Abstract
Basidiomycetes have several biotechnological and industrial applications such as enzyme production, bioremediation, pharmaceutical and functional food production. Due to climatic features, the preservation of several basidiomycetes is threatened, and to guarantee the preservation of this genetic resource, the development of long-term preservation techniques is necessary once there is no universal protocol for the cryopreservation of basidiomycetes. Cryopreservation is a technique in which microorganisms are submitted to ultralow temperatures. Therefore, this study aimed to collect information on the main conditions for long-term cryopreservation of basidiomycetes in the last 20 years. Scientific articles on cryopreservation of basidiomycetes published from 1997 to 2016, were researched, and only the studies on two intervals of cryopreservation were considered: from 1 to 2 years and for longer than 2 years. The analyzed conditions of basidiomycete cryopreservation were: most studied genera, cryopreservation temperature, substrate, cryoprotectant (and preservation substrate), cryopreservation period, thawing temperature and cultivation medium after thawing, physiological and genetic stability of basidiomycetes after thawing in cryopreservation. In this review, the viability of the main cryopreservation conditions of basidiomycetes studied in the last 20 years are presented and discussed.
Keywords: Preservation, Substrate, Fungi, Mycelial viability, Cryoprotectant
Introduction
Basidiomycetes are utilized to convert agricultural and agroindustrial residues into biomass, enzymes or bioremediation.1, 2, 3 Laccase production by these fungi has been applied to blanching/decoloring and biopulping4, 5, 6 and to biodegradate of xenobiotic pollutants.7, 8 Furthermore, the spent substrate from mushroom production has been used as a highly valuable organic fertilizer, developing the economy of ecological recycling.9
Basidiomycetes are also fundamental to the development of pharmaceutical and functional products10 species such as Grifola frondosa (Dicks.) Gray, Ganoderma lucidum (Curtis) P. Karst, Lentinula edodes (Berk.) Pegler, Pleurotus ostreatus (Jacq. ex Fr.) P. Kumm., Volvariella volvacea (Bulliard ex Fries) Singer11, 12 and Agaricus subrufescens Peck (Agaricus blazei Murrill; Agaricus brasiliensis Wasser et al.)13, 14 among others. These species are characterized by their content of β-d glucans, proteoglycans, triterpenes, phenolic compounds, among others, with immune-stimulating, antitumoral, hypoglycemic, antifungal, antiviral, antibacterial, anti-inflammatory, antiallergenic, antioxidant, and hypocholesterolemic activity.11, 12, 15, 16, 17
It is estimated that the Fungi kingdom consists of 1.5 million species, and 70,000 have been described, 14,000 produce known mushroom and 650 have pharmacological properties.18 However, only 10 species are cultivated in commercial scale.9 This implies that an enormous biodiversity of basidiomycetes has not been explored yet. Thus, it is necessary to preserve these species. However, climatic changes have affected ecological systems changing the life cycle of fungi19, 20, 21 which may cause a loss of genetic diversity. The growing industrial importance of fungi and inherent risks of climatic changes imply vulnerability of genetic diversity which will be reduced with the development of preservation techniques.22
Different preservation methods have been utilized for basidiomycetes and the most versatile technique is cryopreservation, in which standard domestic freezers can be used having proved to be ideal and economical tools to keep frozen culture collections.23 Lyophilization may be one of the most widely used technologies to preserve several fungi and is the primary technique used in most general service culture collections.23 However, these techniques are inappropriate for most basidiomycetes.24
Another widely used method is periodical subculture but it takes up more time, space, reagents and is susceptible to contamination, genetic and physiological long-term changes.25, 26, 27, 28 Techniques for fungal preservation in distilled water, silica, and mineral oil are less effective in the long run.26, 27, 29 Depending on the utilized preservation technique, basidiomycetes can reduce productivity, lose morphological characteristics, and pharmacological activity.30
Several factors affect cryopreservation of basidiomycetes such as species, growth phase, substrate, cultivation conditions, cryoprotectant, among others.31, 32 Few microorganisms survive after freezing and thawing,32 mainly due to cryoinjuries caused to cell membrane and cell wall.32
Cryoinjuries are related to two main aspects: the formation of intracellular ice crystals, and the water flow outward the cell (dehydration) increasing the intracellular concentration of solutes.32, 33, 34 Despite that, cryopreservation at several freezing temperatures35 is the most recommended method by several microorganism culture collections such as the American Type Culture Collection (ATCC) and the National Collection of Types Cultures (NCTC).36, 37 Cryopreservation allows that most cultures remain stable for a long period of time, and may reach up to 30 years, due to the low occurrence of metabolic activity at these temperatures; that is why it is the most utilized technique by most microbial banks.38 However, few studies on cryopreservation have been carried out with basidiomycetes.
The main studies on fungal cryopreservation suggest that there is not a universal technique or protocol and that each species has to be studied separately,39 even though there are generalizations for the cryopreservation procedures for basidiomycetes.40 Also, basidiomycete preservation is difficult due to the predominance of vegetative mycelia, which are sensitive to environmental variations, and absence of asexual spores in many species.25 Therefore, because of the importance of basidiomycetes in biotechnology and the need to verify favorable conditions for the cryopreservation of basidiomycetes in the long run, this study aims to collect information about the main conditions for long-term cryopreservation of basidiomycetes in the last 20 years.
Methodology
This bibliographical review collected scientific articles on cryopreservation of basidiomycetes published from 1997 to 2016 in the databank of Web of Science, World Wide Web and Google Scholar. The search tags were cryopreservation, mushroom, basidiomycete, preservation and fungi. The mycelial viability was verified after cryopreservation, and the treatments where mycelial growth was equal or greater than 75% of the replications were considered viable.41 The studies on cryopreservation of basidiomycetes were divided into two groups: one-year to two-year cryopreservation (short term) and cryopreservation of more than two years (long term). Both groups were divided into subgroups to evaluate genus, final freezing temperature of −20 °C, from −70 to −86 °C and −196 °C, substrate and preservation substrate used in cryopreservation, cryoprotectant type and concentration, and cryopreservation period. In this review, any substrate that works preserving mycelia in cryopreservation without cryoprotectants was considered a preservation substrate. Only for the cryopreservation period, the treatments were divided into cryopreservation of 1–2 years, 2–3 years, 3–5 years, or more than 5 years.
In general, short-term cryopreservation periods are more successful regarding mycelial viability recovery. However, short time periods are not significant to differentiate physiological and genetic characteristics, but they are effective to verify when a technique is unviable. Mantovani et al.42 cryopreserved L. edodes at −70 °C for one, two and three years and verified loss of mycelial viability in most treatments, between the first and second year; however, after the second and third year of cryopreservation, the viability was kept. Colauto et al.41 also observed loss of viability after four years of cryopreservation at −70 °C in 8 out of 12 evaluated treatments. Nevertheless, Singh et al.43 cryopreserved different strains of basidiomycetes in liquid nitrogen and observed viability maintenance for most strains.
According to Nakasone et al.,44 long-term preservation occurs when cultures are preserved for longer than one year using a specific technique. However, for Ryan et al.,39 long-term fungal preservation occurs when the culture preservation period is longer than two years. According to Mantovani et al.,42 only after two years it is possible to verify differences in cryopreservation efficiency. Therefore, in this review, we will consider only basidiomycete cryopreservation of 1-2 years (short term) and of more than 2 years (long term). Cryopreservation studies shorter than a year were not considered due to the insufficient time to evaluate the long-term technical efficiency.
Literature survey results
The search for cryopreservation articles resulted in a total of 17 articles regarding basidiomycete cryopreservation. Eleven articles on cryopreservation of basidiomycetes were not included in this study because they were studies shorter than one-year cryopreservation. The information collected from the articles was organized according to their treatments and cryopreservation period.
