Abstract
As functional liquid media, natural deep eutectic solvent (NADES) species can dissolve natural or synthetic chemicals of low water solubility. Moreover, the special properties of NADES, such as biodegradability and biocompatibility, suggest that they are alternative candidates for concepts and applications involving some organic solvents and ionic liquids. Owing to the growing comprehension of the eutectic mechanisms and the advancing interest in the natural eutectic phenomenon, many NADES applications have been developed in the past several years. However, unlike organic solvents, the basic structural unit of NADES media primarily depends on the intermolecular interactions among their components. This makes NADES matrices readily influenced by various factors, such as water content, temperature, and component ratio and, thus, extends the metabolomic challenge of natural products (NPs). To enhance the understanding of the importance of NADES in biological systems, this review focuses on NADES properties and applications in NP research. The present thorough chronological and statistical analysis of existing report adds to the recognition of the distinctiveness of (NA)DES, involves a discussion of NADES-related observations in NP research, and reportes applications of these eutectic mixtures. The work identifies potential areas for future studies of (NA)DES by evaluating relevant applications, including their use as extraction and chromatographic media as well as their biomedical relevance. The chemical diversity of natural metabolites that generate or participate in NADES formation highlights the growing insight that biosynthetically primordial metabolites (PRIMs) are as essential to the biological function and bioactivity of unrefined natural products as the biosynthetically more highly evolutionary metabolites (HEVOs) that can be isolated from crude mixtures.
Graphical abstract
INTRODUCTION
The term “eutectic” is derived from the Greek word for low melting and applies to either an alloy or liquid medium. In general terms, a eutectic system represents a mixture of components that by virtue of specific proportions have the lowest melting point. This review focuses on natural product (NP) research related eutectics and, thus, only covers eutectic solvents. As evident from the phase diagram shown in Figure 1, temperature and component ratio are two major determinants in a binary eutectic system. These components interact via intermolecular forces, but not through covalent or ionic bonds.1,2 Theoretically, the involvement of other component molecules delocalizes the component molecular lattice units, causing a melting point depression of the corresponding eutectic mixture.3 Compared to the covalent or ionic bonding in a chemical entity such as ionic liquids (Table 1), a eutectic mixture with low thermodynamic stability tends to be influenced by geometry and chemical environment4 and can dissociate into the individual substances. Owing to this thermodynamic transition, the existence of a eutectic state is not readily recognized in natural materials, which may explain why it is a relatively under-represented topic in NP research. As shown by the following examples, eutectic phenomena are encountered in our daily life. Classical examples are honey and syrup, representing mixtures of sugars that are highly viscous media at ambient temperature. They have been used as dietary supplements and as ingredients for traditional medical concoctions for hundreds of years.5,6 Another prominent example is the mixture of the two solids menthol and camphor, which can form a eutectic liquid that is used as a vehicle for dissolving drugs.7
Figure 1.
Schematic diagram of the eutectic point on a two-component (1 and 2) phase, where EC means eutectic component. The dashed curve represents the melting points of a binary NADES family under different molar ratios. All unified liquid media are located in A, while applied NADES species are at or under ambient temperature as a part of A. This area should be the shape of a del operator, for which one angle point in the valley is the eutectic point. B and C represent the mixtures of EC1 and EC2 (solid/liquid or liquid/solid), and D is a mixture of EC1 and EC2 (solid/solid). The eutectic point is remarkable in that two or more compounds may combine in precise and fortuitous proportions to become mutually compatible in such as way that dramatically lowers the melting point of the mixture.
Table 1.
Comparative Properties of Ionic Liquids and Deep Eutectic Solvents Including NADES
| ionic liquids | deep eutectic solvents | |
|---|---|---|
| Distinguished Characterizations | ||
| constituent | salt | salt, sugar, organic acid, amino acid, etc. |
| composition | single salt | mixture |
| intermolecular force | ionic bonding | hydrogen bonding |
| molecular force | strong | weak |
| free energy content | 1–5 kcal/mol | |
| intermolecular distance | <1.2 Å | 2–5 Å |
| conductivity | often moderate to poor | |
| vapor pressure | low | low |
| cytotoxicity | positive for many | hard to detect |
| Identical Characterizations | ||
| melting point | below arbitrary temperature, e.g., 100 °C, and some are liquid at or below room temperature | |
| viscosity | positive linear correlation with temperature | |
| solvating properties | a range of polar and nonpolar compounds | |
| mobility | depends on the size of mobile species and the availability of holes of appropriate dimensions | |
Evolution of Eutectic to Natural Deep Eutectic Solvents
As illustrated in Figure 2, the eutectic precipitate of NaNH2–KNH2 (1:2, mol/mol) was initially observed as an entity in ammonia solution in 1918.8 This eutectic phenomenon was studied by establishing a melting point curve of the NaNH2–KNH2 mixture, as modeled in Figure 1. The eutectic point of NaNH2–KNH2 was found to be 97 °C under the molar ratio of 1:2.9 Due to its chemical stability and being nonvolatile at a high temperature, the eutectic mixture of sodium amide and potassium amide was first employed as an organic synthesis medium in 1946.10 Since then, it took over 30 years before studies of eutectic mixtures entered exponential growth (Figure 2). A series of eutectic media and their properties have been uncovered, and the quantity of their relevant eutectic applications continues to grow. More examples can be summarized as follows: (i) to overcome the compatibility issue between enzymes and organic solvents, eutectic media have been used as alternatives to organic solvents to catalyze reactions.11,12 (ii) Eutectic phenomena have been considered systematically using two liquid–solid phase diagrams (often called refining zones, Figure 1B and C), where a crystal can be obtained by changing the temperature, e.g., a melt- or cold-crystallization procedure.13 This eutectic property has been utilized in purification studies.14 (iii) Lipophilic NPs have also been recognized as eutectic components. For instance, a mixture of ibuprofen and certain terpenes, such as 1,8-cineole, p-cymene, d-limonene, menthol, menthone, or thymol, can be developed into a medical eutectic mixture. Eventually, these eutectic mixtures were advanced into transdermal drug delivery systems.15 (iv) Due to its ecological friendliness and low toxicity, the concept of a deep eutectic solvent was proposed in 2004 to describe a group of eutectic mixtures.16 As alternative candidates to ionic liquids (Table 1), the deep eutectic solvents (DESs) represent a group of more universally applicable green media. (v) In a key contribution by Choi, Verpoorte, and co-workers on eutectic mixtures occurring in Nature, the concept of a natural deep eutectic solvent (NADES; used as both singular and plural in the following) was advanced recently.1 As NADES components always refer to NPs, NADES media gradually have received the attention of the NP scientific community.
Figure 2.
Number of publications on key topics since 1950: eutectic (● in blue, 1700 items), eutectic mixture (■ in orange, 652 items), deep eutectic solvent (× in gray, 224 items), and natural deep eutectic solvent (+ in yellow, 37 items). The analyzed data were retrieved from PubMed (National Center for Biotechnology Information, NCBI) on November 7, 2016. The bottom shows the chronological milestone eutectic events.
Natural Deep Eutectic Solvents
The NADES concept has been proposed via the observation of both diversity of redundant metabolites in natural resources and the occurrence of certain natural eutectic mixtures.1 For example, many plant constituents that have been classified as so-called “primary metabolites” (here: “biosynthetically primordial metabolites”, or PRIM; see the section on “Metabolic Naming”) are also recognized as being functionally overproduced in their source organisms. This disproportionality seems to conflict with the natural roles of energy expenditures by living systems. Hence, it is only logical to hypothesize that these metabolites may also play other physiological roles, e.g., as NADES components in Nature. Moreover, certain NPs considered to be lipophilic metabolites may also be produced in copious quantities. For instance, quercetin, which recently has been recognized as a likely invalid metabolic panacea (IMP),17 is highly produced in several plant species, mainly in the form of its glycosides. Therefore, one might assume that it serves a useful purpose in the organisms that produce it. Although this does not imply that all IMPs are NADES components, some lipophilic metabolites may still be part of NADES matrices. Considering the polarities of both hydrophilic and many lipophilic metabolites, NADES media can achieve a broad polarity range. Hence, the diversity of NADES components can enrich NADES properties and expand the pool of NADES applications.
