Despite insufficiencies in concentrations of antimicrobial peptides, cervical mucus plug proteins can enhance opsonophagocytic killing of group B streptococcus.
Keywords: cervical mucus plugs, group B streptococcus, growth inhibition, pregnancy
Abstract
Preterm birth is a leading cause of neonatal mortality and lacks an effective therapy. Ascending microbial infections from the lower genital tract lead to infection of the placenta, amniotic fluid, and fetus causing preterm birth or stillbirth. Directly in the path of an ascending infection is the cervical mucus plug (CMP), a dense mucoid structure in the cervical canal with potential antimicrobial properties. In this study, we aimed to define the components of CMP responsible for antimicrobial activity against a common lower genital tract organism associated with preterm birth and stillbirths, namely, group B streptococcus (GBS). Using a quantitative proteomic approach, we identified antimicrobial factors in CMPs that were collected from healthy human pregnancies. However, we noted that the concentration of antimicrobial peptides present in the human CMPs were insufficient to directly kill GBS, and antimicrobial activity, when observed, was due to antibiotics retained in the CMPs. Despite this insufficiency, CMP proteins were able to activate leukocytes in whole blood resulting in increased rates of bacterial killing, suggesting a role for the CMP in enhancing complement-mediated killing or leukocyte activation. This study provides new insight into how the human CMP may limit ascending bacterial infection.
Preterm birth, defined as delivery before 37 weeks gestation, is the leading cause of neonatal morbidity and mortality [1–3]. Globally, preterm birth contributes to an estimated 4 million neonatal deaths and the incidence is rising [2, 4]. Cases of early preterm birth have a strong association with infection of the placenta and amniotic fluid [3, 5] by bacteria that normally reside in the vagina [6, 7]. These lower genital tract bacteria ascend into the uterus [3] and induce tissue damage leading to increased levels of proinflammatory cytokines, preterm premature rupture of membranes, cervical ripening, and uterine contractions [3, 8, 9]. A better understanding of mechanisms that prevent bacterial trafficking is necessary to design strategies for the prevention of infection-associated preterm birth.
The human cervical mucus plug (CMP) represents a unique, structural barrier between the lower genital tract and uterus. The CMP is a dense viscoelastic mass of mucoid material (~10 grams) that fills the cervical canal during human pregnancy and is produced by endocervical secretory cells [10]. Mucous glycoproteins (mucins) provide the structural framework for CMPs and act as binding partners for cytokines, larger proteins, and positively charged molecules [10]. Cervical mucus plugs can also contain a variety of antimicrobial compounds, such as lysozyme, lactoferrin, calprotectin, secretory leukoprotease inhibitor 1, and other antimicrobial peptides (AMPs) [11, 12]. Although these compounds are presumed to prevent resident vaginal flora from ascending into the uterus, differences in the biological properties of the CMP may influence clinical outcomes [12, 13]. In addition, it is unclear which of the antimicrobial compounds are critical for preventing ascending microbial infection.
A number of bacteria have been experimentally demonstrated to induce ascending infection and preterm birth [14], yet the mechanisms by which they penetrate and traverse the CMP remains unknown. Bacteria that are frequently associated with infection of the amniotic cavity include Streptococcus agalactiae (or group B streptococcus [GBS]) [14–16], Escherichia coli [14], Mycoplasma hominis [17], and Ureaplasma urealyticum [17–19]. Although previous studies have suggested that CMPs are microbicidal or bacteriostatic towards lower genital tract organisms including GBS [12, 13], the burden of bacterial infection during pregnancy remains high with 3.5 million preterm births attributable to GBS [20]. Recently, CMPs were indicated to be permissive to bacteria such as Ureaplasma [21], which are also commonly detected in placenta and amniotic fluid [7, 9, 22, 23]. Given these observations, further understanding of the CMP components is needed to determine the ability of the CMP to reduce or prevent ascending bacterial infection.
We hypothesized that antimicrobial properties of CMPs varies, and insufficiencies in antimicrobial activity may influence susceptibility to ascending infection and preterm birth. Therefore, we used a quantitative proteomic approach to identify antimicrobial factors that are significantly enriched in human CMP samples and then estimated their physiological concentrations using a large panel of human CMPs (n = 60). We observed that concentrations of antimicrobial factors typically present in CMP samples were insufficient to kill GBS. Although some CMPs exhibited bactericidal activity towards GBS, this could be attributed to the antibiotic that was administered to the patient and retained in the CMP. Despite the apparent insufficiency in direct bactericidal activity, we noted that CMP proteins were able to enhance complement-mediated killing and leukocyte activation. Together, these data shed new light on the ability of the CMP to control ascending bacterial infection.
MATERIALS AND METHODS
Cervical Mucus Plug Sample Collection
Collection of human CMPs was approved by the Central Denmark Region Committee on Biomedical Research Ethics (Project ID: 1-10-72-194-12). Human subjects were enrolled at the Department of Obstetrics & Gynecology at Aarhus University Hospital, Denmark. Informed consent for donation of CMPs from term pregnant women was obtained before labor between 38 and 42 weeks gestation. The CMP specimens were obtained as described [13, 21]. In brief, the area around the external OS was cleaned for any visible mucus or vaginal fluid. Then, a distal specimen of the CMP was aspirated using a sterile 3.1-mm-thick catheter (Aspirette Endocervical Aspirator; Cooper Surgical, Trumbull, CT) inserted into the cervical canal (~3 mm) as described [13, 21]. Samples were immediately frozen at −80°C.
Proteomics
Total protein content from each CMP sample was adjusted to ~1 mg/mL. A 90-μg protein aliquot from each CMP was processed using the Tandem Mass Tag ([TMT] Thermo Fisher Scientific) 10plex kit, and LC-MS/MS analysis was performed (see Supplementary Methods). SEAQUEST [24] was used for database searches. Proteomics data from this study have been deposited to the ProteomeXchange Consortium via the PRIDE [25] partner repository with the dataset identifier PXD008600. Principal component analysis (PCA) on the logged abundance values was performed using the R prcomp function.
