Abstract
Background
Numerous illnesses are associated with bathing in natural waters, although it is assumed that the risk of illness among bathers exposed to relatively clean waters found in high-income countries is negligible. A systematic review was carried out to quantify the increased risk of experiencing a range of adverse health outcomes among bathers exposed to coastal water compared with non-bathers.
Methods
In all 6919 potentially relevant titles and abstracts were screened, and from these 40 studies were eligible for inclusion in the review. Odds ratios (OR) were extracted from 19 of these reports and combined in random-effect meta-analyses for the following adverse health outcomes: incident cases of any illness, ear infections, gastrointestinal illness and infections caused by specific microorganisms.
Results
There is an increased risk of experiencing symptoms of any illness [OR = 1.86, 95% confidence interval (CI): 1.31 to 2.64, P = 0.001] and ear ailments (OR = 2.05, 95% CI: 1.49 to 2.82, P < 0.001) in bathers compared with non-bathers. There is also an increased risk of experiencing gastrointestinal ailments (OR = 1.29, 95% CI: 1.12 to 1.49, P < 0.001).
Conclusions
This is the first systematic review to evaluate evidence on the increased risk of acquiring illnesses from bathing in seawater compared with non-bathers. Our results support the notion that infections are acquired from bathing in coastal waters, and that bathers have a greater risk of experiencing a variety of illnesses compared with non-bathers.
Keywords: Bathing beaches, saline waters, waterborne diseases, sports
Key Messages
Data from 19 studies were included in the meta-analyses.
In high-income countries, bathers are at an increased risk of reporting a variety of illnesses compared with non-bathers.
Head immersion has little effect on the risk to bathers of reporting illnesses.
Introduction
The association between bathing in recreational waters and the risk of illness has been the subject of many epidemiological studies since the 1950s.1 A large number of these investigations report that exposure to water contaminated with faecal material increases a person’s risk of illness, particularly symptoms of gastrointestinal illness.1,2 However, not all studies report such results,3 and the evidence that bathing increases a person’s risk of illness is still uncertain. There is a perception among policy-makers in high-income countries that the risk of ill health associated with bathing is negligible, since many high-income countries report high rates of compliance with bathing water quality regulations.4–6
To develop evidence-based guidelines for ‘safe’ bathing waters where bathers have a low risk of becoming ill, many studies have examined the relationship between the density of faecal indicator organisms (FIOs) in natural waters and the risk of illness in bathers.1 In 2003, a systematic review and meta-analysis of 27 epidemiological studies demonstrated that the FIOs, Escherichia coli and enterococci, were more correlated with the risk of gastrointestinal illness in bathers after exposure to natural waters compared with other FIOs that had been studied.2 Recently, a meta-analysis by Arnold et al. (2016) confirmed that elevated levels of enterococcus were associated with an increased risk of diarrhoea among children.7
Many countries regularly monitor bathing waters for these FIOs to ensure that levels of faecal contamination are not high enough to pose an excess risk to bather health, as recommended by the World Health Organization.6 However, the cost of this activity restricts the agencies responsible for monitoring bathing waters to testing samples taken from a limited number of bathing waters during periods of high use (commonly called ‘the bathing season’).8 Due to the large spatial and temporal fluctuations in FIO densities that occur in natural waters, weekly measurements are unlikely to capture high levels of faecal pollution that occur in between samples.6,9 A number of recent studies have reported that even in bathing waters that were considered to be of good quality (containing low levels of FIOs), bathers were still at an increased risk of experiencing gastrointestinal illness, along with respiratory infections and ear and eye ailments.10–12 Therefore, it is useful to know what risk of illness people face when they bathe in natural waters, regardless of the timing or levels of FIOs.
Current methods of assessing bathing water quality thresholds are limited to an evidence base which has largely focused on gastrointestinal illnesses among bathers. Gastrointestinal symptoms are the most commonly investigated adverse health outcome in epidemiological studies conducted, but bathing in natural waters has been reported to be associated with other illnesses and indicators of infection, including respiratory infections, skin ailments, eye infections and ear infections.1,6,13,14 Furthermore, bathing is an umbrella term for swimming or spending time in the water, encompassing many different activities. Individuals who enjoy high-exposure activities, such as surfing, may be at greater risk of acquiring an infection, since they usually have a greater number of unexpected head immersions and ingest more water.6,15
In this systematic review, the aim was to gather, appraise and synthesize the evidence to answer the following research questions.
Do bathers have an increased risk of experiencing symptoms of infections following recreational use of coastal water, compared with non-bathers?
Does the level of increased risk depend on the nature of exposure to the water?
Having good quality evidence on the risks of infection from using coastal waters for recreation is important for communicating to the public the variety of illnesses they might experience after bathing, and whether certain behaviours (such as doing water sports involving head immersion) alter the risk of experiencing symptoms of ill health. Understanding these risks will allow members of the public to make informed decisions about their bathing habits in coastal waters.
Methods
In August 2013 a protocol was registered with the Prospero database (registration number CRD42013005307). The characteristics of the studies (PECO, see below) to be included in the review were defined and used to develop the search strategy and selection criteria.
Inclusion criteria
The review included the following.
Populations (P). These comprise studies investigating healthy human subjects.
Exposures (E). These comprise any recreational activity done in or on water, involving contact with natural waters. Natural waters are considered to be bodies of water that are not disinfected, like the sea and lakes.
Comparators (C). The main comparator group in this review are non-bathers (people who have not had contact with natural waters). Comparator groups also considered are water users exposed to unpolluted water bodies.
Outcomes (O). These include symptoms of infections likely to be have been caused by infectious microorganisms present in natural waters, particularly sewage-associated microorganisms. Symptoms are not restricted to those related to gastrointestinal illnesses and respiratory, skin, eye and ear infections. Additionally, we consider papers reporting other indicators of infection such as observing infective organisms in stool samples and detecting antibodies against infective agents (serology).
Studies were restricted according to the selection criteria (Table S1, available as Supplementary data at IJE online). Briefly, only studies published in English after 1961 were included. Study designs included were limited to observational epidemiological studies and randomized exposure trials, where the health outcome(s) under investigation had been reported in a suitable comparator group. Studies that were conducted in countries that were not members of the Organisation for Economic Co-operation and Development (OECD)16 were excluded, as were studies that did not investigate health outcomes related to microbial pollution of natural waters.
Information sources
The search strategy aimed to identify published and unpublished literature. This involved using Medical Subject Headings (MeSH) and free-text terms to search databases, hand-searching pertinent journals, asking an expert in the field to suggest articles that might have been missed, looking for unpublished articles and forward- and reverse-citation chasing. Each of these approaches is described in more detail below.
In July 2013, six databases (Table S2, available as Supplementary data at IJE online) were searched for articles. Two of these, BIOSIS and MEDLINE, were searched again in June 2015. The search strategy developed for use in MEDLINE (Table S3) was modified for use in the other databases. In July 2013, the peer-reviewed journal ‘Water Quality, Exposure and Health was hand-searched for suitable articles. This journal was chosen for hand-searching because in 2009 it published a systematic review on skin ailments after recreational exposure to natural waters14 and was not indexed by any of the databases searched.
