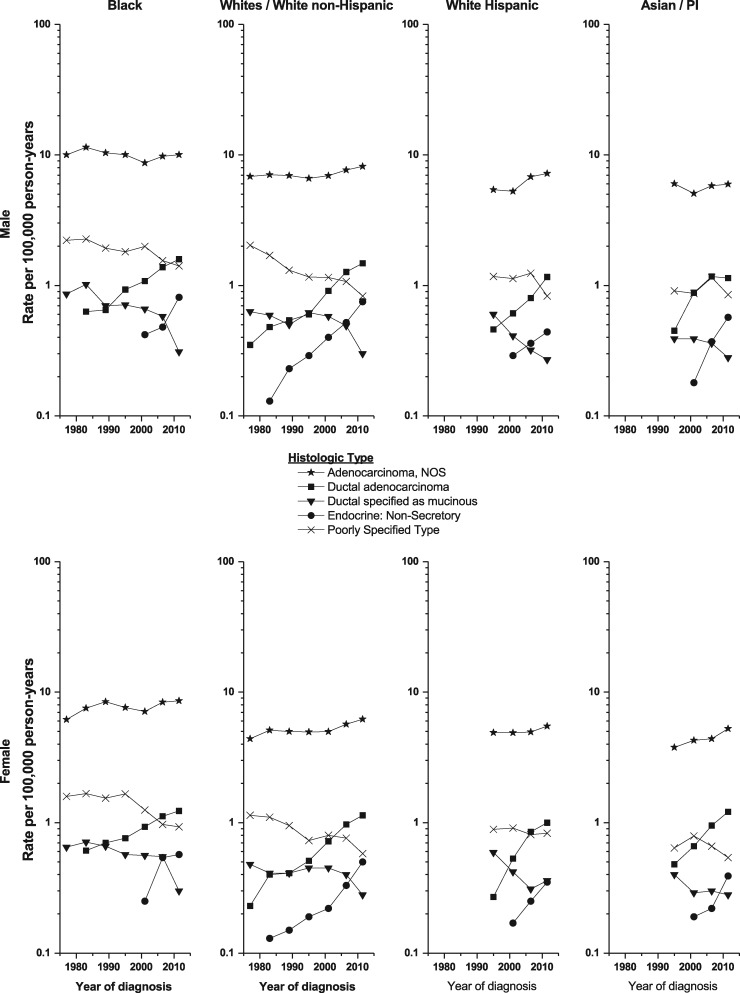
Within SEER 13 (1992–2013), 72 614 (77%) of the 94 501 pancreatic cancer cases diagnosed were microscopically confirmed, which represents an increase compared with the 73% confirmed during the mid-1970s (results not shown). There were five major histologic-type groups with adequate numbers of cases for temporal trends analysis (Table 1 and Figure 3). The numbers of cases were too few for temporal trends analysis by histologic-type groups: cystic (n = 325), ductal specified as arising from an IPMN (211), endocrine: secretory (n = 160) and carcinoid (n = 457) as well for the other specified adenocarcinoma: acinar cell (196), solid pseudopapillary (77), serous (n = 9) and other (243). Rates for the largest histologic type of pancreatic cancer, adenocarcinoma, NOS rose more rapidly during 1992–2013 than the overall pancreatic cancer rate among White non-Hispanic and Hispanic populations. Among the major histologic types, the rates among all racial/ethnic groups rose for ductal adenocarcinoma, NOS not further specified (APC = 3.01% to 5.41% among males and 3.31% to 7.10% among females), but decreased for mucinous adenocarcinoma (APCs = –2.09% to –4.89% among males and –2.28% to –3.14% among females). The rates for endocrine: non-secretory pancreatic cancer rose among all four racial/ethnic groups (Figure 3), and the change in annual incidence for White non-Hispanic and Asian/Pacific Islander males and females was >6.00% (Table 1). There were too few cases among the American Indian/Alaskan Native population to calculate annual incidence by histologic type. Rates for the poorly specified group fell among almost all racial/ethnic groups.
Figure 3.
Pancreatic cancer incidence trends by sex, racial/ethnic group and histological type, SEER 9 (1974–91) and 13 (1992–2013).