The present review focuses on the properties of NADES and their role in natural product research. It extends the classes of NADES’ chemical constituents beyond PRIM (see section on “Metabolic Naming”), statistically analyzes the ratios of NADES components (ranging from 1:1 to 1:4 for binary NADES systems), analyzes known NADES preparation strategies (entropy regulation), provides an update on NADES applications, and discusses an outlook into the future for new uses of NADES in NP research.
COMPONENTS OF NATURAL DEEP EUTECTIC SOLVENTS
Metabolic Naming: PRIM and HEVO
The view of this review on naturally occurring (plant) metabolites, which are often classified as “small molecules”, their roles in Nature, their categorization in biosynthesis and biological function, and their general nomenclature blends well with the result of the elegant work on evolutionary models of metabolism by Firn and Jones, aimed at explaining the chemical diversity of natural products. These authors developed an extension of their framework theory during the period 1990 to 2010 and summarized their results in a series of articles.18–20 One major outcome of their work is the screening hypothesis, which provides a more generalized evolutionary model for the properties of metabolites shaping metabolism and goes beyond the pathway focus of biosynthesis. The screening hypothesis infers that evolutionary selection shapes biochemistry based on the biological properties of the produced molecules and the fitness of its producer.19 The authors devised three main metabolite property classes: component properties (i.e., being a necessary part of an overall pathway; closely matching the definition of “primary” metabolites); biomolecular activity (within the producer as well as in another organism, including human biomedical use, which are typically different); and physiochemical properties. As shown herein, NADES span all three properties and, thus, are not captured adequately by considering their constituents “primary” metabolites. Therefore, and in line with Firn and Jones, the present work avoids the use of two classical, highly abundant, but sufficiently misleading terms, “primary” and “secondary” metabolites. Instead, the terms “biosynthetically primordial metabolites”, abbreviated as PRIM, and “biosynthetically more highly evolutionary metabolites”, HEVO in short, are used and proposed as a more adequate description of current knowledge. Further rationales for this choice and additional considerations are discussed in the section “Future Perspectives”.
Chemical Constituents of NADES
Due to the difficulty of recognizing NADES in natural sources, artificial combinations of presumptive NADES components became a leading strategy for NADES discovery.1,21 Regarding binary eutectic systems, once a mixture of natural metabolites under a certain molar ratio (usually close to 1:1) turns into a unified and transparent liquid at ambient temperature, these NPs are, a priori, NADES components. The corresponding NADES species may coexist in Nature. It is noted that this strategy may somehow distract from the actual discovery of truly multicomponent NADES species in natural sources. Nonetheless, the sets of possible NADES components can be reviewed, as follows.
The most understudied NADES components are PRIM compounds (aka “primary metabolites”). This group of NADES components is characterized by their high polarity and hydrophilicity and comprises sugars, organic acids, amino acids, and choline salts.22 Over 135 PRIM-based binary NADES species have been found;1,21,23,24 see the Supporting Information for further examples. Working combinations of binary eutectic mixtures can almost always be developed further into multicomponent eutectic systems. Accordingly, the PRIM-based NADES species are a rather sizable group of systems. Until now, most NADES studies and applications have been developed via this relatively simplistic group of NADES species.
Whereas many NADES constituents are organic molecules with relatively simple structure, it is important to realize that some other formulations consisting of more complex HEVOs, plus sometimes including designated NPs, are also capable of forming eutectic systems at ambient temperature. Examples are: (i) some essential oil ingredients with low melting points, such as 1,8-cineole, p-cymene, d-limonene, menthol, menthone, or thymol, which tend to form NADES.15 (ii) A eutectic solvent can also consist of a PRIM and a HEVO; for example, a mixture of menthol (HEVO) plus an organic acid (PRIM) is known to form a hydrophobic NADES system.25 In addition, NOE NMR experiments have indicated strong dipole–dipole interactions between the hydroxy groups of quercetin and the components of the NADES consisting of xylitol–choline chloride–water (1:2:3, mol/mol).21 Thus, the designated HEVO, quercetin, could well form part of a NADES matrix. (iii) Glycosidic NPs are characterized by the abundance of functional groups (OH) that are common in both PRIMs and HEVOs. For example, rutin is often found in vascular plants, and although no reports have been found yet, based on the evidence collected during the present study, it is likely that rutin will be recognized as a NADES component.
Water is one of the most abundant and smallest chemical entities on earth and universally present in living organisms. Thus, it is not surprising that water is a NADES component. Until now, at least 39 aqueous NADES species have been described in the literature,26–28 and all of them are ternary NADES systems. The intermolecular interactions between the water and other components can be recognized via NOE NMR measurements. For example, intermolecular interactions between the components of 1,2-propanediol–choline chloride– water (1:1:1, mol/mol) could be distinguished using NOESY experiments.1 To better understand the role of water in a ternary aqueous NADES system, a water loss study was developed as shown in Figure 3. It demonstrated that two major hydrogen-bonding zones, the NADES state and the solution state, existed, where the data points have a linear correlation in either state.22 Compared to the solution state, the NADES state demands more energy to remove water from the NADES matrix.22 According to the Frank model29 for the structure of water around an ion, a closer intermolecular distance (or NADES state) means an ordered zone and, thus, causes a stronger intermolecular force, equivalent to a higher polarity. The NADES state consists of an ordered hydrogen-bonding matrix. In the NADES state matrix, the minute quantities of water enable formation of a higher ordered hydrogen-bonding network. Here, water becomes an integral NADES constituent, at molar proportions similar to those of the other constituents.
Figure 3.
Water loss study on the transition from NADES solution to NADES state. (A) Evaporation of neat distilled water (●; A) and a NADES composed of mannose (0.01 mol), dimethyl urea (0.025 mol), and distilled water (2 mL) (■; B and C) via vacuum centrifugal evaporation. The drying curve of the mannose–dimethyl urea–water NADES is split into a rapid drying zone (initial stage of B) during the first 7 h and the slower, asymptotic loss of water from 12 to 18 h (C). Both time zones exhibit a linear correlation between time and water loss.
Inorganic salts are also universal in Nature in the form of aqueous solutions. Although they form a small group of NADES components, AlCl3, NH4Cl, NaCl, ZnCl2, ZnBr2, FeCl3, SnCl2, and CaCl2 have all been recognized as NADES components.23,24 Regarding this type of NADES, the eutectic donor mates are usually organic alcohols, such as ethylene glycol, or amides such as urea. They function as donors in nonmetal complexation and always coordinate to the cation.23 As such, inorganic salts have become a less studied but still important factor in NP research, e.g., when investigating the water fraction of a crude extract.
Ratios of NADES Components
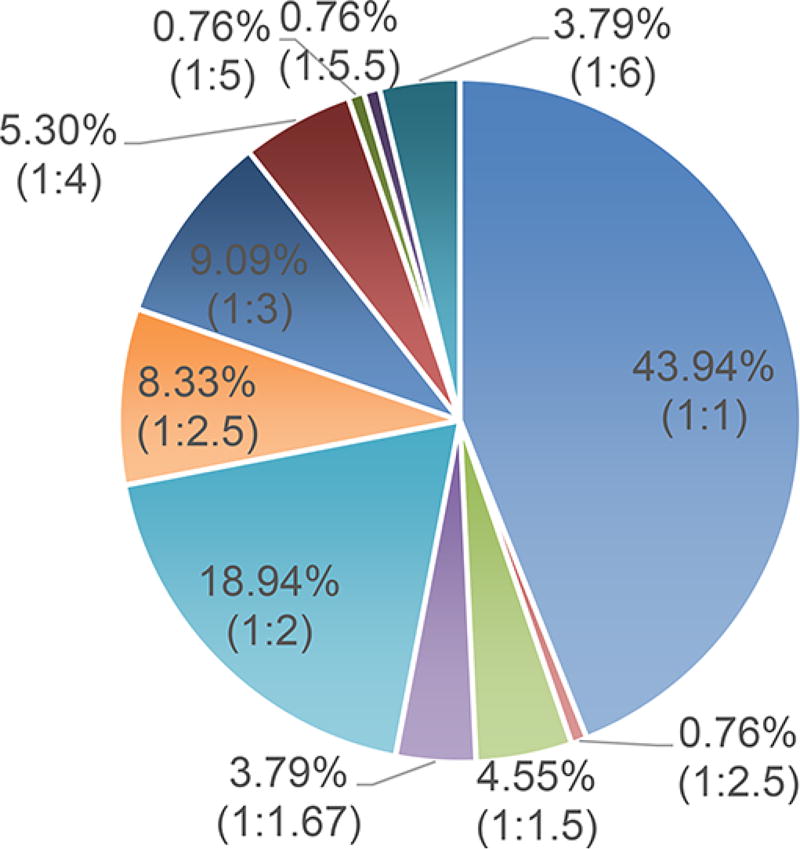
Empirically, the molar ratio of the eutectic components in a binary system should be near unity, because the lattice binding a single component depends on involvement with other components of the lattice.3 Moreover, the ratio of hydrogen-bonding donors and acceptors is another factor that implies unity ratio principles. However, the stoichiometry of hydrogen-bonding donors of a certain NADES component can be inconsistent with that of acceptor functions from other components. This may introduce a deviation from the “theoretical” component ratio of 1:1 (mol/mol). Over 135 PRIM-based binary NADES species were surveyed when preparing this review article. Among all these NADES (Figure 4), those with a molar ratio of 1:1 constitute about 44%, whereas NADES with molar ratios ranging from 1:1 to 1:4 account for almost 95% of all assessed NADES species. No binary NADES species has been found yet, where the molar ratio rises above 1:6. Regarding multicomponent NADES systems, the binary component-based rules continue to apply in principle.