Protein Quantification From Cervical Mucus Plugs
To quantify specific protein concentrations in CMP samples, CMP sections were weighed, lyophilized, and resuspended in phosphate-buffered saline ([PBS] pH 7.4), and total protein content was adjusted to ~1 mg/mL. Enzyme-linked immunoabsorbant assays (ELISAs) from LifeSpan BioSciences, Abcam, or R&D Systems were used. The CMP protein samples were diluted 1:20 for the cathelicin ELISA, 1:100 for elafin and SLP1 ELISAs, and 1:1000 for hNP1 and lysozyme ELISAs in the manufacturer recommended dilution buffers before use.
Cervical Mucus Plug Killing Assays
To determine the antimicrobial activity of CMPs, total CMP protein content was adjusted to ~1 mg/mL as indicated above. Overnight cultures of wild-type (WT) GBS belonging to serotypes Ia, III, and V (A909, COH1, or NCTC10/84 [26]) or Staphylococcus aureus (Newman, LAC, or MW2 [27]) were plated onto Tryptic Soy Agar or Granada agar (Remel), and 1, 3, 5, or 10 μL of either control PBS, CMP, 25 μg/μL erythromycin, or 100 μg/μL ampicillin (Sigma) was spotted. Plates were incubated overnight at 37°C in 5% CO2. For titration experiments, CMP total protein content was adjusted to ~2 mg/mL in (Tryptic Soy Broth [TSB]), then titrated using 2-fold serial dilutions in TSB to obtain a 12-point titration including a blank. Overnight GBS cultures were diluted 1:20 into fresh TSB (OD600 ≤ 0.1), then mixed 1:1 with titrated samples, and grown at 37°C in 5% CO2. For penicillinase experiments, samples were treated with 1 mM penicillinase from Bacillus cereus (Sigma) for 1 hour at 37°C before titration. Optical density was measured at 600 nm (OD600) with a Spectramax i3x plate reader (Molecular Devices).
Bacterial Viability Assays
To determine the antibacterial effects of specific AMPs, the WT GBS COH1 was grown in TSB to OD600 = 0.3. The bacteria were concentrated 10× in sterile PBS, and 100 μL bacterial suspension was mixed with 100 μL 2× AMP concentration or control 100% ethanol or PBS. The AMP-GBS suspension was then incubated at 37°C for 1 (cathelicidin, elafin, hNP1, SLP1) or 3 hours (lysozyme). After incubation, bacterial viability was assessed using the BacLight bacterial viability kit (Molecular Probes) or by plating for colony-forming units (CFUs). Fluorescent intensity was measured with a Spectramax i3x plate reader (Molecular Devices) with excitation at 485 nm and emission at 535 or 635 nm.
Whole Blood Killing Assays
Written informed patient consent for collection of human blood was approved by the Seattle Children’s Research Institute Institutional Review Board (protocol no. 11117). To assess the ability of CMP proteins to amplify opsonophagocytosis, total CMP protein content was adjusted to ~350 mg/mL. Group B streptococcus (WT A909 or isogenic acapsular A909ΔcpsE [28]) were grown in TSB to OD600 = 0.3 and diluted 1:1000 in PBS. A total of 100 μL of GBS (~104 CFU) was mixed with 300 μL fresh human whole blood with or without 10 μg of CMP protein and incubated at 37°C for 3 hours. A different donor and CMP were used for each experimental replicate. After incubation, CFUs were enumerated by dilution plating. Survival index was calculated by dividing output CFUs by input CFUs.
Statistical Analysis
Two-sided Student t test or Bonferroni multiple comparison test after analysis of variance was used, and P < .05 was considered significant. These tests were performed using GraphPad Prism version 5.0, GraphPad Software, La Jolla, CA.
RESULTS
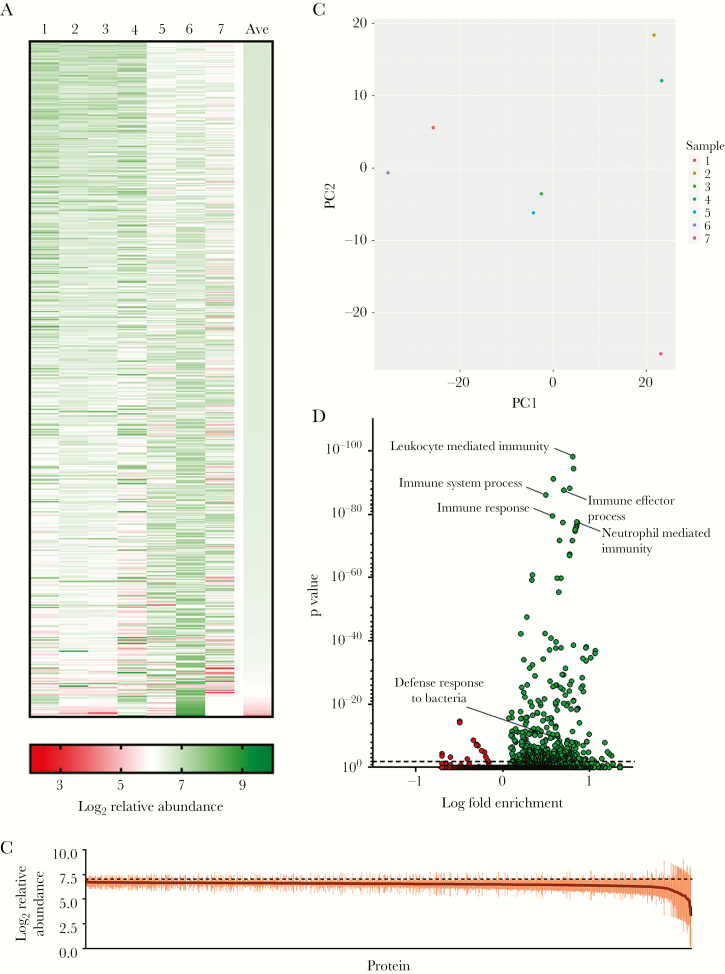
Human CMPs were collected from healthy pregnant women at term, and total proteins were isolated from individual CMPs. A quantitative tandem mass spectrometric approach was used to fully delineate the proteomic profile of the human CMP samples. A total of 1201 consensus proteins were identified from 7 unique CMPs by proteomic mass spectrometry (Figure 1a, Supplementary Table S1). We hypothesized that proteins or peptides with antimicrobial functions may be the most abundant or enriched in the CMPs. To this end, we analyzed the CMP samples to identify proteins with abundance values that were significantly different from the mean abundance of all CMP proteins. Of the 26 proteins identified as significantly enriched (57 identified as significantly depleted) compared with the mean abundance of all CMP proteins, none were characterized as having direct antimicrobial function (Figure 1b, Supplementary Table S1). Thus, we concluded that a specific protein does not confer antimicrobial properties to the CMP. We then aimed to functionally characterize CMP samples using multivariate analysis. To this end, PCA was used to determine the relatedness of our samples (Figure 1c). These data suggest that the CMPs are heterogeneous in protein abundance, indicating that the human CMP proteome is similar in content (Figure 1a) but can differ in composition (Figure 1c). Next, using the Gene Ontology Consortium database [29], we observed that 514 of 15403 biological functions were significantly over- or underrepresented in the CMP samples; the most significant and enriched protein sets were involved in activation of leukocyte-mediated immunity, immune effectors, and control of bacterial infection (Figure 1d, Supplementary Table S2). Together, these data indicate that the CMP is immunological and antibacterial in nature, but this functionality cannot be attributed to a single protein.