Recent rates and male-to-female IRRs
During 2000–13, there were 138 597 cases of pancreatic cancer diagnosed among residents of the SEER 18 (69 049 among males and 69 548 among females) (Table 2)—considerably more than the 95 412 diagnosed among residents of the SEER 13 during the same time period (46 959 among males and 48 454 among females). Despite the larger number of cases among females, rates were higher among males (M/F IRR: 1.28, 95% CI 1.26–1.29). Rates among males compared with females were higher among those aged 35 and older (all IRRs > 1.00); in contrast, rates among women were higher among those younger than age 35 (IRR = age 0–24 years: 0.66 and age 25–34: 0.81). Most cancers were specified only as adenocarcinoma, NOS (∼70%), for which males had an excess (IRR: 1.32). About 15% of pancreatic cancers were ductal adenocarcinomas, with the IRR slightly higher for those specified as arising from an IPMN and slightly lower for those specified as mucinous. In contrast, there was a female excess for ductal adenocarcinoma specified as cystic (IRR: 0.52). About 20% of the cancers were of endocrine origin, and the IRR was higher for non-secretory cancers (IRR 1.47) than for the secretory cancers (IRR: 0.94, CI: 0.69–1.28) and intermediate for the carcinoids (IRR: 1.31). Among the rare other specified adenocarcinoma types, rates among males were elevated for acinar cell adenocarcinoma (IRR: 2.85) and lower for solid pseudopapillary adenocarcinomas (IRR: 0.22).
Table 2.
Pancreatic cancer counts, rates, and male/female incidence rate ratios overall, by age group and histologic type, SEER 18 (2000–2013)1
| Male and Female |
Male |
Female |
Male/Female |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Count | % of total | Count | % of total | Rate | Count | % of total | Rate | IRR | LCI | UCI | |
| Total Pancreas | 138 597 | 100.00 | 69 049 | 100.00 | 13.70 | 69 548 | 100.00 | 10.70 | 1.28 | 1.26 | 1.29 |
| Age Group | |||||||||||
| 0–24 | 160 | 0.12 | 66 | 0.10 | 0.06 | 94 | 0.14 | 0.09 | 0.66 | 0.48 | 0.92 |
| 25–34 | 523 | 0.38 | 237 | 0.34 | 0.29 | 286 | 0.41 | 0.36 | 0.81 | 0.68 | 0.97 |
| 35–44 | 3 069 | 2.22 | 1 726 | 2.50 | 2.02 | 1 343 | 1.93 | 1.56 | 1.29 | 1.20 | 1.39 |
| 45–54 | 13 138 | 9.49 | 7 718 | 11.18 | 9.43 | 5 420 | 7.79 | 6.42 | 1.47 | 1.42 | 1.52 |
| 55–64 | 28 265 | 20.40 | 16 222 | 23.49 | 28.01 | 12 043 | 17.32 | 19.28 | 1.45 | 1.42 | 1.49 |
| 65–74 | 37 137 | 26.80 | 19 587 | 28.37 | 59.17 | 17 550 | 25.23 | 44.93 | 1.32 | 1.29 | 1.34 |
| 75–84 | 38 120 | 27.50 | 17 228 | 24.95 | 90.10 | 20 892 | 30.04 | 75.61 | 1.19 | 1.17 | 1.22 |
| 85 + years | 18 185 | 13.11 | 6 265 | 9.07 | 107.02 | 11 920 | 17.14 | 96.95 | 1.10 | 1.07 | 1.14 |
| Histologic type* | |||||||||||
| Total | 108 952 | 100.00 | 56 202 | 100.00 | 10.89 | 52 750 | 100.00 | 8.29 | 1.31 | 1.30 | 1.33 |
| Adenocarcinoma, NOS | 75 466 | 69.27 | 38 943 | 69.29 | 7.56 | 36 523 | 69.24 | 5.73 | 1.32 | 1.30 | 1.34 |
