The strigolactone analogue GR24 reduces ABA-induced anthocyanin accumulation in Vitis vinifera berries. GR24 treatment does not affect ABA biosynthesis while it activates ABA degradation and possibly ABA membrane transport.
Keywords: ABA conjugation, ABA hydroxylases, ABA transporters, abscisic acid, anthocyanin, grapevine, GR24, ripening, strigolactones
Abstract
Besides signalling to soil organisms, strigolactones (SLs) control above- and below-ground morphology, in particular shoot branching. Furthermore, SLs interact with stress responses, possibly thanks to a crosstalk with the abscisic acid (ABA) signal. In grapevine (Vitis vinifera L.), ABA drives the accumulation of anthocyanins over the ripening season. In this study, we investigated the effects of treatment with a synthetic strigolactone analogue, GR24, on anthocyanin accumulation in grape berries, in the presence or absence of exogenous ABA treatment. Experiments were performed both on severed, incubated berries, and on berries attached to the vine. Furthermore, we analysed the corresponding transcript concentrations of genes involved in anthocyanin biosynthesis, and in ABA biosynthesis, metabolism, and membrane transport. During the experiment time courses, berries showed the expected increase in soluble sugars and anthocyanins. GR24 treatment had no or little effect on anthocyanin accumulation, or on gene expression levels. Exogenous ABA treatment activated soluble sugar and anthocyanin accumulation, and enhanced expression of anthocyanin and ABA biosynthetic genes, and that of genes involved in ABA hydroxylation and membrane transport. Co-treatment of GR24 with ABA delayed anthocyanin accumulation, decreased expression of anthocyanin biosynthetic genes, and negatively affected ABA concentration. GR24 also enhanced the ABA-induced activation of ABA hydroxylase genes, while it down-regulated the ABA-induced activation of ABA transport genes. Our results show that GR24 affects the ABA-induced activation of anthocyanin biosynthesis in this non-climacteric fruit. We discuss possible mechanisms underlying this effect, and the potential role of SLs in ripening of non-ABA-treated berries.
Introduction
Grapevine ranks fourth among major fruit crops worldwide, and first in Europe (http://www.fao.org/faostat/en/#data). Ripe berries are employed for direct consumption and for wine production. At harvest, an optimal balance among berry components (sugars, acids, and secondary metabolites) is an absolute requirement to guarantee consumer preference and commercial success. Grape berry secondary metabolites are represented by many polyphenols (Adams, 2006) and volatile compounds (Kalua and Boss, 2010). Overall, these molecules contribute to the colour, taste, and aroma of grapes, and are involved in wine stabilization and ageing. Anthocyanins are one of the major groups of polyphenols in berry skins of coloured cultivars. Their concentration and diversity control colour intensity and stability in the fruit and in the wine derived therefrom; furthermore, they contribute to seed dispersal and defence against oxidative stress. Anthocyanins are absent in the first stage of berry development, while they accumulate in vacuoles from the start of berry ripening (véraison) (Moskowitz and Hrazdina, 1981).
The molecular and physiological processes controlling ripening and anthocyanin accumulation in the non-climacteric grape berry are still poorly known, although great strides forward have been made, in particular through the application of transcriptomic (Deluc et al., 2007) and proteomic (Giribaldi et al., 2007) approaches. Hormonal control of fruit ripening is a well-described process, and several hormones were shown to interact with some aspects of ripening in grape. Auxins, brassinosteroids, and salicylic acid have an inhibitory effect on berry ripening (Davies et al., 1997; Symons et al., 2006). Disruption of ethylene perception negatively affects anthocyanin accumulation (Chervin et al., 2004), but the relevance of ethylene in berry ripening is debated (Sun et al., 2010). Methyl jasmonate treatments enhance anthocyanin accumulation in suspension cultures (Belhadj et al., 2008) and in whole berries (Symons et al., 2006; Jia et al., 2016). Besides these hormones, abscisic acid (ABA) has been long suspected to be the master controller of ripening in grapevine, as both its biosynthesis (Deluc et al., 2007) and concentration in the berry (Coombe and Hale, 1973; Davies et al., 1997) peak at véraison. This hypothesis is further supported by observation that exogenous ABA activates accumulation of anthocyanins and sugars in the grape berry (Coombe and Hale, 1973; Wheeler et al., 2009), and expression activation of anthocyanin biosynthetic genes and of transcription factors controlling this pathway (Jeong et al., 2004; Gambetta et al., 2010; Giribaldi et al., 2010; Villalobos-González et al., 2016). The role of ABA in the induction of anthocyanin accumulation is not limited to the grape berry, indeed it has been demonstrated in other non-climacteric fruits (Kadomura-Ishikawa et al., 2015) and in Arabidopsis and maize seed vegetative tissues (McCarty et al., 1989).
Strigolactones (SLs) were first discovered for their ability to induce seed germination of root parasite plants when exuded in soil (Bradow and Connick, 1988). Later on, they were demonstrated to play an essential role as plant signals for other soil organisms, such as arbuscular mycorrhizal (AM) fungi (Akiyama et al., 2005) and symbiotic nitrogen-fixing bacteria (Peláez-Vico et al., 2016). The study of Arabidopsis and rice branching mutants showed, however, that SLs also strongly repress the growth of axillary buds (Gomez-Roldan et al., 2008; Umehara et al., 2008). The action of SL on shoot branching may be mediated by complex interaction with other hormones, namely auxin and cytokinins (Ruyter-Spira et al., 2013).
SL concentration is responsive to nutrient deprivation, in particular of phosphorus and nitrogen (Yoneyama et al., 2007). This is seen as an adaptive strategy to regulate interaction with AM fungi: plants increase SL production under nutrient starvation, in order to minimize shoot branching and promote AM colonization (Gomez-Roldan et al., 2008; Umehara et al., 2008). Recent studies have demonstrated that SLs are also involved in responses to other abiotic stresses, in particular drought. Arabidopsis, Lotus, and tomato genotypes with reduced SL levels are hypersensitive to drought stress (Ha et al., 2014; Liu et al., 2015; Visentin et al., 2016; Lv et al., 2018), while SL supplementation abolishes the drought-sensitive genotype. In most of these studies, SL-dependent changes in stress susceptibility were mainly linked to an ABA signalling-dependent modulation of stomatal closure, suggesting that SLs may interact with the ABA signal upon stress. These observations raise the question of whether SLs can also interact with ABA in developmentally regulated processes, such as ripening of the non-climacteric grape berries.
In this study, we investigated the effect of modifications of exogenous SL on ABA-induced ripening of grapevine berries. By application of the SL analogue GR24 (Besserer et al., 2008) to berries at véraison in the presence and absence of exogenous ABA, we demonstrate that exogenous SL down-regulates the effects of exogenous (but not endogenous) ABA, possibly by affecting its metabolism and transport.
Materials and methods
Plant material and experimental set-up
Experiments were performed on Vitis vinifera cultivar Barbera, whose anthocyanin profile is dominated by mono- and di-methylated forms (Ferrandino et al., 2012).
Treatments were applied in a first experiment on detached, in vitro incubated berries. This technique has often been used to study ripening processes in grape; however, the berries at this stage are exchanging substances with the plant via the vascular system and, to take this into account, we replicated our treatments in a second experiment on intact berries attached to the plant.
For the in vitro experiment, non-coloured, field-grown berries were collected at the start of ripening (véraison) 2015 from vines at the Grugliasco campus vineyard (Piedmont, Italy, 45°03'55''N, 7°35'35''E) by severing the apical end of their pedicel. Vines were trellised and Guyot-pruned, subjected to standard management techniques, and véraison started on 22 July 2015 (52 d after flowering). Berries were surface-sterilized with 70% ethanol followed by a 20% (w/v) NaClO solution, then rinsed with sterile water. Berries were laid in sterile Petri dishes (~10 berries per dish) in close contact (on the petiole side) with agar containing 8% (w/v) sucrose and the following combinations of ±ABA (Sigma) and rac-GR24 (Strigolab, Turin, Italy): no hormones; ±ABA 200 µM; rac-GR24 10−5 M; ±ABA 200 µM; and rac-GR24 10−5 M. To prevent contamination, the whole procedure was conducted under sterile conditions in a laminar hood. Sixty berries per treatment were collected 0, 24, 72, and 144 h after the start of the experiment, frozen in liquid nitrogen, and stored at –80 °C.