Results of unpublished studies were also included in the review, and a variety of information sources providing unpublished results of studies were incorporated into the search strategy to combat publication bias:17 In July 2013, Dr Nick Ashbolt, a scientist who has published studies on the topic of water quality and human health, was contacted and asked for a list of key papers. Additionally, in August 2013, the websites of 11 environment and health authorities (see Table S4, available as Supplementary data at IJE online) were searched using the phrases ‘bathing water quality’ and ‘human health’. Results were sorted by relevance and the first 50 hits were evaluated for their suitability for the review. The web-based search engine [www.google.com] was used in a similar way to identify additional studies. Finally, the reference lists of identified reports and reviews were searched (reverse-citation chasing), along with the list of papers that had cited included studies (forward-citation chasing) to identify further relevant papers.
Study selection
After removing duplicate records, selection criteria were used to screen titles and abstracts for inclusion in the review. Independent double-screening was shared among four members of the review team (A.L., R.G., A.S. and W.G.). Disagreements about which records should be retrieved for full-text review were discussed, and if a decision could not be reached, the record was discussed by all members of the review team.
Full texts for the records identified in the first round of screening were obtained and screened using these same selection criteria. Independent double-screening was shared among four members of the review team (A.L., R.G., A.S. and W.G.). Any discrepancies were discussed, and if a decision could not be reached the record was discussed by all members of the team.
Data extraction and quality appraisal
Data extraction forms for four different study designs were drafted and piloted on a selection of different study designs: randomized controlled trials (RCTs), cohort studies, cross-sectional studies and case-control studies (Table S5, available as Supplementary data at IJE online). The following data were extracted, where available, from each record:
description of the study: the study location, year(s) of study, study design and sample size;
study population: eligibility criteria and methods of recruitment;
exposure assessment: the recreational exposure and how this was defined and assessed, and the comparator groups’ exposure and how this was defined and assessed;
outcome assessment: the health outcomes investigated in the study, how this information was collected, and how these outcomes were diagnosed.
The numbers of cases among exposed and unexposed individuals were extracted, or were calculated if enough data were provided. This information was used to calculate an odds ratio (OR) and 95% confidence intervals (95% CI) using STATA statistical analysis software.18 If fewer than one case or control was reported in the exposed or unexposed group, 0.5 was added to each cell of the 2-by-2 table to estimate crude ORs and 95% CI.19 If reported, adjusted ORs and 95% CI were also extracted.
If articles had not provided enough information to adequately appraise the quality of the study, authors of the reports were contacted in order to get additional information about their investigation. Final and technical reports, as well as results from pilot studies, were obtained where possible and used to fill in information where it was missing or unclear in published records.
Each study was assessed for quality and sources of bias using a relevant version of the Critical Appraisal Skills Programme (CASP) tool. Studies were recorded as being of good quality (the design and methods of the study were sufficient to make the results reliable), moderate quality (the design and methods of the study were sufficient to make the results fairly reliable) or poor quality (the design and methods of the study were insufficient to make the results reliable). A quality appraisal tool interpretation instrument (Table S7, available as Supplementary data at IJE online) was developed to ensure the same assessment criteria were applied to all studies. Data extraction and quality appraisal were conducted by one reviewer (A.L.) and were double-checked by one of three members of the review team (AS., R.G. and W.G.). Any disagreements were discussed and if a decision could not be made, the paper was discussed as a team.
Data analysis
Given the scale of the evidence base and resource constraints, the freshwater papers were not fully data-extracted or quality-appraised, and have not been included in this review. The results presented here focus on the risks of incident cases of the following four health outcome categories among bathers exposed to coastal waters compared with non-bathers (beach-going non-bathers and people who had not been to the beach): (i) cases of any illness; (ii) ear ailments; (iii) gastrointestinal illness; and (iv) infections caused by specific microorganisms. The selected outcome categories were chosen after data extraction but before data analysis in order to: (i) get a sensitive measure of the risk of illness by meta-analysing the risk of experiencing any illness; (ii) investigate an outcome, ear ailments, for which there is intense interest among water users but which has not been systematically reviewed before; (iii) compare these risks with an outcome, gastrointestinal illness, that has been reviewed before; and (iv) provide insight into some of the pathogens that might be causing these symptoms. Data were synthesized in order to answer the following research questions.
Do bathers have an increased risk of experiencing symptoms of infections following recreational use of coastal water, compared with non-bathers?
The OR comparing the risk of infection in bathers with the risk of infection in non-bathers was pooled across studies. If the adjusted OR was available, this were used in the meta-analyses instead of the crude OR. Random-effects meta-analyses were conducted using a single OR estimate per included study.20 Two included studies estimated effects for separate subgroups (stratified analyses) but not an overall effect. As the raw data in these papers were not available, fixed-effect meta-analysis was used to combine stratified results reported in a given paper into a single overall estimate which was then subsequently pooled in the main analyses with results from the other papers using random-effects meta-analysis.21 For studies that stratified results by the extent of immersion in the water (for example, any water contact, paddling, water up to the waist, head immersion or swallowing water), the most inclusive definition was used (i.e. any water contact). Heterogeneity among studies was quantified using the I-squared (I2) statistic,22,23 and publication bias was assessed using funnel plots. Meta-regression was used to investigate whether the odds ratio for infection differed across three geographical regions (Europe, North America and Oceania).
Does the level of increased risk depend on the nature of exposure to the water?
Recreational exposures were categorized into two groups based on how the authors defined exposure: (i) any water contact, which was also considered to be exposures that were not defined in the study, and those which encompassed many different exposures including getting the head wet and not getting the head wet; and (ii) exposures where bathers got their head or face wet. Whereas the meta-analysis described above combined data from the most inclusive definitions of water contact (i.e. any water contact), the impact of high levels of contact (head immersion) were also assessed. The same methods as the first analysis were used. The results of the head immersion meta-analyses are reported in the Supplementary material (available as Supplementary data at IJE online).
Results
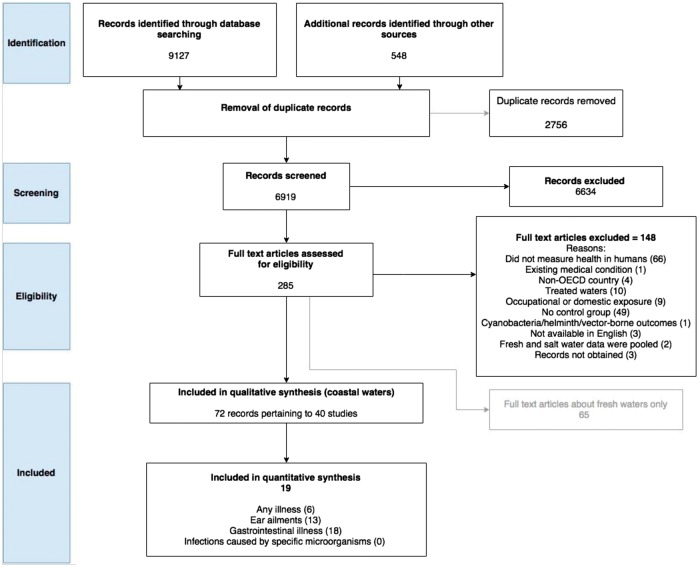
A total of 6919 titles and abstracts were screened for relevance. Of the 285 selected for full text review, 148 were excluded; 65 records for inclusion were put aside because they reported the results of studies conducted in freshwater environments (Table S9, available as Supplementary data at IJE online). This left 72 full texts pertaining to 40 separate studies for inclusion in the coastal water review (Figure 1). A reference list of the articles reporting the 40 included studies is presented in Table S8 (available as Supplementary data at IJE online).
Figure 1.
PRISMA flow chart of the number of records considered at each stage of the systematic review.