Figure 1.
Summary of quantitative proteomic analysis of human cervical mucus plugs (CMPs). Total proteins from 7 human CMPs were analyzed by quantitative tandem mass spectrometry. (a) Heat map representing relative abundance values of proteins from 7 CMP samples. (b) Protein relative abundance values for all consensus proteins were compared with determine proteins that were significantly enriched or depleted in CMP samples (for complete dataset, see Table S1). Thirty-five of 1201 proteins were determined to be significantly above or below the mean abundance value for all proteins (dashed line indicates mean relative abundance, Bonferroni multiple-comparison test after one-way analysis of variance [ANOVA]). (c) Principle co-ordinate analysis of protein abundance values from CMPs samples. (d) Gene Ontology analysis of biological functions of all consensus proteins identified in the CMP samples. A total of 514 of 15403 biological functions were found to be significantly over- or underrepresented in CMP samples (Bonferroni multiple-comparison test after one-way ANOVA; for complete dataset, see Table S2).
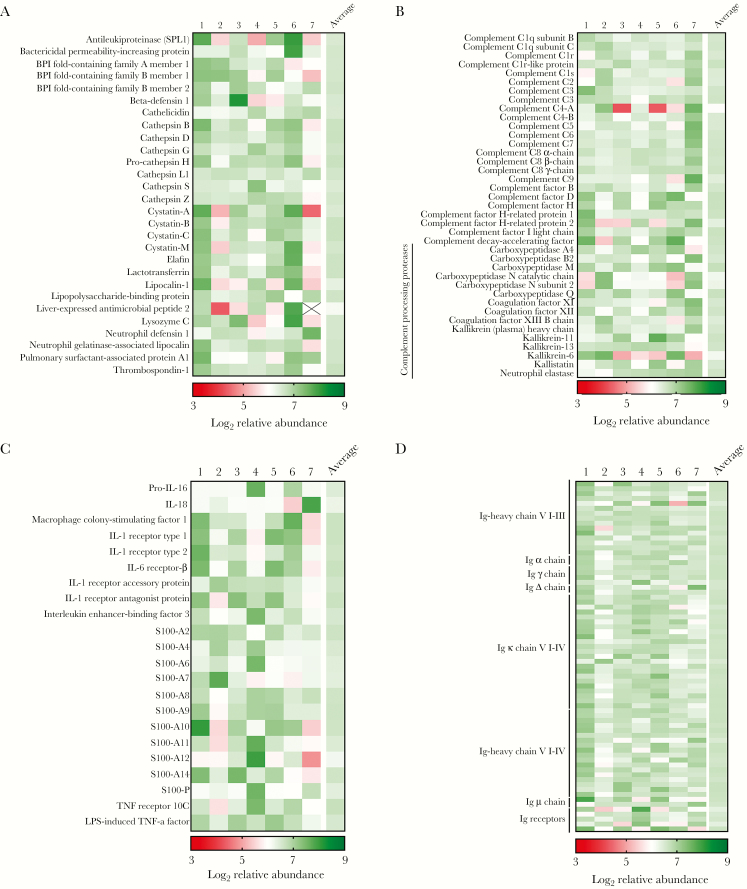
Given the enrichment of protein families involved in the host immune response to bacteria, we aimed to further identify proteins with immune functions that were present in CMP samples. To identify immune proteins in the CMP, we analyzed the proteomic dataset for AMPs, complement, complement-activating proteins, cytokines, chemokines, immunoglobulin, and immunoglobulin-related proteins. We identified 28 AMPs in the CMP samples including antileukoproteinase (SPL1), β-defensin 1, cathelicidin (LL-37), lactotransferrin (lactoferrin), lipocalin 1 and 2, and lysozyme C (Figure 2a). In addition, we identified 39 complement or complement-related proteins (Figure 2b), including proteases involved in proteolytic activation and complement maturation. We also detected 22 cytokines, chemokines, or related receptors (Figure 2c) that can affect leukocyte activation and chemotaxis. In addition, multiple members of the S100 protein family were present in CMP samples, which act as chemoattractants and for iron sequestration [30, 31]. Finally, 71 immunoglobulin or immunoglobulin-receptor proteins (Figure 2d) were identified. Collectively, these data suggest that the human CMP may be immunologically dynamic and that antimicrobial activity may not be limited to the action of AMPs but involve a spectrum of peptides and proteins acting in concert to opsonize bacterial pathogens and activate innate and adaptive immunity.
Figure 2.
Quantitative proteomic analysis reveals the immunological nature of the human cervical mucus plugs (CMPs). After complete proteomic profiling (see Figure 1), consensus CMP proteins were further analyzed for proteins that have been characterized to have immunological function. Heat maps representing relative abundance values of antimicrobial peptides (AMPs [a]), complement or complement-related proteins (b), cytokines, chemokines, or related receptors (c), and immunoglobulin or immunoglobulin-receptor proteins (d) identified in 7 human CMP samples. Boxes containing an “X” indicate that an individual protein was not identified in a given sample.
