Abstract
Background
Maintaining population-based registries requires adequate and sustained resources; however, to date there has been no systematic evaluation to identify the resource needs for cancer registration in most countries, including Colombia. A systematic assessment of the costs can quantify the funding required and identify processes to improve efficiency of cancer registries.
Methods
The Centers for Disease Control and Prevention’s (CDC’s) International Registry Costing Tool (IntRegCosting Tool) was tailored specifically for the Colombian registries and was used to collect resource use data from five regional population-based cancer registries: Barranquilla, Bucaramanga, Cali, Manizales, and Pasto. The registries provided cost data for the year 2013 and cancer cases corresponding to the year 2010.
Results
We identified an almost threefold variation in the average cost per case (77,932 to 214,082 Colombian pesos or US $41 to US $113 in 2013) across the registries, but there were also substantial differences in data collection approaches, types of data collected, and activities performed. Cost per inhabitant varied between 95 and 415 Colombian pesos (US $0.05 to US $0.22). Between 20% and 45% of the total cost was due to fixed cost activities.
Conclusions
The detailed economic information presented in this study constitutes a valuable source of activity-based cost data that registries can use to compare operations, assess key factors that lead to differences in cost per case, and identify potential approaches to improve efficiencies. Furthermore, the knowledge gained from studying the Colombian registries can help inform the planning and operations of other registries in the region.
Keywords: Colombia, Evaluation, Cancer registry, Cost
1. Introduction
1.1. History and current situation of cancer registration in Colombia
Colombia, an upper-middle income country [1], has a long history of cancer registration activities. The Cali population-based cancer registry (PBCR) was the first such cancer registry to be established in South America and has functioned without interruption since 1962. Furthermore, its data have been published in all 10 volumes of Cancer Incidence in Five Continents (CI5) [2]. In the late 1980s, Colombia’s National Cancer Institute and the Ministry of Health developed a national cancer registry plan to include cancer registration in additional areas of the country. Four municipal PBCRs were created, and they cover the cities of Barranquilla, Bucaramanga, Manizales, and Pasto (Fig. 1). Together with the Cali registry, these registries cover about 12% of the total population [3]. These registries are in different developmental and quality stages, having been established between 1962 and 2007. Among the registries, the percentage of microscopically verified cases varies between 78.3% and 85.4%, and the percentage of death certificate–only cases varies between 4.2% and 11.5% [4,5]. All of the registries follow the methodology recommended by the International Agency for Research on Cancer (IARC) [6]. Four of the five registries, the ones located in Bucaramanga, Cali, Manizales, and Pasto, have achieved international completeness and quality standards and published their high-quality data in the 10th volume of CI5 [2]. The Colombian National Cancer Institute provides financial support to these municipal registries and believes that the registries’ integration with universities is a sustainable model for producing high-quality cancer incidence data. Colombia’s National Cancer Institute, which can only provide partial funds to contribute to the registries’ sustainability, has decided that the maintenance of a few high-quality PBCRs in representative areas of the country is sufficient to have detailed information on specific regions and to be able to produce national and regional estimates that form a good basis for cancer control purposes. Colombia is a very diverse country, with regions that differ by population density, ethnicity, and culture and dietary habits; the areas with the PBCR largely represent these differences. Based on the data from these selected registries, in combination with mortality statistics, the Colombian National Cancer Institute regularly produces estimates of cancer incidence using the established Globocan methodology [7–9]. In South America, the National Cancer Institute of Brazil and the Ministry of Health of Chile take the same approach and produce national and subnational cancer incidence estimates based on a few representative regional registries [10,11].
Fig. 1.
Geographic coverage of Colombian municipal population-based cancer registries.
Note: The dark shaded areas represent the geographic coverage of Colombia’s population-based cancer registries.
Until 2010, the reporting of malignant neoplasms was not obligatory in Colombia, except for childhood leukemia. In 2010, because of the associated high economic burden, cancer was declared a national priority public health problem, and several information systems relying on passive mandatory reporting of cancer patients were established at a national level [4]. However, these systems do not align with the methods and definitions for PBCRs and are far from being complete; for example, the cancer registry abstraction form excludes patients diagnosed only postmortem [12]. Therefore, the well-established and rigorous methodology used by the PBCR, which is based on active case finding [6], remains very important as a source of reliable information on cancer burden in Colombia. All PBCRs manage their own databases and send their data to the Colombian National Cancer Institute, which makes national estimates every 5 years. PBCRs have not been provided with access to electronic databases; therefore, the registries must manually enter data instead of linking automatically with the cause-of-death databases or other sources.