Genera of cryopreserved basidiomycetes
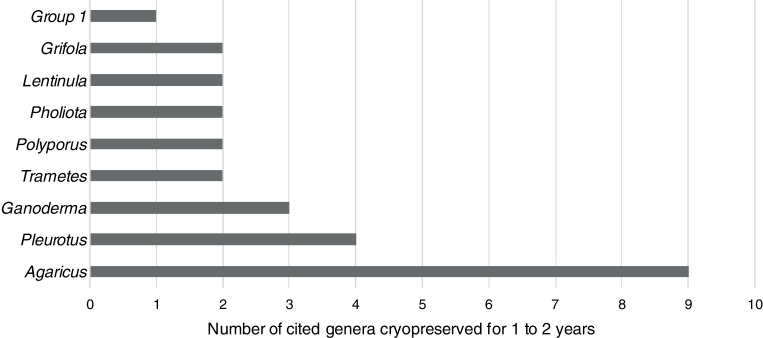
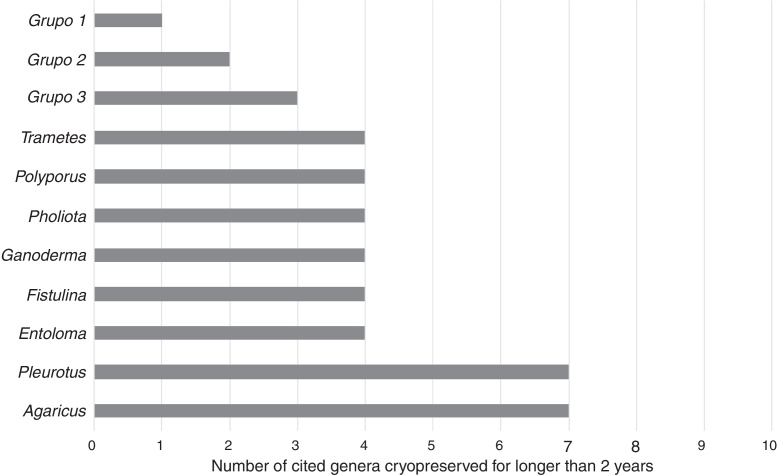
Thirty-seven genera were evaluated for 1-year to 2-year cryopreservation and 114 genera for longer than 2-year cryopreservation. Agaricus, Pleurotus and Ganoderma were the most cited genera (Fig. 1, Fig. 2).
Fig. 1.
Most cited genera of basidiomycetes cryopreserved for 1–2 years. Group 1 = Boletus, Coprinopsis, Cryptoporus, Cystoderma, Entoloma, Fistulina, Flammulina, Fomes, Hebeloma, Inonotus, Lampteromyces, Langermannia, Lepista, Lycoperdon, Micromphale, Microporus, Neolentinus, Paxillus, Pleurocybella, Pluteus, Psathyrella, Pycnoporus, Sarcodontia, Schizophyllum, Scleroderma, Serpula, Sparassis, Tephrocybe and Trichaptum genus.
Fig. 2.
Most cited genera of basidiomycetes cryopreserved for longer than 2 years. Group 1 = Anthurus, Auricularia, Bolbitius, Boletus, Bondarzewia, Bovista, Calocybe, Campanella, Ceriporia, Clavariadelphus, Coprinopsis, Coprinus, Cryptoporus, Cystoderma, Hebeloma, Hymenochaete, Irpex, Laccaria, Lacrymaria, Langermannia, Leucocoprinus, Leucopaxillus, Lycoperdon, Macrolepiota, Microporus, Neolentinus, Pachykytospora, Strobilurus, Stropharia, Tephrocybe and Volvariella genus. Group 2 = Aurantioporus, Bjerkandera, Calvatia, Clitopilus, Coniophora, Coriolopsis, Creolophus, Cyathus, Daedalea, Daedaleopsis, Dichomitus, Faerberia, Fomes, Gloeophyllum, Gymnopilus, Hapalopilus, Hericium, Hohenbuehelia, Hypholoma, Hypsizygus, Ischnoderma, Kuehneromyces, Laetiporus, Lampteromyces, Laricifomes, Lentinellus, Lentinus, Lenzites, Lyophyllum, Meripilus, Merulius, Micromphale, Onnia, Panellus, Phallus, Phanerochaete, Phlebiopsis, Piptoporus, Psilocybe, Pyrofomes, Rhodocybe, Rhodotus, Rigidoporus, Sarcodontia, Scleroderma, Serpula, Sparassis, Stereum, Tubaria, Tyromyces, Vascellum, Xerula and Xylobolus genus. Group 3 = Abortiporus, Agrocybe, Antrodia, Armillaria, Clitocybe, Collybia, Flammulina, Fomitopsis, Grifola, Inonotus, Lentinula, Lepista, Oudemansiella, Paxillus, Phaeolus, Phellinus, Pleurocybella, Pluteus, Psathyrella, Pycnoporus, Schizophyllum and Trichaptum genus.
The most preserved genera are also the most cultivated due to their nutritional, functional and/or biotechnological interest such as Agaricus bisporus, A. subrufescens, P. ostreatus and G. lucidum. In China, the greatest worldwide producer, 60 different types of mushrooms are cultivated and the most popular genera are Pleurotus, Lentinula and Auricularia.10 However, A. bisporus is the world most commonly cultivated species, mainly in United States of America, Poland, Netherland and Spain.57, 58 The great number of commercially cultivated mushrooms in the world and the continuous discovery and introduction of new strains of basidiomycetes make the development of cryopreservation essential for the maintenance of this biological material. Despite that, there is a mismatch between the increase in mushroom type in the world production and the number of techniques of basidiomycete preservation.
Mushrooms are an important product in global trade with an estimated production of 3.4 million metric tons in 2007. China is the biggest producer, consumer, and exporter of mushrooms in the world, with a market of US$ 82.9 million in 2008 and 2009.10 The main produced and commercialized mushrooms in Brazil are A. bisporus, P. ostreatus, A. subrufescens and L. edodes.59 A. bisporus mushrooms are the most cultivated and consumed worldwide10 and in Brazil.60 However, there are few and conflicting data about Brazilian mushroom consumption and production, and this makes difficult to prioritize cryopreservation studies.
In conclusion, the most studied genus in short-term cryopreservation is Agaricus and in long-term cryopreservation are Agaricus and Pleurotus genera. A. bisporus and A. subrufescens have worldwide importance and are the main species studied in Agaricus genus. However, A. subrufescens has been utilized in cryopreservation in most recent studies due to its recent commercialization, importance and sensitivity to cryopreservation process. P. ostreatus is the most cited species in this genus because of its workability and worldwide importance.
Cryopreservation temperature for basidiomycetes
Cryopreservation at −20 °C has had reduced success in basidiomycete recovery (Table 1, Table 2). For 2-year cryopreservation at −20 °C there were 134 treatments and there was recovery of mycelial viability only in 7.5% (Table 1). For cryopreservation longer than 2 years at −20 °C there were 132 treatments with only 1.5% of recovery (Table 2). Thus, despite being possible for some species such P. ostreatus preserved until three years,52 cryopreservation at a −20 °C is not a viable technique for the preservation of basidiomycetes.42, 51
Table 1.
Freezing temperature, treatments with mycelial viability recovery ≥ 75%, and number of treatments of basidiomycetes submitted to different cryopreservation temperatures from 1 to 2 years.
Table 2.
Freezing temperature, treatments with mycelial viability recovery ≥ 75%, and number of treatments of basidiomycetes submitted to different cryopreservation temperatures for longer than 2 years.
Freezing temperatures from −15 to −60 °C are a challenge to cell cryopreservation as they often result in cryoinjuries caused by ice formation, water migration and/or ion concentration. One of the main cell injuries to overcome in cryopreservation at −20 °C is slow freezing that forms long ice crystals in the cell interstice causing cellular membrane injuries.61 Besides the loss of cellular structure, slow freezing of external water causes cell dehydration and increase in cytoplasmic ion concentration. This process reduces the freezing temperature of cytoplasm turning it in a highly viscous concentration mixture with low metabolic activity but with irreversible damages to cell and/or cell proteins.62 However, Mantovani et al.52 demonstrated that cryopreservation at −20 °C is efficient for the maintenance of P. ostreatus until three years with 87% of mycelial viability when wheat grains are used as substrate and 10% sucrose or 4% glucose are utilized as cryoprotectant. Thus, the temperature of −20 °C to preserve basidiomycetes is possible if the cryoprotection conditions to reduce the effect of cryoinjuries are found. These conditions still need to be determined for a lot of species and would enable long-term maintenance of the viability of basidiomycetes with low cost equipment in the long run.
The most studied freezing temperature in cryopreservation of basidiomycetes in several articles ranged from −70 °C to −86 °C (Table 1, Table 2). The number of studies is greater for cryopreservation at −70 °C to −86 °C in a period ranging from 1 year to 2 years (Table 1), and equivalent at −70 °C to −86 °C and −196 °C when cryopreservation is longer than 2 years, and despite the numbers of treatments, the studies are greater at −196 °C (Table 2). The mycelial viability for cryopreservation conducted from −70 °C to −86 °C is similar for 1-year to 2-year cryopreservation but greater at −196 °C when cryopreservation is longer than 2 years (Table 1, Table 2). This suggests that, because it is a technique (liquid nitrogen) that has been established longer, it has been preferably utilized in cryopreservation studies, and because ultrafreezers from −70 °C to −86 °C are a technique that more recently has become more viable economically, it has been less utilized without being necessarily less effective in the long run.