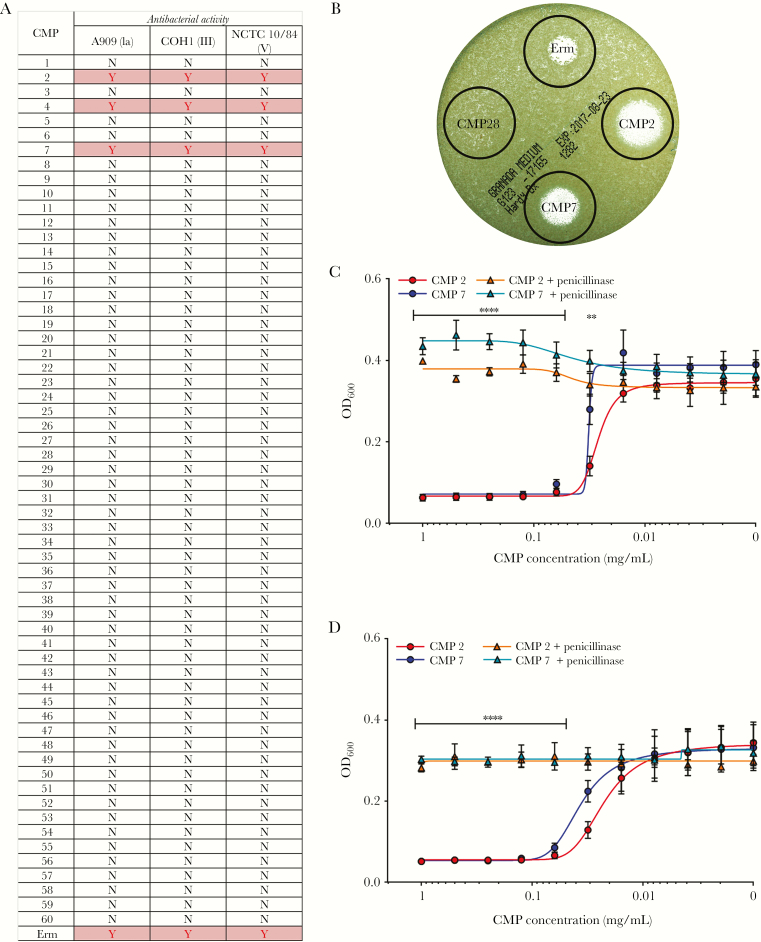
Our next objective was to test the specificity of antimicrobial activity of CMPs towards pathogens associated with ascending infection such as GBS [32–35]. Although prior studies indicated that some CMPs are antimicrobial to GBS [12, 13], the incidence of preterm births and stillbirths associated with GBS infection remains high [20], leading us to re-evaluate the antimicrobial properties of CMPs to GBS. To this end, CMP protein extracts were prepared from 60 individual CMPs that also included those used in the mass spectrometry studies above (CMPs 1–7; Figure 3a), and antimicrobial activity against GBS was examined by testing their ability to prevent GBS growth. Of the 60 CMPs tested, 3 CMPs displayed direct antimicrobial activity against GBS (Figure 3a and b).
Figure 3.
Antibacterial activity of cervical mucus plugs (CMPs) against group B streptococcus (GBS) is due to antibiotics. To assess antimicrobial activity of CMPs against GBS, CMPs were sectioned, lyophilized, and resuspended in phosphate-buffered saline to a concentration of 1 mg/mL. Cervical mucus plug extracts (1 mg/mL) or erythromycin (Erm, 25 mg/mL) were spotted onto TSA plates containing GBS strains (either serotype Ia strain A909, serotype III strain COH1, or serotype V strain NCTC10/84). Antimicrobial activity was determined by the ability of the CMP extracts to prevent GBS growth at the respective spots (a and b); “Y” indicates growth inhibition and “N” indicated no growth inhibition (also see zone of clearing in b). To determine whether antibacterial activity in CMP 2 and CMP 7 could be attributed to antibiotics provided to the patient that was retained in the CMP before collection, extracts from CMP 2 and CMP 7 were treated with 1 mM penicillinase from Bacillus cereus. Growth was determined by measuring OD600 at 4 ([c] n = 3, mean displayed ± standard error of the mean [SEM], ****P > .00001, **P > .001, Bonferroni multiple-comparison test after two-way analysis of variance [ANOVA] comparing sample to sample + penicillinase) and 24 hours ([d] n = 3, mean displayed ± SEM, ****P > .00001, Bonferroni multiple-comparison test after one-way ANOVA comparing sample to sample + penicillinase).
Since it is not uncommon for antibiotics to be administered to the patient during labor, we sought to determine whether antimicrobial activity observed in the above CMPs could be attributed to antibiotic exposure. It is notable that the women from whom the 3 antimicrobial CMPs (CMPs 2, 4, and 7) were collected had received the antibiotic benzylpenicillin (or penicillin G), approximately 3 hours before CMP collection. Of note, other CMPs in our study were obtained from women who had not received antibiotics before CMP collection. To test whether the antimicrobial activity in these CMPs was attributable to retained antibiotics, we treated CMP 2 and CMP 7 with penicillinase from B cereus. Treatment with penicillinase completely abrogated the antibacterial activity of these CMPs (Figure 3c and d). In addition, a methicillin-susceptible S. aureus strain (Newman) was sensitive to killing by CMP 2 and CMP 7 in contrast to 2 different methicillin-resistant S. aureus strains (LAC and MW-2; Figure S1). Together, these data indicate that the CMP has the ability to retain antibiotics, and that the antimicrobial activity of the above CMP samples against GBS was due to antibiotics administered before CMP collection, rather than the activity of antimicrobial proteins or peptides present in the CMP.