1.2. The importance of economic analysis for operating cancer registries in Colombia
In Colombia, the National Cancer Institute receives funds on an annual basis from the national government to support cancer registries, though these funds are not necessarily guaranteed. Cancer registries apply annually to the Colombian National Cancer Institute for these funds and risk discontinuing data collection if they do not receive the funds. A systematic assessment of the costs can justify the required funding and potentially identify processes to improve efficiency. Indeed, it is important to know the costs of setting up, maintaining, and/or extending cancer registry activities for any funding organization. However, currently, the real costs by type of activity and cost per case are unknown. In Colombia, the five municipal PBCRs are based in universities, which frequently provide in-kind contributions, including the use of office space and equipment, which makes determining the real costs of a registry based on received funding and documented expenses very difficult. Moreover, to obtain funding and justify costs, the cancer registries must know their real annual costs. In addition, knowing the costs-per-cancer case registered can help a PBCR compare cost over-time and with other PBCRs. Authorities can use more detailed cost information to estimate the sustainability of the support or to decide on whether to finance the initiation of new registries or the expansion of existing registries under similar circumstances.
2. Materials and methods
A cost data collection tool [13] that was initially developed to evaluate the costs of operating PBCRs in the United States was tailored for use in a non-US setting. Details on the development and testing of the Centers for Disease Control and Prevention’s (CDC’s) International Registry Costing Tool (IntRegCosting Tool) are reported in a separate manuscript [14]. In brief, the IntRegCosting Tool is an activity-based tool that can be used to calculate the costs for specific cancer registry activities using the collected data. The tool consists of 10 data collection modules that collect the following information: general registry information; total expenditure by funding source; in-kind contributions; personnel expenditures; personnel activities; consultant expenditures; costs associated with computers, travel, and training; software licensing costs; and administrative costs and information on factors that may affect costs of registry operation and effectiveness. The tool also collects information on incident cases and number of reporting sources for each registry. Reporting sources include any facility in which a patient receives cancer care, such as hospitals, outpatient clinics, hospice, as well as pathology laboratories and death certificates sources.
For this study, the IntRegCosting Tool and the user’s guide were translated into Spanish and were adapted for use in Latin America. The Spanish translations of the activities included in the tool were kept as close as possible to the original English versions to allow for international comparisons. Because Colombian PBCRs receive a substantial proportion of their funding as in-kind contributions from their host universities, extra care was taken to capture these costs as accurately as possible. The registries provided feedback before and during the initial phase of data collection. Based on the questions and comments received, the tool and user’s guide were modified as necessary to clarify issues relating to in-kind contributions and indirect costs. The tool was further modified in an interactive manner as registries raised questions or issues that required minor adaptations of the types of data collected or the definitions used.
All five municipal PBCRs that received funding and technical support from the National Cancer Institute of Colombia were invited, and all chose to participate. In addition to the user’s guide, the registries received training via webinars on collecting the requested resource use and cost data using the translated tool. Face-to-face training and discussion were also provided during two site visits before beginning data collection. Technical support for completing the tool was provided by the Colombian country coordinator overseeing the use of the IntRegCosting Tool, who was familiar with both the cost data collection tool and the registries’ operations. The Colombian country coordinator was continuously available to provide technical assistance to resolve any remaining uncertainties. Because of the methods used to collect PBCR data, cases from several years are updated at any given time period. That is, some cases are in the initial abstraction stage, while others have their treatment and follow-up information updated. It generally takes 2 years to report a case; therefore, we focused on cases that were completed and reported during the fiscal year for which cost data were reported. The Colombian municipal PBCR provided cost data for financial year 2013 and cancer cases corresponding to the year 2010.
3. Calculations
Registry expenditures based on all funding sources, including in-kind contributions (both labor and nonlabor) from the host universities, were reviewed to ensure consistency in reporting. The registries provided detailed data to allow for comparative assess-ments of the differences between them. For example, a registry employee enters their percentage time devoted to specific registry activities into the tool, which is then multiplied by that employee’s wage to derive the total labor cost for each activity. Labor costs, in addition to other nonlabor costs, were aggregated for each registry for specific activities. The specific registry activities and detailed methods that the registries used to complete the IntRegCosting Tool are described in Subramanian et al. [14]. In-kind contributions, such as information technology (IT) support, were valued based on best-case estimates and were compared between registries to ensure consistency in reporting the resources provided by universities. Descriptive statistics were generated, and each registry received a summary of their data and queries on potential discrepancies identified during the review process. This process identified some misinterpretations, which were corrected after receiving updated information from the registries. Using this interactive process to finalize the analytical database ensured that any identifiable differences in interpretation were resolved, thereby increasing the comparability of the results between registries.
We report descriptive statistics of the registry characteristics based on the data collected. To facilitate comparing the activity-based cost information among the registries, activities were consolidated and categorized as “fixed cost activities,” “variable cost core activities,” and “variable cost other activities” such as conducting research. Activities considered to have fixed costs were those whose costs do not vary (in the short run) as the volume of cases changes. Fixed cost activities include management, indirect or administrative costs, training of registry staff, IT support, and reporting requirements. In contrast, variable cost activities are those that vary with the volume of cases collected and are classified into core and other activities. The core registry activities were all related to data collection and processing, including case ascertainment and development of analytic files. Variable cost other activities included research studies and enhanced registry activities related to data linkages and analysis.
