Abstract
Background
Patient-reported outcomes are integral in benefit–risk assessments of new treatment regimens. The PALOMA-2 study provides the largest body of evidence for patient-reported health-related quality of life (QOL) for patients with metastatic breast cancer (MBC) receiving first-line endocrine-based therapy (palbociclib plus letrozole and letrozole alone).
Patients and methods
Treatment-naïve postmenopausal women with estrogen receptor-positive (ER+)/human epidermal growth factor receptor 2-negative (HER2−) MBC were randomized 2 : 1 to palbociclib plus letrozole (n = 444) or placebo plus letrozole (n = 222). Patient-reported outcomes were assessed at baseline, day 1 of cycles 2 and 3, and day 1 of every other cycle from cycle 5 using the Functional Assessment of Cancer Therapy (FACT)-Breast and EuroQOL 5 dimensions (EQ-5D) questionnaires.
Results
As of 26 February 2016, the median duration of follow-up was 23 months. Baseline scores were comparable between the two treatment arms. No significant between-arm differences were observed in change from baseline in FACT-Breast Total, FACT-General Total, or EQ-5D scores. Significantly greater improvement in pain scores was observed in the palbociclib plus letrozole arm (−0.256 versus −0.098; P = 0.0183). In both arms, deterioration of FACT-Breast Total score was significantly delayed in patients without progression versus those with progression and patients with partial or complete response versus those without. No significant difference was observed in FACT-Breast and EQ-5D index scores in patients with and without neutropenia.
Conclusions
Overall, women with MBC receiving first-line endocrine therapy have a good QOL. The addition of palbociclib to letrozole maintains health-related QOL and improves pain scores in treatment-naïve postmenopausal patients with ER+/HER2− MBC compared with letrozole alone. Significantly greater delay in deterioration of health-related QOL was observed in patients without progression versus those who progressed and in patients with an objective response versus non-responders.
ClinicalTrials.gov: NCT01740427 (https://clinicaltrials.gov/ct2/show/NCT01740427)
Keywords: palbociclib, letrozole, health-related quality of life, metastatic breast cancer, ER+/HER2‒
Key Message
In PALOMA-2, adding palbociclib to letrozole maintained health-related quality of life (HRQOL) in treatment-naïve postmenopausal patients with estrogen receptor-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer. This observation is maintained regardless of the occurrence of neutropenia while deterioration of HRQOL is delayed in patients without progression.
Introduction
Endocrine therapy is the primary treatment option for patients with treatment-naïve estrogen receptor-positive (ER+)/human epidermal growth factor receptor 2-negative (HER2−) metastatic breast cancer (MBC). However, all patients eventually develop tumor progression or have primary tumor resistance to endocrine treatments. Improving response and prolonging the duration of response to endocrine-based therapy while maintaining or improving quality of life (QOL) is an important treatment goal. The addition of agents targeted to pathways contributing to resistance may improve response and delay progression, but it is critical to understand both the safety profile and the impact of these therapies on QOL [1, 2]. As such, patient-reported outcomes (PROs) are an integral component of benefit–risk assessments in the evaluation of new treatment regimens.
Palbociclib (Ibrance, Pfizer, New York, NY) is an orally bioavailable small-molecule inhibitor of cyclin-dependent kinases (CDKs) 4 and 6 [3]. The recently reported results from the primary end point of the phase III study (PALOMA-2) demonstrated a significant improvement in progression-free survival (PFS) with palbociclib plus letrozole [median 24.8 versus 14.5 months; hazard ratio (HR): 0.58; 95% confidence interval (CI), 0.46–0.72; P < 0.001] versus placebo plus letrozole [4]. The main objective of the current report describes the impact of palbociclib and letrozole combination therapy on patient-reported QOL in PALOMA-2 (NCT01740427).
Patients and methods
Study design and participants
PALOMA-2 is an international (17 countries), randomized, double-blind, placebo-controlled, parallel-group, phase III study in which postmenopausal women with ER+/HER2‒ advanced/metastatic breast cancer who had not received systemic anticancer treatment of advanced disease (ie, treatment-naïve) were randomized 2 : 1 to palbociclib plus letrozole (n = 444) or placebo plus letrozole (n = 222). All randomized patients received study therapy (supplementary Figure S1, available at Annals of Oncology online); additional methods are published elsewhere [4]. The study was carried out in compliance with ethical principles outlined in the Declaration of Helsinki; the study protocol was approved by local review boards at each investigational center.
Treatment
Palbociclib or placebo was administered orally 125 mg daily for 21 days of every 28-day cycle followed by 7 days off. Letrozole was administered orally 2.5 mg on a continuous daily dosing schedule.
