ABSTRACT
Drosophila melanogaster is an outstanding model for studying host antipathogen defense. Although substantial progress has been made in understanding how metabolism and immunity are interrelated in flies, little information has been obtained on the molecular players that regulate metabolism and inflammation in Drosophila during pathogenic infection. Recently, we reported that the inactivation of thioester-containing protein 2 (Tep2) and Tep4 promotes survival and decreases the bacterial burden in flies upon infection with the virulent pathogens Photorhabdus luminescens and Photorhabdus asymbiotica. Here, we investigated physiological and pathological defects in tep mutant flies in response to Photorhabdus challenge. We find that tep2 and tep4 loss-of-function mutant flies contain increased levels of carbohydrates and triglycerides in the presence or absence of Photorhabdus infection. We also report that Photorhabdus infection leads to higher levels of nitric oxide and reduced transcript levels of the apical caspase-encoding gene Dronc in tep2 and tep4 mutants. We show that Tep2 and Tep4 are upregulated mainly in the fat body rather than the gut in Photorhabdus-infected wild-type flies and that tep mutants contain decreased numbers of Photorhabdus bacteria in both tissue types. We propose that the inactivation of Tep2 or Tep4 in adult Drosophila flies results in lower levels of inflammation and increased energy reserves in response to Photorhabdus, which could confer a survival-protective effect during the initial hours of infection.
KEYWORDS: Drosophila, innate immunity, insect, metabolism, Photorhabdus, thioester-containing protein
INTRODUCTION
A central question in insect immunology involves the identification of the pathological defects that lead to insect death following a microbial infection (1). The fruit fly Drosophila melanogaster is an established model to interrogate the pathology of infection and inflammation (2–5). Drosophila activates distinct immune responses against microbial pathogens. These responses include the activation of NF-κB signaling pathways that lead to the production of antimicrobial peptides and cellular immune reactions that involve phagocytosis, nodulation, and coagulation (6). Infection also induces stress signaling cascades, resulting in the synthesis of nitric oxide (6). To accomplish these immune functions, Drosophila relies on its stored reservoirs of energy (7). Metabolism and immunity share a complex relationship depending on the nature of the pathogen that the fly encounters. For example, Drosophila flies undergo anorexia after pathogenic infection with Listeria monocytogenes and Salmonella enterica serovar Typhimurium (8). L. monocytogenes infection also depletes the stored energy pools of glycogen and triglycerides, which in turn leads to fly death (9).
The Drosophila-Photorhabdus model forms a flexible system for understanding the molecular and mechanistic basis of host-pathogen interactions (10–13). Photorhabdus bacteria are Gram-negative bacteria that belong to the family Enterobacteriaceae, which includes several other important bacterial pathogens. The main characteristic of Photorhabdus is that the bacteria form a mutualistic association with their nematode partner Heterorhabditis (14). The bacteria are present in the gut of infective juvenile nematodes that have the ability to attack and invade susceptible insects (15). Following entry, infective juveniles regurgitate Photorhabdus in the insect hemocoel, where the bacteria divide exponentially and produce a wide range of toxins and hydrolytic enzymes that cause rapid insect death (16).
The bacteria secrete multiple virulence factors in addition to molecules that interfere with the insect host immune system (17). They can replicate in the insect gut, and as a consequence, the insect ceases feeding and dies due to septicemia (14). Photorhabdus also resides and multiplies in insect fat body tissue, an infection strategy that results in the evasion or deactivation of the insect humoral immune response (18). To suppress the cellular immune response, Photorhabdus secretes virulence factors that interfere with hemocyte function or morphology, while other secreted molecules promote the death of gut and immune cells (19–21). Although exciting findings have been obtained from studying Photorhabdus infection processes in the insect models Galleria mellonella and Manduca sexta (22–24), the physiological changes caused by these pathogens in Drosophila flies have yet to be explored (25).