Ito63 and Ito and Yokoyama64 preserved non-spore forming basidiomycetes and ascomycetes in aqueous solution with 10% glycerol for longer than 5 years in a mechanic ultrafreezer at −80 °C. This procedure causes smaller damages to the cell due to a smaller dehydration and membrane rupture.62 Cryopreservation at −80 °C is appropriate for several fungi, including basidiomycetes.43, 65 However, to completely stabilize frozen cultures, the temperature has to be sufficiently reduced to decrease the metabolism and prevent the formation of ice crystals.66 According to Homolka,40 the limit temperature to prevent ice crystal formation is −139 °C and this is why a lot of culture collections keep their samples at −150 °C in ultrafreezers equipped with liquid nitrogen.
Freezing in liquid nitrogen (−196 °C) is considered one of the best preservation methods of microorganisms in general.67 Despite that, Colauto et al.46 verified that A. subrufescens strains respond differently to cryopreservation at −196 °C, presenting a recovery of mycelial viability of two out of five strains. For those authors, the fast freezing of A. subrufescens mycelia in liquid nitrogen is not the most appropriate method to preserve this species. Eichlerová et al.68 verified no significant loss of laccase activity in the samples of Trametes versicolor after short-time (24 h) storage at −80 °C but the storage in liquid nitrogen caused 10.6–21.7% laccase activity decrease. The authors described that controlled freezing in liquid nitrogen, using a cooling rate of 1 or 3 °C per min, reduced laccase activity changes from 1.47 to 18.42% after short-time storage depending on the kind and concentration of the cryoprotectant. After 6 months storage in liquid nitrogen, a decrease of laccase activity of 21–44% was found; however, when preserved at −80 °C, laccase activity decrease was from 19 to 28%. Thus, fast freezing in liquid nitrogen can cause cryoinjuries and the utilization of this technique must be rigorously followed. Cooling rate is a parameter that strongly affects the viability of living cells during freezing process.68, 69 The damage principle is that crystallization of water in extracellular medium increases instantaneously concentration of internal cytoplasm and the osmotic pressure, damaging the enzymatic activity.69 Another interesting aspect is that the recovery of viability after freezing at −80 °C is faster than in liquid nitrogen which may take days or weeks.70
Cryopreservation in liquid nitrogen is still an effective way to preserve several organisms, including basidiomycetes that cannot be lyophilized, at least not yet. The main disadvantages of liquid nitrogen are the high cost of equipment, constant refilling of liquid nitrogen (every two days), separate large space for storage, and the need of constant surveillance.44 However, due to the risk of biological material loss by rupture of containers, other techniques must be utilized to guarantee the maintenance of culture collections. Cryopreservation at −80 °C in mechanic ultrafreezers is a lower cost alternative and that can be coupled to an automated energy generator in case of failure of electric energy supply. A second backup ultrafreezer should be considered in case of mechanic failure of the equipment. Therefore, it is possible to obtain similar results of long-term cryopreservation for basidiomycetes, mainly in centers where the supply of liquid nitrogen is not regular.
Few studies report basidiomycete cryopreservation by vitrification or encapsulation.71, 72 In the vitrification system, the vitrification solution surrounds the cells forming an amorphous glass and this prevents cryoinjuries. Samples may be rapidly cooled in liquid nitrogen without a system to control cooling rate. In the encapsulation process, the cells are embedded in calcium alginate beads prior to cryopreservation.
The utilization of encapsulation has two main benefits: firstly, the water content of cells can be reduced decreasing the risk of ice damages or concentration effects during the cooling stage of the procedure; secondly, it allows cells to be easily handled and manipulated by providing a suitable suspending matrix.73 Wood et al.71 reported simultaneous preservation of orchid seeds and its fungal symbiont, Ceratobasidium cornigerum, using alginate encapsulation with no adverse effects after cryopreservation. Serpula lacrymans was immobilized on calcium alginate beads and cryopreserved at −20 °C for one month with success compared to control.72
In conclusion, cryopreservation at −20 °C is still a challenge, mainly in long term. However, P. ostreatus has been cryopreserved successfully for 3 years when wheat grains were used as substrate and sucrose or glucose as cryoprotectant. In short term, cryopreservation between −70 °C and −86 °C and −196 °C has been equivalently successful on mycelial viability recovery, but in long term, cryopreservation at −196 °C has been 30% greater than between −70 °C and −86 °C, although the number of treatments at −196 °C is three times greater. Few studies have evaluated basidiomycete cryopreservation using vitrification and encapsulation techniques, opening new possibilities for the preservation of this biological material.
Substrates for basidiomycete cryopreservation
The substrates utilized for mycelial growth before cryopreservation are several such as agar-based culture media, mainly potato dextrose agar (PDA) and malt extract agar (MEA), up to cereal grains or perlite-based (Table 3, Table 4). The most cited substrate treatments for basidiomycetes cryopreserved from 1 to 2 years were those with grains, perlite, agar, sawdust, and grains and perlite were the most recently tested in greater amounts (Table 3). The most cited substrate treatments for basidiomycetes cryopreserved for longer than 2 years were those based on agar, wort, grain and sawdust (Table 4). Probably, the agar-based substrate is predominant because they are established procedures and are always used as control instead of treatment variations; however, new treatments with grains have come up as an alternative (Table 3, Table 4).
Table 3.
Substrate, treatments with mycelial viability recovery ≥ 75%, and number of treatments of basidiomycetes submitted to different substrates for 1–2 year cryopreservation.
| Substrate | Mycelial viability treatments (%) | Number of treatments | Source |
|---|---|---|---|
| Agar | |||
| Ground wheat grain | 0 | 10 | 50 |
| Potato-dextrose | 26.9 | 26 | 42, 47, 52 |
| Malt-extract | 42.4 | 33 | 41, 46, 50, 51 |
| Ground hard endosperm wheat grain + glycerol | 0 | 6 | 51 |
| Ground hard endosperm wheat grain + glycerol | 16.7 | 6 | 51 |
| Wheat grain ground semi-hard endosperm + glycerol | 33.3 | 6 | 51 |
| Ground semi-hard endosperm wheat grain + glycerol | 16.7 | 6 | 51 |
| Ground semi-hard endosperm wheat grain + glycerol | 33.3 | 6 | 51 |
| Ground hard endosperm rye grain + glycerol | 33.3 | 6 | 51 |
| Ground hard endosperm rye grain + glycerol | 33.3 | 6 | 51 |
| Barley flour yeast extract + sucrose | 37.5 | 8 | 49 |
| Grain | |||
| Rice with hull | 41.7 | 12 | 45, 47 |
| Rice without hull | 54.2 | 24 | 42, 52 |
| Oat | 50 | 24 | 42, 52 |
| Wheat | 75 | 24 | 42, 52 |
| Hard endosperm wheat | 33.3 | 6 | 51 |
| Semi-hard endosperm wheat | 33.3 | 6 | 51 |
| Hard endosperm wheat + glycerol | 33.3 | 6 | 51 |
| Semi-hard endosperm wheat + glycerol | 33.3 | 6 | 51 |
| Sorghum | 50 | 12 | 48 |
| Hard endosperm rye | 33.3 | 6 | 51 |
| Hard endosperm rye + glycerol | 33.3 | 6 | 51 |
| Millet | 54.2 | 24 | 42, 52 |
| Perlite | |||
| Wort + 5% glycerol | 96.8 | 155 | 24 |
| Sawdust | |||
| Rice bran + 10% glycerol | 100 | 69 | 27 |
Table 4.
