Abstract
Since the early 1990s it has been postulated that hypofunction of N-methyl-D-aspartate (NMDA) receptors in brain networks supporting perception and cognition underlies schizophrenic psychosis. Recently, NMDA receptor hypofunction was described in patients with psychotic manifestations who exhibited autoantibodies binding the GluN1 subunit of the receptor, and who improved when the level of these antibodies was lowered by immunomodulation. In this disorder, NMDA receptor antibodies decrease the availability of NMDA receptors by internalizing them. Here we review this mechanism as well as data supporting or refuting the possibility that this disorder, or similar autoimmune disorders affecting synaptic proteins which are therefore treatable with immunomodulation, could account for some cases of idiopathic psychosis. We also suggest methodological approaches to clarify this issue.
Keywords: Psychosis, antibodies, NMDA receptor, immunotherapy, schizophrenia, synapsis
Autoantibodies against the NMDA receptor: Relevance to psychotic symptoms
Most excitatory neurotransmission in the brain is mediated by L-glutamate, an amino acid that activates several types of receptors, including the N-methyl-D-aspartate (NMDA) receptors (NMDARs). Most native NMDARs are heterotetramers composed of an obligatory GluN1 subunit and various combinations of GluN2 (A–D) and sometimes GluN3 A–B subunits, with various arrangements depending on developmental stage, function and brain location [1–6]. NMDARs cluster at the core of postsynaptic densities, where they mediate synaptic signaling involved in persistent forms of synaptic plasticity thought to underlie learning and memory [7, 8]. Not surprisingly, NMDARs play a key role in human cognition and behavior. For example, both working and episodic memory rely on NMDAR-regulated networks [9–12]. The perception of auditory and visual stimuli, even at the early sensory level, is also modulated by NMDARs [13]. As a consequence, pharmacological interventions that interfere with the function of NMDARs result in striking behavioral abnormalities. In healthy volunteers, the NMDARs-blocking agents phencyclidine and ketamine cause not only impaired working memory and perceptual abnormalities, but also other symptoms characteristic of schizophrenic psychosis, such as paranoia, dissociative states and impaired executive function [14, 15].
Decreased function of the synaptic NMDARs has also been described in naturally-occurring human disorders associated with psychotic symptoms [16, 17]. The most interesting among these disorders, because it is treatable, is produced by IgG autoantibodies targeting the GluN1 subunit of the NMDA receptor [18]. This disorder, which in this review we call NMDAR antibody synaptopathy (NMDARAS), was first reported in 2007 [19], affects both sexes, and debuts most often in persons 12–29-years old [20], an age range that includes the age of onset for schizophrenic psychosis. So far NMDARAS has been detected mostly in patients with a severe course, often requiring intensive care [20–22], but cases with a milder form consisting only of psychotic symptoms have also been reported (see for instance [23–25]). This disorder is eminently treatable. Even with severe NMDARAS, about 80 % of the patients recover after immunotherapy: they not only cease to require intensive care, but their psychotic symptoms disappear [20].
Idiopathic psychoses are not unusual: they have been estimated to affect 0.1–0.5 % of the population [26, 27]. The possibility of finding among patients with psychosis some with NMDARAS, amenable to treatment, has recently spurred several groups to look for NMDAR antibodies in the sera of patients diagnosed with schizophrenia. The results have been disappointing. Some studies have not found them [28–30]. Others have found antibodies against the NMDAR, but generally not of the IgG type [31, 32]; furthermore, these non-IgG antibodies could also be detected in patients without psychosis or even in healthy individuals and therefore lacked any specificity for psychosis, suggesting that the association of antibodies with psychosis was purely coincidental [33]. However, there are compelling reasons, which we articulate in this review, to continue searching for antibodies against the NMDAR in such patients. We review evidence suggesting hypofunction of the NMDA receptor, long postulated to underlie schizophrenic psychosis, could be explained by an autoimmune mechanism in some patients with psychosis.
Hypofunction of the NMDA receptor: The glutamate hypothesis of psychosis
The term psychosis refers to an array of symptoms including hallucinations, delusions, paranoia and thought disorder. Psychosis may be caused by the ingestion of drugs, such as LSD, or by metabolic derangements of the brain, as in liver failure. Most often the cause of psychosis is unknown, that is, idiopathic, as exemplified by schizophrenia. Largely because dopamine D2-receptor-blocking agents improve the positive symptoms of schizophrenia [34], and because dopamine striatal synthesis and release are increased in this disorder [35, 36] characterized by dysfunction of dopamine-regulated frontotemporal cognitive networks [37, 38], schizophrenia has long been considered primarily a disorder of the dopaminergic system.
However, more recent formulations of the pathophysiology of schizophrenia include an important role for dysfunction of the glutamatergic system, which may actually underlie the hyperdopaminergic state in schizophrenic psychosis [39]. The glutamate hypothesis was first postulated in the early 1990s following observations of the similarity between the positive and negative symptoms of schizophrenia and the symptoms developed by healthy subjects given the NMDA receptor-blocking agents phencyclidine and ketamine [14, 15]. To the evidence provided by these initial studies in humans, were later added genetic [16, 40, 41], electrophysiologic [42–45], imaging [46–48] and post-mortem [49, 50] findings supporting hypofunction of the NMDA system in schizophrenia, as well as in animal models of psychoses [51, 52]. However, the NMDA system does not appear to be hypofunctional in schizophrenia as a result of glutamate production being decreased; on the contrary, glutamate may be increased in schizophrenia [39, 48], and hypofunction of NMDARs or of signaling pathways modulating NMDARs (Fig 2) is more likely [39, 48].
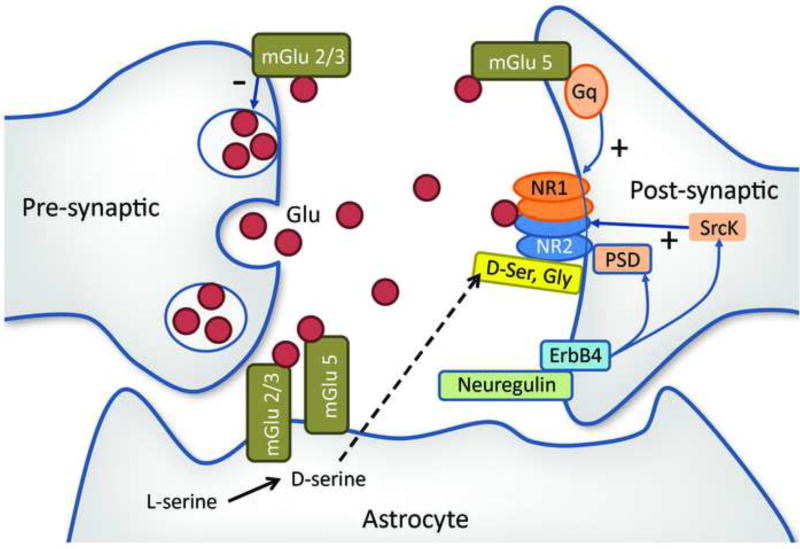
Figure 2.