Patient-reported outcomes
Patient-reported health-related QOL (HRQOL) outcomes, a secondary objective in the PALOMA-2 trial, were assessed using the fully validated Functional Assessment of Cancer Therapy-Breast (FACT-B) and EuroQOL 5 dimensions (EQ-5D) questionnaires at baseline, day 1 of cycles 2 and 3, and day 1 of every alternate cycle from cycle 5 until disease progression or end of study treatment [5–7]. Patients completed the self-assessment questionnaires at the study site. The FACT-B was chosen as it is a well-validated questionnaire to assess breast cancer-specific HRQOL. The majority of patients in the validation study for this instrument had stage IV breast cancer; hence, the instrument was considered appropriate to assess HRQOL in the PALOMA-2 study.
The FACT-B (version 4, 1997) measures multidimensional breast cancer-specific HRQOL using a 37-item self-reporting instrument containing the 27-question FACT-General (FACT-G) survey and a 10-question breast cancer-additional concerns scale [5]. Patients are asked to respond to each question on a Likert scale where 0 = not at all, 1 = a little bit, 2 = somewhat, 3 = quite a bit, and 4 = very much. FACT-B produces five subscale scores that reflect the patient’s QOL: physical well-being (PWB), social/family well-being (SWB), emotional well-being (EWB), functional well-being (FWB), and a breast cancer subscale (BCS). The BCS was calculated using the first 9 of the 10 items in our analyses with the last item assessing pain excluded as recommended by the scoring guidelines for FACT-B v4.0. These subscale scores were used to derive three assessment outcomes—FACT-B total score, FACT-G total score, and FACT-B Trial Outcome Index (TOI), which are defined as follows:
FACT-B total score = PWB + SWB + EWB + FWB + BCS
FACT-G total score = PWB + SWB + EWB + FWB
TOI score = PWB + FWB + BCS
Subscale scores were calculated if over 50% of the component individual items in that subscale are non-missing; the total scores were calculated if over 80% of the component items are non-missing and all the component subscales have valid scores.
A higher score in any FACT-B assessment indicates better QOL. A meaningful change in QOL is defined as a change in baseline score equal to or greater than the established minimally important differences: FACT-G = 5–6 points and FACT-B = 7–8 points [8]. The tenth item of the BCS (P2) assessing severity of pain in certain parts of the body was analyzed as an individual item separate from the BCS. A higher raw score on the pain item indicates higher pain severity.
The EQ-5D questionnaire [6] is composed of a 5-item health status measure and a visual analog scale (VAS) administered separately and used to generate two different scores, the EQ-5D index and the VAS general health status. The EQ-5D index score is based on answers to a 5-item questionnaire evaluating mobility, self-care, usual activities, pain, discomfort, and anxiety/depression. Answers received a score ranging from 1 to 3, depending on whether patients perceived no problems (=1), some problems (=2), or extreme problems (=3) in that aspect of their health. Summary scores were calculated using an algorithm based on societal preferences from general population-based valuation studies in the UK (0 = death, 1.0 = perfect health) [6]. A higher EQ-5D index score indicated a better QOL. The EQ-5D VAS generates a single health status score in which patients are asked to rate their current health on a VAS ranging from 100 (best imaginable health state) to 0 (worst imaginable health state).
Statistical analyses
PRO analyses were based on the PRO-evaluable population (i.e., patients in the intent-to-treat [ITT] population with a baseline and ≥1 post-baseline assessment before the end of study treatment). Completion rates were summarized by cycle in the ITT population. The primary prespecified PRO end point was HRQOL assessed by the FACT-B total score. Other PRO end points included FACT-G total score, FACT-G subscale scores, BCS, TOI, EQ-5D index score, and EQ-VAS general health status score. For each questionnaire (FACT-B and EQ-5D), a completion status table was generated showing the numbers and percentages of patients at each visit and the numbers and percentages of patients completing ≥1 item of the questionnaire at that cycle. Changes from baseline in total FACT-B and subscale scores occurring during treatment were assessed by subtracting baseline QOL score from follow-up visit scores. The primary prespecified PRO analysis to compare the 2 treatment groups was based on a longitudinal, mixed-effects, random-intercept, random-slope model [9]. The variables in the model are treatment, time, treatment by time, and baseline used as covariates. In fitting the mixed-effects model, time was used as a continuous variable and the method of restricted maximum likelihood was used and an unstructured covariance matrix was assumed. A linear function was used because the slope of line graph across timepoints was linear and the time trend did not differ by treatment assignment. Similar analyses were carried out to compare the overall EQ-5D index and VAS scores between treatment arms. No adjustments for multiple comparisons were made.