For the experiment on attached berries, grape bunches from 10 vines were sprayed once at the start of véraison until run-off, in the late afternoon and with the same hormone combinations, omitting sucrose (two bunches per treatment, each from a different vine). During the period of treatment, bunches were protected from direct sunlight by shading nets. Sixty berries per treatment were collected 0, 48, and 144 h after spraying, by severing the apical end of the pedicel. Berries were frozen in liquid nitrogen, and stored at –80 °C.
Additional samples of non-treated berries were taken at different stages of development to assess expression of SL biosynthetic genes.
Frozen berries were quickly peeled, and berry skins were powdered in liquid nitrogen and stored at –80 °C until analysis, while flesh was used for measurement of soluble solids.
Soluble sugars, total anthocyanin, and ABA concentration
Soluble sugars were assessed in triplicate with a refractometer on 10 extracts of berry flesh obtained by pressing.
Anthocyanin content was quantified in triplicate on ~1.5 g of powdered skin tissue, diluted 1:10 with acidic ethanol chloride (CH3CH2OH:H2O:HCl 70:30:1 v/v/v), by spectrophotometric analysis, reading the absorbance at 520 nm (Ferrandino and Guidoni, 2010).
ABA was quantified by LC-MS (Floková et al., 2014). A 15 mg sample from powdered berry skins was extracted using 1 ml of cold extraction solvent (10% methanol). In the same tube, 10 µl of stable isotope-labelled standard (D6-ABA 10−6 M) were added together with ceramic beads, in order to facilitate the homogenization with a Tissue Lyser (Qiagen) for 5 min at 27 Hz. The homogenates were sonicated for 3 min at 4 °C and shaken for 30 min at 4 °C. Samples were then centrifuged for 15 min at 20 000 rpm (4 °C). The supernatant was filtered using Oasis HLB extraction cartridges (30 μm cut-off) previously conditioned with 2 ml of 100% CH3OH and 1 ml of redistilled water. For the elution, 3 ml of 80% CH3OH were used, evaporated to dryness under a gentle stream of nitrogen at 30 °C for ~2 h. The dried residue was resuspended in 40 ml of 15% acetonitrile+85% HCOOH, filtered using 2 ml filtration tubes of 0.2 µm, and analysed with an Acquity UPLC® system (Waters, Milford, MA, USA) coupled to a quadrupole mass spectrometer Xevo™ TQ MS (Waters MS Technologies, Manchester, UK). Each sample (10 µl) was first separated on an RP column (Acquity® UPLC CSH™ C18; 1.7 µm, 2.1 × 100 mm) at a flow rate of 0.4 ml min−1, using the following solvents: 10 mM HCOOH (A) and acetonitrile (B). The gradient elution over 35 min was as follows: 0–5 min isocratic elution (15% A; v/v); 5–15 min linear gradient to 45% A; 15–28 min, logarithmic gradient to 48.6% A; 28–29 min linear gradient to 100% A. Finally, the column was washed with 100% acetonitrile and then equilibrated to the initial conditions (15% A, v/v) for 5 min. The effluent was introduced into the ESI (electrospray ionization) ion source of a tandem MS analyser with a cone/desolvation gas temperature of 120/550 °C at a flow of 70/650 l h−1, with the capillary voltage set to 3 kV; cone voltage, 23–30 V; collision energy, 12–23 eV; and collision gas flow (argon), 0.21 ml min−1. Detection was performed by multiple reaction monitoring (MRM) in positive ion mode. Optimization of fragmentation was done with labelled standards using the MAssLynx™ software package (version 4.1, Waters).
Matrix effects were calculated as the ratio of the mean peak area of the analyte spiked post-extraction to the mean peak area of the same analyte standards multiplied by 100. The process efficiency was determined as the mean peak area of the added standards before sample preparation divided by the known mean peak area of standard solutions. For assessment of the validation method, the concentration of the analyte was calculated using the standard isotope dilution method for each plant extract spiked before extraction and compared with the concentration of a proper standard solution. Each measurement was performed in quadruplicate.
In silico and quantitative reverse transcription–PCR analysis
Two putative biosynthetic genes for SL, namely the Carotenoid Cleavage Dioxygenases (CCD) 7 and 8, were identified by BLAST searching the grapevine ‘PN40024’ 12X genome draft, V1 annotation, at the Grape Genome Database (http://genomes.cribi.unipd.it/grape/) with the Arabidopsis sequences.
Concentration changes of target transcripts were quantified on powdered berry skin samples (1.5 g) by quantitative reverse transcription–PCR (RT–qPCR). Total RNA was extracted following a cetyltrimethylammonium bromide (CTAB)-based protocol (Carra et al., 2007). RNA integrity and quantity were checked using a 2100 Bioanalyzer (Agilent Technologies). RNA samples were treated with RNase-free DNase I (Fermentas: 50 U µl−1 UAB, Vilnius, Lithuania) to avoid any risk of genomic DNA contamination, and first-strand cDNA was synthesized starting from 5 µg of total RNA using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA, USA) following the manufacturer’s instructions. cDNA integrity and primer specificity were then checked by gradient PCR and agarose gel electrophoresis. RT–qPCR was conducted in triplicate using a StepOnePlus™ System (Applied Biosystems), and the SYBR Green method (Power SYBR® Green PCR Master Mix, Applied Biosystems) was used for quantifying amplification results (Giordano et al., 2016; Pagliarani et al., 2017). Each reaction contained 1 μl of 5 μM primer mix, 100 ng of template cDNA, 5 μl of 2× SYBR Green mix, and 3 μl of diethylpyrocarbonate (DEPC)-treated water for a total reaction volume of 10 μl. Thermal cycling conditions were as follows: 95 °C for 10 min before the beginning of the amplification (holding stage), followed by 40 cycles at 95 °C for 15 s and 60 °C for 1 min. Specific annealing of primers was further checked on dissociation kinetics at the end of each RT–qPCR run. Expression of target transcripts was quantified after normalization to the geometric mean of the Ubiquitin (VvUBI) and Actin (VvACT1) transcripts used as endogenous controls. Expression changes were analysed for VvMybA1 (encoding a Myb transcription factor controlling anthocyanin biosynthesis in grapevine: Walker et al., 2007), VvUFGT (terminal gene of anthocyanin biosynthesis in grapevine, encoding UDP-glucose:flavonoid 3-O-glucosyltransferase: Ford et al., 1998), VvNCED1 (rate-limiting gene of ABA biosynthesis, encoding 9-cis-epoxycarotenoid dioxygenase: Wheeler et al., 2009), two genes encoding ABA 8'-hydroxylases (VvHYD1 and VvHYD2; Speirs et al., 2013), an ABA-UDPG glycosyl transferase (VvGT1; Sun et al., 2015), a β-glucosidase that hydrolyses ABA-glucose ester (VvBG1; Sun et al., 2015), and the grapevine orthologues of the Arabidopsis ABC Transporter G Family Protein (ABCG) ABA membrane transporters VvABCG25 (Kuromori et al., 2010) and VvABCG40 (Kang et al., 2010). Transcript quantification of the putative grapevine CCD7 and CCD8 was performed on non-treated berry samples only. Gene-specific primer pairs used in RT–qPCR experiments are listed in Supplementary Table S1 at JXB online.
Statistical analyses
For all measurements, three replicates of 10 berries were extracted and analysed independently for each treatment and sampling time. Significant differences among treatments were statistically evaluated by applying a one-way ANOVA using the Tukey’s HSD post-hoc test for separating means when ANOVA results were significant (P<0.05). The SPSS statistical software package was used for the analysis (SPSS Inc., Cary, NC, USA, v.22).
