Abstract
Background
An increased prevalence of gastric premalignant abnormalities was reported among relatives of gastric cancer (GC) patients, with rather unexplored clinical significance.
Methods
In Swedish computerized pathology registers, we identified, as ‘index’ persons, 232 681 patients who were born after 1931 and underwent endoscopic examination with stomach biopsy between 1979 and 2014. Through linkage with the Multi-Generation Register, we compiled a cohort consisting of 903 337 first-degree relatives of these biopsied patients. The relatives were grouped according to their ‘family histories’, defined as the first gastric mucosal diagnosis of the index person or GC family history known before that. Standardized incidence ratios (SIRs) provided comparisons with the matched general population. For internal comparisons with relatives with ‘normal/minor changes’ mucosal family history, hazard ratios (HRs) were derived from adjusted Cox regression modelling.
Results
During follow-up, 1302 relatives developed GC. Crude incidence rates of non-cardia GC were 7.7 × 10−5 year−1 for the ‘normal/minor changes’ family history group (SIR = 1.0), 11.2 to 12.6 × 10−5 year−1 for precancerous changes groups (atrophic gastritis/intestinal metaplasia/dysplasia, SIR = 1.5 to 1.6), and 18.4 × 10−5 year−1 for those with a family history of GC (SIR = 2.3). HRs derived from Cox models corroborated the family history-related risk pattern, with the most conspicuous trend observed among siblings—a family history of any precancerous changes and GC was associated with, respectively, a 2.5-fold and a 3.8-fold increment in non-cardia GC hazard, compared with siblings of index persons with ‘normal/minor mucosal changes’.
Conclusions
The precancerous mucosal abnormalities recorded in a person’s first-degree relatives may improve GC risk stratification for this person.
Keywords: Gastric cancer, family history, gastric biopsy, observational study, first-degree relatives, epidemiology
Introduction
Although corroborating prospective longitudinal data in humans are scarce, it has long been postulated that gastric cancer (GC) is preceded by a sequence of gastric mucosal changes (superficial gastritis → atrophic gastritis [AG] → intestinal metaplasia [IM] → dysplasia → GC), known as Correa’s cascade.1 In a previous prospective observational study,2 we demonstrated that the incidence of GC increased monotonically with each step in this cascade among affected persons.
Key Messages
To the best of our knowledge, this is the first population-based cohort study concerning familial clustering of gastric mucosal abnormality and its association with gastric cancer risk.
Our results indicate that, in addition to family history of gastric cancer, information about precancerous gastric mucosal changes in first-degree relatives may also have a discriminatory value in gastric cancer risk stratification of apparently healthy individuals as well as individuals who themselves have precancerous mucosal abnormalities.
Although this study cannot differentiate between genetic and shared environmental factors as mediators of this familial co-occurrence, it further corroborates Correa’s model of gastric carcinogenesis.
Further studies might be able to optimize the gastric cancer stratification by referring to the clinical records of precancerous mucosal abnormalities in a person’s first-degree relatives.
About 10% of all GC shows familial aggregation,3–5 which translates into a 50–250% excess risk of GC among subjects with a positive GC family history.6,7 In addition to genetic susceptibility, such familial clustering may also reflect shared environmental and lifestyle-related factors7 such as carcinogenic Helicobacter pylori (H. pylori) infection,8 smoking, and a diet rich in salt and low in fruits and vegetables.7
Previous studies, typically using cross-sectional or case-control designs with limited sample sizes, reported a significantly increased prevalence of precancerous mucosal abnormality (e.g. AG,9 IM10 or dysplasia11) among first-degree relatives of GC patients, in particular for the relatives with H. pylori infection,9 compared with controls without a GC family history. This was interpreted as evidence of a particularly high risk of GC among family members of GC cases. Consequently, H. pylori eradication therapy was recommended for these individuals.12 However, whether or not a family history of gastric premalignant abnormalities has any implications for the unaffected relatives remains largely unknown. Therefore, we aimed to elucidate the association between family history of gastric mucosal abnormality and GC risk among biopsied patients and their relatives.
Methods
Data sources
Taking advantage of the computerized registers held by all 24 pathology departments in Sweden (the first one was initiated in 1979 and the last in 1998), we established the Swedish Stomach Biopsy Cohort (SBC), consisting of all electronically registered patients who had undergone endoscopy with gastric biopsies. The details of this cohort (until 2011) were described previously.2 In brief, up until 31 December 2014, 442 899 subjects who received at least one endoscopic examination with stomach biopsy were identified in the pathology registers. After exclusion of 10 209 patients with missing/conflicting information, as well as 7107 patients who had undergone gastric resection or gastrectomy for non-cancer disease before their first biopsy, 425 583 patients remained. Data regarding the patients’ national registration numbers (NRNs: unique identifiers for all Swedish residents), biopsy date, age, sex and pathological-anatomical diagnosis using the Systematized NOmenclature of MEDicine Morphology (SNOMED M) codes13 were documented.