As part of the post hoc analyses, an examination of the time to deterioration (TTD) in FACT-B total score was conducted using survival analysis methods including Kaplan–Meier plots, calculation of HRs, and log-rank tests to compare the two treatment arms. TTD was defined as the duration between baseline and first occurrence of a decrease of ≥7 points in FACT-B score with no subsequent observation of a <7-point decrease. In addition, TTD in FACT-B scores was compared between the following groups in both treatment arms combined as well as in each treatment arm separately: patients with versus without objective response [complete response or partial response according to Response Evaluation Criteria in Solid Tumors (RECIST) v 1.1] and patients with a progression event versus those who did not progress. Although a one-sided test at a significance level of 0.05 was used for consistency with the primary and key secondary time-to-event end points, the critical value used for interpretation for TTD was 0.025.
In addition, as part of post hoc analyses, the change from baseline in the individual item assessing pain in body parts was carried out using repeated-measures mixed-effects analyses with baseline as a covariate. To assess the effect of neutropenia, patients with and without neutropenia were identified at each cycle and compared on change from baseline FACT-B and observed EQ-5D index scores using analysis of variance with baseline as a covariate. This analysis was carried out only for the patients in the palbociclib plus letrozole arm as few patients in the placebo plus letrozole arm had neutropenia.
All analyses were conducted using Statistical Analysis System software (SAS Institute, Cary, NC). All P values are two-sided unless stated otherwise.
Results
Patients and disease characteristics
Baseline characteristics in the ITT population were well-balanced between the two treatment arms as previously reported [4]. Mean age was 62 years in the palbociclib plus letrozole arm and 61 years in the placebo plus letrozole arm (supplementary Table S1, available at Annals of Oncology online). A total of 338 (76.1%) and 171 (77.0%) patients had measurable disease at baseline in the palbociclib plus letrozole and placebo plus letrozole arms, respectively. Most patients in both treatment arms had Eastern Cooperative Oncology Group performance status of 0 or 1 (98%). More than one third of patients in each treatment arm had de novo disease and about 40% had 3 or more metastatic sites (supplementary Table S1, available at Annals of Oncology online).
Completion rates of PROs
The percentage of patients completing at least 1 question from baseline to cycle 37 for FACT-B ranged from 95% to 100% in each treatment arm except for cycle 33 in the placebo plus letrozole arm, where an 80% completion rate was observed among 5 eligible patients (at the time of data cut-off with 22.3 months median follow-up). A summary of completion rates for FACT-B by treatment arm and cycle and the number of eligible patients available to complete the questionnaire is presented in supplementary Table S2, available at Annals of Oncology online; reasons for discontinuation overall and by treatment arm are presented in supplementary Table S3, available at Annals of Oncology online.
FACT-B total scores
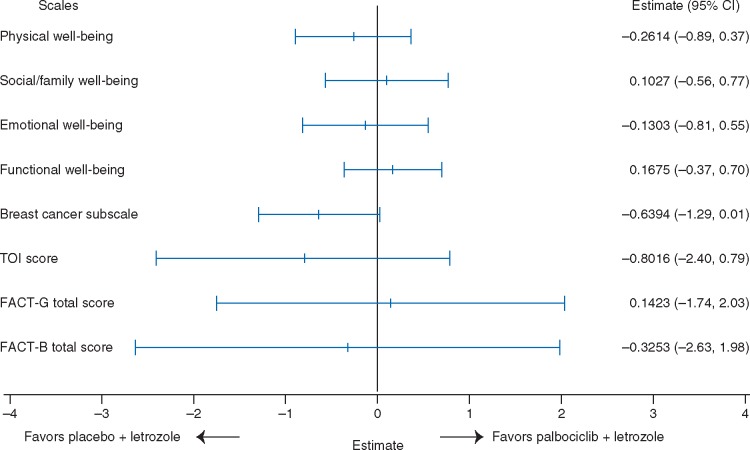
Baseline mean [±standard deviation (SD)] scores for FACT-B total scores were similar for the palbociclib and the placebo plus letrozole arms (101.5 ± 19.1 versus 103.2 ± 18.7) and comparable to healthy individuals (Table 1) [10]. The overall change from baseline in FACT-B scores from the repeated-measures mixed-effects model was not found to be significantly different (P = 0.782) between the palbociclib plus letrozole arm [−0.11 (95% CI −1.42 to 1.21)] and the placebo plus letrozole arm [0.22 (−1.68 to 2.12)]; the treatment-by-time interaction term was not found to be significant (P = 0.610). The change from baseline value did not reach the threshold of seven points in either treatment arm indicating that addition of palbociclib to letrozole did not have a clinically meaningful adverse impact on patient QOL. A Forest plot of between-treatment comparisons of change from baseline in total FACT-B and subscale scores is displayed in Figure 1.