Description of the included studies
Location
Nearly half of the all studies (19/40) included in the coastal waters review were conducted in the USA. Eight studies were in the UK, four in Australia, two in New Zealand, two in Spain and one each in Denmark, Greece, Mexico, Norway and Turkey (Table 1).
Table 1.
Summary of key characteristics of the included studies
| Study ID | Quality | Location | Sample size | Health outcome categories reported |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any | Ear | GI | Eye | Other | Resp | Skin | U | Specific | ||||
| Randomized controlled trials | ||||||||||||
| Fleisher 2010 | G | USA | 1303 | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Kay 1994 | G | UK | 1216 | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Prospective cohort studies | ||||||||||||
| Alexander 1992 | P | UK | 703 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Arnold 2013 | G | USA | 5674 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Balarajan 1991 | M | UK | 1460 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Bonilla 2007 | P | USA | 1491 | ✓ | ✓ | |||||||
| Cabelli 1982 | M | USA | 26 686 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Colford 2005 | G | USA | 8797 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Colford 2012 | G | USA | 9525 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Corbett 1993 | M | Australia | 2968 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Fewtrell 1994 | M | UK | 450 | ✓ | ||||||||
| Fleming 2004 | P | USA | 208 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Haile 1999 | G | USA | 11 686 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Harrington 1993 | M | Australia | 1529 | ✓ | ✓ | ✓ | ✓ | |||||
| Kocasoy 1995 | G | Turkey | 3661 | ✓ | ✓ | |||||||
| Lepesteur 2006 | M | Australia | 340 | ✓ | ||||||||
| McBride 1998 | P | NZ | 3884 | ✓ | ✓ | |||||||
| Morens 1994 | P | USA | 2556 | ✓ | ✓ | |||||||
| Nelson 1997 | P | UK | NR | ✓ | ||||||||
| NJSDH 1988 | M | USA | 11 447 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Papastergiou 2011 | M | Greece | 4377 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Prieto 2001 | M | Spain | 1805 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| UNEP 1991 | M | Spain | 9691 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Wade 2010 | G | USA | 6350 | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Wade 2013 | G | USA | 11 159 | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Yau 2014 | G | USA | 6165 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Retrospective cohort study | ||||||||||||
| Harder-Lauridsen 2013 | M | Denmark | 1769 | ✓ | ✓ | ✓ | ||||||
| Cross-sectional studies | ||||||||||||
| Brown 1987 | P | UK | 1903 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Dale 2009 | M | Australia | 2794 | ✓ | ||||||||
| Dwight 2004 | M | USA | 1873 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Gammie 1997 | M | UK | 3533 | ✓ | ||||||||
| Harding 2015 | M | USA | 510 | ✓ | ✓ | ✓ | ✓ | |||||
| Reed 2006 | M | USA | 447 | ✓ | ||||||||
| Case-control studies | ||||||||||||
| Calderon 1982 | P | USA | 58 | ✓ | ||||||||
| Charoenca 1995 | P | USA | 106 | ✓ | ||||||||
| Hoque 2002 | P | NZ | 519 | ✓ | ||||||||
| Roy 2004 | P | USA | 772 | ✓ | ||||||||
| Soraas 2013 | M | Norway | 290 | ✓ | ||||||||
| Case-control studies of outbreaks | ||||||||||||
| Begier 2008 | P | Mexico | 25 | ✓ | ||||||||
| Ihekweazu 2006 | P | UK | 33 | ✓ | ||||||||
| Total | 147 583 | 10 | 21 | 28 | 20 | 19 | 25 | 19 | 5 | 9 | ||
Health outcomes investigated include: A, any illness; E, ear ailments; GI, gastrointestinal illness; Eye, eye ailments; Other, other illness; Resp, respiratory illness; S, skin ailment; U, urogenital complaint; Specific, infections caused by a specific microorganism; NZ, New Zealand; NR, Not reported; G, Good quality studies; M, Moderate quality studies; P, Poor quality studies.
Study size
The size of the 40 studies ranged from 25 subjects to 26 686.
Study designs
Four major study designs were identified: two RCTs, 25 cohort studies (24 prospective cohort and one retrospective cohort), six cross-sectional studies and seven case-control studies. Table 2 presents PECO descriptions for each study, and these are summarized briefly here.
Table 2.
Study characteristics (PECO) of the studies included in the coastal water review
| Study ID | Population | Exposure (assessment method) | Comparator group | Outcome assessment |
|---|---|---|---|---|
| Alexander 1992 | Beach-goers | Any water contact (self-reported) | Beach-going non-bathers | Self-reported by telephone 10 to 14 days after exposure |
| Children only (aged 6–11 years) | ||||
| Arnold 2013 | Beach-goers | Any water contact, body immersion, head immersion and swallowing water (self-reported) | Beach-going non-bathers | Self-reported by telephone at 10 to 19 days after exposure |
| Adults and children | ||||
| Balarajan 1991 | Beach-goers | Any water contact, wading, paddling, swimming, surfing, windsurfing, diving (self-reported) | Beach-going non-bathers | Self-reported by telephone in the week after exposure |
| Adults and children (aged ≥ 5 years) | ||||
| Begier 2008 | Holiday-makers to Mexico | Swimming (self-reported, 24-day recall period) | Non-bathers | Laboratory-diagnosed |
| Adults and children | ||||
| Bonilla 2007 | Beach-goers and people from the local population | Swimming, snorkelling, diving (self-reported) | Non-beach goers | Self-reported by questionnaire 4 days after exposure |
| Age range not reported | ||||
| Brown 1987 | Beach-goers | Swimming (self-reported in an interview, recall period not reported) | Beach-going non-bathers | Self-reported |
| Age range not reported | ||||
| Cabelli 1982 | Beach-goers | Swimming (self-reported) | Beach-going non-bathers | Self-reported by telephone 7 to 10 days after exposure |
| Adults and children (families with children < 10 years old) | ||||
| Calderon 1982 | Children attending a health care facility (aged 4 years to 17 years) | Swimming (self-reported, 7-day recall period) | No swimming in the sea | Physician-diagnosed |
| Charoenca 1995 | Children attending a health care facility (aged 4 months to 16 years) | Any water contact, swimming, boogie boarding, wading (self-reported, 10-day recall period) | No water contact | Physician-diagnosed |
| Colford 2005 | Beach-goers | Any water contact, body immersion, head immersion and swallowing water (self-reported) | Beach going non-bathers | Self-reported by telephone 10 to 14 days after exposure |
| Adults and children | ||||
| Colford 2012 | Beach-goers | Any water contact, body immersion, head immersion and swallowing water (self-reported) | Beach-going non-bathers | Self-reported by telephone 10 to 14 days after exposure |
| Adults and children | ||||
| Corbett 1993 | Beach-goers | Swimming (self-reported) | Beach-going non-bathers. | Self-reported by telephone 7 to 10 days after exposure |
| Adults only (aged ≥ 15 years) | ||||
| Dale 2009 | Community | Swimming (self-reported) | Non-bathers | Self-reported in a weekly diary kept for 68 weeks |
| Adults and children (families with children 1–15 years old) | ||||
| Dwight 2004 | Adult surfers (aged ≥ 18 years) | Surfing (self-reported , 3-month recall period) | Surfers exposed to coastal water affected by diffuse pollution | Self-reported in an interview |
| Fewtrell 1994 | People attending a sports event (rowing) | Rowing (participation in a sporting event) | Event spectators (non-rowers) | Self-reported by telephone 5 to 7 days after exposure, and a postal questionnaire 1 to 4 weeks after exposure |
| Fleisher 2010 | Beach-goers | Swimming (randomized and observed) | Beach-going non-bathers | Self-reported by telephone 7 days after exposure |
| Adults only (aged ≥18 years) | ||||
