Abstract
Growing evidence suggests that glutamate neurotransmission plays a critical role in alcohol addiction. Cue-induced change of glutamate has been observed in animal studies but never been investigated in humans. This work investigates cue-induced change in forebrain glutamate in individuals with alcohol use disorder (AUD). A total of 35 subjects (17 individuals with AUD and 18 healthy controls) participated in this study. The glutamate concentration was measured with single-voxel 1H-MR spectroscopy at the dorsal anterior cingulate. Two MRS sessions were performed in succession, the first to establish basal glutamate levels and the second to measure the change in response to alcohol cues. The changes in glutamate were quantified for both AUD subjects and controls. A mixed model ANOVA and t-tests were performed for statistical analysis. ANOVA revealed a main effect of cue-induced decrease of glutamate level in the anterior cingulate cortex (ACC). A significant interaction revealed that only AUD subjects showed significant decrease of glutamate in the ACC. There were no significant group differences in the level of basal glutamate. However, a negative correlation was found between the basal glutamate level and the number of drinking days in the past 2 weeks for the AUD subjects. Collectively, our results indicate that glutamate in key areas of the forebrain reward circuit is modulated by alcohol cues in early alcohol dependence.
INTRODUCTION
With a prevalence of lifetime alcohol abuse of 17.8% and a prevalence of lifetime alcohol dependence of 12.5%, alcohol use disorder (AUD) not only impacts a significant number of Americans but also impacts both the economy and healthcare (Hasin et al., 2007). A better understanding of the neural substrates of AUD may prove to be important in improving treatment. Although evidence suggests that alcohol dependence involves several major neurotransmitter systems, including dopamine, gamma-aminobutyric acid (GABA) and glutamate (Clapp et al., 2008; Hillmer et al., 2015), the glutamatergic system is sensitive to acute and chronic alcohol exposure and is believed to play a critical role in cue- and alcohol-induced reinstatement of drinking during withdrawal (Clapp et al., 2008; Fliegel et al., 2013). As a result, glutamate has become a key pharmacological target for the treatment of AUD (Rao et al., 2015).
The majority of research investigating the relationship between brain glutamate and alcohol addiction has focused on animal models. Increased glutamate from acute and repeated ethanol exposure was found at various brain regions including the prefrontal cortex, and the striatum (nucleus accumbens (NAc), caudate and putamen) (Fliegel et al., 2013). Significant enhancements of extracellular glutamate levels were also associated with acute ethanol withdrawal (Fliegel et al., 2013). Moreover, systemically administered glutamate receptor antagonists attenuated cue-induced reinstatement of alcohol-seeking behavior (Adams et al., 2008) and pharmacological manipulations that enhance the mechanisms responsible for removing glutamate after its release also decreased alcohol intake in alcohol-preferring rats (Sari et al., 2011). Together, these results suggest that glutamate plays a role in alcohol-seeking behaviors.
Studies of alcohol-dependent (AD) humans have primarily focused on glutamate changes during withdrawal (Hillmer et al., 2015). Magnetic resonance spectroscopy (MRS) is used to measure glutamate in humans. Although it is generally hard to resolve the MRS peaks of glutamate and glutamine at lower magnetic field, quantified glutamate concentration with high accuracy and repeatability can be achieved at 3 T or higher (Schubert et al., 2004; Hancu, 2009; Wijtenburg and Knight-Scott, 2011). As the key components in the reward/motivation circuit, the anterior cingulate cortex (ACC) and the NAc have been the focus of these MRS studies. Yeo et al. (2013) reported a trend for higher glutamate + glutamine (Glx) levels in the ACC of AD participants relative to controls. A glutamate increase in the ACC was also found during acute alcohol withdrawal (Hermann et al., 2012). However, Bauer et al. (2013) failed to show glutamate differences in the ACC after detoxification but did find increases in glutamate in the NAc. In contrast, one study reported lower concentration of glutamate in the ACC for the alcohol dependence subjects as compared to light/non-drinking controls (Mon et al., 2012). Prisciandaro et al. (2016) found a negative correlation between the glutamate concentration at dorsal ACC and the number of heavy drinking days in the 14 days preceding the MRS scan in non-treatment-seeking individuals with alcohol dependence. Prolonged withdrawal was also reported to decrease glutamate in medial frontal cortex (Thoma et al., 2011) and occipital cortex (Bagga et al., 2014). Thus, although glutamate changes have been recorded in both the ACC and NAc in AD individuals, an increase or decrease appears to depend on brain region and duration of withdrawal.