The data were then cross-linked with the Swedish Multi-Generation Register14 which contains information about parents and offspring of all individuals born in 1932 or later and who were alive in 1961. All first-degree family members of biopsied patients satisfying the above prerequisites were identified in the register, using the NRNs as identifiers. We obtained information about all incident GC cases (presenting as the 7th version of the International Classification of Diseases [ICD-7] code 151) by linking data in the Swedish nationwide Cancer Register. Established in 1958, the Swedish Cancer Register is 98% complete for gastrointestinal cancers.15 GC was subdivided into cardia (151.1) and non-cardia GC (all 151 except 151.1). Further linkages with the registers of causes of death, hospital care and emigration provided the necessary eligibility and censoring information for both biopsied patients and their first-degree relatives.
Study design
Cohort of relatives
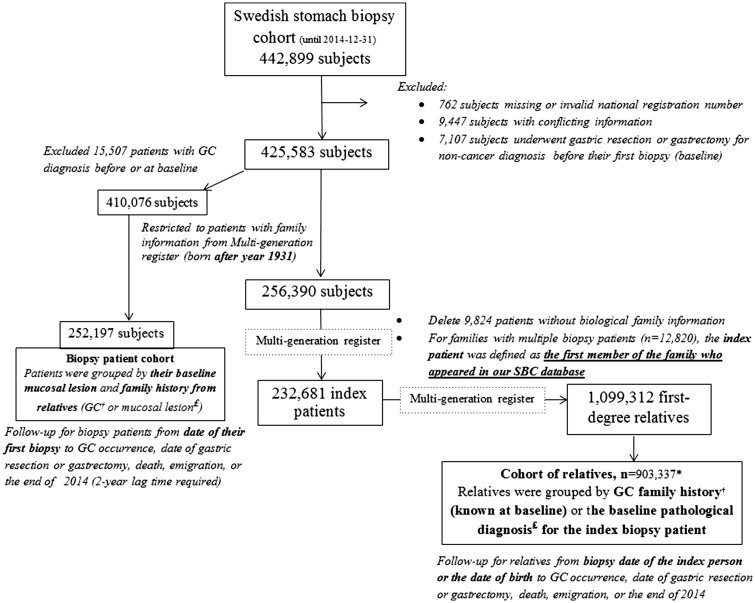
With the main interest of exploring variation in incidence of GC among first-degree relatives of biopsied patients, we compiled a ‘cohort of relatives’ through the procedure illustrated in Figure 1. First, we restricted the SBC cohort to 256 390 patients who were born after 1931 and thus had information about first-degree relatives in the Multi-Generation Register. Then, 232 681 biopsied patients who had recorded biological relatives and appeared in our SBC cohort as the first member of a family were defined as the ‘index’ patients. We accordingly identified 1 099 312 first-degree relatives, of whom 903 337 entered the ‘cohort of relatives’ either from the date of the first biopsy of the index patient (baseline), or the date of birth (if born after baseline). ‘Family history’ of interest was determined by GC family history (through record linkages with the Cancer Register) or the index patient’s pathological diagnosis at baseline, with the principle of grouping according to the most severe finding (see Table S1 at IJE online, classified as normal/minor changes, gastritis, other unspecified changes, AG, IM, dysplasia or GC, in order of severity). Individuals in this cohort were followed from study entry until a diagnosis of GC or censoring due to death, date of gastric resection or gastrectomy, emigration or the end of follow-up (31 December 2014), whichever came first.
Figure 1.
Study design (GC, gastric cancer; SBC Stomach biopsy cohort). *Relatives with negative follow-up time (received gastric resection/gastrectomy or GC diagnosis/died/emigrated before the first visit of the index patient) were excluded. †Gastric cancer family history was identified through linked data from Cancer Register. £Mucosal lesion (normal/minor change, gastritis, atrophic gastritis, intestinal metaplasia and dysplasia) was detected by referring to the pathological diagnosis from Swedish stomach biopsy cohort.
SBC biopsy patient cohort
In addition, to investigate whether the family history of gastric mucosal abnormality could offer extra information for GC risk assessment regarding the biopsied index patient in addition to the biopsy diagnosis, we calculated GC incidence rates among a subset of biopsy patients (n = 252 197) who were born after 1931 and had no GC diagnosis before/at the time of their first biopsy (baseline) (Figure 1). There, the exposure group was defined by both the patients’ SNOMED M diagnosis at baseline and the family history of GC or most severe gastric mucosal changes detected in their first-degree relatives (Table S1, available as Supplementary data at IJE online). Follow-up for biopsied patients started from the baseline biopsy until GC occurrence, date of gastric resection or gastrectomy, emigration, death or the end of follow-up, whichever occurred first.