Table 1.
Baseline FACT-B scores
| Domain | Palbociclib + letrozole Mean (SD) | Placebo + letrozole Mean (SD) | Normative scores [10] Mean (SD) |
|---|---|---|---|
| Physical well-being | 21.9 (5.5) | 21.8 (5.4) | 22.1 (5.4) |
| Social/family well-being | 21.8 (5.9) | 22.2 (5.6) | 19.8 (6.8) |
| Emotional well-being | 16.3 (4.7) | 16.6 (4.7) | 19.4 (5.1) |
| Functional well-being | 17.5 (6.0) | 18.3 (6.0) | 18.3 (6.9) |
| Breast cancer subscale | 24 (5.6) | 24.2 (5.5) | NA |
| Trial Outcome Index | 63.4 (13.6) | 64.3 (13.3) | NA |
| FACT-G total | 77.7 (15.5) | 79.1 (15.4) | 79.6 (18.6) |
| FACT-B total | 101.5 (19.1) | 103.2 (18.7) | NA |
| Pain | 1.8 (1.3) | 1.8 (1.3) | NA |
For FACT-B total, FACT-G total, and each of the subscales, a higher score indicates better well-being or quality of life. For the pain item, higher scores indicate greater pain severity. FACT-B, Functional Assessment of Cancer Therapy-Breast; FACT-G, Functional Assessment of Cancer Therapy-General; NA, not available; SD, standard deviation.
Figure 1.
Between-treatment comparison of overall change from baseline in FACT-B scores. FACT-B, Functional Assessment of Cancer Therapy-Breast; FACT-G, Functional Assessment of Cancer Therapy-General; TOI, Trial Outcome Index.
FACT-G total and subscale scores
Baseline mean (±SD) scores for FACT-G total scores were similar for the palbociclib plus letrozole arm and placebo plus letrozole arm (77.7 ± 15.5 versus 79.1 ± 15.4; Table 1). The overall change from baseline in FACT-G scores was not found to be significantly different (P =0.883) between the palbociclib plus letrozole [−0.39 (95% CI −1.46 to 0.68)] and placebo plus letrozole [−0.53 (−2.08 to 1.02)] treatment arms (Figure 1).
The overall change from baseline scores for PWB for the palbociclib plus letrozole arm versus the placebo plus letrozole arm was not found to be statistically significantly different between the two treatment arms [−0.50 (95% CI −0.90 to −0.20) versus −0.30 (−0.80 to 0.30)], SWB [−0.60 (−1.00 to −0.20) versus −0.70 (−1.20 to −0.10)], EWB [0.70 (0.40–1.00) versus 0.50 (0.10–0.90)], and FWB [0.20 (−0.20 to 0.60) versus 0.30 (−0.30 to 0.90)]. Similarly no statistically significant difference was observed in overall change from baseline in the BCS [0.19 (95% CI −0.18 to 0.56) versus 0.83 (0.29 to 1.37)] and FACT-B TOI [−0.10 (−1.00 to 0.81) versus 0.71 (−0.61 to 2.02)] scores between the palbociclib plus letrozole and the placebo plus letrozole arms (Figure 1). The treatment-by-time interaction term was not statistically significant in models for any scale (except for emotional functioning) indicating a similar time trend for PRO scores for both arms after adjusting for covariates. A significantly greater improvement from baseline was observed in the scores of the item assessing pain in body parts [−0.256 (95% CI −0.33 to −0.18) versus −0.098 (−0.21 to 0.01); P = 0.018].