While glutamate changes during withdrawal are of great research interest, its role in alcohol relapse is another intriguing topic. Approximately 90% of individuals with AUD experience at least one relapse within the first 4 years of treatment (Polich et al., 1981). The relapse rate is even higher without treatment (Moos and Moos, 2006). Relapse to alcohol seeking is triggered by stress, alcohol-related cues and alcohol itself (Katner et al., 1999; Maccioni et al., 2007; Sinha and Li, 2007). Many fMRI studies have shown that cue-induced alcohol craving is linked to activation in multiple forebrain regions. For instance, brain activity in the left NAc, left orbitofrontal cortex, and ACC was significantly correlated with subjective craving ratings in AD but not control subjects (Myrick et al., 2004). In addition, the ACC, and adjacent medial prefrontal cortex were activated in response to alcohol-related visual stimuli versus neutral stimuli in alcoholics (Grüsser et al., 2004). Moreover, the intensity of cue-induced activation of these brain areas predicted subsequent relapse (Grüsser et al., 2004). Similar findings were reported for addiction to nicotine and illicit drugs (Bonson et al., 2002; Wilson et al., 2005). Interestingly, the regions found to respond to alcohol cues overlap with those involved in the glutamatergic signaling system related to addiction, namely, the anterior cingulate and the ventral striatum. However, none of these studies have examined the underlying neurotransmitter changes related to the cues in humans. How the glutamate system interacts with craving effects over the course of alcohol dependence remains unknown in humans. The aim of this study is to explore dynamic changes of glutamate associated with craving in the anterior cingulate in a young cohort of individuals with AUD. To this end, 1H-MR spectroscopy (MRS) was employed to measure glutamate level changes in response to alcohol cues. A mixed model ANOVA design was adopted by assessing the basal and alcohol cue-induced glutamate in the ACC of individuals with and without AUD using 1H-MRS.
METHODS
Subjects
The study was approved by the Institutional Review Board of Indiana University. Subjects were recruited from a large ongoing study of decision making in young adults with AUD who had been recruited from the community (Finn et al., 2014). AUD subjects met criteria for moderate or severe AUD based on DSM-V and DSM-IV Alcohol Dependence diagnoses criteria using the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA) (Bucholz et al., 1994); control subjects had no history of AUD. The severity of alcohol problems was indexed as total count of all positive responses to all SSAGA questions in the Alcohol Diagnosis section. This measure has good validity as a measure of severity of alcohol problems in terms of its association with higher levels of other externalizing psychopathology, increased excessive drinking, reduced cognitive function and increased delay discounting (Bucholz et al., 1994; Finn et al., 2014). Recent alcohol consumptions were quantified for the past 2 weeks and 3 months as measures of frequency (number of drinking days in the past 2 weeks and the average number of drinking days per week in the past 3 months) and drinking amount (total number of drinks consumed in the past 2 weeks and average number of drinks per week over the past 3 months). Lifetime alcohol problem counts (APC), marijuana problem counts, and other drug problem counts were calculated as the sum of positive responses pertaining to corresponding substance use, respectively, as reported on the SSAGA. Alcohol craving symptom counts (ACSC) was calculated as a sum of endorsed alcohol craving-related items in the SSAGA, on the scale between 0 and 8. A total of 17 individuals with AUD (22.7 ± 2.4 years old, 8 females) and 18 controls (individuals without AUD diagnosis, 22.0 ± 3.0 years old, 8 females) participated in the study. The age of the subjects was between 18 and 30 years old.
All participants provided written informed consent. All participants were required to refrain from drinking alcohol 12 h prior to the MR scan. A breath alcohol test was performed at the MRI facility using a breathalyzer (Alco-Sensor IV made by Intoximeters®).
Imaging protocols
All subjects were scanned on a 3 T Siemens TIM Trio scanner. A T1-weighted high-resolution anatomical image was acquired with the MP-RAGE sequence (160 sagittal slices, FOV = 256 mm, matrix = 256 × 256, TR/TE = 2300/2.91 ms, TI = 900 ms, flip angle = 9°, slice thickness = 1 mm, iPAT factor = 3), followed by four scan sessions of single-voxel MRS. The voxel was selected at the region of the dorsal ACC (voxel size 15 × 20 × 30 mm3) as shown in Fig. 1 or at the NAc. The ACC voxel was positioned in the following way: scroll the sagittal slices to find the mid-slice of the corpus callosum, then place the voxel right above the superior and posterior genu of the corpus callosum with the long axis aligned with them as much as possible. The PRESS sequence was used for MRS: TR/TE = 2000/30 ms, bandwidth = 2000 Hz, 2048 data points, number of measurements = 120, scan time = 4 min, followed by a water reference scan (8 averages). Manual shimming was performed to obtain good spectra. The linewidth of the water signal after shimming was below 14 Hz at the ACC and below 26 Hz at the NAc. During the first two MRS scans, a total of 60 furniture pictures (Fig. 2A) were shown to the subjects in a random order repeatedly. Each picture was displayed for 2 s. These pictures were changed to pictures of alcoholic beverages (Lovett et al., 2015) (Fig. 2B) and were displayed in the same fashion in the last two MRS scans until the scan ended. The order of the MRS scan was ACC–NAc–NAc–ACC to minimize the shimming effort when switching voxel location. After the last MRS scan, the subjects were asked to rate their craving on a scale of 1–5 with 1 meaning little to no craving and 5 meaning extreme craving. Because of the difficulty in positioning the voxel in the NAc that allows for non-noisy data, only the ACC data are presented due to poor spectra quality of the NAc data.