| Fleming 2004 | Beach-goers | Swimming (self-reported) | Bathers exposed to historically poor quality bathing waters | Self-reported by telephone 8 to 10 days after exposure |
| Adults and children | ||||
| Gammie 1997 | Participants at water surfing event | Surfing (participation in a sporting event, recall period not reported) | Territorial army (no exposure) | Laboratory-diagnosed |
| Adults and children | ||||
| Haile 1999 | Beach-goers | Swimming (head immersion) Observed by study investigators | Bathers exposed to less polluted water | Self-reported by telephone 9 to 14 days after exposure |
| Adults and children | ||||
| Harder-Lauridsen 2013 | Athletes participating in a triathlon | Swimming (participation in a sporting event) | Bathers exposed to less polluted water | Self-reported via online questionnaire 14 to 30 days after exposure; laboratory-diagnosed |
| Adults only | ||||
| Harding 2015 | Surfers | Surfing (self-reported, recall period not reported) | Surfers exposed to less polluted water | Self-reported via online questionnaire |
| Adults only (aged ≥ 18 years) | ||||
| Harrington 1993 | Beach-goers | Swimming (self-reported) | Beach-going non-bathers | Self-reported in a daily diary kept for 2 months |
| Adults only (aged 14 years to 40 years) | ||||
| Hoque 2002 | Community | Swimming (self-reported by telephone, 21-day recall period) | Non-bathers | Laboratory-diagnosed |
| Adults only (aged 15 years to 64 years) | ||||
| Ihekweazu 2006 | Children holidaying in Cornwall (aged 1–10 years) | Water contact (self-reported) | Non-bathers | Laboratory-diagnosed |
| Kay 1994 | Beach-goers | Swimming (randomized and observed) | Beach-going non-bathers | Self-reported in face-to-face interview 7 days after exposure, and by post 21 days after exposure |
| Adults only (aged ≥ 18 years) | ||||
| Kocasoy 1995 | Beach-goers | Water contact (self-reported) | Beach-going non-bathers. Also, bathers exposed to relatively less polluted waters | Self-reported by telephone 10 to 15 days after exposure |
| Adults and children | ||||
| Lepesteur 2006 | Beach-goers, | Water contact (self-reported) | Beach-going non-bathers | Self-reported by telephone 14 days after exposure |
| Adults and children | ||||
| McBride 1998 | Beach-goers | Any water contact, body immersion and head immersion (self-reported) | Beach-going non-bathers | Self-reported by telephone or postal questionnaire 3 to 5 days after exposure |
| Adults and children (aged ≥5 years) | ||||
| Morens 1994 | Beach-goers | Swimming (self-reported) | Beach-going non-bathers | Self-reported by telephone 3 days after exposure |
| Adults and children | ||||
| Nelson | Beach-goers | Swimming and wading (self-reported) | Beach-going non-bathers | Self-reported by telephone 10 days after exposure |
| Adults and children | ||||
| New Jersey State Department of Health (NJSDH) 1988 | Beach-goers | Any water contact, body immersion, and head immersion (self-reported) | Beach-going non-bathers and members of community who had not been to the beach | Self-reported by telephone 3 to 5 days after exposure |
| Adults and children (families with children < 10 years old) | ||||
| Papastergiou 2011 | Beach-goers and members of community (non-beach-goers) | Any water contact, body immersion and head immersion (self-reported) | Beach-going non-bathers and members of community who had not been to the beach | Self-reported by telephone 10 days after exposure |
| Adults and children | ||||
| Prieto 2001 | Beach-goers | Swimming (self-reported) | Beach-going non bathers | Self-reported by telephone 7 days after exposure |
| Adults and children | ||||
| Reed 2006 | Community | Swimming (self-reported in questionnaire) | Never swimming in the ocean | Physician-diagnosed |
| Adults and children (aged > 1 year) | ||||
| Roy 2004 | Community | Swimming (self-reported by telephone, 14-day recall period) | Non-bathers | Lab-diagnosed |
| Adults and children | ||||
| Soraas 2013 | Community | Swimming (self-reported in face-to-face interview or by telephone, 1-year recall period) | Non-bathers | Laboratory-diagnosed |
| Adults only (aged ≥ 18 years) | ||||
| UNEP 1991 | Beach-goers | Swimming (self-reported, and validated by investigators observing subjects) | Beach-going non-bathers, as well as bathers exposed to less polluted waters | Self-reported by telephone 5 to 10 days after exposure |
| Adults and children | ||||
| Wade 2010 | Beach-goers | Swimming (self-reported) | Beach-going non-bathers | Self-reported by telephone 10 to 12 days after exposure |
| Adults and children | ||||
| Wade 2013 | Beach-goers | Swimming, including body immersion, head immersion and swallowing water (self-reported) | Beach-going non-bathers | Self-reported by telephone 10 to 14 days after exposure |
| Adults and children | ||||
| Yau 2014 | Beach-goers | Swimming, including body immersion, head immersion and swallowing water (self-reported) | Beach-going non-bathers | Self-reported by telephone at 10 to 14 days after exposure |
| Adults and children |
Populations
Nearly two-thirds of studies (25/40) recruited beach-goers by approaching potential participants at the beach. Four sourced participants from the local community, three identified subjects from patients attending health care facilities, three recruited participants at water sports events and three recruited participants who self-identified as being regular water users. The two studies investigating outbreaks recruited holiday-makers who had visited the same region. Twenty-four studies recruited both adults and children, nine studies recruited adults only, four recruited children only and three did not report this information.
Exposures
Definitions of bathing varied greatly among the studies, from any kind of contact with seawater to body immersion, head immersion and swallowing water. Six studies investigated people doing specific water sports, including surfing, diving and rowing. The majority of studies (34/40) had participants self-report their exposure to seawater during an interview with investigators or in a questionnaire. The exceptions to this were: two RCTs randomized participants into bather and non-bather groups, three studies recruited participants at water sports events and one study had investigators observe the location of bathers in the water.
Comparator groups
The comparator groups in 35 studies were non-bathers. In 23 studies, these participants were recruited at the beach, although in other studies non-bathers were recruited from members of the community who had not been to the beach, or who had not taken part in a water sport event. The comparator groups in five of the studies comprised bathers who had been exposed to water with lower levels of pollution.
Outcomes
The outcome categories reported by each study are presented in Table 1. The majority of studies (31/40) relied upon participants self-reporting symptoms of illness. This information was usually collected in a telephone interview 3 to 15 days after exposure to seawater, although people also reported symptoms in questionnaires or diaries. Seven studies analysed specimens from subjects using laboratory-based methods, and three studies had cases diagnosed by health care professionals.
The quality of studies
The CASP tool was used to assess the presence of various types of bias in the studies, such as misclassification bias. The overall quality of a study was evaluated by posing the question: ‘Are the results reliable?’, to which the answer could be ‘yes’ (good quality), ‘partially’ (moderate quality) or ‘no’ (poor quality). The overall quality of each study is reported in Table 1. Few high quality studies were found, and were found exclusively among the prospective cohort studies and RCTs. Other study designs (cross-sectional and case-control studies) were generally found to be of moderate or poor quality.