Time to deterioration analyses
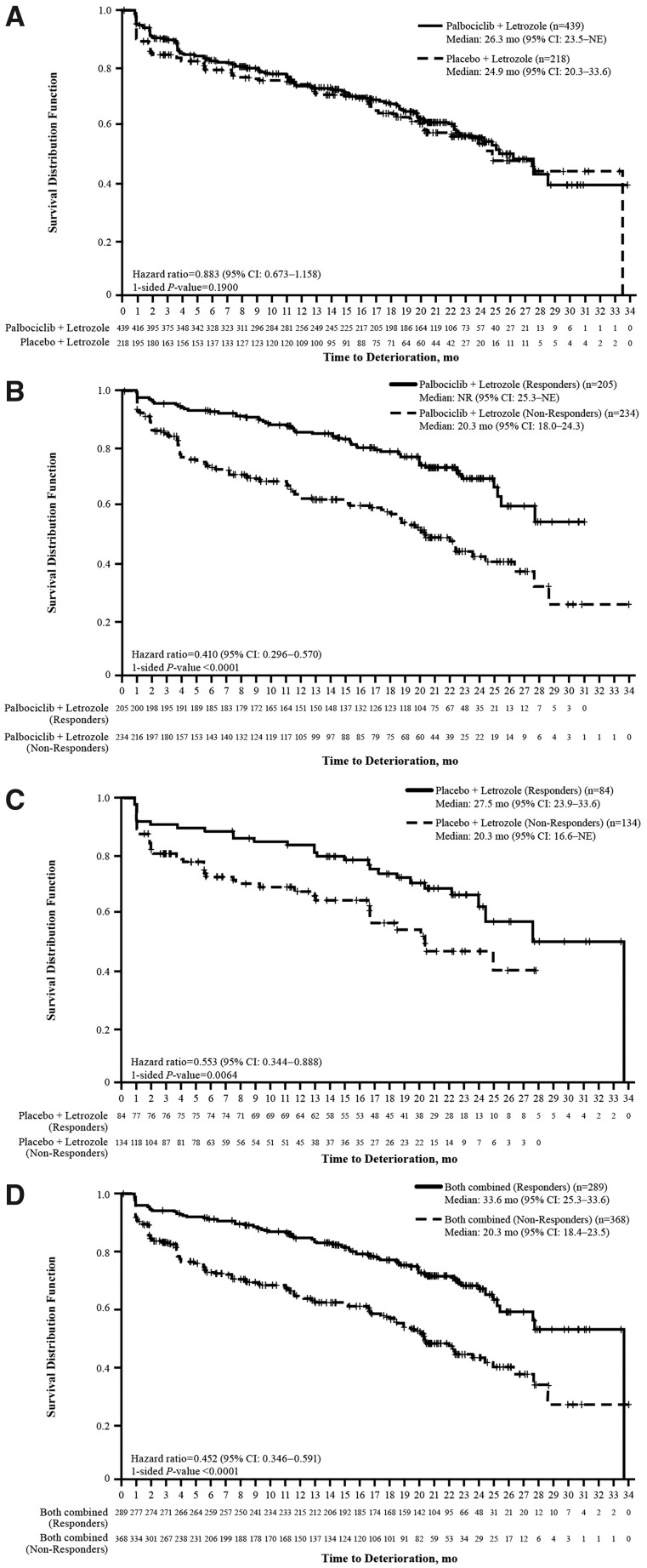
An analysis of TTD in FACT-B showed a positive trend (HR < 1) favoring the palbociclib plus letrozole arm but was not statistically significant [HR: 0.883 (95% CI 0.673−1.158); one-sided P =0.1900]. A Kaplan–Meier graph is provided in Figure 2A.
Figure 2.
Time to deterioration in Functional Assessment of Cancer Therapy-Breast (FACT-B) score. Between-treatment comparison of time to deterioration in FACT-B score among all patients (A), comparison of time to deterioration in FACT-B score between responders and non-responders (based on objective response status) in (B) the palbociclib plus letrozole arm, (C) the placebo plus letrozole arm, and (D) both treatment arms combined. CI, confidence interval; mo, months; NE, not estimable; NR, not reached.
Time to deterioration analyses based on progression status and objective response
The TTD analysis by progression status demonstrated a significantly greater TTD in HRQOL as assessed by the FACT-B total score in patients who had not progressed compared with those who did in the palbociclib plus letrozole arm (HR: 0.53; one-sided P < 0.001; supplementary Figure S2A, available at Annals of Oncology online) and the placebo plus letrozole arm (HR: 0.57; one-sided P = 0.009; supplementary Figure S2B, available at Annals of Oncology online) as well as in both treatment arms combined (HR: 0.53; one-sided P < 0.001; supplementary Figure S2C, available at Annals of Oncology online).
The TTD analysis of patients with an objective response compared with those without demonstrated a significantly greater TTD in HRQOL as assessed by the FACT-B total score in responders compared with non-responders. This was true based on individual treatment arms: in the palbociclib plus letrozole arm (HR: 0.410; one-sided P < 0.0001; Figure 2B) and in the placebo arm (HR: 0.553; one-sided P = 0.0064; Figure 2C) as well as based on all patients (HR: 0.452; one-sided P < 0.0001; Figure 2D).
EQ-5D health state profile
The percentage of patients reporting ‘extreme problems’ for any descriptors was low in both treatment arms at baseline (Table 2) and did not change notably from baseline over the course of treatment.