4. Results
Table 1 presents key characteristics of the five participating Colombian PBCRs in terms of their data collection approach and coverage areas. The table indicates that substantial differences exist between registries in the total population and geographic area covered. Cali covers the largest population, followed by Barranquilla, Bucaramanga, Pasto, and then Manizales. However, Bucaramanga has the greatest coverage area in square kilometers, followed by Pasto, Manizales, Barranquilla, and then Cali. Cali has the largest number of cancer cases but the smallest coverage area by size. Cali’s population is 5.6 times larger than Pasto’s; however, Pasto covers a geographic area 9.8 times larger than Cali. The registries in Pasto and Manizales include the smallest numbers of cases, about 6–7 times smaller than Cali’s number of cases, but their coverage areas are quite different: Pasto’s coverage area is more than twice the size of Manizales’s coverage area.
Table 1.
Characteristics of Colombian population-based cancer registries.
| Barranquilla | Bucaramanga | Cali | Manizales | Pasto | |
|---|---|---|---|---|---|
| Date established | 2007 | 2000 | 1962 | 2001 | 1998 |
| Organization type | Private university | Private university | Public university | Public university | Public university |
| Total population covered (2011) | 1,189,503 | 1,084,699 | 2,333,016 | 390,084 | 417,484 |
| Geography covered (sq km)a | 166 | 1479 | 121 | 572 | 1181 |
| Incident cases (2010) | 1450 | 2124 | 5027 | 726 | 809 |
| Number of reporting/data sources (2013) | 50 | 40 | 180 | 33 | 37 |
| Coverage of sources (2013)b | 60% | 90% | 100% | 90% | 86.4% |
| Methods of data reporting/collection (2013) | |||||
| Paper | 65% | 30% | 30% | 75% | 95% |
| Diskettesc | 35% | – | – | – | – |
| Paper and electronic | – | 60% | 29% | 25% | 5% |
| Other electronic linkages | – | 10% | 41% | – | – |
| Does the registry collect and report nonresident cases? (2013) | N | N | Y | N | Y |
| If yes, total number of cases exchanged (2013) | – | – | 3551 | – | 1331 |
| Collects in situ cancers | N | Y | Yd | N | Y |
| Performs searches for cases based on death certificates | N | Y | Y | N | Y |
| Performs active follow-up for vital status (2013) | N | Y | Y | N | N |
| Included in CI5, 10th edition? | N | Y | Y | Y | Y |
| Registry staff: Full-time equivalent (FTE)e (2013) | |||||
| Management and administrative | 2 | 1.5 | 2 | 0.5 | 1 |
| Registrar and data collection | 3 | 5 | 9 | 1 | 4 |
| Database management and IT supportf | 1 | 1 | 1 | 2 | 2 |
| Research, investigation, and medical | 0.12 | 1 | 1.35 | 0.1 | 0 |
For Bucaramanga, Pasto, and Cali, we used square kilometers covered from the CI5 registry background page at http://ci5.iarc.fr/CI5-X/Pages/Table2.aspx. The registries directly provided square kilometers covered for Barranquilla and Manizales.
Coverage of sources equals the number of cancer case sources visited and reported to the registry divided by the total number of sources.
Dashes indicate a zero.
Cali collects in situ cancers for cervix and breast cancer only.
FTEs were assigned to the labor category based on registry staff allocation of time spent on cancer registry activities. Management and administrative FTEs include staff who spend a majority of time performing management and administrative tasks (e.g., addressing personnel and staffing issues, serving as liaisons to other registries and organizations, preparing registry applications and reports, mailing, filing, logging, and other clerical tasks).
IT support = Information technology support.
The number of reporting sources providing data to the registries (33–180) and the total number of incident cases collected in 2010 (726–5027) vary across the registries. The coverage of sources indicates the number of cancer case sources visited and reported to the registry out of the total number of sources. Some sources do not give access or are not visited because they do not contribute substantially to additional cases. Cali, Colombia’s longest estab-lished registry, collects data from all sources (100%), while Barranquilla, the most recently established registry, collects data from 60% of sources. The registries rely heavily on manual data extraction from either paper medical records or digitally available medical files. Several key features related to data collection also vary among the registries. Only two of the five registries collect and share nonresident cases or perform active follow-up, and three of the five registries include in situ cases in the incidence counts. Additionally, three of the five registries consistently revise death certificates to verify mortality and identify new cases.
Four of the five cancer registries published their data (2003– 2007) in the 10th volume of CI5 and have continued to provide more recent data for inclusion in a registry tool that IARC manages. Only Barranquilla, the registry that has been operational for the shortest time, has not yet submitted data to CI5.