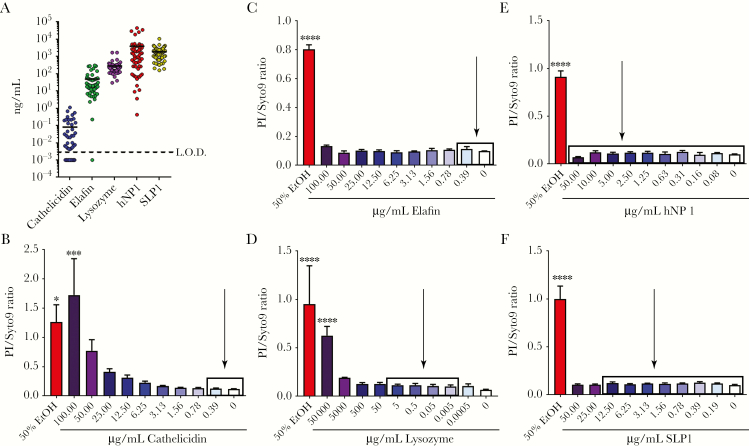
Previous studies suggested that some human CMPs have direct antimicrobial activity against GBS due to the presence of AMPs in CMP samples [10, 12]. To further probe the role of AMPs in controlling GBS, specific AMP concentrations were measured using all 60 CMP samples and were found to be as follows: cathelicidin (0.00418–1.11 ng/mL), elafin (0.221–301 ng/mL), lysozyme (29.6–1687 ng/mL), hNP1 (0.423–44426 ng/mL), and SLP1 (157–10613 ng/mL) (Figure 4a). Once the physiological range of AMPs in CMPs were determined (see above and Figure 4a), we used recombinant AMPs in in vitro assays to determine whether the AMP at this concentration range was sufficient to kill GBS. The results shown in Figure 4b–e indicate that these AMP concentrations were insufficient to kill GBS. These data were also confirmed by bacterial CFU enumeration (data not shown). Although the role of cathelicidin and lysozyme in GBS killing has been studied [36, 37], the ability of elafin, hNP1, and SLP1 to kill GBS is not known. Our results indicate that none of these AMPs have antimicrobial activity towards GBS at the physiological levels found in the CMPs used in our study, suggesting that an alternative defense against ascending GBS infections may exist.
Figure 4.
Physiologically relevant concentrations of antimicrobial peptides (AMPs) present in cervical mucus plugs (CMPs) are insufficient to kill group B streptococcus (GBS). To assess AMP concentration in CMPs against GBS, CMPs were sectioned, lyophilized, and resuspended in phosphate-buffered saline to a concentration of 1 mg/mL. Concentrations of specific AMPs (cathelicidin, elafin, lysozyme, hNP1, and SLP1) were determined from 60 individual CMPs by enzyme-linked immunosorbent assay ([a] mean displayed). These concentrations were then used in bacterial viability assays to determine the antibacterial activity of AMPs against GBS (cathelicidin [b], elafin [c], lysozyme [d], hNP1 [e], and SLP1 [f]; n = 3, mean displayed ± standard error of the mean, *P < .05, ***P < .0005, ****P < .00005, Bonferroni multiple-comparison test after one-way analysis of variance). Higher PI/Syto9 ratio indicates lower bacterial viability, and 50% ethanol was included as a positive control. Boxes on bar graphs indicate range of detected AMP concentration in the CMPs, and arrows indicate approximate mean detected AMP concentration.
Although initially surprising, our results concerning AMP-mediated killing of GBS are consistent with another study demonstrating that fetal fecal and meconium samples displayed minimal AMP-attributable activity against GBS [38]. However, if CMPs are completely unable to control ascending GBS infection, adverse pregnancy rates due to GBS would be expected to be much higher. Given the presence of complement-, neutrophil-, and macrophage-activating cytokines and immunoglobulins, we hypothesized that proteins in the human CMP may enhance opsonophagocytic killing of bacteria. To test this hypothesis, whole blood killing assays were performed. Group B streptococcus were incubated with human whole blood spiked with 25 μg/mL CMP proteins or an equivalent volume of control PBS. The results shown in Figure 5 indicate that the presence of CMP proteins enhanced killing of GBS, resulting in an approximate 2-fold enhancement of bacterial killing. To validate these results, we repeated the experiment with the GBS mutant deficient for capsule production (GBSΔcpsE). Group B streptococcus use their sialic acid capsule to prevent deposition of complement and antibodies on their cell surface, thus preventing opsonophagocytic killing or killing through the membrane attack complex in a serotype-specific manner [39–44], and reduction of capsule results in decreased fitness. Indeed, deletion of cpsE reduced survival in whole blood, and microbial killing was enhanced in the presence of CMP protein (Figure 5). Together, these data suggest that despite inefficiencies by AMPs, the CMP has the ability to act as an immunological barrier for ascending infection through amplification of opsonophagocytic killing.
Figure 5.