Table 2.
EQ-5D scores at baseline and overall on treatment scores
| Palbociclib + letrozole (n = 437) |
Placebo + letrozole (n = 218) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Health state (at baseline) | n | No problem n (%) | Some problem n (%) | Extreme problem n (%) | n | No problem n (%) | Some problem n (%) | Extreme problem n (%) |
| Mobility | 436 | 268 (61.5) | 166 (38.1) | 2 (0.5) | 215 | 132 (61.4) | 83 (38.6) | 0 |
| Self-care | 436 | 383 (87.8) | 50 (11.5) | 3 (0.7) | 215 | 189 (87.9) | 25 (11.6) | 1 (0.5) |
| Usual activities | 436 | 244 (56.0) | 173 (39.7) | 19 (4.4) | 215 | 131 (60.9) | 78 (36.3) | 6 (2.8) |
| Pain/discomfort | 436 | 135 (31.0) | 277 (63.5) | 24 (5.5) | 215 | 76 (35.3) | 132 (61.4) | 7 (3.3) |
| Anxiety/depression | 436 | 202 (46.3) | 212 (48.6) | 22 (5.0) | 215 | 99 (46.0) | 111 (51.6) | 5 (2.3) |
| EQ-5D index scorea | Mean | SD | 95% CI | Mean | SD | 95% CI | ||
| Baseline | 436 | 0.697 | 0.25 | 0.67–0.72 | 215 | 0.730 | 0.21 | 0.70–0.76 |
| Overall on treatmentb | NA | 0.736 | NA | 0.72–0.75 | NA | 0.712 | NA | 0.69–0.73 |
| End of treatment | 181 | 0.630 | 0.30 | 0.59–0.67 | 129 | 0.662 | 0.30 | 0.61–0.71 |
| EQ-5D VASa | ||||||||
| Baseline | 432 | 71.3 | 21.16 | 69.3–73.3 | 216 | 72.3 | 19.83 | 69.7–75.0 |
| Overall on treatmentb | NA | 75.07 | NA | 73.87–76.27 | NA | 75.25 | NA | 73.51–76.99 |
| End of treatment | 181 | 68.0 | 21.81 | 64.8–71.2 | 131 | 70.1 | 21.11 | 66.5–73.8 |
Higher EQ-5D index and VAS scores indicate better health status/quality of life.
Estimated from a repeated-measures mixed-effects model with baseline, treatment, time, and treatment × time interaction terms as covariates.
CI, confidence interval; EQ-5D, EuroQOL 5 dimensions questionnaire; SD, standard deviation; VAS, visual analog scale.
EQ-5D index and VAS scores
Baseline EQ-5D index scores were similar between the palbociclib plus letrozole and the placebo plus letrozole arms (Table 2). Based on the longitudinal mixed-effects models, no statistically significant differences were observed in the overall EQ-5D index score on treatment between the palbociclib plus letrozole and placebo plus letrozole arms [0.74 (95% CI 0.72–0.75) versus 0.71 (0.69–0.73); P = 0.093].
Baseline EQ-5D VAS scores were also found to be similar between both study arms, and no statistically significant difference was observed between treatment arms during treatment [75.07 (95% CI 73.87–76.27) versus 75.25 (73.51–76.99)] (Table 2).
FACT-B and EQ-5D index scores between patients with and without neutropenia in the palbociclib plus letrozole arm
No statistically significant differences were observed in the change from baseline in FACT-B total score at any cycle between patients with and without neutropenia in the palbociclib plus letrozole arm (supplementary Figure S3, available at Annals of Oncology online). No statistically significant differences were observed in the EQ-5D index score at any cycle between patients with and without neutropenia except cycles 11, 13, and 15 (P < 0.05), in which the scores were higher in the patients with neutropenia (supplementary Figure S4, available at Annals of Oncology online).
Discussion
Hormone receptor-positive (HR+) MBC is not curable but can have a long natural history with multiple lines of sequential effective regimens. For this reason, it is critical when introducing new therapies with increased efficacy to demonstrate that QOL is not compromised. Although treatment can prolong time to disease progression, thereby forestalling the effects of disease, toxicity associated with treatment can adversely affect QOL. In general, patients receiving first-line therapy for HR+/HER2− disease have a high functional level of QOL at the time of diagnosis, comparable to the general healthy population, and our current analysis provides confirmation of this finding. It is therefore even more important to understand the effect of treatment on patients’ overall QOL, and balance risk versus benefit. In the current study, we utilized two widely known and validated PRO questionnaires, one assessing breast cancer-specific HRQOL (FACT-B) and the other assessing general health status (EQ-5D). To our knowledge, the results presented here represent the longest period of follow-up to date in the published literature on PROs in women receiving endocrine-based therapy for HR+/HER2− MBC in the most contemporary care setting. This analysis shows that women on endocrine therapy are doing exceedingly well as evaluated by PROs, comparable to healthy individuals [10]. More importantly, results of outcomes assessed using FACT-B showed maintenance of QOL in both arms, without detriment from the addition of palbociclib.
