This study tested topiramate and aripiprazole alone and in combination. The results replicate past findings and suggest that topiramate may be an effective treatment for alcohol use disorder. The present results suggest that the combination of topiramate and aripiprazole do not warrant further evaluation.
Abstract
Aims
The goal of this study was to evaluate the efficacy of topiramate up to 200 mg/day and of aripiprazole up to 15 mg/day, alone and combined, in reducing alcohol-related outcomes in a human laboratory study.
Method
This was a 5 week, between-subject, double-blind, placebo-controlled human laboratory study with topiramate [0 mg/day (placebo), 100 mg/day, 200 mg/day] and aripiprazole [0 mg/day (placebo), 7.5 mg/day, 15 mg/day] in 90 non-treatment seeking, heavy drinking, alcohol-dependent individuals. Main outcomes were the efficacy of 200 mg/day topiramate and 15 mg/day aripiprazole, alone and combined, in reducing drinks consumed during an alcohol self-administration procedure (human laboratory phase) and while receiving the study medications prior to the laboratory session (naturalistic drinking phase). Other outcomes in the laboratory phase included alcohol craving, and alcohol biphasic effects.
Results
In the human laboratory phase, topiramate 200 mg/day reduced alcohol craving [**P < 0.01] and amplified alcohol-induced stimulation [*P < 0.05], but did not reduce the number of drinks consumed. Topiramate 200 mg/day was also effective in reducing drinking days [*P < 0.05], and alcohol craving [*P < 0.05], in the naturalistic drinking phase. No significant findings were found for aripiprazole for any of the outcomes analyzed.
Conclusion
Participants receiving 200 mg/day topiramate reported reduced alcohol drinking and craving, and increased alcohol-related stimulation. These findings provide further support for the role of topiramate as a pharmacological treatment for AUD.
ClinicalTrial.gov Identifier
Short Summary
This study tested topiramate and aripiprazole alone and in combination. The results replicate past findings and suggest that topiramate may be an effective treatment for alcohol use disorder. The present results suggest that the combination of topiramate and aripiprazole do not warrant further evaluation.
INTRODUCTION
Combining medications with different mechanisms of action may lead to novel pharmacological approaches to treat patients with alcohol use disorder (AUD) (Lee and Leggio, 2014). Numerous animal and human studies demonstrate that γ-aminobutyric acid (GABA)-ergic, glutamatergic, dopaminergic and serotonergic neurobiological pathways play important roles in the development and maintenance of AUD. Topiramate is an antiepileptic, with several mechanisms of action, including actions on GABA-ergic/glutamatergic pathways (Kenna et al., 2009b). Aripiprazole is an antipsychotic that targets dopamine/serotonin pathways (Kenna, 2003). Thus, the combined administration of these two medications that act on multiple and different pharmacological targets may have additive and possibly even synergisitc effects on alcohol-related outcomes. In fact, topiramate targets GABA-ergic/glutamergic systems to reduce both withdrawal and protracted withdrawal, and facilitate the initiation of alcohol abstinence (Johnson et al., 2004; Ait-Daoud et al., 2006), while aripiprazole targets the dopamine/serotonin systems to reduce alcohol craving and compulsive alcohol-seeking, thereby reducing alcohol consumption (Anton et al., 2008; Haass-Koffler et al., 2014).
Previous randomized clinical trials (RCTs) indicate a role of topiramate in reducing heavy drinking in treatment-seeking patients with AUD (Johnson et al., 2003, 2008). Additionally, a human laboratory study reported that topiramate reduced alcohol drinking during the naturalistic drinking phase (Miranda et al., 2016). As for aripiprazole, a RCT with treatment-seeking patients with AUD indicated that, although there was no difference between aripiprazole and placebo in the primary drinking outcomes, individuals in the aripiprazole group reported more positive subjective treatment effects and less alcohol dependence severity than the placebo group (Anton et al., 2008). In a human laboratory study, aripiprazole was more effective than placebo in reducing alcohol drinking both in the human laboratory and in the naturalistic drinking phases (Voronin et al., 2008).
Taken together, these studies suggest that the individual administration of either topiramate or aripiprazole may be effective in alcohol-related outcomes. Given their different mechanisms of action, combining these medications could result in more beneficial effects on alcohol-related drinking outcomes. This study was designed to test this hypothesis.
Our team previously demonstrated the safety of 300 mg/day topiramate and 30 mg/day aripiprazole combined and co-administered with alcohol (Kenna et al., 2009a). These safety data provided the basis for further investigating the efficacy of the combination of topiramate and aripiprazole. Furthermore, evidence suggests that lower doses of aripiprazole (Martinotti et al., 2007) and topiramate (Johnson et al., 2007; Miranda et al., 2017) are as effective as the higher doses for alcohol dependence, while improving the safety profile and decreasing the risk of attrition. The goal of this study was to investigate the effect of 200 mg/day topiramate and 15 mg/day aripiprazole, alone and in combination, on alcohol drinking assessed in an alcohol self-administration (ASA) experimental session (human laboratory phase) and during the naturalistic drinking phases preceding the ASA.
MATERIALS AND METHODS
Study design
This was a 5 week, between-subject, double-blind, placebo-controlled human laboratory study with topiramate and aripiprazole (N = 90). The study was conducted at Brown University, Providence, RI, USA, at the Center for Alcohol and Addiction Studies (ClinicalTrial.gov: NCT00884884). The study was approved by the local Institutional Review Board and conducted under a Food and Drug Administration (FDA) Investigational New Drug (IND: 70,603).
