Abstract
The aim of this study was evaluated the biofilm formation by Staphylococcus aureus 4E and Salmonella spp. under mono and dual-species biofilms, onto stainless steel 316 (SS) and polypropylene B (PP), and their sensitivity to cetrimonium bromide, peracetic acid and sodium hypochlorite. The biofilms were developed by immersion of the surfaces in TSB by 10 d at 37 °C. The results showed that in monospecies biofilms the type of surface not affected the cellular density (p > 0.05). However, in dual-species biofilms on PP the adhesion of Salmonella spp. was favored, 7.61 ± 0.13 Log10 CFU/cm2, compared with monospecies biofilms onto the same surface, 5.91 ± 0.44 Log10 CFU/cm2 (p < 0.05). The mono and dual-species biofilms were subjected to disinfection treatments; and the most effective disinfectant was peracetic acid (3500 ppm), reducing by more than 5 Log10 CFU/cm2, while the least effective was cetrimonium bromide. In addition, S. aureus 4E and Salmonella spp. were more resistant to the disinfectants in mono than in dual-species biofilms (p < 0.05). Therefore, the interspecies interactions between S. aureus 4E and Salmonella spp. had a negative effect on the antimicrobial resistance of each microorganism, compared with the monospecies biofilms.
Keywords: Biofilms, Salmonella spp., Staphylococcus aureus, Disinfectants, Sensitivity
Introduction
Salmonella is a zoonotic bacteria, which is one of the most significant enteric foodborne bacterial pathogens1 that caused losses estimated at 3.3 billion dollars per year in the Unites States.2 Extra-animal survival is an important parameter for the environmental dissemination of salmonellae, with the ability of these bacteria to survive in the food chain to be largely due to their ability to sense and adapt to a diverse range of adverse environmental conditions.1 Salmonella is able to adhere and form biofilms on a wide range of surfaces, including metal, plastic and rubber, due to their aggregative fimbriae and lipopolysaccharides; it is also able to produce cellulose, leading to bacterial cells being immersed in a hydrophobic network.3 On the other hand, Staphylococcus aureus is a ubiquitous bacterial species commonly found on the skin and hair, as well as in the noses and throats of people and animals.4, 5 S. aureus can produce heat-stable enterotoxins, which cause 13–40% of poisonings in countries like the United States, Canada and Japan.6, 7, 8 The ability of S. aureus to develop biofilms is strongly linked to the production of polysaccharide intracellular adhesion (PIA), and adhesins called MSCRAMM (microbial surface components recognizing adhesive molecules matrix) on the surface of the microorganism, which have been implicated as major factors in biofilm formation by the ica-independent pathway.9, 10
The biofilms are the dominant lifestyle of bacteria in all environments, either natural or man-made (e.g. food processing).11 Biofilms can develop on a wide variety of surfaces, including those used within the food industry. The biofilms have been implicated in food spoilage, foodborne diseases and damage to processing equipment in the food industry, including the meat industry, brewing, dairy, fisheries, and other industries.12, 13, 14 In the food industry, the presence of microorganisms, inorganic and organic debris on the surfaces favored biofilm formation.15 Therefore, without a suitable cleaning treatment followed by the application of disinfectants, the microorganisms can colonize and persist on food contact and non-food contact surfaces. Thus, the food might be contaminated by contact with the contaminated surface.16, 17, 18 Few studies have focused on the evaluation of resistance to disinfectants by multispecies biofilms; most of these studies did not include an assessment of the resistance from each microorganism in the single-species models; therefore, it is impossible to judge whether the interspecies effect affects the individual resistance of each species in the multispecies communities.14, 19, 20, 21 Even when these communities in the environment are mainly multi-specie; and the interactions between the microorganisms can affect the biofilm structure and function.22 In addition, the biofilm formation by bacterial pathogens is important due to its a potential risk, the antimicrobial resistance and bacterial persistence increase.14, 23, 24 For this reason, the aim of this study was evaluate the biofilm formation of S. aureus 4E and Salmonella spp. under mono and dual-species biofilms, onto stainless steel 316 and polypropylene B, and their sensitivity to cetrimonium bromide, peracetic acid and sodium hypochlorite.
Materials and methods
Bacterial strains
The bacterial strains used were S. aureus 4E isolated from a stainless steel table from the dairy industry. The strain was confirmed by 23S rDNA according to Straub et al.25 In addition, the presence of ica ABCD operon26 and gen of bap protein were determined,27 which are important factors for ica dependent and independent biofilm formation, respectively.8 Salmonella spp. were isolated from the meat industry, and was confirmed by PCR using the primer pair ST11 and ST15, specific for Salmonella spp.28 As positive controls for biofilm formation, S. aureus ATCC 259236, 29 and Salmonella Enteritidis ATCC 1307630 were used. Before utilization, the microorganisms were incubated in tryptic soy broth (TSB; Bioxon, Le Pont de Claix, France) for 24 h at 37 °C to give a final concentration of 108 CFU/mL.
Biofilm formation of S. aureus and Salmonella spp.
Contact surfaces
Stainless Steel (SS, AISI 316, 0.7 × 0.8 × 0.1 cm) and polypropylene B coupons (PP, 0.8 × 2 × 0.1 cm) were cleaned according to the method described by Rossoni and Gaylarde (2000), modified by Marques et al. (2007). Briefly, the surfaces were immersed in pure acetone (Fermont) for 1 h to remove any debris and grease, immersed in neutral detergent (30 mL/L, provide by CIP & GROUP) for 1 h, rinsed with sterile distilled water, cleaned with ethanol (70%, Hycel), dried for 2 h at 60 °C, and sterilized in autoclave (121 °C for 15 min).
Development of mono and dual-species biofilms and quantification
For the biofilm formation, each coupon was individually introduced into a glass test containing 5 mL of TSB. The monospecies biofilms were inoculated with 50 μL of cultures incubated at 37 °C for 24 h of the corresponding strain and the dual-species biofilms were inoculated with 25 μL of each bacterial suspension, after that, the biofilms were incubated at 37 °C for 10 d.32, 33 Finally, after the incubation period, the coupons were removed from the glass test using sterile forces, and rinsed two times by pipetting 2 mL of Dulbecco's phosphate buffered saline (PBS; Sigma-Aldrich) in order to remove the loosely attached cells.14 Each coupon was introduced individually into a glass test with 10 mL of casein peptone (BD, Bioxon, Becton Dickinson) (1 g/L), and the biofilms were removed by sonication (1 min, Sonicor Model SC-100th operating 50–60 Hz). Serial dilutions and conventional plating on tryptic soy agar (TSA; Becton Dickinson, Le Pont de Claix, France) for monospecies biofilms and TSA with lactose (10 g/L, Sigma–Aldrich) and phenol red (0.025 g/L, Hycel) for dual-species biofilms were used to estimate the number of microorganisms in the biofilm. The Petri dishes were incubated at 37 °C for 24 h.34 The colonies of S. aureus 4E were yellow due to lactose fermentation and Salmonella spp. colonies were colorless.
