Abstract
Nearly 100 years have passed since Frederick Banting and Charles Best first discovered and purified insulin. Their discovery and subsequent improvements revolutionized the treatment of diabetes, and the field continues to move at an ever-faster pace with respect to unique treatments for both type 1 and type 2 diabetes. Despite these advances, we still do not fully understand how apoptosis of the insulin-producing β-cells is triggered, presenting a challenge in the development of preventative measures. In recent years, the process of autophagy has generated substantial interest in this realm due to discoveries highlighting its clear role in the maintenance of cellular homeostasis. As a result, the number of studies focused on islet and β-cell autophagy has increased substantially in recent years. In this review, we will discuss what is currently known regarding the role of β-cell autophagy in type 1 and type 2 diabetes pathogenesis, with an emphasis on new and exciting developments over the past 5 years. Further, we will discuss how these discoveries might be translated into unique treatments in the coming years.
This review discusses recent developments regarding the role of autophagy in diabetes pathogenesis and outlines how these discoveries might be translated into novel treatments in the coming years.
The failure to maintain glucose homeostasis underlies both type 1 diabetes (T1D) and type 2 diabetes (T2D). Although these two forms of diabetes are fundamentally very different, β-cell failure and death play a key role in the pathogenesis of both diseases, leading to hyperglycemia resulting from a reduced capacity to produce insulin. In T1D, β-cell apoptosis is the underlying event leading to dysglycemia, whereas in T2D, insulin resistance leads to an inability to keep up with insulin demand. Endoplasmic reticulum (ER) stress and oxidative stress are major contributors to β-cell dysfunction and death under a variety of conditions found in both T1D and T2D, and a large body of literature is dedicated to understanding what triggers these pathways as well as the endogenous cellular responses to restore homeostasis.
It was recently proposed that autophagy promotes β-cell survival by delaying apoptosis and enabling adaptive responses to mitigate the detrimental effects of ER stress and DNA damage (1), the latter of which has been directly linked to oxidative stress. Thus, identifying mechanisms of β-cell autophagy regulation under these conditions is an important area of research for better understanding β-cell survival and developing therapies that target the β-cell directly. Watada and Fujitani (2) comprehensively reviewed β-cell autophagy in the context of T2D in 2015. The following year, the Nobel Prize in Physiology or Medicine was awarded to Yoshinori Ohsumi for “discoveries of the mechanisms for autophagy” (3), prompting additional enthusiasm for how this fundamental cellular process could play a role in diabetes development. There have been many recent discoveries in this context, yielding several dozen primary research publications on islet and β-cell autophagy since 2015. Therefore, in this review, we will provide an update of the latest research on β-cell autophagy in both T1D and T2D pathogenesis and treatment.
Autophagy and Cellular Homeostasis
Molecular details of the activation and the process of autophagy have been covered in excellent detail in recent reviews (2, 4, 5). Therefore, we will provide an overview of mechanisms of autophagy with a focus on how this fundamental process regulates cellular function, homeostasis, and survival (Fig. 1). Autophagy is a highly conserved catabolic process that enables excess or damaged contents of cells to be recycled, promoting cellular homeostasis and survival under conditions of stress. During autophagy, cellular components are taken up by acid hydrolase-containing lysosomes for degradation. There are four predominant mechanisms for lysosomal uptake of cellular components that have been described, each with its own specific classification: macroautophagy, microautophagy, crinophagy, and chaperone-mediated autophagy (6–8). We will use the term “autophagy” to describe the process of macroautophagy, the focus of most autophagy literature in the β-cell field, which is the formation of autophagosomes containing cellular material that subsequently fuse with lysosomes to degrade the autophagosome cargo. This is a distinct process from microautophagy, where cellular components are directly taken up via invagination of the lysosomal membrane. Crinophagy is a functionally related, targeted mechanism of direct uptake into the lysosome, where secretory vesicles are specifically engulfed by lysosomes. Chaperone-mediated autophagy directly targets cytosolic proteins to lysosomes for degradation via the recognition of specific amino acid sequence motifs by the chaperone protein hsc70 (9).
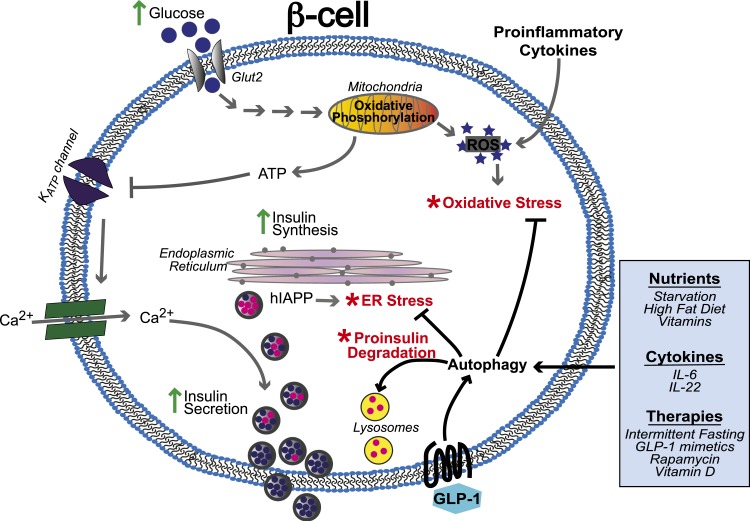
Figure 1.
Autophagy in the context of β-cell dysfunction. The core function of the pancreatic β-cell is to regulate systemic insulin levels in response to blood glucose levels. Metabolism of glucose by the mitochondria generates adenosine triphosphate (ATP) and reactive oxygen species (ROS) that are important signals for glucose-stimulated insulin secretion. Red asterisks indicate points where the system can get overwhelmed or defects can lead to cellular stress that can be resolved by autophagy. Hyperglycemia can lead to excessive ROS levels leading to oxidative stress, and the increased burden of chronically high levels of insulin secretion on the ER can lead to ER stress. Autophagy is stimulated by oxidative stress and ER stress to protect the β-cell from apoptosis. Autophagy can also be stimulated by external factors such as nutrients, vitamins, glucagonlike peptide-1 (GLP-1), and cytokines. Defects in autophagy can lead to a buildup of stress, decrease in insulin secretion, and cell death. IL, interleukin.
Autophagy can be selective or nonselective. Nonselective autophagy is a bulk degradative type of autophagy in which autophagosomes simply engulf materials in their surrounding environment (10). Nutrient deprivation often stimulates this type of autophagy in which nearly any component of the cytoplasm can be degraded. Autophagy can also be selective, in which specific targets are recruited for degradation by autophagosomes via interactions with autophagy adaptor proteins (sometimes referred to as autophagy receptors) such as p62 (SQSTM1). Autophagy adaptors bind to specific ubiquitinated target proteins or organelles and recruit them to autophagosomes through the interaction of the autophagy adaptor with LC3 molecules located in the phagophore membrane. More than 15 types of selective autophagy have been described, often functioning to maintain or restore cellular homeostasis under stress conditions, and these are defined by the specific target being degraded (11). For example, lipophagy is the autophagic degradation of lipid droplets, mitophagy is the degradation of mitochondria, ER-phagy is the recycling of the ER, and proteaphagy is the degradation of the proteasome by autophagy. In response to nutrient deprivation, upregulation of lipophagy, ER-phagy, and proteaphagy functions to provide energy and nutrients, as well as counteract stress induced by starvation (12–16). Likewise, mitophagy prevents and ameliorates oxidative cell stress through the degradation of dysfunctional mitochondria. In an environment of oxidative stress, such as those observed in the pathogenesis of both T1D and T2D, selective autophagy can also mediate the activation of the antioxidant response through targeted degradation of a key inhibitor of the antioxidant response, KEAP1 (17).
The autophagic response to cellular stressors such as the lack of amino acids, growth factors, oxygen, or adenosine triphosphate (ATP) is primarily integrated through the mammalian target of rapamycin complex 1 (mTORC1) signaling pathway (18–20) (Fig. 2). Under normal conditions, mTORC1 is active and negatively regulates autophagy initiation through inhibitory phosphorylation of proteins in the ULK1 complex. There are multiple upstream regulators that function to convey nutrient sensing signals through mTORC1 in the regulation of autophagy (18). Under conditions of starvation or energy depletion, adenosine 5′-monophosphate–activated protein kinase responds to changes in the cellular ATP/adenosine 5′-diphosphate ratio and activates autophagy through the inhibition of mTORC1. In response to starvation and hypoxia, the deacetylase SIRT1 activates autophagy through several mechanisms, including the inhibition of mTORC1 and the deacetylation of several components of the autophagy machinery (Atg5, Atg7, and LC3) (18, 21–23). Importantly, polymorphisms in SIRT1 have been associated with increased incidence of T1D (24), suggesting the importance of this pathway in β-cell survival. Several additional factors that regulate autophagy through mTORC1 in response to environmental cues include AKT and ERK1/2 that sense growth factors, as well as Rag guanosine triphosphatases that respond to amino acid levels (18). Thus, the inhibition of mTORC1 is a critical point upon which multiple types of cellular stressors converge, resulting in the activation of autophagy as an adaptive response to promote cell survival.
Figure 2.
Regulation of β-cell autophagy. Autophagy functions to maintain β-cell homeostasis in response to cellular stressors [e.g., inflammation, reactive oxygen species (ROS), and ER stress] and in changes in the external environment such as growth factors and nutrient levels. β-cell autophagy is regulated at multiple stages, including at phagophore formation and lysosome function, and many of the stressors and environmental conditions regulate the autophagy pathway through multiple molecular mechanisms. Inhibition of mTORC1 is a central mechanism by which many cellular conditions stimulate the activation of autophagy. IFN, interferon; IL, interleukin; TNF, tumor necrosis factor.
Challenges in the Experimental Detection and Measurement of Autophagy
Comprehensive guidelines for the analysis of autophagy have been published (25). The gold standard for autophagy measurement is analysis of the autophagosome component LC3, which becomes processed and lipidated as a necessary step in autophagosome biogenesis. The immature form of LC3 is commonly referred to as LC3-I, whereas the mature form present in autophagosomes is called LC3-II. LC3 processing/modification can be detected by the appearance of a faster-migrating protein species by western blot as well as the appearance of cytosolic puncta that are indicative of autophagosome formation.
However, care must be taken in the interpretation of these measurements as they cannot be used alone to indicate flux through the autophagic pathway. As autophagy proceeds, the autophagosomes containing materials to be degraded fuse with lysosomes containing acid hydrolases that function in organelle and protein breakdown. During this process, the structural machinery that makes up the autophagosome (e.g., LC3) are also degraded. Therefore, experimental methods that give a static picture of levels of players in the autophagy pathway are not suitable for measuring autophagic activity (26, 27). This problem is not, however, intractable, as several solutions exist and have been used effectively. LC3 and the autophagy adaptor p62 can be analyzed in tandem, where one would expect both an increase in the ratio of LC3-II to LC3-I and a reduction in p62 protein levels as an indicator of autophagy. Alternatively, autophagy inhibitors such as bafilomycin A or LC3 can be used to induce a blockade leading to increased LC3-II and p62 under conditions where autophagic flux were intact. More recently, dual-color, pH-sensitive LC3 expression vectors have also been developed that enable analysis of autophagic flux during live-cell imaging, and these methods have been successfully applied in cultured β-cells (28). Detection of autophagy stimulation in vivo remains a challenge. However, this same dual-color LC3 vector has been successfully coupled to intravital microscopy to analyze autophagic flux in the nervous system (29). Therefore, we propose that adaptation of these methods to study β-cell autophagy are now within reach.
Autophagy Is Critical for Proper β-Cell Function and Survival
Autophagy has been proposed to play many important roles in the β-cell, including supporting proper differentiation during development (30) and contributing to β-cell function (31). Importantly, autophagy promotes survival under conditions of β-cell stress that can lead to cell death, including nutrient depletion, oxidative stress, ER stress, mitochondrial damage, and hypoxia (1, 18). Loss of autophagy in the β-cell was described a number of years ago in mice with a β-cell-specific deficiency for a key enzyme in autophagosome biogenesis, autophagy-related (ATG)7. atg7 knockout mice exhibit reduced β-cell mass due to increased apoptosis and decreased proliferation that ultimately leads to reduced insulin secretion and impaired glucose tolerance (32).
Autophagy appears to play an active role in the regulation of insulin homeostasis in addition to its role in β-cell survival. Acute inhibition of β-cell autophagy by small interfering RNA–mediated knockdown of atg5/7 leads to increased secretion of proinsulin and insulin (31). Likewise, stimulation of autophagy through rapamycin-mediated inhibition of mTORC1 also reduces insulin secretion from mouse and human islets (33). The importance of balanced regulation of autophagy for β-cell survival is further highlighted by the observations that both activation and inhibition of autophagy through knockout or hyperactivation of mTORC1, respectively, lead to increased β-cell apoptosis and a decline in β-cell mass (33, 34). These studies collectively show that autophagy plays a critical role in the maintenance of β-cell function and survival through multiple mechanisms.
A Role for Autophagy in the Resolution of β-Cell ER Stress
A functional ER is critical for stimulus-secretion coupling in the β-cell, allowing the β-cell to secrete insulin in response to external stimuli. The high demand of β-cells to synthesize and secrete insulin in response to environmental changes makes them particularly susceptible to ER stress. The β-cell must rapidly ramp up insulin synthesis in response to increases in glucose while maintaining the ability to properly convert proinsulin to the mature form of insulin. Disruptions to this delicate balance can trigger β-cell dysfunction. Under these conditions, if normal ER homeostasis is not restored, then cells eventually undergo apoptosis that is triggered by the unfolded protein response. Chronically elevated glucose and fatty acids associated with T2D have been shown to trigger β-cell ER stress (35–39), and mutations in components of the unfolded protein response pathway have been linked to the development of diabetes in rodents and humans (40, 41).