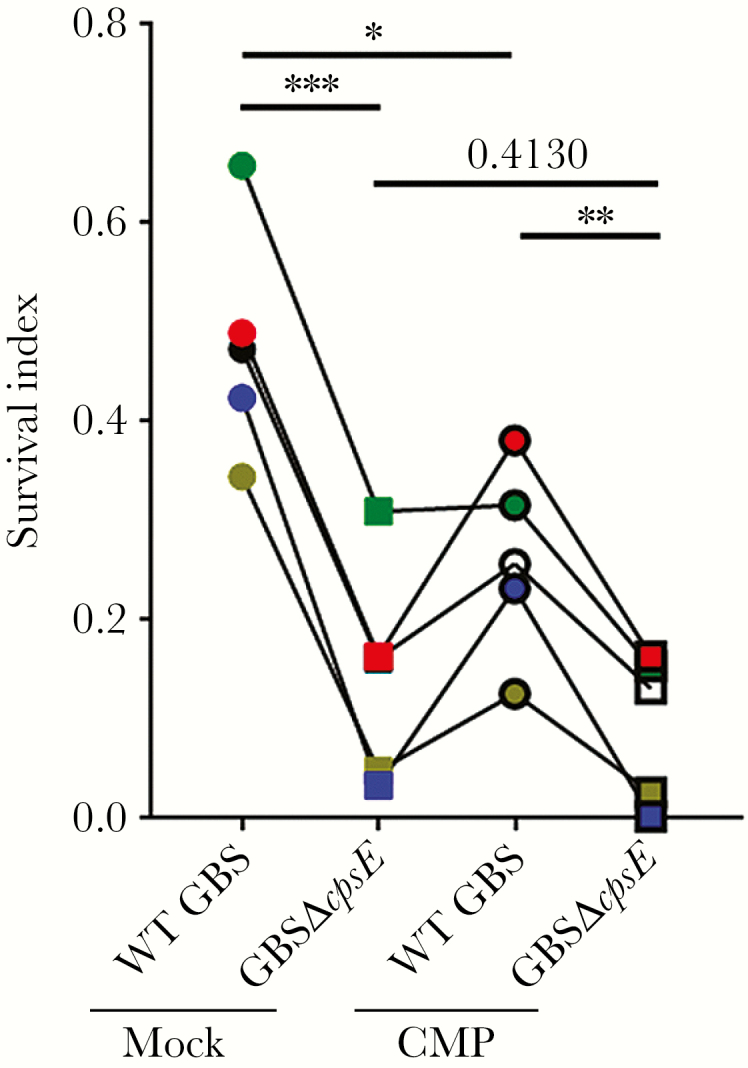
Cervical mucus plug (CMP) protein enhances killing of group B streptococcus (GBS) in whole blood. To assess CMP extract enhancement of antibacterial activity against GBS in whole blood, CMPs were sectioned, lyophilized, and resuspended in phosphate-buffered saline (PBS) to a concentration of 1 mg/mL. Wild-type (WT) GBS serotype Ia (strain A909) or isogenic GBSΔcpsE were incubated with human whole blood with 25 μg/mL CMP protein or an equivalent volume of PBS. Survival index was estimated 3 hours after incubation and compares colony-forming units at 3 hours postincubation to initial inoculum (n = 5, *P < .05, **P < .005, ***P < .0005, Bonferroni multiple comparison test after one-way analysis of variance). Each assay was performed with an independent CMP and blood donor, indicated by different symbol color and connecting line. Cervical mucus plugs 2, 4, and 7 were not used in these experiments due to retained antibiotics.
DISCUSSION
The CMP represents the physical barrier through which vaginal bacteria must traffic to invade the uterus and infect the amniotic fluid and fetus. Little is known about this critical structure, and our work provides significant insight into its composition and also why it may fail as a “gate-keeper” in some women to prevent bacterial trafficking into the uterus. Despite 60 years of research, the susceptibility of some groups of women to preterm birth, which is both age- and ethnicity-dependent, is still unknown. Difficulties in studying the CMP have hindered development of this literature. Our work provides a novel direction and methodology for investigating susceptibility to preterm birth. To date, only 1 other study has attempted to study the CMP holistically, and studies that have attempted to merge systematic and functional experimentation are few if not none. Thus, much of our understanding of the human CMP and its role in preventing ascending infection is extrapolated from unconnected studies. Here, we attempt to address this critical knowledge gap.
The data presented here reveals novel insight into the immunological complexity of the human CMP and provides a comprehensive proteomic view of the CMP. We have identified 1201 consensus proteins that define the CMP, which map to more than 500 functional pathways. These represent the most comprehensive proteomic profile of the human CMP, because we have identified more than 4 times as many proteins and over 15 times as many significantly over- or underrepresented biological functions than previous studies [11]. We observed that AMPs present in the CMPs are not sufficient to kill GBS, but instead CMP proteins can amplify the antibacterial cellular immune response. Bacterial viability was reduced in the presence of CMP proteins in whole blood killing assays, indicating enhanced leukocyte activation or complement-mediated killing. We also show that the CMP has the ability to retain antibiotics, which can amplify its antibacterial properties. In women who were administered antibiotics during labor or delivery, the CMP was able to retain those antibiotics and confer antibacterial activity that was abolished upon enzymatic degradation. Together, these data help refine our understanding of how the CMP prevents ascending infection of microbes and consequent preterm birth.
Little is known about the interactions between vaginal bacteria and the CMP. The recovery of vaginal bacteria from placental membranes and amniotic fluid from women in preterm labor indicates that microbial trafficking from the lower genital tract into the uterus likely occurs despite the presence of the CMP [7, 14, 15, 17, 18, 45, 46]. Modulation of the cellular immune response has been shown to impact ascending bacterial infection [15, 19, 34], where ineffective leukocyte-mediated killing of bacteria or diversion of leukocytes away from an antibacterial phenotype lead to increased rates of ascending infection. This study and other proteomic studies [47] describe the presence of immune proteins in the human CMP, which could potentially modify the cellular host immune response. The presence of various cytokines, complement proteins, and metal-chelating proteins, immune cell-derived proteins, and even immune cells themselves [48] dictate a critical role for the CMP in activation of the antimicrobial immune response; however, many facets of the role of these proteins in immune activation, such as the spatial distribution, temporal expression, and downstream effects, have yet to be explored. Given the role of cellular immunity in conferring antimicrobial activity of the CMP, studies aimed at defining the role of immune proteins and correlating immune insufficiencies and adverse pregnancy outcomes in the context of the CMP are needed. A defect in the cellular immune response associated with the CMP, and an immunologic and anatomic barrier to microbial trafficking may underlie some cases of preterm birth.
Our study provides comprehensive insight into the human CMP proteome and reveals a role for cellular immunity. However, limitations of our study include the fact that the CMPs used in this study were collected from a single geographic region. It is plausible that CMPs from other geographic regions may exhibit differences in proteomic profiles and forms of biological functionality due to variations in genetics, epigenetics, and environment. Additional studies from other geographic locations are needed to identify the core CMP proteome and for a better understanding of the role of CMPs during human pregnancy. Our studies evaluated antimicrobial activity of the human CMP to 3 GBS strains representing serotypes that are responsible for the highest burden of disease. Although our conclusions were applicable to these GBS strains, our experiments were not exhaustive. Given the biological differences between GBS capsular serotypes and sequence types, more research is needed to refine our conclusions about the ability of the human CMP to kill GBS. In addition, although GBS is an important cause of ascending infection and adverse pregnancy outcomes, other bacterial species including E coli, U urealyticum, and those associated with bacterial vaginosis may have differential responses to CMP-stimulated immunity. Expansion of these studies to include other pathogen(s) associated with adverse pregnancy outcomes may reveal novel and important aspects of CMP biology.