Eligible non-abstinent, non-treatment seeking, heavy drinking, alcohol-dependent (AD) individuals were randomly assigned to one of the nine cells [0 mg/day (placebo), 100 mg/day, 200 mg/day topiramate; 0 mg/day (placebo), 7.5 mg/day, 15 mg/day aripiprazole]. For inclusion/exclusion criteria, see Supplement (Table S1).
Study drugs and titration schedule
Study medications or matched placebo were prepared as opaque capsules by a local compounding pharmacy. The study medications, dosing by cell and titration by dose, are depicted in SupplementTables S2 and S3. Study medications were initially dispensed at the Week 1 visit, and participants began taking the pills the following day. The study physician monitored side effects to evaluate re-titration of the study dose. Participants remained eligible and included in the analyses if they exceeded thresholds of 7.5 mg/day of aripiprazole and/or 100 mg/day of topiramate. Study medication was inserted in blister packs and adherence was assessed by pill count and self-report. Additionally, capsules contained 25 mg riboflavin as a marker of adherence through UV light analysis of urine sample (Del Boca et al., 1996).
Study procedures
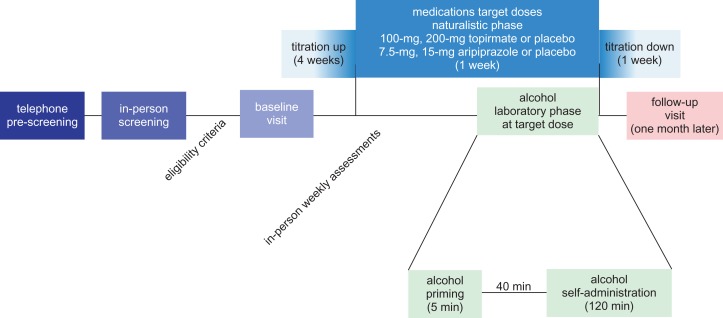
The study consisted of the following phases (Fig. 1): (1) telephone pre-screening, (2) in-person screening, (3) naturalistic phase (5-week outpatient dosing of the study medications), (4) human laboratory phase (ASA experimental session), (5) 1-week tapering of the study medication(s) and (6) 1-month follow-up.
Fig. 1.
Study design. The study included a telephone pre-screening, in-person screening, naturalistic phase (5-week outpatient dosing of the study medications), human laboratory phase (ASA experimental session), 1-week medications down titration and finally 1-month follow-up.
Potential participants were recruited from the community primarily via advertisements in public transportation, websites and mass media. They were first phone screened, and then invited for an in-person screening. At the in-person screening, after breath alcohol concentration was measured and indicated 0.0 g/dl., written informed consent was discussed and obtained. Then, individuals were assessed for eligibility by psychological questionnaires, including the Structured Clinical Interview for DSM Disorders (SCID) version IV-TR, medical history, physical exam, electrocardiogram, clinical laboratory tests (e.g. blood liver and kidney function tests, urine drug and pregnancy tests), and 90-day alcohol consumption history collected using the Timeline Follow-back (TLFB) (Sobell et al., 1988).
If eligible, participants were randomized to receive daily doses of topiramate [0 mg/day (placebo), 100 mg/day or 200 mg/day] and/or aripiprazole [0 mg/day (placebo), 7.5 mg/day or 15 mg/day], titrated over 4 weeks.
Weekly assessments were conducted during the 4 weeks of medication titration and dosing to monitor and address possible adverse events (AEs), study medications compliance and alcohol drinking.
Alcohol administration procedure
The laboratory phase was conducted after 5 weeks of medication(s) dosing, including 1 week at the full-medication dose.
Alcohol priming
Consistent with other studies (O’Malley et al., 2002; Anton et al., 2008; Kenna et al., 2009a, 2014), we included a priming drink that was designed to raise blood alcohol concentrations (BACs) to 0.03 g/dl (Watson, 1989), followed by the ASA phase.
The priming drink was consumed within 5 min. At 10-, 30- and 40-min time-points, breath alcohol content (BrAC) and vital signs were taken and participants rated their alcohol craving and subjective responses to alcohol, as detailed in the study outcomes and assessments session below.
Alcohol self-administration
About 40-min after consuming the priming drink, a tray containing four drinks was offered. The volume of each drink was designed to raise BAC by 0.015 g/dl. Sixty minutes later, the first tray was removed and another tray of four drinks was presented. The participant could choose to drink any or all eight drinks. The equivalent cost of a drink in a bar ($3.00) was provided as an alternative reinforcer for each mini-drink not consumed. Every 30 min, BrAC and vital signs were taken and participants rated their alcohol craving and possible AEs, as detailed below.
Study outcomes and assessments
Drinking outcomes
The primary outcomes, as detailed in the protocol, were the efficacy of 200 mg/day of topiramate and 15 mg/day of aripiprazole compared to placebo, both alone and combined, in reducing the number of drinks during the human laboratory phase. We also measured self-reported alcohol drinking during the naturalistic drinking phase (5-week outpatient dosing of the study medications) preceding the human laboratory phase. Standard Drinking Units (SDUs) were used to express drinks consumed during both the human laboratory and naturalistic phases. Drinking data were collected using the TLFB (Sobell et al., 1996) and the potential effect of the study medication(s) on the SDUs per day, reported as Drinking Day (DD), was analyzed based on our pilot safety study (Kenna et al., 2009a). The lower doses of topiramate (100 mg/day) and aripiprazole (7.5 mg/day) were also explored to determine similar efficacy, with reduction of AEs both in the human laboratory phase and in the naturalistic phase.