| Ductal Adenocarcinoma | 11 189 | 10.27 | 5 661 | 10.07 | 1.09 | 5 528 | 10.48 | 0.87 | 1.24 | 1.20 | 1.29 |
| Ductal specified as Mucinous | 4 603 | 4.22 | 2 239 | 3.98 | 0.44 | 2 364 | 4.48 | 0.37 | 1.17 | 1.10 | 1.24 |
| Ductal specified as Papillary | 522 | 0.48 | 256 | 0.46 | 0.05 | 266 | 0.50 | 0.04 | 1.18 | 0.99 | 1.41 |
| Ductal specified as Cystic | 371 | 0.34 | 109 | 0.19 | 0.02 | 262 | 0.50 | 0.04 | 0.52 | 0.41 | 0.65 |
| Endocrine: Non-Secretory | 4 892 | 4.49 | 2 746 | 4.89 | 0.51 | 2 146 | 4.07 | 0.34 | 1.47 | 1.39 | 1.56 |
| Endocrine: Secretory | 174 | 0.16 | 80 | 0.14 | 0.01 | 94 | 0.18 | 0.02 | 0.94 | 0.69 | 1.28 |
| Endocrine: Carcinoid | 756 | 0.69 | 400 | 0.71 | 0.07 | 356 | 0.67 | 0.06 | 1.31 | 1.13 | 1.51 |
| Other Specified Adenocarcinoma: Acinar cell | 305 | 0.28 | 213 | 0.38 | 0.04 | 92 | 0.17 | 0.01 | 2.85 | 2.21 | 3.68 |
| Other Specified Adenocarcinoma: Solid Pseudopapillary | 157 | 0.14 | 27 | 0.05 | 0.00 | 130 | 0.25 | 0.02 | 0.22 | 0.14 | 0.34 |
| Other Specified Adenocarcinoma: Other | 188 | 0.17 | 113 | 0.20 | 0.02 | 75 | 0.14 | 0.01 | 1.94 | 1.41 | 2.67 |
| Specified Non-Carcinomas | 193 | 0.18 | 103 | 0.18 | 0.02 | 90 | 0.17 | 0.01 | 1.35 | 1.00 | 1.82 |
| Poorly Specified Type | 10 136 | 9.30 | 5 312 | 9.45 | 1.06 | 4 824 | 9.15 | 0.75 | 1.41 | 1.36 | 1.47 |
1Rates are per 100 000 person-years, age-adjusted to the 2000 US Standard Population (19 age groups - Census P25–1130).
IRR = Incidence Rate Ratios (based on unrounded rates); LCI, UCI = lower, upper 95% confidence interval.
Confidence intervals (Tiwari mod) are 95% for the ratios.
*Includes microscopically confirmed cases only.
Recent IRRs by racial/ethnic group
Among residents of the SEER 18 during 2000–13, Black males and females had higher pancreatic cancer rates compared with those who were White non-Hispanic (IRR = 1.24 and 1.37, respectively) (Table 3). In contrast, compared with those identified as White non-Hispanic, rates were lower among Asian/Pacific Islander residents (IRR: males 0.78 and females 0.85), White Hispanic males (IRR = 0.88) and American Indian/Alaskan Native males (IRR = 0.79). The rates among White non-Hispanic and White Hispanic females were similar.
Table 3.
Pancreatic cancer counts, rates, and incidence rate ratios by gender and racial/ethnic group, SEER 18 (2000–2013)1
| Total pancreas | Male |
Female |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Count | Rate | IRR | LCI | UCI | Count | Rate | IRR | LCI | UCI | |
| White Non-Hispanic | 50 429 | 13.85 | 49 136 | 10.50 | ||||||
| White Hispanic | 5 967 | 12.25 | 0.88 | 0.86 | 0.91 | 6 393 | 10.73 | 1.02 | 0.99 | 1.05 |
| Black | 7 726 | 17.12 | 1.24 | 1.20 | 1.27 | 8 730 | 14.38 | 1.37 | 1.34 | 1.40 |
| Asian/Pacific Islander | 4 386 | 10.73 | 0.78 | 0.75 | 0.80 | 4 735 | 8.94 | 0.85 | 0.83 | 0.88 |
| American Indian/Alaskan Native (CHSDA)^ | 284 | 10.98 | 0.79 | 0.69 | 0.90 | 295 | 9.47 | 0.90 | 0.80 | 1.02 |
1Rates are per 100 000 person-years, age-adjusted to the 2000 US Standard Population (19 age groups - Census P25–1130).