Simplified diagram of some of the synaptic receptors known to modulate NMDARs. The excitatory neurotransmitter glutamate (Glu) is secreted at the presynaptic terminal and activates the NMDA receptor on the postsynaptic membrane. The NMDAR is a tetramer composed of two GluN1 and two NR2 subunits. It is modulated by a number of synaptic proteins. On the presynaptic membrane, metabotropic glutamate type 2/3 receptors (Glu 2/3), which bind glutamate, downregulate Glu production, while in the postsynaptic membrane metabotropic glutamate type 5 receptors (Glu 5) upregulate the NMDAR via the Gq/11 protein and phospholipase C enzyme [106]. Src kinase (Src kinase) contributes to stabilize NR2 though its action on the postsynaptic density (PSD) complex [107, 108]. Enhanced ErbB4 signaling through PSD-95 and neuregulin 1 may cause NMDAR hypofunction [109]. NMDAR activation requires the presence of glycine (Gly) or D-serine (D-ser) occupying a binding site in the GluN1 subunit [110].
Effect of NMDAR hypofunction on the GABAergic and dopaminergic systems
Hypofunction of NMDARs has repercussions critical for cognitive neuronal networks. In animal models, NMDAR hypofunction has been associated with decreased activity of gamma-aminobutyric acid (GABA) synthesis in the parvalbumin-containing subpopulation of inhibitory GABA neurons [43]. NMDA receptor-GABA hypofunction in the dorsolateral prefrontal cortex leads to a diminished capacity for the gamma-frequency synchronized neuronal activity that is required for working memory function [43, 53], and NMDA receptor-GABA hypofunction impairs pyramidal inhibition (Figure 1). Overactive pyramidal cells, notably those in the hippocampal formation, can drive a hyperdopaminergic state [10, 54] (Figure 1). The resultant enhanced release of dopamine by midbrain neurons from the ventral tegmental area could cause some of the behavioral and cognitive manifestations of schizophrenia [54] while excessive dopamine release by nigral neurons could cause the dyskinetic movements described in up to a quarter of antipsychotic–naïve patients with schizophrenia [55].
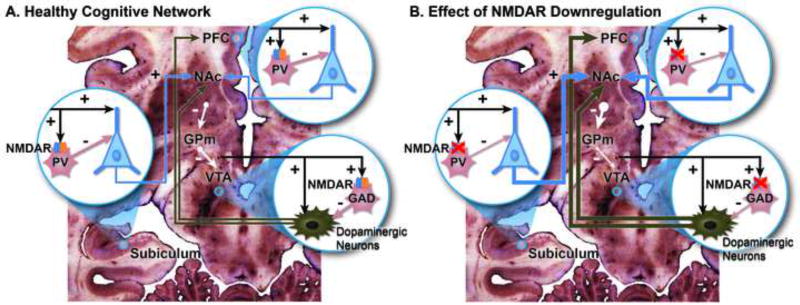
Figure 1.
Effect of NMDA receptor (NMDAR) hypofunction. This is a simplified diagram of some of the cognitive networks that are affected by NMDAR hypofunction. Three critical nodes are shown: (1) The subiculum, effector region of the hippocampal formation; (2) the prefrontal cortex (PFC), supporting working memory and executive function; and (3) the ventral tegmental area (VTA), which houses dopaminergic neurons involved in facilitating episodic and working memory, as well as motivation. (A) At each of these nodes, glutamatergic excitatory inputs to pyramidal or dopaminergic neurons (these from multiple brain regions, prominently lateral hypothalamus [101]) provide collaterals to NMDARs on gabaergic neurons, which in their turn inhibit excessive pyramidal firing. Gabaergic interneurons in the subiculum and PFC are parvalbumin positive (PV) and, in the PFC are identified with the fast-spiking cells, critical for the generation of synchronous gamma oscillations [53, 102]. In the VTA, gabaergic interneurons also harbor NMDARs [103] and stain with glutamic acid decarboxylase (GAD); by contrast VTA PV neurons are mostly projection neurons [104]. Dopaminergic VTA neurons project to the nucleus accumbens (NAc), PFC and hippocampus (not shown). The NAc inhibits the globus pallidus medialis (GPm), which in turn inhibits tonically VTA dopaminergic neurons [10, 54]. (B) NMDAR hypofunction is associated with increased pyramidal firing, which in turn increases the inhibitory activity of the NAc over the GPm and lessens its inhibitory tone over the VTA dopaminergic neurons. As a result, there is an increased production of dopamine, as found in psychoses, which are also attended by impaired working memory associated with abnormal functioning of PV interneurons [39, 54]. Increased pyramidal firing has also been documented with NMDAR ablation restricted to the NMDARs on frontal pyramidal neurons [105].
Synaptic autoimmunity against the NMDAR in humans
One of the mechanisms that may lead to hypofunction of the NMDARs is an autoimmune attack on these receptors. As they are located mostly in the postsynaptic membrane, with a large extracellular component [3], NMDARs have the potential to present antigenic epitopes outside the neuron. These epitopes could bind antibodies produced by one’s own immune system, ultimately resulting in autoimmune damage to the NMDAR.
Remarkably, in the past few years, antibodies have been described that target extracellular neuronal domains involving receptors on the synaptic surface [19, 56–59]. Brain biopsies of patients with these autoimmune disorders have shown anti-receptor-antibody-producing plasma cells, derived from B lymphocytes, but no complement production or neuronal loss [19, 60–62]. In this situation, the affected receptor is damaged but the neuron remains viable. Furthermore, the affected receptor can be restored by lowering antibody titers; therefore immunotherapeutic approaches to decrease antibody titers can be effective in reversing the symptoms [19, 56, 58, 59]. Among these disorders, the most frequently described to date involves IgG antibodies directed against the GluN1 subunit of the NMDA receptor [20, 63].
Molecular mechanisms of synaptic autoimmunity against the NMDAR GluN1 subunit
In NMDARAS, IgG antibodies are directed to the N-terminal extracellular domain of the GluN1 subunit of the NMDA receptor, specifically an epitope region at GluN1 aa369 [18, 64, 65]. Cultures of dissociated rat hippocampal neurons and antibody-containing cerebrospinal fluid (CSF) from patients with NMDARAS have been used to study the molecular mechanism by which IgG antibodies cause hypofunction of the NMDAR [66, 67]. Antibodies decrease the levels of synaptic NMDA receptor and disrupt NMDA receptor currents in cultured neurons [18, 66]. In addition, the antibodies disrupt the interaction between NMDAR and the ephrin B2 receptor (EphB2R), a major stabilizer of NMDARs at postsynaptic sites, facilitating the displacement of NMDARs from the synapse [68]. The antibody does not act as a receptor antagonist, by modulating the physiologic receptor binding domain, but causes capping and internalization of the receptor [66–68]. This effect is specific to the antibody isolated from the serum or CSF of patients: by itself alone, the Fab fragment of the antibody does not cause receptor internalization, but this effect is recovered when the entire antibody is reconstituted [66]. Complement is not necessary: antibodies within patient CSF are both necessary and sufficient to cause the loss of surface NMDARs [66, 67]. Antibody-mediated internalization is independent of NMDAR activity and does not occur as a compensatory response to agonism of the receptor, suggesting that the mechanism of internalization is primarily NMDAR crosslinking by patient antibodies [67]. In NMDARAS, NMDA receptors are bound by immunoglobulins during endocytosis, a scenario that would not occur under physiological conditions of receptor internalization. However, the postendocytic trafficking of NMDARs is not affected by IgG binding the receptor [67].