Craving outcomes
Alcohol craving was assessed by the Alcohol Urge Questionnaire (AUQ) (Bohn et al., 1995) administered at baseline, at Week 3 and at Week 5 during the naturalistic phase. In the human laboratory phase, AUQ was measured after the priming drink.
Alcohol-related subjective effects
Secondary outcomes were alcohol-related subjective effects for topiramate and aripiprazole, alone and combined. The Biphasic Alcohol Effects Scale (BAES) was utilized to measure alcohol-related subjective effects, i.e. stimulation within 10 min after priming drink and sedation after 10 min after priming drink (Martin et al., 1993).
Severity of alcohol dependence
The severity of alcohol dependence was assessed at baseline by the Alcohol Dependence Scale (ADS) (Skinner and Allen, 1982) and used as a direct measure of severity of dependence.
Profile mood state and adverse events
Profile mood state (POMS) (Pollock et al., 1979) was administered at every visit and used to assess safety during the trial. Clinical and laboratory assessments were used to identify other AEs.
Statistical analyses
In the preliminary analyses, we examined the presence of attrition bias on demographics and other baseline variables. A second set of preliminary analyses examined any bias in the randomization procedure on these same variables of the study-retained participants. Distributional characteristics of outcome measures were examined to evaluate similarity to the normal distribution. We found a randomization bias for topiramate on the ADS. Those receiving topiramate had greater alcohol dependence. Therefore, in all outcome analyses ADS was entered as a covariate. The baseline value of the respective outcome measured was also entered as a covariate. The two primary outcome variables, drinking days (DD) the week before the laboratory session, and drinks consumed after the priming drink during the laboratory session were subjected to a square root transformation to improve distributional characteristics.
Our statistical strategy parallels the approach used in the COMBINE study (Anton et al., 2006), where effects for naltrexone and acamprosate were tested in separate statistical models. We utilized a full 3 × 3 design to analyze dose response for both topiramate and aripiprazole. The single medication effect and the interaction between topiramate and aripiprazole was analyzed by collapsing the two doses of each medication and performing separate 2 × 2 design using each medication separately and their interaction as covariate in the model.
Secondary outcome measures were analyzed as described above. We included the AUQ scale that measures alcohol craving and the biphasic alcohol effects scale (BAES) that assesses the stimulation and sedation response. Given the multiple time-points, these DVs were analyzed using mixed model analysis, which accommodates missing data.
All other AEs items were analyzed by collapsing the doses of each medication and performing separate 2 × 2 analysis of variance (ANOVAs) for each item. All statistical tests were two-sided, and statistical significance was accepted if a P-value <0.05 was obtained.
All results are reported in M ± SEM with exception of demographic, which are reported in M ± SD or percentage (%). Cohen’s f was calculated for all effect sizes. The analyses were conducted using SPSS (v.22) (Armonk, NY, USA) and GraphPad Prism (v.7) was used to generate figures (La Jolla, CA, USA).
RESULTS
Participants’ characteristics and retention
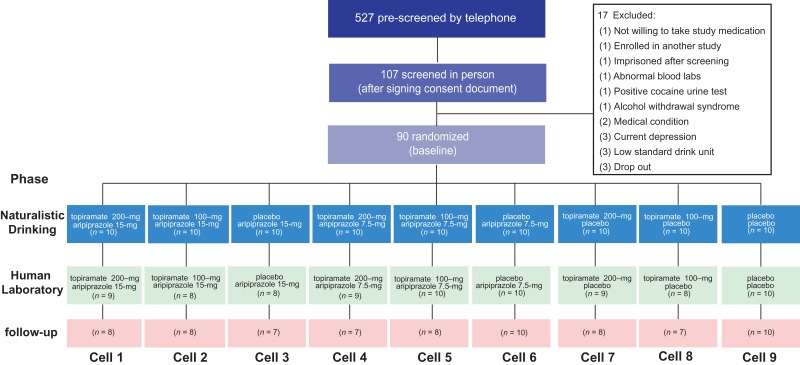
The CONSORT diagram is depicted in Fig. 2.
Fig. 2.
CONSORT diagram showing the flow of participants through each phase of the study.
No differences between groups were found for gender, ethnicity, race, age, body mass index (BMI), age of onset, baseline DD or CIWA-Ar [P’s > 0.05] (Table 1).
Table 1.