Thioester-containing proteins (TEPs) participate in the opsonization and elimination of invading microbes in both vertebrate and invertebrate animals, and they are involved in augmenting inflammatory responses in vertebrates (26–28). Substantial information on the function of TEPs in Anopheles mosquitoes has been acquired, but only a few studies have examined the contribution of TEPs to the antimicrobial immune response of Drosophila (11, 29–34). Recently, we reported that Tep2, Tep4, and Tep6 in Drosophila are transcriptionally upregulated after infection with Photorhabdus luminescens or Photorhabdus asymbiotica (11, 35, 36). In particular, the inactivation of Tep2, Tep4, and Tep6 in Drosophila prolongs the survival of the mutants in response to Photorhabdus infection, which is accompanied by a lower level of persistence of the pathogens in infected flies. Here, we have hypothesized that the absence of TEP2 or TEP4 molecules results in lower levels of inflammation in the fly upon Photorhabdus infection. For this, we examined the physiological responses of tep mutant flies to the two Photorhabdus pathogens. We report that tep2 and tep4 mutants display increased metabolic reserves and low levels of inflammation in gut and fat body compared to background control flies. Our findings reveal that TEP2 and TEP4 molecules are associated with the regulation of pathophysiological effects, programmed cell death, and metabolic activities in flies in response to Photorhabdus challenge.
RESULTS
Drosophila tep2 mutants exhibit high carbohydrate levels after bacterial infection.
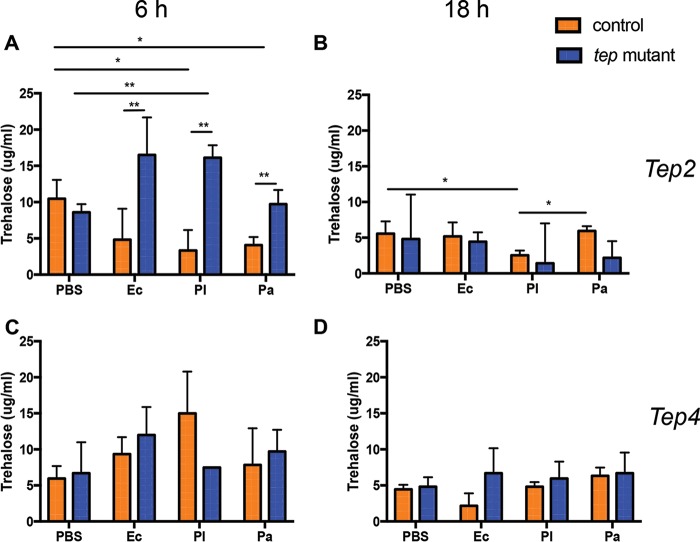
Previous studies have shown that infection with Listeria monocytogenes or Mycobacterium marinum leads to significant metabolic changes in the fly (9, 37). To understand the prolonged survival of tep2 and tep4 mutants, we measured the levels of the carbohydrates trehalose, glycogen, and glucose in flies infected with either pathogenic Photorhabdus or nonpathogenic Escherichia coli bacteria at early (6 h) and late (18 h) time points postinfection and before fly death occurred (11, 35). We first tested changes in trehalose levels in tep mutants and background controls because trehalose is one of the major circulating sugars in fruit flies (38). The levels of trehalose in w1118 flies were decreased 6 h after infection with either Photorhabdus species and 18 h after infection with P. luminescens only compared to phosphate-buffered saline (PBS)-injected controls (Fig. 1A and B). We further observed that tep2 mutants had significantly higher levels of trehalose than did their background control flies (w1118) 6 h after infection with E. coli and Photorhabdus (Fig. 1A), whereas no differences were observed between the two mutants 18 h after infection with any of the bacteria or after PBS injection (Fig. 1B). Interestingly, we found no significant changes in trehalose levels between the tep4 mutants and their background controls (yw) at any time point after bacterial infection (Fig. 1C and D). These results demonstrate that the inactivation of Tep2 affects trehalose levels during pathogenic infection with Photorhabdus or nonpathogenic infection with E. coli bacteria.
FIG 1.
Inactivation of Tep2 and Tep4 modulates trehalose levels in Drosophila in the presence or absence of Photorhabdus infection. Trehalose levels (micrograms per milliliter) in tep2 (A and B) and tep4 (C and D) loss-of-function mutants are compared to those in the corresponding background control flies (w1118 and yw, respectively) (n = 5) 6 and 18 h after infection with E. coli (Ec), P. luminescens (Pl), or P. asymbiotica (Pa) or injection with 1× PBS (negative control). The means from three independent experiments are shown, and error bars represent standard deviations. Significant differences are shown with asterisks (*, P < 0.05; **, P < 0.01).