A recent study showed the pathogenic effects of the antibodies in a model of cerebroventricular passive transfer of CSF antibodies from NMDARAS patients to mice [69]. The resulting findings, which fulfill the Witebsky’s criteria [70] for an antibody-mediated disease, included: 1) the development of symptoms in animals infused with patients’ CSF, but not control CSF; 2) the infused antibodies reacted predominantly with brain regions with high density of NMDAR (e.g., hippocampus) and specifically recognized these receptors; 3) in proportion to their concentration, brain-bound antibodies decreased the density of total and synaptic NMDAR clusters and total NMDAR protein concentration without affecting PSD95; and 4) the intensity of the above-mentioned findings correlated with the time-course of patients’ antibody infusion and symptoms resolved in parallel to the restoration of NMDAR levels after stopping the infusion of CSF antibodies [69].
Detection and manifestations of NMDA-receptor-antibody synaptopathy (NMDARAS) in humans
Prevalence
Being a recently recognized disorder, the true prevalence of NMDARAS is unknown. Case discovery in the reported series [20] may have been biased toward sicker patients. But even this potentially more serious manifestation of NMDARAS is not rare, accounting for 1% of all admissions to one intensive care unit (ICU) of women aged 18 – 35 years [71]. A multicenter, population-based prospective study of causes of encephalitis in the UK showed that 4% of patients had anti-NMDA receptor encephalitis and that this was the second most common immune-mediated cause, after acute disseminated encephalomyelitis [72]. In a center focused on the etiology and epidemiology of encephalitis (California encephalitis project), NMDARAS was found to be more frequent than any single viral encephalitis [73]. NMDARAS is much more frequent than the classical paraneoplastic syndromes (compare refs [84] and [74]).
Demographics and presentation
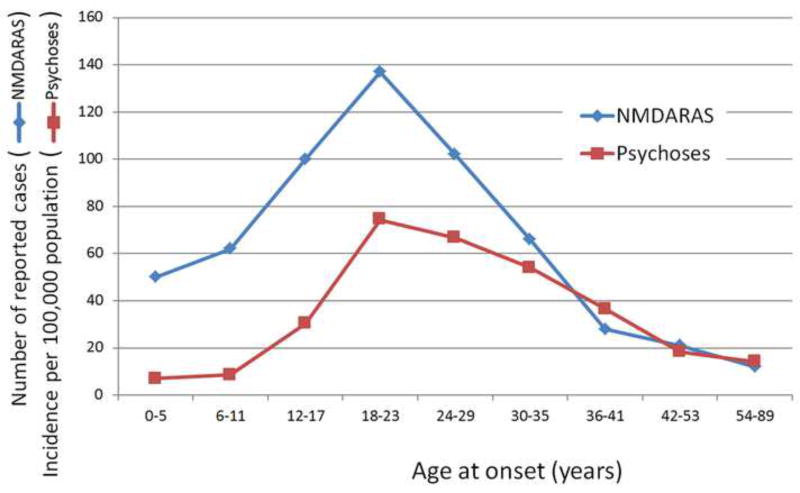
Eighty percent of the patients reported were women [20]. The higher discovery in women may be explained by the association of NMDARAS with ovarian teratomas, which contain neuronal elements expressing NMDA receptors that act as antigens [20]. The age of onset of NMDARAS ranges between eight months and 85 years, but the median age of onset is 21 years, and the majority of cases cluster in the 12–29 age range with a peak from 18–23 years [20]. This peak is strikingly similar to the age of onset of schizophrenic psychosis (Figure 3).
Figure 3.
Age at onset of NMDA receptor antibody synaptopathy (NMDARAS) and of schizophrenic psychosis. Graphic built with data from [20] for NMDARAS and from [111, 112] for schizophrenic psychoses.
Patients with NMDARAS in the first decade of life tend to present with seizures or movement disorders, while psychiatric abnormalities are the usual mode of presentation for those over 12, perhaps reflecting a developmental vulnerability of those neural circuits most important in psychiatric disease. Behavioral manifestations occurred during the first month of the disease in over 90% of the patients, regardless of age group [20]. Psychiatric manifestations were not circumscribed to schizophrenia-like psychosis, but included manic psychosis and other mood disorders [24]. Mood symptoms are frequent in the months preceding the diagnosis of schizophrenia spectrum disorder and throughout the course of the disease [75]. Most of the patients described with NMDARAS worsened, developing catatonia, dyskinesias and central hypoventilation that required intensive care.
The possibility of NMDARAS causing idiopathic psychoses goes well beyond being academically interesting and may have profound clinical implications. About 80% of patients with NMDARAS in a sample that included 70% requiring intensive care improved greatly after immunotheraphy [20]. First-line immunotherapy used a combination of steroids and intravenous immunoglobulin or plasmapheresis. Of patients who did not improve with first-line treatment, the majority improved with second-line immunotherapy, most often rituximab or cyclophosphamide [20]. Judging from the experience with other autoimmune brain disorders, such as multiple sclerosis [76], an even more favorable outcome may be likely if the immunological attack is milder, resulting only in psychotic symptoms.
Correlation between symptoms and antibody titers: parallels with pharmacologic NMDA receptor antagonists
Informative parallels can be drawn between the clinical effects of antibodies against NMDA receptors and the clinical effects of pharmacologic NMDA receptor non-competitive antagonists, such as phencyclidine and ketamine. These drugs induce behaviors similar to the positive and negative symptoms of schizophrenia, along with repetitive orofacial and limb movements, autonomic instability, and seizures. This profile of symptoms caused by antagonists of NMDA receptor is dose dependent: at low doses, NMDA receptor antagonists cause illusionary perceptions, ideas of reference and paranoia, coupled with impaired performance on the Wisconsin Card Sorting and other tasks requiring executive function, but no substantial memory loss [14]; higher doses of these drugs manifest with psychosis, agitation, memory disturbance, and decreased responsiveness to pain [15]; and at even higher doses these substances cause dissociative anesthesia, a state of profound unresponsiveness with catatonic features, and coma [15]. The low-dose pharmacological effect resembles the symptoms in patients with milder forms of NMDARAS and lower CSF anti-NMDA receptor antibody titers, so far best documented in the process of recovery of the serious illness, when patients again manifest psychotic symptoms similar to the initial presentation [74]. The high-dose drug effect mimics the more severe stages of NMDARAS, characterized by catatonia, impaired respiratory drive and coma [74]. In NMDARAS, NMDA receptor availability correlates strongly and inversely with CSF antibody titers [18]. Abundant antibodies cause internalization of a larger proportion of NMDA receptors, decreasing the availability of these receptors in a manner similar to their binding by non-competitive antagonists (Figure 4).