Demographic and baseline characteristics of randomized and retained at the laboratory phase sample [M ± (SD) or percentage (%)]
| Enrolled | Completers (i.e. they performed the laboratory phase) | |
|---|---|---|
| Number (n) | 90 | 81 |
| Female (%) | 33 | 32 |
| Hispanic (%) | 10 | 9 |
| White (%) | 68 | 67 |
| Age | 40.6 ± 11.9 | 39.9 ± 12.2a |
| BMI | 29.0 ± 6.0 | 29.2 ± 6.1 |
| Onset | 25.1 ± 10.4 | 25.4 ± 10.4 |
| Baseline DDb | 20.8 ± 6.0 | 20.4 ± 6.0c |
| ADS | 10.4 ± 6.6 | 10.5 ± 6.7 |
| CIWA-Ar | 0.80 + 1.07 | 0.75 + 1.01 |
ADS, alcohol dependence scale; BMI, body mass index; CIWA-Ar, Clinical Institute Withdrawal Assessment for Alcohol—revised (CIWA-Ar); DD, drinks per day.
aYounger participants were more likely to be retained [attriters: 46.9 ± 6.3 years old, t(16) = 2.80, *P = 0.013].
bBaseline drinking days were calculated for a 90-day baseline period.
cLess frequent drinkers were more likely to be retained [attriters DD: 24.8 ± 4.1, t(88) = 2.16, *P = 0.033].
The least tolerated dose was 100 mg of topiramate, with 77% of those assigned to this medication completing this condition. No differences in retention were found among all cells [P > 0.05].
The full summary of the drinking, craving and alcohol-related outcomes in the laboratory and naturalistic phase are reported on Table 2.
Table 2.
Summary of drinking, craving and alcohol-related outcomes analysis
| Phase | Laboratory | Naturalistic | Statistical model | |||||
|---|---|---|---|---|---|---|---|---|
| Drinking outcomes | Number of SDU | DD | ||||||
| Medication | Dose (mg) | n | P | f | n | P | f | |
| Aripiprazole | 15 | 25 | n.s. | – | 30 | n.s. | – | Alone with topiramate as covariate |
| 7.5 | 29 | n.s. | – | 30 | n.s. | – | ||
| Collapsed | 54 | n.s. | – | 60 | n.s. | – | ||
| Topiramate | 200 | 27 | n.s. | – | 30 | 0.048 | 0.36 | Alone with aripiprazole as covariate |
| 100 | 26 | n.s. | – | 30 | n.s. | – | ||
| Collapsed | 53 | n.s. | – | 60 | 0.03 | 0.26 | ||
| Aripiprazole | Collapsed | 54 | n.s. | – | 60 | n.s. | – | Interaction with topiramate |
| Topiramate | Collapsed | 53 | n.s. | – | 60 | 0.015 | 0.26 | Interaction with aripiprazole |
| Craving outcomes | AUQ | AUQ | ||||||
| Medication | Dose (mg) | n | p | f | n | p | f | |
| Aripiprazole | 15 | 25 | n.s. | – | 30 | n.s. | – | Alone with topiramate as covariate and interaction with time |
| 7.5 | 29 | n.s. | – | 30 | n.s. | – | ||
| Collapsed | 54 | n.s. | – | 60 | n.s. | – | ||
| Topiramate | 200 | 27 | 0.04 | 0.36 | 30 | 0.029 | 0.27 | Alone with aripiprazole as covariate |
| 200 | 27 | n.s. | – | 30 | 0.013 | 0.31 | Alone with aripiprazole as covariate and interaction with time | |
| 100 | 26 | n.s. | – | 30 | n.s. | – | Alone with aripiprazole as covariate and interaction with time | |
| Collapsed | 53 | n.s. | – | 60 | n.s. | – | ||
| Aripiprazole | Collapsed | 54 | n.s. | – | 60 | n.s. | – | Interaction with topiramate and time |
| Topiramate | Collapsed | 53 | n.s. | – | 60 | n.s. | – | Interaction with aripiprazole and time |
| Alcohol-related outcomes | BAES Stimulation/sedation | |||||||
| Medication | Dose (mg) | n | p | f | n | p | f | |
| Aripiprazole | 15 | 25 | n.s./n.s. | – | – | – | – | Alone with topiramate as covariate and interaction with time |
| 7.5 | 29 | n.s./n.s. | – | – | – | – | ||
| Collapsed | 54 | n.s./n.s. | – | – | – | – | ||
| Topiramate | 200 | 27 | 0.041/n.s. | 0.25 | – | – | – | Alone with aripiprazole as covariate and interaction with time |
| 100 | 26 | n.s./n.s. | – | – | – | – | ||
| Collapsed | 53 | n.s./n.s. | – | – | – | – | ||
| Aripiprazole | Collapsed | 54 | n.s./n.s. | – | – | – | – | Interaction with topiramate and time |
Source: AUQ, Alcohol Urge Questionnaire; BAES, Biphasic Alcohol Effect Scale; DD, Drinking Days; SDU, Standard Drink Unit; n.s.: no significant. Bold values indicate statistical significance.
Drinking outcomes
Human laboratory phase
No significant findings were found for topiramate or aripiprazole, alone or in combination, for any of the ASA outcomes analyzed.