ER stress has been implicated as one of the mechanisms contributing to the development of human islet amyloid polypeptide (hIAPP) oligomers (42), likely through defects in the processing of the protein precursor, ProhIAPP, to hIAPP. Islet amyloid polypeptide is cosecreted with insulin and plays a key role in glycemic regulation. In humans, but not mice or rats, islet amyloid polypeptide can form amyloid aggregates that have been associated with β-cell death and the development of T2D (43–45). Recent studies provide evidence that autophagy is important for ameliorating the cytotoxic effects of hIAPP. In β-cells, blocking autophagy increased the toxicity of hIAPP (46). In addition, mice expressing hIAPP in autophagy-deficient β-cells developed diabetes, whereas neither hIAPP nor loss of autophagy alone was sufficient to induce disease (47). This suggests that autophagy plays an important role in clearing hIAPP oligomers, thus preventing cytotoxicity, β-cell failure/death, and the development of diabetes.
ER stress results from the accumulation of unfolded and misfolded proteins and can initiate apoptosis if the stress conditions are not resolved. Stimulation of autophagy can degrade the misfolded proteins or dysfunctional regions of the ER, thus restoring structural and functional integrity (48–50). Recent studies suggest that ER stress itself stimulates autophagy, perhaps as a feedback mechanism to protect the cell. For example, Kong et al. (51) found that blocking ER stress blocked autophagy and blocking autophagy increased ER stress. Further, Quan et al. (52) found that loss of autophagy in the β-cell compromised the unfolded protein response, resulting in an increased incidence of diabetes. Although the mechanisms by which autophagy responds to ER stress are not fully understood, some connections are becoming clear. Activation of ER stress leads to the phosphorylation and inactivation of the eukaryotic translation initiation factor eIF2α, thus preventing ribosome assembly and resulting in the global downregulation of protein translation. In parallel, the expression of some protein chaperones and transcription factors like ATF4 and CHOP is upregulated. It has been reported that ATF4 and CHOP regulate the expression of autophagy genes, increasing the transcription of genes required for autophagosome formation and function (53). In addition, Takatani et al. (54) found that signaling through eEF2K, which phosphorylates eEF2, thus contributing to the translation block induced by ER stress, also regulates autophagy. These results provide insight into the potential molecular links between ER stress and the stimulation of autophagy. Further research into how autophagy alleviates ER stress will be required to understand the mechanisms by which autophagy promotes cell survival under conditions of stress.
The Oxidative Stress and Autophagy Feedback Loop
β-Cells are also highly susceptible to oxidative stress. Interestingly, some of the same proteins involved in ER stress response, such as eIF2α, have also been reported to play a role in the response to oxidative stress (55). Reactive oxygen species (ROS) are generated during the metabolism of glucose and serve as important signaling messengers to trigger insulin secretion and β-cell expansion in response to elevated levels of glucose (56, 57). However, chronic exposure to ROS under hyperglycemic conditions can lead to cellular damage, impaired glucose-stimulated insulin secretion, and, eventually, cell death (58). Thus, hyperglycemia during diabetes pathogenesis can exacerbate β-cell apoptosis through ROS-driven oxidative stress.
The transcription factor NRF2 is a key player in the antioxidant pathway and has been shown to have a cytoprotective function in the β-cell, in part through the stimulation of autophagy. Activation of NRF2 stimulates the expression of its antioxidant factor target genes in human islets and increases survival of the islets following induction of oxidative stress (59). Similarly, in mouse models, induction of NRF2 protects from oxidative stress and has been shown to prevent diabetes, whereas knockout of NRF2 in the β-cell resulted in decreased expression of antioxidant genes, decreased β-cell mass, and impaired glucose tolerance (60, 61). NRF2 expression is increased early in diabetes in human and rodent islets and functions to reduce apoptosis to attempt to preserve β-cell mass and maintain glucose homeostasis (62). In rat islets, NRF2-mediated protection from apoptosis is associated with the stimulation of autophagy (62). NRF2-regulated antioxidant response elements have also been identified in the promoters of at least nine genes whose products function in autophagy, including the autophagy chaperone p62 that mediates the recruitment of targets to the autophagosome (63, 64). Autophagy can also promote NRF2 activation through p62, which disrupts the interaction between NRF2 and its inhibitor, KEAP1, targeting KEAP1 to the autophagosome for degradation (65, 66). Thus, autophagy and the antioxidant response participate in a feedback loop in which they mutually reinforce one another to promote cellular homeostasis and survival.
Autophagy also combats oxidative stress through the degradation of dysfunctional mitochondria via mitophagy. PINK1-PARKIN-mediated mitophagy is stimulated by moderate elevations in ROS that lead to a reduction in mitochondrial membrane potential (67–69). The kinase PINK1 accumulates on the outer membrane of depolarized mitochondria, resulting in the recruitment of its target, the E3 ubiquitin ligase PARKIN. PARKIN then ubiquitinates multiple proteins in the outer mitochondrial membrane, leading to the recruitment of proteasomes and autophagosomes for degradation of the mitochondria. Hyperglycemia and glucolipotoxicity can impair mitophagy. Hyperglycemia can stimulate high levels of ROS that overwhelm the mitophagy machinery, resulting in accumulation of dysfunctional mitochondria (70). Glucolipotoxicity also causes accumulation of cytoplasmic p53, which blocks the mitochondrial translocation of PARKIN and inhibits mitophagy (71). Disruption of mitophagy in rodent β-cells can then lead to impaired insulin secretion (72, 73). Human subjects with T2D exhibit decreased expression of components of the mitophagy pathway (74), and mutations in genes whose products function in mitophagy, including PINK1, PARKIN, CLEC16A, and PDX1, have been associated with both T1D and T2D in humans (72, 75–77). One of these mitophagy components, CLEC16a, plays a key role in the proteasome-mediated degradation of PARKIN. Mice with pancreas-specific knockout of clec16a have abnormal mitochondrial function and reduced glucose-stimulated insulin secretion, and humans with a T1D-associated SNP in the CLEC16a gene have reduced insulin secretion (75). PDX1, a T2D-associated gene, regulates expression of clec16a, and loss of PDX1 leads to a blockade of mitophagy in which autophagosomes containing mitochondria fail to fuse with lysosomes (76). Thus, defects in multiple aspects of mitophagy have been shown to disrupt β-cell function and lead to increased diabetes risk. Together, these data highlight the important role that autophagy plays as an endogenous adaptive mechanism to maintain β-cell function, prevent oxidative stress, and, ultimately, protect against diabetes development.
Extracellular Signals That Regulate β-Cell Autophagy
Multiple factors have been reported to stimulate autophagy in the β-cell, including dietary components, cytokines, and GLP-1. Importantly, although context is important, all of these factors may contribute to the regulation of β-cell autophagy during both T1D and T2D pathogenesis. Starvation and amino acid deprivation are well-known activators of autophagy in multiple cell types. However, there have been mixed reports about whether nutrient deprivation induces β-cell autophagy. Fasting and calorie-restricted diet has been shown to stimulate autophagy both in vitro and in vivo in some reports (78–81) but not in others (80, 82). It is possible that these discrepancies could be due to differences in duration of starvation and in the type of autophagy that is stimulated. Studies reporting stimulation of autophagy by nutrient deprivation used longer durations of starvation than studies that did not observe induction of autophagy. In addition, Goginashvili et al. (82) observed that starvation may actually inhibit autophagy and, instead, stimulate crinophagy, in which insulin granules directly fuse to lysosomes. Therefore, perhaps short periods of nutrient deprivation may stimulate β-cell crinophagy, whereas longer durations of starvation may be required to stimulate classical macroautophagy in the β-cell.
Excess lipids appear to play a role in autophagy regulation, with varying effects that are context dependent. For example, autophagy protects against β-cell apoptosis induced solely by fatty acids such as palmitate and cholesterol in vitro (83–85), whereas excess fatty acids and glucose that generate conditions of glucolipotoxicity can inhibit β-cell autophagic flux and lead to cell death (86–88). This inhibition of autophagy is likely due to lysosomal defects under these conditions, as reacidification of lysosomes using photoactivatable nanoparticles was able to restore autophagic flux in cells exposed to lipotoxicity (88). Multiple studies have also reported that mice and rats fed high-fat diets (HFDs) exhibit upregulated autophagy in the β-cell (78, 80, 89–91), and inhibition of β-cell autophagy exacerbated the metabolic defects caused by a combined high-fat and high-glucose diet (91). Together, these data suggest that chronic, excessive exposure to glucose and fatty acids may block the endogenous adaptive mechanism by which β-cells would protect themselves from these sources of stress and toxicity, which then contributes to the development of diabetes.
Interestingly, both excess and restricted caloric intake appear to stimulate autophagy in the β-cell. Gao et al. (89) performed an interesting study combining these observations by switching the caloric content of the food provided to mice and measuring autophagy. When mice were switched from HFDs to a calorie-restricted diet or from normal chow to a calorie-restricted diet, autophagy markers like LC3II/LC3I ratio and p62 degradation were stimulated to the same degree as a continuous HFD. However, mice that were switched to standard chow diets exhibited lower levels of autophagy markers. Interestingly, a recent study demonstrated that activation of the antioxidant factor NRF2 is critical for repair of the β-cell after short-term HFD and subsequent return to chow (92). Thus, it appears that cells respond to stress from drastic changes in nutrient levels by activating autophagy to promote cell survival, and NRF2-mediated effects may play a key role in this process.
An active area of diabetes research focuses on identifying dietary components that can be modulated and thus developed into diabetes therapies. Several recent studies have identified specific vitamins and other dietary components that promote autophagy in β-cells and play a protective role in stress response using both T1D and T2D models. For example, it was recently reported that autophagy stimulated by kaempferol, a flavonol found in fruit and vegetables, could exert a cytoprotective effect against exposure to palmitic acid, which is often used to mimic lipotoxic conditions observed in T2D (93). Alternatively, the selective β-cell toxin streptozotocin (STZ) has relevance to T1D due to its propensity to initiate β-cell oxidative damage and lead to immune infiltration that exacerbates β-cell death. Recent work demonstrated that vitamin D supplementation reduced the incidence of diabetes in STZ-treated mice and protected STZ-treated β-cells in vitro from apoptosis while coordinately upregulating expression of autophagy genes (94). Similarly, Hwang et al. (95) found that omega-3 fatty acids increased autophagy and protected fat-1 mice against STZ-induced β-cell death. These results suggest that the protective roles of vitamin D and omega-3 fatty acids in the STZ model of diabetes function, at least in part, through the stimulation of autophagy. Collectively, these data also suggest that specific nutrients or antioxidants present in foods could be selectively used to stimulate β-cell autophagy. Future developments in the field with respect to diet modulation will likely yield exciting results.
Proinflammatory cytokines such as tumor necrosis factor-α, interleukin (IL)-1β, and interferon (IFN)-γ have been linked to T1D pathogenesis through mechanisms involving elevated ROS levels and the induction of ER stress (40, 96–100). IL-1β and IFN-γ were recently shown to stimulate early phases of autophagy through ER stress–dependent activation of adenosine 5′-monophosphate–activated protein kinase while also inhibiting β-cell autophagic flux due to impaired lysosomal function, which contributed to β-cell apoptosis (101). However, select cytokines have also recently been shown to stimulate autophagy and play a protective role in β-cell survival. For example, interleukin-22 stimulates autophagy and protects cells from palmitate-induced damage caused by ER stress (102). We have also recently found that IL-6 stimulates autophagy under basal conditions and protects β-cells from apoptosis induced by tumor necrosis factor-α, IL-1β, and IFN-γ (103). These protective roles of cytokines are somewhat surprising given the evidence, especially in the case of IL-6, for elevation in both T1D and T2D (104, 105). Evidence is building to suggest that the proinflammatory vs anti-inflammatory effects of cytokines are context dependent (106), though it is still unclear how this switch in function is regulated. One consideration may be whether the cytokine is produced by the islet, immune cells, or elsewhere, as the source of the cytokine could affect its posttranslational modifications and/or the expression of other factors and signaling pathways. It is also possible that the duration of exposure to the cytokine could affect its proinflammatory or anti-inflammatory consequences and whether it stimulates autophagy. Thus, the definitive effects of cytokine production on autophagy and in the pathogenesis of diabetes is not completely clear, and additional studies must be undertaken to identify their role.
Glucagonlike peptide-1 (GLP-1) is a new player in the field of autophagy. In addition to its critical role in stimulating insulin secretion in response to glucose, GLP-1 has also been shown to play a role in increasing β-cell proliferation (107). A number of recent reports in multiple models of diabetes have shown that the protective role of GLP-1 on the β-cell is mediated in part by the stimulation of autophagy. The GLP-1 receptor agonists exendin-4 and liraglutide have been shown to stimulate autophagy in INS-1 β-cells and isolated islets and protect from cell death induced by glucolipotoxicity and fatty acid treatment, respectively (28, 108). In vivo studies with mice fed HFDs and rats with tacrolimus-induced diabetes showed similar results in which GLP-1 receptor agonists or blockade of the GLP-1 inhibitor DPP-4 stimulated autophagy and improved β-cell function (108–110). In a few of these models of diabetes (glucolipotoxicity in β-cells and islets, as well as in rats with tacrolimus-induced diabetes), it was shown that defects in lysosomal function and autophagy contributed to the β-cell dysfunction and death and that the ability of GLP-1 to restore normal lysosomal activity and autophagy was required for its protective effect in the β-cell (28, 109). Interestingly, the cytokine IL-6 has been shown to regulate α-cell GLP-1 production (111), and we have observed that IL-6 is also able to stimulate β-cell autophagy (103). Given the overlapping stimulation of β-cell autophagy by both IL-6 and GLP-1 and their regulatory links, further exploration of this pathway will certainly yield exciting data.