Figure 4.
Distribution (percentage, %) of the component molar ratios (mol/mol) in reported NADES.1,19,21,22
Physicochemical Interactions of NADES Components
The most prominent functional groups of NADES constituents are carboxylic acids, hydroxy groups, and carbonyl groups.24 In a NADES matrix, these groups can form a hydrogen-bonding network via intermolecular interactions that modify their physicochemical environment. At least two basic strategies can be used to determine the physicochemical interactions: one strategy involves referencing to functional group properties. As shown in Table 2, FT-IR spectrometry26,30–32 and 1H NMR spectroscopy30 have been used to recognize the functional group modifications by comparing them to the individual isolated components. The hydroxy and carbonyl groups are two major functional groups used in this strategy. The second method involves the detection of NOE effects via 1H,1H dipole–dipole relaxation. A NOE between small molecules may be observed between hydrogen nuclei that are 2–5 Å apart.33 Typically, NOESY1,21,34 or HOESY21 experiments are applied to measure the intermolecular 1H dipole–dipole interaction (Table 2). In this strategy, the signals of interacting protons are the investigated subjects. However, as the high viscosity of some NADES species may lower the resolution of 1H NMR spectra, it becomes difficult to distinguish the 1H signals of the corresponding functional groups, especially for mixtures of sugars and/or amino acids. To overcome this challenge, two major approaches can be used (see also the proposed entropy models in Figure 5). (i) Temperature regulation: usually, a higher temperature can increase the entropy level in the NADES matrix and thereby reduce viscosity effects (see section on “Viscosity”). Thus, NOESY experiments used in NADES studies should be acquired at a temperature of ≥40 °C.1 Depending on the capabilities of the NMR instruments, particularly the probe, temperatures up to 80 °C are typically practical. However, the boiling point of the NMR solvent (deuterated, for lock purposes) is another consideration. (ii) Dilution effect: as discussed for the water loss study below, an appropriate dilution can reduce the viscosity of the NADES matrix. This entropy regulation (Figure 5) can produce an increase in the NMR spectral resolution. However, because overdilution causes NADES disruption, the optimum degree of dilution has to be determined for each individual NADES when using this approach.
Table 2.
Intermolecular Interactions in NADES As Determined by FT-IR and NMRa
| NADES system | functional groups | ref | |
|---|---|---|---|
| FT-IR | proline–malic acid | νC═O and νO–H | 26 |
| trehalose–choline chloride | νC═O, νC–H, νCH3, and νO–H | 30 | |
| quercetin–glucose–choline chloride | νC═O, νO–H, and aromatic ring | 31 | |
| 1,2-propanediol–choline chloride | νCH3 and νO–H | 32 | |
| NMR | |||
| 1H NMR | trehalose–choline chloride | dilution effect (νCH2) | 30 |
| NOESYa | sucrose–malic acid | νH, νO–H and water | 1 |
| 1,2-propanediol–choline chloride–water | νH and νH | 21 | |
| xylitol-choline chloride-quercetin | νO–H | 21 | |
| glycerol–choline chloride(–water) | 34 | ||
| HOESYb | 1,2-propanediol–choline chloride–water | νC and νH | 21 |
NOESY: nuclear Overhauser effect (NOE) spectroscopy.
HOESY: heteronuclear NOE experiment.
Figure 5.
Kinetic energy models for a binary NADES (A, temperature administration) and a ternary aqueous system (B, water content administration). As entropy rises, the strength of intermolecular bonding decreases, which subsequently impacts the relevant physical parameters.
PREPARATION OF NATURAL DEEP EUTECTIC SOLVENTS
NADES Preparation
Essential NADES components are all commercially available. Usually, they are treated under vacuum and/or at high temperature for water removal before use.35 As shown in Figure 1, NADES preparation is about administration of the NADES component in a given ratio and establishing entropy in the matrix via heating and/or homogenizing treatments (Table 3). Nevertheless, reported NADES preparation methods are not uniform. Unless highly dispersive methods are used, the neat mixing of NADES components is comparatively inefficient for transforming solid state NADES components into a liquid state. Hence, novice practitioners may find the preparation of a documented NADES challenging.
Table 3.
Summary of Standardized Preparation Strategies for NADES Species
This may be overcome by entropy regulation (Figure 5) during NADES preparation, in which heating and vacuum evaporation are two major NADES preparation strategies (Table 3). In the heating strategy,21,28,36,37 the NADES components are mixed at the designated component ratio and then stirred at a certain temperature (e.g., 50–100 °C). The increased entropy readily enables eutectic formation. Depending on the NADES properties, a homogeneous transparent liquid can be formed. After the mixture cools to ambient temperature, a transparent uniform mixture indicates that the designed NADES has been obtained. The vacuum evaporation strategy21,22,38,39 includes two basic steps. In the first stage, the NADES components are dissolved in water, with heating applied, vortexing motion, and/or ultrasound to accelerate dissolution. In the second step, water is removed through evaporation in vacuo, most commonly by centrifugal vacuum or rotatory evaporators. Centrifugal vacuum evaporation provides comparatively low throughput and, thus, is more appropriate for small-scale preparation up to the level of hundreds of milligrams.22 Rotary in vacuo evaporation can accommodate medium-scale (gram-level) preparation.1 Optimization of water removal conditions can be explored via a water loss study: due to the linear correlation of data points in the NADES state (Figure 3), a water loss equation can be established to obtain the proper NADES preparation time.22
NADES components are usually specified as a molar ratio (mol/mol),1 which directly reflects the contribution of hydrogen bond donors and acceptors. A weight ratio (w/w)23 can be more practical for the preparation procedure. When developing a new NADES, it is best to start with a component molar ratio of 1:1 and, in subsequent steps, adjust this ratio according to the number of hydrogen acceptors and donors in the matrix.
NADES Solutions
Most NADES described in the literature are PRIM-based and characterized by high polarity and hydrophilicity. However, NADES can dissolve analytes possessing a broad polarity range. This indicates that the dissolution behavior of NADES species may not follow the “like dissolves like” principle that is implied by the physiochemical characteristics of the neat PRIM compounds. While screening NADES species as potential solvents remains an empirical procedure, a limited number of tools exist for making this process more targeted. For example, organic dyes such as Reichardt’s dye can be used to determine the NADES polarity. When preparing NADES solutions of other solutes, viscosity has to be kept in mind as an important factor of solubilization. According to the Flory–Huggins equation,40,41 the NADES viscosity delays the analyte’s penetration of the NADES lattice. Compared to conventional organic solvents, some NADES with high viscosity will be slow to dissolve an analyte. However, it is important to note that speed of dissolution is not equivalent to solubility. Heating and/or stirring commonly increase the rate of dissolution.
PROPERTIES OF NATURAL DEEP EUTECTIC SOLVENTS
Expanding both the applications and overall understanding of NADES in the future will depend on the observation, comprehension, and interpretation of observed NADES phenomena. The study of NADES properties lies mainly in the fields of physical chemistry and thermodynamics. Herein, several important properties are reviewed to summarize the current knowledge and provide guidance for NADES applications relevant to NP research.