Naturalistic phase
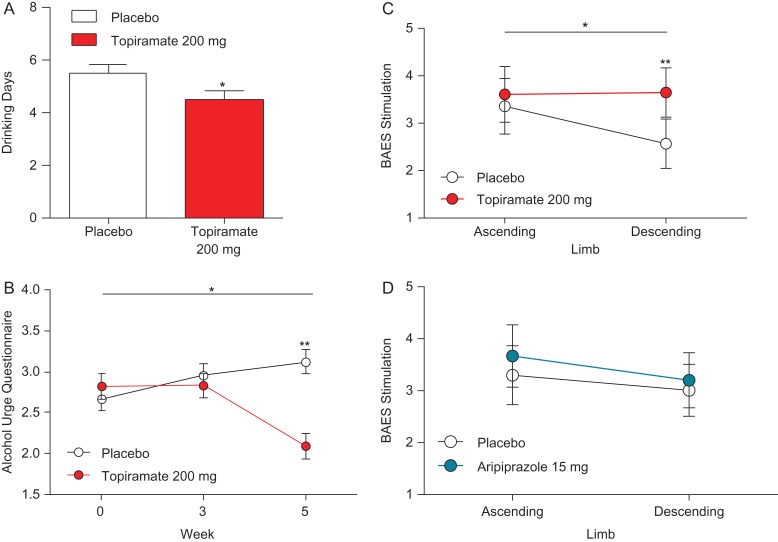
There was a significant effect for topiramate 200 mg/day, compared to placebo, on DD [*P < 0.05] (Fig. 3A). The main effect of topiramate was also observed when the two doses were collapsed [*P < 0.05], and in combination aripiprazole [*P < 0.05]. There was no main effect for aripiprazole alone or in combination with topiramate [P > 0.05] for DD.
Fig. 3.
Topiramate 200 mg/day effect for drinking days (DD), alcohol craving during the naturalistic drinking phase and effects on alcohol craving and on BAES in the laboratory. In the naturalistic phase, (A) there was a significant main effect for 200 mg/day topiramate [F(1,29) = 9.44, *P = 0.042, f = 0.26]. In the laboratory before alcohol prime, (B) there was a 200 mg/day topiramate main effect on AUQ [F(1,35) = 5.21, *P = 0.029, f = 0.27], there was also a 200 mg/day topiramate × time interaction [F(1,33) = 6.93, *P = 0.013, f = 0.31]. In the laboratory after alcohol prime, there was a significant 200 mg/day topiramate × time interaction [F(1,35) = 4.51, *P = 0.041, f = 0.25]. Post hoc testing indicated no difference between the two groups on the descending limb [t(34) = 1.47, P > 0.05]. (D) There was no 15 mg/day aripiprazole × time for subscale stimulation score and no significant differences between groups in the subscale sedation score [P’s ns]. Results are reported as M and error bars indicate SEM; not significant [P > 0.05].
Craving outcomes
Naturalistic phase
topiramate 200 mg/day was effective in reducing alcohol craving only when the medication reached target dose. We found a topiramate 200 mg/day main effect for AUQ [*P < 0.05] and a topiramate 200 mg/day × time interaction [*P < 0.05] (Fig. 3B). Time was represented by Week 0, 3 and 5, including the titration period. There were no significant effects on alcohol craving for aripiprazole, aripiprazole × time and topiramate × time interaction [P’s > 0.05].
Human laboratory phase
At Week 5, there was an effect for AUQ prior to the alcohol prime, when 200 mg/day topiramate was compared to placebo [**P = 0.01] (Fig. 3B). However, topiramate or aripiprazole, alone or combined, had no effect on craving after the alcohol priming at any time-points (every 10 min) during the alcohol ascending and descending limbs [P’s > 0.05].
Alcohol-related outcomes in the human laboratory phase
The stimulant BAES subscale scores, assessed after the priming drink, before the ASA, showed a significant topiramate 200 mg/day × time interaction on stimulation [*P < 0.05]. Post hoc testing revealed significant differences between placebo and topiramate 200 mg/day [**P < 0.001] but not between placebo and aripiprazole 15 mg/day [P > 0.05] (Fig. 3C). There was no aripiprazole × time interaction for stimulation and no significant differences between groups in the sedation subscale score [P’s > 0.05] (Fig. 3D).
Profile mood state and adverse events
In the POMS scale, three items in the impairment and one item in the mood category showed significant differences, all with lower effects for aripiprazole than placebo [difficulty with concentration: F(1,76) = 4.13, P < 0.05; memory difficulties: *P < 0.05; confusion: F(1,76) = 7.99, **P < 0.01 and irritability: F(1,76) = 4.72, *P < 0.05]. When we examined medication effects before, immediately after, and 30-min after the priming alcohol drink, we found that both in the pre-priming and after priming there was an aripiprazole effect on fatigue in the POMS subscale [pre-priming: F(1,77) = 4.31, *P < 0.05; post-priming: F(1,77) = 4.22, *P < 0.05], with aripiprazole associated with greater fatigue. There was no significant difference in fatigue after 30-min, following the priming [P > 0.05] and no significant differences for topiramate or placebo for any POMS subscale [P’s > 0.05].
There was a significant main effect for topiramate [F(1,37) = 6.07, *P < 0.05] in decreasing BMI. There were no serious adverse events (SAEs) during the entire study.