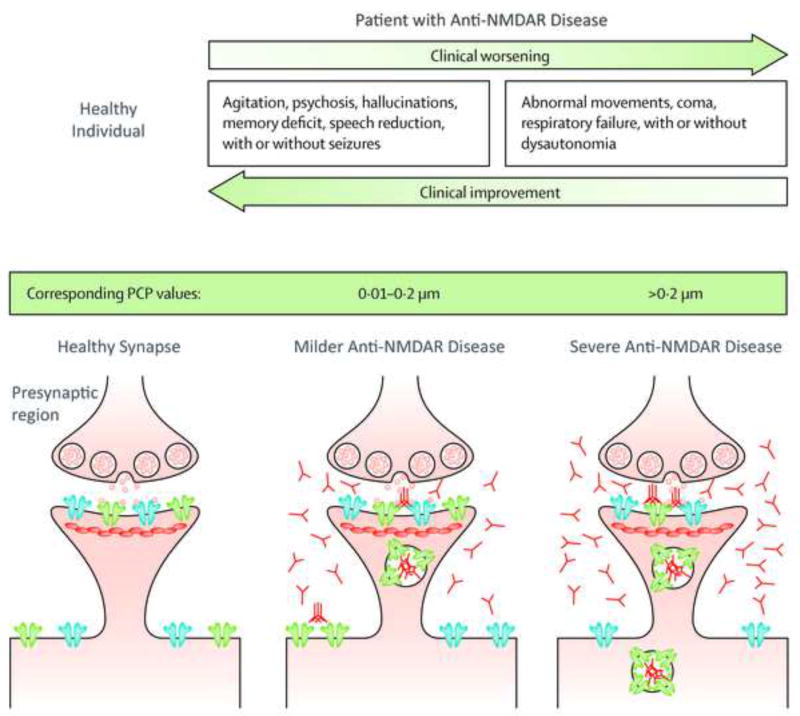
Figure 4.
Diagram illustrating how more severe clinical findings in patients with anti-NMDAR disease are associated with lesser NMDA receptor availability on the postsynaptic region and higher levels of brain antibodies. The clinical picture resembles that caused by phencyclidine (PCP, green bar), in which the profile of symptoms correlates with the circulating concentration of the drug. Post-synaptic receptors depicted in blue represent α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPAR), those in green N-methyl-D-aspartate receptors (NMDAR). The small round vesicles that are released in the synapse represent glutamate. Modified from Dalmau et al [74].
Determination of anti-NMDAR antibodies in patients with idiopathic psychoses
NMDARAS has been identified in single cases or small series of patients with psychosis resembling schizophrenia (see for instance [77–79]). In addition, the similarity between some of the symptoms of NMDARAS and idiopathic psychosis such as schizophrenia has added renewed interest to the hypothesis that some cases of schizophrenia have an autoimmune basis [80] [81]. Autoimmunity could explain the clinical course of remissions and exacerbations in about one-third of the patients with schizophrenia [82], as well as the upregulation of inflammation-related genes in brain tissue [83, 84], brain inflammation detected with positron emission tomography [85], and the association of schizophrenia with genes expressed in tissues that have important roles in immunity [86]. The tantalizing prospect of finding a reversible disorder has prompted a number of investigators to search systematically for NMDA receptor antibodies in patients with psychoses [87]. To date, all studies have been performed using patient sera, not CSF. Three studies failed to detect any cases with raised levels of IgG anti-NMDA receptor antibodies among a total of 505 patients with schizophrenia [28–30]. Other studies detected a small proportion of patients with antibodies. Some studies found a small proportion of patients with NMDAR antibodies among first episode psychosis (2/46, [25]) or in schizophrenia (4/51 [88]) in adults or children (6/43 patients [23]). The last study included a negative healthy control group. Other studies [33, 89], using a methodology different from Dalmau’s three-step procedure [18], have found similar proportions of antibodies among patients with schizophrenia and controls with neurological disorders such as Parkinson’s disease and even healthy controls (See Box 1). These reports highlight the complexity of serologic findings and, perhaps, their less robust relationship to clinical status, as compared to CSF findings, which are more likely to reflect immunological events inside the blood-brain-barrier [33, 65, 90].
Concluding remarks: The case for continuing the search for synaptic autoantibodies in psychoses
NMDARAS provides a tantalizing model of a human disease causing psychosis for which highly specific biomarkers—increased serum and CSF levels of IgG antibodies directed against the GluN1 subunit of the NMDA receptor—are already available, thus facilitating genetic and other studies. However, despite the evidence reviewed linking NMDAR hypofunction and autoimmune disease to the idiopathic psychoses, direct measurements of antibodies in serum have failed to identify even a subgroup of patients with schizophrenia who harbor pathogenic antibodies. Scenarios that may explain this discrepancy include:
It is possible that offending antibodies may be present in serum in amounts too small to be detected. Even in patients with severe NMDARAS, antibodies are more likely to be detected in CSF than in serum [65]. Intrathecal antibody synthesis has been documented in NMDARAS, and CSF antibody levels correlated better with the severity of clinical symptoms than did serum levels [65]. CSF analysis was crucial in the identification of novel antigens, including NMDA receptor, AMPAR, GABAB receptor, GABAA receptor, mGluR5, DPPX, and LGI1 and Caspr2 [91]. Serum negativity is more likely with a milder form of the disease, presenting with psychosis but not requiring intensive care, particularly if the antibodies are predominantly generated in the brain [90, 92]. For these reasons several investigators have emphasized the importance of testing CSF in patients with psychoses (see for instance [29, 33, 93]).
Hypofunction of NMDARs may be due to antibodies directed to proteins that modulate NMDARs (Fig 2), rather than to the NMDARs themselves. It is instructive that some of the antibodies recently described to be associated with psychotic symptoms target, not NMDA receptors, but AMPA receptors [94, 95], mGluR5 [96], or the potassium-channel complexes [97], all of which modulate NMDA receptors [57]. Any of the proteins of the complex system, only partially understood, that preserves intact NMDAR function could in theory be targeted by specific antibodies and result in NMDAR hypofunction.