IRR = Incidence Rate Ratios (based on unrounded rates); LCI, UCI = lower, upper 95% confidence interval.
Confidence intervals (Tiwari mod) are 95% for the ratios.
^Excludes 74 male and 89 female American Indian/Alaskan Native cases who live outside a CHSDA area.
The reference group is White non-Hispanic.
Discussion
Our analysis showed increases in pancreatic cancer rates since the 1990s though 2013 and found important differences by gender, race/ethnicity, age and histologic subtype. Pancreatic cancer incidence rates rose among White non-Hispanic and White Hispanic males and females, and Asian/Pacific Islander females but not Black or American Indian/Alaskan Native individuals, although rates of pancreatic cancer consistently were higher overall among the Black population. The overall increases in incidence were primarily due to pancreatic adenocarcinoma, which represents most cases. However, the most rapid increases were in the non-secretory endocrine cancers but, because of their rarity, they accounted for only a small proportion of the overall increases. Such increases in rates of pancreatic cancer have also been observed internationally27,28 and, although a number of studies have demonstrated increasing incidence for pancreatic cancer by histologic type,29 further exploration of pancreatic cancer by detailed groupings globally would enable a greater understanding of the changing rates of the disease.
Pancreatic cancer rates were notably higher among males than females overall and within most age groups, although a female predominance at ages <35 years was apparent. Similar gender differences have been found in studies that explored pancreatic cancer in Europe and Asia.27,30 Rates for most histologic types were higher among males, except for solid pseudopapillary adenocarcinomas, cystic adenocarcinomas and secretory endocrine cancers. The excess in young women is likely due to solid pseudopapillary pancreatic cancer and cystic adenocarcinoma—the types for which women were found to have an overall excess compared with men. Case series of solid pseudopapillary pancreatic cancer have reported nearly 90% occurring in females with a F:M ratio of close to 10:1 and a majority diagnosed at ages less than 35 years.31,32 Within this current study, we also found the majority of solid pseudopapillary cases were diagnosed in women, with 84% diagnosed at ages <45 years. In our analysis using SEER 18 data, females also have an excess incidence of cystic adenocarcinoma compared with males at all ages except 85 years or older, with peak incidence between 65 and 84 years old (data not shown), and our results are also mostly consistent with previous reports. A systematic review of 52 papers published between 1970 and 2015 showed mucinous cystic neoplasm occurring more in middle-aged women (F:M 20:1) with a peak incidence in the fifth decade of life.33
Rates for secretory endocrine tumours were similar among men and women. Secretory endocrine tumours are functional neuroendocrine tumours and are extremely rare, representing 0.16% of total pancreatic cancer; little is known about their epidemiology and aetiology.34,35 Similarly, acinar cell cancer of the pancreas is a rare tumour, accounting for 0.3% of all histologically confirmed cases. Rare familial susceptibility syndromes may contributing to acinar cell cancer;36 however, beyond this, there is a paucity of evidence related to its causes. The excess acinar cell carcinomas among males is consistent with results of an earlier study that showed a M:F ratio of 2:1.37,38
Cigarette smoking is an established risk factor for pancreatic adenocarcinoma.39,40 The decreases in pancreatic cancer incidence since 1974 through the early 1990s may in part be explained by the dramatic decline in cigarette smoking in the USA since the mid-1960s.41 Smoking rates among White and Black males were 50% and 59%, respectively, in the 1960s and consistently declined to a recent low of 22% among both racial groups.42 Smoking rates among White and Black females exceeded 30% through the mid-1970s and then declined to recent rates of 19% and 15%, respectively.13 Asian females presently have the lowest current smoking rates compared with women of other racial/ethnic groups, whereas Asian men have the second lowest rate after Hispanic men, which may contribute to their lower pancreatic cancer rates.13 The notable declines in smoking rates among all racial/ethnic groups and most age groups, in recent decades, contrasts with the stable or rising rates of pancreatic cancer in recent years. In terms of histologic type, the evidence for a positive association between smoking and adenocarcinoma, NOS and ductal adenocarcinoma appears consistent, whereas limited earlier evidence for cystic or mucinous pancreatic cancer is suggestive of no association.43–45