Results
Ripening and colour turning
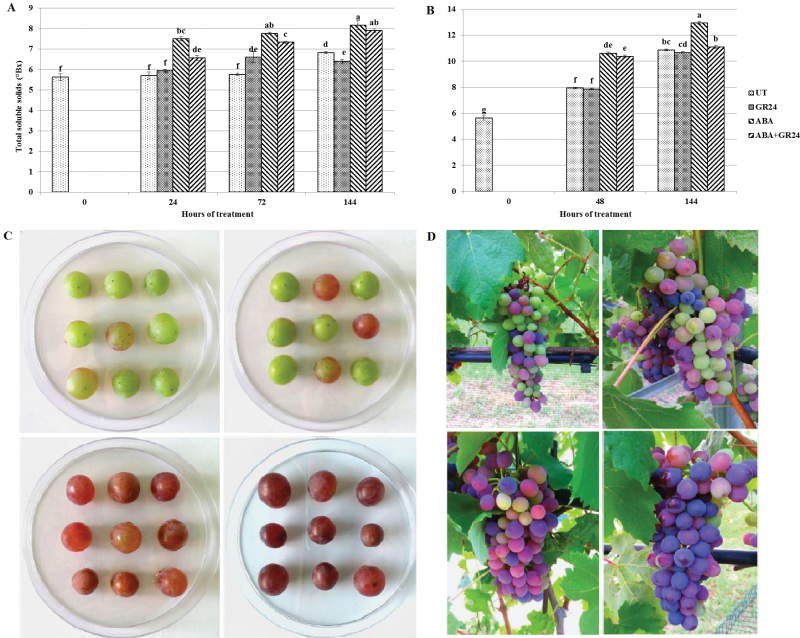
In order to investigate both specific and combined effects of GR24 and ABA on ripening of grape berries, we incubated detached berries in vitro on medium supplied with sucrose and hormones. Furthermore, in a second experiment, the same hormone treatments were applied to intact berries at véraison, to avoid the possible interference by exogenous sucrose and to allow for transport processes to the berry via the intact vasculature. Ripening, as shown by the accumulation of soluble sugars, proceeded as expected in untreated berries, in particular in those attached to the plant that were able to import phloematic sugar. Accumulation of soluble solids was slightly (but not significantly) hampered by GR24; it was significantly enhanced by exogenous ABA; however, this effect was counteracted by GR24 co-treatment (Fig. 1A, B). Also in both experiments, ABA induced colour turning; effects of treatment with GR24 in the absence of ABA were not visible, while GR24 administered together with ABA delayed colour accumulation compared with the samples treated with ABA alone (Fig 1C, D).
Fig. 1.
Accumulation of soluble solids (A, B) and colour turning (C, D) in V. vinifera berries (A, C) severed from the vine and incubated at véraison in the presence of different hormones, or (B, D) attached to the vine and sprayed at véraison with the same hormone combinations. UT, untreated control (no hormones); GR24, rac-GR24 10−5 M; ABA, ±ABA 200 µM; ABA+GR24, rac-GR24 10−5 M and ABA 200 µM. (C and D) Pictures were taken 6 d after treatment; treatments are displayed clockwise starting from the upper left panel. Values marked by the same letter do not significantly differ at P=0.05; bars are SEs.
Anthocyanin accumulation
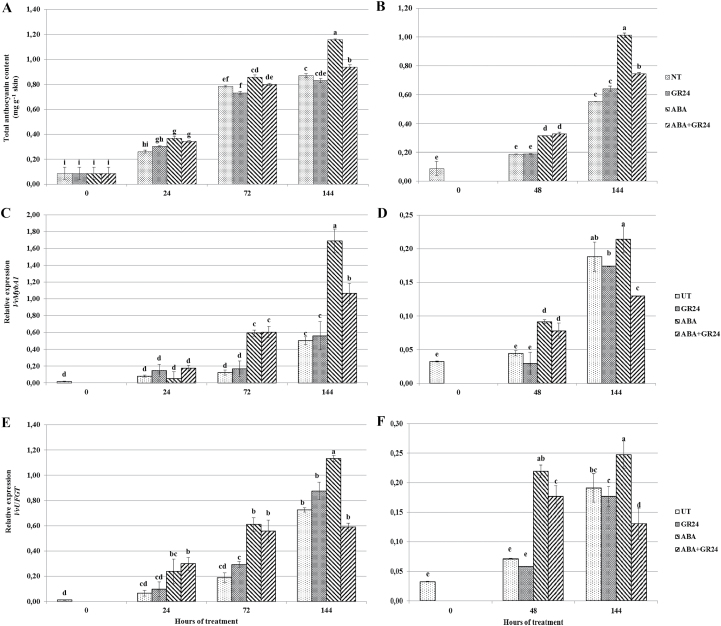
Colour changes were reflected in anthocyanin concentrations, which increased above those of untreated control following ABA treatment from the first sampling time onwards in both experiments. When berries were treated with GR24 only, the anthocyanin concentration was in some cases slightly lower, but never differed significantly from that measured in untreated control samples. When combined ABA and GR24 were supplied to the medium, anthocyanin accumulation was significantly lower than in the case of berries treated with ABA alone; this trend was observed in both experiments, and was particularly evident at the end of the time course (Fig. 2A, B).
Fig 2.
Anthocyanin accumulation (A, B) and transcript accumulation of regulatory (VvMybA1: C, D) and biosynthetic (VvUFGT: E, F) genes of anthocyanin biosynthesis in V. vinifera berry skins (A, C, E) severed from the vine and incubated at véraison in the presence of different hormones, or (B, D, F) attached to the vine and sprayed at véraison with the same hormone combinations. For treatment labels and significance of differences, see legend to Fig. 1.
The transcript concentrations of VvMybA1 (Fig 2B, C) and VvUFGT (Fig 2E, F) followed the pattern of anthocyanin accumulation well. In untreated controls, transcripts progressively accumulated to reach significantly higher amounts at the end of the experiment. In berries treated with GR24, transcript levels of these genes showed no difference from untreated controls at the same sampling times. In ABA-treated berries, the concentration of VvMybA1 and VvUFGT transcripts underwent a significant increase above that of the untreated control from 48 h (in vitro) or 72 h after treatment (in intact berries), confirming that expression of these genes is induced by exogenous ABA. The combined application of ABA and GR24 negatively affected the expression of both genes compared with treatment with ABA alone, in most cases limiting transcript accumulation to the level observed in untreated berries.
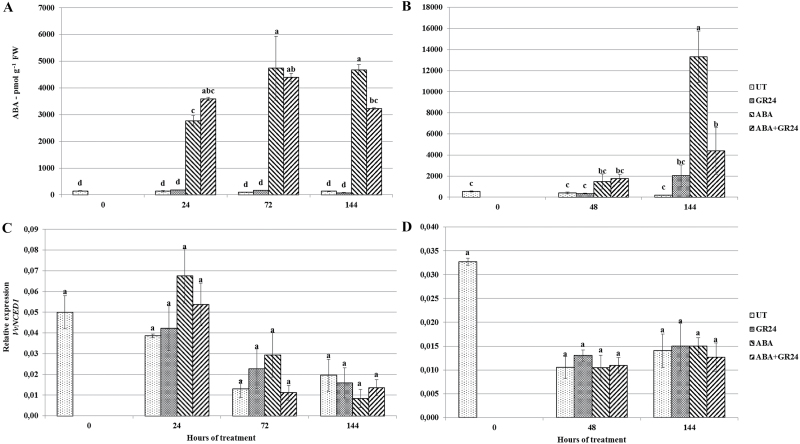
ABA concentration and biosynthesis
We explored whether GR24 could act on the anthocyanin concentration by modulating ABA concentrations. ABA levels showed no significant changes over time in the untreated control samples; average concentrations across all sampling times were significantly higher in attached than in in vitro incubated berries (391 pmol g FW versus 125 pmol g−1 FW), consistent with ABA phloematic transport to the berry (Fig. 3A, B). No significant effects of treatment with GR24 alone were detected. As expected, in ABA-treated berry skins, ABA concentration drastically increased at the first sampling time and remained stable in incubated berries (Fig. 3A), while the increase was slower in attached berries (Fig. 3B). GR24 co-treatment induced no significant effects on ABA skin concentration in the intermediate measurements, while at the end of both experiments these berries contained significantly less ABA than berries treated with ABA alone (Fig. 3A, B).