DISCUSSION
In this study, we aimed to assess the effect of topiramate and aripiprazole, alone and in combination, on alcohol drinking outcomes in a human laboratory study. We found no effect of combining topiramate and aripiprazole; however, our results indicate that topiramate alone, but not aripiprazole, may be effective for reducing alcohol drinking. The main findings of this study were that topiramate at 200 mg/day reduced alcohol consumption and lessened alcohol craving in the naturalistic phase. It also increased alcohol-related stimulation on the descending limb during the alcohol laboratory phase. Aripiprazole, both as an individual medication and in combination with topiramate, did not affect alcohol drinking or craving in the naturalistic phase or in the human laboratory phase. In the laboratory phase, aripiprazole did not alter the biphasic effects of alcohol. The lack of aripiprazole efficacy is consistent with the negative results of the large multi-site RCTs (Anton et al., 2008). The lack of aripiprazole effect in our study could be potentially explained by evidence that aripiprazole may be better suited for individuals with an alcohol dependence and mood or psychotic disorder comorbidity, due to its mood stabilizing and anti-depressive properties (Janiri et al., 2007). Furthermore, a recent review has suggested that aripiprazole may be most effective at lower dosages (e.g. 5 mg), such that the anti-depressive properties of aripiprazole can be observed (Martinotti et al., 2016). On the other hand, this study provides additional evidence supporting a role for topiramate in AD patients. Consistent with our pilot safety study (Kenna et al., 2009a), participants in this trial did not report any serious AEs either during the human laboratory phase, when the medications were coadministered with alcohol, or in naturalistic drinking phase, when the medications were titrated up to target dose and participants were still drinking alcohol.
Topiramate did not reduce the amount of alcohol consumed in the human laboratory phase, for which several reasons can be hypothesized. First, the ASA paradigm applies a behavioral, economic approach (for review, see Mackillop, 2016), where alcohol is considered a merchandise whose reinforcing value is estimated by the cost that a buyer is prepared to pay. As such, the ASA paradigm evaluates a discrete aspect of alcohol-driven behavior (Mackillop et al., 2010). Second, it is possible that ASA could be better at measuring impulse control, or other similar phenotypes, that explain alcohol use versus actual change in the number of drinks, per se. Third, we cannot rule out the possibility that there is a difference in drinking behaviors between the self-reported alcohol drinking during the naturalistic phase and the alcohol drinking during the ASA in the human laboratory phase. This difference in behaviors may create a floor effect during the ASA session, preventing a difference in findings between the two groups during the two phases. It is important to highlight that the environment plays a strong role in alcohol consumption and many factors contribute to excessive drinking behavior in AD individuals, and perhaps the ASA paradigm did not capture these other factors. Contrary to a recent study (Anton et al., 2017), we did not find aripiprazole to affect any of the alcohol variables. Specifically, in this study we found that aripiprazole reduced alcohol consumption in the laboratory only in individuals with low self-control and increased latency to consume alcohol in individuals with high impulsivity (Anton et al., 2017). Potential reasons for the discrepancy between studies may relate to differences in the laboratory paradigm and in the assessments procedures. We did not use a forced choice, between immediate drinking reward and we did not assess impulsivity and self-control in our population.
In the naturalistic phase, topiramate 200 mg/day reduced the quantity of alcohol consumed before the human laboratory phase. This result is consistent with previous human laboratory studies with higher target doses (up to 300 mg/day) (Miranda et al., 2008, 2016), which reported that topiramate might be already effective at 200 mg/day. Furthermore, the larger and longer RCT with topiramate (Johnson et al., 2007) showed that topiramate’s therapeutic effect was already noticeable during the titration period (at 200 mg/day); however, these results cannot be confirmed since topiramate was then titrated up to 300 mg.
We hypothesized that, in the naturalistic phase, topiramate affects drinks per day by reducing alcohol craving. This is consistent with the effects of topiramate observed during the titration period in other studies (Johnson et al., 2008). Changes in drinking were accompanied by changes in weekly reports of craving for alcohol only after the 3-week period, when the target dose was reached. These results are consistent with a previous RCT with treatment-seeking alcohol-dependent individuals (Johnson et al., 2008) and with a human laboratory study that assessed craving in naturalistic condition using ecological momentary assessment (EMA) methods, which showed that topiramate reduced craving in a naturalistic environment (Miranda et al., 2016).
The lack of topiramate’s efficacy in reducing alcohol craving in the presence of alcohol cues is consistent with previous studies (Miranda et al., 2008). In our study, we used a priming drink designed to raise BACs to 0.03 g/dl (Watson, 1989), while Miranda et al. administered a drink to reach a BAC level of 0.06 g/dl (Miranda et al., 2008). Our results indicate that topiramate may be more effective in reducing craving before the actual alcohol priming occurs, i.e. in anticipation of a subsequent planned alcohol drinking. As such, one methodological consideration would be to measure craving before the priming session. Taken together, these data suggest that topiramate is not effective in reducing alcohol craving in the laboratory in the presence of cues and/or moderate amounts of alcohol. However, this effect was only seen for alcohol, and there may be craving differences when individuals are primed with different types of substances.
In the human laboratory phase, topiramate at 200 mg/day increased alcohol-related stimulation effects on the descending limb, without affecting alcohol-related sedation. This effect is not consistent with a previous pilot study that tested both 200 mg and 300 mg topiramate doses (Miranda et al., 2008), and reported that the 200 mg/day dose, but not the 300 mg/day dose, was able to reduce the stimulation, but not the sedation effects of alcohol. Our findings, however, stand also in contrast to the negative results on alcohol-induced stimulation reported later (Miranda et al., 2016). Potential reasons for the discrepancy between these studies may relate to differences in the experimental procedures. In the human laboratory studies reported here, and previously by Miranda et al. (2008), we measured alcohol-stimulation effects in real-time, by administering a fixed priming dose of alcohol in the laboratory. In the later study (Miranda et al., 2016), the alcohol-related effects were collected, retrospectively, in the naturalistic environment. Taken together, these results, however, consistently suggested that of three topiramate doses tested in previous studies (Miranda et al., 2008), and in the present study, the 200 mg/day dose, did not affect sedation. Our results should be, however, considered with caution. First, as for the topiramate pilot study (Miranda et al., 2008), our results also reported effects relatively small in magnitude, suggesting that these findings do not fully explain the effects of topiramate on alcohol intoxication. Furthermore, both studies reported topiramate’s effect studied only at estimated peak BrAC, rather than a complete PK/PD-modeling (Haass-Koffler et al., 2015) that would link alcohol concentration (pharmacokinetics) and alcohol effects (pharmacodynamics) during the complete alcohol ascending/descending phases.