The quality assessment tool also considered how well each study was able to demonstrate a causal relationship between exposure and the outcomes investigated. This was based upon Hill’s considerations for causation.24 None of the studies met all of the criteria, a major limitation among all studies being lack of specificity (health outcomes specific to swimming in seawater or demonstrating that infectious organisms are present in both the environment and exposed individuals). One study analysed water samples and human specimens (ear and throat swabs) for a variety of bacteria to identify pathogenic organisms responsible for the symptoms observed among bathers.25 However, the authors were unable to identify specific pathogens in symptomatic participants, and none of the studies succeeded in identifying the microorganisms causing symptoms of ill health as well as isolating the causal organisms from seawater at the time of exposure.
Risk of illness or infection
Characteristics of the included studies have been summarized as part of the quantitative synthesis for the four outcomes: (i) any illness; (ii) ear ailments; (iii) gastrointestinal illness; and (iv) infections caused by specific microorganisms. Case definitions reported by studies included in the meta-analysis have been provided in the Supplementary materials (Table S10, available as Supplementary data at IJE online). Due to the size of the review, only the risks of experiencing incident cases, as opposed to prevalent cases, of these infections among bathers compared with non-bathers were meta-analysed. Forest plots for each random-effects analysis are available in the Supplementary material (Figures S1 to S12, available as Supplementary data at IJE online). Additionally, the effects that head immersion specifically has on the risks of these infections were assessed, and these results are presented in the Supplementary material (Figures S11 to S18, available as Supplementary data at IJE online). Estimates of association based on prevalence data were not pooled since any differences in the occurrence of prevalent cases of illness between bathers and non-bathers might not be due to exposure to coastal waters.
Risk of experiencing symptoms of any illness
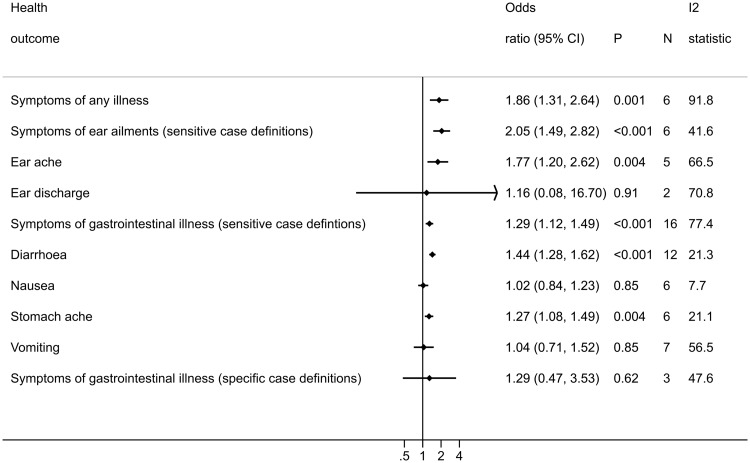
Ten studies reported the outcome ‘any illness’ (Table 1). Four of these could not be included in this meta-analysis because it was either not possible to obtain an OR and 95% CI from the data reported (Harrington 1993, Nelson 1997), or the comparator group were not non-bathers (Dwight 2004, Fleming 2004). This left six studies that were suitable for meta-analysis. The results show that there is an increase in the risk to bathers of experiencing any illness compared with non-bathers (OR = 1.86, 95% CI: 1.31 to 2.64, P = 0.001: Figure 2). Despite considerable heterogeneity between the included studies (I2 = 91.8%), all the studies included in this analysis report an increased risk of experiencing symptoms of any illness compared with non-bathers (Figure S1). Meta-regression findings indicated that there was little evidence to suggest that the odds ratio differs across regional subgroups (P = 0.99) (Table S13, available as Supplementary data at IJE online).
Figure 2.
Forest plot displaying the results of random-effects meta-analyses conducted for each health outcome investigated. Forest plots for each individual meta-analysis are in the Supplementary materials (Figures S1 to S12, available as Supplementary data at IJE online).
Risk of experiencing ear ailments
A total of 21 studies reported on the risk of ear ailments (Table 1). Eight studies could not be pooled in a meta-analysis because the comparator group were not non-bathers (Dwight 2004, Fleming 2004, Haile 1999, Yau 2014), or prevalence was measured (Alexander 1992, Brown 1987, Dwight 2004, Harding 2015). The nine remaining studies reported data that were suitable for pooling.
Sensitive case definition
Among bathers there is an increased risk of experiencing ear ailments compared with non-bathers (OR = 2.05, 95% CI: 1.49 to 2.82, P < 0.001: Figure 2). The results reported by all studies were reasonably consistent based on effect size, and heterogeneity was moderate (I2 = 41.6%: Figure S2).23 There was little evidence to suggest that the odds ratio differs across regions (P = 0.10) (Table S13, available as Supplementary data at IJE online).
Single symptom case definitions
Bathers were at increased risk of experiencing earache compared with non-bathers (OR = 1.77, 95% CI: 1.20 to 2.63, P = 0.004: Figure 2). The point estimates reported by all the included studies were greater than one, with the exception of Colford 2005 (Figure S3), and the I2 value (66.5%) indicated moderate heterogeneity. Conversely, there was little evidence of an increase in the risk to bathers of experiencing ear discharge (OR = 1.16, 95% CI: 0.08 to 16.6, P = 0.91: Figure 2). Two studies were included in this analysis and the I2 value indicates high heterogeneity (70.8%: Figure S4). There was little evidence to suggest that differences in risk of earache among bathers observed across regional subgroups was due to region (P = 0.31). There were too few studies to conduct this analysis on ear discharge (Table S13).
Specific case definition
One study reported data for the reporting of ear ailments which had a specific case definition (Papastergiou 2011). This study reported an odds ratio of 8.6 (95% CI: 0.52 to 140.5, P = 0.13), suggesting little evidence of an association between bathing and ear ailments.
Case definition not reported
Five studies estimated the risk of bathers experiencing undefined cases of ear ailments compared with non-bathers (Figure S5).
Risk of reporting gastrointestinal illness
A total of 28 studies reported the risk of symptoms of gastrointestinal illness (Table 1). Nine of these could not be included in the meta-analyses because the comparator group were not non-bathers (Dwight 2004, Fleming 2004, Haile 1999, Harder-Lauridsen 2013, Harding 2015), prevalence was measured (Alexander 1992, Brown 1987, Dwight 2004, Harding 2015) or ORs were not available (Harrington 1993, Morens 1994). The 18 remaining studies reported data that were suitable for pooling.
Sensitive case definitions
There was an increased risk of bathers experiencing gastrointestinal illness compared with non-bathers (OR = 1.29, 95% CI: 1.12 to 1.49, P < 0.001: Figure 2). All but one of the pooled studies make the same observation: bathers are at an increased risk of experiencing gastrointestinal illness (Figure S6a). However, the results reported in Bonilla 2007 are noticeably different from the rest of the studies, reporting that bathers have a lower risk of experiencing gastrointestinal symptoms compared with non-bathers. This is most likely due to the comparator group recruited in this study being members of the general population who did not go to the beach, rather than (as is the case with the other studies) being beach-goers who did not go in the water. The comparator group in the Bonilla 2007 study could consist of people with poorer health overall, experiencing higher rates of illness than people who chose to go to the beach. This could be responsible for the high heterogeneity observed in this meta-analysis (I2 = 77.4%). Removing Bonilla 2007, as well as other studies where the comparator group could include people who had not been to the beach (Dale 2009 and Papastergiou 2011), from this meta-analysis slightly increased the point estimate (OR = 1.40, 95% CI: 1.29 to 1.52, P< 0.001), and reduced the heterogeneity (I2 = 17.6%) (Figure S6b). There was little evidence that the odds ratio differs across regions (P = 0.19) (Table S13).