Statistical analysis
For both the cohort of relatives and the biopsy patient cohort, we calculated standardized incidence ratio (SIR; the ratio of the observed to the expected number of newly diagnosed GC cases) with its 95% confidence intervals (CIs) to estimate the relative risk of GC for each exposure group. The expected number of cases was calculated by multiplying the observed number of person-years by sex-, calendar year- and age (5-year strata)-specific incidence rates derived from the entire Swedish population. We used the chi2 test to examine trends in excess GC risks with the increasing severity of mucosal family history (excluding the ‘other unspecified changes’ category).16 In particular, to reduce the possible selection bias in the biopsy patient cohort, we discarded the first 2 years of follow-up and outcomes detected during this period.2
Internal comparisons between exposure groups were conducted within the cohort of relatives. Associations between family history of gastric mucosal changes and risk of GC were assessed by hazard ratios (HRs) with 95% CIs, using the Cox proportional hazards regression model with attained age as underlying time scale. The model was adjusted for sex, number of relatives and year of birth (to control for the birth cohort effects), and stratified by the pathology department. The proportional hazards assumption was checked by assessing the correlation between Schoenfeld partial residuals and the time scale, both graphically and through a significance test; neither of them revealed any indication of violation. The trend in excess GC risks with mucosal family history of increasing severity was tested by including the grouping variable (1 to 5 stands for ‘Normal/minor changes’, ‘Gastritis’, ‘AG’, ‘IM’ and ‘Gastric cancer’) as a continuous variable in the model. We further performed subgroup analyses by type of relatives (parents/ siblings/ children), sex and period of birth (before 1936/37–67/68–74/after 1974). Cumulative incidence curves of non-cardia GC for relatives with different family history groups were graphically illustrated using the Nelson-Aalen method. All analyses were conducted in SAS statistical software, version 9.4 (Cary, NC).
Results
GC risk among first-degree relatives of biopsied patients
The cohort of relatives contained 903 337 participants, who contributed 10 914 200 person-years at risk (Table 1). The male:female ratio was 1:1 in all of the exposure groups with different family histories of gastric mucosal changes. Longest mean duration of follow-up (14.3 years), together with the earliest mean date of study entry (3 September 1999), was observed for relatives of index patients with dysplasia, whereas the shortest mean duration (9.9 years) and the latest mean date of entry (22 July 2004) were noted for relatives of patients with IM.
Table 1.
Characteristics of first-degree relatives of biopsy patients
| Family history of gastric mucosal changes* | Age of the index persons at their first biopsies, mean (yr) ± SD | Number of relatives | Family size, mean ± SD | Gender (% male) | Mean date of entry | Age at entry, mean (yr) ± SD | Follow-up duration, mean (yr) ± SD | Accumulated person-years |
|---|---|---|---|---|---|---|---|---|
| Normal/minor changes | 43.0 ± 17.4 | 306832 | 6.6 ± 2.3 | 49.1 | 7 Oct 2002 | 38.0 ± 23.1 | 11.4 ± 6.4 | 3486126 |
| Gastritis | 47.8 ± 15.5 | 371940 | 6.9 ± 2.7 | 49.3 | 7 Oct 2000 | 37.3 ± 22.4 | 13.2 ± 7.0 | 4922798 |
| Other unspecified changes | 53.2 ± 13.9 | 120599 | 6.9 ± 2.4 | 49.0 | 14 Oct 2003 | 42.2 ± 21.9 | 10.4 ± 5.8 | 1251661 |
| Atrophic gastritis | 48.9 ± 16.9 | 29738 | 7.0 ± 2.7 | 48.9 | 27 Dec 2001 | 39.7 ± 21.9 | 12.0 ± 6.7 | 355686 |
| Intestinal metaplasia | 56.9 ± 12.7 | 21533 | 7.0 ± 2.7 | 49.1 | 22 July 2004 | 42.7 ± 20.5 | 9.9 ± 5.2 | 213432 |
| Dysplasia | 53.7 ± 12.5 | 15487 | 7.1 ± 2.5 | 48.7 | 3 Sept 1999 | 41.1 ± 21.2 | 14.3 ± 7.4 | 221788 |
| Gastric cancer | 57.8 ± 10.6 | 37208 | 7.5 ± 2.7 | 48.4 | 6 Dec 2001 | 43.4 ± 20.0 | 12.4 ± 6.8 | 462710 |
Yr, years.
*Defined by the gastric cancer family history known at baseline (Cancer Register) or the mucosal change diagnosis of the index biopsy patient.
During follow-up, 1302 relatives developed GC—1 020 non-cardia GC and 282 cardia GC. The distribution of GC cases across mucosal family history groups is exhibited in Table 2, together with crude incidence rates and SIRs. The incidence of total GC in the ‘normal/minor changes’ family history group was 10.2 × 10−5 year−1 (95% CI 9.2–11.3), equal to the average level of age-, sex- and calendar period-matched Swedish population (SIR = 1.0). Whereas no clear trend towards increasing or decreasing incidence of cardia GC was observed among relatives of index patients with increasingly advanced gastric mucosal changes (albeit estimates were unstable due to small numbers), the relatives’ incidence of non-cardia GC increased almost monotonically with each step in Correa’s cascade (not including the poorly defined category ‘other unspecified changes’). The crude incidence rate increased from 9.6 × 10−5 year−1 for relatives of index patients with gastritis to 18.4 × 10−5 year−1 for relatives with a family history of GC, corresponding to a 30–130% excess risk of non-cardia GC in relation to the matched Swedish population (SIR = 1.3 for gastritis group, and 2.3 for GC group, P for trend <0.0001).
Table 2.