Targeting Autophagy for Diabetes Therapy
The established role of autophagy in maintaining β-cell homeostasis under conditions of stress makes components of this pathway attractive targets for the prevention and treatment of diabetes. Intervention early in the development of diabetes could help patients preserve β-cell mass and prevent progression of the disease. As outlined above, dietary modulation could provide one feasible route for prevention and or treatment. In the case of T2D, caloric restriction has clearly established clinical benefits for reducing dysglycemia. However, studies of dietary modulation in individuals at risk for development of T1D are continually developing and have shown promise. Recently, Cheng et al. (112) demonstrated that a fasting-mimicking diet could promote the regeneration of β-cells in both T2D and T1D mouse models. Parallel “fasting” experiments in islets from human organ donors with T1D were also able to restore insulin production, suggesting that dietary modulation in humans might have similar effects. This effect could be mimicked by simultaneous inhibition of the autophagy inhibitors mTORC1 and PKA, prompting the hypothesis that autophagy may play a role in this reprogramming of islets that restored insulin production to type 1 diabetic islets. In support of this, other studies have shown a role for autophagy and mTOR modulation in β-cell differentiation and islet reprogramming and maturation, respectively (30, 113–115). The use of specific dietary components such as vitamin D, as outlined previously, may also prove useful and is currently under investigation to determine if the addition of vitamin D to standard insulin therapy for patients with T1D could have beneficial effects on residual β-cell function (e.g., ClinicalTrials.gov no. NCT03046927). Given the central role of autophagy in coordinating cell function with cues from the external environment, future research into the identification of dietary components that stimulate autophagy and protect pancreatic β-cells may yield unique treatments for diabetes.
Metformin, rosiglitazone, and GLP-1 mimetics are all highly effective treatments for diabetes currently on the market that have been shown to stimulate autophagy in the β-cell. Metformin stimulates β-cell autophagy and prevents apoptosis under conditions of lipotoxicity in vitro (116), suggesting that some of the beneficial effects of metformin on islet function could be mediated through autophagy stimulation in persons with diabetes. Rosiglitazone has also been shown to induce β-cell autophagy and to protect from palmitate-induced apoptosis (117), and although it is less-widely used than GLP-1-based therapies, it is still prescribed by many providers as an effective T2D therapy. GLP-1 mimetics, including both GLP-1 receptor agonists and DPP-IV inhibitors, have also begun to be explored for the treatment of T1D (118). Considering the recent discovery that GLP-1 exerts some of its β-cell protective effects through the stimulation of autophagy, the outcome of clinical studies in this realm will be especially illuminating with respect to T1D pathophysiology.
As discussed above, mTORC1 is a key inhibitor of autophagy. Therefore, there is great interest in determining if inhibition mTORC1 can be developed as a therapy for diabetes. Rapamycin is an mTORC1 inhibitor that is currently used as an immunosuppressant in allogeneic organ transplantation and in the treatment of some types of cancer. Rapamycin has been shown to stimulate β-cell autophagy in vitro and in vivo (119–121), but there are contradictory reports on its effects on the development of diabetes. Several rodent studies have reported that rapamycin decreased weight gain, blood glucose levels, and/or insulin resistance in some diabetic and HFD-fed models (122–127). However, rapamycin treatment resulted in decreased β-cell function and viability in vivo and in vitro (119) and has been widely reported to cause decreased glucose tolerance, increased insulin resistance, and frank diabetes in rodent models, even in some of the same studies where a reduction in adiposity or a stimulation of autophagy was observed (121, 122, 126–131). Further, the incidence of hyperglycemia and new-onset diabetes in rapamycin-treated patients in human cancer clinical trials ranges from 13% to 50% (132). Because of this conflicting evidence, further research is needed to better evaluate the suitability of rapamycin for the treatment of diabetes. Intermittent rapamycin treatment, rather than daily treatment, eliminated the negative metabolic effects of rapamycin treatment while still extending life span in mice (133, 134). Therefore, it is possible that intermittent treatment with rapamycin may be more beneficial in the treatment of diabetes and may be analogous to the benefits of intermittent fasting, which are also, at least partially, mediated through mTORC1 inhibition.
Overall, caution and thoughtful design should be taken in the approach to diabetes therapies that target autophagy. In this review, we described recent results that highlight the cytoprotective role of autophagy in the β-cell; however, none of the treatments being tested or on the horizon specifically target the β-cell. An important consideration for the development of diabetes therapies, which will often be administered chronically over an extended period of time, is that autophagy has been reported to promote the survival of cancerous cells (135), and inhibition of autophagy is of great clinical interest for the treatment of several types of cancer (136, 137). It is critical that efforts to prevent and treat diabetes do not simultaneously promote transformation and/or inappropriate cell growth that have the potential to lead to malignant transformation. More research is needed into the mechanisms underlying the role that autophagy plays in cell survival vs apoptosis to develop therapies that can selectively promote β-cell survival without the risks associated with chronic systemic activation of autophagy.
Acknowledgments
Financial Support: This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant K01DK102492 (to A.K.L.) and startup funds to A.K.L. from the Indiana University School of Medicine and the Herman B. Wells Center for Pediatric Research.
Disclosure Summary:
The authors have nothing to disclose.
Glossary
Abbreviations:
- ATG
autophagy-related
- ATP
adenosine triphosphate
- ER
endoplasmic reticulum
- GLP-1
glucagonlike peptide-1
- HFD
high-fat diet
- hIAPP
human islet amyloid polypeptide
- IFN
interferon
- IL
interleukin
- mTORC1
mammalian target of rapamycin complex 1
- ROS
reactive oxygen species
- STZ
streptozotocin
- T1D
type 1 diabetes
- T2D
type 2 diabetes
References
- 1. Hayes HL, Peterson BS, Haldeman JM, Newgard CB, Hohmeier HE, Stephens SB. Delayed apoptosis allows islet β-cells to implement an autophagic mechanism to promote cell survival. PLoS One. 2017;12(2):e0172567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Watada H, Fujitani Y. Minireview: autophagy in pancreatic β-cells and its implication in diabetes. Mol Endocrinol. 2015;29(3):338–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Levine B, Klionsky DJ. Autophagy wins the 2016 Nobel Prize in Physiology or Medicine: breakthroughs in baker’s yeast fuel advances in biomedical research. Proc Natl Acad Sci USA. 2017;114(2):201–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bento CF, Renna M, Ghislat G, Puri C, Ashkenazi A, Vicinanza M, Menzies FM, Rubinsztein DC. Mammalian autophagy: how does it work? Annu Rev Biochem. 2016;85(1):685–713. [DOI] [PubMed] [Google Scholar]
- 5. Hurley JH, Young LN. Mechanisms of autophagy initiation. Annu Rev Biochem. 2017;86(1):225–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010;221(1):3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weckman A, Di Ieva A, Rotondo F, Syro LV, Ortiz LD, Kovacs K, Cusimano MD. Autophagy in the endocrine glands. J Mol Endocrinol. 2014;52(2):R151–R163. [DOI] [PubMed] [Google Scholar]
- 8. Csizmadia T, Lőrincz P, Hegedűs K, Széplaki S, Lőw P, Juhász G. Molecular mechanisms of developmentally programmed crinophagy in Drosophila. J Cell Biol. 2018;217(1):361–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cuervo AM, Wong E. Chaperone-mediated autophagy: roles in disease and aging. Cell Res. 2014;24(1):92–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yin Z, Pascual C, Klionsky DJ. Autophagy: machinery and regulation. Microb Cell. 2016;3(12):588–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hayat MA. Autophagy: Cancer, Other Pathologies, Inflammation, Immunity, Infection, and Aging. Cambridge, MA: Academic Press; 2016. Human Diseases and Autophagosome; vol 9.
- 12. Hamasaki M, Noda T, Baba M, Ohsumi Y. Starvation triggers the delivery of the endoplasmic reticulum to the vacuole via autophagy in yeast. Traffic. 2005;6(1):56–65. [DOI] [PubMed] [Google Scholar]
- 13. Cohen-Kaplan V, Livneh I, Avni N, Fabre B, Ziv T, Kwon YT, Ciechanover A. p62- and ubiquitin-dependent stress-induced autophagy of the mammalian 26S proteasome. Proc Natl Acad Sci USA. 2016;113(47):E7490–E7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khaminets A, Heinrich T, Mari M, Grumati P, Huebner AK, Akutsu M, Liebmann L, Stolz A, Nietzsche S, Koch N, Mauthe M, Katona I, Qualmann B, Weis J, Reggiori F, Kurth I, Hübner CA, Dikic I. Regulation of endoplasmic reticulum turnover by selective autophagy. Nature. 2015;522(7556):354–358. [DOI] [PubMed] [Google Scholar]
- 15. Singh R, Cuervo AM. Autophagy in the cellular energetic balance. Cell Metab. 2011;13(5):495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Singh R, Cuervo AM. Lipophagy: connecting autophagy and lipid metabolism. Int J Cell Biol. 2012;2012:282041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ichimura Y, Waguri S, Sou YS, Kageyama S, Hasegawa J, Ishimura R, Saito T, Yang Y, Kouno T, Fukutomi T, Hoshii T, Hirao A, Takagi K, Mizushima T, Motohashi H, Lee MS, Yoshimori T, Tanaka K, Yamamoto M, Komatsu M. Phosphorylation of p62 activates the Keap1-Nrf2 pathway during selective autophagy. Mol Cell. 2013;51(5):618–631. [DOI] [PubMed] [Google Scholar]
- 18. Kroemer G, Mariño G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40(2):280–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ravanan P, Srikumar IF, Talwar P. Autophagy: the spotlight for cellular stress responses. Life Sci. 2017;188:53–67. [DOI] [PubMed] [Google Scholar]
- 20. Jung CH, Ro S-H, Cao J, Otto NM, Kim D-H. mTOR regulation of autophagy. FEBS Lett. 2010;584(7):1287–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE, Tsokos M, Alt FW, Finkel T. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci USA. 2008;105(9):3374–3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kume S, Uzu T, Horiike K, Chin-Kanasaki M, Isshiki K, Araki S, Sugimoto T, Haneda M, Kashiwagi A, Koya D. Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney. J Clin Invest. 2010;120(4):1043–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ma L, Fu R, Duan Z, Lu J, Gao J, Tian L, Lv Z, Chen Z, Han J, Jia L, Wang L. Sirt1 is essential for resveratrol enhancement of hypoxia-induced autophagy in the type 2 diabetic nephropathy rat. Pathol Res Pract. 2016;212(4):310–318. [DOI] [PubMed] [Google Scholar]