Whereas smoking has decreased, the prevalence of overweight and obesity have been increasing in the USA since the 1970s13,39 across all racial/ethnic groups42 and may in part account for the recent rising pancreatic cancer rates.46 Because of the rarity of some histologic types, there is a paucity of evidence regarding how overweight and obesity impact disease risk.29 Among males, overweight/obesity rates have been highest among Hispanic (including Black and White) and similar among White non-Hispanic and Black individuals—trends that are in contrast with pancreatic cancer rates (highest among Black, and lower among Hispanic, compared with White non-Hispanic males). The prevalence of overweight adults is substantially higher among Black and Hispanic females compared with White47 and this is reflected in their pancreatic cancer rates. Similarly, Asian populations have the lowest prevalence of overweight and obesity compared with the other racial groups, which may be a factor contributing to their lower pancreatic cancer rates.42 The prevalence of diabetes mellitus has also increased, with rates higher among males than females and among Black and Hispanic individuals compared with non-Hispanic White persons42 and is likely to be another factor contributing to the pancreatic cancer trends in recent decades. Similarly, the prevalence of prediabetes has also been increasing, with an estimated 37% of US adults living with the condition,48 and is thought to also be a risk factor for pancreatic cancer.49 Changing rates and trends of such lifestyle factors likely explain some of the differences in rates of pancreatic cancer by racial/ethnic group.50
Our findings also contrast with an earlier study that showed increasing pancreatic cancer mortality rates among the US Black population from 1970 to the late 1980s (women) or early 1990s (men)—trends that were not observed in our current incidence study.51 As there is no cure for pancreatic adenocarcinoma, which represents the majority of pancreatic cancer, incidence and mortality trends should have similar patterns. The mortality analyses were based on national mortality data so have more power to detect changing trends compared with our analyses of SEER, which are based on smaller proportions of the US population. In addition, the former started in the early 1970s, whereas our analysis started in the mid-1970s. The recent CSR also shows increases in national pancreatic cancer mortality rates during 1975–89 among Black men and 1975–86 among women, in contrast to decreases among Black men and no change in rates among women in SEER 9 incidence during 1975–2013.52
The rates of mucinous adenocarcinoma have declined since 1992 (Figure 3), as have those for cystic mucinous adenocarcinoma (data not shown). Classification of these tumours has also improved since the 1990s.53 Improved diagnostic imaging in recent years may have enabled the detection and treatment of cystic or mucinous precursor lesions prior to malignant transformation.54 Only patients with malignant lesions are included in our analysis. Alternatively, cases that previously were described as invasive mucinous ductal adenocarcinoma more recently may be described only as ductal adenocarcinoma or adenocarcinoma, although this seems unlikely as the tendency generally is towards more detailed specificity.
Finally, non-secretory endocrine tumour rates rose among all demographic groups, with the increases among White non-Hispanic and Asian/Pacific Islander males and females exceeding 6% per year. This may be at least partially related to increases in imaging and subsequent diagnosis. This histologic type includes primarily non-functional neuroendocrine tumours (4.5% of histologically confirmed pancreatic cancer in SEER 18) and are indolent. Hereditary syndromes such as multiple endocrine neoplasia, Type 1 (MEN1) increase susceptibility for neuroendocrine tumours, whereas the aetiology of sporadic tumours is largely unknown.11,29
The key strengths of our study were the use of large population-based datasets, with SEER 18 covering 30% of the US population, which allowed the analysis of recent pancreatic cancer rates by histologic type. Studies that report only total pancreatic cancer may miss important differences for less frequent histologic types—this is particularly relevant when seeking to explore the associations of lifestyle risk factors by detailed histologic type. Our study also has limitations. We can only speculate about the aetiology of the trends—this is particularly true of the rarer types of pancreatic cancer for which risk factors and aetiology are largely unknown. Although we suggest that coinciding changes in the prevalence of cigarette smoking, overweight and obesity, and diabetes may account for pancreatic cancer trends over the past 40 years, unknown factors are also likely to be involved. Heavy alcohol use, high meat and fat intake, and dietary patterns suspected to be associated with exocrine pancreatic cancer that lack reliable long-term data could additionally be contributing to trends in the disease.55–57 The inclusion of only those cases that were microscopically confirmed within our analysis of pancreatic cancer by histologic type increases the validity of case diagnoses but may have excluded cases of more severe or aggressive disease. Nonetheless, the SEER registry data are of good quality, and population-based statistics reveal risk patterns and trends over time.