As expected, the average number and types of staff members per cancer registry in 2013 varied. Overall, Cali employed the most staff, including significantly higher numbers of staff performing data collection and slightly higher numbers performing research activities. This finding is expected because Cali has the highest populated coverage area, performs active follow-up, and collects information on nonresident cases. Manizales, with the lowest population in its coverage area, employed the fewest staff, while the other three registries employed a relatively similar number of staff. In the context of the registries’ overall resources, and as described in Tangka et al. [15], Bucaramanga and Cali each devoted a little over three-fourths of registry costs to labor. Barranquilla and Pasto each devoted slightly less than three-fourths of registry costs to labor, while Manizales devoted just over half of its total costs to labor [15].
To support ongoing operations, the registries received a proportion of their funding—5% (Cali) to 50% (Barranquilla) of the total budget—from the Colombian National Cancer Institute. Additionally, host universities supported the registries by paying the salaries of many staff, such as the director, and providing in-kind contributions, such as office space, IT support, and other administrative capacities. The contributions of host institutions were estimated to vary between 57% (Pasto) and 70% (Bucaramanga) of the total costs of operating each registry [15]. Local or international organizations other than the host or Colombian National Cancer Institute provided about 6% of Pasto’s total funding and 32% of Cali’s total funding. Cali, serving as Colombia’s longest established registry, receives funding from several external organizations for research activities.
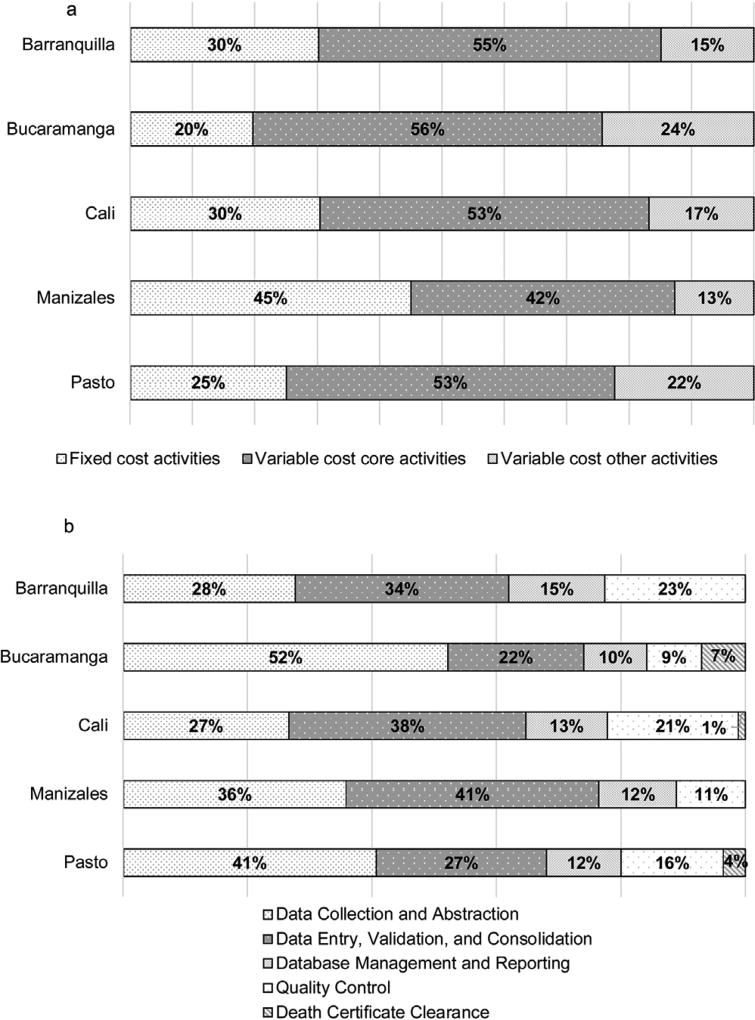
Fig. 2a shows a wide range in the proportion of registry expenditures (20 to 45%) related to fixed cost activities. Much less variation is evident in the proportion spent on variable cost activities, with the costs of core activities ranging from 42 to 56% and other activities ranging from 13 to 24% of the total variable costs.
Fig. 2.
(a) Colombian cancer registries’ distributions of fixed and variable cost activities, 2013. (b) Colombian cancer registries’ distributions of variable cost core activities, 2013.
Note: For Cali, “Data Collection and Abstraction” includes the registry activity of sharing cases.
Fig. 2b presents the distribution of the variable cost core activities. The largest variations were found for “data collection and abstraction” and “data entry, validation, and consolidation” activities, which are interlinked activities and combined account for approximately 62 to 77% of the variable cost core activities. Therefore, across all registries, data collection and entry activities account for most of the variable cost core activities, as expected. The remainder of the resources towards variable cost core activities was spent on database management and reporting, and quality control, with only three registries performing searches for cases based on death certificate information.