- 24. Biason-Lauber A, Böni-Schnetzler M, Hubbard BP, Bouzakri K, Brunner A, Cavelti-Weder C, Keller C, Meyer-Böni M, Meier DT, Brorsson C, Timper K, Leibowitz G, Patrignani A, Bruggmann R, Boily G, Zulewski H, Geier A, Cermak JM, Elliott P, Ellis JL, Westphal C, Knobel U, Eloranta JJ, Kerr-Conte J, Pattou F, Konrad D, Matter CM, Fontana A, Rogler G, Schlapbach R, Regairaz C, Carballido JM, Glaser B, McBurney MW, Pociot F, Sinclair DA, Donath MY. Identification of a SIRT1 mutation in a family with type 1 diabetes. Cell Metab. 2013;17(3):448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Acevedo Arozena A, Adachi H, Adams CM, Adams PD, Adeli K, Adhihetty PJ, Adler SG, Agam G, Agarwal R, Aghi MK, Agnello M, Agostinis P, Aguilar PV, Aguirre-Ghiso J, Airoldi EM, Ait-Si-Ali S, Akematsu T, Akporiaye ET, Al-Rubeai M, Albaiceta GM, Albanese C, Albani D, Albert ML, Aldudo J, Algül H, Alirezaei M, Alloza I, Almasan A, Almonte-Beceril M, Alnemri ES, Alonso C, Altan-Bonnet N, Altieri DC, Alvarez S, Alvarez-Erviti L, Alves S, Amadoro G, Amano A, Amantini C, Ambrosio S, Amelio I, Amer AO, Amessou M, Amon A, An Z, Anania FA, Andersen SU, Andley UP, Andreadi CK, Andrieu-Abadie N, Anel A, Ann DK, Anoopkumar-Dukie S, Antonioli M, Aoki H, Apostolova N, Aquila S, Aquilano K, Araki K, Arama E, Aranda A, Araya J, Arcaro A, Arias E, Arimoto H, Ariosa AR, Armstrong JL, Arnould T, Arsov I, Asanuma K, Askanas V, Asselin E, Atarashi R, Atherton SS, Atkin JD, Attardi LD, Auberger P, Auburger G, Aurelian L, Autelli R, Avagliano L, Avantaggiati ML, Avrahami L, Awale S, Azad N, Bachetti T, Backer JM, Bae DH, Bae JS, Bae ON, Bae SH, Baehrecke EH, Baek SH, Baghdiguian S, Bagniewska-Zadworna A, Bai H, Bai J, Bai XY, Bailly Y, Balaji KN, Balduini W, Ballabio A, Balzan R, Banerjee R, Bánhegyi G, Bao H, Barbeau B, Barrachina MD, Barreiro E, Bartel B, Bartolomé A, Bassham DC, Bassi MT, Bast RC Jr, Basu A, Batista MT, Batoko H, Battino M, Bauckman K, Baumgarner BL, Bayer KU, Beale R, Beaulieu JF, Beck GR Jr, Becker C, Beckham JD, Bédard PA, Bednarski PJ, Begley TJ, Behl C, Behrends C, Behrens GM, Behrns KE, Bejarano E, Belaid A, Belleudi F, Bénard G, Berchem G, Bergamaschi D, Bergami M, Berkhout B, Berliocchi L, Bernard A, Bernard M, Bernassola F, Bertolotti A, Bess AS, Besteiro S, Bettuzzi S, Bhalla S, Bhattacharyya S, Bhutia SK, Biagosch C, Bianchi MW, Biard-Piechaczyk M, Billes V, Bincoletto C, Bingol B, Bird SW, Bitoun M, Bjedov I, Blackstone C, Blanc L, Blanco GA, Blomhoff HK, Boada-Romero E, Böckler S, Boes M, Boesze-Battaglia K, Boise LH, Bolino A, Boman A, Bonaldo P, Bordi M, Bosch J, Botana LM, Botti J, Bou G, Bouché M, Bouchecareilh M, Boucher MJ, Boulton ME, Bouret SG, Boya P, Boyer-Guittaut M, Bozhkov PV, Brady N, Braga VM, Brancolini C, Braus GH, Bravo-San Pedro JM, Brennan LA, Bresnick EH, Brest P, Bridges D, Bringer MA, Brini M, Brito GC, Brodin B, Brookes PS, Brown EJ, Brown K, Broxmeyer HE, Bruhat A, Brum PC, Brumell JH, Brunetti-Pierri N, Bryson-Richardson RJ, Buch S, Buchan AM, Budak H, Bulavin DV, Bultman SJ, Bultynck G, Bumbasirevic V, Burelle Y, Burke RE, Burmeister M, Bütikofer P, Caberlotto L, Cadwell K, Cahova M, Cai D, Cai J, Cai Q, Calatayud S, Camougrand N, Campanella M, Campbell GR, Campbell M, Campello S, Candau R, Caniggia I, Cantoni L, Cao L, Caplan AB, Caraglia M, Cardinali C, Cardoso SM, Carew JS, Carleton LA, Carlin CR, Carloni S, Carlsson SR, Carmona-Gutierrez D, Carneiro LA, Carnevali O, Carra S, Carrier A, Carroll B, Casas C, Casas J, Cassinelli G, Castets P, Castro-Obregon S, Cavallini G, Ceccherini I, Cecconi F, Cederbaum AI, Ceña V, Cenci S, Cerella C, Cervia D, Cetrullo S, Chaachouay H, Chae HJ, Chagin AS, Chai CY, Chakrabarti G, Chamilos G, Chan EY, Chan MT, Chandra D, Chandra P, Chang CP, Chang RC, Chang TY, Chatham JC, Chatterjee S, Chauhan S, Che Y, Cheetham ME, Cheluvappa R, Chen CJ, Chen G, Chen GC, Chen G, Chen H, Chen JW, Chen JK, Chen M, Chen M, Chen P, Chen Q, Chen Q, Chen SD, Chen S, Chen SS, Chen W, Chen WJ, Chen WQ, Chen W, Chen X, Chen YH, Chen YG, Chen Y, Chen Y, Chen Y, Chen YJ, Chen YQ, Chen Y, Chen Z, Chen Z, Cheng A, Cheng CH, Cheng H, Cheong H, Cherry S, Chesney J, Cheung CH, Chevet E, Chi HC, Chi SG, Chiacchiera F, Chiang HL, Chiarelli R, Chiariello M, Chieppa M, Chin LS, Chiong M, Chiu GN, Cho DH, Cho SG, Cho WC, Cho YY, Cho YS, Choi AM, Choi EJ, Choi EK, Choi J, Choi ME, Choi SI, Chou TF, Chouaib S, Choubey D, Choubey V, Chow KC, Chowdhury K, Chu CT, Chuang TH, Chun T, Chung H, Chung T, Chung YL, Chwae YJ, Cianfanelli V, Ciarcia R, Ciechomska IA, Ciriolo MR, Cirone M, Claerhout S, Clague MJ, Clària J, Clarke PG, Clarke R, Clementi E, Cleyrat C, Cnop M, Coccia EM, Cocco T, Codogno P, Coers J, Cohen EE, Colecchia D, Coletto L, Coll NS, Colucci-Guyon E, Comincini S, Condello M, Cook KL, Coombs GH, Cooper CD, Cooper JM, Coppens I, Corasaniti MT, Corazzari M, Corbalan R, Corcelle-Termeau E, Cordero MD, Corral-Ramos C, Corti O, Cossarizza A, Costelli P, Costes S, Cotman SL, Coto-Montes A, Cottet S, Couve E, Covey LR, Cowart LA, Cox JS, Coxon FP, Coyne CB, Cragg MS, Craven RJ, Crepaldi T, Crespo JL, Criollo A, Crippa V, Cruz MT, Cuervo AM, Cuezva JM, Cui T, Cutillas PR, Czaja MJ, Czyzyk-Krzeska MF, Dagda RK, Dahmen U, Dai C, Dai W, Dai Y, Dalby KN, Dalla Valle L, Dalmasso G, D’Amelio M, Damme M, Darfeuille-Michaud A, Dargemont C, Darley-Usmar VM, Dasarathy S, Dasgupta B, Dash S, Dass CR, Davey HM, Davids LM, Dávila D, Davis RJ, Dawson TM, Dawson VL, Daza P, de Belleroche J, de Figueiredo P, de Figueiredo RC, de la Fuente J, De Martino L, De Matteis A, De Meyer GR, De Milito A, De Santi M, de Souza W, De Tata V, De Zio D, Debnath J, Dechant R, Decuypere JP, Deegan S, Dehay B, Del Bello B, Del Re DP, Delage-Mourroux R, Delbridge LM, Deldicque L, Delorme-Axford E, Deng Y, Dengjel J, Denizot M, Dent P, Der CJ, Deretic V, Derrien B, Deutsch E, Devarenne TP, Devenish RJ, Di Bartolomeo S, Di Daniele N, Di Domenico F, Di Nardo A, Di Paola S, Di Pietro A, Di Renzo L, DiAntonio A, Díaz-Araya G, Díaz-Laviada I, Diaz-Meco MT, Diaz-Nido J, Dickey CA, Dickson RC, Diederich M, Digard P, Dikic I, Dinesh-Kumar SP, Ding C, Ding WX, Ding Z, Dini L, Distler JH, Diwan A, Djavaheri-Mergny M, Dmytruk K, Dobson RC, Doetsch V, Dokladny K, Dokudovskaya S, Donadelli M, Dong XC, Dong X, Dong Z, Donohue TM Jr, Doran KS, D’Orazi G, Dorn GW II, Dosenko V, Dridi S, Drucker L, Du J, Du LL, Du L, du Toit A, Dua P, Duan L, Duann P, Dubey VK, Duchen MR, Duchosal MA, Duez H, Dugail I, Dumit VI, Duncan MC, Dunlop EA, Dunn WA Jr, Dupont N, Dupuis L, Durán RV, Durcan TM, Duvezin-Caubet S, Duvvuri U, Eapen V, Ebrahimi-Fakhari D, Echard A, Eckhart L, Edelstein CL, Edinger AL, Eichinger L, Eisenberg T, Eisenberg-Lerner A, Eissa NT, El-Deiry WS, El-Khoury V, Elazar Z, Eldar-Finkelman H, Elliott CJ, Emanuele E, Emmenegger U, Engedal N, Engelbrecht AM, Engelender S, Enserink JM, Erdmann R, Erenpreisa J, Eri R, Eriksen JL, Erman A, Escalante R, Eskelinen EL, Espert L, Esteban-Martínez L, Evans TJ, Fabri M, Fabrias G, Fabrizi C, Facchiano A, Færgeman NJ, Faggioni A, Fairlie WD, Fan C, Fan D, Fan J, Fang S, Fanto M, Fanzani A, Farkas T, Faure M, Favier FB, Fearnhead H, Federici M, Fei E, Felizardo TC, Feng H, Feng Y, Feng Y, Ferguson TA, Fernández ÁF, Fernandez-Barrena MG, Fernandez-Checa JC, Fernández-López A, Fernandez-Zapico ME, Feron O, Ferraro E, Ferreira-Halder CV, Fesus L, Feuer R, Fiesel FC, Filippi-Chiela EC, Filomeni G, Fimia GM, Fingert JH, Finkbeiner S, Finkel T, Fiorito F, Fisher PB, Flajolet M, Flamigni F, Florey O, Florio S, Floto RA, Folini M, Follo C, Fon EA, Fornai F, Fortunato F, Fraldi A, Franco R, Francois A, François A, Frankel LB, Fraser ID, Frey N, Freyssenet DG, Frezza C, Friedman SL, Frigo DE, Fu D, Fuentes JM, Fueyo J, Fujitani Y, Fujiwara Y, Fujiya M, Fukuda M, Fulda S, Fusco C, Gabryel B, Gaestel M, Gailly P, Gajewska M, Galadari S, Galili G, Galindo I, Galindo MF, Galliciotti G, Galluzzi L, Galluzzi L, Galy V, Gammoh N, Gandy S, Ganesan AK, Ganesan S, Ganley IG, Gannagé M, Gao FB, Gao F, Gao JX, García Nannig L, García Véscovi E, Garcia-Macía M, Garcia-Ruiz C, Garg AD, Garg PK, Gargini R, Gassen NC, Gatica D, Gatti E, Gavard J, Gavathiotis E, Ge L, Ge P, Ge S, Gean PW, Gelmetti V, Genazzani AA, Geng J, Genschik P, Gerner L, Gestwicki JE, Gewirtz DA, Ghavami S, Ghigo E, Ghosh D, Giammarioli AM, Giampieri F, Giampietri C, Giatromanolaki A, Gibbings DJ, Gibellini L, Gibson SB, Ginet V, Giordano A, Giorgini F, Giovannetti E, Girardin SE, Gispert S, Giuliano S, Gladson CL, Glavic A, Gleave M, Godefroy N, Gogal RM Jr, Gokulan K, Goldman GH, Goletti D, Goligorsky MS, Gomes AV, Gomes LC, Gomez H, Gomez-Manzano C, Gómez-Sánchez R, Gonçalves DA, Goncu E, Gong Q, Gongora C, Gonzalez CB, Gonzalez-Alegre P, Gonzalez-Cabo P, González-Polo RA, Goping IS, Gorbea C, Gorbunov NV, Goring DR, Gorman AM, Gorski SM, Goruppi S, Goto-Yamada S, Gotor C, Gottlieb RA, Gozes I, Gozuacik D, Graba Y, Graef M, Granato GE, Grant GD, Grant S, Gravina GL, Green DR, Greenhough A, Greenwood MT, Grimaldi B, Gros F, Grose C, Groulx JF, Gruber F, Grumati P, Grune T, Guan JL, Guan KL, Guerra B, Guillen C, Gulshan K, Gunst J, Guo C, Guo L, Guo M, Guo W, Guo XG, Gust AA, Gustafsson ÅB, Gutierrez E, Gutierrez MG, Gwak HS, Haas A, Haber JE, Hadano S, Hagedorn M, Hahn DR, Halayko AJ, Hamacher-Brady A, Hamada K, Hamai A, Hamann A, Hamasaki M, Hamer I, Hamid Q, Hammond EM, Han F, Han W, Handa JT, Hanover JA, Hansen M, Harada M, Harhaji-Trajkovic L, Harper JW, Harrath AH, Harris AL, Harris J, Hasler U, Hasselblatt P, Hasui K, Hawley RG, Hawley TS, He C, He CY, He F, He G, He RR, He XH, He YW, He YY, Heath JK, Hébert MJ, Heinzen RA, Helgason GV, Hensel M, Henske EP, Her C, Herman PK, Hernández A, Hernandez C, Hernández-Tiedra S, Hetz C, Hiesinger PR, Higaki K, Hilfiker S, Hill BG, Hill JA, Hill WD, Hino K, Hofius D, Hofman P, Höglinger GU, Höhfeld J, Holz MK, Hong Y, Hood DA, Hoozemans JJ, Hoppe T, Hsu C, Hsu CY, Hsu LC, Hu D, Hu G, Hu HM, Hu H, Hu MC, Hu YC, Hu ZW, Hua F, Hua Y, Huang C, Huang HL, Huang KH, Huang KY, Huang S, Huang S, Huang WP, Huang YR, Huang Y, Huang Y, Huber TB, Huebbe P, Huh WK, Hulmi JJ, Hur GM, Hurley JH, Husak Z, Hussain SN, Hussain S, Hwang JJ, Hwang S, Hwang TI, Ichihara A, Imai Y, Imbriano C, Inomata M, Into T, Iovane V, Iovanna JL, Iozzo RV, Ip NY, Irazoqui JE, Iribarren P, Isaka Y, Isakovic AJ, Ischiropoulos H, Isenberg JS, Ishaq M, Ishida H, Ishii I, Ishmael JE, Isidoro C, Isobe K, Isono E, Issazadeh-Navikas S, Itahana K, Itakura E, Ivanov AI, Iyer AK, Izquierdo JM, Izumi Y, Izzo V, Jäättelä M, Jaber N, Jackson DJ, Jackson WT, Jacob TG, Jacques TS, Jagannath C, Jain A, Jana NR, Jang BK, Jani A, Janji B, Jannig PR, Jansson PJ, Jean S, Jendrach M, Jeon JH, Jessen N, Jeung EB, Jia K, Jia L, Jiang H, Jiang H, Jiang L, Jiang T, Jiang X, Jiang X, Jiang X, Jiang Y, Jiang Y, Jiménez A, Jin C, Jin H, Jin L, Jin M, Jin S, Jinwal UK, Jo EK, Johansen T, Johnson DE, Johnson GV, Johnson JD, Jonasch E, Jones C, Joosten LA, Jordan J, Joseph AM, Joseph B, Joubert AM, Ju D, Ju