Biocide resistance assays
The coupons with the biofilms were removed from the culture media described above, and immersed individually in 2 mL aqueous solutions of disinfectants: i) cetrimonium bromide (CB; Sigma–Aldrich) at 100 and 200 ppm, ii) peracetic acid (PAA; Sigma–Aldrich) at 10 and 3500 ppm and iii) sodium hypochlorite (NaClO) at 100 and 200 ppm; the disinfectants were prepared in sterile distilled water. The CB and PAA were applied at 25 °C and 50 °C, while NaClO was assessed at 25 °C and 37 °C; the three disinfectants were evaluated at two times of exposure (10 and 15 min). After the exposure period, each coupon was transferred into 1.5 mL or 3 mL of neutralizer solution (SS and PP, respectively): sodium thiosulfate 1 mol L−1 for NaClO, Letheen broth for CB, and Gibson neutralizer (3 g soy lecithin, 30 mL Tween-80, 5 g sodium thiosulfate, 1 g l-histidine and 10 mL PBS per liter of distilled water pH 7.2) for PAA.16 After 30 min of contact with the neutralizer, the surviving cells were estimated by sonication and counting plate as described above.34
Epifluorescent microscopy
Before and after disinfections treatment all coupons of SS and PP were stained with 5(6)-carboxyfluorescein diacetate (CFDA, 10 μg/mL), and dried in a level II cabinet; the CFDA excess was rinsed with SDS. The coupons were observed in a Nikon Eclipse E400 Epifluorescent Microscope using 100× oil immersion lens and filter BA 515 B-2ª at 450–490 nm; at least eighteen fields were observed.35 With this technique, it is possible to observe the presence of metabolically active living cells, the CFDA is a lipophilic substrate that is moderately permeable to most cell membranes.35 Once inside the cell, the diacetate is hydrolyzed by intracellular non-specific esterases, producing carboxy-fluorescein (CF), which is retained by live cells with an intact plasma membrane. Hence, the conversion to CF by the cells indicates the integrity of the plasma membrane and the esterase activity.35
Statistical analysis
All of the experiments were performed in triplicate; the statistical analysis was carried out using ANOVA, and the variances were examined by a least significant difference (LDS) test in the software Statgraphics Centurion XV (Statpoint Technologies, Inc., Warrenton, USA).
Results
Biofilm formation and quantification
The cell density in the biofilm and cellular viability were evaluated using standard plate counting and epifluorescent microscopy. The biofilm formation by S. aureus 4E at 10 d of incubation at 37 °C reached 6.26 ± 0.15 and 6.17 ± 0.15 Log10 CFU/cm2 on PP and SS, respectively (p > 0.05). On the other hand the cellular density recovered from Salmonella spp. monospecies biofilms was 6.24 ± 0.56 and 5.91 ± 0.44 Log10 CFU/cm2 from SS and PP (p > 0.05), respectively. Hence the type of surface had not effect on biofilm development by S. aureus 4E and Salmonella spp. under monospecies conditions and both microorganisms shown the same ability to develop biofilms (p > 0.05). In dual-species biofilms, Salmonella spp. was present in approximately 1 Log10 CFU/cm2 more that S. aureus 4E (p < 0.05) in both surfaces. Onto SS the cellular density enhanced 6.65 ± 0.06 Log10 CFU/cm2, of which 5.60 ± 0.28 Log10 CFU/cm2 was S. aureus 4E and 6.61 ± 0.10 Log10 CFU/cm2 Salmonella spp. in this case the S. aureus 4E decreased in comparison with monospecies biofilms (p < 0.05) while Salmonella spp. maintained their density (p > 0.05). In contrast, on PP 7.67 ± 0.10 Log10 CFU/cm2 were recovered, of which 6.77 ± 0.32 Log10 CFU/cm2 was S. aureus 4E and 7.61 ± 0.13 Log10 CFU/cm2 of Salmonella spp.; therefore both microorganisms increased their cellular densities in comparison with monospecies biofilms in the same surface (p < 0.05). Hence, in dual-species biofilms on PP both microorganisms were favored than in the others models evaluated (p < 0.05). Through epifluorescent microscopy, the biofilms were observed. In the micrographs of the negative controls SS (Fig. 1A) and PP (Fig. 1E), only the surface topography was observed without bacterial cells, in mono and dual-species biofilms of S. aureus 4E and Salmonella spp. metabolically active cells were observed on both surfaces (Fig. 1).
Fig. 1.
Epifluorescence photomicrographs of mono and dual-species biofilms of S. aureus 4E and Salmonella spp. Biofilms were develop on SS (B–D) and PP (F–H) by 10 d of incubation. (A) and (E) represent negative controls of SS and PP respectively, (B) S. aureus 4E, (C) Salmonella spp., and (D) dual-species biofilms on SS, (F) S. aureus 4E, (G) Salmonella spp. and (H) dual-species biofilms onto PP. The white bar scale indicates 10 μm.
Disinfectants test
Effectiveness of peracetic acid
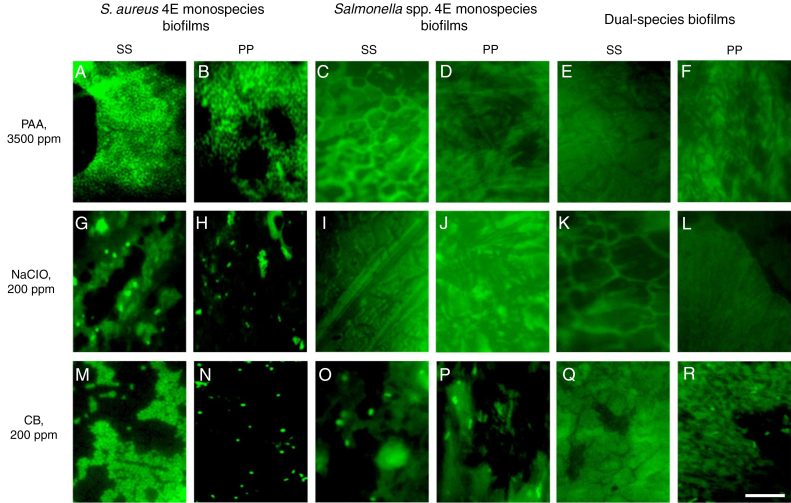
The disinfectants were evaluated on monospecies biofilms of S. aureus 4E develop and Salmonella spp. monospecies biofilms and dual-species biofilms developed in TSB, at 10 d of incubation, because these conditions guaranteed more than 5 Log10 CFU/cm2. Using the PAA at 3500 ppm at 10 and 15 min of time exposure and 25 and 50 °C, it was not possible to recover bacterial cells in any of the studied models (Table 1). Through epifluorescence microscopy, it was not possible to observe viable cells in the coupons, with the three biofilms evaluated, subjected to disinfection with PAA at 3500 ppm at 25 °C for 15 min (Fig. 3A–F); however, in the surfaces with S. aureus 4E biofilms, the presence of dead cells was observed (Fig. 3A and B). Hence, PAA 3500 ppm was able to kill S. aureus 4E cells in the biofilm but was not able to remove them from the SS and PP surfaces.