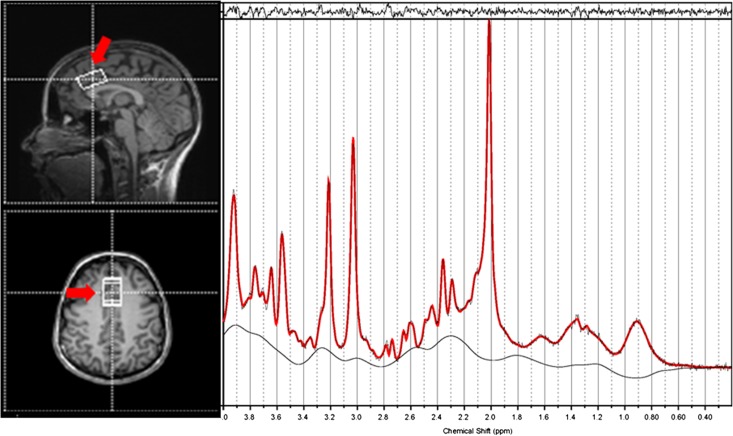
Fig. 1.
An example of the location of the voxel for MRS in the dorsal anterior cingulate along with the resultant spectra processed by LCModel (right). The fitted spectrum (red) is superimposed on the original spectrum (black); the residual of fitting is on the top while the baseline is at the bottom. The FWHM value is 0.033 ppm.
Fig. 2.

Example of the pictures shown to the subjects during MRS scans. (A) Furniture and (B) alcoholic beverage.
Data analysis
First, the T1 MP-RAGE image was segmented in SPM12 (The Wellcome Trust Centre for Neuroimaging at University College London, http://www.fil.ion.ucl.ac.uk/spm/) to obtain the gray matter (GM), white matter (WM) and CSF maps. Then we registered the MRS voxel to the T1 image using an in-house matlab (MathWorks Inc, Natick, MA) code and computed the relative volume for GM, WM and CSF in the voxel. The MRS data were processed with LCModel (http://www.s-provencher.com/). LCModel was used to fit each spectrum as a weighted linear combination of a basis set of in vitro spectra from individual metabolite solutions. The unsuppressed water signal was used for Eddy current correction and scaling the metabolite concentrations. Default settings for water attenuation, estimated water concentration and baseline modeling were applied. LCModel also reports the Crame´r-Rao lower bounds (CRLB) estimated as the relative standard deviation for each fitted component. Only fitting results with the CRLB values for above metabolites <20% were used for further statistical analysis. The glutamate concentration was expressed as a ratio with respect to the total creatine concentration. Based on our results and the literature (Henry et al., 2011), the glutamine quantified from LCModel is likely underestimated. Hence, it was not included in our analysis.
A two-way mixed ANOVA was performed using SPSS (IBM Corp., Armonk, NY, USA). In the model, there are two groups (AUD versus control) and the within-subjects factor was cue (furniture versus alcohol). With the model one can evaluate the main effect of alcohol cues, the difference between AUD and controls, and the interaction between cue and group on the brain glutamate levels. We also ran paired comparisons for groups and conditions (furniture and alcohol cues) with Bonferroni adjustment for P-values.
RESULTS
All subjects had a breathalyzer test of 0.0% prior to being scanned. The demographic information of the subjects is listed in Table 1. The average age of onset of AUD symptoms was 20.2 years. The AUD subjects reported significantly more counts of alcohol-related craving symptoms and APC than the controls. AUD subjects also reported higher craving ratings after viewing alcohol-related pictures than controls (P = 0.012).
Table 1.