Renewability and Biodegradability
One of the main attractive properties of NADES is that their components are all NPs. Thus, NADES can be biosynthesized as well as metabolized by essentially all organisms, explaining why they are ultimately highly “biocompatible”. Compared to organic solvents and ionic liquids, the biodegradability of NADES helps avoid potential environmental hazards. Therefore, NADES systems are generally considered to be environmentally friendly and “green” solvents.42 For applications as functional solvents, NADES can be recycled and reused. So far, many recovery strategies, such as supercritical carbon dioxide, antisolvents, back extraction, chromatography (anion-exchange resin and countercurrent separation)22,24 have been used for this purpose. According to one study,43 dilution of a NADES will not influence its chromatographic behavior. This suggests that the separation of a target analyte and the recycling of a NADES can be achieved simultaneously. In order to maintain the quality of a reconditioned NADES, it can be analyzed quantitatively by MS, IR, and/or NMR methods.22,44 Sometimes, the composition needs readjustment by addition of precise amounts of certain individual components. This renewable cycle can reduce the total cost of NADES applications.
Conductivity and Viscosity
As the conductivity and viscosity of NADES are influenced by similar physicochemical factors, they can be discussed together. In an ionic (or electrolyte) solution model, temperature (eq 1) is one major factor determining conductivity. As the kinetic energy rises, the conductivity of a binary NADES system can increase significantly.28 On the other hand, the intermolecular cohesive forces contribute to liquid viscosity.45 As temperature can regulate the cohesive forces in a NADES matrix,16 the viscosity of a NADES species is very sensitive to kinetic energy.28 Large kinetic energy will overcome the strength of intermolecular forces and, thus, lessen the matrix viscosity. This indicates that conductivity is inversely proportional to viscosity, consistent with the results of a study on a group of choline chloride-based NADES species.23,28
| (1) |
where α is the temperature coefficient of resistivity, T0 is a fixed reference temperature (usually ambient temperature), and ρ0 is the resistivity at temperature T0. The parameter α is an empirical parameter fitted from measured data. Due to the approximate nature of the linearity, α differs as the reference temperature changes.
For a ternary aqueous NADES species, the water content is another determining factor for both conductivity and viscosity. As only a relatively small quantity of water is involved in the building of a NADES matrix, its conductivity will increase. However, as the water content increases, resistivity may also rise.26 Thus, the correlation between conductivity and water content in NADES (solutions) follows a nearly Gaussian shape. Parallel to the effect of temperature, NADES viscosity decreases as the amount of water increases.28 Within the range of typical experimental temperatures, conductivity and viscosity exhibit a positive linear correlation.27
Polarity
Generally, the greater the intermolecular attractions, the larger the polarity. Thus, polarity is generally a solubilization property. To study NADES polarity, solvatochromic studies have been performed using Reichardt’s organic dyes46,47 (eq 2) as well as Nile red48 (eq 3). Although solvatochromism in organic solvents covers almost the entire visual spectrum,49 solvatochromic effects of PRIM-based NADES species are limited to parts of the visual spectrum. The parameter ETN (eq 4)50 can be quantitatively applied for a polarity assessment. Polarity intervals can be identified by referencing against a standard.
| (2) |
| (3) |
where 30 means the standard solvatochromic betaine dye No. 30 (Reichardt’s dye) and NR represents Nile red. In turn, h is Planck’s constant, c is light speed, and λmax = wavelength of a maximum of UV absorbance.
| (4) |
where ETN is a normalized parameter. Both water and tetramethylsilane (TMS) are reference solvents. For water, ET(Water) equals 1, whereas for TMS, ET(TMS) is defined as 0.
For a ternary aqueous NADES matrix, a water loss study can reflect the strength of the intermolecular forces and, thus, provide a measure of polarity. In the linear equation of the NADES state (Figure 3), the corresponding slope displays intermolecular forces and their arrangement in the NADES matrix.22 When comparing NADES with different components, the slopes can be used to compare polarities. When the slope is high, it indicates that the intermolecular forces are comparatively low. In a ternary aqueous NADES family, the water quantity can be used to estimate the corresponding polarity.27 As less water content means that a higher order arrangement can be formed in the matrix, this corresponds to a higher polarity of the NADES.
Hydrophilicity and Hydrophobicity
Assessment of the hydrophilicity/hydrophobicity balance of NADES can be divided into two categories: the state of its mostly hydrophilic components and a consideration of extraordinary solubilities of the more hydrophilic NADES species for lipophilic metabolites. NADES systems include both hydrophilic and lipophilic components. Hydrophilic components with highly electronegative groups can form hydrogen bonding via special cases of dipole–dipole interactions. This explains the tendency of hydrophilic NADES species to be miscible with polar solvents such as water and MeOH.16 Conversely, due to differences in their capability of hydrogen bonding through the electrostatic forces, lipophilic compounds remain relatively passive participants in the hydrophilicity/lipophilicity balance phenomenon. Some NADES species, such as menthol-7 or fatty-acid-based51 NADES, hold the components with the electronegative groups and, thus, mainly induce electrostatic interactions between different component molecules. These NADES species are immiscible with water,3,52 a behavior that appears to be counterintuitive at first. Examples for these (perceived) exceptions are eutectic mixtures such as citric acid–menthol25 that contain both a hydrophilic and a lipophilic component to create a hydrophobic NADES species. The hydrophilicity/lipophilicity balance of such eutectic mixtures has to be measured individually. Interestingly, the hydrophilic, aqueous NADES species can exhibit a remarkable solubilizing ability for some lipophilic NPs.21 Compared to conventional neat polar solvents such as water, the hydrophobic net effect reflects the observed tendency of nonpolar substances to aggregate in aqueous solution under the exclusion of water molecules. In this way, the segregation maximizes the hydrogen bonding between water molecules and minimizes the area of contact between water and nonpolar molecules. However, some hydrophilic NADES differ from this water-like property and show an intriguing duality of lipophilicity and hydrophilicity.22
Solubilizing and Stabilizing Abilities
Solvents can have an effect on solubility and stability of a solute by inducing or preventing certain molecular associations. The unique intermolecular interactions or arrangements of a NADES matrix generate its special solubilizing22,53 and stabilizing31,43 properties. Two main theories have been proposed to explain the extraordinary features of NADES: the hole or liquid crystal theory1 and the binding theory. According to the hole theory, the inner structure of a NADES forms a polymer-like matrix, and the solute can dissolve into the space (or holes) of this molecular network. As the component ratio changes, the inner arrangement such as the size and shape of the matrix hole of the NADES may be modified significantly. For example, as the water content in a NADES matrix changes, the matrix hole size can alter from micrometer to nanometer levels.54,55 Following the binding theory, the target solutes actually become segments/parts of the NADES matrix through intermolecular interactions. For example, xylitol–choline chloride–water (XChWat) exhibits an extraordinary solubilizing ability for quercetin.21 According to NOE measurements, the components of XChWat have a significant intermolecular correlation with the solute, quercetin. Moreover, due to the influence of the intermolecular bonding, some properties of the target analyte may be greatly changed. For example, while proline is only sparingly soluble in DMSO, a proline-based multiplecomponent NADES can be fully miscible with the same organic solvent.
Biocompatibility
Researchers working on eutectics uniformly consider NADES as nontoxic media.28,56,57 The collective experience of research on NPs over the past decades has repeatedly led to the observation that NADES-enriched water fractions are most often devoid of pharmacological activity.58 While being a general indicator of lack of (cyto)toxicity, this observation may also require a more critical interpretation due to the extremely shifted relative concentrations of solutes in these polar materials. However, PRIMs are usually dietary, food additives, or drug excipients and serve as the main NADES constituents. Still, many PRIM-based NADES species can exhibit side-effects in biological tests. Table 4 provides the details on the test models, including cell line,37,59,60 bacterial,38,39,57,61 fungal,57,62 shrimp,63 carp,62 and murine37 models. These two general observations are not necessarily in conflict. For example, honey is a widely used food and dietary supplement. On the other hand, it is also capable of inhibiting bacterial growth and serves as an important ingredient in preparations used for human health services.