Fig. 3.
ABA concentration (A, B) and transcript accumulation of the ABA biosynthetic gene VvNCED1 (C, D) in V. vinifera berry skins (A, C) incubated at véraison in the presence of different hormones, or (B, D) attached to the vine and sprayed at véraison with the same hormone combinations. For treatment labels and significance of differences, see legend to Fig. 1.
The expression trend of the ABA biosynthetic gene VvNCED1 featured a decline in transcript levels over time in both experiments, and was not affected by treatment with the different hormone combinations (Fig. 3C, D).
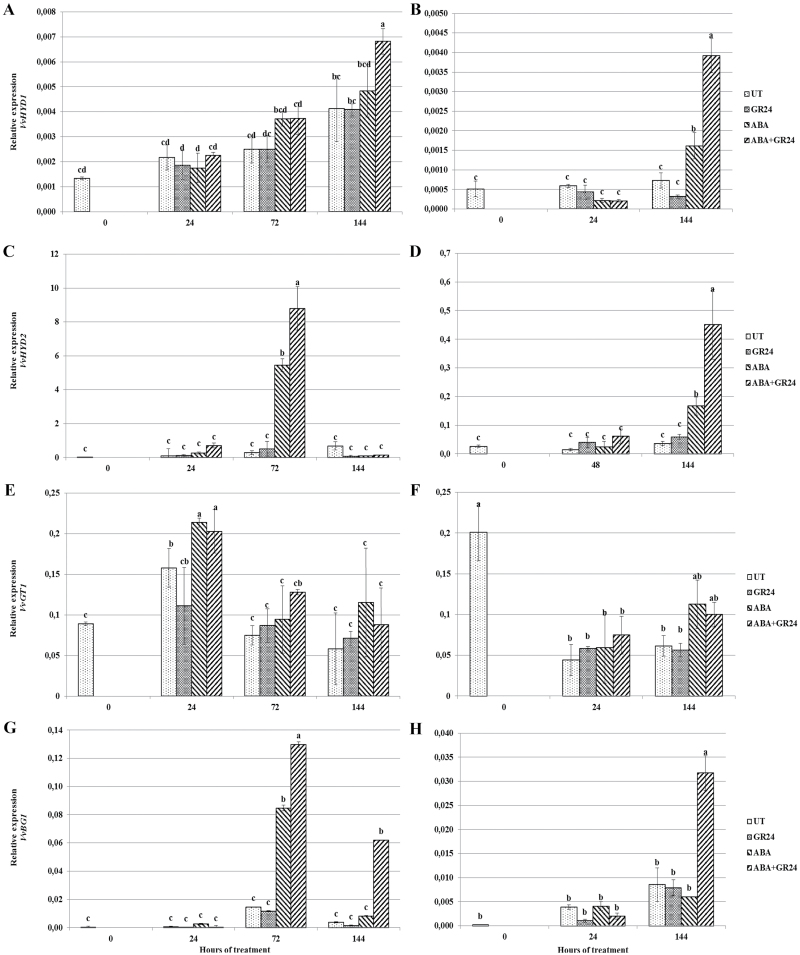
ABA metabolism and transport
The effect of exogenous GR24 on ABA metabolism was further explored by analysing the expression of genes involved in ABA hydroxylation (VvHYD1 and VvHYD2), conjugation (VvGT1), and de-conjugation (VvBG1). Expression of VvHYD1 increased during both time courses, and was significantly higher at the end of the experiment in attached ABA-treated berries than in untreated controls, and even significantly higher following co-treatment with the two hormones in both experiments (Fig. 4A, B). Similar transcript profiles were observed for VvHYD2 in attached berries (Fig 4C, D), while in vitro the concentration peak was anticipated at 72 h after the start of the experiment. Expression of VvGT1 did not significantly differ among treatments at each sampling time (Fig 4E, F). Transcript accumulation of VvBG1 was enhanced by ABA only in incubated berries at 72 h from the beginning of the experiment, whereas ABA+GR24 co-treatment consistently and significantly increased expression in both experiments (Fig 4H, G).
Fig. 4.
Transcript accumulation of genes involved in ABA metabolism. Relative expression of VvHYD1 (A, B), VvHYD2 (C, D), VvGT1 (E, F), and VvBG1 (G, H) in V. vinifera berry skins (A, C, E, G) incubated at véraison in the presence of different hormones, or (B, D, F, H) attached to the vine and sprayed at véraison with the same hormone combinations. For treatment labels and significance of differences, see legend to Fig. 1.
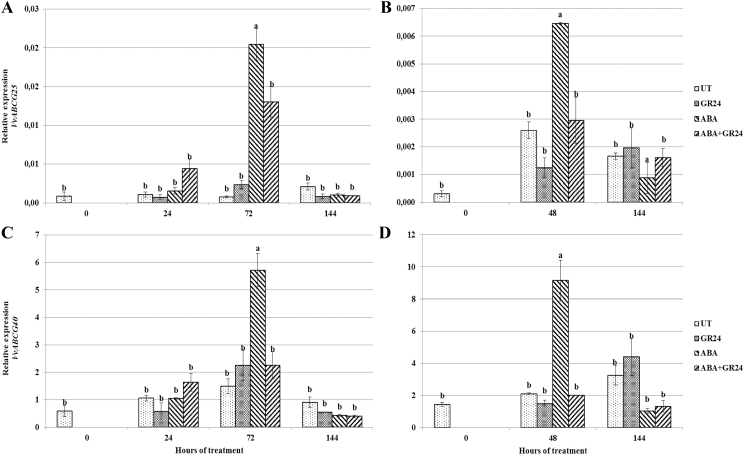
ABA transporters tune the level of cytosolic ABA and thus the responses due to ABA recognition by PYR-like/Regulatory Component of ABA Receptor (PYL/RCAR) cytosolic receptors. The transcript levels of the putative ABA transporter-encoding genes VvABCG25 (Fig 5A, B) and VvABCG40 (Fig 5C, D) were thus monitored, showing no significant concentration changes in either untreated or GR24-treated berries throughout the experiments. In contrast, transcript levels of these genes increased significantly following ABA treatment, peaking at 72 h and 48 h in the berries treated with ABA in vitro and in vivo, respectively, and decreasing afterwards. Co-treatment with GR24 and ABA significantly limited this increase or hindered it completely.
Fig. 5.
Transcript accumulation of genes involved in ABA transport. Relative expression of VvABC25 (A, B) and of VvABCG40 (C, D) in V. vinifera berry skins (A, C) incubated at véraison in the presence of different hormones, or (B, D) attached to the vine and sprayed at véraison with the same hormone combinations. For treatment labels and significance of differences, see legend to Fig. 1.
Discussion
Exogenous SL negatively interacts with ABA-induced anthocyanin accumulation in grape berries
Accumulation of soluble sugars and, in coloured varieties, of anthocyanins are main facets of grape berry ripening. Grape berries contain glucose and fructose as soluble sugars, and glucosides of cyanidin, delphinidin, peonidin, petunidin, and malvidin, the latter predominant in the majority of coloured cultivars, such as Barbera (Ferrandino et al., 2012). Total soluble sugar content increases from about 5°Brix at véraison (start of ripening) to well above 20°Brix at the end of ripening; anthocyanins accumulate from véraison during 20–40 d (Hrazdina et al., 1984) to reach final concentrations >1.2 mg g−1 in skin tissue in Barbera (Ferrandino et al., 2012).
Exogenous ABA supplemented via the severed pedicel or sprayed on intact grape berries enhances sugar content and anthocyanin accumulation (Pirie and Mullins, 1976; Wheeler et al., 2009; Sandhu et al., 2011). In both our experiments, ABA-treated berries followed this pattern, and reacted to exogenous ABA with an increase in soluble sugars and anthocyanins. Some molecular markers of anthocyanin accumulation are well known in grape berries: expression of the MYB transcription factor VvMybA1, encoding a transcriptional regulator that activates anthocyanin biosynthesis (Walker et al., 2007), and of the UDP-glucose:flavonoid 3-O-glucosyltransferase (VvUFGT) gene, encoding the last step of the anthocyanin biosynthetic pathway (Ford et al., 1998), closely follows the pattern of anthocyanin accumulation, and they are correspondingly activated by exogenous ABA (Jeong et al., 2004), as confirmed in our experiments.