Substrate, treatments with mycelial viability recovery ≥ 75%, and number of treatments of basidiomycetes submitted to different substrates for longer than 2 year cryopreservation.
| Substrate | Mycelial viability treatments (%) | Number of treatments | Source |
|---|---|---|---|
| Agar | |||
| Potato-dextrose | 22.2 | 36 | 42, 52 |
| Malt-extract | 27.8 | 18 | 41, 51 |
| Malt-extract + glycerol | 0 | 6 | 51 |
| Malt-extract + yeast extract + glycerol | 93.2 | 176 | 53 |
| Ground hard endosperm wheat grain | 0 | 6 | 51 |
| Ground hard endosperm wheat grain + glycerol | 0 | 6 | 51 |
| Ground semi-hard endosperm wheat grain | 0 | 6 | 51 |
| Ground semi-hard endosperm wheat grain + glycerol | 0 | 6 | 51 |
| Ground hard endosperm rye grain | 0 | 6 | 51 |
| Ground hard endosperm rye grain + glycerol | 0 | 6 | 51 |
| Wort | 61.3 | 243 | 53, 54 |
| Grain | |||
| Rice with hull | 27.8 | 36 | 42, 52 |
| Oat | 27.8 | 36 | 42, 52 |
| Wheat | 60.9 | 46 | 42, 43, 52 |
| Hard endosperm wheat | 16.7 | 6 | 51 |
| Semi-hard endosperm wheat | 16.7 | 6 | 51 |
| Hard endosperm wheat + glycerol | 16.7 | 6 | 51 |
| Semi-hard endosperm wheat + glycerol | 16.7 | 6 | 51 |
| Hard endosperm barley | 0 | 6 | 51 |
| Hard endosperm barley + glycerol | 0 | 6 | 51 |
| Millet | 36.1 | 36 | 42, 52 |
| Sorghum | 100 | 4 | 56 |
| Sawdust | |||
| Rice bran + 10% glycerol | 97.1 | 70 | 27 |
| Perlite | |||
| Wort + 5% glycerol | 100 | 265 | 54, 55 |
Wheat grain has been effective as a substrate even without cryoprotectant to maintain the mycelial viability after cryopreservation (Table 3, Table 4). It is possible that the positive effect of wheat grain is related to carbohydrates and proteins that are capable of bonding water to the grain,74 reducing free water and formation of intracellular ice crystals. Another possibility is that the wheat grain structure, consisting of a capillarity network, provides physical protection to the mycelium.51 Mata and Rodríguez-Estrada75 evaluated the viability of A. bisporus in wheat grains with and without glycerol as a cryoprotectant and reported mycelial growth even in the absence of the cryoprotectant, suggesting that wheat grains have physical attributes that allow mycelia to survive liquid nitrogen cryopreservation process. Another possibility is that a specific compound in the seed, such as starch or sugar, might act as a non-permeating cryoprotectant,76 thereby reducing the injuries associated with freezing and thawing.
Mantovani et al.52 reported the maintenance of P. ostreatus viability grown in wheat grains and cryopreserved for 3 years at −20 °C and −70 °C. Another genus such as Rhizoctonia has been preserved for more than 10 years in seeds of oats, barley, wheat, rye, millet and sorghum.77 Mata et al.56 reported 100% mycelial viability of Pleurotus spp. grown in sorghum and frozen with 10% glycerol for eight years in liquid nitrogen. Tanaka et al.51 showed that the whole grains of hard endosperm wheat had the highest mycelial growth recovery (87%) after two-year cryopreservation at −70 °C than on agar ground hard endosperm wheat. They demonstrated that grain capillary physical structure is more important than grain chemical composition in substrate for keeping mycelial viability after cryopreservation. The grain capillary net provides reduced physical space that limits the presence of water molecules78, 79 and protects mycelia from ice formation. Although the capillarity in the substrate is a fundamental factor in cryopreservation, there is still the presence of anti-freeze proteins produced by hard endosperm wheat grains and used as an alternative for A. subrufescens cryopreservation at −70 °C.51 Grains have been working like a preservation substrate in cryopreservation even without cryoprotectants.
Perlite with wort and 5% glycerol, and sawdust with rice bran and glycerol, also presented treatments with greater mycelial viability from 1 to 2 years and after 2 years (Table 3, Table 4). However, the treatment of agar-based cryopreservation after 2 years with wort or malt extract + yeast extract + glycerol was very promising (Table 4). On the other hand, a series of combinations of agar-based substrate with malt extract, wheat grain or ground yeast were not effective for the recovery of mycelial viability after 2 years of cryopreservation (Table 3, Table 4).
The cryopreservation method using perlite54 with more than 400 tested strains, or sawdust27 with more than 30 evaluated strains or sorghum grain56 with four strains, for more than 5 years, was effective to maintain mycelial viability of basidiomycetes. Perlite is the only volcanic mineral of aluminum silicate that retains large quantities of water to release as needed. The protocol with perlite can be used to cryopreserve groups of taxonomically different fungi, besides basidiomycetes, including yeast,26 and is as an alternative for other fungi that did not survive routine procedures of preservation.
In conclusion, substrates such as perlite + wort + glycerol, followed by sawdust + rice bran + glycerol and cereal grains (wheat, rice, sorghum, oat or millet), without cryoprotectant added to the substrate, have been more effective in short-term cryopreservation, mainly when compared to agar-based culture medium. Long-term cryopreservation have been more effective in substrates such as perlite + wort + glycerol, followed by sawdust + rice bran + glycerol, sorghum grains with glycerol, and wheat grains with or without cryoprotectant added to the substrate. However, the agar-based substrate of malt-extract + yeast-extract + glycerol has been also effective in long-term cryopreservation when yeast and glycerol were added to the substrate.
Cryoprotectants for basidiomycete cryopreservation
A cryoprotectant is utilized to reduce cryoinjuries in the cell during freezing and cryopreservation. The mechanisms in which these cryoprotectants act have been reviewed by Meryman,80 Calcott,81 Heckly82 and Hubálek.32 It is believed that the cellular membrane is the primary location of the injury due to formation of ice crystals during freezing, causing physical stress and cellular rupture.83 Furthermore, ice concentrates solutes that cause chemical stress in cellular molecules.32
Different cryoprotectants are utilized in cryopreservation of basidiomycetes, and the most cited treatments use glycerol, followed by dimethyl sulfoxide, glucose and sucrose for cryopreservation after 2 years (Table 5, Table 6). Hubálek32 classified the cryoprotectants according to the rate of penetration: those that penetrate quickly, usually within 30 min, the ones that penetrate more slowly, and non-penetrating or non-permeating compounds that cause extracellular cryoprotection when present at concentrations of 10–40%. Also, the author classified cryoprotectants as those that penetrate only the cell wall and not the cytoplasmic membrane and those that penetrate both cell wall and cytoplasmic membrane.
Table 5.
Cryoprotectant, treatments with mycelial viability recovery ≥ 75%, and number of treatments of basidiomycetes submitted to different cryoprotectants from 1-2 year cryopreservation.
| Cryoprotectant | Mycelial viability treatment (%) | Number of treatments | Source |
|---|---|---|---|
| Ultrapure water | 0 | 32 | 50, 51 |
| Dimethyl sulfoxide | |||
| 1% | 59 | 22 | 41, 42, 52 |
| 10% | 40 | 5 | 46 |
| Malt extract | |||
| 4% | 50 | 10 | 42 |
| 5% | 58.3 | 12 | 41, 52 |
| Glycerol | |||
| 5% | 83.6 | 201 | 24, 47, 50, 51, 52 |
| 6% | 100 | 2 | 41 |
| 10% | 47.1 | 34 | 42, 45, 48, 49 |
| Glucose | |||
| 4% | 50 | 26 | 41, 42, 50, 52 |
| Polyethylene glycol | |||
| 6% | 35 | 20 | 42, 52 |
| 8% | 100 | 2 | 41 |
| Sucrose | |||
| 10% | 65 | 20 | 42, 52 |
| 15% | 33.3 | 6 | 41, 50 |
| Without cryoprotectant | 34.2 | 38 | 48, 50, 51 |
Table 6.
Cryoprotectant, treatments with mycelial viability recovery ≥ 75%, and number of treatments of basidiomycetes submitted to different cryoprotectants for longer than 2 year cryopreservation.
| Cryoprotectant | Mycelial viability treatments (%) | Number of treatments | Source |
|---|---|---|---|
| Ultrapure water | 0 | 28 | 51 |
| Dimethyl sulfoxide | |||
| 1% | 28.1 | 32 | 41, 42, 52 |
| Malt extract | |||
| 4% | 20 | 20 | 42 |
| 5% | 33.3 | 12 | 41, 52 |
| Glycerol | |||
| 5% | 91.2 | 479 | 51, 52, 53, 54, 55 |
| 6% | 0 | 2 | 41 |
| 10% | 68 | 337 | 27, 42, 53, 54, 56 |
| 15% | 100 | 10 | 43 |
| Glucose | |||
| 4% | 46.9 | 32 | 41, 42, 52 |
| Polyethylene glycol | |||
| 6% | 10 | 30 | 42, 52 |
| 8% | 0 | 2 | 41 |
| Sucrose | |||
| 10% | 50 | 30 | 42, 52 |
| 15% | 100 | 2 | 41 |
| Without cryoprotectant | 0 | 28 | 51 |
Glycerol is the most cited cryoprotectant in treatments with cryopreservation of 1–2 years and it was also the most effective to keep mycelial viability after cryopreservation, differently from dimethyl sulfoxide that is not so effective to basidiomycetes when compared with glycerol (Table 5). In general, those without cryoprotectants that had cereal grains as substrate (preservation substrate) or when the number of treatments was not representative, even with a greater mycelial viability, were not considered in our analysis. Tanaka et al.51 suggested that the use of glycerol in association with whole grains could improve the mycelial viability of A. subrufescens cryopreserved at −80 °C and −20 °C.