Table 4.
Summary of NADES Test Methods, Study Models, and Sample Treatments
| methodology | study model | sample treatment | refs | |
|---|---|---|---|---|
| cytotoxicity | MTT cell viability (IC50)a | A375, B16F10, CaOV3, H413, HelaS3, HepG2, HT29, MCF-7, OKF6, and PC3 | a 2-fold dilution series of six concentrations of the solvents | 37, 59 |
| others | Artemia salina (brine shrimp) | – | 57, 63 | |
| differentiated T37i brown adipocytes | 0.01–10% of culture medium | 60 | ||
| bacterial phototoxicityb | E. coli, E. faecalis, S. aureus | 0–200 times in PBS or water of dilution | 38, 39 | |
| bacterial inhibitionb | E. coli, S. aureus, P. aeruginosa, and B. subtilis | – | 57 | |
| toxicity (Microtox test; luminescence inhibition)b | V. fischeri | diluted aqueous solution of tested compound (from 0 to 81.9%)c | 61 | |
| fungal toxicity | Aspergillus niger | 0–4500 mM (or 0–650 mg/mL) | 62 | |
| acute toxicity (toxicological symptoms) | Cyprinus carpio (common carp) | 25–14 000 mg/L | 62 | |
| imprinting control region mice | vehicle (dH2O), high (20 g/kg), medium (10 g/kg), and low (5 g/kg) doses | 37 | ||
Mouse: B16F10, skin cell line; human: A375, malignant melanoma cell line; CaOV3, ovarian cell line; H413, carcinoma-derived oral keratinocyte cell; HeLaS3, cervical cell line; HepG2, liver hepatocellular cell line; HT29, colon adenocarcinoma cell line; MCF-7, breast cell line; OKF6, oral keratinocyte cell line; PC3, prostate cell line.
B. subtilis, Bacillus subtilis; E. coli, Escherichia coli; E. faecalis, Enterococcus faecalis; P. aeruginosa, Pseudomonas aeruginosa; S. aureus, Staphylococcus aureus; V. fischeri, Vibrio fischeri.
The unit of % (v/v, wt/wt, or mol/mol) was not reported unclear; – indicated suitable data unavailable.
Notwithstanding their general biocompatibility, unexplained molecular interactions with NADES during sample treatment can be a potential cause of concern for some assays. For example, it is known that in diluted NADES species such as mannose–dimethylurea–water (2:5:5, mol/mol) over five times, no intermolecular interactions among the components can be detected by an NOE 1H NMR experiment. However, for some test studies, the samples were diluted over 100-fold (Table 4). Theoretically, this overdilution leads to matrix disruption into the individual components (Figure 5) and, thus, will cease to function as a NADES species. Accordingly, the observed outcomes of tested NADES principally depend on the individual components of the NADES and related synergistic effects.28
APPLICATIONS OF NATURAL DEEP EUTECTIC SOLVENTS
NADES as Extraction Media
Any NADES extraction strategy includes two basic steps: (i) the separation of the target analytes from the source tissues or a chemically complex matrix. As NADES species exhibit a superior solubilizing ability for NPs, this provides a special advantage for NADES as extraction media. Practitioners interested in the extraction capabilities of NADES are referred to a pair of excellent recent reviews.24,64 Table 5 provides an update on some of the literature findings, covering 29 compounds belonging to nine different classes that were extracted using NADES media.28,32,43,53,65–67 Chalcones, anthocyanins, and phenolic acids are the major target NP classes covered in the literature. The most commonly used NADES components are lactic acid, choline chloride, and 1,2-propanediol (used in eight of the nine NADES systems). Compared to organic solvents, many NADES species are relatively viscous at ambient temperature.68 This may weaken the capability of target analytes to diffuse passively from the tissues, through cell walls, into the NADES media and, thus, impede the recovery of the target compound from the NADES matrix. (ii) As extraction media, most NADES species are PRIM-based and, therefore, usually nonvolatile.69 NADES cannot be evaporated, thus requiring additional chromatographic treatment for neat analyte recovery. According to a previous study,43 NADES do not disrupt LC systems. Usually, the chromatographic step enables a separation of the target analytes from the (NA)DES matrix.22,24 To overcome the absence of UV absorption in most PRIM constituents, a conductivity-based device22 has been developed for chromatographic monitoring. Hence, NADES media can even be recycled. Compared to fresh NADES media, reconditioned systems have been shown to exhibit consistent extraction yields for the target analytes.70
Table 5.
Update of NADES Applications as Extraction Media
| NADES system | class | analytes | resource | ref |
|---|---|---|---|---|
| 1,2-propanediol–choline chloride | xanthonoid | α-mangostin | Garcinia mangostana | 32 |
| lactic acid–glucose 1,2-propanediol–choline chloride | anthocyanins | delphinidin, cyanidin, peonidin, pelargonidin, petunidin, and malvidin | Catharanthus roseus | 43 |
| proline–malic acid–water sucrose–choline chloride–water lactic acid–glucose | chalconoid | carthamin, cartormin, hydroxysafflor yellow A, N1,N10,N5-(Z)-tri-p-coumaroylspermidine, N1-(E)-N5,N10-(Z)-tri-p-coumaroylspermidine, N1,N10-(E)-N5-(Z)-tri-p-coumaroylspermidine, stereoisomer of tri-p-coumaroylspermidine, N1,N10,N5-(E)-tri-p-coumaroylspermidine | Carthamus tinctorius | 53 |
| lactic acid–glucose–water | phenolic acid | hydroxybenzoic acid, protocathecuic acid, vanillic acid; p-coumaric acid; caffeic acid | olive oil | 65 |
| phenethyl alcohol | tyrosol | |||
| flavone | apigenin | |||
| lignan | pinoresinol | |||
| lactic acid–choline chloride | biopolymer | lignin, cellulose, hemicellulose, holocellulose | rice straw | 66 |
| proline–lactic acid | zingerone | gingerol, shogaol | Zingiber officinale | 67 |
NADES as Chromatographic Media
An ideal chromatographic system can separate the target analytes into separate concentrated zones at an appropriate volume distance away from each other. As NADES species are capable of selectively extracting NPs, in high yields, they may exhibit an efficient chromatographic selectivity for the separation of NPs. In addition, a polarity gradient of NADES species can be designed (see section on “Polarity”). Due to the development of hydrophobic NADES species,25 the diversity of NADES candidates as chromatographic eluents is likely to increase over time. The compatibility of the thermodynamic properties of NADES with those of LC systems suggests that certain NADES species are suitable chromatographic media. Altogether, NADES media may function as a proper mobile phase in different chromatographic strategies. For example, regarding a liquid-only countercurrent separation, one documented biphasic solvent system represents the behavior of an individual column.71 As with all solvents, the development of the biphasic solvent system becomes a priority task for countercurrent separation,72 including for NADES. Recently, biphasic solvent systems with NADES were successfully employed to separate natural product mixtures.73
Biomedical Applications of NADES
Investigations of the biocompatibility of NADES have shown that NADES species can replace organic solvents such as DMSO as dissolving media in biological assays. In the reported applications, the NADES solutions of the test samples were diluted during the biological studies, typically to the point where the DES matrix was disrupted, meaning that the NADES species eventually lost their solubilizing and/or stabilizing abilities. Therefore, reported protocols have been carrying the inherent risk of not determining the true bioactivity of the target analyte, because disruption of the NADES matrix may cause the loaded NPs to precipitate and/or change their physiochemical stage relevant to the bioassay. However, the relative ease of disruption of NADES matrices together with the NADES duality (lipophilicity and hydrophilicity) feature suggests that NADES are capable of serving a helpful role in small-molecule formulations. For example, NADES can carry lipophilic bioactive ingredients for subsequent loading into an otherwise unduly hydrophilic polymer such as a hydrogel. This is feasible, as spontaneous diffusion by movement along a concentration gradient removes NADES components from the polymer, whereas a sizable portion of the lipophilic molecules passively remains inside the biopolymer construct. This advantageous behavior indicates that NADES species may be structurally analogous to cyclodextrin, a commonly used excipient in hydrophobic drug delivery systems.19
In the context of biomedical applications, it is important to remember that synthetic-based DES or HEVO-based NADES can form biomedical delivery systems by themselves. For example, lidocaine hydrochloride–acrylic acid and lidocaine hydrochloride–methacrylic acid can form polymerized eutectic systems.74 Considering the structural similarity between lidocaine and the capsaicins, eutectic formation is likely a shared property of these types of amides. Other examples are the eutectic ibuprofen–menthone, ibuprofen–1,8-cineole, ibuprofen–d-limonene, and ibuprofen–p-cymene systems, which were developed as transdermal delivery systems.15 Ibuprofen–menthol–poly-ε-caprolactone has even been advanced to create eutectic controlled release systems.75
Basic Research on NADES
The electrochemical properties of NADES including their conductivity allow their ohmmetric monitoring. For example, when studying the recovery of NPs from a NADES matrix, a phase metering apparatus—originally constructed to monitor the composition of countercurrent liquid phases—was employed to differentiate NADES (as a negative/dispersive signal) from the absorptive signals of eluting analytes.22 With the assistance of a buffer solution, NADES as electrochemical reagents can also enhance the electrochemical detection of NPs.76 Moreover, dipeptide-based eutectic solvents have been shown to catalyze organic reactions.66 An unexpected property of some amine-based NADES species was their high CO2 solubility, compared to aqueous amines.77–79 Amine-based NADES species can avoid elevated vapor pressure in the procedure of CO2 dissolution. This suggests that NADES have the potential capability of capturing gaseous molecules from the air and can possibly be developed into novel absorbent materials.