The main finding of this study is that GR24 modified this pattern as it markedly inhibited the ABA-induced accumulation of both sugars and anthocyanins, and the transcriptional increase of VvMybA1 and VvUFGT. GR24 is a synthetic SL analogue widely used to simulate the action of natural compounds, also due to its ability to permeate plant tissues, as shown by the fact that it efficiently reverts the effects of genetic SL depletion (Ruyter-Spira et al., 2011; Visentin et al., 2016; Ito et al., 2017), and that it can be detected within treated tissues (Liu et al., 2015). We thus assume that GR24 concentration increased in GR24-treated berries, as was the case for ABA following ABA treatment.
The effects of GR24 were accompanied by a significant reduction of ABA concentration in ABA-treated berries, compared with those treated with ABA only, suggesting that the effects of GR24 were mediated by changes in the ABA signal. Bi-directional hormone interactions involving ABA and SL have been reported in other experimental systems. In tomato, chemically or genetically induced reduction of ABA concentration inhibits SL biosynthesis (López-Ráez et al., 2010). Conversely, changes in SL levels or sensitivity affect ABA concentration and responses: SL-depleted or SL-insensitive Arabidopsis mutants in the adult stage are drought stress hypersensitive and lack correct physiological and molecular responses to ABA (Ha et al., 2014), while max2 (SL-insensitive) mutants are hypersensitive to ABA at the seedling stage (Bu et al., 2014). The SL–ABA relationship seems to be organ dependent: Lotus japonicus and tomato SL biosynthetic mutants show a decrease in the drought stress-induced ABA surge in leaves, suggesting a positive interaction (Liu et al., 2015). In contrast, in Lotus roots, treatment with GR24 inhibits the osmotic stress-triggered increase of ABA concentration (Liu et al., 2015), and drought stress decreases SL and increases ABA concentration in non-mycorrhizal roots of Lotus, tomato, and lettuce (Liu et al., 2015; Ruiz-Lozano et al., 2016), as would be the case for a negative interaction. Clearly, the interactions at the biosynthetic, catabolic, membrane transport, and signalling levels may be intricate and diverse in the different plant organs.
Although our results strongly suggest that GR24 affected sugar and anthocyanin accumulation through modulation of ABA concentration, other possibilities exist. Lv et al. (2018) recently showed that in Arabidopsis leaves GR24 induces stomatal closure also in ABA-depleted mutants, and that this ABA-independent effect could be triggered by an oxidative burst. A transcriptomic study suggested that an oxidative burst takes place at véraison in grape berries (Pilati et al., 2007), and this could represent an additional mechanism of action of GR24 in grape berries.
GR24 controls the expression of ABA metabolic but not of biosynthetic genes
We observed that the GR24 treatment significantly reduced ABA concentration in ABA-treated berries, compared with those treated with ABA only. The concentration of ABA is regulated by its biosynthesis, controlled by NCED genes, and by catabolism, which can follow both oxidation and conjugation pathways (Nambara and Marion-Poll, 2005). Oxidation reactions are catalysed by cytochrome P450 monooxygenases such as ABA 8'-hydroxylase (CYP707A gene family; Kushiro et al., 2004; Saito et al., 2004). In grapevine, three members of this gene family are described, among which VvHYD1 and VvHYD2 are most expressed in root and leaf (Speirs et al., 2013). ABA oxidation to inactive compounds controls the drop in ABA concentration observed in leaves upon rehydration (Okamoto et al., 2009) and in seeds upon imbibition (Okamoto et al., 2006). ABA conjugation to ABA-glucose ester is performed by ABA-GlucosylTransferase (AGT) (Xu et al., 2002). The grapevine homologue VvGT1 is down-regulated after véraison (Sun et al., 2015). In Arabidopsis, ABA-glucose ester is hydrolysed by a β-glucosidase (BG1) (Lee et al., 2006). The grapevine homologue of this gene (VvBG1) was biochemically characterized and is up-regulated in berries at véraison (Sun et al., 2015).
A straightforward hypothesis to explain the lower ABA concentration following GR24 co-treatment of ABA-treated berries is the activation of ABA catabolism. CYP707A genes are transcriptionally up-regulated following ABA treatment, suggesting an active contribution to homeostasis of free ABA levels (Cutler and Krochko, 1999; Saito et al., 2004). We correspondingly observed a marked peak of VvHYD1 and VvHYD2 expression following ABA treatment. In the in vitro experiment, this peak, observed 72 h after treatment, did not cause a significant reduction of ABA concentration thereafter, probably due either to the high ABA levels induced by the treatment or to a relatively low amount of cytosolic ABA, the potential substrate of the cytosolic CYP707A gene products. Most interestingly, co-treatment with GR24 induced a further, significant expression increase of both hydroxylases, which could have elevated the enzyme activity to levels sufficient to observe the decrease of ABA at later sampling times. This finding, considering that GR24 application activates CYP707A1 expression and enhances germination of Phelipanche ramosa seeds (Lechat et al., 2012), while Arabidopsis CYP707A3 is up-regulated by gibberellin and brassinolide (Saito et al., 2004), suggests that this gene family may mediate several hormone interactions in plants.
The effect of GR24 treatment on ABA conjugation is less clear: we observed no significant changes in expression of VvGT1 (encoding a conjugating enzyme), and an activation of VvBG1 (encoding a de-conjugating enzyme) transcript concentration, which could represent a homeostatic control of free ABA levels induced by the increase of ABA hydroxylation observed upon GR24 treatment. However, as VvBG1 is two orders of magnitude less expressed than VvGT1, the contribution of de-conjugation to free ABA levels might be negligible.
Members of the NCED gene family are considered to be the main control point of ABA biosynthesis in Arabidopsis (Nambara and Marion-Poll, 2005) and are activated by ABA in some ecotypes (Cheng et al., 2002). A second possible mechanism underlying the effect of GR24 on ABA-treated berries could thus be due to changes in ABA-induced ABA biosynthesis rate, which could contribute to the rise in ABA concentration, particularly in the cytosolic compartment. Two NCED genes were cloned from grapevine, NCED1 being the most expressed in berries (Deluc et al., 2007; Wheeler et al., 2009; Zhang et al., 2009). However, while VvNCED1 expression decreased throughout the experiments, it was not significantly affected by ABA, as previously observed in tomato (Thompson et al., 2000), suggesting that GR24 does not lower the free ABA concentration in ABA-treated samples by inhibiting biosynthesis at the transcriptional level.
Membrane transport of ABA is regulated by GR24
Besides direct effects on ABA concentration, GR24 could control the expression of ABA membrane transport genes (Boursiac et al., 2013). In Arabidopsis, ABCG40 controls ABA cellular uptake: it is expressed in leaves, roots, and seed, and its down-regulation dampens physiological responses to ABA (Kang et al., 2010). The ABA-induced ABCG25, localized to the vasculature, and in the endosperm, mediates ATP-dependent extrusion of ABA (Kuromori et al., 2010; Kang et al., 2015). Expression of these transport genes may affect the concentration of cytosolic free ABA, which interacts with the cytosolic PYL/RCAR receptors (Park et al., 2009). In the grape berry, ABA transport genes have not been studied in detail yet, while PYL/RCAR genes have been identified and are expressed in vegetative tissue and in berries (Li et al., 2012). We observed an early (i.e. 72 h and 48 h after treatment in the in vitro and in vivo experiments, respectively), transient induction of VvABCG25 and VvABCG40 transcript levels following ABA treatment, which was abolished upon GR24 co-treatment. These changes suggest that the cellular/apoplastic ABA concentration ratio may be affected by GR24 in ABA-treated berry skins by a decrease of import coupled to an increase of export activity. Additionally, since VvABCG25 is two orders of magnitude less expressed than VvABCG40 with respect to the same housekeeping genes, the dampening of ABA import might contribute more than the decreased export, resulting in a lower free ABA cellular concentration in ABA- and GR24-treated berry skins, compared with those treated with ABA alone.