Demographic information of the subjects
| Controls | AUD | P-value | |
|---|---|---|---|
| Number of subjects | 18 (8 females) | 17 (8 females) | |
| Age | 22.0 ± 3.0 | 22.7 ± 2.4 | 0.807 |
| Alcohol craving symptom countsa | 0.06 ± 0.24 | 2.88 ± 2.78 | 1.4e-4 |
| Craving score after viewing picturesb | 2.08 ± 0.94 | 2.91 ± 0.89 | 0.012 |
| Alcohol problem counts | 2.50 ± 3.38 | 39.71 ± 15.97 | 1.0e-10 |
| Drinking days in the past 2 weeks | 1.61 ± 1.29 | 7.71 ± 3.55 | 8.6e-8 |
| Drinking amount in the past 2 weeks | 6.17 ± 6.62 | 64.65 ± 53.13 | 5.4e-5 |
| Weekly drinking counts in the past 3 months | 1.67 ± 1.14 | 5.00 ± 1.58 | 3.0e-8 |
| Weekly alcohol consumption in the past 3 months | 5.44 ± 3.68 | 48.82 ± 32.34 | 2.7e-6 |
| Onset age for AUD symptoms | 20.2 ± 2.2 |
aCalculated as a sum of endorsed alcohol craving-related items in the SSAGA, on the scale between 0 and 8.
bScores are on the scale between 1 and 5, 5 for the highest craving.
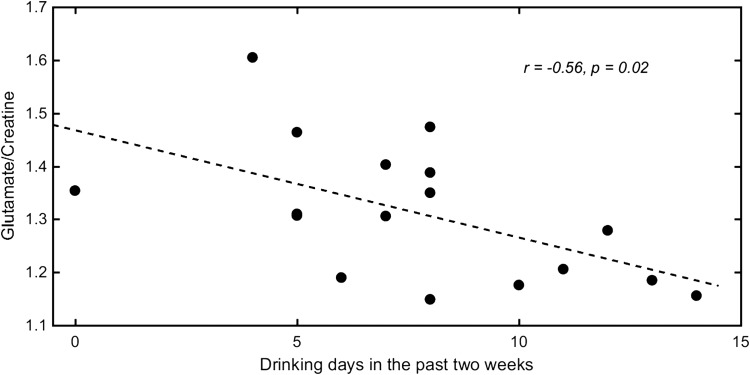
An example MRS spectrum at the ACC voxel and the fitting from LCModel is shown in Fig. 1. No subject was excluded from the CRLB’s > 20% criterion. There was no significant group difference for the partial volume ratio of GM (P = 0.78), WM (P = 0.61) or CSF (P = 0.07). The mixed model ANOVA revealed a main effect of alcohol cue [F(1, 33) = 8.40, P = 0.007, η2 effect size = 0.202]. There was no significant effect of group (F = 0.589, P = 0.448, η2 effect size = 0.017) but a significant interaction between cue and group was observed (F = 5.32, P = 0.027, η2 effect size = 0.138). The significant interaction implies that the alcohol cue impacted glutamate levels in AUD subjects differently than in the control group. The subsequent pairwise comparisons showed a significant decrease in glutamate for AUD subjects after viewing alcohol cues (P = 0.001) while the decrease was not significant for the control group (P = 0.674). No significant difference was observed in the basal glutamate level or after viewing the cues between the two groups (P = 0.10 and 0.44, respectively; see Table 2). A negative correlation between basal glutamate level at the ACC and number of drinking days of past 2 weeks prior to being scanned was found for the AUD subjects (r = −0.56, P = 0.02). A scatter plot of the relationship is shown in Fig. 3. It should be noted that for both controls and AUD subjects, no significant difference of the SNR of the spectra or CRLB values for glutamate was found between the conditions.
Table 2.
Mean concentration and standard error of glutamate expressed as the ratio to total creatine in the ACC for control subjects and AUD subjects at different conditions. P-values were computed from multivariate tests between the two conditions of furniture cue and alcohol cue with Bonferroni adjustment for multiple correction. Only AUD subjects show a significant difference between the two conditions (P = 0.001). The CRLB values are also shown for all conditions/cohorts with P-values from paired two-sample t-tests
| Glu/tCr (Control) | Glu/tCr (AUD) | CRLB (Control) | CRLB (AUD) | |
|---|---|---|---|---|
| Furniture | 1.245 ± 0.104 | 1.312 ± 0.129 | 5.50 ± 0.92% | 5.71 ± 0.91% |
| Alcohol | 1.234 ± 0.096 | 1.211 ± 0.075 | 5.33 ± 1.03% | 5.50 ± 1.02% |
| P-value | 0.674 | 0.001 | 0.381 | 0.385 |
Fig. 3.