FUTURE PERSPECTIVES
Due to demands for green solvents in the global scientific and manufacturing communities, NADES studies have rapidly multiplied in the chemical and natural product literature since 2011. An increasing variety of basic properties and applications have been investigated and developed. A primarily chronological approach was chosen for this review, as it provides the reader with an additional layer of understanding of the evolution of NADES research starting from early eutectic studies. The authors found this approach a helpful means of grasping the continuous chemical extension of NADES as multicomponent mixtures, which represents a timely topic in NP research. Statistically, the literature survey performed for this study revealed that the component ratio in NADES is rarely over 1:6 in binary systems (major components).
This review also sheds light on the recent directions toward a series of NADES applications, which facilitates a discussion around the roles of NADES in NP research and biological systems in general. The quest for determining their natural role and the appeal of finding novel applications create six major areas for future scientific inquiry regarding NADES research and applications.
NADES properties are directly associated with their components. Although at least 174 NADES species have already been described, the discovery of new NADES components and/or species will likely continue to be a major focus in the future. Currently, NADES components are considered to be derived primarily from botanical resources. Nevertheless, all metabolites produced by natural sources, including microorganisms and animals, have to be considered as potential NADES components.
NADES species exhibit significant solubilizing selectivity for some NPs, which represents both opportunities and challenges in their applications. This partially explains how Nature formulates lipophilic ingredients without assistance of organic solvents. However, this is also equivalent to solubilizing exclusivity for others. Following this logic, although it is still unclear how such a mechanism works in Nature, it may be helpful for NP enrichment in a specific tissue or organ in a living organism. For natural product research, such NADES species may not be good solvent candidates for a universal extraction purpose.
In order to develop efficient NADES experiments and applications, practitioners must consider the inherent properties of NADES. For example, due to the relatively weak chemical force of intermolecular interactions among NADES components, it is challenging to preserve an entire NADES matrix after a dilution. The ability of NADES to act as solubilizing agents or solvents in bioassays for the improvement of hydrophobic analyte solubility will require validation of the impact of NADES in any given experiment/application.
Documented observations of NADES in Nature are still limited, in particular with regard to their chemical diversity. Accordingly, study models are still in high demand that can reveal the biologically relevant and/or “synergistic” functions of NADES in natural resources, dietary supplements, and traditional medicine formulas and whether they are physical or biochemical in type, or both. New experimental designs will need to be developed to observe NADES behavior more directly, to further our understanding of known or uncover new NADES phenomena and develop potential applications.
The intriguing role of water molecules in NADES triggers the reasonable hypothesis that certain liquid/gaseous ingredients may also be able to serve as NADES components. However, this type of NADES component is reported only rarely. Interestingly, results from a study aimed at the purification of licochalcone A from a plant extract serve to validate the general feasibility of this hypothesis. Performing a countercurrent separation using the solvent system of n-hexane– EtOAc–MeOH–water (4:6:5:5, v/v), the fraction containing the target compound was concentrated using a centrifugal vacuum evaporator (16 h, 37 °C). Figure 6 shows that the sample exhibited the typical physical appearance of a eutectic, and it contained two major components: licochalcone A and EtOAc. Their molar ratio as determined by quantitative 1H NMR spectroscopy was close to 1:1. Although some minor impurities could be distinguished, the molar ratio of licochalcone A (or EtOAc) to any impurity was >7:1. The low abundance of the impurities made it unlikely that they serve as eutectic components (see section on “Ratio of NADES Components”). While the sample mainly contained licochalcone A, n-hexane, EtOAc, MeOH, and water prior to the evaporation, no solvents other than EtOAc were observed in the concentrated, eutectic sample. Considering that water has a higher boiling point than EtOAc, this eutectic system may result from the similar polarities (electrostatic forces) between licochalcone A and EtOAc. This suggests that other organic solvents such as 1,3-propanediol, EtOAc, and n-BuOH, which are used widely in NP studies, may also serve as eutectic components.80 This piece of evidence is in line with the general experience of the authors in the NP chemistry laboratory with regard to two broader observations: (i) the frequent occurrence of (semi)liquids as isolation products of compounds that should form solids at RT and (ii) the frequent occurrence of EtOAc as residual solvent in purified NPs and commercial reference materials, which can be readily detected by quantitative 1H NMR (qHNMR) spectroscopy. It is quite conceivable that NADES may influence target analyte properties such as solubility, stability, and even pharmacokinetic profiles. The potential broader impact of such phenomena on NP research makes it an important consideration for future work.
NADES components are very common in organisms (most studied for plants) and their extracts. They frequently are major constituents in crude NP-derived materials, and, thus, NADES could be a dominant form of biomass in Nature. Considering the validity of the mass balance approach to the analytical description of biomedical intervention materials, in particular drugs, this suggests that NADES components should be considered more widely as candidate marker compounds for NP-derived materials. For example, extending botanical quality control to NADES will be a means of capturing the chemistry of these complex materials with a much expanded latitude.
Figure 6.
Quantitative 1H NMR spectrum (360 MHz) of licochalcone A (A) vs the same compound purified by countercurrent separation (B) (DMSO-d6, 60 s relaxation delay [D1]). The image insets show the physical appearance of corresponding samples, especially the liquid nature of the otherwise “pure” licochalcone A in sample (B).
The chemical diversity of the metabolites with reported NADES-generating qualities nurtures the idea that the difference between biosynthetically primordial metabolites and biosynthetically more highly evolutionary metabolites is a very fine or even nonexisting line. It could even be a border that biological systems cross intentionally, and in very delicate ways, in order to function. This expands the recognition of metabolomes as snapshots of the physiological stage and function of an organism, while highlighting the importance of the distinctiveness of NADES components in metabolomic analysis and research on the bioactivity of NPs.
A review of existing evidence already provides strong indication that PRIMs not merely are essential, biosynthetically primordial molecules for organisms to function but also play a major role in the bioactivity of unrefined NPs as well as in the function of cells and cellular compartments. The former domain is currently occupied almost exclusively by HEVOs, which typically are isolated from crude mixtures, with varying degrees of residual complexity (see http://go.uic.edu/residualcomplexity). The excessively high dynamic range yet limited chemical complexity of typical PRIM components of NADES stands in contrast to the chemically more demanding HEVO constituents, which are contained or participate in the formation of NADES matrices. This situation engenders a challenging set of analytical and biological questions for future NP research involving natural eutectics.
In each cell and organism, the biosynthetic pathways that produce metabolites must be connected with the biological functions of these products. While the pathways are known to have underlying genomic and proteomic blueprints, and whereas feedback mechanisms such as quorum sensing and hormonal effects are increasingly understood, the overarching mechanisms and forces (“vis vitalis”) used by living systems to initiate and regulate biological function chemically are (and may remain) essentially unknown. Considering that one metabolite can have multiple functions and that a certain function can be fulfilled by a variety of metabolites, redundancy is likely an intrinsic part of Nature’s function. In addition, and as indicated by the broad solubilizing characteristics of NADES, it is very likely that not only PRIM but also HEVO compounds can participate in the formation of NADES and/or modify NADES behavior.