Standardized incidence ratios (SIRs) with 95% confidence intervals (CIs) for relatives of biopsy patients, grouped by the mucosal changes of index biopsy patient
| Family history of gastric mucosal changes* | All gastric cancer |
Non-cardia gastric cancer |
Cardia gastric cancer |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Observed cases | Crude incidence rate± | SIR§ (95% CI) | Observed cases | Crude incidence rate± | SIR§ (95% CI) | Observed cases | Crude incidence rate± | SIR§ (95% CI) | |
| Normal/minor changes | 357 | 10.2 | 1.0 (0.9–1.1) | 268 | 7.7 | 1.0 (0.9–1.1) | 89 | 2.5 | 1.0 (0.8–1.3) |
| Gastritis | 592 | 12.0 | 1.2 (1.2–1.4) | 475 | 9.6 | 1.3 (1.2–1.4) | 117 | 2.4 | 1.0 (0.9–1.3) |
| Other unspecified changes | 145 | 11.6 | 1.1 (0.9–1.3) | 99 | 7.9 | 1.0 (0.8–1.2) | 46 | 3.7 | 1.4 (1.0–1.9) |
| Atrophic gastritis | 47 | 13.2 | 1.3 (1.0–1.8) | 40 | 11.2 | 1.5 (1.1–2.0) | 7 | 2.0 | 0.8 (0.3–1.7) |
| Intestinal metaplasia | 27 | 12.7 | 1.3 (0.8–1.9) | 25 | 11.7 | 1.6 (1.0–2.4) | 2 | 1.0 | 0.4 (0.0–1.3) |
| Dysplasia | 29 | 13.1 | 1.2 (0.8–1.8) | 28 | 12.6 | 1.5 (1.0–2.2) | 1 | 0.5 | 0.2 (0.0–1.0) |
| Gastric cancer | 105 | 22.7 | 2.2 (1.8–2.6) | 85 | 18.4 | 2.3 (1.9–2.9) | 20 | 4.3 | 1.7 (1.0–2.6) |
| Chi2 test for trend€ | P < 0.0001 | P < 0.0001 | P = 0.63 | ||||||
*Defined by the gastric cancer family history known at baseline (Cancer Register) or the mucosal change diagnosis of the index biopsy patient.
±Per 100 000 person-years.
§Observed to expected number of GC cases, based on age- (5-year strata), calendar year- (5-year strata) and sex-specific incidence data in the total Swedish population; 95% CIs of SIRs were calculated by assuming that observed cancer occurrence followed a Poisson distribution.
€Excluded the ‘other unspecified changes’ category.
Table 3 shows adjusted HRs describing the association between mucosal family history and the occurrence of GC in the cohort of relatives, using Cox regression modelling with the ‘normal/minor changes’ group as reference. With essentially the same trend as that observed in our SIR calculations (P for trend < 0.0001), the results linked positive GC family history with a doubled hazard for non-cardia GC (HR 2.2, 95% CI 1.7–2.8), and the HR for family history of IM/dysplasia was 1.5 (95% CI 1.0–2.3). Of note, the most conspicuous excesses were seen for those who were siblings of the biopsied patients with mucosal changes (Table 4). Having a sibling with precancerous lesions (AG/IM/dysplasia), or with GC, was associated with, a 2.3-fold (95% CI 1.3–4.2) to a 3.8-fold (95% CI 2.6–5.6) increment in non-cardia GC hazard, compared with having siblings with ‘normal/minor changes’. For the biopsy patients’ offspring, the small number of GC outcomes prohibited a meaningful interpretation. Subgroup analyses by sex and year of birth showed similar results as the main analyses (data not shown).
Table 3.
Observed number, hazard ratios (HRs) and 95% confidence intervals (CIs) for gastric cancer among relatives of biopsy patients with different pathological changes in the stomach, compared with relatives of patients with normal gastric mucosa
| Family history of gastric mucosal changes* | All gastric cancer |
Non-cardia gastric cancer |
Cardia gastric cancer |
|||
|---|---|---|---|---|---|---|
| Number of cases | HRs and 95% CIs± | Number of cases | HRs and 95% CIs± | Number of cases | HRs and 95% CIs± | |
| Normal/minor changes | 357 | Reference | 268 | Reference | 89 | Reference |
| Gastritis | 592 | 1.1 (1.0–1.3) | 475 | 1.2 (1.0–1.4) | 117 | 0.9 (0.7–1.2) |
| Other unspecified changes | 145 | 1.1 (0.9–1.3) | 99 | 1.0 (0.8–1.3) | 46 | 1.4 (1.0–2.0) |
| Atrophic gastritis | 47 | 1.2(0.9–1.7) | 40 | 1.3 (0.9–1.8) | 7 | 0.8 (0.4–1.7) |
| Intestinal metaplasia | 27 | 1.4 (0.9–2.0) | 25 | 1.5 (1.0–2.3) | 2 | 0.4 (0.1–1.8) |
| Dysplasia | 29 | 1.1 (0.8–1.7) | 28 | 1.5 (1.0–2.2) | 1 | 0.2 (0.0–1.2) |
| Gastric cancer | 105 | 2.1 (1.6–2.6) | 85 | 2.2 (1.7–2.8) | 20 | 1.6 (1.0–2.7) |
| Test for trend§ | P < 0.0001 | P < 0.0001 | P = 0.60 | |||
*Defined by the gastric cancer family history known at baseline (Cancer Register) or the mucosal change diagnosis of the index biopsy patient.