Single symptom case definitions
Compared with non-bathers, bathers are at an increased risk of reporting diarrhoea (OR = 1.44, 95% CI: 1.28 to 1.63, P < 0.001: Figure 2), and stomach ache (OR = 1.27, 95% CI: 1.08 to 1.49, P = 0.004: Figure 2). The results reported by all studies for these two outcomes were reasonably consistent, in that they generally report OR greater than one (Figures S7 and S9). I2 values reported for these meta-analyses were low ( 21.3% and 21.1%). There was little evidence of an association between sea bathing and the risk of experiencing nausea (OR = 1.02, 95% CI: 0.84 to 1.23, P = 0.85: Figure 2) or vomiting (OR = 1.04, 95% CI: 0.71 to 1.51, P = 0.85: Figure 2). I2 values reported for these meta-analyses were low (7.7% for nausea) to moderate (56.6% for vomiting) (Figures S8 and S10). There was little evidence to suggest that differences in risk of diarrhoea, nausea, stomach ache or vomiting among bathers observed across regional subgroups were due to region (all P-values > 0.21) (Table S13).
Specific case definitions
There was no evidence of an association between sea bathing and the risk of experiencing cases of gastrointestinal illnesses that require two or more symptoms to be reported together (OR = 1.29, 95% CI: 0.47 to 3.52, P = 0.62: Figure 2). The results from the studies included in this meta-analysis were inconsistent based upon effect sizes (Figure S11), and statistical heterogeneity was moderate (I2 = 47.6%). There was little evidence to suggest that the odds ratios differ across regions (Table S13).
Case definition not reported
Four studies reported the odds ratios of bathers reporting undefined cases of gastrointestinal illness compared with non-bathers (Figure S12).
Infections caused by specific microorganisms
The above results focused on the reporting of symptomatic infections without identifying the causative agent. However, nine studies reported on the risks of infections caused by specific microorganisms (Table 1), including those caused by bacteria, viruses and protozoans (Table 3).
Table 3.
Infections caused by specific microorganisms, sorted by type of causative agent
| Health outcome | Study ID |
|---|---|
| Bacterial pathogens | |
| Campylobacter infections, E. coli infections (including diarrhoeagenic E. coli, enteropathic E. coli, enterotoxigenic E. coli) | Harder-Lauridsen 2013 |
| Community-acquired urinary tract infections caused by ESBL-producing E. coli or Klebsiella pneumoniae | Soraas 2013 |
| E. coli O157 infections | Ihekweazu 2006 |
| Mycobacterium avium complex infection | Reed 2006 |
| Staphylococcal skin infections | Charoenca 1995 |
| Protozoan pathogens | |
| Cryptosporidium infections | Roy 2004 |
| Giardia infections | Hoque 2002, Harder-Lauridsen 2013 |
| Viral pathogens | |
| Echovirus infection | Begier 2008 |
| Hepatitis A | Gammie 1997 |
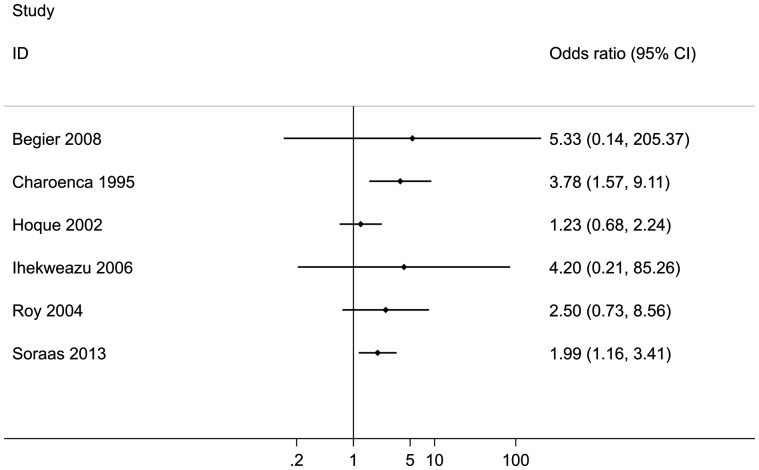
Due to the variety of infections reported, it was not sensible to combine the results of any of these studies in a meta-analysis. Instead, the quantitative results reported by the individual papers are presented separately in a forest plot (Figure 3). Point estimates for ORs were greater than one (indicating an increase in risk), although the precision of the ORs reported varied greatly. Two of these studies reported a statistically significant increase in the odds of acquiring bacterial infections among bathers compared with non-bathers. These were staphylococcal skin infections (Charoenca 1995), and community-acquired (CA) urinary tract infections (UTI) caused by the antibiotic-resistant bacteria, extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli and Klebsiella pneumoniae (Soraas 2013).
Figure 3.
Forest plot with the outcomes of studies investigating the risk of infections caused by specific organisms (outcome) associated with recreational exposure to coastal waters. A pooled estimate is not reported.
Discussion
This is the first study that has systematically reviewed the literature reporting on the association between recreational exposure to coastal waters and the incidence of non-enteric illnesses. Among bathers there is an increase in the risk of experiencing any symptoms of illness (OR = 1.86, 95% CI: 1.31 to 2.64, P = 0.001) and ear ailments (OR = 2.05, 95% CI: 1.49 to 2.82, P < 0.001), including symptoms like earache (OR = 1.77, 95% CI: 1.20 to 2.63, P = 0.004). In addition, the odds ratio of acquiring gastrointestinal illness between bathers and non-bathers, which has been systematically reviewed and pooled previously, was assessed. The results confirm that there is an increase in the risk of experiencing gastrointestinal symptoms (OR = 1.29, 95% CI: 1.12 to 1.49, P < 0.001), including diarrhoea (OR = 1.44, 95% CI: 1.28 to 1.63, P < 0.001) and stomach ache (OR = 1.27, 95% CI: 1.08 to 1.49, P = 0.004).
The risk of gastrointestinal illness among bathers has received a lot of attention in the literature. Recently, a meta-analysis has estimated the risk of gastroenteritis in children reported by 13 cohort studies conducted in the USA.7 Like our study, the Arnold paper indicates an increased risk for diarrhoea among bathers compared with non-bathers. In contrast, a recent rapid evidence assessment of the epidemiological literature, conducted since 2003, reported that although most papers report an increase in the odds of gastrointestinal illness among bathers at marine beaches compared with non-bathers, there is little evidence of a true difference.26 The most plausible reason for the contradictory results of this review is that the rapid evidence assessment conducted by King et al. was limited to reviewing studies published in the previous 10 years (from 2003 to 2013), and a pooled estimate was not produced as the authors considered their included studies too heterogeneous to combine. In a sensitivity analysis, studies conducted before 2006 were excluded from the meta-analyses. This was done because one might assume that water quality, as measured by FIOs, has improved over the years to comply with water quality regulations,4 and therefore one might expect that the risk of illness in recent years would be reduced since FIOs (such as enterococci) are associated with increased risk of illnesses.2,6,11,14,27 Pooling estimates only from studies conducted since 2006 did not greatly reduce the risk estimates for bathers experiencing ear ailments or gastrointestinal illnesses; the risk of ear ailments (including earache) remained higher in bathers compared with non-bathers, with small differences to the overall effect size (Table S12, available as Supplementary data at IJE online). The risk of experiencing gastrointestinal illness, including diarrhoea, also remained elevated in bathers compared with non-bathers (Table S12). However, after removing studies conducted before 2006, the increase in the risk of stomach ache was no longer statistically significant. While the included studies reported similar effect sizes to studies conducted before 2006, the small number of studies available for this analysis meant that the confidence intervals around the point estimates were wider. For the risk of nausea, no association was found between bathing and this particular outcome (OR = 1.02, 95% CI 0.84 to 1.23, P = 0.85). Likewise, there was no association between bathing and the incidence of vomiting (OR = 1.04, 95% CI 0.71 to 1.51, P = 0.85).