The recently gained knowledge about NADES reviewed herein calls attention to the importance of differentiating between biosynthetic assembly and biological function: whereas metabolite form(ation) has historically been assigned to “primary” and “secondary” metabolic pathways, metabolite function is not correlated directly to chemical genesis, nor are the biological roles evident from biosynthetic origin. The growing insight about the physiological roles of NADES and their biomedical applications indicates that the metabolites involved in their formation have a range of unknown functions in Nature. Furthermore, it should be kept in mind that metabolite function is not unideterminant, as multiple functions per metabolite coexist with multiple metabolites per function, nor can metabolite function be generalized across phyla, as unified metabolites for certain functions vs multiple metabolites for other functions occurr in parallel. Considering this dichotomy of metabolite form vs function, the question of designating NADES constituents as PRIM/HEVO vs “primary/secondary” metabolites might inspire important discussions about a modernized terminology as well as future searches of NADES chemistry, biology, and biomedical applications.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge support for their research through grants P50 AT000155 and U41 AT008706 from the Office of Dietary Supplements (ODS) and the National Center for Complementary and Integrative Health (NCCIH) of the National Institutes of Health (NIH). Furthermore, Y.L. gratefully acknowledges support from the United States Pharmacopeial Convention in the form of a USP Global Fellowship.
Footnotes
ASSOCIATED CONTENT
- Review of the NADES components in the literature and survey of the frequency of their occurrence; compilation and origin of abbreviations of NADES components (PDF)
The authors declare no competing financial interest.
DEDICATION
Dedicated to Dr. Susan Band Horwitz, of Albert Einstein College of Medicine, Bronx, NY, for her pioneering work on bioactive natural products.
References
- 1.Choi YH, van Spronsen J, Dai Y, Verberne M, Hollmann F, Arends IW, Witkamp GJ, Verpoorte R. Plant Physiol. 2011;156:1701–1705. doi: 10.1104/pp.111.178426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pandey A, Bhawna, Dhingra D, Pandey S. J. Phys. Chem. B. 2017;121:4202–4212. doi: 10.1021/acs.jpcb.7b01724. [DOI] [PubMed] [Google Scholar]
- 3.Abbott AP, Capper G, Davies DL, Rasheed RK, Tambyrajah V. Chem. Commun. 2003:70–71. doi: 10.1039/b210714g. [DOI] [PubMed] [Google Scholar]
- 4.Okuniewski M, Paduszyński K, Domańska U. J. Phys. Chem. B. 2016;120:12928–12936. doi: 10.1021/acs.jpcb.6b10034. [DOI] [PubMed] [Google Scholar]
- 5.Jain K, Ghai B, Saxena AK, Saini D, Khandelwal N. Paediatr. Anaesth. 2010;20:330–337. doi: 10.1111/j.1460-9592.2010.03279.x. [DOI] [PubMed] [Google Scholar]
- 6.Günergun F. Osmanlı Bilimi Araştırmaları. 2013;XIV/2:41–68. [Google Scholar]
- 7.Tuntarawongsa S, Phaechamud T. Adv. Mater. Res. 2012;506:355–358. [Google Scholar]
- 8.Franklin EC. J. Phys. Chem. 1918;23:36–53. [Google Scholar]
- 9.Kraus CA, Cuy EJ. J. Am. Chem. Soc. 1923;45:712–715. [Google Scholar]
- 10.Bergstrom FW, Sturz HG, Tracy HW. J. Org. Chem. 1946;11:239–246. doi: 10.1021/jo01173a005. [DOI] [PubMed] [Google Scholar]
- 11.Gill I, Vulfson E. J. Am. Chem. Soc. 1993;115:3348–3349. [Google Scholar]
- 12.Gill I, Vulfson E. Trends Biotechnol. 1994;12:118–122. doi: 10.1016/0167-7799(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 13.Murrell JN, Jenkins AD. Properties of Liquids and Solutions. 2. University of Sussex; Brighton, UK: 1994. [Google Scholar]
- 14.Davey RJ, Garside J, Hilton AM, McEwan D, Morrison JW. Nature. 1995;375:664–666. [Google Scholar]
- 15.Stott PW, Williams AC, Barry BW. J. Controlled Release. 1998;50:297–308. doi: 10.1016/s0168-3659(97)00153-3. [DOI] [PubMed] [Google Scholar]
- 16.Abbott AP, Boothby D, Capper G, Davies DL, Rasheed RK. J. Am. Chem. Soc. 2004;126:9142–9147. doi: 10.1021/ja048266j. [DOI] [PubMed] [Google Scholar]
- 17.Bisson J, McAlpine JB, Friesen JB, Chen S-N, Graham J, Pauli GF. J. Med. Chem. 2016;59:1671–1690. doi: 10.1021/acs.jmedchem.5b01009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Firn R. Nature’s Chemicals: The Natural Products that Shaped Our World. Oxford University Press; Oxford, UK: 2011. [Google Scholar]
- 19.Firn RD, Jones CG. Nat. Prod. Rep. 2003;20:382–391. doi: 10.1039/b208815k. [DOI] [PubMed] [Google Scholar]
- 20.Firn RD, Jones CG. Mol. Microbiol. 2000;37:989–994. doi: 10.1046/j.1365-2958.2000.02098.x. [DOI] [PubMed] [Google Scholar]
- 21.Dai Y, van Spronsen J, Witkamp GJ, Verpoorte R, Choi YH. Anal. Chim. Acta. 2013;766:61–68. doi: 10.1016/j.aca.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Garzon J, Friesen JB, Zhang Y, McAlpine JB, Lankin DC, Chen SN, Pauli GF. Fitoterapia. 2016;112:30–37. doi: 10.1016/j.fitote.2016.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruß C, Konig B. Green Chem. 2012;14:2969–2982. [Google Scholar]
- 24.Dai Y, van Spronsen J, Witkamp GJ, Verpoorte R, Choi YH. J. Nat. Prod. 2013;76:2162–2173. doi: 10.1021/np400051w. [DOI] [PubMed] [Google Scholar]
- 25.Ribeiro BD, Florindo C, Iff LC, Coelho MAZ, Marrucho IM. ACS Sustainable Chem. Eng. 2015;3:2469–2477. [Google Scholar]
- 26.Dai Y, Witkamp GJ, Verpoorte R, Choi YH. Food Chem. 2015;187:14–19. doi: 10.1016/j.foodchem.2015.03.123. [DOI] [PubMed] [Google Scholar]
- 27.Craveiro R, Aroso I, Flammia V, Carvalho T, Viciosa MT, Dionísio M, Barreiros S, Reis RL, Duarte ARC, Paiva A. J. Mol. Liq. 2016;215:534–540. [Google Scholar]
- 28.Zhao B-Y, Xu P, Yang F-X, Wu H, Zong M-H, Lou W-Y. ACS Sustainable Chem. Eng. 2015;3:2746–2755. [Google Scholar]
- 29.Frank HS, Wen W-Y. Discuss. Faraday Soc. 1957;24:133–140. [Google Scholar]
- 30.Xin R, Qi S, Zeng C, Khan FI, Yang B, Wang Y. Food Chem. 2017;217:560–567. doi: 10.1016/j.foodchem.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 31.Dai Y, Verpoorte R, Choi YH. Food Chem. 2014;159:116–121. doi: 10.1016/j.foodchem.2014.02.155. [DOI] [PubMed] [Google Scholar]
- 32.Mulia K, Krisanti E, Terahadi F, Putri S. Int. J. Technol. 2015;6:1211–1220. [Google Scholar]