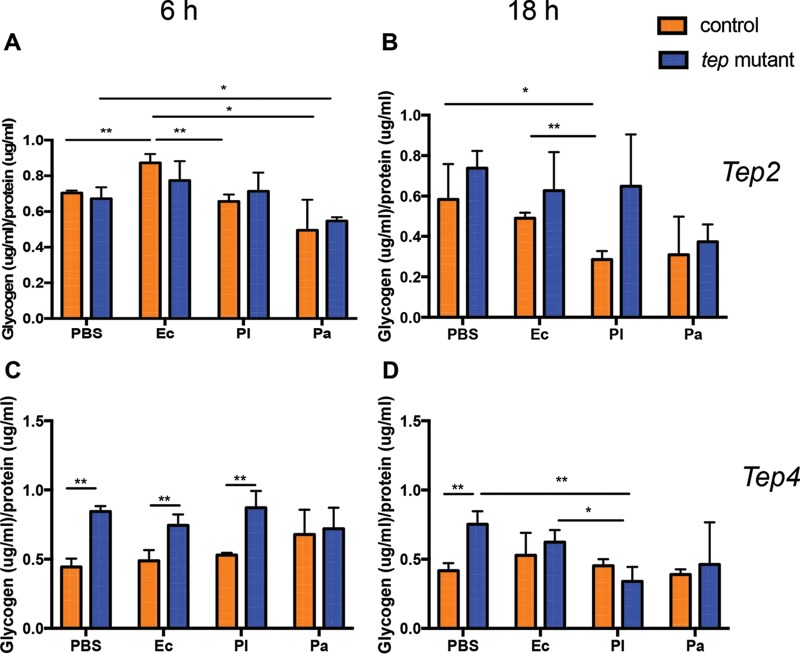
Next, we examined the levels of glycogen in tep mutants and background controls in the presence or absence of bacterial infection. We found no changes in glycogen levels between the tep2 mutants and w1118 flies at any of the time points (Fig. 2A and B). We also noticed low glycogen levels in w1118 flies 6 h after infection with either Photorhabdus species and 18 h after infection with P. luminescens only compared to the PBS or E. coli treatments (Fig. 2A and B). We further observed significantly higher glycogen levels in tep4 mutants than in their background controls (yw) 6 h after infection with P. luminescens or E. coli or injection with PBS (Fig. 2C and D). In addition, we found that tep4 mutants had significantly more glycogen than did yw flies injected with PBS at 18 h (Fig. 2D). However, tep4 mutants contained less glycogen 18 h after infection with P. luminescens than did those infected with E. coli or given control injections with PBS (Fig. 2D). These results indicate that the inactivation of Tep4 in Drosophila affects the utilization of glycogen during the early and late stages of infection with pathogenic and nonpathogenic bacteria.
FIG 2.
Drosophila mutants for Tep2 and Tep4 display differential glycogen levels in the presence or absence of Photorhabdus infection. Shown are glycogen levels in tep loss-of-function mutants and background control flies (n = 5) injected with 1× PBS (negative control), E. coli (Ec), P. luminescens (Pl), or P. asymbiotica (Pa). Glycogen levels (micrograms) are normalized to the protein content (micrograms) and represented as a ratio in tep2 mutants (A and B) and tep4 mutants (C and D) compared to those of their background control strains (w1118 and yw, respectively) at 6 and 18 h postinjection. The means from three independent experiments are shown, and error bars represent standard deviations. Significant differences are shown with asterisks (*, P < 0.05; **, P < 0.01).