J, Juan HF, Juenemann K, Juhász G, Jung HS, Jung JU, Jung YK, Jungbluth H, Justice MJ, Jutten B, Kaakoush NO, Kaarniranta K, Kaasik A, Kabuta T, Kaeffer B, Kågedal K, Kahana A, Kajimura S, Kakhlon O, Kalia M, Kalvakolanu DV, Kamada Y, Kambas K, Kaminskyy VO, Kampinga HH, Kandouz M, Kang C, Kang R, Kang TC, Kanki T, Kanneganti TD, Kanno H, Kanthasamy AG, Kantorow M, Kaparakis-Liaskos M, Kapuy O, Karantza V, Karim MR, Karmakar P, Kaser A, Kaushik S, Kawula T, Kaynar AM, Ke PY, Ke ZJ, Kehrl JH, Keller KE, Kemper JK, Kenworthy AK, Kepp O, Kern A, Kesari S, Kessel D, Ketteler R, Kettelhut IdoC, Khambu B, Khan MM, Khandelwal VK, Khare S, Kiang JG, Kiger AA, Kihara A, Kim AL, Kim CH, Kim DR, Kim DH, Kim EK, Kim HY, Kim HR, Kim JS, Kim JH, Kim JC, Kim JH, Kim KW, Kim MD, Kim MM, Kim PK, Kim SW, Kim SY, Kim YS, Kim Y, Kimchi A, Kimmelman AC, Kimura T, King JS, Kirkegaard K, Kirkin V, Kirshenbaum LA, Kishi S, Kitajima Y, Kitamoto K, Kitaoka Y, Kitazato K, Kley RA, Klimecki WT, Klinkenberg M, Klucken J, Knævelsrud H, Knecht E, Knuppertz L, Ko JL, Kobayashi S, Koch JC, Koechlin-Ramonatxo C, Koenig U, Koh YH, Köhler K, Kohlwein SD, Koike M, Komatsu M, Kominami E, Kong D, Kong HJ, Konstantakou EG, Kopp BT, Korcsmaros T, Korhonen L, Korolchuk VI, Koshkina NV, Kou Y, Koukourakis MI, Koumenis C, Kovács AL, Kovács T, Kovacs WJ, Koya D, Kraft C, Krainc D, Kramer H, Kravic-Stevovic T, Krek W, Kretz-Remy C, Krick R, Krishnamurthy M, Kriston-Vizi J, Kroemer G, Kruer MC, Kruger R, Ktistakis NT, Kuchitsu K, Kuhn C, Kumar AP, Kumar A, Kumar A, Kumar D, Kumar D, Kumar R, Kumar S, Kundu M, Kung HJ, Kuno A, Kuo SH, Kuret J, Kurz T, Kwok T, Kwon TK, Kwon YT, Kyrmizi I, La Spada AR, Lafont F, Lahm T, Lakkaraju A, Lam T, Lamark T, Lancel S, Landowski TH, Lane DJ, Lane JD, Lanzi C, Lapaquette P, Lapierre LR, Laporte J, Laukkarinen J, Laurie GW, Lavandero S, Lavie L, LaVoie MJ, Law BY, Law HK, Law KB, Layfield R, Lazo PA, Le Cam L, Le Roch KG, Le Stunff H, Leardkamolkarn V, Lecuit M, Lee BH, Lee CH, Lee EF, Lee GM, Lee HJ, Lee H, Lee JK, Lee J, Lee JH, Lee JH, Lee M, Lee MS, Lee PJ, Lee SW, Lee SJ, Lee SJ, Lee SY, Lee SH, Lee SS, Lee SJ, Lee S, Lee YR, Lee YJ, Lee YH, Leeuwenburgh C, Lefort S, Legouis R, Lei J, Lei QY, Leib DA, Leibowitz G, Lekli I, Lemaire SD, Lemasters JJ, Lemberg MK, Lemoine A, Leng S, Lenz G, Lenzi P, Lerman LO, Lettieri Barbato D, Leu JI, Leung HY, Levine B, Lewis PA, Lezoualc’h F, Li C, Li F, Li FJ, Li J, Li K, Li L, Li M, Li M, Li Q, Li R, Li S, Li W, Li W, Li X, Li Y, Lian J, Liang C, Liang Q, Liao Y, Liberal J, Liberski PP, Lie P, Lieberman AP, Lim HJ, Lim KL, Lim K, Lima RT, Lin CS, Lin CF, Lin F, Lin F, Lin FC, Lin K, Lin KH, Lin PH, Lin T, Lin WW, Lin YS, Lin Y, Linden R, Lindholm D, Lindqvist LM, Lingor P, Linkermann A, Liotta LA, Lipinski MM, Lira VA, Lisanti MP, Liton PB, Liu B, Liu C, Liu CF, Liu F, Liu HJ, Liu J, Liu JJ, Liu JL, Liu K, Liu L, Liu L, Liu Q, Liu RY, Liu S, Liu S, Liu W, Liu XD, Liu X, Liu XH, Liu X, Liu X, Liu X, Liu Y, Liu Y, Liu Z, Liu Z, Liuzzi JP, Lizard G, Ljujic M, Lodhi IJ, Logue SE, Lokeshwar BL, Long YC, Lonial S, Loos B, López-Otín C, López-Vicario C, Lorente M, Lorenzi PL, Lõrincz P, Los M, Lotze MT, Lovat PE, Lu B, Lu B, Lu J, Lu Q, Lu SM, Lu S, Lu Y, Luciano F, Luckhart S, Lucocq JM, Ludovico P, Lugea A, Lukacs NW, Lum JJ, Lund AH, Luo H, Luo J, Luo S, Luparello C, Lyons T, Ma J, Ma Y, Ma Y, Ma Z, Machado J, Machado-Santelli GM, Macian F, MacIntosh GC, MacKeigan JP, Macleod KF, MacMicking JD, MacMillan-Crow LA, Madeo F, Madesh M, Madrigal-Matute J, Maeda A, Maeda T, Maegawa G, Maellaro E, Maes H, Magariños M, Maiese K, Maiti TK, Maiuri L, Maiuri MC, Maki CG, Malli R, Malorni W, Maloyan A, Mami-Chouaib F, Man N, Mancias JD, Mandelkow EM, Mandell MA, Manfredi AA, Manié SN, Manzoni C, Mao K, Mao Z, Mao ZW, Marambaud P, Marconi AM, Marelja Z, Marfe G, Margeta M, Margittai E, Mari M, Mariani FV, Marin C, Marinelli S, Mariño G, Markovic I, Marquez R, Martelli AM, Martens S, Martin KR, Martin SJ, Martin S, Martin-Acebes MA, Martín-Sanz P, Martinand-Mari C, Martinet W, Martinez J, Martinez-Lopez N, Martinez-Outschoorn U, Martínez-Velázquez M, Martinez-Vicente M, Martins WK, Mashima H, Mastrianni JA, Matarese G, Matarrese P, Mateo R, Matoba S, Matsumoto N, Matsushita T, Matsuura A, Matsuzawa T, Mattson MP, Matus S, Maugeri N, Mauvezin C, Mayer A, Maysinger D, Mazzolini GD, McBrayer MK, McCall K, McCormick C, McInerney GM, McIver SC, McKenna S, McMahon JJ, McNeish IA, Mechta-Grigoriou F, Medema JP, Medina DL, Megyeri K, Mehrpour M, Mehta JL, Mei Y, Meier UC, Meijer AJ, Meléndez A, Melino G, Melino S, de Melo EJ, Mena MA, Meneghini MD, Menendez JA, Menezes R, Meng L, Meng LH, Meng S, Menghini R, Menko AS, Menna-Barreto RF, Menon MB, Meraz-Ríos MA, Merla G, Merlini L, Merlot AM, Meryk A, Meschini S, Meyer JN, Mi MT, Miao CY, Micale L, Michaeli S, Michiels C, Migliaccio AR, Mihailidou AS, Mijaljica D, Mikoshiba K, Milan E, Miller-Fleming L, Mills GB, Mills IG, Minakaki G, Minassian BA, Ming XF, Minibayeva F, Minina EA, Mintern JD, Minucci S, Miranda-Vizuete A, Mitchell CH, Miyamoto S, Miyazawa K, Mizushima N, Mnich K, Mograbi B, Mohseni S, Moita LF, Molinari M, Molinari M, Møller AB, Mollereau B, Mollinedo F, Mongillo M, Monick MM, Montagnaro S, Montell C, Moore DJ, Moore MN, Mora-Rodriguez R, Moreira PI, Morel E, Morelli MB, Moreno S, Morgan MJ, Moris A, Moriyasu Y, Morrison JL, Morrison LA, Morselli E, Moscat J, Moseley PL, Mostowy S, Motori E, Mottet D, Mottram JC, Moussa CE, Mpakou VE, Mukhtar H, Mulcahy Levy JM, Muller S, Muñoz-Moreno R, Muñoz-Pinedo C, Münz C, Murphy ME, Murray JT, Murthy A, Mysorekar IU, Nabi IR, Nabissi M, Nader GA, Nagahara Y, Nagai Y, Nagata K, Nagelkerke A, Nagy P, Naidu SR, Nair S, Nakano H, Nakatogawa H, Nanjundan M, Napolitano G, Naqvi NI, Nardacci R, Narendra DP, Narita M, Nascimbeni AC, Natarajan R, Navegantes LC, Nawrocki ST, Nazarko TY, Nazarko VY, Neill T, Neri LM, Netea MG, Netea-Maier RT, Neves BM, Ney PA, Nezis IP, Nguyen HT, Nguyen HP, Nicot AS, Nilsen H, Nilsson P, Nishimura M, Nishino I, Niso-Santano M, Niu H, Nixon RA, Njar VC, Noda T, Noegel AA, Nolte EM, Norberg E, Norga KK, Noureini SK, Notomi S, Notterpek L, Nowikovsky K, Nukina N, Nürnberger T, O’Donnell VB, O’Donovan T, O’Dwyer PJ, Oehme I, Oeste CL, Ogawa M, Ogretmen B, Ogura Y, Oh YJ, Ohmuraya M, Ohshima T, Ojha R, Okamoto K, Okazaki T, Oliver FJ, Ollinger K, Olsson S, Orban DP, Ordonez P, Orhon I, Orosz L, O’Rourke EJ, Orozco H, Ortega AL, Ortona E, Osellame LD, Oshima J, Oshima S, Osiewacz HD, Otomo T, Otsu K, Ou JH, Outeiro TF, Ouyang DY, Ouyang H, Overholtzer M, Ozbun MA, Ozdinler PH, Ozpolat B, Pacelli C, Paganetti P, Page G, Pages G, Pagnini U, Pajak B, Pak SC, Pakos-Zebrucka K, Pakpour N, Palková Z, Palladino F, Pallauf K, Pallet N, Palmieri M, Paludan SR, Palumbo C, Palumbo S, Pampliega O, Pan H, Pan W, Panaretakis T, Pandey A, Pantazopoulou A, Papackova Z, Papademetrio DL, Papassideri I, Papini A, Parajuli N, Pardo J, Parekh VV, Parenti G, Park JI, Park J, Park OK, Parker R, Parlato R, Parys JB, Parzych KR, Pasquet JM, Pasquier B, Pasumarthi KB, Patschan D, Patterson C, Pattingre S, Pattison S, Pause A, Pavenstädt H, Pavone F, Pedrozo Z, Peña FJ, Peñalva MA, Pende M, Peng J, Penna F, Penninger JM, Pensalfini A, Pepe S, Pereira GJ, Pereira PC, Pérez-de la Cruz V, Pérez-Pérez ME, Pérez-Rodríguez D, Pérez-Sala D, Perier C, Perl A, Perlmutter DH, Perrotta I, Pervaiz S, Pesonen M, Pessin JE, Peters GJ, Petersen M, Petrache I, Petrof BJ, Petrovski G, Phang JM, Piacentini M, Pierdominici M, Pierre P, Pierrefite-Carle V, Pietrocola F, Pimentel-Muiños FX, Pinar M, Pineda B, Pinkas-Kramarski R, Pinti M, Pinton P, Piperdi B, Piret JM, Platanias LC, Platta HW, Plowey ED, Pöggeler S, Poirot M, Polčic P, Poletti A, Poon AH, Popelka H, Popova B, Poprawa I, Poulose SM, Poulton J, Powers SK, Powers T, Pozuelo-Rubio M, Prak K, Prange R, Prescott M, Priault M, Prince S, Proia RL, Proikas-Cezanne T, Prokisch H, Promponas VJ, Przyklenk K, Puertollano R, Pugazhenthi S, Puglielli L, Pujol A, Puyal J, Pyeon D, Qi X, Qian WB, Qin ZH, Qiu Y, Qu Z, Quadrilatero J, Quinn F, Raben N, Rabinowich H, Radogna F, Ragusa MJ, Rahmani M, Raina K, Ramanadham S, Ramesh R, Rami A, Randall-Demllo S, Randow F, Rao H, Rao VA, Rasmussen BB, Rasse TM, Ratovitski EA, Rautou PE, Ray SK, Razani B, Reed BH, Reggiori F, Rehm M, Reichert AS, Rein T, Reiner DJ, Reits E, Ren J, Ren X, Renna M, Reusch JE, Revuelta JL, Reyes L, Rezaie AR, Richards RI, Richardson DR, Richetta C, Riehle MA, Rihn BH, Rikihisa Y, Riley BE, Rimbach G, Rippo MR, Ritis K, Rizzi F, Rizzo E, Roach PJ, Robbins J, Roberge M, Roca G, Roccheri MC, Rocha S, Rodrigues CM, Rodríguez CI, de Cordoba SR, Rodriguez-Muela N, Roelofs J, Rogov VV, Rohn TT, Rohrer B, Romanelli D, Romani L, Romano PS, Roncero MI, Rosa JL, Rosello A, Rosen KV, Rosenstiel P, Rost-Roszkowska M, Roth KA, Roué G, Rouis M, Rouschop KM, Ruan DT, Ruano D, Rubinsztein DC, Rucker EB III, Rudich A, Rudolf E, Rudolf R, Ruegg MA, Ruiz-Roldan C, Ruparelia AA, Rusmini P, Russ DW, Russo GL, Russo G, Russo R, Rusten TE, Ryabovol V, Ryan KM, Ryter SW, Sabatini DM, Sacher M, Sachse C, Sack MN, Sadoshima J, Saftig P, Sagi-Eisenberg R, Sahni S, Saikumar P, Saito T, Saitoh T, Sakakura K, Sakoh-Nakatogawa M, Sakuraba Y, Salazar-Roa M, Salomoni P, Saluja AK, Salvaterra PM, Salvioli R, Samali A, Sanchez AM, Sánchez-Alcázar JA, Sanchez-Prieto R, Sandri M, Sanjuan MA, Santaguida S, Santambrogio L, Santoni G, Dos Santos CN, Saran S, Sardiello M, Sargent G, Sarkar P, Sarkar S, Sarrias MR, Sarwal MM, Sasakawa C, Sasaki M, Sass M, Sato K, Sato M, Satriano J, Savaraj N, Saveljeva S, Schaefer L, Schaible UE, Scharl M, Schatzl HM, Schekman R, Scheper W, Schiavi A, Schipper HM, Schmeisser H, Schmidt J, Schmitz I, Schneider BE, Schneider EM, Schneider JL, Schon EA, Schönenberger MJ, Schönthal AH, Schorderet DF, Schröder B, Schuck S, Schulze RJ, Schwarten M, Schwarz TL, Sciarretta S, Scotto K, Scovassi AI, Screaton RA, Screen M, Seca H, Sedej S, Segatori L, Segev N, Seglen PO, Seguí-Simarro JM, Segura-Aguilar J, Seki E, Sell C, Seiliez I, Semenkovich CF, Semenza GL, Sen U, Serra AL, Serrano-Puebla A, Sesaki H, Setoguchi T, Settembre C, Shacka JJ, Shajahan-Haq AN, Shapiro IM, Sharma S, She H, Shen CK, Shen CC, Shen HM, Shen S, Shen W, Sheng R, Sheng X, Sheng ZH, Shepherd TG, Shi J, Shi Q, Shi Q, Shi Y, Shibutani S, Shibuya K, Shidoji Y, Shieh JJ, Shih CM, Shimada Y, Shimizu S, Shin DW, Shinohara ML, Shintani M, Shintani T, Shioi T, Shirabe K, Shiri-Sverdlov R, Shirihai O, Shore GC, Shu CW, Shukla D, Sibirny AA, Sica V, Sigurdson CJ, Sigurdsson EM, Sijwali PS, Sikorska B, Silveira WA, Silvente-Poirot S, Silverman GA, Simak J, Simmet T, Simon AK, Simon HU, Simone C, Simons M, Simonsen A, Singh R, Singh SV, Singh SK, Sinha D, Sinha S, Sinicrope FA, Sirko A, Sirohi K, Sishi BJ, Sittler A, Siu PM, Sivridis E, Skwarska A, Slack R, Slaninová I, Slavov N, Smaili SS, Smalley KS, Smith DR, Soenen SJ, Soleimanpour SA, Solhaug A, Somasundaram K, Son JH, Sonawane A, Song C, Song F, Song HK, Song JX, Song W, Soo KY, Sood AK, Soong TW, Soontornniyomkij V, Sorice M, Sotgia F, Soto-Pantoja DR, Sotthibundhu A, Sousa MJ, Spaink