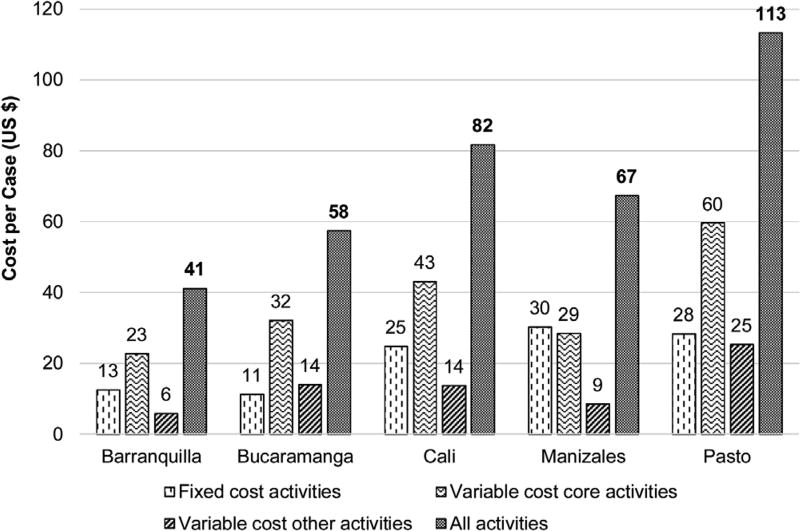
Fig. 3 shows the total costs per reported case, which varied almost threefold from 77,933 to 214,082 Colombian pesos (COP) (US $41 to US $113 in 2013 [used average exchange rate in 2013: 1 US $ = 1888 COP]). This figure also presents the cost per case for fixed, variable core, and variable other activities costs. Barranquilla had the lowest costs, with a total cost per case of US $41, which included US $13 for fixed cost activities, US $23 for variable cost core activities, and US $6 for variable cost other activities. Bucaramanga had a total cost per case of US $58, which included US $11 for fixed cost activities, US $32 for variable cost core activities, and US $14 for variable cost other activities. Cali had a total cost per case of US $82, which included US $25 for fixed cost activities, US $43 for variable cost core activities, and US $14 for variable cost other activities. Manizales had a total cost per case of US $67, which included US $30 for fixed cost activities, US $29 for variable cost core activities, and US $9 for variable cost other activities. Pasto had the highest costs, with total cost per case of US $113, which included US $28 for fixed cost activities, US $60 for variable cost core activities, and US $25 for variable cost other activities. The fixed activities cost per case was the highest for the registries with the smallest case volumes: Manizales and Pasto. Although the variable core activities cost per case was the key driver of total costs for most registries, variations in the relative costs per case classified as fixed, variable core, and variable other activities costs were noted across registries. The cost per inhabitant fluctuated between US $0.05 and US $0.22 [15].
Fig. 3.
Colombian cancer registries’ fixed, variable, and total cost per case, 2013.
Notes: In 2013, 1 US $ = 1888 Colombian pesos.
Cost per case is based on incidence cases during year 2010: 1450 Barranquilla cases; 2124 Bucaramanga cases; 5027 Cali cases; 726 Manizales cases; and 809 Pasto cases.
5. Discussion
This is the first study to report the activity-based costs incurred by five municipal Colombian PBCRs and to allow for comparative analysis using the cost per registered case. The registries included in this study vary in terms of the volume of cases collected, number of reporting sources, whether active case follow-up was performed, and the geographic area that must be covered to report the population-based incidence. Some differences in the data collection approaches, types of data collected, and activities performed also exist.
We identified an almost threefold variation in the average cost per case, which was also observed in a previous study [5]. Observations from studies on the costs of operating cancer registries in the United States and Europe [5,16–18] have shown that the case volume is strongly associated with the cost per case. Although no clear associations have been reported between population size, case volume, or number of abstracts handled and the cost per case in our study, some evidence supports potential economies of scale. In this current analysis, Barranquilla and Bucaramanga handled larger volumes than Manizales or Pasto and had lower fixed costs per case, likely because they can distribute these costs over larger numbers of cases. Fixed costs were found to constitute at least 20% of the total registry costs. The total fixed costs of Barranquilla, Bucaramanga, Manizales, and Pasto are very similar, given that each registry will require management and basic overhead resources, such as rent or electricity. However, because of Manizales’s small size and low variable costs, the proportion of its total resources for fixed cost activities is relatively high (45%).
An association between volume and cost per case is not apparent among these registries and is most likely due to the small number of registries included and variations in other registry characteristics. For example, the two smallest registries—Man-izales and Pasto—share some similarities but differ in the geographic areas covered and the method of data collection. Cali has the largest budget, partly because of its many research activities, intensive quality control (responsible for 20% of their expenses), and large number of reporting sources. However, it also handles the highest volume of cases and has a lower cost per case than Pasto. Since Cali received the death certificate database, they have spent much less of their budget (only 1%) on completing the registry based on death certificate cases compared with Bucaramanga, which spent 4% on this activity because it does not have access to the local database. Pasto, on the other hand, does all data collection on paper and has a large area to cover, which likely increased its costs. These results suggest that many interlinked characteristics likely affect the costs of operating registries; therefore, in the future, a more in-depth assessment is needed to systematically identify the factors that affect costs.