This study provides both scientifically and clinically important insight for the use of topiramate in AD individuals; however, this study has several limitations. First, the number of participants for each cell was small. Nonetheless, the effect size of the significant results was all ranging from medium to large. Second, as seen with many alcohol laboratory studies, our enrolled participants do not entirely represent the general population. For instance, our participants were not seeking treatment and, therefore, our sample may not reflect the type of patients who would seek help for their alcohol use disorder. Notably, recent clinical work points to some potentially important phenotypic differences between non-treatment-seeking vs. treatment-seeking individuals with alcohol dependence (Ray et al., 2017; Rohn et al., 2017). Then, reliance on TLFB data for assessing amount of alcohol consumed in the naturalistic phase of the study may be problematic without biochemical or ecological momentary assessment validation. Finally, the fictitious naturalistic environment created in our human laboratory, and the temporal sequence of drinking procedures designed in the alcohol paradigm, may be insufficient to reflect the real-life settings where AD individuals consume alcohol. Given recent pharmacogenetic findings on topiramate (Kranzler et al., 2014; Feinn et al., 2016), future pharmacogenetic studies may be helpful to further understand its role in AUD.
In conclusion, this study found no evidence supporting that the combination of topiramate and aripiprazole, or aripiprazole alone, has any effect on drinking outcomes. The results from our study do suggest, however, that topiramate remains a promising and effective medication for individuals with AUD.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Dr Molly Magill for her statistical advice.
SUPPLEMENTARY MATERIAL
Supplementary data are available at Alcohol and Alcoholism online.
FUNDING
This study was funded by the National Institute on Alcohol Abuse and Alcoholism (NIAAA), Grant R01AA015753 (PI: Swift). Dr Haass-Koffler’s work is currently supported by the NIAAA K01AA023867 and, previously, by the 5T32AA007459 training grant. Dr Goodyear is funded by the 5T32AA007459 training grant. Dr Leggio’s current work is supported by the NIAAA Division of Intramural Clinical and Biological Research and the National Institute on Drug Abuse Intramural Research Program.
CONFLICT OF INTEREST STATEMENT
Dr Swift has received travel and honorarium from D&A Pharma, Lundbeck and consultant fees from CT Laboratories. The other authors report no biomedical financial interests or potential conflicts of interest.
REFERENCES
- Ait-Daoud N, Malcolm RJ Jr, Johnson BA (2006) An overview of medications for the treatment of alcohol withdrawal and alcohol dependence with an emphasis on the use of older and newer anticonvulsants. Addict Behav 31:1628–49. [DOI] [PubMed] [Google Scholar]
- Anton RF, Kranzler H, Breder C, et al. (2008) A randomized, multicenter, double-blind, placebo-controlled study of the efficacy and safety of aripiprazole for the treatment of alcohol dependence. J Clin Psychopharmacol 28:5–12. [DOI] [PubMed] [Google Scholar]
- Anton RF, O’Malley SS, Ciraulo DA, et al. (2006) Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA 295:2003–17. [DOI] [PubMed] [Google Scholar]
- Anton RF, Schacht JP, Voronin KE, et al. (2017) Aripiprazole suppression of drinking in a clinical laboratory paradigm: influence of impulsivity and self-control. Alcohol Clin Exp Res 41:1370–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn MJ, Krahn DD, Staehler BA (1995) Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol Clin Exp Res 19:600–6. [DOI] [PubMed] [Google Scholar]
- Del Boca FK, Kranzler HR, Brown J, et al. (1996) Assessment of medication compliance in alcoholics through UV light detection of a riboflavin tracer. Alcohol Clin Exp Res 20:1412–7. [DOI] [PubMed] [Google Scholar]
- Feinn R, Curtis B, Kranzler HR (2016) Balancing risk and benefit in heavy drinkers treated with topiramate: implications for personalized care. J Clin Psychiatry 77:e278–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass-Koffler CL, Leggio L, Davidson D, et al. (2015) Effects of idazoxan on alcohol pharmacokinetics and intoxication: a preliminary human laboratory study. Alcohol Clin Exp Res 39:594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass-Koffler CL, Leggio L, Kenna GA (2014) Pharmacological approaches to reducing craving in patients with alcohol use disorders. CNS Drugs 28:343–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janiri L, Martinotti G, Di Nicola M (2007) Aripiprazole for relapse prevention and craving in alcohol-dependent subjects: results from a pilot study. J Clin Psychopharmacol 27:519–20. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N, Akhtar FZ, et al. (2004) Oral topiramate reduces the consequences of drinking and improves the quality of life of alcohol-dependent individuals: a randomized controlled trial. Arch Gen Psychiatry 61:905–12. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N, Bowden CL, et al. (2003) Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet 361:1677–85. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Rosenthal N, Capece JA, et al. Topiramate for Alcoholism Advisory Board & Topiramate for Alcoholism Study Group (2008) Improvement of physical health and quality of life of alcohol-dependent individuals with topiramate treatment: US multisite randomized controlled trial. Arch Intern Med 168:1188–99. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Rosenthal N, Capece JA, et al. Topiramate For Alcoholism Advisory Board & Topiramate For Alcoholism Study Group (2007) Topiramate for treating alcohol dependence: a randomized controlled trial. JAMA 298:1641–51. [DOI] [PubMed] [Google Scholar]