Scatter plot of baseline glutamate concentration (as a ratio to creatine) versus drinking days in the past 2 weeks for AUD subjects.
DISCUSSION
This is the first in vivo study of neurochemical changes related to alcohol cues in young AD individuals. The average age of our AUD subjects was 22.7 years and the onset of AUD was within 3 years prior to their participation. We found that alcohol cues elicited a glutamate decrease in the ACC, indicating that glutamate in a key area of the forebrain reward circuit is modulated by alcohol cues in early alcohol dependence. Additionally, the effect of cues was different between the AUD subjects and the control group in spite of a lack of difference in the baseline glutamate levels.
Glutamate is one of the important neurotransmitters linked to cue-induced relapse in animal models (Adams et al., 2010; Gass and Olive, 2012). When alcohol-preferring rats were presented with an alcohol cue after withdrawal from alcohol access, presynaptic glutamate receptor antagonists inhibited cue-induced reinstatement of alcohol-seeking behavior (Bäckström and Hyytiä, 2004; Sanchis-Segura et al., 2006; Schroeder et al., 2008). The ACC, particularly dorsal ACC, has been linked to inhibitory control and appraisal (Botvinick et al., 2001; Venkatraman and Huettel, 2012). Additionally, the ACC has been implicated in craving (Schacht et al., 2013; Seo et al., 2013; Sjoerds et al., 2014). For example, Seo et al. (2013) showed that fMRI measured activation of the medial prefrontal cortex was associated with high cue-induced alcohol craving and alcohol relapse outcomes in abstinent AD patients. More specifically, Seo and colleagues found that AD patients showed higher prefrontal activation during the neutral condition and hypoactivation during the presentation of alcohol cues; also the subjects who showed this activation profile used alcohol on a greater number of days after treatment than those who did not, suggesting that prefrontal cortex, including the ACC is closely linked to cue-induced craving. Our results support this view. The AUD group reported higher alcohol craving. Additionally, the AUD group showed a significant decrease in ACC glutamate after viewing alcohol cues while the control group revealed a non-significant, smaller decrease.
It has been reported by Lovett et al. (2015) that alcohol craving ratings obtained when viewing the alcohol pictures used in the current study were positively correlated with the Alcohol Use Disorder Identification Test (AUDIT) scores, even after controlling for neutral cue craving ratings and other related variables. This finding suggests that the AUD group likely responded stronger to the alcohol cues than did the control group. This stronger response to the alcohol pictures may then be linked to the stronger change of ACC glutamate levels. This conclusion is supported by a significant interaction between the alcohol cue and group (P = 0.027) as well as the lack of a significant change in glutamate in the controls in response to the alcohol cues (P = 0.674).
While differences in alcohol craving may account for the observed results, another hypothesis is that frequent craving in those who are addicted to alcohol might induce an imbalance in glutamate homeostasis and ultimately change the basal concentration of glutamate. We found that glutamate levels were negatively correlated with drinking days in the past 2 weeks in individuals with AUD (r = −0.56, P = 0.02), similar to the results previously reported by Prisciandaro et al. (2016) when examining non-treatment-seeking individuals with alcohol dependence. Likewise, a negative association of glutamate level with severity of alcohol dependence was found in frontal white matter for non-treatment-seeking heavy drinkers (Ende et al., 2013). A number of animal studies reported higher baseline glutamate levels in alcohol seeking rats (Fliegel et al., 2013) and a higher Glu/Cr ratio was reported in the patients with alcohol dependence (Lee et al., 2007). Additionally, increased glutamate in the ACC during acute withdrawal was found for patients seeking treatments but the glutamate levels normalized after 2 weeks of abstinence (Hermann et al., 2012). This observation was confirmed by their parallel animal study using MRS that showed increased glutamate in the prefrontal cortex during the withdrawal stage. Another study using 2D J-resolved MRS found higher glutamate levels in the high-craving group of AUD patients versus low-craving group in the left dorsolateral prefrontal cortex (Frye et al., 2016). We did not observe a significant difference in baseline glutamate concentration between the AUD subjects and controls (P = 0.1), although the mean glutamate level of the AUD group was higher. This non-significant difference could be attributed to the short period of alcohol dependence in the AUD subjects examined in this study. In fact, studies using MRS to measure glutamate levels in humans have reported mixed results. For example, Thoma et al. (2011) reported no glutamate difference in the ACC for AUD subjects compared to controls. This lack of a difference was also reported by others for AD patients after detoxification (Bauer et al., 2013). Although there have been some discrepancies in the human data, the results presented here along with previous results do seem to support an important role of glutamate in drug craving and demonstrates the importance of further research in this area.