Similar to the FACT-B total scores, the general health status scores assessed using the EQ-5D VAS suggest that the addition of palbociclib to letrozole does not have a significant adverse impact on a patient’s health status compared with letrozole alone. A significantly greater improvement in pain scores was observed in the palbociclib plus letrozole arm. These results are consistent with an earlier report for the palbociclib plus fulvestrant regimen as later-line therapy [11].
Overall, maintenance of QOL in patients with MBC, at a level close to healthy individuals in the general population with a median duration of treatment of almost 2 years is a significant improvement over previous therapies, including targeted therapy over similar or shorter periods of time, and is clinically meaningful for patients [12].
In both arms, deterioration of FACT-B Total score was significantly delayed in patients without progression versus those with progression and patients with partial or complete response versus those without. It is worth noting that disease progression and tumor response in this study were assessed by RECIST, which is heavily reliant on radiographic imaging assessment. These data emphasize the value of radiological assessment on treatment effect and are supported by patients’ direct reported outcomes demonstrating the impact of differences in progression and response status (a physician-reported outcome) and QOL. The knowledge obtained from this analysis will add to the ongoing debate in the medical community regarding the implications of radiologically assessed versus clinically assessed disease progression for clinical practice and research.
Neutropenia is the main treatment-related adverse event associated with CDK4/6-based therapy, requiring complete blood count monitoring in the palbociclib plus letrozole arm; however, neutropenia has been shown to have a neutral and/or no negative impact, if any, on patients’ QOL (supplementary Figures S3 and S4, available at Annals of Oncology online).
Potential limitations in the design of PRO analyses should be considered when interpreting these results. The assumption that missing data were missing at random in the PRO analysis may be incorrect, and bias could occur as a result. To address this limitation, the mixed-model approach was chosen because it is robust as long as the proportion of missing data that cannot be ignored is reasonably small (<5%) as was the case in this study.
Despite these limitations, the patient-reported QOL in this study provides clear evidence about a critical component of the risk-benefit profile from first-line palbociclib plus letrozole. These data demonstrate that overall QOL in patients receiving first-line endocrine-based therapy is maintained at high level, and that patients’ QOL is not negatively affected by the addition of palbociclib to letrozole. Maintenance of QOL close to general healthy population norms for more than 2 years is clinically meaningful. In conclusion, the addition of palbociclib to letrozole significantly improves PFS while maintaining HRQOL and general health status in treatment-naïve, postmenopausal women with ER+/HER2− MBC. This observation is maintained regardless of the occurrence of neutropenia while deterioration of QOL is delayed in patients with response and/or no progression.
Supplementary Material
Acknowledgements
We thank all of the patients, investigators, nurses, and site staff who participated in the PALOMA-2 study. This study was sponsored by Pfizer Inc. Editorial assistance was provided by Johna Van Stelten, PhD, and Lauren D’Angelo, PhD, of Complete Healthcare Communications, LLC, and was funded by Pfizer.
Funding
Pfizer Inc. (no grant numbers applied).