The most cited cryoprotectant in treatments with cryopreservation after 2 years, glycerol, was also the most effective for the maintenance of mycelial viability after cryopreservation; however, other cryoprotectants such as sucrose and glucose are good alternatives that have shown to be efficient while dimethyl sulfoxide has shown lower efficiency (Table 6). Glycerol is classified as a penetrating or intracellular cryoprotectant and has the property to bond itself to water molecules, minimizing the formation and size of ice crystals as well as reducing solute concentrations in the extracellular or intracellular medium.32, 84 This cryoprotectant penetrates the cellular membrane through passive diffusion, getting into the membrane as well as in the cytoplasm85; its protective effects are represented by colligative properties, reducing the freezing point with consequent reduction of electrolyte concentration in the non-frozen fraction of the sample.86
Although glycerol is the most utilized cryoprotectant in the preservation of frozen basidiomycetes, it can have adverse effects like physical and chemical alterations, rupture of plasmatic membrane, removal of important membrane proteins, and it can induce modifications in the stability of the lipid structure.87, 88
The concentration of glycerol ranged from 5 to 15%, with greater mycelial viability at 5% for cryopreservation after 2 years (Table 6). Ito and Nakagiri89 examined the viability of 939 strains of basidiomycetes cryopreserved at −80 °C for one, five and 15 years with effective mycelial viability of 88% of these strains in agar plugs with mycelia submersed in cryoprotectant solution of 10% glycerol. Tanaka et al.51 suggested that the use of glycerol in association with whole grains could improve the mycelial viability of A. subrufescens cryopreserved at −80 °C and −20 °C. Colauto et al.41 reported that concentrations of 10–30% glycerol caused inhibition of mycelial growth of A. subrufescens. However Mata et al.56 reported that 10% glycerol with sorghum grown with Pleurotus spp. had mycelial viability of after eight years of cryopreservation in liquid nitrogen.
The cryoprotective effect of glycerol is probably due to the capacity of this cryoprotectant to penetrate the cellular wall and the plasmatic membrane and to link water in the interstice between the wall and the plasmatic membrane,90 increasing the membrane elasticity, which allows the molecular accommodation during the volume expansion in freezing.60, 91
Sucrose as well as glucose showed effective mycelial viability in cryopreserved treatments of more than 2 years (Table 6). Colauto et al.45 evaluated A. subrufescens cryopreservation at −70 °C using different cryoprotectants. For those authors, the cryoprotective agent – and not the freezing protocol – was the most important variable for effective mycelial viability after cryopreservation. Long-term cryopreservation (4 years) of A. subrufescens was effective when sucrose or glucose was used as cryoprotectant, regardless of the freezing protocol, and glycerol, polyethylene glycol, and malt extract were ineffective, dimethyl sulfoxide was effective only when a slow freezing protocol was used.41 However, for cryopreservation of 1 year, all cryoprotectants were effective to keep mycelial viability of A. subrufescens.45 Sucrose and glucose are not able to diffuse themselves through the plasmatic membrane, but they increase the external osmotic strength and promote the cellular dehydration, reducing the formation of ice crystals within the cell.32 These sugars can be utilized as a source of carbon in the recovery of the cellular growth. Sucrose is frequently used in cryopreservation of microorganisms at concentrations from 1 to 68% (average of 10%).32 Colauto et al.41 reported concentrations of 5, 15 and 30% sucrose in cultivation media with inhibition of mycelial growth after 15%.
Cryoprotectant agents can be utilized as an additive to the substrate. Tanaka et al.51 added glycerol to the substrate before cryopreservation of A. subrufescens with effective increase of mycelial viability after two-year cryopreservation at −70 °C. The utilization of cryoprotectants as a solution in the cryotube or an addition to the substrate is important to long-term basidiomycete cryopreservation. However, the choice of concentration and cryoprotectant depends on the cell type, strain, mycelial age, substrate, cultivation conditions, among other factors that affect the permeability and elasticity of membranes.41 Despite the importance of the cryoprotectant for the maintenance of cellular viability throughout the cryopreservation, this compound can cause mycelial growth inhibition. Therefore, the type of cryoprotectant, the concentration and inhibition or adverse effects on mycelial growth must be defined for each case, depending on strain and the cryopreservation method. Colauto et al.41 evaluated the adverse effects of cryoprotectants used in cryopreservation of A. subrufescens and reported that the increase of the concentration inhibit mycelial growth. According to these authors, dimethyl sulfoxide and glycerol inhibited mycelial growth, mainly dimethyl sulfoxide at concentrations of 2.5% and 5%, completely inhibiting mycelial growth, but sucrose, glucose, polyethylene glycol and malt extract showed low inhibition of mycelial growth.
In conclusion, the use of cryoprotectants in short-term cryopreservation has been effective mainly with glycerol, followed by sucrose, dimethyl sulfoxide, malt extract and glucose. However, in long-term cryopreservation the most effective cryoprotectant has been glycerol, followed by sucrose and glucose. Dimethyl sulfoxide has not been effective in long-term cryopreservation for basidiomycetes.
Cryopreservation period for basidiomycetes
The interval of 1 to <2 years of cryopreservation had the greatest number of articles and the interval of 5 years or longer had the smallest number of articles (Table 7). This is due to the difficulty to carry out long-term treatments with a lot of replications, running the risk of losing the genetic material because of failure in the equipment and the supply of liquid nitrogen during the cryopreservation. Moreover, long-term studies demand planning and professional stability in the workplace. Therefore, a greater amount of studies in a shorter cryopreservation time interval, mainly a year at the most, is common.25, 28, 45, 46, 48, 55, 92 Some cryopreservation between 2 and less than 5 years were effective for several basidiomycetes whereas others were effective for 5 years or longer as 15 years (Table 7). However, each species and even each strain of biotechnological importance must have a protocol and a specific cryopreservation method because it is not possible to meet all the specifications for all basidiomycetes with only one method.39, 93 The cryopreservation of more than 5 years with basidiomycetes is a challenge. Besides the cryopreservation period varying according to each method and species, the method has to provide the long-term maintenance of the mycelial viability and the genetic and physiological stability of the fungus, and the substrate for basidiomycete recovery after freezing.
Table 7.
Cryopreservation period, treatments with mycelial viability recovery ≥ 75%, and number of treatments of basidiomycetes submitted to different cryopreservation periods.
The number of basidiomycete cryopreservation studies is greater for periods shorter than 5 years (Table 7). Only three articles successfully described basidiomycete cryopreservation for 5 years or more, probably due to the difficulty of maintaining a culture collection for so long or even due to technique failures after 5 years. The scarcity of cryopreservation reports after 5 years shows the difficulty of maintaining studies for such long periods and the limits of the technique. In conclusion, basidiomycete cryopreservation for more than 5 years is still a challenge.
Cultivation medium and temperature for the recovery of cryopreserved basidiomycetes
The thawing temperature and the cultivation medium utilized in the recovery of basidiomycetes after freezing are important parameters in the cryopreservation process. The thawing temperature has been standardized and utilized successfully through the immersion in water at 30 °C for 15 min.41, 42, 45, 51, 52 The choice of the recovery cultivation medium is generally based on the one in which the fungus is usually initially grown before freezing, but it is not always the best choice.
PDA cultivation medium is the most utilized in frozen basidiomycete recovery.27, 47, 48, 52 The second most utilized cultivation medium is MEA,41, 43, 45, 56 followed by other agar-based culture media such as wort-agar,24, 53 MEYA (malt extract, yeast extract, glycerol and agar)53 and MGL1 (barley flour, yeast extract, sucrose and agar).49 Tanaka et al. (unpublished data) verified differences in the mycelial viability of A. subrufescens after 5 years of cryopreservation at −70 °C in MEA with different concentrations of agar and verified that the water activity in the cultivation medium affects the recovery of mycelial viability.
In conclusion, culture media, temperature and other inherent aspects to the recovery of cryopreserved basidiomycetes are still little studied with a greater concentration of studies in the initial and intermediate stages of the cryopreservation process.