±Using attained age as underlying time scale, estimated by Cox proportional hazards regression model, adjusted for sex, family size and year of birth and stratified by pathology department.
§The trends in excess risks with the progression of mucosal family history (excluding the ‘other unspecified changes’ category) were examined by including grouping variable (1 to 5 stands for ‘Normal/minor change’ to ‘Gastric cancer’ group, respectively) as a continuous variable in the model.
Table 4.
Hazard ratios (HRs) and 95% confidence intervals (CIs) for non-cardia gastric cancer among relatives of biopsy patients with different pathological changes in the stomach compared with relatives of patients with normal gastric mucosa, by classes of first-degree relatives
| Family history of gastric mucosal changes | Parents (n = 195 704) |
Siblings (n = 283 371) |
Children (n = 424 262) |
|||
|---|---|---|---|---|---|---|
| Number of cases | HRs and 95% CIs* | Number of cases | HRs and 95% CIs* | Number of cases | HRs and 95% CIs* | |
| Normal/minor changes | 204 | Reference | 55 | Reference | 9 | Reference |
| Gastritis | 267 | 1.2 (1.0–1.4) | 184 | 1.7 (1.3–2.3) | 24 | 1.3 (0.6–2.7) |
| Other unspecified changes | 52 | 0.9 (0.7–1.2) | 46 | 1.7 (1.2–2.5) | 1 | 0.2 (0.0–1.5) |
| Atrophic gastritis | 20 | 1.2 (0.7–1.9) | 20 | 2.5 (1.5–4.2) | 0 | – |
| Intestinal metaplasia | 10 | 1.4 (0.8–2.7) | 13 | 2.7 (1.5–5.0) | 2 | 1.7 (0.4–8.1) |
| Dysplasia | 11 | 1.1 (0.6–2.1) | 14 | 2.3 (1.3–4.2) | 3 | 2.6 (0.7–9.9) |
| Gastric cancer | 25 | 1.7 (1.1–2.6) | 56 | 3.8 (2.6–5.6) | 4 | 1.7 (0.5–5.6) |
*Using attained age as underlying time scale, estimated by Cox proportional hazards regression model, adjusted for sex, family size and year of birth and stratified by pathology department.
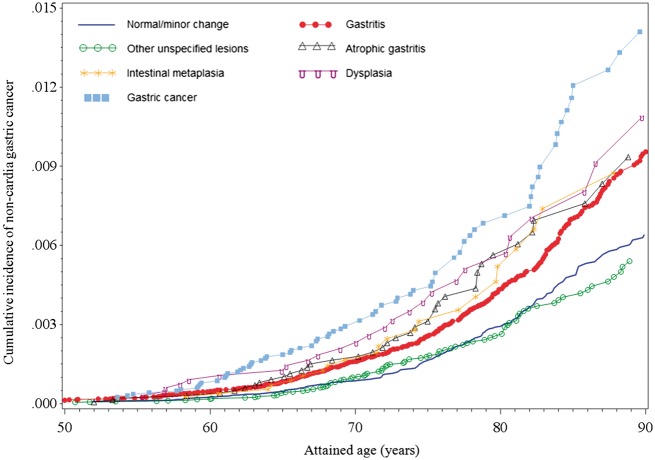
The Nelson-Aalen cumulative incidence plot in Figure 2 illustrates the probability of developing non-cardia GC with increasing age of individuals with different family histories of gastric mucosal changes. Whereas there were only minor differences between curves for the ‘normal/minor changes’ and ‘other unspecified changes’ groups, increased incidence proportions were discerned from the age of 55–60 for all other groups. Although the curves for ‘atrophic gastritis’ and ‘intestinal metaplasia’ were somewhat intertwined, the ranking of group-specific incidence proportions remained essentially constant across ages and coincided well with current ranking of the severity of gastric mucosal changes.
Figure 2.
Cumulative incidence of non-cardia gastric cancer among relatives, grouped by the different mucosal changes of the index biopsy patient.
GC risk among biopsied patients according to family history of gastric mucosal changes
After excluding the first 2 years of follow-up, 240 101 individuals remained in the biopsy patient cohort. They contributed 2 399 130 person-years at risk. Mean age at baseline biopsy was 47 years (Table S2, available as Supplementary data at IJE online). SIR calculations, where the exposure status was determined by both the patient’s own baseline mucosal changes and mucosal family history derived from his/her first-degree relatives, indicated that SIRs generally increased with more advanced baseline mucosal changes in the biopsied patients (Table 5). Furthermore, for both the ‘gastritis’ and ‘AG/IM/dysplasia’ baseline groups, the incidence of non-cardia GC was further elevated in the presence of a family history of GC or precancerous changes. Among biopsied patients in whom precancerous mucosal changes (AG/IM/dysplasia) were detected at baseline, the SIR for GC was 3.8 when the family history was negative (minor changes detected at most), 6.8 when one or more first-degree relatives had either AG, IM or dysplasia and 7.9 when relatives had GC. The latter estimates were, however, based on small numbers. Unfortunately, the scarcity of observed cases prohibited deeper analyses of the type of statistical interaction between own mucosal changes and relatives’ mucosal changes.
Table 5.