HP, Span PN, Spang A, Sparks JD, Speck PG, Spector SA, Spies CD, Springer W, Clair DS, Stacchiotti A, Staels B, Stang MT, Starczynowski DT, Starokadomskyy P, Steegborn C, Steele JW, Stefanis L, Steffan J, Stellrecht CM, Stenmark H, Stepkowski TM, Stern ST, Stevens C, Stockwell BR, Stoka V, Storchova Z, Stork B, Stratoulias V, Stravopodis DJ, Strnad P, Strohecker AM, Ström AL, Stromhaug P, Stulik J, Su YX, Su Z, Subauste CS, Subramaniam S, Sue CM, Suh SW, Sui X, Sukseree S, Sulzer D, Sun FL, Sun J, Sun J, Sun SY, Sun Y, Sun Y, Sun Y, Sundaramoorthy V, Sung J, Suzuki H, Suzuki K, Suzuki N, Suzuki T, Suzuki YJ, Swanson MS, Swanton C, Swärd K, Swarup G, Sweeney ST, Sylvester PW, Szatmari Z, Szegezdi E, Szlosarek PW, Taegtmeyer H, Tafani M, Taillebourg E, Tait SW, Takacs-Vellai K, Takahashi Y, Takáts S, Takemura G, Takigawa N, Talbot NJ, Tamagno E, Tamburini J, Tan CP, Tan L, Tan ML, Tan M, Tan YJ, Tanaka K, Tanaka M, Tang D, Tang D, Tang G, Tanida I, Tanji K, Tannous BA, Tapia JA, Tasset-Cuevas I, Tatar M, Tavassoly I, Tavernarakis N, Taylor A, Taylor GS, Taylor GA, Taylor JP, Taylor MJ, Tchetina EV, Tee AR, Teixeira-Clerc F, Telang S, Tencomnao T, Teng BB, Teng RJ, Terro F, Tettamanti G, Theiss AL, Theron AE, Thomas KJ, Thomé MP, Thomes PG, Thorburn A, Thorner J, Thum T, Thumm M, Thurston TL, Tian L, Till A, Ting JP, Titorenko VI, Toker L, Toldo S, Tooze SA, Topisirovic I, Torgersen ML, Torosantucci L, Torriglia A, Torrisi MR, Tournier C, Towns R, Trajkovic V, Travassos LH, Triola G, Tripathi DN, Trisciuoglio D, Troncoso R, Trougakos IP, Truttmann AC, Tsai KJ, Tschan MP, Tseng YH, Tsukuba T, Tsung A, Tsvetkov AS, Tu S, Tuan HY, Tucci M, Tumbarello DA, Turk B, Turk V, Turner RF, Tveita AA, Tyagi SC, Ubukata M, Uchiyama Y, Udelnow A, Ueno T, Umekawa M, Umemiya-Shirafuji R, Underwood BR, Ungermann C, Ureshino RP, Ushioda R, Uversky VN, Uzcátegui NL, Vaccari T, Vaccaro MI, Váchová L, Vakifahmetoglu-Norberg H, Valdor R, Valente EM, Vallette F, Valverde AM, Van den Berghe G, Van Den Bosch L, van den Brink GR, van der Goot FG, van der Klei IJ, van der Laan LJ, van Doorn WG, van Egmond M, van Golen KL, Van Kaer L, van Lookeren Campagne M, Vandenabeele P, Vandenberghe W, Vanhorebeek I, Varela-Nieto I, Vasconcelos MH, Vasko R, Vavvas DG, Vega-Naredo I, Velasco G, Velentzas AD, Velentzas PD, Vellai T, Vellenga E, Vendelbo MH, Venkatachalam K, Ventura N, Ventura S, Veras PS, Verdier M, Vertessy BG, Viale A, Vidal M, Vieira HL, Vierstra RD, Vigneswaran N, Vij N, Vila M, Villar M, Villar VH, Villarroya J, Vindis C, Viola G, Viscomi MT, Vitale G, Vogl DT, Voitsekhovskaja OV, von Haefen C, von Schwarzenberg K, Voth DE, Vouret-Craviari V, Vuori K, Vyas JM, Waeber C, Walker CL, Walker MJ, Walter J, Wan L, Wan X, Wang B, Wang C, Wang CY, Wang C, Wang C, Wang C, Wang D, Wang F, Wang F, Wang G, Wang HJ, Wang H, Wang HG, Wang H, Wang HD, Wang J, Wang J, Wang M, Wang MQ, Wang PY, Wang P, Wang RC, Wang S, Wang TF, Wang X, Wang XJ, Wang XW, Wang X, Wang X, Wang Y, Wang Y, Wang Y, Wang YJ, Wang Y, Wang Y, Wang YT, Wang Y, Wang ZN, Wappner P, Ward C, Ward DM, Warnes G, Watada H, Watanabe Y, Watase K, Weaver TE, Weekes CD, Wei J, Weide T, Weihl CC, Weindl G, Weis SN, Wen L, Wen X, Wen Y, Westermann B, Weyand CM, White AR, White E, Whitton JL, Whitworth AJ, Wiels J, Wild F, Wildenberg ME, Wileman T, Wilkinson DS, Wilkinson S, Willbold D, Williams C, Williams K, Williamson PR, Winklhofer KF, Witkin SS, Wohlgemuth SE, Wollert T, Wolvetang EJ, Wong E, Wong GW, Wong RW, Wong VK, Woodcock EA, Wright KL, Wu C, Wu D, Wu GS, Wu J, Wu J, Wu M, Wu M, Wu S, Wu WK, Wu Y, Wu Z, Xavier CP, Xavier RJ, Xia GX, Xia T, Xia W, Xia Y, Xiao H, Xiao J, Xiao S, Xiao W, Xie CM, Xie Z, Xie Z, Xilouri M, Xiong Y, Xu C, Xu C, Xu F, Xu H, Xu H, Xu J, Xu J, Xu J, Xu L, Xu X, Xu Y, Xu Y, Xu ZX, Xu Z, Xue Y, Yamada T, Yamamoto A, Yamanaka K, Yamashina S, Yamashiro S, Yan B, Yan B, Yan X, Yan Z, Yanagi Y, Yang DS, Yang JM, Yang L, Yang M, Yang PM, Yang P, Yang Q, Yang W, Yang WY, Yang X, Yang Y, Yang Y, Yang Z, Yang Z, Yao MC, Yao PJ, Yao X, Yao Z, Yao Z, Yasui LS, Ye M, Yedvobnick B, Yeganeh B, Yeh ES, Yeyati PL, Yi F, Yi L, Yin XM, Yip CK, Yoo YM, Yoo YH, Yoon SY, Yoshida K, Yoshimori T, Young KH, Yu H, Yu JJ, Yu JT, Yu J, Yu L, Yu WH, Yu XF, Yu Z, Yuan J, Yuan ZM, Yue BY, Yue J, Yue Z, Zacks DN, Zacksenhaus E, Zaffaroni N, Zaglia T, Zakeri Z, Zecchini V, Zeng J, Zeng M, Zeng Q, Zervos AS, Zhang DD, Zhang F, Zhang G, Zhang GC, Zhang H, Zhang H, Zhang H, Zhang H, Zhang J, Zhang J, Zhang J, Zhang J, Zhang JP, Zhang L, Zhang L, Zhang L, Zhang L, Zhang MY, Zhang X, Zhang XD, Zhang Y, Zhang Y, Zhang Y, Zhang Y, Zhang Y, Zhao M, Zhao WL, Zhao X, Zhao YG, Zhao Y, Zhao Y, Zhao YX, Zhao Z, Zhao ZJ, Zheng D, Zheng XL, Zheng X, Zhivotovsky B, Zhong Q, Zhou GZ, Zhou G, Zhou H, Zhou SF, Zhou XJ, Zhu H, Zhu H, Zhu WG, Zhu W, Zhu XF, Zhu Y, Zhuang SM, Zhuang X, Ziparo E, Zois CE, Zoladek T, Zong WX, Zorzano A, Zughaier SM. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy. 2016;12(1):1–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Martinet W, Roth L, De Meyer GRY. Standard immunohistochemical assays to assess autophagy in mammalian tissue. Cells. 2017;6(3):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140(3):313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zummo FP, Cullen KS, Honkanen-Scott M, Shaw JAM, Lovat PE, Arden C. Glucagon-like peptide 1 protects pancreatic β-cells from death by increasing autophagic flux and restoring lysosomal function. Diabetes. 2017;66(5):1272–1285. [DOI] [PubMed] [Google Scholar]
- 29. Castillo K, Valenzuela V, Matus S, Nassif M, Oñate M, Fuentealba Y, Encina G, Irrazabal T, Parsons G, Court FA, Schneider BL, Armentano D, Hetz C. Measurement of autophagy flux in the nervous system in vivo. Cell Death Dis. 2013;4(11):e917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ren L, Yang H, Cui Y, Xu S, Sun F, Tian N, Hua J, Peng S. Autophagy is essential for the differentiation of porcine PSCs into insulin-producing cells. Biochem Biophys Res Commun. 2017;488(3):471–476. [DOI] [PubMed] [Google Scholar]
- 31. Riahi Y, Wikstrom JD, Bachar-Wikstrom E, Polin N, Zucker H, Lee M-S, Quan W, Haataja L, Liu M, Arvan P, Cerasi E, Leibowitz G. Autophagy is a major regulator of beta cell insulin homeostasis. Diabetologia. 2016;59(7):1480–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jung HS, Chung KW, Won Kim J, Kim J, Komatsu M, Tanaka K, Nguyen YH, Kang TM, Yoon KH, Kim JW, Jeong YT, Han MS, Lee MK, Kim KW, Shin J, Lee MS. Loss of autophagy diminishes pancreatic β cell mass and function with resultant hyperglycemia. Cell Metab. 2008;8(4):318–324. [DOI] [PubMed] [Google Scholar]
- 33. Blandino-Rosano M, Barbaresso R, Jimenez-Palomares M, Bozadjieva N, Werneck-de-Castro JP, Hatanaka M, Mirmira RG, Sonenberg N, Liu M, Rüegg MA, Hall MN, Bernal-Mizrachi E. Loss of mTORC1 signalling impairs β-cell homeostasis and insulin processing. Nat Commun. 2017;8:16014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bartolomé A, Kimura-Koyanagi M, Asahara S, Guillén C, Inoue H, Teruyama K, Shimizu S, Kanno A, García-Aguilar A, Koike M, Uchiyama Y, Benito M, Noda T, Kido Y. Pancreatic β-cell failure mediated by mTORC1 hyperactivity and autophagic impairment. Diabetes. 2014;63(9):2996–3008. [DOI] [PubMed] [Google Scholar]
- 35. Elouil H, Bensellam M, Guiot Y, Vander Mierde D, Pascal SMA, Schuit FC, Jonas JC. Acute nutrient regulation of the unfolded protein response and integrated stress response in cultured rat pancreatic islets. Diabetologia. 2007;50(7):1442–1452. [DOI] [PubMed] [Google Scholar]
- 36. Cnop M, Ladriere L, Hekerman P, Ortis F, Cardozo AK, Dogusan Z, Flamez D, Boyce M, Yuan J, Eizirik DL. Selective inhibition of eukaryotic translation initiation factor 2 α dephosphorylation potentiates fatty acid-induced endoplasmic reticulum stress and causes pancreatic β-cell dysfunction and apoptosis. J Biol Chem. 2007;282(6):3989–3997. [DOI] [PubMed] [Google Scholar]
- 37. Laybutt DR, Preston AM, Åkerfeldt MC, Kench JG, Busch AK, Biankin AV, Biden TJ. Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia. 2007;50(4):752–763. [DOI] [PubMed] [Google Scholar]
- 38. Kharroubi I, Ladrière L, Cardozo AK, Dogusan Z, Cnop M, Eizirik DL. Free fatty acids and cytokines induce pancreatic β-cell apoptosis by different mechanisms: role of nuclear factor-kappaB and endoplasmic reticulum stress. Endocrinology. 2004;145(11):5087–5096. [DOI] [PubMed] [Google Scholar]
- 39. Karaskov E, Scott C, Zhang L, Teodoro T, Ravazzola M, Volchuk A. Chronic palmitate but not oleate exposure induces endoplasmic reticulum stress, which may contribute to INS-1 pancreatic β-cell apoptosis. Endocrinology. 2006;147(7):3398–3407. [DOI] [PubMed] [Google Scholar]
- 40. Eizirik DL, Cardozo AK, Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev. 2008;29(1):42–61. [DOI] [PubMed] [Google Scholar]
- 41. Cnop M, Foufelle F, Velloso LA. Endoplasmic reticulum stress, obesity and diabetes. Trends Mol Med. 2012;18(1):59–68. [DOI] [PubMed] [Google Scholar]
- 42. Abedini A, Schmidt AM. Mechanisms of islet amyloidosis toxicity in type 2 diabetes. FEBS Lett. 2013;587(8):1119–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Saafi EL, Konarkowska B, Zhang S, Kistler J, Cooper GJ. Ultrastructural evidence that apoptosis is the mechanism by which human amylin evokes death in RINm5F pancreatic islet β-cells. Cell Biol Int. 2001;25(4):339–350. [DOI] [PubMed] [Google Scholar]
- 44. Matveyenko AV, Butler PC. Beta-cell deficit due to increased apoptosis in the human islet amyloid polypeptide transgenic (HIP) rat recapitulates the metabolic defects present in type 2 diabetes. Diabetes. 2006;55(7):2106–2114. [DOI] [PubMed] [Google Scholar]
- 45. Kahn SE, Andrikopoulos S, Verchere CB. Islet amyloid: a long-recognized but underappreciated pathological feature of type 2 diabetes. Diabetes. 1999;48(2):241–253. [DOI] [PubMed] [Google Scholar]
- 46. Shigihara N, Fukunaka A, Hara A, Komiya K, Honda A, Uchida T, Abe H, Toyofuku Y, Tamaki M, Ogihara T, Miyatsuka T, Hiddinga HJ, Sakagashira S, Koike M, Uchiyama Y, Yoshimori T, Eberhardt NL, Fujitani Y, Watada H. Human IAPP-induced pancreatic β cell toxicity and its regulation by autophagy. J Clin Invest. 2014;124(8):3634–3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kim J, Cheon H, Jeong YT, Quan W, Kim KH, Cho JM, Lim YM, Oh SH, Jin SM, Kim JH, Lee MK, Kim S, Komatsu M, Kang SW, Lee MS. Amyloidogenic peptide oligomer accumulation in autophagy-deficient β cells induces diabetes. J Clin Invest. 2014;124(8):3311–3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bernales S, McDonald KL, Walter P. Autophagy counterbalances endoplasmic reticulum expansion during the unfolded protein response. PLoS Biol. 2006;4(12):e423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, Murakami T, Taniguchi M, Tanii I, Yoshinaga K, Shiosaka S, Hammarback JA, Urano F, Imaizumi K. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol. 2006;26(24):9220–9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yorimitsu T, Nair U, Yang Z, Klionsky DJ. Endoplasmic reticulum stress triggers autophagy. J Biol Chem. 2006;281(40):30299–30304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kong F-J, Wu J-H, Sun S-Y, Zhou J-Q. The endoplasmic reticulum stress/autophagy pathway is involved in cholesterol-induced pancreatic β-cell injury. Sci Rep. 2017;7:44746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Quan W, Hur KY, Lim Y, Oh SH, Lee J-C, Kim KH, Kim GH, Kim SW, Kim HL, Lee MK, Kim KW, Kim J, Komatsu M, Lee MS. Autophagy deficiency in beta cells leads to compromised unfolded protein response and progression from obesity to diabetes in mice. Diabetologia. 2012;55(2):392–403. [DOI] [PubMed] [Google Scholar]