- 33.Jones CR, Butts CP, Harvey JN. Beilstein J. Org. Chem. 2011;7:145–150. doi: 10.3762/bjoc.7.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hadj-Kali MK, Al-khidir KE, Wazeer I, El-blidi L, Mulyono S, AlNashef IM. Colloids Surf., A. 2015;487:221–231. [Google Scholar]
- 35.Radošević K, Ćurko N, Gaurina Srček V, Cvjetko Bubalo M, Tomašević M, Kovačević Ganić K, Radojčić Redovniković I. LWT - Food Sci. Technol. 2016;73:45–51. [Google Scholar]
- 36.Jablonský M, Škulcová A, Kamenská L, Vrška M, Šíma J. BioResources. 2015;10:8039–8047. [Google Scholar]
- 37.Hayyan M, Looi CY, Hayyan A, Wong WF, Hashim MA. PLoS One. 2015;10:e0117934. doi: 10.1371/journal.pone.0117934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wikene KO, Bruzell E, Tønnesen HH. J. Photochem. Photobiol., B. 2015;148:188–196. doi: 10.1016/j.jphotobiol.2015.04.022. [DOI] [PubMed] [Google Scholar]
- 39.Wikene KO, Rukke HV, Bruzell E, Tønnesen HH. Eur. J. Pharm. Biopharm. 2016;105:75–84. doi: 10.1016/j.ejpb.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 40.Huggins ML. Polym. J. 1973;4:511–516. [Google Scholar]
- 41.Huggins ML. Polym. J. 1973;4:502–510. [Google Scholar]
- 42.Paiva A, Craveiro R, Aroso I, Martins M, Reis RL, Duarte ARC. ACS Sustainable Chem. Eng. 2014;2:1063–1071. [Google Scholar]
- 43.Dai Y, Rozema E, Verpoorte R, Choi YH. J. Chromatogr. A. 2016;1434:50–56. doi: 10.1016/j.chroma.2016.01.037. [DOI] [PubMed] [Google Scholar]
- 44.Florindo C, Oliveira FS, Rebelo LPN, Fernandes AM, Marrucho IM. ACS Sustainable Chem. Eng. 2014;2:2416–2425. [Google Scholar]
- 45.Stefanovic R, Ludwig M, Webber GB, Atkin R, Page AJ. Phys. Chem. Chem. Phys. 2017;19:3297–3306. doi: 10.1039/c6cp07932f. [DOI] [PubMed] [Google Scholar]
- 46.Osterby BR, McKelvey RD. J. Chem. Educ. 1996;73:260. [Google Scholar]
- 47.Machado VG, Machado C. J. Chem. Educ. 2001;78:649. [Google Scholar]
- 48.Fowler SD, Greenspan P. J. Histochem. Cytochem. 1985;33:833–836. doi: 10.1177/33.8.4020099. [DOI] [PubMed] [Google Scholar]
- 49.Reichardt C. Chem. Rev. 1994;94:2319–2358. [Google Scholar]
- 50.Hernandez-Perni G, Stengele A, Leuenberger H. Int. J. Pharm. 2005;291:189–195. doi: 10.1016/j.ijpharm.2004.07.056. [DOI] [PubMed] [Google Scholar]
- 51.van Osch DJGP, Zubeir LF, van den Bruinhorst A, Rocha MAA, Kroon MC. Green Chem. 2015;17:4518–4521. [Google Scholar]
- 52.Li C, Li D, Zou S, Li Z, Yin J, Wang A, Cui Y, Yao Z, Zhao Q. Green Chem. 2013;15:2793–2799. [Google Scholar]
- 53.Dai Y, Witkamp G-J, Verpoorte R, Choi YHN. Anal. Chem. 2013;85:6272–6278. doi: 10.1021/ac400432p. [DOI] [PubMed] [Google Scholar]
- 54.Ma BL, Yin C, Zhang BK, Dai Y, Jia YQ, Yang Y, Li Q, Shi R, Wang TM, Wu JS, Li YY, Lin G, Ma YM. Sci. Rep. 2016;6:20110. doi: 10.1038/srep20110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hammond OS, Bowron DT, Edler KJ. Angew. Chem., Int. Ed. 2017;56:9782–9785. doi: 10.1002/anie.201702486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Faggian M, S S, Perissutti B, Baldan V, Grabnar I, Dall’Acqua S. Molecules. 2016;21:1531. doi: 10.3390/molecules21111531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hayyan M, Hashim MA, Hayyan A, Al-Saadi MA, AlNashef IM, Mirghani MES, Saheed OK. Chemosphere. 2013;90:2193–2195. doi: 10.1016/j.chemosphere.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 58.Zhijun L. Endocr., Metab. Immune Disord.: Drug Targets. 2008;8:112–121. doi: 10.2174/187153008784534358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hayyan M, Mbous YP, Looi CY, Wong WF, Hayyan A, Salleh Z, Mohd-Ali O. SpringerPlus. 2016;5:913. doi: 10.1186/s40064-016-2575-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rozema E, van Dam AD, Sips HCM, Verpoorte R, Meijer OC, Kooijman S, Choi YH. RSC Adv. 2015;5:61398–61401. [Google Scholar]
- 61.de Morais P, Gonçalves F, Coutinho JAP, Ventura SPM. ACS Sustainable Chem. Eng. 2015;3:3398–3404. [Google Scholar]
- 62.Juneidi I, Hayyan M, Hashim MA. RSC Adv. 2015;5:83636–83647. [Google Scholar]
- 63.Hayyan M, Hashim MA, Al-Saadi MA, Hayyan A, AlNashef IM, Mirghani MES. Chemosphere. 2013;93:455–459. doi: 10.1016/j.chemosphere.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 64.Ruesgas-Ramón M, Figueroa-Espinoza MC, Durand E. J. Agric. Food Chem. 2017;65:3591–3601. doi: 10.1021/acs.jafc.7b01054. [DOI] [PubMed] [Google Scholar]
- 65.Paradiso VM, Clemente A, Summo C, Pasqualone A, Caponio F. Food Chem. 2016;212:43–47. doi: 10.1016/j.foodchem.2016.05.082. [DOI] [PubMed] [Google Scholar]
- 66.Kumar AK, Parikh BS, Pravakar M. Environ. Sci. Pollut. Res. 2016;23:9265–9275. doi: 10.1007/s11356-015-4780-4. [DOI] [PubMed] [Google Scholar]
- 67.Mariappan R, Ayyappan P, Uthandakalaipandian R. Nat. Prod. J. 2015;5:3–13. [Google Scholar]
- 68.Duan L, Dou L-L, Guo L, Li P, Liu EH. ACS Sustainable Chem. Eng. 2016;4:2405–2411. [Google Scholar]
- 69.Liu P, Hao J-W, Mo L-P, Zhang Z-H. RSC Adv. 2015;5:48675–48704. [Google Scholar]
- 70.Martinez R, Berbegal L, Guillena G, Ramon DJ. Green Chem. 2016;18:1724–1730. [Google Scholar]
- 71.Friesen JB, McAlpine JB, Chen S-N, Pauli GF. J. Nat. Prod. 2015;78:1765–1796. doi: 10.1021/np501065h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu Y, Friesen JB, McAlpine JB, Pauli GF. Planta Med. 2015;81:1582–1591. doi: 10.1055/s-0035-1546246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Roehrer S, Bezold F, García EM, Minceva M. J. Chromatogr. A. 2016;1434:102–110. doi: 10.1016/j.chroma.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 74.Sanchez-Leija RJ, Pojman JA, Luna-Barcenas G, Mota-Morales JD. J. Mater. Chem. B. 2014;2:7495–7501. doi: 10.1039/c4tb01407c. [DOI] [PubMed] [Google Scholar]
- 75.Aroso IM, Craveiro R, Rocha Â, Dionísio M, Barreiros S, Reis RL, Paiva A, Duarte ARC. Int. J. Pharm. 2015;492:73–79. doi: 10.1016/j.ijpharm.2015.06.038. [DOI] [PubMed] [Google Scholar]
- 76.Gomez FJV, Espino M, de los Angeles Fernandez M, Raba J, Silva MF. Anal. Chim. Acta. 2016;936:91–96. doi: 10.1016/j.aca.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 77.Trivedi TJ, Lee JH, Lee HJ, Jeong YK, Choi JW. Green Chem. 2016;18:2834–2842. [Google Scholar]
- 78.Sze LL, Pandey S, Ravula S, Pandey S, Zhao H, Baker GA, Baker SN. ACS Sustainable Chem. Eng. 2014;2:2117–2123. [Google Scholar]
- 79.Uma Maheswari A, Palanivelu K. Ind. Eng. Chem. Res. 2015;54:11383–11392. [Google Scholar]
- 80.Garcia JI, Garcia-Marin H, Pires E. Green Chem. 2014;16:1007–1033. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.