Genetic and physiological stability of basidiomycetes after cryopreservation
The expansion of the biotechnological applications of basidiomycetes requires maintenance of the physiological, morphological and therapeutic characteristics, and the genomic stability after thawing of the biological material, besides the long-term preservation of the mycelial viability. All articles in this review evaluated the mycelial viability of basidiomycetes after thawing, 23.5% of them also evaluated characteristics like the production of enzymes such as laccase and peroxidase manganese,24, 53, 54, 55 29.4% of them characteristics like mushroom production,27, 43, 47, 48, 56 11.7% of them characteristics such as the genetic stability with the utilization of molecular markers43, 55 and 17.6% of them macro and micromorphological characteristics such as texture, coloring and density of mycelium, presence or absence of connection clamps among others.24, 54, 55 Only one article analyzed biomass production, exopolysaccharides and intrapolysaccharides.49
Overall, the articles have shown that mycelial viability and morphological, genetic or physiological characteristics may be preserved by several cryopreservation methods while some others are less effective. Eichlerová and Homolka24 did not observe loss in the laccase production for most analyzed basidiomycetes. Homolka et al.53 reported similar results when evaluating 250 strains of basidiomycetes in which only two did not keep the activity of laccase after thawing. Positive results regarding the maintenance of the enzyme production activity were also observed by Homolka et al.54
Singh et al.43 and Homolka et al.55 assessed the mycelial viability and other parameters like maintenance of morphological and enzymatic characteristics and genetic stability of cryopreserved basidiomycetes and did not observe changes in these characteristics. Homolka et al.55 verified the morphological, physiological and genetic stability of 30 cryopreserved basidiomycetes without finding significant differences. Zaghi Junior et al. (unpublished data) did not verify alteration by RAPD molecular markers in the pattern of amplicons among A. subrufescens strains cryopreserved for two years at −75 °C, either.
In conclusion, mycelial viability analysis of cryopreserved basidiomycete is still the main and most important way to verify the efficacy of the technique. However, other aspects such as analysis of recovery period after thawing, morphological, physiological and genetic stability are important parameters of analysis of the biological material after cryopreservation. For genetic stability analysis, techniques that evaluate the greater part of the genome are more representative as RAPD in detriment of ITS. However, due to the long time of the study and the number of treatments and the microbial isolates, the evaluation of all characteristics becomes impracticable. Therefore, reducing the amount of analyzes for one of each type such as viability, recovery period, morphological, physiological and genetic stability are a possible analysis until one of the treatments on cryopreservation becomes more effective, and then reducing the number of treatments and increasing the number of analysis. It is recommended to reduce the number of species per study as there is no universal cryopreservation technique for basidiomycetes.
Conclusion
In the last 20 years of basidiomycete cryopreservation studies, only 37 genera have been published and the most studied genera in short-term cryopreservation are Agaricus, Pleurotus and Ganoderma, and 114 genera have been published and the most studied genera in long-term cryopreservation are Agaricus and Pleurotus. Cryopreservation at −20 °C is still a challenge but wheat grains could be an alternative to improve long-term cryopreservation. Cryopreservation between −70 °C and −86 °C and −196 °C are equivalently in short- and long-term cryopreservation but in long term at −196 °C there is a greater number of positive results. The most effective substrates are perlite-based, sawdust-based and cereal grains in short- and long-term cryopreservation; however, agar-based substrate of malt and extract with glycerol has been reported as effective in long-term cryopreservation. Glycerol mainly, but sucrose, dimethyl sulfoxide, malt extract and glucose has been effective cryoprotectants in short-term cryopreservation. However, in long-term cryopreservation the most effective cryoprotectant has been glycerol firstly and then sucrose and glucose. Dimethyl sulfoxide has not been effective in long-term cryopreservation for basidiomycetes. Basidiomycete cryopreservation for more than 5 years is still a challenge. Culture media, temperature and other inherent aspects to the recovery of cryopreserved basidiomycetes are still little studied with a greater concentration of studies in the initial and intermediate stages of the cryopreservation process. Mycelial viability analysis of cryopreserved basidiomycete is still the main and most important way to verify the efficacy of preservation. However, other aspects such as analysis of recovery period after thawing, morphological, physiological and genetic stability are important parameters of analysis of the biological material after cryopreservation. It is recommended to reduce the number of species per study as there is no universal cryopreservation technique for basidiomycetes.
Conflicts of interest
The authors have not declared any conflicts of interest.
Acknowledgements
The authors thank Paranaense University, Posgraduate Program in Biotechnology Applied to Agriculture of Paranaense University, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Associate Editor: Andre Rodrigues
References
- 1.Cohen R., Persky L., Hadar Y. Biotechonological applications and potencial of wood-degrafing mushrooms of the genus Pleurotus. Appl Microbiol Biotechnol. 2002;58(1):582–594. doi: 10.1007/s00253-002-0930-y. [DOI] [PubMed] [Google Scholar]
- 2.Sánchez C. Lignocellulosic residues: biodegradation and bioconversion by fungi. Biotechnol Adv. 2009;27(2):185–194. doi: 10.1016/j.biotechadv.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Seto M., Nishibori K., Masai E., Fukuda M., Ohdaira Y. Degradation of polychlorinated biphenyls by a “Maitake” mushroom, Grifola frondosa. Biotechnol Lett. 1999;21(1):27–31. [Google Scholar]
- 4.Onysko K.A. Biological bleaching of chemical pulps: a review. Biotechnol Adv. 1993;11(2):179–198. doi: 10.1016/0734-9750(93)90040-t. [DOI] [PubMed] [Google Scholar]
- 5.Reid I.D., Paice M.G. Biological bleaching of the kraft pulps by white-rot fungi and their enzymes. FEMS Microbiol Rev. 1994;13:369–376. [Google Scholar]
- 6.Messner K., Srebotnik E. Biopulping: an overview of developments in an environmentally safe paper-making technology. FEMS Microbiol Rev. 1994;13(2–3):351–364. [Google Scholar]
- 7.Field J.A., De Jong E., Feijoo-Costa G., De Bont J.A.M. Screening for ligninolytic fungi applicable to the biodegradation of xenobiotics. Trends Biotechnol. 1993;11(2):44–49. [Google Scholar]
- 8.Barr D.P., Aust S.D. Mechanisms white-rot fungi use to degrade pollutants. Environ Sci Technol. 1994;28(2):78A–87A. doi: 10.1021/es00051a724. [DOI] [PubMed] [Google Scholar]
- 9.Li Y. Present development situation and tendency of edible mushroom industry in China. In: Zhang J., Wang H., Chen M., editors. Mushroom Science XVIII. Proceedings of the 18th Congress of the International Society for Mushroom Science, China; Agriculture; 2012. pp. 3–9. [Google Scholar]
- 10.Ghorai S., Banik S.P., Verma D., Chowdhury S., Mukherjee S., Khowala S. Fungal biotechnology in food and feed processing. Food Res Int. 2009;42(5–6):577–587. [Google Scholar]
- 11.Zhang M., Cui S.W., Cheung P.C.K., Wan Q. Antitumor polysaccharides from mushrooms: a review on their isolation process, structural characteristics and antitumor activity. Trends Food Sci Technol. 2007;18(1):4–19. [Google Scholar]
- 12.Rathee S., Rathee D., Rathee D., Kumar V., Rathee P. Mushrooms as therapeutic agents. Braz J Pharm. 2012;22(2):459–474. [Google Scholar]
- 13.Colauto N.B., Linde G.A. Avances sobre el cultivo de “cogumelo-do-sol” em Brasil. In: Sánchez J.E., Mata G., editors. Hongos comestibles y medicinales in Iberoamérica: investigación y desarrollo en un entorno multicultural. Colegio de la Frontera Sur; Tapachula: 2012. pp. 53–67. [Google Scholar]
- 14.Wasser S.P. Medicinal mushroom science: current perspectives, advances, evidences, and challenges. Biomed J. 2014;37(6):345–356. doi: 10.4103/2319-4170.138318. [DOI] [PubMed] [Google Scholar]
- 15.Chen S.Y., Ho K.J., Hsieh Y.J., Wang L.T., Mau J.L. Contents of lovastatin, γ-aminobutyric acid and ergothioneine in mushroom fruiting bodies and mycelia. LWT Food Sci Technol. 2012;47(2):274–278. [Google Scholar]