Based on models of the reward network the decrease of glutmate in the ACC suggests a projection of glutamate from the ACC to other brain regions. As a regulator of the reward circuit, the ACC has been associated with determining whether a response will be emitted to a salient stimulus as well as the intensity of that response. In the cognitive domain the ACC has been also linked to inhibitory control processing (Kalivas and Volkow, 2005). Moreover, the ACC together with the NAc have been associated with alcohol craving (Bell et al., 2016). An alcohol cue induced increase in glutamate has been found in basolateral amygdala and nucleus accumbens of alcohol seeking rats (Gass et al., 2011). The increase of glutamate in the NAc in that experiment along with decreased glutamate in the ACC from our study implicates the glutamate projection from the ACC to NAc in alcohol craving, which is in line with the neurobiological model of addiction and other addiction studies. In fact, a recent study reported that in ethanol administered rats the ablation of medial prefrontal cortex neurons projecting to the NAc blocked cue-induced reinstatement of alcohol seeking (Keistler et al., 2017), showing that the mPFC → NAc pathway is involved and necessary for cue-induced alcohol craving. A direct translation of that result into human studies will be a projection of glutamate from ACC to the NAc upon alcohol craving, which agrees well with our observation of decrease in glutamate in the ACC in response to the alcohol cues in the human subjects.
One limitation of our study is the relatively small sample size. In addition, the design of the study did not incorporate counterbalancing cue conditions across participants. Instead, the neutral cues were always presented first. While counterbalancing is the preferred design and one that has been used successfully in fMRI studies of alcohol cues (Wrase et al., 2002; Myrick et al., 2004), we opted to not use it because the duration of the effect of viewing alcohol cues on glutamate is unknown. To rule out external effects that could lead to global signal change due to head motion or scanner drift, we have examined other metabolites including total NAA (tNAA) = N-acetylaspartate (NAA) + N-acetylaspartylglutamate, total choline (Cho) = glycerophosphocholine + phosphocholine, Myo-inositol (mI), and total creatine (tCr) = creatine + phosphocreatine. There is no difference between the two scans for the AUD subjects for any of these metabolites (see Supplementary Material). However, we cannot rule out the possibility that glutamate changes were invoked by increased stress or physical discomfort. Finally, we did not have a baseline craving score before viewing the pictures, therefore, the craving score obtained at the end of the MRS scans cannot be entirely attributed to the alcohol cues. Another potential limitation is related to the potential differential durations between AUD particpant’s last alcoholic drink and the scan. Each participant was asked to refrain from drinking 12 h prior to the scan. Differences in time of last drink may affect the response to the alcohol cue and glutamate levels. Unfortunately, we do not have data on time of last drink and future studies should control for this difference.
In summary, the current study investigated the effect of alcohol cues on glutamate levels in AUD subjects and controls. Overall, alcohol cues show a main effect of decreasing the glutamate level in the ACC. The glutamate concentration significantly decreased for AUD subjects but not for the controls after they viewed pictures of alcoholic beverages. No difference was found in the baseline glutamate level between the two groups. These results taken together suggest that, like in animal models, glutamate levels are modulated by alcohol cues and may play an important role in cue-induced relapse in humans.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr Lindsay Ham and David Lovett from the University of Arkansas for providing the pictures of alcoholic beverages as alcohol cues. We are grateful for Dr Brian O’Donnell for his insightful comments and advice.
SUPPLEMENTARY MATERIAL
Supplementary data are available at Alcohol And Alcoholism online.
FUNDING
This research was supported in part by NIAAA grant R01 AA13650 to Finn. This project was funded, in part, with support from the Indiana Clinical and Translational Sciences Institute funded, in part by Project Development Teams (PDT) pilot grants (Grant number 586) from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award.
CONFLICT OF INTEREST STATEMENT
All the authors have no conflict of interest to declare relevant to the subject matter of this article.