If NMDARs in the human brain present antigens that differ from those of rodents, screening studies using rat brain tissue may fail to detect specific anti-human antibodies. NMDAR structure varies across species, particularly in regions of the brain highly evolved, such as the frontal lobe [9]. GluN1 shows high sequence identity between human and rat, but even for this subunit there are seven non-identical amino acids [98]. The other NMDAR subunits are even more different across species [98]. Supporting the possibility that current methodology may lack sensitivity, some patients with psychotic manifestations, but negative sera and CSF antibody testing, improved after immunotherapy [99, 100]. Testing using marmoset or other model closer to human may provide a more sensitive paradigm to test for still undiscovered antibodies targeting human NMDAR or other synaptic receptors.
Trends Box.
Hypofunction of N-methyl-D-aspartate (NMDA) receptors occurs in schizophrenia.
Recently, autoantibodies have been found to cause NMDA receptor hypofunction.
These autoantibodies target the GluN1 subunit of the receptor, internalizing it, and can be detected in the cerebrospinal fluid and, less often, in the sera of affected patients.
These patients have psychotic manifestations in the course of their disease; cerebrospinal fluid was obtained when they worsened, often requiring a respirator.
Despite the severity of their disease they recovered with immunotherapy.
Within in the context of rigorously controlled studies, antibodies against NMDA receptors, or other proteins modulating them, should be sought in the cerebrospinal fluid of patients with schizophrenic or manic psychoses unexplained by psychotropic drug ingestion or other metabolic factors.
Outstanding questions.
To clarify to what extent, if any, autoantibodies cause idiopathic psychoses at least the following three questions should be addressed:
What is the prevalence of elevated IgG antibodies targeting the GluN1 subunit of the NMDA receptor in cerebrospinal fluid, in addition to sera, of people with idiopathic psychosis?
Could NMDA receptor hypofunction in some idiopathic psychoses be caused not by direct autoimmune attack on NMDA receptors but by antibodies directed to other still poorly characterized proteins modulating NMDA receptors?
Are there antigens in human NMDA receptors that are not found in rodents and therefore may generate antibodies not detectable with some of the methods that use rodent brain tissue for antibody detection?
Acknowledgments
JCM and KFB were supported by the Houston Methodist Research Institute and by the NIH Intramural Research Program respectively; JD by NIH grants RO1NS077851, RO1MH094741, FIS, 11/01780, Fundació la Marató de TV3, a McKnight Neuroscience of Brain Disorders award and a research grant from Euroimmun Inc. Dr. Isabel Pérez Otaño provided valuable comments on the manuscript.
JD has a patent on a procedure to determine anti-NMDA antibodies and receives royalties from Euroimmun, a company that provides this procedure commercially.
Glossary
- Autoimmunity
is the system of immune responses of an organism against its own tissues. Any disease that results from such an aberrant immune response is termed an autoimmune disease.
- Episodic memory
Memory of autobiographical events (times, places, associated emotions, and other contextual who, what, when, where, why knowledge) that can be explicitly stated.
- Executive function
is an umbrella term for the management (regulation, control) of cognitive processes, including working memory, reasoning, task flexibility, and problem solving as well as planning and execution.
- Dyskinesias
are excessive movements of the face or extremities.
- Glutamate
is the main excitatory neurotransmitter in the brain.
- Idiopathic disease
A disease of unknown cause.
- IgG, Immunoglobulin G
is a type of antibody, a protein complex composed of four peptide chains—two identical heavy chains and two identical light chains arranged in a Y-shape. They are produced by B lymphocytes evolved to plasma cells.
- N-methyl-D-aspartate receptor (NMDAR)
a glutamate receptor and ion channel protein found in nerve cells. It is involved in synaptic transmission and plays an important role in learning and memory.
- NMDAR hypofunction hypothesis
a glutamate-based hypothesis which postulates that reduced NMDAR activation underlies the development of different schizophrenia symptoms.
- NMDARAS (NMDA Receptor Antibody Synaptopathy)
A disorder in humans caused by IgG antibodies against the GluN1 subunit of the receptor that cause internalization of the receptor.
- Psychosis
An array of symptoms of mental illness, including hallucinations, delusions, paranoia and disordered thinking.
- Prevalence
is the proportion of the population who harbors a given disease at a given time.
- Working memory
enables the temporary holding of information for perceptual, linguistic and other cognitive processing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: JCM and KFB report no conflicts of interest.
References
- 1.Hansen KB, et al. Distinct functional and pharmacological properties of Triheteromeric GluN1/GluN2A/GluN2B NMDA receptors. Neuron. 2014;81:1084–1096. doi: 10.1016/j.neuron.2014.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karakas E, Furukawa H. Crystal structure of a heterotetrameric NMDA receptor ion channel. Science. 2014;344:992–997. doi: 10.1126/science.1251915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee CH, et al. NMDA receptor structures reveal subunit arrangement and pore architecture. Nature. 2014;511:191–197. doi: 10.1038/nature13548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paoletti P, et al. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci. 2013;14:383–400. doi: 10.1038/nrn3504. [DOI] [PubMed] [Google Scholar]
- 5.Wyllie DJ, et al. Influence of GluN2 subunit identity on NMDA receptor function. Neuropharmacology. 2013;74:4–17. doi: 10.1016/j.neuropharm.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pachernegg S, et al. GluN3 subunit-containing NMDA receptors: not just one-trick ponies. Trends Neurosci. 2012;35:240–249. doi: 10.1016/j.tins.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Lau CG, Zukin RS. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci. 2007;8:413–426. doi: 10.1038/nrn2153. [DOI] [PubMed] [Google Scholar]
- 8.Traynelis SF, et al. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang M, Arnsten AF. Contribution of NMDA receptors to dorsolateral prefrontal cortical networks in primates. Neurosci Bull. 2015;31:191–197. doi: 10.1007/s12264-014-1504-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Modinos G, et al. Translating the MAM model of psychosis to humans. Trends Neurosci. 2015;38:129–138. doi: 10.1016/j.tins.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Souza Silva MA, et al. Evidence for a specific integrative mechanism for episodic memory mediated by AMPA/kainate receptors in a circuit involving medial prefrontal cortex and hippocampal CA3 region. Cereb Cortex. 2015 doi: 10.1093/cercor/bhv112. [DOI] [PubMed] [Google Scholar]