- 53. B’chir W, Maurin A-C, Carraro V, Averous J, Jousse C, Muranishi Y, Parry L, Stepien G, Fafournoux P, Bruhat A. The eIF2α/ATF4 pathway is essential for stress-induced autophagy gene expression. Nucleic Acids Res. 2013;41(16):7683–7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Takatani T, Shirakawa J, Roe MW, Leech CA, Maier BF, Mirmira RG, Kulkarni RN. IRS1 deficiency protects β-cells against ER stress-induced apoptosis by modulating sXBP-1 stability and protein translation. Sci Rep. 2016;5(6):28177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rajesh K, Krishnamoorthy J, Kazimierczak U, Tenkerian C, Papadakis AI, Wang S, Huang S, Koromilas AE. Phosphorylation of the translation initiation factor eIF2α at serine 51 determines the cell fate decisions of Akt in response to oxidative stress. Cell Death Dis. 2015;6(1):e1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Leloup C, Tourrel-Cuzin C, Magnan C, Karaca M, Castel J, Carneiro L, Colombani AL, Ktorza A, Casteilla L, Pénicaud L. Mitochondrial reactive oxygen species are obligatory signals for glucose-induced insulin secretion. Diabetes. 2009;58(3):673–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ahmed Alfar E, Kirova D, Konantz J, Birke S, Mansfeld J, Ninov N. Distinct levels of reactive oxygen species coordinate metabolic activity with beta-cell mass plasticity. Sci Rep. 2017;7(1):3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Robertson RP. Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in diabetes. J Biol Chem. 2004;279(41):42351–42354. [DOI] [PubMed] [Google Scholar]
- 59. Masuda Y, Vaziri ND, Li S, Le A, Hajighasemi-Ossareh M, Robles L, Foster CE, Stamos MJ, Al-Abodullah I, Ricordi C, Ichii H. The effect of Nrf2 pathway activation on human pancreatic islet cells. PLoS One. 2015;10(6):e0131012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yagishita Y, Fukutomi T, Sugawara A, Kawamura H, Takahashi T, Pi J, Uruno A, Yamamoto M. Nrf2 protects pancreatic β-cells from oxidative and nitrosative stress in diabetic model mice. Diabetes. 2014;63(2):605–618. [DOI] [PubMed] [Google Scholar]
- 61. Uruno A, Furusawa Y, Yagishita Y, Fukutomi T, Muramatsu H, Negishi T, Sugawara A, Kensler TW, Yamamoto M. The Keap1-Nrf2 system prevents onset of diabetes mellitus. Mol Cell Biol. 2013;33(15):2996–3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Li W, Wu W, Song H, Wang F, Li H, Chen L, Lai Y, Janicki JS, Ward KW, Meyer CJ, Wang XL, Tang D, Cui T. Targeting Nrf2 by dihydro-CDDO-trifluoroethyl amide enhances autophagic clearance and viability of β-cells in a setting of oxidative stress. FEBS Lett. 2014;588(12):2115–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pajares M, Jiménez-Moreno N, García-Yagüe ÁJ, Escoll M, de Ceballos ML, Van Leuven F, Rábano A, Yamamoto M, Rojo AI, Cuadrado A. Transcription factor NFE2L2/NRF2 is a regulator of macroautophagy genes. Autophagy. 2016;12(10):1902–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jain A, Lamark T, Sjøttem E, Larsen KB, Awuh JA, Øvervatn A, McMahon M, Hayes JD, Johansen T. p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. J Biol Chem. 2010;285(29):22576–22591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lau A, Wang X-J, Zhao F, Villeneuve NF, Wu T, Jiang T, Sun Z, White E, Zhang DD. A noncanonical mechanism of Nrf2 activation by autophagy deficiency: direct interaction between Keap1 and p62. Mol Cell Biol. 2010;30(13):3275–3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Komatsu M, Kurokawa H, Waguri S, Taguchi K, Kobayashi A, Ichimura Y, Sou YS, Ueno I, Sakamoto A, Tong KI, Kim M, Nishito Y, Iemura S, Natsume T, Ueno T, Kominami E, Motohashi H, Tanaka K, Yamamoto M. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol. 2010;12(3):213–223. [DOI] [PubMed] [Google Scholar]
- 67. Xiao B, Goh J-Y, Xiao L, Xian H, Lim K-L, Liou Y-C. Reactive oxygen species trigger Parkin/PINK1 pathway-dependent mitophagy by inducing mitochondrial recruitment of Parkin. J Biol Chem. 2017;292(40):16697–16708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wang Y, Nartiss Y, Steipe B, McQuibban GA, Kim PK. ROS-induced mitochondrial depolarization initiates PARK2/PARKIN-dependent mitochondrial degradation by autophagy. Autophagy. 2012;8(10):1462–1476. [DOI] [PubMed] [Google Scholar]
- 69. Frank M, Duvezin-Caubet S, Koob S, Occhipinti A, Jagasia R, Petcherski A, Ruonala MO, Priault M, Salin B, Reichert AS. Mitophagy is triggered by mild oxidative stress in a mitochondrial fission dependent manner. Biochim Biophys Acta. 2012;1823(12):2297–2310. [DOI] [PubMed] [Google Scholar]
- 70. Scherz-Shouval R, Elazar Z. ROS, mitochondria and the regulation of autophagy. Trends Cell Biol. 2007;17(9):422–427. [DOI] [PubMed] [Google Scholar]
- 71. Hoshino A, Ariyoshi M, Okawa Y, Kaimoto S, Uchihashi M, Fukai K, Iwai-Kanai E, Ikeda K, Ueyama T, Ogata T, Matoba S. Inhibition of p53 preserves Parkin-mediated mitophagy and pancreatic β-cell function in diabetes. Proc Natl Acad Sci USA. 2014;111(8):3116–3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Jin H-S, Kim J, Lee S-J, Kim K, Go MJ, Lee J-Y, Lee HJ, Song J, Jeon BT, Roh GS, Kim SJ, Kim BY, Hong KW, Yoo YH, Oh B, Kang Y, Jeong SY. The PARK2 gene is involved in the maintenance of pancreatic β-cell functions related to insulin production and secretion. Mol Cell Endocrinol. 2014;382(1):178–189. [DOI] [PubMed] [Google Scholar]
- 73. Chen L, Liu C, Gao J, Xie Z, Chan LWC, Keating DJ, Yang Y, Sun J, Zhou F, Wei Y, Men X, Yang S. Inhibition of Miro1 disturbs mitophagy and pancreatic βnhibition of Miro1 disturbs mitophagy and pancreatic etion ncreatic ssio. Oncotarget. 2017;8(53):90693–90705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bhansali S, Bhansali A, Walia R, Saikia UN, Dhawan V. Alterations in mitochondrial oxidative stress and mitophagy in subjects with prediabetes and type 2 diabetes mellitus. Front Endocrinol. 2017;15(8):347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Soleimanpour SA, Gupta A, Bakay M, Ferrari AM, Groff DN, Fadista J, Spruce LA, Kushner JA, Groop L, Seeholzer SH, Kaufman BA, Hakonarson H, Stoffers DA. The diabetes susceptibility gene Clec16a regulates mitophagy. Cell. 2014;157(7):1577–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Soleimanpour SA, Ferrari AM, Raum JC, Groff DN, Yang J, Kaufman BA, Stoffers DA. Diabetes susceptibility genes Pdx1 and Clec16a function in a pathway regulating mitophagy in β-cells. Diabetes. 2015;64(10):3475–3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Qu Y, Sun L, Yang Z, Han R. Variation in the PTEN-induced putative kinase 1 gene associated with the increase risk of type 2 diabetes in northern Chinese. J Genet. 2011;90(1):125–128. [DOI] [PubMed] [Google Scholar]
- 78. Sun Q, Nie S, Wang L, Yang F, Meng Z, Xiao H, Xiang B, Li X, Fu X, Wang S. Factors that affect pancreatic islet cell autophagy in adult rats: evaluation of a calorie-restricted diet and a high-fat diet. PLoS One. 2016;11(3):e0151104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Fujimoto K, Hanson PT, Tran H, Ford EL, Han Z, Johnson JD, Schmidt RE, Green KG, Wice BM, Polonsky KS. Autophagy regulates pancreatic beta cell death in response to Pdx1 deficiency and nutrient deprivation. J Biol Chem. 2009;284(40):27664–27673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ebato C, Uchida T, Arakawa M, Komatsu M, Ueno T, Komiya K, Azuma K, Hirose T, Tanaka K, Kominami E, Kawamori R, Fujitani Y, Watada H. Autophagy is important in islet homeostasis and compensatory increase of beta cell mass in response to high-fat diet. Cell Metab. 2008;8(4):325–332. [DOI] [PubMed] [Google Scholar]
- 81. Liu H, Javaheri A, Godar RJ, Murphy J, Ma X, Rohatgi N, Mahadevan J, Hyrc K, Saftig P, Marshall C, McDaniel ML, Remedi MS, Razani B, Urano F, Diwan A. Intermittent fasting preserves beta-cell mass in obesity-induced diabetes via the autophagy-lysosome pathway. Autophagy. 2017;13(11):1952–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Goginashvili A, Zhang Z, Erbs E, Spiegelhalter C, Kessler P, Mihlan M, Pasquier A, Krupina K, Schieber N, Cinque L, Morvan J, Sumara I, Schwab Y, Settembre C, Ricci R. Insulin granules. Insulin secretory granules control autophagy in pancreatic β cells. Science. 2015;347(6224):878–882. [DOI] [PubMed] [Google Scholar]
- 83. Choi S-E, Lee S-M, Lee Y-J, Li L-J, Lee S-J, Lee J-H, Kim Y, Jun HS, Lee KW, Kang Y. Protective role of autophagy in palmitate-induced INS-1 β-cell death. Endocrinology. 2009;150(1):126–134. [DOI] [PubMed] [Google Scholar]
- 84. Martino L, Masini M, Novelli M, Beffy P, Bugliani M, Marselli L, Masiello P, Marchetti P, De Tata V. Palmitate activates autophagy in INS-1E βtcells and in isolated rat and human pancreatic islets. PLoS One. 2012;7(5):e36188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wu J, Kong F, Pan Q, Du Y, Ye J, Zheng F, Li H, Zhou J. Autophagy protects against cholesterol-induced apoptosis in pancreatic β-cells. Biochem Biophys Res Commun. 2017;482(4):678–685. [DOI] [PubMed] [Google Scholar]
- 86. Mir SUR, George NM, Zahoor L, Harms R, Guinn Z, Sarvetnick NE. Inhibition of autophagic turnover in β-cells by fatty acids and glucose leads to apoptotic cell death. J Biol Chem. 2015;290(10):6071–6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Las G, Serada SB, Wikstrom JD, Twig G, Shirihai OS. Fatty acids suppress autophagic turnover in β-cells. J Biol Chem. 2011;286(49):42534–42544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Trudeau KM, Colby AH, Zeng J, Las G, Feng JH, Grinstaff MW, Shirihai OS. Lysosome acidification by photoactivated nanoparticles restores autophagy under lipotoxicity. J Cell Biol. 2016;214(1):25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Gao X, Yan D, Zhao Y, Tao H, Zhou Y. Moderate calorie restriction to achieve normal weight reverses β-cell dysfunction in diet-induced obese mice: involvement of autophagy. Nutr Metab (Lond). 2015;12:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Chu KY, O’Reilly L, Ramm G, Biden TJ. High-fat diet increases autophagic flux in pancreatic beta cells in vivo and ex vivo in mice. Diabetologia. 2015;58(9):2074–2078. [DOI] [PubMed] [Google Scholar]