- Kenna GA. (2003) Rationale for use of aripiprazole for alcohol dependence treatment. Drugs Future 12:1227. [Google Scholar]
- Kenna GA, Leggio L, Swift RM (2009. a) A safety and tolerability laboratory study of the combination of aripiprazole and topiramate in volunteers who drink alcohol. Hum Psychopharmacol 24:465–72. [DOI] [PubMed] [Google Scholar]
- Kenna GA, Lomastro TL, Schiesl A, et al. (2009. b) Review of topiramate: an antiepileptic for the treatment of alcohol dependence. Curr Drug Abuse Rev 2:135–42. [DOI] [PubMed] [Google Scholar]
- Kenna GA, Zywiak WH, Swift RM, et al. (2014) Ondansetron reduces naturalistic drinking in nontreatment-seeking alcohol-dependent individuals with the LL 5’-HTTLPR genotype: a laboratory study. Alcohol Clin Exp Res 38:1567–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Covault J, Feinn R, et al. (2014) Topiramate treatment for heavy drinkers: moderation by a GRIK1 polymorphism. Am J Psychiatry 171:445–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MR, Leggio L (2014) Combined pharmacotherapies for the management of alcoholism: rationale and evidence to date. CNS Drugs 28:107–19. [DOI] [PubMed] [Google Scholar]
- Mackillop J. (2016) The behavioral economics and neuroeconomics of alcohol use disorders. Alcohol Clin Exp Res 40:672–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackillop J, O’hagen S, Lisman SA, et al. (2010) Behavioral economic analysis of cue-elicited craving for alcohol. Addiction 105:1599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, et al. (1993) Development and validation of the biphasic alcohol effects scale. Alcohol Clin Exp Res 17:140–6. [DOI] [PubMed] [Google Scholar]
- Martinotti G, Di Nicola M, Janiri L (2007) Efficacy and safety of aripiprazole in alcohol dependence. Am J Drug Alcohol Abuse 33:393–401. [DOI] [PubMed] [Google Scholar]
- Martinotti G, Orsolini L, Fornaro M, et al. (2016) Aripiprazole for relapse prevention and craving in alcohol use disorder: current evidence and future perspectives. Expert Opin Investig Drugs 25:719–28. [DOI] [PubMed] [Google Scholar]
- Miranda R Jr, Mackillop J, Monti PM, et al. (2008) Effects of topiramate on urge to drink and the subjective effects of alcohol: a preliminary laboratory study. Alcohol Clin Exp Res 32:489–97. [DOI] [PubMed] [Google Scholar]
- Miranda R Jr, Mackillop J, Treloar H, et al. (2016) Biobehavioral mechanisms of topiramate’s effects on alcohol use: an investigation pairing laboratory and ecological momentary assessments. Addict Biol 21:171–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda R Jr, Treloar H, Blanchard A, et al. (2017) Topiramate and motivational enhancement therapy for cannabis use among youth: a randomized placebo-controlled pilot study. Addict Biol 22:779–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley SS, Krishnan-Sarin S, Farren C, et al. (2002) Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology (Berl) 160:19–29. [DOI] [PubMed] [Google Scholar]
- Pollock V, Cho DW, Reker D, et al. (1979) Profile of mood states: the factors and their physiological correlates. J Nerv Ment Dis 167:612–4. [DOI] [PubMed] [Google Scholar]
- Ray LA, Bujarski S, Yardley MM, et al. (2017) Differences between treatment-seeking and non-treatment-seeking participants in medication studies for alcoholism: do they matter? Am J Drug Alcohol Abuse 43:703–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohn MC, Lee MR, Kleuter SB, et al. (2017) Differences between treatment-seeking and nontreatment-seeking alcohol-dependent research participants: an exploratory analysis. Alcohol Clin Exp Res 41:414–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HA, Allen BA (1982) Alcohol dependence syndrome: measurement and validation. J Abnorm Psychol 91:199–209. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Brown J, Leo GI, et al. (1996) The reliability of the alcohol timeline followback when administered by telephone and by computer. Drug Alcohol Depend 42:49–54. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, Leo GI, et al. (1988) Reliability of a timeline method: assessing normal drinkers’ reports of recent drinking and a comparative evaluation across several populations. Br J Addict 83:393–402. [DOI] [PubMed] [Google Scholar]
- Voronin K, Randall P, Myrick H, et al. (2008) Aripiprazole effects on alcohol consumption and subjective reports in a clinical laboratory paradigm—possible influence of self-control. Alcohol Clin Exp Res 32:1954–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson PE. (1989) Total body water and blood alcohol levels: updating the fundamentals. Hum Metabol Alcohol 1:41–58. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.