- 16.Lavi I., Levinson D., Peri I., Tekoah Y., Hadar Y., Schwartz B. Chemical characterization, antiproliferative and antiadhesive properties of polysaccharides extracted from Pleurotus pulmonarius mycelium and fruiting bodies. Appl Microbiol Biotechnol. 2010;85(6):1977–1990. doi: 10.1007/s00253-009-2296-x. [DOI] [PubMed] [Google Scholar]
- 17.Mourão F., Linde G.A., Messa V. Antineoplasic activity of Agaricus brasiliensis basidiocarps on different maturation phases. Braz J Microbiol. 2009;40(4):901–905. doi: 10.1590/S1517-838220090004000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blackwell M. The fungi: 1, 2, 3 … 5.1 million species? Am J Bot. 2011;98(3):426–438. doi: 10.3732/ajb.1000298. [DOI] [PubMed] [Google Scholar]
- 19.Büntgen U., Kauserud H., Egli S. Linking climate variability to mushroom productivity and phenology. Front Ecol Environ. 2012;10(1):14–19. [Google Scholar]
- 20.A’Bear D., Boddy L., Jones T.H. Impacts of elevated temperature on the growth and functioning of decomposer fungi are influenced by grazing Collembola. Glob Change Biol. 2012;18(6):1823–1832. [Google Scholar]
- 21.Christiansen C.T., Haugwitz M.S., Prieme A. Enhanced summer warming reduces fungal decomposer diversity and litter mass loss more strongly in dry than in wet tundra. Glob Change Biol. 2016;23(1):406–420. doi: 10.1111/gcb.13362. [DOI] [PubMed] [Google Scholar]
- 22.Day J.G., Stacey G.N. 2nd ed. Humana; Totowa: 2007. Methods in Molecular Biology™ 368 – Cryopreservation and Freeze-drying Protocols. [Google Scholar]
- 23.Humber R.A. Fungi: preservation of cultures. In: Lacey LA, editor. Manual of Techniques in Insect Pathology. Academic Press; London: 1997. pp. 269–279. [Google Scholar]
- 24.Eichlerová I., Homolka L. Preservation of basidiomycetes strains on perlite using different protocols. Mycoscience. 2014;55(6):439–448. [Google Scholar]
- 25.Homolka L., Lisá L., Eichlerová I., Nerud F. Cryopreservation of basidiomycete strains using perlite. J Microbiol Methods. 2001;47(3):307–313. doi: 10.1016/s0167-7012(01)00338-4. [DOI] [PubMed] [Google Scholar]
- 26.Homolka L., Lisá L., Kubatova A., Vaňova M., Janderova B., Nerud F. Cryopreservation of filamentous micromycetes and yeasts using perlite. Folia Microbiol. 2007;52:153–157. doi: 10.1007/BF02932154. [DOI] [PubMed] [Google Scholar]
- 27.Kitamoto Y., Suzuki A., Shimada S., Yamanaka K. A new method for the preservation of fungus stock cultures by deep-freezing. Mycoscience. 2002;43(2):143–149. [Google Scholar]
- 28.Mata G., Pérez-Merlo R. Spawn viability in edible mushrooms after freezing in liquid nitrogen without a cryoprotectant. Cryobiology. 2003;47(1):14–20. doi: 10.1016/s0011-2240(03)00064-6. [DOI] [PubMed] [Google Scholar]
- 29.Voyron S., Roussel S., Munaut F. Vitality and genetic fidelity of white-rot fungi mycelia following different methods of preservation. Mycol Res. 2009;113(10):1027–1038. doi: 10.1016/j.mycres.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 30.Homolka L. Preservation of live cultures of basidiomycetes – recent methods. Fungal Biol. 2014;118(2):107–125. doi: 10.1016/j.funbio.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Hubálek Z. Academia Publishing House; Prague: 1996. Cryopreservation of Microorganisms. [Google Scholar]
- 32.Hubálek Z. Protectants used in the cryopreservation of microorganisms. Cryobiology. 2003;46(1):205–229. doi: 10.1016/s0011-2240(03)00046-4. [DOI] [PubMed] [Google Scholar]
- 33.Wolfe J., Bryant G. Cellular cryobiology: thermodynamic and mechanical effects. Int J Refrig. 2001;24(5):438–450. [Google Scholar]
- 34.Acker J.P., Mcgann L.E. Protective effect of intracellular ice during freezing? Cryobiology. 2003;46(2):197–202. doi: 10.1016/s0011-2240(03)00025-7. [DOI] [PubMed] [Google Scholar]
- 35.Bueno L., Gallardo R. Filamentous fungi preservation in distilled water. Rev Iberoam Micol. 1998;15:166–168. [PubMed] [Google Scholar]
- 36.Simione F.P., Brown E.M. 2nd ed. American Type Culture Collection; Rockville: 1991. ATCC Preservation Methods: Freezing and Freeze-drying. [Google Scholar]
- 37.Morgan C.A., Heerman N., White P.A., Vesey G. Preservation of microorganisms by drying: a review. J Microbiol Methods. 2006;66(2):183–193. doi: 10.1016/j.mimet.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 38.Smith D., Ryan M.J. Current status of fungal collections and their role in biotechnology. In: Arora D.K., editor. Handbook of Fungal Biotechnology. Marcel Dekker Inc.; New York: 2004. pp. 527–538. [Google Scholar]
- 39.Ryan M.J., Smith D., Jeffries P. A decision-based key to determine the most appropriate protocol for the preservation of fungi. World J Microbiol Biotechnol. 2000;16(2):183–186. [Google Scholar]
- 40.Homolka L. Methods of cryopreservation in fungi. In: Gupta V.K., Tuohy M.G., Ayyachamy M., Turner K.M., O’Donovan A., editors. Laboratory Protocols in Fungal Biology – Current Methods in Fungal Biology, Fungal Biology. 2013. pp. 9–16. [Google Scholar]
- 41.Colauto N.B., Cordeiro F.A., Geromini K.V.N. Viability of Agaricus blazei after long-term cryopreservation. Ann Microbiol. 2012;62(2):871–876. [Google Scholar]
- 42.Mantovani T.R.D., Tanaka H.S., Umeo S.H. Cereal grains and cryoprotectants in Lentinula edodes cryopreservation at −70 °C. In: Zhang J., Wang H., Chen M., editors. Mushroom Science XVIII Proceedings of the 18th Congress of the International Society for Mushroom Science; China Agriculture; 2012. pp. 887–894. [Google Scholar]
- 43.Singh S.K., Upadhyay R.C., Kamal S., Tiwari M. Mushroom cryopreservation and its effect on survival, yield and genetic stability. Cryo Lett. 2004;25(1):23–32. [PubMed] [Google Scholar]
- 44.Nakasone K.K., Peterson S.W., Jong S.C. Preservation and distribution of fungal cultures. In: Mueller G.M., Bills G.F., Foster M.S., editors. Biodiversity of Fungi. Inventory and Monitoring Methods. Elsevier Academic; London: 2004. pp. 35–47. [Google Scholar]
- 45.Colauto N.B., Eira A.F., Linde G.A. Cryopreservation at −80 °C of Agaricus blazei on rice grains. World J Microbiol Biotechnol. 2011;27(12):3015–3018. [Google Scholar]
- 46.Colauto N.B., Eira A.F., Linde G.A. Criopreservação de Agaricus blazei em nitrogênio líquido usando DMSO como crioprotetor. Biosci J. 2012;28(6):1034–1037. [Google Scholar]
- 47.Maia S.C., Toledo R.C.C., Almeida A.P.M.M., Silva R., Linker D.L., Dias E.S. Low-cost and low maintenance preservation of Agaricus brasiliensis cultures. World J Microbiol Biotechnol. 2012;28(6):2411–2416. doi: 10.1007/s11274-012-1050-1. [DOI] [PubMed] [Google Scholar]
- 48.Mata G., Pérez-Merlo R., Savoie J.M., Salmones D. Proceedings of the 8th Conference on Mushroom Biology Products: Directorate of Mushroom Research, Solan and Mushroom Society of India. 2014. Spawn cryopreservation of Agaricus bisporus and A. subrufescens strains; pp. 108–112. [Google Scholar]
- 49.Palacio A., Gutiérrez Y., Rojas D., Atehortúa L., Zapata P. Viability of basidiomycetes fungal strains under different conservation methods: cryopreservation vs. freeze-drying processes. Actual Biol. 2014;36(100):13–21. [Google Scholar]
- 50.Tanaka H.S., Mantovani T.R.D., Geromini K.V.N. Viability of Agaricus blazei under different preservation conditions. Arq Ciênc Vet Zool Unipar. 2011;14:13–17. [Google Scholar]
- 51.Tanaka H.S., Mantovani T.R.D’.A., Santos M.P., Linde G.A., Colauto N.B. Cereal grains and glycerol in Agaricus blazei cryopreservation. Biosci J. 2013;29(3):627–633. [Google Scholar]
- 52.Mantovani T.R.D’.A., Tanaka H.S., Umeo S.H. Cryopreservation at −20 and −70 °C of Pleurotus ostreatus on grains. Indian J Microbiol. 2012;52(3):484–488. doi: 10.1007/s12088-012-0289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Homolka L., Lisá L., Nerud F. Viability of basidiomycete strains after cryopreservation: comparison of two different freezing protocols. Folia Microbiol. 2003;48(2):219–226. doi: 10.1007/BF02930959. [DOI] [PubMed] [Google Scholar]
- 54.Homolka L., Lisá L., Nerud F. Basidiomycete cryopreservation on perlite: evaluation of a new method. Cryobiology. 2006;52(3):446–453. doi: 10.1016/j.cryobiol.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 55.Homolka L., Lisá L., Eichlerová I., Valásková V., Baldrian P. Effect of long-term preservation of basidiomycetes on perlite in liquid nitrogen on their growth, morphlogical, enzimatic and genetic characteristics. Fungal Biol. 2010;114(11–12):929–935. doi: 10.1016/j.funbio.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 56.Mata G., Salmones D., Gaitán-Hernández R. Spawn viability and mushroom production in Pleurotus strains frozen for eight years in liquid nitrogen. Mushroom Sci. 2004;16:185–191. [Google Scholar]
- 57.FAOSAT, 2015. http://faostat3.fao.org/home/E.