Disclosure
HSR received honoraria from Genomic Health, served on the speakers’ bureau for Genomic Health, received research funding from Plexxikon (Inst), Macrogenics (Inst), OBI Pharma (Inst), Eisai (Inst), Pfizer (Inst), Novartis (Inst), Eli Lilly (Inst), GlaxoSmithKline (Inst), Genentech (Inst), Celsion (Inst), Merck (Inst), and received travel, accommodations, and expenses from Novartis, Roche/Genentech, OBI Pharma, Bayer, Pfizer. VD has served as a consultant/advisor for Pfizer, Roche, Genentech, Lilly, Novartis, AbbVie, served on the speakers’ bureau for Pfizer, Roche, Novartis, and received travel, accommodations, and expenses from Roche, Novartis, Pfizer, AstraZeneca. KAG has served as a consultant/advisor for Pfizer, Roche, AstraZeneca, Novartis, Merck. RSF received honoraria from Bayer, Pfizer, Bristol-Myers Squibb, Novartis, Eisai and received research funding from Bayer (Inst), Pfizer (Inst), Bristol-Myers Squibb (Inst), Novartis (Inst), Eisai (Inst). DJS served in a leadership role for BioMarin, has stock or other ownership in Pfizer, and received travel, accommodations, and expenses from Pfizer, BioMarin. MM has served as a consultant/advisor for Amgen, Lilly, Novartis, Pfizer, Roche, Genentech and has received research funding from Novartis (Inst). PN has no conflicts to declare. YS has received research funding from Pfizer. AM has stock or other ownership in Pfizer and is an employee of Pfizer. DRL has stock or other ownership in Pfizer and is an employee of Pfizer. HB has stock or other ownership in Pfizer and is an employee of Pfizer. CH-B has stock or other ownership in Pfizer and is an employee of Pfizer. SI has stock or other ownership in Pfizer and is an employee of Pfizer. SRJ has served as a consultant/advisor for Eli Lilly, AstraZeneca, Novartis, has served on the speakers’ bureau for GlaxoSmithKline and Roche, and has received research funding from Pfizer (Inst). JE has served as a consultant/advisor for GlaxoSmithKline, Novartis, Pfizer, Pierre Fabre, Roche Pharma AG, Teva, has served on the speakers' bureau for GlaxoSmithKline, Novartis, Pfizer, Pierre Fabre, Roche Pharma AG, Teva, and has received travel, accommodations, and expenses from GlaxoSmithKline, Novartis, Pfizer, Pierre Fabre, Roche Pharma AG, Teva. NH has received honoraria from Amgen, Celgene, Genomic Health, NanoString Technologies, Novartis, Pfizer, Roche, has served as a consultant/advisor for AstraZeneca, Celgene, Novartis, Pfizer, Roche, Sandoz, and has received research funding from Novartis (Inst), Pfizer (Inst), Roche (Inst), MSD (Inst).
References
- 1. Butters DJ, Ghersi D, Wilcken N. et al. Addition of drug/s to a chemotherapy regimen for metastatic breast cancer. Cochrane Database Syst Rev 2010; CD003368. [DOI] [PMC free article] [PubMed]
- 2. Miles D, von Minckwitz G, Seidman AD.. Combination versus sequential single-agent therapy in metastatic breast cancer. Oncologist 2002; 7(Suppl 6): 13–19. [PubMed] [Google Scholar]
- 3. Toogood PL, Harvey PJ, Repine JT. et al. Discovery of a potent and selective inhibitor of cyclin-dependent kinase 4/6. J Med Chem 2005; 48(7): 2388–2406. [DOI] [PubMed] [Google Scholar]
- 4. Finn RS, Martin M, Rugo HS. et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med 2016; 375(20): 1925–1936. [DOI] [PubMed] [Google Scholar]
- 5. Brady MJ, Cella DF, Mo F. et al. Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J Clin Oncol 1997; 15(3): 974–986. [DOI] [PubMed] [Google Scholar]
- 6. EuroQoL Group. EuroQol—a new facility for the measurement of health-related quality of life. Health Policy 1990; 16(3): 199–208. [DOI] [PubMed] [Google Scholar]
- 7. Zhou X, Cella D, Cameron D. et al. Lapatinib plus capecitabine versus capecitabine alone for HER2+ (ErbB2+) metastatic breast cancer: quality-of-life assessment. Breast Cancer Res Treat 2009; 117(3): 577–589. [DOI] [PubMed] [Google Scholar]
- 8. Eton DT, Cella D, Yost KJ. et al. A combination of distribution- and anchor-based approaches determined minimally important differences (MIDs) for four endpoints in a breast cancer scale. J Clin Epidemiol 2004; 57(9): 898–910. [DOI] [PubMed] [Google Scholar]
- 9. Fitzmaurice GM, Laird NM, Ware JH.. Applied Longitudinal Analysis. Hoboken, NJ: John Wiley & Sons, 2011. [Google Scholar]
- 10. Brucker PS, Yost K, Cashy J. et al. General population and cancer patient norms for the Functional Assessment of Cancer Therapy-General (FACT-G). Eval Health Prof 2005; 28(2): 192–211. [DOI] [PubMed] [Google Scholar]
- 11. Harbeck N, Iyer S, Turner N. et al. Quality of life with palbociclib plus fulvestrant in previously treated hormone receptor-positive, HER2-negative metastatic breast cancer: patient-reported outcomes from the PALOMA-3 trial. Ann Oncol 2016; 27(6): 1047–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Campone M, Beck JT, Gnant M. et al. Health-related quality of life and disease symptoms in postmenopausal women with HR(+), HER2(-) advanced breast cancer treated with everolimus plus exemestane versus exemestane monotherapy. Curr Med Res Opin 2013; 29(11): 1463–1473. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.