- 12.Wang M, et al. NMDA receptors subserve persistent neuronal firing during working memory in dorsolateral prefrontal cortex. Neuron. 2013;77:736–749. doi: 10.1016/j.neuron.2012.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Javitt DC, Freedman R. Sensory processing dysfunction in the personal experience and neuronal machinery of schizophrenia. Am J Psychiatry. 2015;172:17–31. doi: 10.1176/appi.ajp.2014.13121691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krystal JH, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 15.Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- 16.Fromer M, et al. De novo mutations in schizophrenia implicate synaptic networks. Nature. 2014;506:179–184. doi: 10.1038/nature12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wen Z, et al. Synaptic dysregulation in a human iPS cell model of mental disorders. Nature. 2014;515:414–418. doi: 10.1038/nature13716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dalmau J, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091–1098. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dalmau J, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61:25–36. doi: 10.1002/ana.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Titulaer MJ, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12:157–165. doi: 10.1016/S1474-4422(12)70310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Irani SR, et al. N-methyl-D-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain. 2010;133:1655–1667. doi: 10.1093/brain/awq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Viaccoz A, et al. Clinical specificities of adult male patients with NMDA receptor antibodies encephalitis. Neurology. 2014;82:556–563. doi: 10.1212/WNL.0000000000000126. [DOI] [PubMed] [Google Scholar]
- 23.Pathmanandavel K, et al. Antibodies to surface dopamine-2 receptor and N-methyl-d-aspartate receptor in the first episode of acute psychosis in children. Biol Psychiatry. 2015;77:537–547. doi: 10.1016/j.biopsych.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 24.Kayser MS, et al. Frequency and characteristics of isolated psychiatric episodes in anti-N-methyl-d-aspartate receptor encephalitis. JAMA Neurol. 2013;70:1133–1139. doi: 10.1001/jamaneurol.2013.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zandi MS, et al. Disease-relevant autoantibodies in first episode schizophrenia. J Neurol. 2011;258:686–688. doi: 10.1007/s00415-010-5788-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kendler KS, et al. Lifetime prevalence, demographic risk factors, and diagnostic validity of nonaffective psychosis as assessed in a US community sample. The National Comorbidity Survey. Arch Gen Psychiatry. 1996;53:1022–1031. doi: 10.1001/archpsyc.1996.01830110060007. [DOI] [PubMed] [Google Scholar]
- 27.Jablensky A, et al. Psychotic disorders in urban areas: an overview of the Study on Low Prevalence Disorders. Aust N Z J Psychiatry. 2000;34:221–236. doi: 10.1080/j.1440-1614.2000.00728.x. [DOI] [PubMed] [Google Scholar]
- 28.de Witte LD, et al. Absence of N-methyl-D-aspartate receptor IgG autoantibodies in schizophrenia: the importance of cross-validation studies. JAMA Psychiatry. 2015;72:731–733. doi: 10.1001/jamapsychiatry.2015.0526. [DOI] [PubMed] [Google Scholar]
- 29.Masdeu JC, et al. Serum IgG antibodies against the NR1 subunit of the NMDA receptor not detected in schizophrenia. Am J Psychiatry. 2012;169:1120–1121. doi: 10.1176/appi.ajp.2012.12050646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rhoads J, et al. Lack of anti-NMDA receptor autoantibodies in the serum of subjects with schizophrenia. Schizophr Res. 2011;129:213–214. doi: 10.1016/j.schres.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 31.Hammer C, et al. Neuropsychiatric disease relevance of circulating anti-NMDA receptor autoantibodies depends on blood-brain barrier integrity. Mol Psychiatry. 2013 doi: 10.1038/mp.2013.110. [DOI] [PubMed] [Google Scholar]
- 32.Steiner J, et al. Increased prevalence of diverse N-methyl-D-aspartate glutamate receptor antibodies in patients with an initial diagnosis of schizophrenia: specific relevance of IgG NR1a antibodies for distinction from N-methyl-D-aspartate glutamate receptor encephalitis. JAMA Psychiatry. 2013;70:271–278. doi: 10.1001/2013.jamapsychiatry.86. [DOI] [PubMed] [Google Scholar]
- 33.Dahm L, et al. Seroprevalence of autoantibodies against brain antigens in health and disease. Ann Neurol. 2014;76:82–94. doi: 10.1002/ana.24189. [DOI] [PubMed] [Google Scholar]
- 34.Kapur S, et al. Relationship between dopamine D(2) occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry. 2000;157:514–520. doi: 10.1176/appi.ajp.157.4.514. [DOI] [PubMed] [Google Scholar]
- 35.Kegeles LS, et al. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch Gen Psychiatry. 2010;67:231–239. doi: 10.1001/archgenpsychiatry.2010.10. [DOI] [PubMed] [Google Scholar]
- 36.Meyer-Lindenberg A, et al. Reduced prefrontal activity predicts exaggerated striatal dopaminergic function in schizophrenia. Nat Neurosci. 2002;5:267–271. doi: 10.1038/nn804. [DOI] [PubMed] [Google Scholar]
- 37.Fusar-Poli P, et al. Abnormal prefrontal activation directly related to pre-synaptic striatal dopamine dysfunction in people at clinical high risk for psychosis. Mol Psychiatry. 2011;16:67–75. doi: 10.1038/mp.2009.108. [DOI] [PubMed] [Google Scholar]
- 38.Eisenberg DP, et al. Catechol-o-methyltransferase valine(158)methionine genotype and resting regional cerebral blood flow in medication-free patients with schizophrenia. Biol Psychiatry. 2010;67:287–290. doi: 10.1016/j.biopsych.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moghaddam B, Javitt D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology. 2012;37:4–15. doi: 10.1038/npp.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodriguez-Santiago B, et al. Association of common copy number variants at the glutathione S-transferase genes and rare novel genomic changes with schizophrenia. Mol Psychiatry. 2010;15:1023–1033. doi: 10.1038/mp.2009.53. [DOI] [PubMed] [Google Scholar]
- 41.Martucci L, et al. N-methyl-D-aspartate receptor NR2B subunit gene GRIN2B in schizophrenia and bipolar disorder: Polymorphisms and mRNA levels. Schizophr Res. 2006;84:214–221. doi: 10.1016/j.schres.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 42.Javitt DC. Neurophysiological models for new treatment development in schizophrenia: early sensory approaches. Ann N Y Acad Sci. 2015;1344:92–104. doi: 10.1111/nyas.12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lewis DA, et al. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 44.Naatanen R, Kahkonen S. Central auditory dysfunction in schizophrenia as revealed by the mismatch negativity (MMN) and its magnetic equivalent MMNm: a review. Int J Neuropsychopharmacol. 2009;12:125–135. doi: 10.1017/S1461145708009322. [DOI] [PubMed] [Google Scholar]