REFERENCES
- Adams C, Cowen M, Short J, et al. (2008) Combined antagonism of glutamate mGlu5 and adenosine A2A receptors interact to regulate alcohol-seeking in rats. Int J Neuropsychopharmacol 11:229–41. [DOI] [PubMed] [Google Scholar]
- Adams C, Short J, Lawrence A (2010) Cue-conditioned alcohol seeking in rats following abstinence: involvement of metabotropic glutamate 5 receptors. Br J Pharmacol 159:534–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckström P, Hyytiä P (2004) Ionotropic glutamate receptor antagonists modulate cue-induced reinstatement of ethanol-seeking behavior. Alcohol Clin Exp Res 28:558–65. [DOI] [PubMed] [Google Scholar]
- Bagga D, Khushu S, Modi S, et al. (2014) Impaired visual information processing in alcohol-dependent subjects: a proton magnetic resonance spectroscopy study of the primary visual cortex. J Stud Alcohol Drugs 75:817–26. [DOI] [PubMed] [Google Scholar]
- Bauer J, Pedersen A, Scherbaum N, et al. (2013) Craving in alcohol-dependent patients after detoxification is related to glutamatergic dysfunction in the nucleus accumbens and the anterior cingulate cortex. Neuropsychopharmacology 38:1401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R, Hauser S, McClintick J, et al. (2016) Ethanol-associated changes in glutamate reward neurocircuitry: a minireview of clinical and preclinical genetic findings. Prog Mol Biol Transl Sci 137:41–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonson K, Grant S, Contoreggi C, et al. (2002) Neural systems and cue-induced cocaine craving. Neuropsychopharmacology 26:376–86. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Braver T, Barch D, et al. (2001) Conflict monitoring and cognitive control. Psychol Rev 108:624–52. [DOI] [PubMed] [Google Scholar]
- Bucholz K, Cadoret R, Cloninger C, et al. (1994) A new semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol 55:149–58. [DOI] [PubMed] [Google Scholar]
- Clapp P, Bhave S, Hoffman P (2008) How adaptation of the brain to alcohol leads to dependence: a pharmacological perspective. Alcohol Res Health 31:310–39. [PMC free article] [PubMed] [Google Scholar]
- Ende G, Hermann D, Demirakca T, et al. (2013) Loss of control of alcohol use and severity of alcohol dependence in non-treatment-seeking heavy drinkers are related to lower glutamate in frontal white matter. Alcohol Clin Exp Res 37:1643–9. [DOI] [PubMed] [Google Scholar]
- Finn P, Gunn R, Gerst K (2014) The effects of a working memory load on delay discounting in those with externalizing psychopathology. Clin Psychol Sci 3:202–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliegel S, Brand I, Spanagel R, et al. (2013) Ethanol-induced alterations of amino acids measured by in vivo microdialysis in rats: a meta-analysis. In Silico Pharmacol 1:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye M, Hinton D, Karpyak V, et al. (2016) Elevated glutamate levels in the left dorsolateral prefrontal cortex are associated with higher cravings for alcohol. Alcohol Clin Exp Res 40:1609–16. [DOI] [PubMed] [Google Scholar]
- Gass J, Olive M (2012) Neurochemical and neurostructural plasticity in alcoholism. ACS Chem Neurosci 3:494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass J, Sinclair C, Cleva R, et al. (2011) Alcohol-seeking behavior is associated with increased glutamate transmission in basolateral amygdala and nucleus accumbens as measured by glutamate-oxidase-coated biosensors. Addict Biol 16:215–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grüsser S, Wrase J, Klein S, et al. (2004) Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology (Berl) 175:296–302. [DOI] [PubMed] [Google Scholar]
- Hancu I. (2009) Optimized glutamate detection at 3T. J Magn Reson Imaging 30:1155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin D, Stinson F, Ogburn E, Grant B (2007) Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry 64:830–42. [DOI] [PubMed] [Google Scholar]
- Henry M, Lauriat T, Shanahan M, et al. (2011) Accuracy and stability of measuring GABA, glutamate, and glutamine by proton magnetic resonance spectroscopy: a phantom study at 4 Tesla. J Magn Reson 208:210–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann D, Weber-Fahr W, Sartorius A, et al. (2012) Translational magnetic resonance spectroscopy reveals excessive central glutamate levels during alcohol withdrawal in humans and rats. Biol Psychiatry 71:1015–21. [DOI] [PubMed] [Google Scholar]
- Hillmer A, Mason G, Fucito L, et al. (2015) How imaging glutamate, c-aminobutyric acid, and dopamine can inform the clinical treatment of alcohol dependence and withdrawal. Alcohol Clin Exp Res 39:2268–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas P, Volkow N (2005) The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry 162:1403–13. [DOI] [PubMed] [Google Scholar]
- Katner S, Magalong J, Weiss F (1999) Reinstatement of alcohol-seeking behavior by drug-associated discriminative stimuli after prolonged extinction in the rat. Neuropsychopharmacology 20:471–9. [DOI] [PubMed] [Google Scholar]