CONCLUSIONS
A better understanding of key antimicrobial components of the CMP is necessary to define mechanisms that contribute to infection associated adverse pregnancy outcomes and to develop strategies to decrease and/or prevent these outcomes. These studies have expanded our knowledge of the proteomic profile of the human CMP and provided insight into additional mechanisms on how the CMP can control ascending bacterial infection. However, more studies are needed to successfully prevent ascending bacterial infection. The consequences of adverse pregnancy outcomes cannot be overstated. Successful pregnancies have substantial impacts on maternal health, influence the long-term outcomes of offspring [49], and alleviate the economic effects of complicated pregnancies [50]. The development of successful interventions to improve the health of the mother and fetus in utero rely on a comprehensive understanding of the various biological defenses for healthy pregnancies, including the understudied CMP.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. Proteomics was performed using the Proteomic core at the Fred Hutch Cancer Research Center, Seattle. We thank Dr. Philip Gafken for help with the proteomic data and Sean Merillat for technical assistance.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other funders. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This study was funded by the National Institutes of Health (NIH), Grants R21AI125907 and R01AI112619 (to L. R.) and Grants R01AI33976 and R01AI100989 (to L. R. and K. M. A. W.); and NIH training Grant T32 AI07509 (to J. V. [Principal Investigator: Lee Ann Campbell]).
Potential conflicts of interest All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Blencowe H, Cousens S, Chou D et al. Born too soon: the global epidemiology of 15 million preterm births. Reprod Health 2013; 10(Suppl 1):S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Katz J, Lee AC, Kozuki N et al. Mortality risk in preterm and small-for-gestational-age infants in low-income and middle-income countries: a pooled country analysis. Lancet 2013; 382:417–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science 2014; 345:760–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rubens CE, Gravett MG, Victora CG et al. Global report on preterm birth and stillbirth (7 of 7): mobilizing resources to accelerate innovative solutions (Global Action Agenda). BMC Pregnancy Childbirth 2010; 10(Suppl 1):S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nold C, Anton L, Brown A, Elovitz M. Inflammation promotes a cytokine response and disrupts the cervical epithelial barrier: a possible mechanism of premature cervical remodeling and preterm birth. Am J Obstet Gynecol 2012; 206:208.e1–7. [DOI] [PubMed] [Google Scholar]
- 6. Romero R, Gonzalez R, Sepulveda W et al. Infection and labor. VIII. Microbial invasion of the amniotic cavity in patients with suspected cervical incompetence: prevalence and clinical significance. Am J Obstet Gynecol 1992; 167:1086–91. [DOI] [PubMed] [Google Scholar]
- 7. Hillier SL, Krohn MA, Kiviat NB, Watts DH, Eschenbach DA. Microbiologic causes and neonatal outcomes associated with chorioamnion infection. Am J Obstet Gynecol 1991; 165:955–61. [DOI] [PubMed] [Google Scholar]
- 8. Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med 2000; 342:1500–7. [DOI] [PubMed] [Google Scholar]
- 9. Whidbey C, Harrell MI, Burnside K et al. A hemolytic pigment of group B streptococcus allows bacterial penetration of human placenta. J Exp Med 2013; 210:1265–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Becher N, Adams Waldorf K, Hein M, Uldbjerg N. The cervical mucus plug: structured review of the literature. Acta Obstet Gynecol Scand 2009; 88:502–13. [DOI] [PubMed] [Google Scholar]
- 11. Arko D, Dovnik A, Fokter N, Takač I. The role of genital pathogens in morbidity following diathermy loop excision of the transformation zone of the uterine cervix. Int J Gynaecol Obstet 2012; 117:27–9. [DOI] [PubMed] [Google Scholar]
- 12. Hein M, Valore EV, Helmig RB, Uldbjerg N, Ganz T. Antimicrobial factors in the cervical mucus plug. Am J Obstet Gynecol 2002; 187:137–44. [DOI] [PubMed] [Google Scholar]
- 13. Hein M, Helmig RB, Schønheyder HC, Ganz T, Uldbjerg N. An in vitro study of antibacterial properties of the cervical mucus plug in pregnancy. Am J Obstet Gynecol 2001; 185:586–92. [DOI] [PubMed] [Google Scholar]
- 14. Mendz GL, Kaakoush NO, Quinlivan JA. Bacterial aetiological agents of intra-amniotic infections and preterm birth in pregnant women. Front Cell Infect Microbiol 2013; 3:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boldenow E, Gendrin C, Ngo L et al. Group B streptococcus circumvents neutrophils and neutrophil extracellular traps during amniotic cavity invasion and preterm labor. Sci Immunol 2016; 1:eaah4576. doi:10.1126/sciimmunol.aah4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Randis TM, Gelber SE, Hooven TA et al. Group B streptococcus β-hemolysin/cytolysin breaches maternal-fetal barriers to cause preterm birth and intrauterine fetal demise in vivo. J Infect Dis 2014; 210:265–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Berg TG, Philpot KL, Welsh MS, Sanger WG, Smith CV. Ureaplasma/Mycoplasma-infected amniotic fluid: pregnancy outcome in treated and nontreated patients. J Perinatol 1999; 19:275–7. [DOI] [PubMed] [Google Scholar]
- 18. Jalava J, Mäntymaa ML, Ekblad U et al. Bacterial 16S rDNA polymerase chain reaction in the detection of intra-amniotic infection. Br J Obstet Gynaecol 1996; 103:664–9. [DOI] [PubMed] [Google Scholar]
- 19. Racicot K, Cardenas I, Wünsche V et al. Viral infection of the pregnant cervix predisposes to ascending bacterial infection. J Immunol 2013; 191:934–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Seale AC, Bianchi-Jassir F, Russell NJ et al. Estimates of the burden of group B streptococcal disease worldwide for pregnant women, stillbirths, and children. Clin Infect Dis 2017; 65:200–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hansen LK, Becher N, Bastholm S et al. The cervical mucus plug inhibits, but does not block, the passage of ascending bacteria from the vagina during pregnancy. Acta Obstet Gynecol Scand 2014; 93:102–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hillier SL, Martius J, Krohn M, Kiviat N, Holmes KK, Eschenbach DA. A case-control study of chorioamnionic infection and histologic chorioamnionitis in prematurity. N Engl J Med 1988; 319:972–8. [DOI] [PubMed] [Google Scholar]