- 45.Lisman JE, et al. A thalamo-hippocampal-ventral tegmental area loop may produce the positive feedback that underlies the psychotic break in schizophrenia. Biol Psychiatry. 2010;68:17–24. doi: 10.1016/j.biopsych.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anticevic A, et al. NMDA receptor function in large-scale anticorrelated neural systems with implications for cognition and schizophrenia. Proc Natl Acad Sci U S A. 2012;109:16720–16725. doi: 10.1073/pnas.1208494109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pilowsky LS, et al. First in vivo evidence of an NMDA receptor deficit in medication-free schizophrenic patients. Mol Psychiatry. 2006;11:118–119. doi: 10.1038/sj.mp.4001751. [DOI] [PubMed] [Google Scholar]
- 48.Poels EM, et al. Imaging glutamate in schizophrenia: review of findings and implications for drug discovery. Mol Psychiatry. 2014;19:20–29. doi: 10.1038/mp.2013.136. [DOI] [PubMed] [Google Scholar]
- 49.Weickert CS, et al. Molecular evidence of N-methyl-D-aspartate receptor hypofunction in schizophrenia. Mol Psychiatry. 2013;18:1185–1192. doi: 10.1038/mp.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Law AJ, Deakin JF. Asymmetrical reductions of hippocampal NMDAR1 glutamate receptor mRNA in the psychoses. Neuroreport. 2001;12:2971–2974. doi: 10.1097/00001756-200109170-00043. [DOI] [PubMed] [Google Scholar]
- 51.Mohn AR, et al. Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell. 1999;98:427–436. doi: 10.1016/s0092-8674(00)81972-8. [DOI] [PubMed] [Google Scholar]
- 52.Papaleo F, et al. Mouse models of genetic effects on cognition: relevance to schizophrenia. Neuropharmacology. 2012;62:1204–1220. doi: 10.1016/j.neuropharm.2011.04.025. [DOI] [PubMed] [Google Scholar]
- 53.Rotaru DC, et al. The role of glutamatergic inputs onto parvalbumin-positive interneurons: relevance for schizophrenia. Rev Neurosci. 2012;23:97–109. doi: 10.1515/revneuro-2011-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lisman JE, et al. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tandon R, et al. Schizophrenia, "just the facts" 4. Clinical features and conceptualization. Schizophr Res. 2009;110:1–23. doi: 10.1016/j.schres.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 56.Buckley C, et al. Potassium channel antibodies in two patients with reversible limbic encephalitis. Ann Neurol. 2001;50:73–78. doi: 10.1002/ana.1097. [DOI] [PubMed] [Google Scholar]
- 57.Lancaster E, Dalmau J. Neuronal autoantigens--pathogenesis, associated disorders and antibody testing. Nat Rev Neurol. 2012;8:380–390. doi: 10.1038/nrneurol.2012.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lai M, et al. AMPA receptor antibodies in limbic encephalitis alter synaptic receptor location. Ann Neurol. 2009;65:424–434. doi: 10.1002/ana.21589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Petit-Pedrol M, et al. Encephalitis with refractory seizures, status epilepticus, and antibodies to the GABAA receptor: a case series, characterization of the antigen, and analysis of the effects of antibodies. Lancet Neurol. 2014;13 doi: 10.1016/S1474-4422(13)70299-0. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bien CG, et al. Immunopathology of autoantibody-associated encephalitides: clues for pathogenesis. Brain. 2012;135:1622–1638. doi: 10.1093/brain/aws082. [DOI] [PubMed] [Google Scholar]
- 61.Camdessanche JP, et al. Brain immunohistopathological study in a patient with anti-NMDAR encephalitis. Eur J Neurol. 2011;18:929–931. doi: 10.1111/j.1468-1331.2010.03180.x. [DOI] [PubMed] [Google Scholar]
- 62.Martinez-Hernandez E, et al. Analysis of complement and plasma cells in the brain of patients with anti-NMDAR encephalitis. Neurology. 2011;77:589–593. doi: 10.1212/WNL.0b013e318228c136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lim M, et al. Autoimmune encephalopathies. Pediatr Clin North Am. 2015;62:667–685. doi: 10.1016/j.pcl.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 64.Gleichman AJ, et al. Anti-NMDA receptor encephalitis antibody binding is dependent on amino acid identity of a small region within the GluN1 amino terminal domain. J Neurosci. 2012;32:11082–11094. doi: 10.1523/JNEUROSCI.0064-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gresa-Arribas N, et al. Antibody titres at diagnosis and during follow-up of anti-NMDA receptor encephalitis: a retrospective study. Lancet Neurol. 2014;13:167–177. doi: 10.1016/S1474-4422(13)70282-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hughes EG, et al. Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J Neurosci. 2010;30:5866–5875. doi: 10.1523/JNEUROSCI.0167-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moscato EH, et al. Acute mechanisms underlying antibody effects in anti-N-methyl-D-aspartate receptor encephalitis. Ann Neurol. 2014;76:108–119. doi: 10.1002/ana.24195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mikasova L, et al. Disrupted surface cross-talk between NMDA and Ephrin-B2 receptors in anti-NMDA encephalitis. Brain. 2012;135:1606–1621. doi: 10.1093/brain/aws092. [DOI] [PubMed] [Google Scholar]
- 69.Planaguma J, et al. Human N-methyl D-aspartate receptor antibodies alter memory and behaviour in mice. Brain. 2015;138:94–109. doi: 10.1093/brain/awu310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rose NR, Bona C. Defining criteria for autoimmune diseases (Witebsky's postulates revisited) Immunol Today. 1993;14:426–430. doi: 10.1016/0167-5699(93)90244-F. [DOI] [PubMed] [Google Scholar]
- 71.Pruss H, et al. Retrospective analysis of NMDA receptor antibodies in encephalitis of unknown origin. Neurology. 2010;75:1735–1739. doi: 10.1212/WNL.0b013e3181fc2a06. [DOI] [PubMed] [Google Scholar]
- 72.Granerod J, et al. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect Dis. 2010;10:835–844. doi: 10.1016/S1473-3099(10)70222-X. [DOI] [PubMed] [Google Scholar]
- 73.Gable MS, et al. The frequency of autoimmune N-methyl-D-aspartate receptor encephalitis surpasses that of individual viral etiologies in young individuals enrolled in the California Encephalitis Project. Clin Infect Dis. 2012;54:899–904. doi: 10.1093/cid/cir1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dalmau J, et al. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10:63–74. doi: 10.1016/S1474-4422(10)70253-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hafner H, et al. ABC Schizophrenia study: an overview of results since 1996. Soc Psychiatry Psychiatr Epidemiol. 2013;48:1021–1031. doi: 10.1007/s00127-013-0700-4. [DOI] [PubMed] [Google Scholar]
- 76.Novotna M, et al. Poor early relapse recovery affects onset of progressive disease course in multiple sclerosis. Neurology. 2015;85:722–729. doi: 10.1212/WNL.0000000000001856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lebon S, et al. Anti-N-methyl-D-aspartate (NMDA) receptor encephalitis mimicking a primary psychiatric disorder in an adolescent. J Child Neurol. 2012;27:1607–1610. doi: 10.1177/0883073812438099. [DOI] [PubMed] [Google Scholar]