Observed number, crude incidence rate of non-cardia gastric cancers (GC) and standardized incidence ratios (SIRs) with 95% confidence intervals (CIs) for biopsy patients, grouped by family history of gastric mucosal changes
| Mucosal status at baseline | Exposure group | Biopsy patients (n = 240 101) |
||
|---|---|---|---|---|
| Family history of gastric mucosal changes | Observed cases* | Crude incidence rate± | SIR§ (95% CI) | |
| Normal/minor mucosal changes | No/minor changes detected | 66 | 9.9 | 1.1 (0.9–1–4) |
| Gastritis | 6 | 7.7 | 1.1 (0.4–2.3) | |
| AG/IM/dysplasia | 1 | 5.2 | 0.8 (0.0–4.2) | |
| GC | 0 | 0.0 | – | |
| Gastritis | No/minor changes detected | 238 | 25.0 | 2.4 (2.1–2.7) |
| Gastritis | 25 | 20.0 | 2.1 (1.4–3.7) | |
| AG/IM/dysplasia | 11 | 33.2 | 3.4 (1.7–6.1) | |
| GC | 13 | 43.4 | 3.3 (1.8–5.7) | |
| Other unspecified diagnoses | No/minor changes detected | 85 | 31.1 | 2.6 (1.11–3.3) |
| Gastritis | 6 | 18.3 | 1.8 (0.7–3.8) | |
| AG/IM/dysplasia | 2 | 21.4 | 2.0 (0.3–7.4) | |
| GC | 4 | 37.0 | 2.8 (0.8–7.2) | |
| AG/IM/ dysplasia | No/minor changes detected | 68 | 64.8 | 3.8 (3.0–4.7) |
| Gastritis | 13 | 88.8 | 5.4 (3.0–9.1) | |
| AG/IM/dysplasia | 4 | 97.1 | 6.8 (2.2–9.1) | |
| GC | 5 | 115.4 | 7.9 (2.6–18.5) | |
AG, atrophic gastritis; IM, intestinal metaplasia; GC, gastric cancer.
*The first 2 years of observation and corresponding events were excluded.
±Per 100 000 person-years.
§Observed to expected number of GC cases, based on age- (5-year strata), calendar year- (5-year strata) and sex-specific incidence data in the total Swedish population; 95% CIs of SIRs were calculated by assuming that observed cancer occurrence followed a Poisson distribution.
Discussion
To the best of our knowledge, this is the first population-based cohort study concerning familial clustering of gastric mucosal abnormality and its association with GC risk. Our results indicate that, in addition to family history of GC, information about precancerous gastric mucosal changes in first-degree relatives may also have a discriminatory value in GC risk stratification of apparently healthy individuals as well as of individuals who themselves have precancerous mucosal abnormalities. The incidence of non-cardia GC among first-degree relatives of biopsied patients with either of AG, IM or dysplasia was elevated by 50–60%, compared with the age-, sex- and calendar period-matched general population; and individuals who had siblings with AG, IM or dysplasia had a more than 2-fold excess hazard compared with individuals whose siblings had normal findings or only minor changes. Reciprocally, the SIR estimates for non-cardia GC in biopsied patients with AG, IM or dysplasia, who had first-degree relatives with AG, IM, dysplasia or GC, were approximately doubled compared with corresponding biopsied patients without such family history.
Although varying in strength, a positive link between a previous history of GC in the family and subsequent development of GC in yet unaffected individuals has been consistently demonstrated in earlier studies of GC patients and cancer-free controls.4,7 The slightly more than doubled GC hazard among first-degree relatives of GC patients in the present study, compared with first-degree relatives of biopsied patients with ‘normal/minor changes’, was on a par with what was found previously,17–19 albeit both lower20 and higher21,22 estimates have also been reported. However, the possible familial aggregation of gastric mucosal abnormalities consistent with the Correa’s cascade has remained less investigated.23 Whereas previous case-control studies have demonstrated a more frequent presence of precancerous changes (AG, IM and dysplasia)10,11,24,25 among first-degree relatives of GC patients compared with controls of mixed type,26 no published data could be found regarding the clinical significance of having a relative with precancerous changes, either for seemingly healthy persons or for persons who themselves have precancerous changes.
Similar to the potential causes for familial aggregation of GC, familial aggregation of gastric precancerous changes could be due to genetic/inherited predisposition (e.g. germline E-cadherin/CDH1 mutations5) or exposure to similar environmental factors within a family, such as carcinogenic H. pylori strains (if any),27 common dietary habits or other cancer-promoting exposures. Interestingly, compared with a parental history of precancerous changes/GC, such a history in a sibling was considerably more predictive of non-cardia GC risk. This is in line with the results of a Turkish case-control study, which reported a 9.1-fold increased odds of having GC for siblings of GC patients compared with non-GC controls,21 whereas this excess was only 5.6-fold for parents.22 This is consistent with the notion that the critical causal exposure(s) are shared between siblings and need to occur during childhood in order to result in GC development.28,29 There is accumulating evidence supporting a crucial role of long-term host-environmental interaction in non-cardia GC development.29,30
The major strength of the present study is the prospective study design with essentially complete follow-up via precise linkages to well-managed nationwide registers, using the individually unique NRNs as identifiers. The prospective design minimizes the risk of differential misclassification of the exposure (i.e. that the pathological diagnosis in biopsied patients is affected by the outcome in the relatives). Also, we argue that the risk of differential misclassification of the outcome is small. This argument derives from three circumstances: (i) the dismal and clinically evident course of the outcome (GC); (ii) pathologists are typically unaware of family histories (if any); and (iii) the proportion of ‘early GC’ (where there is scope for misclassification) is comparably low in Sweden (84% of all GC was diagnosed at stage III-IV according to the Swedish GC quality register). Additionally, the fact that all eligible first-degree relatives were included reduces the risk of selection bias among relatives; among biopsied patients, however, an imminent or subclinical cancer could potentially affect the decision to undergo gastroscopy and thus to enter the biopsy cohort. This possible selection bias was reduced by the application of a 2-year lag-time in our analysis.