- 91. Sheng Q, Xiao X, Prasadan K, Chen C, Ming Y, Fusco J, Gangopadhyay NN, Ricks D, Gittes GK. Autophagy protects pancreatic beta cell mass and function in the setting of a high-fat and high-glucose diet. Sci Rep. 2017;7(1):16348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Abebe T, Mahadevan J, Bogachus L, Hahn S, Black M, Oseid E, Urano F, Cirulli V, Robertson RP. Nrf2/antioxidant pathway mediates β cell self-repair after damage by high-fat diet-induced oxidative stress. JCI Insight. 2017;2(24):92854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Varshney R, Gupta S, Roy P. Cytoprotective effect of kaempferol against palmitic acid-induced pancreatic β-cell death through modulation of autophagy via AMPK/mTOR signaling pathway. Mol Cell Endocrinol. 2017;448:1–20. [DOI] [PubMed] [Google Scholar]
- 94. Wang Y, He D, Ni C, Zhou H, Wu S, Xue Z, Zhou Z. Vitamin D induces autophagy of pancreatic β-cells and enhances insulin secretion. Mol Med Rep. 2016;14(3):2644–2650. [DOI] [PubMed] [Google Scholar]
- 95. Hwang W-M, Bak D-H, Kim DH, Hong JY, Han S-Y, Park K-Y, Lim K, Lim DM, Kang JG. Omega-3 polyunsaturated fatty acids may attenuate streptozotocin-induced pancreatic β-cell death via autophagy activation in Fat1 transgenic mice. Endocrinol Metab (Seoul). 2015;30(4):569–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Padgett LE, Broniowska KA, Hansen PA, Corbett JA, Tse HM. The role of reactive oxygen species and proinflammatory cytokines in type 1 diabetes pathogenesis. Ann N Y Acad Sci. 2013;1281:16–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Eizirik DL, Colli ML, Ortis F. The role of inflammation in insulitis and β-cell loss in type 1 diabetes. Nat Rev Endocrinol. 2009;5(4):219–226. [DOI] [PubMed] [Google Scholar]
- 98. Brozzi F, Nardelli TR, Lopes M, Millard I, Barthson J, Igoillo-Esteve M, Grieco FA, Villate O, Oliveira JM, Casimir M, Bugliani M, Engin F, Hotamisligil GS, Marchetti P, Eizirik DL. Cytokines induce endoplasmic reticulum stress in human, rat and mouse beta cells via different mechanisms. Diabetologia. 2015;58(10):2307–2316. [DOI] [PubMed] [Google Scholar]
- 99. Tersey SA, Maier B, Nishiki Y, Maganti AV, Nadler JL, Mirmira RG. 12-Lipoxygenase promotes obesity-induced oxidative stress in pancreatic islets. Mol Cell Biol. 2014;34(19):3735–3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Weaver JR, Holman TR, Imai Y, Jadhav A, Kenyon V, Maloney DJ, Nadler JL, Rai G, Simeonov A, Taylor-Fishwick DA. Integration of pro-inflammatory cytokines, 12-lipoxygenase and NOX-1 in pancreatic islet beta cell dysfunction. Mol Cell Endocrinol. 2012;358(1):88–95. [DOI] [PubMed] [Google Scholar]
- 101. Lambelet M, Terra LF, Fukaya M, Meyerovich K, Labriola L, Cardozo AK, Allagnat F. Dysfunctional autophagy following exposure to pro-inflammatory cytokines contributes to pancreatic β-cell apoptosis. Cell Death Dis. 2018;9(2):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Hu M, Yang S, Yang L, Cheng Y, Zhang H. Interleukin-22 alleviated palmitate-Induced Endoplasmic Reticulum Stress in INS-1 cells through activation of autophagy. PLoS One. 2016;11(1):e0146818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Linnemann AK, Blumer J, Marasco MR, Battiola TJ, Umhoefer HM, Han JY, Lamming DW, Davis DB. Interleukin 6 protects pancreatic β cells from apoptosis by stimulation of autophagy. FASEB J. 2017;31(9):4140–4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Chen Y-L, Qiao Y-C, Pan Y-H, Xu Y, Huang Y-C, Wang Y-H, Geng LJ, Zhao HL, Zhang XX. Correlation between serum interleukin-6 level and type 1 diabetes mellitus: A systematic review and meta-analysis. Cytokine. 2017;94:14–20. [DOI] [PubMed] [Google Scholar]
- 105. Liu C, Feng X, Li Q, Wang Y, Li Q, Hua M. Adiponectin, TNF-α and inflammatory cytokines and risk of type 2 diabetes: a systematic review and meta-analysis. Cytokine. 2016;86:100–109. [DOI] [PubMed] [Google Scholar]
- 106. Cavaillon JM. Pro- versus anti-inflammatory cytokines: myth or reality. Cell Mol Biol. 2001;47(4):695–702. [PubMed] [Google Scholar]
- 107. Ma X, Guan Y, Hua X. Glucagon-like peptide 1-potentiated insulin secretion and proliferation of pancreatic β-cells. J Diabetes. 2014;6(5):394–402. [DOI] [PubMed] [Google Scholar]
- 108. Wang J, Wu J, Wu H, Liu X, Chen Y, Wu J, Hu C, Zou D. Liraglutide protects pancreatic β-cells against free fatty acids in vitro and affects glucolipid metabolism in apolipoprotein E-/- mice by activating autophagy. Mol Med Rep. 2015;12(3):4210–4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Lim SW, Jin L, Jin J, Yang CW. Effect of exendin-4 on autophagy clearance in beta cell of rats with tacrolimus-induced diabetes mellitus. Sci Rep. 2016;20(6):29921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Liu L, Liu J, Yu X. Dipeptidyl peptidase-4 inhibitor MK-626 restores insulin secretion through enhancing autophagy in high fat diet-induced mice. Biochem Biophys Res Commun. 2016;470(3):516–520. [DOI] [PubMed] [Google Scholar]
- 111. Ellingsgaard H, Hauselmann I, Schuler B, Habib AM, Baggio LL, Meier DT, Eppler E, Bouzakri K, Wueest S, Muller YD, Hansen AM, Reinecke M, Konrad D, Gassmann M, Reimann F, Halban PA, Gromada J, Drucker DJ, Gribble FM, Ehses JA, Donath MY. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat Med. 2011;17(11):1481–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Cheng C-W, Villani V, Buono R, Wei M, Kumar S, Yilmaz OH, Cohen P, Sneddon JB, Perin L, Longo VD. Fasting-mimicking diet promotes Ngn3-driven β-cell regeneration to reverse diabetes. Cell. 2017;168(5):775–788.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Ni Q, Gu Y, Xie Y, Yin Q, Zhang H, Nie A, Li W, Wang Y, Ning G, Wang W, Wang Q. Raptor regulates functional maturation of murine beta cells. Nat Commun. 2017;8:15755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ding L, Yin Y, Han L, Li Y, Zhao J, Zhang W. TSC1-mTOR signaling determines the differentiation of islet cells. J Endocrinol. 2017;232(1):59–70. [DOI] [PubMed] [Google Scholar]
- 115. Bozadjieva N, Blandino-Rosano M, Chase J, Dai X-Q, Cummings K, Gimeno J, Dean D, Powers AC, Gittes GK, Rüegg MA, Hall MN, MacDonald PE, Bernal-Mizrachi E. Loss of mTORC1 signaling alters pancreatic α cell mass and impairs glucagon secretion. J Clin Invest. 2017;127(12):4379–4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Jiang Y, Huang W, Wang J, Xu Z, He J, Lin X, Zhou Z, Zhang J. Metformin plays a dual role in MIN6 pancreatic β cell function through AMPK-dependent autophagy. Int J Biol Sci. 2014;10(3):268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Wu J, Wu JJ, Yang LJ, Wei LX, Zou DJ. Rosiglitazone protects against palmitate-induced pancreatic beta-cell death by activation of autophagy via 5′-AMP-activated protein kinase modulation. Endocrine. 2013;44(1):87–98. [DOI] [PubMed] [Google Scholar]
- 118. Janzen KM, Steuber TD, Nisly SA. GLP-1 agonists in type 1 diabetes mellitus. Ann Pharmacother. 2016;50(8):656–665. [DOI] [PubMed] [Google Scholar]
- 119. Tanemura M, Ohmura Y, Deguchi T, Machida T, Tsukamoto R, Wada H, Kobayashi S, Marubashi S, Eguchi H, Ito T, Nagano H, Mori M, Doki Y. Rapamycin causes upregulation of autophagy and impairs islets function both in vitro and in vivo. Am J Transplant. 2012;12(1):102–114. [DOI] [PubMed] [Google Scholar]
- 120. Tanemura M, Saga A, Kawamoto K, Machida T, Deguchi T, Nishida T, Sawa Y, Doki Y, Mori M, Ito T. Rapamycin induces autophagy in islets: relevance in islet transplantation. Transplant Proc. 2009;41(1):334–338. [DOI] [PubMed] [Google Scholar]
- 121. Zhou Z, Wu S, Li X, Xue Z, Tong J. Rapamycin induces autophagy and exacerbates metabolism associated complications in a mouse model of type 1 diabetes. Indian J Exp Biol. 2010;48(1):31–38. [PubMed] [Google Scholar]
- 122. Chang G-R, Wu Y-Y, Chiu Y-S, Chen W-Y, Liao J-W, Hsu H-M, Chao TH, Hung SW, Mao FC. Long-term administration of rapamycin reduces adiposity, but impairs glucose tolerance in high-fat diet-fed KK/HlJ mice. Basic Clin Pharmacol Toxicol. 2009;105(3):188–198. [DOI] [PubMed] [Google Scholar]
- 123. Chang G-R, Chiu Y-S, Wu Y-Y, Chen W-Y, Liao J-W, Chao T-H, Mao FC. Rapamycin protects against high fat diet-induced obesity in C57BL/6J mice. J Pharmacol Sci. 2009;109(4):496–503. [DOI] [PubMed] [Google Scholar]
- 124. Gong F-H, Ye Y-N, Li J-M, Zhao H-Y, Li X-K. Rapamycin-ameliorated diabetic symptoms involved in increasing adiponectin expression in diabetic mice on a high-fat diet. Kaohsiung J Med Sci. 2017;33(7):321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Reifsnyder PC, Flurkey K, Te A, Harrison DE. Rapamycin treatment benefits glucose metabolism in mouse models of type 2 diabetes. Aging (Albany NY). 2016;8(11):3120–3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Fang Y, Westbrook R, Hill C, Boparai RK, Arum O, Spong A, Wang F, Javors MA, Chen J, Sun LY, Bartke A. Duration of rapamycin treatment has differential effects on metabolism in mice. Cell Metab. 2013;17(3):456–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Fraenkel M, Ketzinel-Gilad M, Ariav Y, Pappo O, Karaca M, Castel J, Berthault MF, Magnan C, Cerasi E, Kaiser N, Leibowitz G. mTOR inhibition by rapamycin prevents beta-cell adaptation to hyperglycemia and exacerbates the metabolic state in type 2 diabetes. Diabetes. 2008;57(4):945–957. [DOI] [PubMed] [Google Scholar]
- 128. Schindler CE, Partap U, Patchen BK, Swoap SJ. Chronic rapamycin treatment causes diabetes in male mice. Am J Physiol Regul Integr Comp Physiol. 2014;307(4):R434–R443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Lamming DW, Ye L, Astle CM, Baur JA, Sabatini DM, Harrison DE. Young and old genetically heterogeneous HET3 mice on a rapamycin diet are glucose intolerant but insulin sensitive. Aging Cell. 2013;12(4):712–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Lamming DW, Ye L, Katajisto P, Goncalves MD, Saitoh M, Stevens DM, Davis JG, Salmon AB, Richardson A, Ahima RS, Guertin DA, Sabatini DM, Baur JA. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science. 2012;335(6076):1638–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Barlow AD, Nicholson ML, Herbert TP. Evidence for rapamycin toxicity in pancreatic β-cells and a review of the underlying molecular mechanisms. Diabetes. 2013;62(8):2674–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Vergès B, Cariou B. mTOR inhibitors and diabetes. Diabetes Res Clin Pract. 2015;110(2):101–108. [DOI] [PubMed] [Google Scholar]
- 133. Arriola Apelo SI, Neuman JC, Baar EL, Syed FA, Cummings NE, Brar HK, Pumper CP, Kimple ME, Lamming DW. Alternative rapamycin treatment regimens mitigate the impact of rapamycin on glucose homeostasis and the immune system. Aging Cell. 2016;15(1):28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Arriola Apelo SI, Pumper CP, Baar EL, Cummings NE, Lamming DW. Intermittent administration of rapamycin extends the life span of female C57BL/6J mice. J Gerontol Ser A. 2016;71(7):876–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. White E. The role for autophagy in cancer. J Clin Invest. 2015;125(1):42–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Morel E, Mehrpour M, Botti J, Dupont N, Hamaï A, Nascimbeni AC, Codogno P. Autophagy: a druggable process. Annu Rev Pharmacol Toxicol. 2017;57(1):375–398. [DOI] [PubMed] [Google Scholar]
- 137. Galluzzi L, Bravo-San Pedro JM, Levine B, Green DR, Kroemer G. Pharmacological modulation of autophagy: therapeutic potential and persisting obstacles. Nat Rev Drug Discov. 2017;16(7):487–511. [DOI] [PMC free article] [PubMed] [Google Scholar]