Conclusion
Pancreatic cancer incidence rates have been increasing in recent years in the USA. Our study reveals differing patterns by sex, race, age and histologic type. The secular trends in the incidence of pancreatic cancer match trends in the prevalence in known risk factors for pancreatic adenocarcinoma such as smoking, overweight and obesity, and diabetes particularly for pancreatic adenocarcinoma, as well as improvements in detection, diagnosis and classification accuracy for rarer tumour types such as endocrine cancers and those specified as cystic.
Funding
This work was supported by the Intramural Research Program of the National Institutes of Health, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Department of Health and Human Services. Parts of this study have been presented as a poster at the 2017 AACR Annual Meeting.
Conflict of interest: The authors have no conflicts of interest to declare.
References
- 1. Howlader N, Noone A-M, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2014. Bethesda: National Cancer Institute, 2017. [Google Scholar]
- 2. Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014. http://cancerres.aacrjournals.org/content/74/11/2913.long (11 March 2017, date last accessed). [DOI] [PubMed] [Google Scholar]
- 3. American Cancer Society. Cancer Facts & Figures 2016. Atlanta, GA: American Cancer Society, 2016. [Google Scholar]
- 4. Kamisawa T, Wood LD, Itoi T, et al. Pancreatic cancer. Lancet Lond Engl 2016;388:73–85. [DOI] [PubMed] [Google Scholar]
- 5. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- 6. Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol 2016;22:9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology 2013;144:1252–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhou J, Enewold L, Stojadinovic A, et al. Incidence rates of exocrine and endocrine pancreatic cancers in the United States. Cancer Causes Control 2010;21:853–61. [DOI] [PubMed] [Google Scholar]
- 9. Siegel RL, Jacobs EJ, Newton CC, et al. Deaths due to cigarette smoking for 12 smoking-related cancers in the United States. JAMA Intern Med 2015;175:1574. [DOI] [PubMed] [Google Scholar]
- 10. Stolzenberg-Solomon RZ, Schairer C, Moore S, et al. Lifetime adiposity and risk of pancreatic cancer in the NIH-AARP Diet and Health Study cohort. Am J Clin Nutr 2013;98:1057–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ben Q, Zhong J, Fei J, et al. Risk factors for sporadic pancreatic neuroendocrine tumors: a case-control study. Sci Rep 2016;6:36073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hassan MM, Phan A, Li D, et al. Risk factors associated with neuroendocrine tumors: a U.S.-based case-control study. Int J Cancer 2008;123:867–73. [DOI] [PubMed] [Google Scholar]
- 13. National Center for Health Statistics (US). Health, United States, 2015: With Special Feature on Racial and Ethnic Health Disparities. Hyattsville, MD: National Center for Health Statistics (US), 2016. http://www.ncbi.nlm.nih.gov/books/NBK367640/ (20 October 2017, date last accessed). [PubMed] [Google Scholar]
- 14. Surveillance Research Program, NCIs Division of Cancer Control and Population Sciences. SEER Registries: Population Characteristics. https://seer.cancer.gov/registries/characteristics.html (20 October 2017, date last accessed). [Google Scholar]
- 15. Jemal A, Simard EP, Dorell C, et al. Annual Report to the Nation on the Status of Cancer, 1975–2009, featuring the burden and trends in human papillomavirus (HPV)-associated cancers and HPV vaccination coverage levels. J Natl Cancer Inst 2013;105:175–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Percy C, Berg J, Thomas L (eds). Manual of Tumor Nomenclature and Coding, 1968 edn New York: American Cancer Society, 1968. [Google Scholar]
- 17. World Health Organization. ICD-O International Classification of Diseases for Oncology, 1st edn Geneva: World Health Organization, 1976. [Google Scholar]
- 18. Percy C, Van Holten V, Muir C (eds). ICD- O International Classification of Diseases for Oncology, 2nd edn Geneva: World Health Organization, 1990. [Google Scholar]