The findings that the cost per case in 2013 varies between US $41 and US $113 differ only slightly from those of a more basic study performed previously, which found that in 2011 the cost per case varied between US $28 and US $115 [5]. This small variation in costs using different methodologies indicates a certain level of robustness of the estimates. These differences, especially for the lower range, may be attributable to a failure to include all in-kind costs, funding from all sources, and all registry operation expenses. Because both studies were based on only 1 year of data, fluctuations in costs could also be a factor [5]. Because a cancer registry serves the whole population, an interesting result reported in Tangka et al. is that the cost per inhabitant fluctuated between US $0.05 and US $0.22 [15], lower than numbers observed for European PBCRs, where the average cost of cancer registration per inhabitant in the year 2010 was US $0.37 (range US $0.04 to US $1.34) [18]. Another study used direct funding sources and estimated the cost per case for registries in the African Cancer Registry Network to be between US $8 and US $9: this study did not include in-kind contributions and therefore understates the true cost of operating cancer registries [19].
Because we only have 1 year of data, an in-depth analysis of the factors that influence costs was not possible. The population size and number of cases registered could be influential factors, and smaller populations and smaller numbers of cases could be associated with relatively expensive cancer registries. Similarly, the geographical area and number of reporting sources covered by the registry could exert strong effects because of the time and financial resources spent on traveling to distant sources. Because the active case finding in Colombia is performed in each of the sources, coverage area is an important factor in data collection costs, which is a key driver of the overall costs of running cancer registries. Our findings show that the registries covering the largest geographic areas have the largest proportion of costs allocated to data collection and abstraction. Some of the cancer registries are based in large cities and receive many nonresident patient referrals. These nonresidents are often initially registered and their registrations are subsequently discarded for being nonres-idents, thereby consuming time and effort. Although all registries in principle record stage-related items and perform passive follow-up, data on these variables are somewhat incomplete. Some registries perform death certificate clearance, and some collect data on in situ cancers. Three of the five registries collect information on nonmelanoma skin cancers, although these data are not included in their formal reports. Additionally, the research activities, which are primarily performed by “mature” cancer registries, contribute substantially to the cost per registered case and are sometimes financed by external sources. Finally, the quality of the data initially submitted to the cancer registries from reporting sources and the number of data sources visited by the cancer registry staff must be explored in more detail to assess their impacts on the cost of operating the registry [16].
Although substantial differences in population and case volume exist among Colombian cancer registries, it is important to note that all of these registries—with a maximum of 5000 cases annually—can be considered low-volume registries based on a previously established threshold of fewer than 10,000 cases annually [17]. Furthermore, a study performed in the United States found that smaller registries had a higher cost per case [16,17]. However, compared with US and European estimates, the costs per case found in this study are relatively low, despite the low case volumes. This is most likely because of the much lower salary costs for staff in Colombia, where the minimum monthly salary in 2013 was about 589,000 COP (in 2013, this salary was equivalent to US $312).
All Colombian PBCRs operate under the same national legislation, which unlike the US legislation, does not specifically require the reporting of cancer cases to cancer registries. However, the municipality of Pasto has regional legislation that requires sources to report cancer incidence cases to the Pasto Cancer Registry. These laws, which many Latin American countries have, can benefit registries by increasing the access to sources and reducing the costs associated with active data collection. All other cancer registries included in the international study of which Colombia takes part are located in countries where cancer is not a reportable disease by legislation, with the exception of the Nairobi Cancer Registry, located in Kenya. [15].
PBCRs in Colombia have not been provided with access to electronic databases from childhood and adult cancers following the mandatory legislations from 2010 and 2014, respectively. If electronic database access were obtained, this access could substantially reduce the amount of manual work currently performed by the cancer registrars and reduce the cost of operations, as observed elsewhere [17]. Manual searches though will still be needed to review and correct routinely reported data, as was recently illustrated in a comparison between registry-based incidence data and routinely reported data [12].
One of the main limitations of this study is that it represents only one round of data collection. If a large investment related to specific features occurred or if costs were particularly low in the year in which the data were collected, it will have heavily influenced our conclusions. However, it is unlikely that all five cancer registries would have experienced these fluctuations in the same direction in 2013. Furthermore, many factors affect costs; therefore, 1 years’ worth of data is not adequate to elucidate all potential effects. A second limitation is that despite our efforts to standardize data collection, it is likely inevitable that registries interpreted certain items in the tool differently. Although the detailed user’s guide, ongoing technical support, and in-person presentation of preliminary results helped ensure accuracy of the cost results, we cannot exclude the possibility that variation in interpretation influenced our findings. Additionally, although we were as thorough as possible in our efforts to identify the resources needed, we were not always able to quantify the in-kind contributions accurately. In-kind contributions cover most IT and equipment costs, and IARC provides much of the software used by these cancer registries free of charge.