Limitations to be highlighted include the fact that our classification of mucosal changes was entirely based on the pathologists’ SNOMED classification. There was no information about the anatomical distribution of the changes or the presence of visible lesions. In addition, we could not ascertain GC before 1958 or mucosal changes before 1979. This could lead to misclassification of the family history of gastric mucosal changes. But since our additional analyses restricted to family history identified from only siblings and offspring revealed a very similar GC risk pattern (data not shown), this shortcoming should have had limited effects on our estimates.
Further, it must be emphasized that the SBC cohort is not a representative random sample of the general Swedish population, but more likely a subpopulation at an increased risk (biopsies were prescribed for reasons). Consequently, their close relatives could differ from the general population somehow (e.g. genetic predisposition, lifestyle, living environment). However, the consistent results from internal comparisons and SIR analyses partly alleviate such concerns.
Last, because the familial clustering of gastric mucosal changes could, to a large extent, be driven by factors like lifestyle, H. pylori prevalence and ethnicity-specific genetics, the generalizability of these findings beyond Sweden remains to be proven. Nevertheless, our data seem to constitute proof of the principle that familial history of gastric precancerous changes consistent with ‘Correa’s cascade’ matters, both for healthy people and for biopsied patients who themselves have such changes. This seems to confirm the robustness of the ‘Correa’s cascade’ hypothesis.
In conclusion, our investigation demonstrated that, in addition to family history of GC, familial gastric mucosal abnormalities consistent with ‘Correa’s cascade’ in first-degree relatives are associated with an increased risk of non-cardia GC. Although this study cannot differentiate between genetic and shared environmental factors as mediators of this familial co-occurrence, it further corroborates Correa’s model of gastric carcinogenesis.
Supplementary Data
Supplementary data are available at IJE online.
Funding
This work was supported by the European Research Council (Consolidator grant, no.: 682663). I.G.E. is partly supported by a scholarship from the Karolinska Institutet MD/PhD programme.
Author Contributions
Study concept and design: W.Y., H.S., O.N.; data collection: I.G.E., H.S; data analysis: H.S., W.Y., A.P., O.N.; data interpretation: H.S., W.Y., O.N., A.P., J.E.; drafting of the manuscript: H.S., O.N., W.Y., A.P., I.G.E., J.E.
Conflict of interest: None.
Supplementary Material
References
- 1. Correa P. Human gastric carcinogenesis - a multistep and multifactorial process. 1st American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res 1992;52:6735–40. [PubMed] [Google Scholar]
- 2. Song H, Ekheden IG, Zheng ZL, Ericsson J, Nyren O, Ye WM. Incidence of gastric cancer among patients with gastric precancerous lesions: observational cohort study in a low risk Western population. BMJ 2015;351:h4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Corso G, Roncalli F, Marrelli D, Carneiro F, Roviello F. History, pathogenesis, and management of familial gastric cancer: original study of John XXIII’s family. BioMed Res Int 2013;2013:385132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dinis-Ribeiro M, Areia M, de Vries AC, et al. Management of precancerous conditions and lesions in the stomach (MAPS): guideline from the European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of Pathology (ESP), and the Sociedade Portuguesa de Endoscopia Digestiva (SPED). Endoscopy 2012;44:74–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carneiro F, Oliveira C, Suriano G, Seruca R. Molecular pathology of familial gastric cancer, with an emphasis on hereditary diffuse gastric cancer. J Clin Pathol 2008;61:25–30. [DOI] [PubMed] [Google Scholar]
- 6. Nyrén O, Adami H-O. Stomach cancer. In: Adami Hans-Olov, Hunter David, Trichopoulos Dimitrios (eds). Textbook of Cancer Epidemiology. Oxford, UK: Oxford University Press, 2008. [Google Scholar]
- 7. Yaghoobi M, Bijarchi R, Narod SA. Family history and the risk of gastric cancer. Br J Cancer 2010;102:237–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Song H, Michel A, Nyren O, Ekstrom AM, Pawlita M, Ye WM. A CagA-independent cluster of antigens related to the risk of noncardia gastric cancer: Associations between Helicobacter pylori antibodies and gastric adenocarcinoma explored by multiplex serology. Int J Cancer 2014;134:2942–50. [DOI] [PubMed] [Google Scholar]