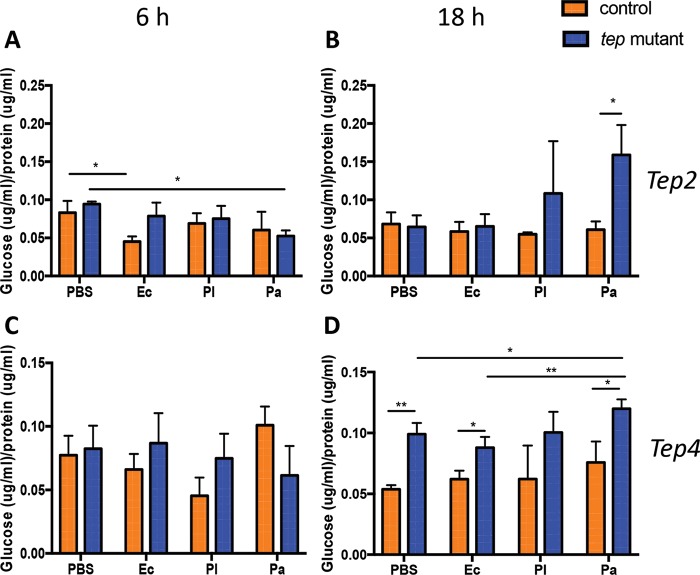
We next examined the levels of free glucose before and after infection with E. coli or Photorhabdus. We recorded increased glucose levels in tep2 mutants compared to w1118 flies 18 h after infection with P. asymbiotica only (Fig. 3A and B). We also noticed that free glucose levels were decreased in w1118 flies 6 h after infection with E. coli compared to those in w1118 flies injected with PBS (Fig. 3A). There were no changes in free glucose levels between tep4 mutants and yw flies 6 h after the injection of bacteria or PBS (Fig. 3C). In contrast, glucose levels were significantly higher in tep4 mutant flies injected with E. coli, P. asymbiotica, or PBS at 18 h than in yw flies (Fig. 3D). These results indicate that dysregulation of the expression of Tep2 results in increased free glucose reserves during P. asymbiotica infection, whereas the inactivation of Tep4 promotes increased free glucose reserves during E. coli or P. asymbiotica infection.
FIG 3.
Drosophila mutants for Tep2 and Tep4 have altered glucose levels upon infection with Photorhabdus. Shown are free glucose levels in tep2 (A and B) and tep4 (C and D) loss-of-function mutant flies compared to those in the corresponding background controls (w1118 and yw, respectively) (n = 5) 6 and 18 h after infection with E. coli (Ec), P. luminescens (Pl), or P. asymbiotica (Pa) or injection with 1× PBS (negative control). Glucose levels are normalized to the total protein content and represented as a ratio of the total glucose content to the total protein content. The means from three independent experiments are shown, and error bars represent standard deviations. Significant differences are shown with asterisks (*, P < 0.05; **, P < 0.01).
Drosophila tep2 and tep4 mutants have high triglyceride levels upon bacterial infection.
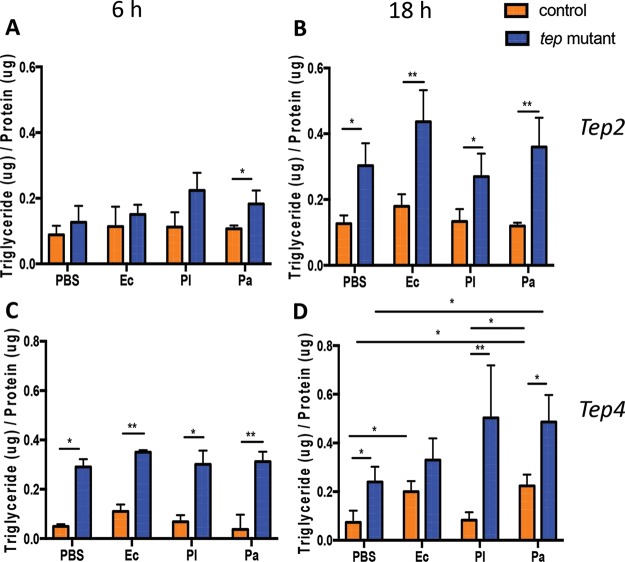
Previous studies have shown that triglyceride levels decrease with bacterial or viral infection in Drosophila (9, 39). Here, we aimed to identify potential changes in triglyceride levels in tep mutant flies in response to Photorhabdus or E. coli infection. We found that tep2 mutants had higher triglyceride levels than those of the background controls 6 h after infection with P. asymbiotica and 18 h after infection with E. coli or Photorhabdus or injection with PBS (Fig. 4A and B). Similarly, tep4 mutants displayed increased levels of triglycerides compared to those in yw flies with any injection treatment at 6 h postinfection (hpi) but only with PBS or Photorhabdus at 18 hpi (Fig. 4C and D). These results imply that the inactivation of Tep2 or Tep4 leads to the increased deposition and storage of triglycerides in uninfected or bacterium-infected flies.