Table 1.
Comparison of the effectiveness of different treatments in the three types of biofilms on SS and PP surfaces.
| Disinfectant | Concentration (ppm) | Temperature (°C) | Time exposure (min) | Log10 CFU/cm2 reduce on SS ± DSa |
Log10 CFU/cm2 reduce on PP ± DSa |
||||
|---|---|---|---|---|---|---|---|---|---|
| S. aureus 4E monospecies biofilms | Salmonella spp. monospecies biofilms | Dual-species biofilms | S. aureus 4E monospecies biofilms | Salmonella spp. monospecies biofilms | Dual-species biofilms | ||||
| PAA | 10 | 25 | 10 | 3.22 ± 0.97 ABbac | 5.86 ± 0.30 ABb | ND | 3.10 ± 0.13 DEFGHa | 3.10 ± 0.69 ABa | ND |
| 10 | 50 | 10 | 5.06 ± 0.51 EFa | 5.70 ± 0.41 ABCa | ND | 2.04 ± 0.06 ABCb | 2.73 ± 0.34 BDEc | 6.14 ± 0.45 ABa | |
| 10 | 25 | 15 | 3.33 ± 0.10 ABa | 5.88 ± 0.23 Ab | 4.86 ± 0.35 Ac | 5.24 ± 0.25 Jc | 2.69 ± 0.37 BDEd | ND | |
| 10 | 50 | 15 | 5.26 ± 0.23 EFa | 5.88 ± 0.25 Ab | ND | 1.67 ± 0.07 ABc | 3.43 ± 0.42 ACd | ND | |
| 3500 | 25 | 10 | ND | ND | ND | ND | ND | ND | |
| 3500 | 50 | 10 | ND | ND | ND | ND | ND | ND | |
| 3500 | 25 | 15 | ND | ND | ND | ND | ND | ND | |
| 3500 | 50 | 15 | ND | ND | ND | ND | ND | ND | |
| NaClO | 100 | 25 | 10 | 1.97 ± 0.09 Ca | 4.91 ± 0.39 BCDEb | 5.07 ± 0.49 ABb | 1.65 ± 0.67 ABa | ND | 6.22 ± 0.50 ABc |
| 100 | 37 | 10 | 3.71 ± 0.38 ABDa | 4.57 ± 0.36 DEFb | 5.52 ± 0.17 Bc | 4.59 ± 0.77 IJabcd | ND | 5.78 ± 0.97 ABbcd | |
| 100 | 25 | 15 | 3.29 ± 0.34 ABa | 4.99 ± 0.81 CDEFb | ND | 2.33 ± 0.88 ABCDEa | ND | ND | |
| 100 | 37 | 15 | 2.95 ± 1.60 ABCa | 4.97 ± 0.30 ABCDbc | 5.62 ± 0.45 Bb | 3.38 ± 0.67 FGHa | ND | 6.67 ± 0.17 Ad | |
| 200 | 25 | 10 | 3.25 ± 0.04 ABa | 4.40 ± 0.23 Fb | ND | 3.24 ± 0.64 EFGHa | ND | ND | |
| 200 | 37 | 10 | 3.39 ± 0.87 ABa | 4.56 ± 0.17 DEFb | 5.64 ± 0.18 Bc | 3.73 ± 0.38 GHIa | ND | ND | |
| 200 | 25 | 15 | 5.34 ± 0.33 Fa | 5.27 ± 0.18 ABCa | ND | 3.90 ± 0.57 HIb | ND | ND | |
| 200 | 37 | 15 | 3.87 ± 0.53 BDa | 5.27 ± 0.51 ABCb | ND | 5.33 ± 0.17 Jb | ND | ND | |
| CB | 100 | 25 | 10 | 1.92 ± 0.13 Ca | 2.10 ± 0.20 Ia | 5.38 ± 0.18 ABb | 1.45 ± 0.17 Ac | 2.20 ± 0.12 EFa | 4.76 ± 0.73 CDb |
| 100 | 50 | 10 | 2.69 ± 0.98 ACa | 4.62 ± 0.17 DEFb | 5.54 ± 0.45 Bc | 2.35 ± 0.72 ABCDEa | 2.41 ± 0.10 DEFa | 4.45 ± 0.49 Db | |
| 100 | 25 | 15 | 2.12 ± 0.24 Ca | 3.48 ± 1.15 GHb | ND | 2.88 ± 0.95 CDEFGabc | 2.50 ± 0.39 DEFa | 4.32 ± 0.42 Dc | |
| 100 | 50 | 15 | 3.23 ± 0.18 ABa | 4.82 ± 0.30 CDEFb | ND | 3.83 ± 0.62 GHIab | 2.81 ± 0.21 BDc | 3.94 ± 0.36 Db | |
| 200 | 25 | 10 | 4.50 ± 0.36 DEa | 4.52 ± 0.08 EFa | ND | 2.62 ± 0.18 BCDEFb | 2.96 ± 0.14 ABDb | 4.49 ± 0.29 Da | |
| 200 | 50 | 10 | 3.68 ± 0.70 ABDa | 2.37 ± 0.05 HIb | ND | 3.76 ± 0.14 GHIa | 2.00 ± 0.07 Fc | 5.61 ± 0.65 BCd | |
| 200 | 25 | 15 | 2.87 ± 0.88 ABCab | 3.43 ± 0.69 Gbc | ND | 2.18 ± 0.15 ABCDa | 2.54 ± 0.24 DEFa | 4.61 ± 0.84 Dc | |
| 200 | 50 | 15 | 3.50 ± 0.42 ABDa | 4.76 ± 0.26 Ib | ND | 2.49 ± 0.57 BCDEFc | 3.98 ± 0.25 Ca | ND | |
Mean of three test of population reduction after disinfection in Log10 ± standard deviation.
Values in the same column with the same capital letter are not significantly different (p > 0.05).
Values in the same row with the same lowercase letter are not significantly different (p > 0.05).
ND: not detected after disinfection treatment.
Fig. 3.
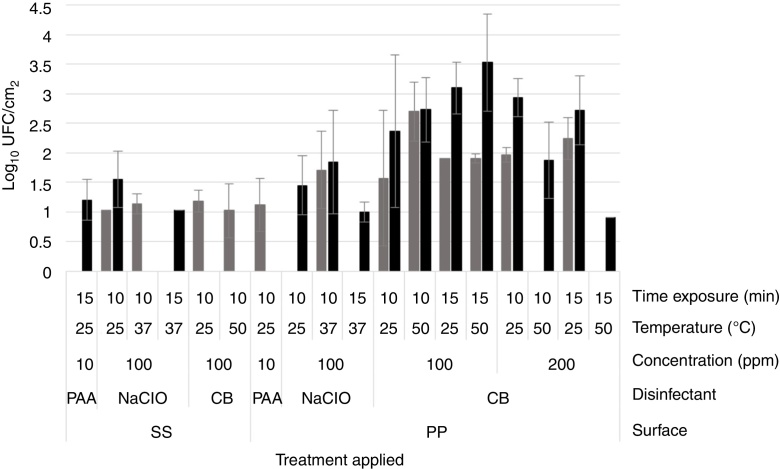
Mean of cells recovered from dual-species biofilms after disinfection. The bars represents the mean of three tests after disinfection: S. aureus 4E ( ) and Salmonella spp. (■). The treatments in which ones just one bar appeared, indicates that just one microorganism were recovered.