This review also investigated the effect that head immersion in seawater has upon the risk of experiencing illness. For most of the outcomes investigated, there were only small differences in the odds of reporting any of the illnesses investigated by immersing heads in seawater, which did not alter the interpretation of the results (Table S11). In the head immersion meta-analyses, point estimates were increased for any illness (OR = 1.91, 95% CI: 1.40 to 2.60, P < 0.001: Figure S13), cases of gastrointestinal illness (sensitive case definitions) (OR = 1.35, 95% CI: 1.17 to 1.55, P < 0.001: Figure S15), diarrhoea (OR = 1.54, 95% CI: 1.30 to 1.82, P < 0.001: Figure S16), nausea (OR = 1.16, 95% CI: 0.97 to 1.40, P = 0.10: Figure S16), stomach ache (OR = 1.31, 95% CI: 1.14 to 1.50, P < 0.001: Figure S16), vomiting (OR = 1.07, 95% CI: 0.74 to 1.54, P = 0.72: Figure S16) and specific case definitions of gastrointestinal illness (OR = 1.37, 95% CI: 1.10 to 1.72, P = 0.006: Figure S17). The OR point estimates were reduced for cases of ear ailments (sensitive case definitions) (OR = 1.79, 95% CI: 1.18 to 2.72, P = 0.006: Figure S14), and earache (OR = 1.64, 95% CI: 1.07 to 2.52, P = 0.02: Figure S14). Regional analyses were conducted to explore whether the odds ratios of the investigated health outcomes are different across geographical regions. The results indicated that there is little evidence of a true difference in the risks of any of the various outcomes investigated across regions (Table S13, available as Supplementary data at IJE online).
This is also the first time that the literature reporting on the association between infections caused by specific microorganisms and sea bathing has been systematically reviewed. All the included studies reported effect sizes greater than one, although the precision of these risk estimates varied greatly, with some of the 95% CIs reported including a value of one (Figure 3). Although viruses, like norovirus, are widely believed to be the most common cause of gastrointestinal illness among bathers,3,28–30 none of the studies included in this review assessed norovirus as the causative agent of symptomatic illness. Among the infections caused by specific microorganisms, the only infections that were significantly increased in bathers compared with non-bathers were caused by bacteria (Charoenca 1995, Soraas 2013).
Most of the studies were limited by selection and confounding bias: beach-goers who elect to go into the sea might comprise a different population of beach-goers to those who choose to stay out of the sea (self-selection bias), and people who volunteer in health studies might be different from those who choose not to (volunteer bias). Therefore, any observed health effects could be due to some other, unmeasured factor, rather than exposure to seawater (confounding bias).31 For these reasons, very few of the studies were scored as being of good quality. Differences in rates of attrition in the exposed and unexposed groups could be another source of bias in the included studies, but this was rarely reported. The RCTs go some way to reducing some of these biases. In a separate set of meta-analyses, the effect estimates reported by the RCTs were compared with those reported by observational studies. Observational studies reported smaller effect sizes compared with the RCTs for all the outcomes investigated (Table S14, available as Supplementary data at IJE online). Nevertheless, meta-analyses restricted to pooling data from only observational studies produced similar effect sizes and lead to the same conclusion being drawn: that bathers are at a higher risk of experiencing symptoms of illness compared with non-bathers.
Misclassification bias was another potential source of bias identified in many of the studies. Most relied upon participants to self-report their exposure and outcomes, the accuracy of which may be influenced by interviewer bias, recall bias and response bias.31 Many of the studies included in the meta-analyses conducted follow-up interviews several days after bathing (Table 2). Two of these studies also conducted analyses to estimate the risk of illness in bathers 3 days after bathing.11,32 Using the risks of illness experienced within 3 days of exposure in the relevant meta-analyses increased the size of the effect estimate slightly (Table S15, available as Supplementary data at IJE online). This is because the excess risk of illness is greatest in the first few days after bathing, and the size of the effect reduces as the risk period is extended further from the day of exposure, during which time both bathers and non-bathers might be exposed to other sources of pathogenic microorganisms.
In our meta-analyses, data were combined from studies regardless of the levels of faecal indicator organisms (FIOs) or type of pollution to which bathers were likely to have been exposed. Variations in FIOs (such as enterococci) and exposure to different sources of pollution (like human faecal contamination) might account for some of the heterogeneity observed in these meta-analyses, since the levels of FIOs and the type of pollution impacting on coastal waters are associated with the risk of illness in bathers.2,7,14,27,33 Another source of heterogeneity in the meta-analyses is likely to be the age of the study participants. Young children have been shown to be at greater risk of experiencing gastrointestinal illnesses compared with adolescents and adults.7 Whereas several included studies provided odds ratios adjusted for confounders (like age and sex), few report age-stratified effects and the ones that did used different age categories, making it difficult to explore the extent to which participants’ ages affected the effect sizes reported.
There are limitations inherent in this systematic review that must be considered. First, our selection criteria for studies excluded those conducted before 1961 and those that were not published in English. One study identified by our search was published before 1961, and three studies were not written in English. Publication bias was assessed using a funnel plot (Figure S19, available as Supplementary data at IJE online). There are few studies occupying the southwest corner of the funnel plot, indicating an under-representation of smaller studies reporting negative findings. The potential for publication bias was anticipated, therefore the search strategy was adapted to include searches for grey literature, and there were no limits on the size of the studies that could be included in the review. One study included in the review was not reported in a peer-reviewed journal (NJSDH 1988). Removing this study from the relevant meta-analyses did not markedly alter the odds ratio (Table S16, available as Supplementary data at IJE online). Moreover, studies that were conducted in countries that were not members of the OECD were excluded. This means that the results reported by this systematic review are not applicable to coastal waters in low- and middle-income countries, nor for landlocked countries and states. The search strategy identified 65 freshwater studies (Table S9), which will be summarized in a future systematic review.
Studies were excluded from the meta-analyses if they did not report an odds ratio and 95% CI or sufficient raw data for this summary statistic to be calculated. Therefore studies reporting other relative effect measures (e.g. risk ratios) were not included in the meta-analysis, as the necessary statistics were not calculable if raw data were unavailable.
Conclusions
This is the first systematic review to evaluate the evidence that bathers are at risk of getting sick from exposure to seawater, and to quantify this risk compared with non-bathers. The findings indicate that bathers are at a higher risk of experiencing a variety of symptoms of illness compared with non-bathers, including ear ailments, gastrointestinal illness and symptoms of any illness. Recreational exposure to coastal waters is likely to cause these illnesses, although future epidemiological studies should aim to identify the microorganisms responsible for observed symptoms, to address the issue of specificity.
Supplementary Data
Supplementary data are available as at IJE online.
Funding
This work was supported by the European Regional Development Fund [grant number 500020]. Funders played no role in the study design, nor in the collection, analysis or interpretation of data.
Data Access Statement
The data informing the random-effects meta-analyses in this paper can be publicly accessed in Open Research Exeter via the following persistent identifier: http://hdl.handle.net/10871/31530.