6. Conclusions
The detailed economic information gathered and presented in this study constitutes a valuable source of activity-based cost data that registries can use to analyze and compare their operations and to identify potential approaches to improve efficiencies. Additionally, the use of standardized approaches to collect cost data can help researchers perform comparative assessments to identify potential differences in costs that may be attributable to different data collection procedures or external environments of the registries. This cost study provides an opportunity for collaboration among registries to improve the efficiency of cancer registration operations in Colombia, particularly if this study is repeated periodically to assess changes. In fact, the Manizales Cancer Registry has already began to review potential changes in registry operations based on results from this project. The Manizales Cancer Registry is using the cost results to benchmark their operations and compare them with other Colombian cancer registries to determine why their costs are so much higher for certain activities. They are in the process of investigating differences across registries and identifying approaches to improve efficiency of their operations.
Colombia has no immediate plans to initiate new PBCRs. However, Central and South America have plans to establish new PBCRs or strengthen existing ones [20]. The knowledge gained from studying the Colombian registries can help inform the planning and operations of other registries in the region.
The adapted IntRegCosting Tool will be useful in evaluating the costs and efficiencies of new registries. Additionally, results from this study enhances our understanding of the current costs of running cancer registries and the potential factors affecting cost. In the future, investigations need to identify factors affecting cost and the most efficient approach to perform and improve cancer registration. Factors affecting the cost of registry procedures could include both internal registry operations and external factors, such as the quality of the data available at provider sites. Ultimately, besides considering the cost per cancer case registered, it is necessary to remember that the benefits of the information generated by the PBCR serve the entire population covered and the country by measuring cancer burden, informing planning and evaluation of cancer prevention and control programs, assessing patient care, and conducting research.
Acknowledgments
The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
This work was funded in part by Centers for Disease Control and Prevention Contract Number 200-2008-27958, Task Order 43, to RTI International.
Abbreviations
- CI5
Cancer Incidence in Five Continents
- CDC
Centers for Disease Control and Prevention
- COP
Colombian peso
- IARC
International Agency for Research on Cancer
- IntRegCosting Tool
International Registry Costing Tool
- IT
information technology
- FTE
full-time equivalent
- PBCR
population-based cancer registry
- US $
United States dollars.
Footnotes
Conflicts of interest
None.
Author contributions
Esther de Vries: Lead author; manuscript conception and design; interpretation of data; drafted the manuscript; revised manuscript for intellectual and scientific content; reviewed and approved final version to be published.
Constanza Pardo: Co-author; interpretation of data; revised manuscript for intellectual and scientific content; reviewed and approved final version to be published.
Nelson Arias: Co-author; interpretation of data; revised manuscript for intellectual and scientific content; reviewed and approved final version to be published.
Luis Eduardo Bravo: Co-author; interpretation of data; revised manuscript for intellectual and scientific content; reviewed and approved final version to be published.
Edgar Navarro: Co-author; interpretation of data; revised manuscript for intellectual and scientific content; reviewed and approved final version to be published.
Claudia Uribe: Co-author; interpretation of data; revised manuscript for intellectual and scientific content; reviewed and approved final version to be published.
Maria Clara Yepez: Co-author; interpretation of data; revised manuscript for intellectual and scientific content; reviewed and approved final version to be published.
Daniel Jurado: Co-author; interpretation of data; revised manuscript for intellectual and scientific content; reviewed and approved final version to be published.
Marion Pineros: Co-author; interpretation of data; revised manuscript for intellectual and scientific content; reviewed and approved final version to be published.
Luz Stella Garci: Co-author; interpretation of data; revised manuscript for intellectual and scientific content; reviewed and approved final version to be published.
Patrick Edwards: Co-author; acquisition, analysis, and interpretation of data; table/figure creation; revised manuscript for intellectual and scientific content; reviewed and approved final version to be published.
Maggie Cole Beebe: Co-author; revised manuscript for intel-lectual and scientific content; reviewed and approved final version to be published.
Florence Tangka: Co-author; manuscript conception and design; acquisition, analysis, and interpretation of data; revised manuscript for intellectual and scientific content; reviewed and approved final version to be published.
Sujha Subramanian: Co-author; manuscript conception and design; acquisition, analysis, and interpretation of data; revised manuscript for intellectual and scientific content; reviewed and approved final version to be published.
References
- 1. [accessed 16.05.20];The World Bank, Country and Lending Groups. http://data.worldbank.org/about/country-and-lending-groups.
- 2. [accessed 16.05.01];Cancer Incidence in Five Continents, International Agency for Research on Cancer. 2013 http://ci5.iarc.fr.
- 3.The World Bank, Population, total. [accessed 16.08.15]; http://data.worldbank.org/indicator/SP.POP.TOTL?locations=CO.