) and Salmonella spp. (■). The treatments in which ones just one bar appeared, indicates that just one microorganism were recovered.
On the other hand, with PAA at 10 ppm on S. aureus 4E monospecies biofilms, the reductions fluctuated from 3 to 6.5 Log10 CFU/cm2 on SS and 1–5 Log10 CFU/cm2 onto PP surfaces, depending of the temperature and time exposure. In Salmonella spp. monospecies biofilms on PP, this was just reduced 2.5–3.5 Log10 CFU/cm2. Instead, the PAA at 10 ppm showed the same effect as PAA at 3500 ppm, on dual-species biofilms on both surfaces and Salmonella spp. monospecies biofilms on SS (p > 0.05). From dual-species biofilms treated with PAA at 10 ppm, Salmonella spp. were recovered from SS (1.12 ± 0.45 Log10 CFU/cm2), and S. aureus 4E from PP coupons (1.20 ± 0.35 Log10 CFU/cm2). By epifluorescent microscopy, metabolically active cells were observed after the treatment, except in the biofilms of Salmonella spp. onto SS even when 1–1.13 Log10 CFU/cm2 was recovered in the standard plate counting. The results obtained demonstrated that PAA has a better effectiveness when it is used on SS surfaces; at low concentrations, their effect is favored at high temperatures, but their effectiveness depends the microorganisms in the biofilm.
Effectiveness of sodium hypochlorite
In the case of NaClO, in all of the conditions evaluated, Salmonella spp. in monospecies biofilms onto PP coupons were abated (Table 1); this agreed with the results obtained from epifluorescent microscopy, in which cells were not observed (Fig. 2I–L). In dual-species biofilms, the results for both concentrations of NaClO (100 and 200 ppm) in each surface are similar, with a greater effect at 200 ppm in both surfaces; however, in all treatments, the abatement achieved was more than 5 Log10 CFU/cm2 (Table 1).
Fig. 2.
Epifluorescence photomicrographs of mono and dual-species biofilms of S. aureus 4E and Salmonella spp after disinfection. The disinfection treatments were applied at different temperature and time exposure: (A–F) 50 °C for 10 min; (G–L) 37 °C for 10 min; and (M–R) 50 °C for 10 min The white bar scale indicates 10 μm.
However, for other models, the NaClO effectiveness was favored at 200 ppm and 15 min exposure (Table 1). Nevertheless, the effect of NaClO under these conditions varied between models, in S. aureus 4E monospecies biofilms on both surfaces SS and PP, only one treatment was able to reduce more than 5 Log10 CFU/cm2, which was applied at 25 °C for 15 min On PP surfaces, the NaClO applied at 200 ppm, for 15 min at 37 °C, reduced by more than 5 Log10 CFU/cm2 (Table 1); nonetheless, metabolically active cells were observed after the treatment (Fig. 2H). Using NaClO, there was non-apparent resistance of either microorganism used in dual-species biofilms; it was possible to recover both in some cases (Fig. 3). With the NaClO at 100 ppm, active cells were observed in Salmonella spp. monospecies biofilms on SS, but not on PP; the same occurs in dual-species biofilms.
Effectiveness of cetrimonium bromide
CB in this study was the least effective disinfectant compared with PAA and NaClO. In monospecies biofilms, the reductions ranged between 2 and 4 Log10 CFU/cm2, so the disinfectant was affected more by the application temperature (50 °C, p < 0.05) and time exposure (15 min; p < 0.05), rather than the concentration (100 or 200 ppm; p > 0.05). Moreover, in monospecies biofilms, metabolically active cells were observed after treatment with CB (Fig. 2M–P). However, in dual-species biofilms on PP coupons, reductions more than 5 Log10 CFU/cm2 were achieved using CB at 200 ppm, at 50 °C (Table 1). Finally, in dual-species biofilms on SS, all microorganisms were abated, using CB under any temperature and concentration; in these conditions, no cells were observed by microscopy (Fig. 2Q). From dual-species biofilms on PP coupons treated with CB, both microorganisms were recovered, although Salmonella spp. predominated (Fig. 2R), while from dual-species biofilms on SS, only cells of S. aureus 4E were recovered after treatment (Fig. 3).
In general, S. aureus 4E seems to be more resistant than Salmonella spp. to the antimicrobial action of all tested disinfectants. The resistance of Salmonella spp. was dependent on the surface; Salmonella spp. monospecies biofilms were more sensitive to PAA at 10 ppm on PP than SS, but showed the same resistance on both surfaces to NaClO and CB. In addition, S. aureus 4E and Salmonella spp. were more sensitive to all disinfectants in dual-species conditions; however, the cell density of Salmonella spp. was favored in this growth condition. Hence, interspecies interactions have a significant effect on the antimicrobial resistance of each species with the disinfectants. Nevertheless, dual-species biofilms on SS were more sensitive to CB than any other model.