Supplementary Material
Acknowledgements
Thanks to information specialists, Morwenna Rogers and Chris Cooper at PenCLAHRC (University of Exeter Medical School) for their assistance in developing the search strategy, and to Dr Nicholas Ashbolt (University of Alberta) for sharing his expertise in this field with us. Obi Ukoumunne is funded, and Ruth Garside part-funded, by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care (CLAHRC) for the South West Peninsula at the Royal Devon and Exeter NHS Foundation Trust. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health in England.
Conflict of interest: We have no competing financial interests to declare.
References
- 1. Pruss A. Review of epidemiological studies on health effects from exposure to recreational water. Int J Epidemiol 1998;27:1–9. [DOI] [PubMed] [Google Scholar]
- 2. Wade TJ, Pai N, Eisenberg JNS, Colford JM Jr. Do U.S. Environmental Protection Agency water quality guidelines for recreational waters prevent gastrointestinal illness? A systematic review and meta-analysis. Environ Health Perspect 2003;111:1102–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Harwood VJ, Boehm AB, Sassoubre LM. et al. Performance of viruses and bacteriophages for fecal source determination in a multi-laboratory, comparative study. Water Res 2013;47:6929–43. [DOI] [PubMed] [Google Scholar]
- 4. European Environment Agency. European Bathing Water Quality in 2016. Luxembourg: European Environment Agency, 2017. [Google Scholar]
- 5. Ministry for the Environment. Microbiological Water Quality Guidelines for Recreational Water - Frequently Asked Questions. 2009http://www.mfe.govt.nz/fresh-water/tools-and-guidelines/microbiological-guidelines-recreational-water (4 May 2016, date last accessed). [Google Scholar]
- 6. World Health Organization. Guidelines for Safe Recreational Water Environments. Vol. 1, Coastal and Fresh Waters. Geneva: World Health Organization, 2003. [Google Scholar]
- 7. Arnold BF, Wade TJ, Benjamin-Chung J. et al. Acute gastroenteritis and recreational water: highest burden among young US children. Am J Public Health 2016;106:1690–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Directive 2006/7/EC of the European Parliament and of the European Council of 15 February 2006 concerning the management of bathing water quality and repealing Directive 76/160/EEC, (2006). https://publications.europa.eu/en/publication-detail/-/publication/…/language-en (16 May 2016, date last accessed).
- 9. Enns AA, Vogel LJ, Abdelzaher AM. et al. Spatial and temporal variation in indicator microbe sampling is influential in beach management decisions. Water Res 2012;46:2237–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Papastergiou P, Mouchtouri V, Pinaka O, Katsiaflaka A, Rachiotis G, Hadjichristodoulou C.. Elevated bathing-associated disease risks despite certified water quality: A cohort study. Int J Environ Res Public Health 2012;9:1548–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arnold BF, Schiff KC, Griffith JF. et al. Swimmer illness associated with marine water exposure and water quality indicators: impact of widely used assumptions. Epidemiology 2013;24:845–53. [DOI] [PubMed] [Google Scholar]
- 12. Colford JM, Wade TJ, Schiff KC. et al. Water quality indicators and the risk of illness at beaches with nonpoint sources of fecal contamination. Epidemiology 2007;18:27–35. [DOI] [PubMed] [Google Scholar]
- 13. Zmirou D, Pena L, Ledrans M, Letertre A.. Risks associated with the microbiological quality of bodies of fresh and marine water used for recreational purposes: Summary estimates based on published epidemiological studies. Arch Environ Health 2003;58:703–11. [DOI] [PubMed] [Google Scholar]
- 14. Yau V, Wade TJ, de Wilde CK, Colford JM Jr. Skin-related symptoms following exposure to recreational water: a systematic review and meta-analysis. Water Qual Expo Health 2009;1:79–103. [Google Scholar]
- 15. Leonard A, Zhang L, Balfour A, Garside R, Gaze W.. Human recreational exposure to antibiotic resistant bacteria in coastal bathing waters. Environ Int 2015;82:92–100. [DOI] [PubMed] [Google Scholar]
- 16. Organisation for Economic Cooperation and Development. List of OECD Member countries - Ratification of the Convention on the OECD 2016. http://www.oecd.org/about/membersandpartners/list-oecd-member-countries.htm (12 May 2016, date last accessed).
- 17. Higgins JPT, Green S.. Including unpublished studies in systematic reviews In: Higgins JPT, Green S (eds). Cochrane Handbook for Systematic Reviews of Intervention Version 5.1.0. London: Cochrane Collaboration, 2011. [Google Scholar]
- 18. StataCorp. Stata Statistical Software. Release 13. College Station, TX: StataCorp LP, 2013. [Google Scholar]
- 19. Deeks JJ, Higgins JPT.. Statistical Algorithms in Review Manager 5: on behalf of the Statistical Methods Group of The Cochrane Collaboration. 2010. http://ims.cochrane.org/ (30 April 2016, date last accessed).
- 20. DerSimonian R, Laird N.. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 21. Mantel N, Haenszel W.. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22:719–48. [PubMed] [Google Scholar]
- 22. Higgins JPT, Green S.. Cochrane Handbook for Systematic Review of Interventions Version 5.1.0. London: Cochrane Collaboration, 2011. [Google Scholar]
- 23. Higgins JP, Thompson SG, Deeks JJ, Altman DG.. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hill AB. The environment and disease: association or causation? Proc R Soc Med 1965;58:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jones F, Kay D, Stanwell-Smith R, Wyer M.. Results of the first pilot-scale controlled cohort epidemiological investigation into the possible health effects of bathing in seawater at Langland Bay, Swansea. J Inst Water Environ Mmanag 1991;5:91–98. [Google Scholar]
- 26. King S, Exley J, Winpenny E, Alves L, Henham M, Larkin J.. The Health Risks of Bathing in Recreational Waters: A Rapid Evidence Assessment of Water Quality and Gastrointestinal Illness. Cambridge, UK: RAND Europe, 2014. [PMC free article] [PubMed] [Google Scholar]
- 27. Arnold BF, Schiff KC, Ercumen A. et al. Acute illness among surfers after exposure to seawater in dry- and wet-weather conditions. Am J Epidemiol 2017;186:866–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Maunula L. Waterborne norovirus outbreaks. Future Virol 2007;2:101–12. [Google Scholar]
- 29. Maunula L, Kalso S, Von Bonsdorff CH, Ponka A.. Wading pool water contaminated with both noroviruses and astroviruses as the source of a gastroenteritis outbreak. Epidemiol Infect 2004;132:737–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sinclair RG, Jones EL, Gerba CP.. Viruses in recreational water-borne disease outbreaks: A review. J Appl Microbiol 2009;107:1769–80. [DOI] [PubMed] [Google Scholar]
- 31. dos Santos Silva I. Interpretation of epidemiological studies In: dos Santos Silva I. (ed). Cancer Epidemiology: Principles and Methods. Lyon, France: International Agency for Research on Cancer, 1999. [Google Scholar]
- 32. Yau VM, Schiff KC, Arnold BF. et al. Effect of submarine groundwater discharge on bacterial indicators and swimmer health at Avalon Beach, CA, USA. Water Res 2014;59:23–36. [DOI] [PubMed] [Google Scholar]
- 33. Benjamin-Chung J, Arnold BF, Wade TJ. et al. Coliphages and gastrointestinal illness in recreational waters: pooled analysis of six coastal beach cohorts. Epidemiology 2017;28:644–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.