- Keistler C, Hammarlund E, Barker J, et al. (2017) Regulation of alcohol extinction and cue-induced reinstatement by specific projections among medial prefrontal cortex, nucleus accumbens, and basolateral amygdala. J Neurosci 37:4462–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Jang D, Kim J, et al. (2007) Alteration of brain metabolites in young alcoholics without structural changes. Neuroreport 18:1511–4. [DOI] [PubMed] [Google Scholar]
- Lovett D, Ham L, Veilleux J (2015) Psychometric evaluation of a standardized set of alcohol cue photographs to assess craving. Addict Behav 48:58–61. [DOI] [PubMed] [Google Scholar]
- Maccioni P, Orrú A, Korkosz A, et al. (2007) Cue-induced reinstatement of ethanol seeking in Sardinian alcohol-preferring rats. Alcohol 41:31–9. [DOI] [PubMed] [Google Scholar]
- Mon A, Durazzo T, Meyerhoff D (2012) Glutamate, GABA, and other cortical metabolite concentrations during early abstinence from alcohol and their associations with neurocognitive changes. Drug Alcohol Depend 125:27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos R, Moos B (2006) Rates and predictors of relapse after natural and treated remission from alcohol use disorders. Addiction 101:212–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrick H, Anton R, Li X, et al. (2004) Differential brain activity in alcoholics and social drinkers to alcohol cues: relationship to craving. Neuropsychopharmacology 29:393–402. [DOI] [PubMed] [Google Scholar]
- Polich J, Armor D, Braiker H (1981) The Course of Alcoholism: Four Years After Treatment. New York: John Wiley & Sons. [Google Scholar]
- Prisciandaro J, Schacht J, Prescot A, et al. (2016) Associations between recent heavy drinking and dorsal anterior cingulate N-acetylaspartate and glutamate concentrations in non-treatment-seeking individuals with alcohol dependence. Alcohol Clin Exp Res 40:491–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao P, Bell R, Engleman E, et al. (2015) Targeting glutamate uptake to treat alcohol use disorders. Front Neurosci 23:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchis-Segura C, Borchardt T, Vengeliene V, et al. (2006) Involvement of the AMPA receptor GluR-C subunit in alcohol-seeking behavior and relapse. J Neurosci 26:1231–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Sakai M, Weedman J, et al. (2011) Ceftriaxone, a beta-lactam antibiotic, reduces ethanol consumption in alcohol-preferring rats. Alcohol Alcohol 46:239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht J, Anton R, Myrick H (2013) Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addict Biol 18:121–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder J, Spanos M, Stevenson J, et al. (2008) Cue-induced reinstatement of alcohol-seeking behavior is associated with increased ERK1/2 phosphorylation in specific limbic brain regions: blockade by the mGluR5 antagonist MPEP. Neuropsychopharmacology 55:546–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert F, Gallinat J, Seifert F, et al. (2004) Glutamate concentrations in human brain using single voxel proton magnetic resonance spectroscopy at 3 Tesla. Neuroimage 21:1762–71. [DOI] [PubMed] [Google Scholar]
- Seo D, Lacadie C, Tuit K, et al. (2013) Disrupted ventromedial prefrontal function, alcohol craving, and subsequent relapse risk. JAMA Psychiatry 70:727–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Li C (2007) Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug Alcohol Rev 26:25–31. [DOI] [PubMed] [Google Scholar]
- Sjoerds Z, van den Brink W, Beekman A, et al. (2014) Cue reactivity is associated with duration and severity of alcohol dependence: an fMRI study. PLoS One 9:e84560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma R, Mullins P, Ruhl D, et al. (2011) Perturbation of the glutamate-glutamine system in alcohol dependence and remission. Neuropsychopharmacology 36:1359–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatraman V, Huettel S (2012) Strategic control in decision making under uncertainty. Eur J Neurosci 35:1075–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijtenburg S, Knight-Scott J (2011) Very short echo time improves the precision of glutamate detection at 3T in 1H magnetic resonance spectroscopy. J Magn Reson Imaging 34:645–52. [DOI] [PubMed] [Google Scholar]
- Wilson S, Sayette M, Delgado M, et al. (2005) Instructed smoking expectancy modulates cue-elicited neural activity: a preliminary study. Nicotine Tob Res 7:637–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrase J, Grüsser S, Klein S, et al. (2002) Development of alcohol-associated cues and cue-induced brain activation in alcoholics. Eur Psychiatry 17:287–91. [DOI] [PubMed] [Google Scholar]
- Yeo R, Thoma R, Gasparovic C, et al. (2013) Neurometabolite concentration and clinical features of chronic alcohol use: a proton magnetic resonance spectroscopy study. Psychiatry Res 211:141–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.