- 78.Tidswell J, et al. Early recognition of anti-N-methyl D-aspartate (NMDA) receptor encephalitis presenting as acute psychosis. Australas Psychiatry. 2013;21:596–599. doi: 10.1177/1039856213506502. [DOI] [PubMed] [Google Scholar]
- 79.Barry H, et al. Anti-NMDA receptor encephalitis: an important differential diagnosis in psychosis. Br J Psychiatry. 2011;199:508–509. doi: 10.1192/bjp.bp.111.092197. [DOI] [PubMed] [Google Scholar]
- 80.Khandaker GM, et al. Inflammation and immunity in schizophrenia: implications for pathophysiology and treatment. Lancet Psychiatry. 2015;2:258–270. doi: 10.1016/S2215-0366(14)00122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Knight JG, et al. Rationale for a trial of immunosuppressive therapy in acute schizophrenia. Mol Psychiatry. 2007;12:424–431. doi: 10.1038/sj.mp.4001959. [DOI] [PubMed] [Google Scholar]
- 82.Häfner H, an der Heiden W. Course and outcome of schizophrenia. In: Hirsch SRI, Weinberger DR, editors. Schizophrenia. Blackwell; 2003. pp. 101–141. [Google Scholar]
- 83.Fillman SG, et al. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry. 2013;18:206–214. doi: 10.1038/mp.2012.110. [DOI] [PubMed] [Google Scholar]
- 84.Saetre P, et al. Inflammation-related genes up-regulated in schizophrenia brains. BMC Psychiatry. 2007;7:46. doi: 10.1186/1471-244X-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bloomfield PS, et al. Microglial activity in people at ultra high risk of psychosis and in schizophrenia: An [C]PBR28 PET brain imaging study. Am J Psychiatry. 2015 doi: 10.1176/appi.ajp.2015.14101358. appiajp201514101358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schizophrenia Working Group of the Psychiatric Genomics C. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pollak TA, et al. Prevalence of anti-N-methyl-D-aspartate (NMDA) receptor [corrected] antibodies in patients with schizophrenia and related psychoses: a systematic review and meta-analysis. Psychol Med. 2014;44:2475–2487. doi: 10.1017/S003329171300295X. [DOI] [PubMed] [Google Scholar]
- 88.Tsutsui K, et al. Anti-NMDA-receptor antibody detected in encephalitis, schizophrenia, and narcolepsy with psychotic features. BMC Psychiatry. 2012;12:37. doi: 10.1186/1471-244X-12-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hammer C, et al. Neuropsychiatric disease relevance of circulating anti-NMDA receptor autoantibodies depends on blood-brain barrier integrity. Mol Psychiatry. 2014;19:1143–1149. doi: 10.1038/mp.2013.110. [DOI] [PubMed] [Google Scholar]
- 90.Ransohoff RM, Engelhardt B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat Rev Immunol. 2012;12:623–635. doi: 10.1038/nri3265. [DOI] [PubMed] [Google Scholar]
- 91.Leypoldt F, et al. Autoimmune encephalopathies. Ann N Y Acad Sci. 2015;1338:94–114. doi: 10.1111/nyas.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Louveau A, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zandi MS, et al. N-methyl-D-aspartate receptor autoantibodies in psychiatric illness. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.03.034. [DOI] [PubMed] [Google Scholar]
- 94.Joubert B, et al. Clinical spectrum of encephalitis associated with antibodies against the alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor: Case series and review of the literature. JAMA Neurol. 2015;72:1163–1169. doi: 10.1001/jamaneurol.2015.1715. [DOI] [PubMed] [Google Scholar]
- 95.Bataller L, et al. Reversible paraneoplastic limbic encephalitis associated with antibodies to the AMPA receptor. Neurology. 2010;74:265–267. doi: 10.1212/WNL.0b013e3181cb3e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lancaster E, et al. Antibodies to metabotropic glutamate receptor 5 in the Ophelia syndrome. Neurology. 2011;77:1698–1701. doi: 10.1212/WNL.0b013e3182364a44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jarius S, et al. Psychotic syndrome associated with anti-Ca/ARHGAP26 and voltage-gated potassium channel antibodies. J Neuroimmunol. 2015;286:79–82. doi: 10.1016/j.jneuroim.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 98.Hedegaard M, et al. Molecular pharmacology of human NMDA receptors. Neurochem Int. 2012;61:601–609. doi: 10.1016/j.neuint.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zandi MS, et al. Clinical relevance of serum antibodies to extracellular N-methyl-D-aspartate receptor epitopes. J Neurol Neurosurg Psychiatry. 2015;86:708–713. doi: 10.1136/jnnp-2014-308736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Najjar S, et al. Neuropsychiatric autoimmune encephalitis without VGKC-complex, NMDAR, and GAD autoantibodies: case report and literature review. Cogn Behav Neurol. 2013;26:36–49. doi: 10.1097/WNN.0b013e31828b6531. [DOI] [PubMed] [Google Scholar]
- 101.Watabe-Uchida M, et al. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron. 2012;74:858–873. doi: 10.1016/j.neuron.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 102.Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci. 2007;27:11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kodangattil JN, et al. Spike timing-dependent plasticity at GABAergic synapses in the ventral tegmental area. J Physiol. 2013;591:4699–4710. doi: 10.1113/jphysiol.2013.257873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Olson VG, Nestler EJ. Topographical organization of GABAergic neurons within the ventral tegmental area of the rat. Synapse. 2007;61:87–95. doi: 10.1002/syn.20345. [DOI] [PubMed] [Google Scholar]
- 105.Tatard-Leitman VM, et al. Pyramidal cell selective ablation of N-methyl-D-aspartate receptor 1 causes increase in cellular and network excitability. Biol Psychiatry. 2015;77:556–568. doi: 10.1016/j.biopsych.2014.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ellaithy A, et al. Positive allosteric modulators of metabotropic glutamate 2 receptors in schizophrenia treatment. Trends Neurosci. 2015 doi: 10.1016/j.tins.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Banerjee A, et al. Src kinase as a mediator of convergent molecular abnormalities leading to NMDAR hypoactivity in schizophrenia. Mol Psychiatry. 2015;20:1091–1100. doi: 10.1038/mp.2014.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pitcher GM, et al. Schizophrenia susceptibility pathway neuregulin 1-ErbB4 suppresses Src upregulation of NMDA receptors. Nat Med. 2011;17:470–478. doi: 10.1038/nm.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hahn CG, et al. Altered neuregulin 1-erbB4 signaling contributes to NMDA receptor hypofunction in schizophrenia. Nat Med. 2006;12:824–828. doi: 10.1038/nm1418. [DOI] [PubMed] [Google Scholar]
- 110.Labrie V, Roder JC. The involvement of the NMDA receptor D-serine/glycine site in the pathophysiology and treatment of schizophrenia. Neurosci Biobehav Rev. 2010;34:351–372. doi: 10.1016/j.neubiorev.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 111.Hafner H, Nowotny B. Epidemiology of early-onset schizophrenia. Eur Arch Psychiatry Clin Neurosci. 1995;245:80–92. doi: 10.1007/BF02190734. [DOI] [PubMed] [Google Scholar]
- 112.Hafner H, et al. Sex differences in schizophrenia. Psychiatria Fennica. 1991;22:123–156. [Google Scholar]