Do natural SLs play a role in grape berry ripening?
SLs are carotenoid-derived hormones, whose core biosynthetic pathway is based on the carotenoid isomerase D27 (Dwarf27), the carotenoid cleavage dioxygenases CCD7 and CCD8, and the P450 monooxygenase MAX1 (More Axillary Growth1) (Ruyter-Spira et al., 2013). They are mostly, though not exclusively, produced in roots, where they are detected in the nanomolar range, and are thought to be transported to the shoots, where their concentration may be two orders of magnitude lower (Liu et al., 2015) and, for most plant species, below the detection threshold. Genetic evidence shows that they are active in above-ground organs at such low concentrations, controlling shoot-specific traits such as axillary bud development (Brewer et al., 2013). Also, reproductive defects of plants compromised in SL biosynthesis or perception suggest a largely unexplored role in flower and fruit development for certain species, besides juvenile to reproductive phase transition (e.g. in tomato, kiwifruit, Lotus, tomato, and petunia) (Snowden et al., 2005; Ledger et al., 2010; Kohlen et al., 2012; Liu et al., 2013).
In the grape berry, DNA microarray data suggest that VvCCD7, VvCCD8, and VvMAX1 are differentially expressed in green and ripening berries (Young et al., 2012), as also shown in tomato fruit for SlCCD7 (Vogel et al., 2010) and in kiwifruit for AcCCD7 and AcCCD8 (Ledger et al., 2010). A reported attempt to quantify expression of putative VvCCD7 and VvCCD8 in above-ground organs of grapevine was not successful (Lashbrooke et al., 2013). We assessed expression of the same two genes in berry skins during berry development by RT–qPCR, and confirmed a very low relative transcript level (Supplementary Fig. S1). Interestingly, expression of both VvCCD7 and VvCCD8 tended to increase in the late stages of ripening, in correspondence with the known decrease in ABA concentration after véraison (Wheeler et al., 2009). In grapevine, no data are available on SL profiles and concentration. It must be noted here that SLs are usually undetectable in the aerial part of plants, and indeed the transcripts of the biosynthetic genes we tested are 10- or even 100-fold less concentrated than in roots, where SLs are more massively produced, especially under phosphate deprivation (data not shown). These preliminary results open up the possibility that changes in SL concentration at véraison may play a regulatory role in grape berry ripening.
While we clearly observed that GR24 limits the ripening effects of exogenous ABA, we were able to detect only very limited, and not significant, effects of GR24 treatments on non-ABA-treated berries. These observations seem contradictory, it being apparently unrealistic that GR24 may have such powerful effects on the signal induced by exogenous ABA, and at the same time to be ineffective on the endogenous ABA signal. A possible reconciling hypothesis is that endogenous SL is only one of several control points of the ABA concentration and/or signalling pathway, possibly co-operating at the molecular level with other effectors. In such a situation, additional, exogenous SL would not further affect the ABA signal in the absence of an increase of such co-operating effectors. It is well demonstrated that ABA can reinforce its own signal by ABA-dependent up-regulation of biosynthetic and signalling genes (Yang and Tan, 2014). Thus ABA treatment could entail an increase in expression of SL-co-operating molecular effectors, finally allowing exogenous SLs to interact with them to control the exogenous ABA concentration and signal.
Supplementary data
Supplementary data are available at JXB online.
Table S1. Oligonucleotides used in this study for RT–PCR analysis
Fig. S1. Expression profiles of VvCCD7 and of VvCCD8 in skins of untreated V. vinifera during berry development
Acknowledgements
The authors wish to acknowledge the Compagnia di San Paolo Foundation (project SLEPS) for financial support.
Abbreviations
- ABA
abscisic acid
- ABCG
ABC Transporter G Family Protein
- PYL/RCAR
PYR-like/Regulatory Component of ABA Receptor
- SL
strigolactone.
References
- Adams DO. 2006. Phenolics and ripening in grape berries. American Journal of Enology and Viticulture 57, 249–256. [Google Scholar]
- Akiyama K, Matsuzaki K, Hayashi H. 2005. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435, 824–827. [DOI] [PubMed] [Google Scholar]
- Belhadj A, Telef N, Saigne C, Cluzet S, Barrieu F, Hamdi S, Mérillon JM. 2008. Effect of methyl jasmonate in combination with carbohydrates on gene expression of PR proteins, stilbene and anthocyanin accumulation in grapevine cell cultures. Plant Physiology and Biochemistry 46, 493–499. [DOI] [PubMed] [Google Scholar]
- Besserer A, Bécard G, Jauneau A, Roux C, Séjalon-Delmas N. 2008. GR24, a synthetic analog of strigolactones, stimulates the mitosis and growth of the arbuscular mycorrhizal fungus Gigaspora rosea by boosting its energy metabolism. Plant Physiology 148, 402–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boursiac Y, Léran S, Corratgé-Faillie C, Gojon A, Krouk G, Lacombe B. 2013. ABA transport and transporters. Trends in Plant Science 18, 325–333. [DOI] [PubMed] [Google Scholar]
- Bradow JM, Connick WJ Jr. 1988. Seed-germination inhibition by volatile alcohols and other compounds associated with Amaranthus palmeri residues. Journal of Chemical Ecology 14, 1633–1648. [DOI] [PubMed] [Google Scholar]
- Brewer PB, Koltai H, Beveridge CA. 2013. Diverse roles of strigolactones in plant development. Molecular Plant 6, 18–28. [DOI] [PubMed] [Google Scholar]
- Bu Q, Lv T, Shen H et al. . 2014. Regulation of drought tolerance by the F-box protein MAX2 in Arabidopsis. Plant Physiology 164, 424–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carra A, Gambino G, Schubert A. 2007. A cetyltrimethylammonium bromide-based method to extract low-molecular-weight RNA from polysaccharide-rich plant tissues. Analytical Biochemistry 360, 318–320. [DOI] [PubMed] [Google Scholar]
- Cheng WH, Endo A, Zhou L et al. . 2002. A unique short-chain dehydrogenase/reductase in Arabidopsis glucose signaling and abscisic acid biosynthesis and functions. The Plant Cell 14, 2723–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chervin C, El-Kereamy A, Roustan J, Latche A, Lamon J, Bouzayen M. 2004. Ethylene seems required for the berry development and ripening in grape, a non-climacteric fruit. Plant Science 167, 1301–1305. [Google Scholar]
- Coombe BG, Hale CR. 1973. The hormone content of ripening grape berries and the effects of growth substance treatments. Plant Physiology 51, 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler AJ, Krochko JE. 1999. Formation and breakdown of ABA. Trends in Plant Science 4, 472–478. [DOI] [PubMed] [Google Scholar]
- Davies C, Boss PK, Robinson SP. 1997. Treatment of grape berries, a nonclimacteric fruit with a synthetic auxin, retards ripening and alters the expression of developmentally regulated genes. Plant Physiology 115, 1155–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deluc LG, Grimplet J, Wheatley MD, Tillett RL, Quilici DR, Osborne C, Schooley DA, Schlauch KA, Cushman JC, Cramer GR. 2007. Transcriptomic and metabolite analyses of Cabernet Sauvignon grape berry development. BMC Genomics 8, 429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrandino A, Carra A, Rolle L, Schneider A, Schubert A. 2012. Profiling of hydroxycinnamoyl tartrates and acylated anthocyanins in the skin of 34 Vitis vinifera genotypes. Journal of Agricultural and Food Chemistry 60, 4931–4945. [DOI] [PubMed] [Google Scholar]