- 4.Arias N. Registros poblacionales de cáncer: Avances en Colombia, Chile y Brasil. Rev. Fac. Nac. Salud Pública. 2013;31(1):127–135. [Google Scholar]
- 5.Pardo C, Bravo LE, Uribe C, Lopez G, Yepez MC, Navarro E, de Vries E, Pineros M. Comprehensive assessment of population-based cancer registries: an experience in Colombia. J. Registry Manag. 2014;41(3):128–134. [PubMed] [Google Scholar]
- 6.Bray F, Znaor A, Cueva P, Korir A, Swaminathan R, Ullrich A, Wang SA, Parkin DM. Planning and developing population-based cancer registration in low- and middle-income settings (IARC Technical Publication No. 43) International Agency for Research on Cancer; Lyon, France: 2014. [PubMed] [Google Scholar]
- 7.Pardo C, Cendales R. Incidencia, mortalidad y prevalencia de cáncer en Colombia, 2007–2011. 1. Instituto Nacional de Cancerología; Bogotá: 2015. [Google Scholar]
- 8. [accessed 16.05.25];Instituto Nacional de Cancerología, Incidencia estimada y mortalidad por cáncer Colombia 1995–1999. 2005 http://www.cancer.gov.co//files/libros/archivos/b105063f4819cf30dc290bc33789000f_Incidencia%20estimada%20y%20mortallidad%20por%20cancer%20colombia%201995%20-%201999.pdf.
- 9. [accessed 16.05.25];Instituto Nacional de Cancerología, Incidencia estimada y mortalidad por cáncer en Colombia, 2002–2006. 2015 http://www.cancer.gov.co//files/libros/archivos/a9412b1cdffddfb228e09f7d31e9e124_Incidencia%20Estimada%20Y%20Mortalidad%202002-2006.pdf.
- 10. [accessed 16.05.25];Instituto Nacional de Cancer Jose Alencar Gomes Da Silva, Estimativa 2016: incidencia de cancer no Brasil. 2016 http://www.inca.gov.br/estimativa/2016/
- 11. [accessed 16.05.25];Chile Ministerio de Salud, Primer informe de registros poblacionales de cancer de Chile: quinquenio 2003–2007. 2012 5596 http://maquetas.ciiet.cl/elgg/file/download/ [Google Scholar]
- 12.de Vries E, Pardo C, Henríquez G, Piñeros M. Discrepancias en manejo de cifras de cáncer en Colombia, Revista Colombiana de Cancerología. 2016;20(1):45–47. doi: http://dx.doi.org/10.1016/j.rccan.2016.02.001. [Google Scholar]
- 13.Subramanian S, Tangka F, Green J, Weir H, Michaud F. Economic assessment of central cancer registry operations. Part II: developing and testing a cost assessment tool. J. Registry Manag. 2009;36(2):47–52. [PubMed] [Google Scholar]
- 14.Subramanian S, Tangka F, Edwards P, Hoover S, Cole-Beebe M. Developing and testing a cost data collection instrument for non-communicable disease registry planning. Cancer Epidemiol. 2016;45S:S4–S12. doi: 10.1016/j.canep.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tangka F, Subramanian S, Edwards P, Cole-Beebe M, Saraiya M, Parkin M, Bray F, Joseph R, Mery L. Resource requirements for cancer registration in areas with limited resources: analysis of cost data from four low - and middle -income countries. Cancer Epidemiol. 2016;45S:S50–S58. doi: 10.1016/j.canep.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Subramanian S, Tangka FK, Beebe MC, Trebino D, Weir HK, Babcock F. The cost of cancer registry operations: impact of volume on cost per case for core and enhanced registry activities. Eval. Program Plann. 2016;55(1):1–8. doi: 10.1016/j.evalprogplan.2015.11.005. doi: http://dx.doi.org/10.1016/j.evalprogplan.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tangka FK, Subramanian S, Beebe MC, Weir HK, Trebino D, Babcock F, Ewing J. Cost of operating central cancer registries and factors that affect cost: findings from an economic evaluation of Centers for Disease Control and Prevention National Program of Cancer Registries. J. Public Health Manag. Pract. 2015;22(5):452–460. doi: 10.1097/PHH.0000000000000349. doi: http://dx.doi.org/10.1097/PHH.0000000000000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zanetti R, Sacchetto L, Calvia M, Bordoni A, Hakulinen T, Znaor A, Møller H, Siesling S, Comber H, Katalinic A, Rosso S. J. Registry Manag. 1. Vol. 41. Spring; 2014. Eurocourse WP3 Working Group. Economic evaluation of cancer registration in Europe; pp. 31–37. [PubMed] [Google Scholar]
- 19.Gakunga R, Parkin DM. African Cancer Registry Network, Cancer registries in Africa 2014: a survey of operational features and uses in cancer control planning. Int. J. Cancer. 2015 Nov;137(9):2045–2052. doi: 10.1002/ijc.29668. doi: http://dx.doi.org/10.1002/ijc.29668. [DOI] [PubMed] [Google Scholar]
- 20. [accessed 26.03.16];International Agency for Research on Cancer, Global Initiative for Cancer Registry Development (GICR) making cancer data count. 2014 http://gicr.iarc.fr.