- 19. Fritz A, Percy C, Jack A, et al. ICD-O International Classification of Diseases for Oncology, 3rd edn Geneva: World Health Organization, 2000. [Google Scholar]
- 20. NCI. SEER*Stat—Surveillance Research Program National Cancer Institute SEER*Stat software (seer.cancer.gov/seerstat) version 8.3.2. seer.cancer.gov/seerstat (20 October 2017, date last accessed). [Google Scholar]
- 21. Surveillance, Epidemiology, and End Results (SEER) Program ( www.seer.cancer.gov ) SEER*Stat Database. Incidence—SEER 13 Regs Research Data (with SEER Delay Factors), Nov 2013 Sub (1992–2011) <Katrina/Rita Population Adjustment>—Linked To County Attributes—Total U.S., 1969–2012 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2014, based on the November 2013 submission. [Google Scholar]
- 22. Surveillance, Epidemiology, and End Results (SEER) Program ( www.seer.cancer.gov ) SEER*Stat Database: Incidence—SEER 9 Regs Research Data, Nov 2015 Sub (1973–2013) <Katrina/Rita Population Adjustment>—Linked To County Attributes—Total U.S., 1969–2014 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2016, based on the November 2015 submission. [Google Scholar]
- 23. Devesa SS, Donaldson J, Fears T. Graphical presentation of trends in rates. Am J Epidemiol 1995;141:300–04. [DOI] [PubMed] [Google Scholar]
- 24. Surveillance, Epidemiology, and End Results (SEER) Program ( www.seer.cancer.gov ) SEER*Stat Database: Incidence—SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2015 Sub (1973–2013 varying)—Linked To County Attributes—Total U.S., 1969–2014 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2016, based on the November 2015 submission. [Google Scholar]
- 25. National Cancer Institute, Statistical Methodology and Applications Branch, Surveillance Research Program: Joinpoint Regression Program, Version 4.5.0.1—June 2017. [Google Scholar]
- 26. Kim HJ, Fay MP, Feuer EJ, et al. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 2000;19:335–51. [DOI] [PubMed] [Google Scholar]
- 27. Lin Q-J. Current status and progress of pancreatic cancer in China. World J Gastroenterol 2015;21:7988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wong MCS, Jiang JY, Liang M, et al. Global temporal patterns of pancreatic cancer and association with socioeconomic development. Sci Rep 2017;7:3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Halfdanarson TR, Bamlet WR, McWilliams RR, et al. Risk factors for pancreatic neuroendocrine tumors: a clinic-based case-control study. Pancreas 2014;43:1219–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Carrato A, Falcone A, Ducreux M, et al. A systematic review of the burden of pancreatic cancer in Europe: real-world impact on survival, quality of life and costs. J Gastrointest Cancer 2015;46:201–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Papavramidis T, Papavramidis S. Solid pseudopapillary tumors of the pancreas: review of 718 patients reported in English literature. J Am Coll Surg 2005;200:965–72. [DOI] [PubMed] [Google Scholar]
- 32. Kim CW, Han DJ, Kim J, et al. Solid pseudopapillary tumor of the pancreas: can malignancy be predicted? Surgery 2011;149:625–34. [DOI] [PubMed] [Google Scholar]
- 33. Nilsson LN, Keane MG, Shamali A, et al. Nature and management of pancreatic mucinous cystic neoplasm (MCN): a systematic review of the literature. Pancreatol Off J Int Assoc Pancreatol IAP Al 2016;16:1028–36. [DOI] [PubMed] [Google Scholar]
- 34. Holzheimer RG. (ed). Surgical Treatment: Evidence-Based and Problem-Oriented. München: Zuckschwerdt, 2001. [PubMed] [Google Scholar]
- 35. Asa SL. Pancreatic endocrine tumors. Mod Pathol 2011;24:S66–77. [DOI] [PubMed] [Google Scholar]
- 36. Lowery MA, Klimstra DS, Shia J, et al. Acinar cell carcinoma of the pancreas: new genetic and treatment insights into a rare malignancy. The Oncologist 2011;16:1714–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. La Rosa S, Sessa F, Capella C. Acinar cell carcinoma of the pancreas: overview of clinicopathologic features and insights into the molecular pathology. Front Med 2 2015. http://journal.frontiersin.org/Article/10.3389/fmed.2015.00041/abstract (20 October 2017, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jun S-Y, Hong S-M. Nonductal pancreatic cancers. Surg Pathol Clin 2016;9:581–93. [DOI] [PubMed] [Google Scholar]