- 9. El-Omar EM, Oien K, Murray LS, et al. Increased prevalence of precancerous changes in relatives of gastric cancer patients: critical role of H. pylori. Gastroenterology 2000;118:22–30. [DOI] [PubMed] [Google Scholar]
- 10. Oh S, Kim N, Yoon H, et al. Risk factors of atrophic gastritis and intestinal metaplasia in first-degree relatives of gastric cancer patients compared with age-sex matched controls. J Cancer Prev 2013;18:149–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. You WC, Ma JL, Liu WD, et al. Blood type and family cancer history in relation to precancerous gastric lesions. Int J Epidemiol 2000;29:405–07. [PubMed] [Google Scholar]
- 12. Malfertheiner P, Megraud F, O’Morain C, et al. Current concepts in the management of Helicobacter pylori infection:the Maastricht III Consensus Report. Gut 2007;56:772–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Côté RA. Systematized Nomenclature of Medicine (SNOMED). 2nd edn Chicago, IL: College of American Pathologists, 1979. [Google Scholar]
- 14. Sweden S. Multi-generation Register 2010, a Description of Contents and Quality. 2011. http://www.scb.se/statistik/_publikationer/BE9999_2011A01_BR_BE96BR1102.pdf (10 April 2017, date last accessed). [Google Scholar]
- 15. Ekstrom AM, Signorello LB, Hansson LE, Bergstrom R, Lindgren A, Nyren O. Evaluating gastric cancer misclassification: a potential explanation for the rise in cardia cancer incidence. J Natl Cancer Inst 1999;91:786–90. [DOI] [PubMed] [Google Scholar]
- 16. Breslow NE, Day NE. The Design and Analysis of Cohort Studies. Lyon, France: International Agency for Research on Cancer, 1987. [Google Scholar]
- 17. Dhillon PK, Farrow DC, Vaughan TL, et al. Family history of cancer and risk of esophageal and gastric cancers in the United States. Int J Cancer 2001;93:148–52. [DOI] [PubMed] [Google Scholar]
- 18. La Vecchia C, Negri E, Franceschi S, Gentile A. Family history and the risk of stomach and colorectal cancer. Cancer 1992;70:50–55. [DOI] [PubMed] [Google Scholar]
- 19. Garcia-Gonzalez MA, Lanas A, Quintero E, et al. Gastric cancer susceptibility is not linked to pro-and anti-inflammatory cytokine gene polymorphisms in whites: a Nationwide Multicenter Study in Spain. Am J Gastroenterol 2007;102:1878–92. [DOI] [PubMed] [Google Scholar]
- 20. Palli D, Galli M, Caporaso NE, et al. Family history and risk of stomach cancer in Italy. Cancer Epidemiol Biomarkers Prev 1994;3:15–18. [PubMed] [Google Scholar]
- 21. Bakir T, Can G, Erkul S, Siviloglu C. Stomach cancer history in the siblings of patients with gastric carcinoma. Eur J Cancer Prev 2000;9:401–08. [DOI] [PubMed] [Google Scholar]
- 22. Bakir T, Can G, Siviloglu C, Erkul S. Gastric cancer and other organ cancer history in the parents of patients with gastric cancer. Eur J Cancer Prev 2003;12:183–89. [DOI] [PubMed] [Google Scholar]
- 23. ECP-EURONUT-Intestinal Metaplasia Study Group, UK Sub-Group. Family history of gastric disease: a risk factor for intestinal metaplasia - a gastric precancerous lesion. Eur J Cancer Prev 1995;4:201–03. [PubMed] [Google Scholar]
- 24. Sheu BS, Yang HB, Sheu SM, Huang AH, Wu JJ. Higher gastric cycloxygenase-2 expression and precancerous change in Helicobacter pylori-infected relatives of gastric cancer patients. Clin Cancer Res 2003;9:5245–51. [PubMed] [Google Scholar]
- 25. Chang YW, Han YS, Lee DK, et al. Role of Helicobacter pylori infection among offspring or siblings of gastric cancer patients. Int J Cancer 2002;101:469–74. [DOI] [PubMed] [Google Scholar]
- 26. Marcos-Pinto R, Dinis-Ribeiro M, Carneiro F, et al. First degree relatives and familial aggregation of gastric cancer:w ho to choose for control in case-control studies? Fam Cancer 2012;11:137–43. [DOI] [PubMed] [Google Scholar]
- 27. Queiroz DM, Silva CI, Goncalves MH, et al. Higher frequency of cagA EPIYA-C phosphorylation sites in H. pylori strains from first-degree relatives of gastric cancer patients. BMC Gastroenterol 2012;12:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ueda M, Kikuchi S, Kasugai T, Shunichi T, Miyake C. Helicobacter pylori risk associated with childhood home environment. Cancer Sci 2003;94:914–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim N, Shin CM. Stomach cancer risk in gastric cancer relatives: interaction between Helicobacter pylori infection and family history of gastric cancer for the risk of stomach cancer. Gastroenterology 2009;136:A462–A. [DOI] [PubMed] [Google Scholar]
- 30. Kim J, Cho YA, Choi WJ, Jeong SH. Gene-diet interactions in gastric cancer risk :a systematic review. World J Gastroenterol 2014;20:9600–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.