- 23. Hitti J, Krohn MA, Patton DL et al. Amniotic fluid tumor necrosis factor-alpha and the risk of respiratory distress syndrome among preterm infants. Am J Obstet Gynecol 1997; 177:50–6. [DOI] [PubMed] [Google Scholar]
- 24. Allen U, Nimrod C, Macdonald N, Toye B, Stephens D, Marchessault V. Relationship between antenatal group B streptococcal vaginal colonization and premature labour. Paediatr Child Health 1999; 4:465–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vizcaíno JA, Csordas A, del-Toro N et al. 2016 update of the PRIDE database and its related tools. Nucleic Acids Res 2016; 44:D447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rajagopal L, Vo A, Silvestroni A, Rubens CE. Regulation of cytotoxin expression by converging eukaryotic-type and two-component signalling mechanisms in Streptococcus agalactiae. Mol Microbiol 2006; 62:941–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bubeck Wardenburg J, Patel RJ, Schneewind O. Surface proteins and exotoxins are required for the pathogenesis of Staphylococcus aureus pneumonia. Infect Immun 2007; 75:1040–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jones AL, Needham RH, Clancy A, Knoll KM, Rubens CE. Penicillin-binding proteins in Streptococcus agalactiae: a novel mechanism for evasion of immune clearance. Mol Microbiol 2003; 47:247–56. [DOI] [PubMed] [Google Scholar]
- 29. Gene Ontology Consortium. Gene Ontology Consortium: going forward. Nucleic Acids Res 2015; 43:D1049–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Donato R, Cannon BR, Sorci G et al. Functions of S100 proteins. Curr Mol Med 2013; 13:24–57. [PMC free article] [PubMed] [Google Scholar]
- 31. Gilston BA, Skaar EP, Chazin WJ. Binding of transition metals to S100 proteins. Sci China Life Sci 2016; 59:792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vornhagen J, Adams Waldorf KM, Rajagopal L. Perinatal group B streptococcal infections: virulence factors, immunity, and prevention strategies. Trends Microbiol 2017; 25:919–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lancefield RC, Hare R. The serological differentiation of pathogenic and non-pathogenic strains of hemolytic streptococci from parturient women. J Exp Med 1935; 61:335–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gendrin C, Vornhagen J, Ngo L et al. Mast cell degranulation by a hemolytic lipid toxin decreases GBS colonization and infection. Sci Adv 2015; 1:e1400225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lawn JE, Gravett MG, Nunes TM, Rubens CE, Stanton C; GAPPS Review Group Global report on preterm birth and stillbirth (1 of 7): definitions, description of the burden and opportunities to improve data. BMC Pregnancy Childbirth 2010; 10(Suppl 1):S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Maisey HC, Quach D, Hensler ME et al. A group B streptococcal pilus protein promotes phagocyte resistance and systemic virulence. FASEB J 2008; 22:1715–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Quach D, van Sorge NM, Kristian SA, Bryan JD, Shelver DW, Doran KS. The CiaR response regulator in group B Streptococcus promotes intracellular survival and resistance to innate immune defenses. J Bacteriol 2009; 191:2023–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kai-Larsen Y, Bergsson G, Gudmundsson GH et al. Antimicrobial components of the neonatal gut affected upon colonization. Pediatr Res 2007; 61:530–6. [DOI] [PubMed] [Google Scholar]
- 39. Marques MB, Kasper DL, Pangburn MK, Wessels MR. Prevention of C3 deposition by capsular polysaccharide is a virulence mechanism of type III group B streptococci. Infect Immun 1992; 60:3986–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Takahashi S, Aoyagi Y, Adderson EE, Okuwaki Y, Bohnsack JF. Capsular sialic acid limits C5a production on type III group B streptococci. Infect Immun 1999; 67:1866–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pietrocola G, Rindi S, Rosini R, Buccato S, Speziale P, Margarit I. The Group B Streptococcus-secreted protein CIP interacts with C4, preventing C3b deposition via the lectin and classical complement pathways. J Immunol 2016; 196:385–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lynch NJ, Roscher S, Hartung T et al. L-ficolin specifically binds to lipoteichoic acid, a cell wall constituent of Gram-positive bacteria, and activates the lectin pathway of complement. J Immunol 2004; 172:1198–202. [DOI] [PubMed] [Google Scholar]
- 43. Butko P, Nicholson-Weller A, Wessels MR. Role of complement component C1q in the IgG-independent opsonophagocytosis of group B streptococcus. J Immunol 1999; 163:2761–8. [PubMed] [Google Scholar]
- 44. Fujieda M, Aoyagi Y, Matsubara K et al. L-ficolin and capsular polysaccharide-specific IgG in cord serum contribute synergistically to opsonophagocytic killing of serotype III and V group B streptococci. Infect Immun 2012; 80:2053–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Russell AN, Zheng X, O’Connell CM et al. Analysis of factors driving incident and ascending infection and the role of serum antibody in Chlamydia trachomatis genital tract infection. J Infect Dis 2016; 213:523–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Horowitz S, Mazor M, Romero R, Horowitz J, Glezerman M. Infection of the amniotic cavity with Ureaplasma urealyticum in the midtrimester of pregnancy. J Reprod Med 1995; 40:375–9. [PubMed] [Google Scholar]
- 47. Lee DC, Hassan SS, Romero R et al. Protein profiling underscores immunological functions of uterine cervical mucus plug in human pregnancy. J Proteomics 2011; 74:817–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hein M, Petersen AC, Helmig RB, Uldbjerg N, Reinholdt J. Immunoglobulin levels and phagocytes in the cervical mucus plug at term of pregnancy. Acta Obstet Gynecol Scand 2005; 84:734–42. [DOI] [PubMed] [Google Scholar]
- 49. Barker DJ, Eriksson JG, Forsén T, Osmond C. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol 2002; 31:1235–9. [DOI] [PubMed] [Google Scholar]
- 50. Lawn JE, Kinney MV, Belizan JM et al. Born too soon: accelerating actions for prevention and care of 15 million newborns born too soon. Reprod Health 2013; 10(Suppl 1):S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.