Discussion
Biofilm formation has become a problem of great importance in a wide range of food industries; including the brewery, dairy, fish and meat industry.16, 36, 37 The biofilms represents a persistent source of microbial contamination. These are associated with food spoilage and foodborne diseases, which result in huge economic losses and threaten food safety; preventing this is the daily priority in the global market.38 The biofilm resistance can be due to different factors, ranging from inhibiting the diffusion of the disinfecting agent by extracellular polymeric substances (EPS), physiological heterogeneity caused by the gradients of nutrients and oxygen generated within the biofilm.17 Therefore, the cleaning and disinfection processes are the most important activities to biofilms control in the food industry.16
Several studies have evaluated the ability of Salmonella and S. aureus to develop biofilms.3, 6, 14, 39, 40, 41, 42 In this study, S. aureus 4E in monospecies biofilms on both surfaces shown similar cellular density, this may be due to S. aureus having the same ability to adhere to hydrophilic or hydrophobic surfaces.42 In Salmonella spp. monospecies biofilms, no differences were observed between cellular densities on both types of surface; however, others studies differ. The adhesion of Salmonella Weltevreden was evaluated on three surfaces at 48 h, and showed increased adhesion on plastic, followed by cement and stainless steel (7.53, 6.20 and 5.48 Log10 CFU/cm2, respectively).40 The difference observed can be attributed to the fact that the bacterial counts in our study were performed in mature biofilms, when the cell population maintained stable levels, while the cells were reversibly adhered after 48 h of incubation.33
In dual-species biofilms Salmonella spp. was found to be 10 times more often than S. aureus 4E, this can be related to the with the high rate of growth of Salmonella; and the predominant microorganisms in the biofilms are those with a higher growth rate, although these can never completely exclude the microorganisms with slow growth.43 Several studies have reported that biofilm production of Salmonella spp. may be promoted by the presence of other bacteria.4, 44, 45, 46 However, Knowles et al. (2005) evaluated the biofilm formation by these microorganisms on SS at 25 °C for 12 d; the results showed that biofilm was constituted mainly by S. aureus (∼99%, 7–8 log CFU/section), while Salmonella was present in lower amounts (6 log CFU/section). In line with this, Gkana et al. (2017) evaluated the biofilm formation in mono and dual-species conditions Salmonella Typhimurium and S. aureus and found that in dual-species biofilms the cells of each microorganism deceased in comparison with monospecies biofilms (p < 0.05). The different results can be related with the fact of the cell density of each microorganism could be dependent on strain ability to biofilm forming in co-culture48; in addition it has been explained that Salmonella require oxygen to produce biofilm in contrast with S. aureus.49 In addition, the ability of six environmental strains of S. aureus to develop mono and dual-species biofilms with L. monocytogenes isolated from rabbit outbreak were evaluated, and the results showed that the effect of L. monocytogenes on the population of S. aureus was strain-dependent. The S. aureus population increased, decreased or was not affected in the presence of L. monocytogenes in dual-species in comparison with monospecies biofilms, while the L. monocytogenes population was not affected.50 These data show that interactions between the microorganisms can stimulate or diminish the colonization of abiotic surfaces; nonetheless, the interactions are mainly influenced by the environmental conditions. In another study, different interactions were found to occur between pathogenic microorganisms, in the biofilm formation by Listeria monocytogenes and Salmonella under mono and dual-species conditions on SS; both species exhibited similar biofilm counts (105 CFU/cm2), independent of culture conditions.14
Additionally, the effect of three disinfectants commonly used in the food industry on mono and dual-species biofilms of S. aureus 4E and Salmonella spp. were evaluated, due to the majority of studies focusing only on monospecies models and dual-species biofilms not often being studied. In the current investigation, PAA was the most effective disinfectant; this disinfectant caused a linear loss of cell viability, demonstrating that higher resistance could not be due to limitations of penetration by the EPS.51 The PAA at 10 ppm on S. aureus 4E monospecies biofilms reduced more than 2 Log10 CFU/cm2 on SS compared with PP, while Salmonella spp. monospecies biofilms were more sensitive on PP than on SS. In this line, it was reported that Salmonella biofilms were more sensitive to disinfectants when they were developed on stainless steel compared to those formed on plastic.40 Due to the stainless steel being hydrophilic, while PP is a hydrophobic surface, for these reasons, the PAA in aqueous solution has less contact with the surface of PP due to the surface tension of the solution.
Regarding the treatments with sodium hypochlorite, the concentration (100 and 200 ppm) had a significant effect in reducing the bacterial cells on both types of surface. However, Rossoni and Gaylarde (2000) found that the concentration of sodium hypochlorite (100 and 200 ppm) did not have a significant effect on S. aureus cell adherence to stainless steel, after 10 min of exposure to the disinfectant at room temperature. The difference between these results could be due to the concentration having an effect on mature biofilms compared with cells in the adhesion process. Mature biofilms are more resistant due to various factors such as high concentration EPS. The EPS limits the spread of disinfectant into the biofilm, with potential interactions existing between antimicrobials and biofilm components; therefore, the state of slow growth of microorganisms in biofilms increases its resistance to disinfectants.17, 52, 53 In the Salmonella spp. monospecies biofilms treated with an aqueous solution of sodium hypochlorite, a total abatement in biofilms formed on PP surfaces was achieved, whereas those formed on SS were recovered to more than 1 Log10 CFU/cm2, with six of the eight treatments applied. Joseph et al. (2001) treated Salmonella Weltevreden monospecies biofilms on plastic surfaces with hypochlorite at 200 ppm of Cl2, for 10 and 15 min, and recovered 4 and 3 Log10 CFU/cm2, respectively, while after 10 min, 3 Log10 CFU/cm2 was recovered from SS surfaces, but no microorganism was detected after 15 min The difference could be attributed to Joseph et al. (2001) having an initial population of 2 Log10 CFU/cm2 more on PP than on SS, while in this study obtained no differences between the counts of Salmonella spp. on SS and PP.
Instead, the CB has a lower effect than PAA on S. aureus 4E monospecies biofilms; in Salmonella spp. monospecies biofilms, the effect was smaller than PAA, even for sodium hypochlorite on both surfaces, while in dual-species biofilms, the CB was less effective when the biofilm was developed on PP. The resistance of S. aureus to quaternary ammonium compound (QAC) is related to the QAC efflux system, which is responsible for the resistance of QAC and cationic biocides in planktonic cells.54 Through this system, bacterial cells are able to free themselves of toxic molecules, allowing them to survive in the presence of these substances.51 In addition, the role of the three-dimensional structure of S. aureus biofilms in QAC resistance is limited and the physiological changes in the biofilm cells are more highly implicated in their resistance.55 In the case of Salmonella, Mangalappalli-Illathu et al.56 reported that resistance of the sessile cells of S. enterica serotype Enteritidis ATCC 4931 on glass surfaces to benzalkonium chloride was due to the phenotypic adaptation to develop biofilm. Another important observation is QAC on S. epidermidis, which caused membrane permeabilization that started on the periphery of cell clusters, then migrated steadily inward; therefore, penetration to the center of the biofilm is 60 times longer than the time estimated for diffusive access in the absence of sorption.57 Moreover, the positive charge and hydrophobic nature of QAC explain the delayed penetration.53 Besides, in Pseudomonas aeruginosa biofilms, the bacterial resistance increased with the C-chain length of QAC55; these data can be related to the low effectiveness of CB in our study, insomuch as the CB has a C-chain of 16C.58
In the present study, biofilm monospecies, treated with PAA at 10 ppm, had greater reductions on SS than on PP coupons in most in the cases (p < 0.05). Due to the effectiveness of the disinfectant can being influenced by the adhesion surface; and this resistance of biofilms can only be observed with certain disinfectants.41, 59 Therefore, the efficiency of disinfectants to the biofilms may vary considerably depending on disinfectant characteristics, the nature of the surface where the biofilm is developed, the type of microorganisms in the biofilm, and other factors such as temperature and time exposure. The dual-species biofilms were more sensitive to disinfectants than monospecies biofilms. Conversely, the majority of studies have demonstrated that multi-species are generally equal or more resistant to disinfection than monospecies biofilms.20, 48, 60, 61, 62 Unfortunately, the resistance mechanisms involved remain unclear.51 However, some possible ways are: chemical interactions between the microorganisms produce a more viscous matrix and the spatial ubication of microorganisms within the biofilm.62, 63 The interaction between the studied strains has a negative effect on their resistance; these data provide a new perspective on the behavior in dual-species biofilms.