- Ferrandino A, Guidoni S. 2010. Anthocyanins, flavonols and hydroxycinnamates: an attempt to use them to discriminate Vitis vinifera L. cv ‘Barbera’ clones. European Food Research and Technology 230, 417–427. [Google Scholar]
- Floková K, Tarkowská D, Miersch O, Strnad M, Wasternack C, Novák O. 2014. UHPLC-MS/MS based target profiling of stress-induced phytohormones. Phytochemistry 105, 147–157. [DOI] [PubMed] [Google Scholar]
- Ford CM, Boss PK, Hoj PB. 1998. Cloning and characterization of Vitis vinifera UDP-glucose:flavonoid 3-O-glucosyltransferase, a homologue of the enzyme encoded by the maize Bronze-1 locus that may primarily serve to glucosylate anthocyanidins in vivo. Journal of Biological Chemistry 273, 9224–9233. [DOI] [PubMed] [Google Scholar]
- Gambetta GA, Matthews MA, Shaghasi TH, McElrone AJ, Castellarin SD. 2010. Sugar and abscisic acid signaling orthologs are activated at the onset of ripening in grape. Planta 232, 219–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano D, Provenzano S, Ferrandino A, Vitali M, Pagliarani C, Roman F, Cardinale F, Castellarin SD, Schubert A. 2016. Characterization of a multifunctional caffeoyl-CoA O-methyltransferase activated in grape berries upon drought stress. Plant Physiology and Biochemistry 101, 23–32. [DOI] [PubMed] [Google Scholar]
- Giribaldi M, Gény L, Delrot S, Schubert A. 2010. Proteomic analysis of the effects of ABA treatments on ripening Vitis vinifera berries. Journal of Experimental Botany 61, 2447–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giribaldi M, Perugini I, Sauvage FX, Schubert A. 2007. Analysis of protein changes during grape berry ripening by 2-DE and MALDI-TOF. Proteomics 7, 3154–3170. [DOI] [PubMed] [Google Scholar]
- Gomez-Roldan V, Fermas S, Brewer PB et al. . 2008. Strigolactone inhibition of shoot branching. Nature 455, 189–194. [DOI] [PubMed] [Google Scholar]
- Ha C, Leyva-Gonzalez M, Osakabe Y et al. . 2014. Positive regulatory role of strigolactone in plant responses to drought and salt stress. Proceedings of the National Academy of Sciences, USA 111, 851–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrazdina G, Parsons GF, Mattick LR. 1984. Physiological and biochemical events during development and maturation of grape berries. Journal of Enology and Viticulture 35, 220–227. [Google Scholar]
- Ito S, Yamagami D, Umehara M et al. . 2017. Regulation of strigolactone biosynthesis by gibberellin signaling. Plant Physiology 174, 1250–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong S, Goto-Yamamoto N, Kobayashi S, Esaka A. 2004. Effects of plant hormones and shading on the accumulation of anthocyanins and the expression of anthocyanin biosynthetic genes in grape berry skins. Plant Science 167, 247–252. [Google Scholar]
- Jia H, Zhang C, Pervaiz T, Zhao P, Liu Z, Wang B, Wang C, Zhang L, Fang J, Qian J. 2016. Jasmonic acid involves in grape fruit ripening and resistance against Botrytis cinerea. Functional and Integrative Genomics 16, 79–94. [DOI] [PubMed] [Google Scholar]
- Kadomura-Ishikawa Y, Miyawaki K, Takahashi A, Masuda T, Noji S. 2015. Light and abscisic acid independently regulated FaMYB10 in Fragaria × ananassa fruit. Planta 241, 953–965. [DOI] [PubMed] [Google Scholar]
- Kalua C, Boss P. 2010. Comparison of major volatile compounds from Riesling and Cabernet Sauvignon grapes (Vitis vinifera L.) from fruitset to harvest. Australian Journal of Grape and Wine Research 16, 337–348. [Google Scholar]
- Kang J, Hwang J, Lee M, Kim Y, Assmann S, Martinoia E, Lee Y. 2010. PDR-type ABC transporter mediates cellular uptake of the phytohormone abscisic acid. Proceedings of the National Academy of Sciences, USA 107, 2355–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Yim S, Choi H, Kim A, Lee KP, Lopez-Molina L, Martinoia E, Lee Y. 2015. Abscisic acid transporters cooperate to control seed germination. Nature Communications 6, 8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlen W, Charnikhova T, Lammers M et al. . 2012. The tomato CAROTENOID CLEAVAGE DIOXYGENASE8 (SlCCD8) regulates rhizosphere signaling, plant architecture and affects reproductive development through strigolactone biosynthesis. New Phytologist 196, 535–547. [DOI] [PubMed] [Google Scholar]
- Kuromori T, Miyaji T, Yabuuchi H, Shimizu H, Sugimoto E, Kamiya A, Moriyama Y, Shinozaki K. 2010. ABC transporter AtABCG25 is involved in abscisic acid transport and responses. Proceedings of the National Academy of Sciences, USA 107, 2361–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushiro T, Okamoto M, Nakabayashi K, Yamagishi K, Kitamura S, Asami T, Hirai N, Koshiba T, Kamiya Y, Nambara E. 2004. The Arabidopsis cytochrome P450 CYP707A encodes ABA 8'-hydroxylases: key enzymes in ABA catabolism. EMBO Journal 23, 1647–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashbrooke JG, Young PR, Dockrall SJ, Vasanth K, Vivier MA. 2013. Functional characterisation of three members of the Vitis vinifera L. carotenoid cleavage dioxygenase gene family. BMC Plant Biology 13, 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechat MM, Pouvreau JB, Péron T et al. . 2012. PrCYP707A1, an ABA catabolic gene, is a key component of Phelipanche ramosa seed germination in response to the strigolactone analogue GR24. Journal of Experimental Botany 63, 5311–5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledger SE, Janssen BJ, Karunairetnam S, Wang T, Snowden KC. 2010. Modified CAROTENOID CLEAVAGE DIOXYGENASE8 expression correlates with altered branching in kiwifruit (Actinidia chinensis). New Phytologist 188, 803–813. [DOI] [PubMed] [Google Scholar]
- Lee KH, Piao HL, Kim HY, Choi SM, Jiang F, Hartung W, Hwang I, Kwak JM, Lee IJ, Hwang I. 2006. Activation of glucosidase via stress-induced polymerization rapidly increases active pools of abscisic acid. Cell 126, 1109–1120. [DOI] [PubMed] [Google Scholar]
- Li G, Xin H, Zheng XF, Li S, Hu Z. 2012. Identification of the abscisic acid receptor VvPYL1 in Vitis vinifera. Plant Biology 14, 244–248. [DOI] [PubMed] [Google Scholar]
- Liu J, He H, Vitali M et al. . 2015. Osmotic stress represses strigolactone biosynthesis in Lotus japonicus roots: exploring the interaction between strigolactones and ABA under abiotic stress. Planta 241, 1435–1451. [DOI] [PubMed] [Google Scholar]
- Liu J, Novero M, Charnikhova T, Ferrandino A, Schubert A, Ruyter-Spira C, Bonfante P, Lovisolo C, Bouwmeester HJ, Cardinale F. 2013. Carotenoid cleavage dioxygenase 7 modulates plant growth, reproduction, senescence, and determinate nodulation in the model legume Lotus japonicus. Journal of Experimental Botany 64, 1967–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Ráez JA, Kohlen W, Charnikhova T et al. . 2010. Does abscisic acid affect strigolactone biosynthesis?New Phytologist 187, 343–354. [DOI] [PubMed] [Google Scholar]
- Lv S, Zhang Y, Li C et al. . 2018. Strigolactone-triggered stomatal closure requires hydrogen peroxide synthesis and nitric oxide production in an abscisic acid-independent manner. New Phytologist 217, 290–304. [DOI] [PubMed] [Google Scholar]
- McCarty DR, Carson CB, Stinard PS, Robertson DS. 1989. Molecular analysis of viviparous-1: an abscisic acid-insensitive mutant of maize. The Plant Cell 1, 523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz AH, Hrazdina G. 1981. Vacuolar contents of fruit subepidermal cells from Vitis species. Plant Physiology 68, 686–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambara E, Marion-Poll A. 2005. Abscisic acid biosynthesis and catabolism. Annual Review of Plant Biology 56, 165–185. [DOI] [PubMed] [Google Scholar]