- 58.IERIGZ, 2015. http://www.ierigz.waw.pl/.
- 59.Dias E.S. Mushroom cultivation in Brazil: challenges and potential for growth. Cienc Agrotecnol. 2010;34(4):795–803. [Google Scholar]
- 60.Bueno F.S. The production and consumption of edible and medicinal mushrooms in Brazil. Hacia un desarrollo sostenible del sistema de producción-consumo de los hongos comestibles y medicinales en latinoamérica: avances y perspectivas en el siglo XXI. Red Latinoamericana de Hongos Comestibles y Medicinales; Puebla; 2010. pp. 359–379. [Google Scholar]
- 61.Mazur P. Freezing of living cells: mechanisms and implications. Am J Physiol. 1984;247(3):C125–C142. doi: 10.1152/ajpcell.1984.247.3.C125. [DOI] [PubMed] [Google Scholar]
- 62.Dumont F., Marechal P.A., Gervais P. Cell size and water permeability as determining factors for cell viability after freezing at different cooling rates. Appl Environ Microbiol. 2004;70(1):268–272. doi: 10.1128/AEM.70.1.268-272.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ito T. Frozen storage of fungal cultures deposited in the IFO culture collection. IFO Res Commun. 1991;15:119–128. [Google Scholar]
- 64.Ito T., Yokoyama T. Preservation of basidiomycete cultures by freezing. IFO Res Commun. 1983;11:60–70. [Google Scholar]
- 65.Stummer B.E., Zanker T., Scott E.S. Cryopreservation of air-dried conidia of Uncinula necator. Australas Plant Pathol. 1999;28(1):82–84. [Google Scholar]
- 66.Leeson E.A., Cann J.P., Morris G.J. Maintenance of algae and protozoa. In: Kirsop B.E., Snell J.J.S., editors. Maintenance of Microorganisms. 1984. pp. 131–160. [Google Scholar]
- 67.Medeiros A.C.S., Cavallari D.A.N. Conservação de germoplasma de aroeira (Astronium urundeuva) germinação de sementes após imersão em nitrogênio líquido (−196 °C) Rev Bras Sementes. 1992;14(1):73–75. [Google Scholar]
- 68.Eichlerová I., Homolka L., Tomsovský M., Lisá L. Long term storage of Pleurotus ostreatus and Trametes versicolor isolates using different cryopreservation techniques and its impact on laccase activity. Fungal Biol. 2015;119(12):1345–1353. doi: 10.1016/j.funbio.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 69.Dumont F., Marechal P.A., Gervais P. Influence of cooling rate on Saccharomyces cerevisiae destruction during freezing: unexpected viability at ultra-rapid cooling rates. Cryobiology. 2003;46(1):33–42. doi: 10.1016/s0011-2240(02)00161-x. [DOI] [PubMed] [Google Scholar]
- 70.Odani M., Komatsu Y., Oka S., Iwahashi H. Screening of genes that respond to cryopreservation stress using yeast DNA microarray. Cryobiology. 2003;47(2):155–164. doi: 10.1016/j.cryobiol.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 71.Wood C.B., Pritchard H.W., Miller A.P. Simultaneous preservation of orchid seed and its fungal symbiont using encapsulation-dehydration is dependent on moisture content and storage temperature. Cryo Lett. 2000;21:125–136. [PubMed] [Google Scholar]
- 72.Ryan M.J. The use of immobilisation for the preservation of Serpula lacrymans. Mycologist. 2001;15:65–67. [Google Scholar]
- 73.Daniele N., Campus M., Pellegrini C., Shkëmbi E., Zinno F. Biobanks and clinical research: an “interesting” connection. Peertechz J Cytol Pathol. 2016;1(1):034–043. [Google Scholar]
- 74.Belitz H.D., Grosch W. Acribia; Zaragoza: 1997. Química de los alimentos. [Google Scholar]
- 75.Mata G., Rodríguez Estrada A.E. Viability in spawn stocks of the white button mushroom, Agaricus bisporus, after freezing in liquid nitrogen without a cryoprotectant. J Agric Technol. 2005;1:153–162. [Google Scholar]
- 76.Jong S.C., Davis E.E. Germoplasm preservation of edible fungi in culture through cryogenic storage. In: West P.J., Royse D.J., Beelman R.B., editors. Cultivating Edible Fungi. Elsevier; New York: 1986. pp. 213–225. [Google Scholar]
- 77.Singleton P.W., Bohlool B.B., Nakao P.L. Legume response to rhizobial inoculation in the tropics: myths and realities. In: Lal R., Sanchez P., editors. Myths and Science of Soils in the Tropics. American Society of Agronomy; Madison: 1992. pp. 135–155. [Google Scholar]
- 78.Hoseney R.C. Acribia; Zaragoza: 1991. Principios de ciencia y tecnología de los cereales. [Google Scholar]
- 79.Lobo A.R., Silva G.M.L. Resistant starch and its physicochemical properties. Rev Nutr. 2003;16(2):219–226. [Google Scholar]
- 80.Meryman H.T. Mechanics of freezing in living cells and tissues. Science. 1956;124(3221):515–521. doi: 10.1126/science.124.3221.515. [DOI] [PubMed] [Google Scholar]
- 81.Calcott P.H. Meadowfield; Durham: 1978. Freezing and Thawing Microbes. [Google Scholar]
- 82.Heckly R.J. Preservation of microorganisms. Adv Appl Microbiol. 1978;24:1–53. doi: 10.1016/s0065-2164(08)70635-x. [DOI] [PubMed] [Google Scholar]
- 83.Mazur P. Cryobiology: the freezing of biological systems. Science. 1970;168(3934):939–949. doi: 10.1126/science.168.3934.939. [DOI] [PubMed] [Google Scholar]
- 84.Nash T. Chemical constitution and physical properties of compounds able to protect living cells against damage due to freezing and thawing. In: Meryman H.T., editor. Cryobiology. Academic; New York: 1966. pp. 179–211. [Google Scholar]
- 85.Parks E.J., Graham J.K. Effects of cryopreservation procedures on sperm membranes. Theriogenology. 1992;38:209–222. doi: 10.1016/0093-691x(92)90231-f. [DOI] [PubMed] [Google Scholar]
- 86.Lovelock J.E., Polge C. The immobilization of spermatozoa by freezing and thawing and the protective action of glycerol. Byochem J. 1954;58(4):318–322. doi: 10.1042/bj0580618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Watson P.F. Recent developments and concepts in the cryopreservation of spermatozoa and the assessment of their post-thawing function. Reprod Fertil Dev. 1995;7:871–891. doi: 10.1071/rd9950871. [DOI] [PubMed] [Google Scholar]
- 88.Curry M.R. Cryopreservation of semen from domestic livestock. Rev Reprod. 2000;5:46–52. doi: 10.1530/ror.0.0050046. [DOI] [PubMed] [Google Scholar]
- 89.Ito T., Nakagiri A. Viability of frozen cultures of basidiomycetes after fifteen-year storage. Microbiol Cult Collect. 1996;12:67–78. [Google Scholar]
- 90.Beatriz A., Araújo Y.J.K., Lima D.P. Glycerol: a brief history and their application in stereoselective syntheses. Quim Nova. 2011;34(2):306–319. [Google Scholar]
- 91.Niemann H. Cryopreservation of ova and embryos from livestock: current status and research needs. Theriogenology. 1991;35(1):109–124. [Google Scholar]
- 92.Sato M., Sukenobe J., Nakagiri A. Cryopreservation of cryosensitive basidiomycete cultures by application and modification of perlite protocol. Cryo Lett. 2012;33(2):86–94. [PubMed] [Google Scholar]
- 93.Challen M.P., Elliot T.J. Polypropylene straw ampoules for the storage of microorganisms in liquid nitrogen. J Microbiol Methods. 1986;5:11–22. [Google Scholar]