It is actually difficult to use epifluorescence microscopy as a counting technique for cells in biofilms, because the bacterial cells in a biofilm usually develop in layers. However, it is possible to observe the presence of surviving cells after decontamination treatments, even in those treatments where it was not possible to achieve expression in the culture medium, either by the detection limit of the technique or by the metabolic state of the bacteria. On the other hand, in those treatments where bacterial cells were recovered through counting technique, but not were observed by microscopy, this might be related to the low presence of bacterial cells per unit area. Additionally, the aerobic plate count is commonly used to detect viable cells; therefore, it does not allow the quantification of sub-lethally damaged cells or non-cultivable but metabolically active cells.60, 64, 65, 66 For these, it is important to use more than one technique for biofilm study as the microorganisms in the biofilms are in different states: metabolically active, metabolically inactive and dead cells.67, 68 The discrepancy between our results and those of other studies14, 31, 40, 47 can be attributed to several factors, including experimental conditions and surface properties. In the case of surface properties, the topography affects bacterial adhesion, particularly if the surface has channels that protect bacteria from the effect of disinfectants.15 Another reason that explains the discrepancy is that the effect of the adhesion surface on the resistance of biofilms might disinfectant-dependent.
In conclusion, the resistance to antimicrobial compounds of microorganisms such as S. aureus 4E and Salmonella spp. in biofilms depends of diverse factors such as type of surface and interspecies interactions. In this study, the surface did not affect the cellular density in monospecies biofilms, but PP surfaces favored de dual-species biofilms. In dual-species biofilms, Salmonella spp. predominated, however both microorganisms were more sensitive to disinfectants than in monospecies biofilms. The PAA was the most effective disinfectant, while the least effective was CB; however, the disinfectant effectiveness was dependent mainly of the microorganisms in the biofilm and the surface on which the biofilm was developed.
Conflict of interest
No conflict of interest declared.
Acknowledgments
We thank the National Council on Science and Technology of Mexico for the scholarship granted to Maricarmen Iñiguez Moreno.
Associate Editor: Solange I. Mussatto
References
- 1.Ruby T., McLaughlin L., Gopinath S., Monack D. Salmonella's long-term relationship with its host. FEMS Microbiol Rev. 2012;36:600–615. doi: 10.1111/j.1574-6976.2012.00332.x. [DOI] [PubMed] [Google Scholar]
- 2.Hoffmann S., Batz M.B., Morris J.G. Annual cost of illness and quality-adjusted life year losses in the United States due to 14 foodborne pathogens. J Food Prot. 2012;75:1292–1302. doi: 10.4315/0362-028X.JFP-11-417. [DOI] [PubMed] [Google Scholar]
- 3.Solomon E.B., Niemira B.A., Sapers G.M., Annous B.A. Biofilm formation, cellulose production, and curli biosynthesis by Salmonella originating from produce, animal, and clinical sources. J Food Prot. 2005;68:906–912. doi: 10.4315/0362-028x-68.5.906. [DOI] [PubMed] [Google Scholar]
- 4.Giaouris E., Heir E., Desvaux M. Intra- and inter-species interactions within biofilms of important foodborne bacterial pathogens. Front Microbiol. 2015;6:841. doi: 10.3389/fmicb.2015.00841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Otto M. Staphylococcal infections: mechanisms of biofilm maturation and detachment as critical determinants of pathogenicity. Annu Rev Med. 2012;64:175–188. doi: 10.1146/annurev-med-042711-140023. [DOI] [PubMed] [Google Scholar]
- 6.Marques S.C., Rezende JDGOS, Alves L.A.D.F. Formation of biofilms by Staphylococcus aureus on stainless steel and glass surfaces and its resistance to some selected chemical sanitizers. Brazilian J Microbiol. 2007;38:538–543. [Google Scholar]
- 7.Marino M., Frigo F., Bartolomeoli I., Maifreni M. Safety-related properties of staphylococci isolated from food and food environments. J Appl Microbiol. 2010;110:550–561. doi: 10.1111/j.1365-2672.2010.04909.x. [DOI] [PubMed] [Google Scholar]
- 8.Archer N.K., Mazaitis M.J., Costerton J.W., Leid J.G., Powers M.E., Shirtliff M.E. Staphylococcus aureus biofilms properties, regulation and roles in human disease. Virulence. 2011;2:445–459. doi: 10.4161/viru.2.5.17724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boles B.R., Horswill A.R. Staphylococcal biofilm disassembly. Trends Microbiol. 2011;19:449–455. doi: 10.1016/j.tim.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang J., Chen J., Li H., Zeng P., Li J. Staphylococcus aureus strains isolated from different sources. Foodborne Pathog Dis. 2013;10:757–763. doi: 10.1089/fpd.2012.1474. [DOI] [PubMed] [Google Scholar]
- 11.Giaouris E., Chorianopoulos N., Skandamis P., Nychas G.-J. Attachment and biofilm formation by Salmonella in food processing environments. In: Mahmoud B.S.M., editor. Salmonella – A Dangerous Foodborne Pathogen. In Tech; 2012. pp. 157–180. Available from: http://www.intechopen.com/books/salmonella-a-dangerous-foodborne-pathogen/attachment-and-biofilm-formation-by-salmonella-in-food-processing-environments. [Google Scholar]
- 12.Donlan R.M. Biofilms: microbial life on surfaces. Emerg Infect Dis. 2002;8:881–890. doi: 10.3201/eid0809.020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brooks J.D., Flint S.H. Biofilms in the food industry: problems and potential solutions. Int J Food Sci Technol. 2008;43:2163–2176. [Google Scholar]
- 14.Kostaki M., Chorianopoulos N., Braxou E., Nychas G.-J, Giaouris E. Differential biofilm formation and chemical disinfection resistance of sessile cells of Listeria monocytogenes strains under monospecies and dual-dpecies (with Salmonella enterica) conditions. Appl Environ Microbiol. 2012;78:2586–2595. doi: 10.1128/AEM.07099-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar C.G., Anand S.K. Significance of microbial biofilms in food industry: a review. Int J Food Microbiol. 1998;42:9–27. doi: 10.1016/s0168-1605(98)00060-9. [DOI] [PubMed] [Google Scholar]
- 16.Gibson H., Taylor J.H., Hall K.E., Holah J.T. Effectiveness of cleaning techniques used in the food industry in terms of the removal of bacterial biofilms. J Appl Microbiol. 1999;87:41–48. doi: 10.1046/j.1365-2672.1999.00790.x. [DOI] [PubMed] [Google Scholar]
- 17.Mah T.F., O’Toole G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2011;9:34–39. doi: 10.1016/s0966-842x(00)01913-2. [DOI] [PubMed] [Google Scholar]