- Okamoto M, Kuwahara A, Seo M, Kushiro T, Asami T, Hirai N, Kamiya Y, Koshiba T, Nambara E. 2006. CYP707A1 and CYP707A2, which encode abscisic acid 8'-hydroxylases, are indispensable for proper control of seed dormancy and germination in Arabidopsis. Plant Physiology 141, 97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M, Tanaka Y, Abrams SR, Kamiya Y, Seki M, Nambara E. 2009. High humidity induces abscisic acid 8'-hydroxylase in stomata and vasculature to regulate local and systemic abscisic acid responses in Arabidopsis. Plant Physiology 149, 825–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliarani C, Vitali M, Ferrero M, Vitulo N, Incarbone M, Lovisolo C, Valle G, Schubert A. 2017. The accumulation of miRNAs differentially modulated by drought stress is affected by grafting in grapevine. Plant Physiology 173, 2180–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Fung P, Nishimura N et al. . 2009. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 324, 1068–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peláez-Vico MA, Bernabéu-Roda L, Kohlen W, Soto MJ, López-Ráez JA. 2016. Strigolactones in the Rhizobium–legume symbiosis: stimulatory effect on bacterial surface motility and down-regulation of their levels in nodulated plants. Plant Science 245, 119–127. [DOI] [PubMed] [Google Scholar]
- Pilati S, Perazzolli M, Malossini A, Cestaro A, Demattè L, Fontana P, Dal Ri A, Viola R, Velasco R, Moser C. 2007. Genome-wide transcriptional analysis of grapevine berry ripening reveals a set of genes similarly modulated during three seasons and the occurrence of an oxidative burst at vèraison. BMC Genomics 8, 428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirie A, Mullins MG. 1976. Changes in anthocyanin and phenolics content of grapevine leaf and fruit tissues treated with sucrose, nitrate, and abscisic acid. Plant Physiology 58, 468–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Lozano JM, Aroca R, Zamarreño ÁM, Molina S, Andreo-Jiménez B, Porcel R, García-Mina JM, Ruyter-Spira C, López-Ráez JA. 2016. Arbuscular mycorrhizal symbiosis induces strigolactone biosynthesis under drought and improves drought tolerance in lettuce and tomato. Plant, Cell and Environment 39, 441–452. [DOI] [PubMed] [Google Scholar]
- Ruyter-Spira C, Al-Babili S, van der Krol S, Bouwmeester H. 2013. The biology of strigolactones. Trends in Plant Science 18, 72–83. [DOI] [PubMed] [Google Scholar]
- Ruyter-Spira C, Kohlen W, Charnikhova T et al. . 2011. Physiological effects of the synthetic strigolactone analog GR24 on root system architecture in Arabidopsis: another belowground role for strigolactones?Plant Physiology 155, 721–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito S, Hirai N, Matsumoto C, Ohigashi H, Ohta D, Sakata K, Mizutani M. 2004. Arabidopsis CYP707As encode (+)-abscisic acid 8'-hydroxylase, a key enzyme in the oxidative catabolism of abscisic acid. Plant Physiology 134, 1439–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu AK, Gray DJ, Lu J, Gu L. 2011. Effect of exogenous abscisic acid on antioxidant capacities, anthocyanin, and flavonol content of muscadine grape (Vitis rotundifolia) skins. Food Chemistry 126, 982–988. [Google Scholar]
- Snowden KC, Simkin AJ, Janssen BJ, Templeton KR, Loucas HM, Simons JL, Karunairetnam S, Gleave AP, Clark DG, Klee HJ. 2005. The Decreased apical dominance1/Petunia hybrida CAROTENOID CLEAVAGE DIOXYGENASE8 gene affects branch production and plays a role in leaf senescence, root growth, and flower development. The Plant Cell 17, 746–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speirs J, Binney A, Collins M, Edwards E, Loveys B. 2013. Expression of ABA synthesis and metabolism genes under different irrigation strategies and atmospheric VPDs is associated with stomatal conductance in grapevine (Vitis vinifera L. cv Cabernet Sauvignon). Journal of Experimental Botany 64, 1907–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Dong Y, Li C, Shen Y. 2015. Transcription and enzymatic analysis of beta-glucosidase VvBG1 in grape berry ripening. Plant Growth Regulation 75, 67–73. [Google Scholar]
- Sun L, Zhang M, Ren J, Qi J, Zhang G, Leng P. 2010. Reciprocity between abscisic acid and ethylene at the onset of berry ripening and after harvest. BMC Plant Biology 10, 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons GM, Davies C, Shavrukov Y, Dry IB, Reid JB, Thomas MR. 2006. Grapes on steroids. Brassinosteroids are involved in grape berry ripening. Plant Physiology 140, 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AJ, Jackson AC, Parker RA, Morpeth DR, Burbidge A, Taylor IB. 2000. Abscisic acid biosynthesis in tomato: regulation of zeaxanthin epoxidase and 9-cis-epoxycarotenoid dioxygenase mRNAs by light/dark cycles, water stress and abscisic acid. Plant Molecular Biology 42, 833–845. [DOI] [PubMed] [Google Scholar]
- Umehara M, Hanada A, Yoshida S et al. . 2008. Inhibition of shoot branching by new terpenoid plant hormones. Nature 455, 195–200. [DOI] [PubMed] [Google Scholar]
- Villalobos-González L, Peña-Neira A, Ibáñez F, Pastenes C. 2016. Long-term effects of abscisic acid (ABA) on the grape berry phenylpropanoid pathway: gene expression and metabolite content. Plant Physiology and Biochemistry 105, 213–223. [DOI] [PubMed] [Google Scholar]
- Visentin I, Vitali M, Ferrero M, Zhang Y, Ruyter-Spira C, Novák O, Strnad M, Lovisolo C, Schubert A, Cardinale F. 2016. Low levels of strigolactones in roots as a component of the systemic signal of drought stress in tomato. New Phytologist 212, 954–963. [DOI] [PubMed] [Google Scholar]
- Vogel JT, Walter MH, Giavalisco P et al. . 2010. SlCCD7 controls strigolactone biosynthesis, shoot branching and mycorrhiza-induced apocarotenoid formation in tomato. The Plant Journal 61, 300–311. [DOI] [PubMed] [Google Scholar]
- Walker AR, Lee E, Bogs J, McDavid DA, Thomas MR, Robinson SP. 2007. White grapes arose through the mutation of two similar and adjacent regulatory genes. The Plant Journal 49, 772–785. [DOI] [PubMed] [Google Scholar]
- Wheeler S, Loveys B, Ford C, Davies C. 2009. The relationship between the expression of abscisic acid biosynthesis genes, accumulation of abscisic acid and the promotion of Vitis vinifera L. berry ripening by abscisic acid. Australian Journal of Grape and Wine Research 15, 195–204. [Google Scholar]
- Xu ZJ, Nakajima M, Suzuki Y, Yamaguchi I. 2002. Cloning and characterization of the abscisic acid-specific glucosyltransferase gene from adzuki bean seedlings. Plant Physiology 129, 1285–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YZ, Tan BC. 2014. A distal ABA responsive element in AtNCED3 promoter is required for positive feedback regulation of ABA biosynthesis in Arabidopsis. PLoS One 9, e87283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama K, Xie X, Kusumoto D, Sekimoto H, Sugimoto Y, Takeuchi Y, Yoneyama K. 2007. Nitrogen deficiency as well as phosphorus deficiency in sorghum promotes the production and exudation of 5-deoxystrigol, the host recognition signal for arbuscular mycorrhizal fungi and root parasites. Planta 227, 125–132. [DOI] [PubMed] [Google Scholar]
- Young PR, Lashbrooke JG, Alexandersson E, Jacobson D, Moser C, Velasco R, Vivier MA. 2012. The genes and enzymes of the carotenoid metabolic pathway in Vitis vinifera L. BMC Genomics 13, 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Leng P, Zhang G, Li X. 2009. Cloning and functional analysis of 9-cis-epoxycarotenoid dioxygenase (NCED) genes encoding a key enzyme during abscisic acid biosynthesis from peach and grape fruits. Journal of Plant Physiology 166, 1241–1252. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.