- 39. Stolzenberg-Solomon RZ, Amundadottir LT. Epidemiology and inherited predisposition for sporadic pancreatic adenocarcinoma. Hematol Oncol Clin North Am 2015;29:619–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Silverman DT, Hoover RN, Brown LM, et al. Why do Black Americans have a higher risk of pancreatic cancer than White Americans? Epidemiol Camb Mass 2003;14:45–54. [DOI] [PubMed] [Google Scholar]
- 41. Schulte A, Pandeya N, Tran B, et al. Cigarette smoking and pancreatic cancer risk: more to the story than just pack-years. Eur J Cancer Oxf Engl 2014;50:997–1003. [DOI] [PubMed] [Google Scholar]
- 42. National Center for Health Statistics (US).Health, United States, 2014: With Special Feature on Adults Aged 55–64. Hyattsville, MD: National Center for Health Statistics (US), 2015. http://www.ncbi.nlm.nih.gov/books/NBK299348/ (20 October 2017, date last accessed). [PubMed] [Google Scholar]
- 43. Becker AE. Pancreatic ductal adenocarcinoma: risk factors, screening, and early detection. World J Gastroenterol 2014;20:11182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Duell EJ, Holly EA, Bracci PM, et al. A population-based, case-control study of polymorphisms in carcinogen-metabolizing genes, smoking, and pancreatic adenocarcinoma risk. JNCI J Natl Cancer Inst 2002;94:297–306. [DOI] [PubMed] [Google Scholar]
- 45. Rezaee N, Khalifian S, Cameron JL, et al. Smoking is not associated with severe dysplasia or invasive carcinoma in resected intraductal papillary mucinous neoplasms. J Gastrointest Surg 2015;19:656–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Genkinger JM, Spiegelman D, Anderson KE, et al. A pooled analysis of 14 cohort studies of anthropometric factors and pancreatic cancer risk. Int J Cancer 2011;129:1708–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. National Center for Health Statistics (US). Health, United States, 2012: With Special Feature on Emergency Care. Hyattsville, MD: National Center for Health Statistics (US), 2013. http://www.ncbi.nlm.nih.gov/books/NBK148940/ (20 October 2017, date last accessed). [PubMed] [Google Scholar]
- 48. Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta, GA: CDC, U.S. Department of Health and Human Services, 2014. http://www.thefdha.org/pdf/diabetes.pdf (11 March 2017, date last accessed). [Google Scholar]
- 49. Liao W-C, Tu Y-K, Wu M-S, et al. Blood glucose concentration and risk of pancreatic cancer: systematic review and dose-response meta-analysis. BMJ 2015;349:g7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pandol S, Gukovskaya A, Edderkoui M, et al. Epidemiology, risk factors, and the promotion of pancreatic cancer: role of the stellate cell: pancreatic cancer. J Gastroenterol Hepatol 2012;27:127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ma J, Siegel R, Jemal A. Pancreatic cancer death rates by race among US men and women, 1970–2009. JNCI J Natl Cancer Inst 2013;105:1694–1700. [DOI] [PubMed] [Google Scholar]
- 52. Howlader N, Noone A-M, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2013. Bethesda, MD: National Cancer Institute, 2016. [Google Scholar]
- 53. Miura F, Takada T, Amano H, et al. Diagnosis of pancreatic cancer. HPB 2005;8:337–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kaur S, Baine MJ, Jain M, et al. Early diagnosis of pancreatic cancer: challenges and new developments. Biomark Med 2012;6:597–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jiao L, Silverman DT, Schairer C, et al. Alcohol use and risk of pancreatic cancer: the NIH-AARP Diet and Health Study. Am J Epidemiol 2009;169:1043–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jiao L, Mitrou PN, Reedy J, et al. A combined healthy lifestyle score and risk of pancreatic cancer in a large cohort study. Arch Intern Med 2009;169:764–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Arem H, Reedy J, Sampson J, et al. The Healthy Eating Index 2005 and risk for pancreatic cancer in the NIH-AARP Study. JNCI J Natl Cancer Inst 2013;105:1298–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]