- 18.Kusumaningrum H.D., Riboldi G., Hazeleger W.C., Beumer R.R. Survival of foodborne pathogens on stainless steel surfaces and cross-contamination to foods. Int J Food Microbiol. 2003;85:227–236. doi: 10.1016/s0168-1605(02)00540-8. [DOI] [PubMed] [Google Scholar]
- 19.Simões M., Simões L.C., Vieira M.J. Species association increases biofilm resistance to chemical and mechanical treatments. Water Res. 2009;43:229–237. doi: 10.1016/j.watres.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 20.van der Veen S., Abee T. Mixed species biofilms of Listeria monocytogenes and Lactobacillus plantarum show enhanced resistance to benzalkonium chloride and peracetic acid. Int J Food Microbiol. 2011;144:421–431. doi: 10.1016/j.ijfoodmicro.2010.10.029. [DOI] [PubMed] [Google Scholar]
- 21.Giaouris E., Heir E., Hébraud M. Attachment and biofilm formation by foodborne bacteria in meat processing environments: causes, implications, role of bacterial interactions and control by alternative novel methods. Meat Sci. 2013;97:298–309. doi: 10.1016/j.meatsci.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 22.Burmølle M., Ren D., Bjarnsholt T., Sørensen S.J. Interactions in multispecies biofilms: do they actually matter? Trends Microbiol. 2014;22:84–91. doi: 10.1016/j.tim.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Giaouris E., Chorianopoulos N., Doulgeraki A., Nychas G.J. Co-culture with Listeria monocytogenes within a dual-species biofilm community strongly increases resistance of Pseudomonas putida to benzalkonium chloride. PLOS ONE. 2013;8:1–14. doi: 10.1371/journal.pone.0077276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chmielewski R.A.N., Frank J.F. Biofilm formation and control in food processing facilities. Compr Rev Food Sci Food Saf. 2003;2:22–32. doi: 10.1111/j.1541-4337.2003.tb00012.x. [DOI] [PubMed] [Google Scholar]
- 25.Straub J.A., Hertel C., Hammes W.P. A 23S rDNA-targeted polymerase chain reaction-based system for detection of Staphylococcus aureus in meat starter cultures and dairy products. J Food Prot. 1999;62:1150–1156. doi: 10.4315/0362-028x-62.10.1150. [DOI] [PubMed] [Google Scholar]
- 26.Arciola C.R., Baldassarri L., Montanario L. Presence of icaA and icaD genes and slime production in a collection of staphylococcal strains from catheter-associated infections. J Clin Microbiol. 2001;39:2151–2156. doi: 10.1128/JCM.39.6.2151-2156.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cucarella C., Tormo MÁ, Úbeda C. Role of biofilm-associated protein bap in the pathogenesis of bovine Staphylococcus aureus. Infect Immun. 2004;72:2177–2185. doi: 10.1128/IAI.72.4.2177-2185.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soumet C., Ermel G., Rose N. Evaluation of a Multiplex PCR assay for simultaneous identification of Salmonella sp., Salmonella Enteritidis and Salmonella Typhimurium from environmental swabs of poultry houses. Lett Appl Microbiol. 1999;28:113–117. doi: 10.1046/j.1365-2672.1999.00488.x. [DOI] [PubMed] [Google Scholar]
- 29.Stepanovic S., Vukovic D., Dakic I., Savic B., Svabic-Vlahovic M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J Microbiol Methods. 2000;40:175–179. doi: 10.1016/s0167-7012(00)00122-6. [DOI] [PubMed] [Google Scholar]
- 30.Orgaz B., Lobete M.M., Puga C.H., San Jose C. Effectiveness of chitosan against mature biofilms formed by food related bacteria. Int J Mol Sci. 2011;12:817–828. doi: 10.3390/ijms12010817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rossoni E.M., Gaylarde C.C. Comparison of sodium hypochlorite and peracetic acid as sanitising agents for stainless steel food processing surfaces using epifluorescence microscopy. Int J Food Microbiol. 2000;61:81–85. doi: 10.1016/s0168-1605(00)00369-x. [DOI] [PubMed] [Google Scholar]
- 32.Heydorn A., Nielsen A.T., Hentzer M. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology. 2000;146:2395–2407. doi: 10.1099/00221287-146-10-2395. [DOI] [PubMed] [Google Scholar]
- 33.Xu H., Zou Y., Lee H., Ahn J. Effect of NaCl on the biofilm formation by foodborne pathogens. J Food Sci. 2010;75:580–585. doi: 10.1111/j.1750-3841.2010.01865.x. [DOI] [PubMed] [Google Scholar]
- 34.Mariscal A., Lopez-Gigosos R.M., Carnero-Varo M., Fernandez-Crehuet J. Fluorescent assay based on resazurin for detection of activity of disinfectants against bacterial biofilm. Appl Microbiol Bitechnol. 2009;82:773–783. doi: 10.1007/s00253-009-1879-x. [DOI] [PubMed] [Google Scholar]
- 35.Gorokhova E., Mattsson L., Sundström A.M. A comparison of TO-PRO-1 iodide and 5-CFDA-AM staining methods for assessing viability of planktonic algae with epifluorescence microscopy. J Microbiol Methods. 2012;89:216–221. doi: 10.1016/j.mimet.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 36.Jessen B., Lammert L. Biofilm and disinfection in meat processing plants. Int Biodeterior Biodegrad. 2003;51:265–269. [Google Scholar]
- 37.Srey S., Jahid I.K., Ha S.-D. Biofilm formation in food industries: a food safety concern. Food Control. 2013;31:572–585. [Google Scholar]
- 38.Van Houdt R., Michiels C.W. Biofilm formation and the food industry, a focus on the bacterial outer surface. J Appl Microbiol. 2010;109:1117–1131. doi: 10.1111/j.1365-2672.2010.04756.x. [DOI] [PubMed] [Google Scholar]
- 39.Oliveira K., Oliveira T., Teixeira P., Azeredo J., Oliveira R. Adhesion of Salmonella Enteritidis to stainless steel surfaces. Brazilian J Microbiol. 2007;38:318–323. [Google Scholar]
- 40.Joseph B., Otta S.K., Karunasagar I. Biofilm formation by Salmonella spp. on food contact surfaces and their sensitivity to sanitizers. Int J Food Microbiol. 2001;64:367–372. doi: 10.1016/s0168-1605(00)00466-9. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen H.D.N., Yuk H.-G. Changes in resistance of Salmonella Typhimurium biofilms formed under various conditions to industrial sanitizers. Food Control. 2013;29:236–240. [Google Scholar]
- 42.Kouidhi B., Zmantar T., Hentati H., Bakhrouf A. Cell surface hydrophobicity, biofilm formation, adhesives properties and molecular detection of adhesins genes in Staphylococcus aureus associated to dental caries. Microb Pathog. 2010;49:14–22. doi: 10.1016/j.micpath.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 43.Banks M.K., Bryers J.D. Bacterial species dominance within a binary culture biofilm. Appl Environ Microbiol. 1991;57:1974–1979. doi: 10.1128/aem.57.7.1974-1979.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Habimana O., Møretrø T., Langsrud S., Vestby L.K., Nesse L.L., Heir E. Micro ecosystems from feed industry surfaces: a survival and biofilm study of Salmonella versus host resident flora strains. BMC Vet Res. 2010;6:48. doi: 10.1186/1746-6148-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jones K., Bradshaw S.B. Synergism in biofilm formation between Salmonella enteritidis and a nitrogen-fixing strain of Klebsiella pneumoniae. J Appl Bacteriol. 1997;82:663–668. doi: 10.1111/j.1365-2672.1997.tb03600.x. [DOI] [PubMed] [Google Scholar]
- 46.Schaefer L.M., Brözel V.S., Venter S.N. Fate of Salmonella Typhimurium in laboratory-scale drinking water biofilms. J Water Health. 2013;11:629–635. doi: 10.2166/wh.2013.208. [DOI] [PubMed] [Google Scholar]
- 47.Knowles J.R., Roller S., Murray D.B., Naidu A.S. Antimicrobial action of carvacrol at different stages of dual-species biofilm development by Staphylococcus aureus and Salmonella enterica Serovar Typhimurium. Appl Environ Microbiol. 2005;71:797–803. doi: 10.1128/AEM.71.2.797-803.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gkana E.N., Giaouris E.D., Doulgeraki A.I., Kathariou S., Nychas G.-J.E. Biofilm formation by Salmonella Typhimurium and Staphylococcus aureus on stainless steel under either mono- or dual-species multi-strain conditions and resistance of sessile communities to sub-lethal chemical disinfection. Food Control. 2017;73:838–846. [Google Scholar]
- 49.Scher K., Romling U., Yaron S. Effect of heat, acidification, and chlorination on Salmonella enterica Serovar Typhimurium cells in a biofilm formed at the air-liquid interface. Appl Environ Microbiol. 2005;71:1163–1168. doi: 10.1128/AEM.71.3.1163-1168.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rieu A., Lemaître J., Guzzo J., Piveteau P. Interactions in dual species biofilms between Listeria monocytogenes EGD-e and several strains of Staphylococcus aureus. Int J Food Microbiol. 2008;126:76–82. doi: 10.1016/j.ijfoodmicro.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 51.Bridier A., Briandet R., Thomas V., Dubois-Brissonnet F. Resistance of bacterial biofilms to disinfectants: a review. Biofouling J Bioadhesion Biofilm Res. 2011;27:1017–1032. doi: 10.1080/08927014.2011.626899. [DOI] [PubMed] [Google Scholar]
- 52.Wingender J., Neu T.R., Flemming H.-C. What are bacterial extracellular polymeric substances? In: Wingender J., Neu T.R., Flemming H.-C., editors. Microbial Extracellular Polymeric Substances. Springer-Verlag; Berlin: 1999. pp. 1–19. [Google Scholar]
- 53.Bridier A., Dubois-Brissonnet F., Greub G., Thomas V., Briandet R. Dynamics of the action of biocides in Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother. 2011;55:2648–2654. doi: 10.1128/AAC.01760-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cervinkova D., Babak V., Marosevic D., Kubikova I., Jaglic Z. The role of the qacA gene in mediating resistance to quaternary ammonium compounds. Microb Drug Resist. 2012;0:1–8. doi: 10.1089/mdr.2012.0154. [DOI] [PubMed] [Google Scholar]
- 55.Campanac C., Pineau L., Payard A., Baziard-Mouysset G., Roques C. Interactions between biocide cationic agents and bacterial biofilms. Antimicrob Agents Chemother. 2002;46:1469–1474. doi: 10.1128/AAC.46.5.1469-1474.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mangalappalli-Illathu A.K., Vidovic S., Korber D.R. Differential adaptive response and survival of Salmonella enterica Serovar Enteritidis planktonic and biofilm cells exposed to benzalkonium chloride. Antimicrob Agents Chemother. 2008;52:3669–3680. doi: 10.1128/AAC.00073-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Davison W.M., Pitts B., Stewart P.S. Spatial and temporal patterns of biocide action against Staphylococcus epidermidis biofilms. Antimicrob Agents Chemother. 2010;54:2920–2927. doi: 10.1128/AAC.01734-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.National Center for Biotechnology Information . 2009. PubChem Substance Database; SID=57651369.52365_SIGMA. Available from: https://pubchem.ncbi.nlm.nih.gov/substance/5765136 Accessed 16.09.09. [Google Scholar]
- 59.Somers E.B., Wong A.L. Efficacy of two cleaning and sanitizing combinations on Listeria monocytogenes biofilms formed at low temperature on a variety of materials in the presence of ready-to-eat meat residue. J Food Prot. 2004;67:2218–2229. doi: 10.4315/0362-028x-67.10.2218. [DOI] [PubMed] [Google Scholar]
- 60.Leriche V., Briandet R., Carpentier B. Ecology of mixed biofilms subjected daily to a chlorinated alkaline solution: spatial distribution of bacterial species suggests a protective effect of one. Environ Microbiol. 2003;5:64–71. doi: 10.1046/j.1462-2920.2003.00394.x. [DOI] [PubMed] [Google Scholar]
- 61.Simões L.C., Simões M., João M. Biofilm interactions between distinct bacterial genera isolated from drinking water. Appl Environ Microbiol. 2007;73:6192–6200. doi: 10.1128/AEM.00837-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bridier A., Sanchez-Vizuete M.P., Le Coq D. Biofilms of a Bacillus subtilis hospital isolate protect Staphylococcus aureus from biocide action. PLOS ONE. 2012;7:e44506. doi: 10.1371/journal.pone.0044506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Burmølle M., Webb J.S., Rao D., Hansen L.H., Sørensen S.J., Kjelleberg S. Enhanced biofilm formation and increased resistance to antimicrobial agents and bacterial invasion are caused by synergistic interactions in multispecies biofilms. Appl Environ Microbiol. 2006;72:3916–3923. doi: 10.1128/AEM.03022-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Giraffa G. Studying the dynamics of microbial populations during food fermentation. FEMS Microbiol Rev. 2004;28:251–260. doi: 10.1016/j.femsre.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 65.Zotta T., Parente E., Ricciardi A. Viability staining and detection of metabolic activity of sourdough lactic acid bacteria under stress conditions. World J Microbiol Biotechnol. 2009;25:1119–1124. [Google Scholar]
- 66.Zotta T., Guidone A., Tremonte P., Parente E., Ricciardi A. A comparison of fluorescent stains for the assessment of viability and metabolic activity of lactic acid bacteria. World J Microbiol Biotechnol. 2012;28:919–927. doi: 10.1007/s11274-011-0889-x. [DOI] [PubMed] [Google Scholar]
- 67.Wentland E.J., Stewart P.S., Huang C., McFeters G.A. Spatial variations in growth rate within Klebsiella pneumoniae colonies and biofilm. Biotechnol Prog. 1996;12:316–321. doi: 10.1021/bp9600243. [DOI] [PubMed] [Google Scholar]
- 68.Huang C.-T., Yu F.P., McFeters G.A., Stewart P.S. Nonuniform spatial patterns of respiratory activity within biofilms during disinfection. Appl Environ Microbiol. 1995;61:2252–2256. doi: 10.1128/aem.61.6.2252-2256.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]