FIG 4.
Infection of Drosophila tep2 and tep4 mutant flies with Photorhabdus alters triglyceride levels. Shown are estimations of triglyceride levels in tep loss-of-function mutants and background controls (n = 5) injected with 1× PBS (negative control), E. coli (Ec), P. luminescens (Pl), or P. asymbiotica (Pa). Triglyceride levels (micrograms) are normalized to the protein content (micrograms) and represented as a ratio of the total triglyceride content to the total protein content in tep2 mutants (A and B) and tep4 mutants (C and D) compared to the background control strains (w1118 and yw, respectively) at 6 and 18 h postinfection. The means from three independent experiments are shown, and error bars represent standard deviations. Significant differences are shown with asterisks (*, P < 0.05; **, P < 0.01).
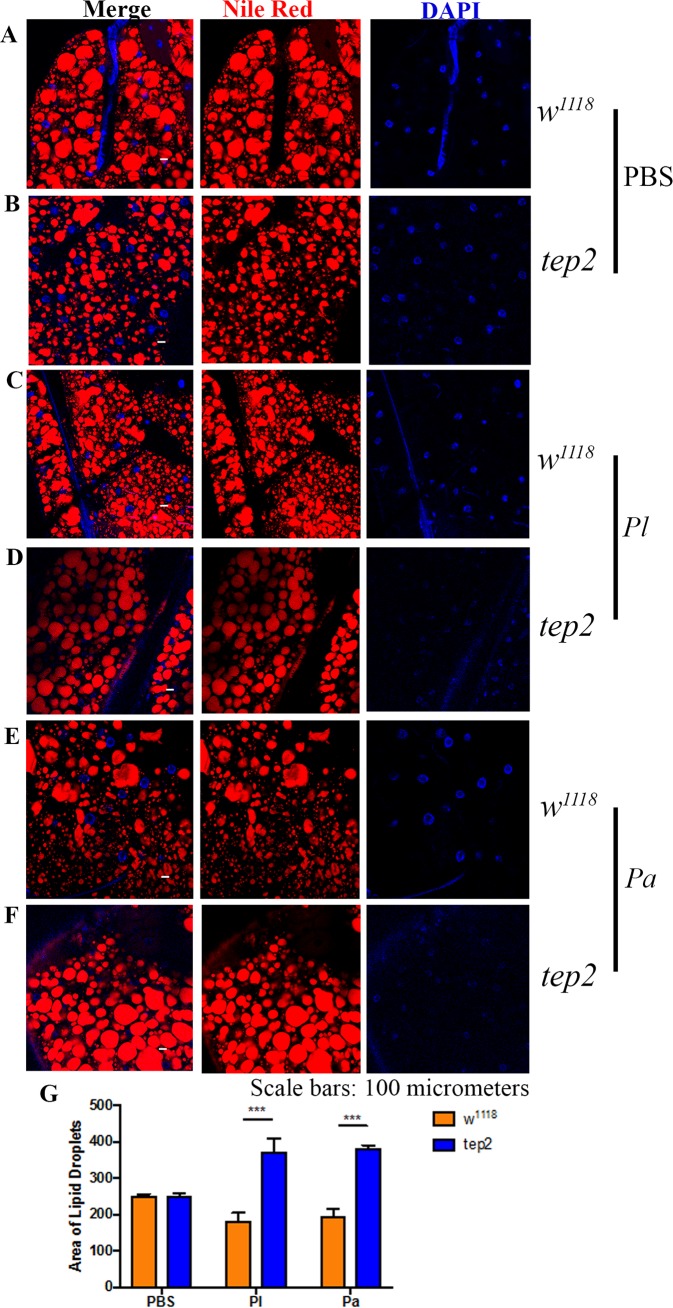
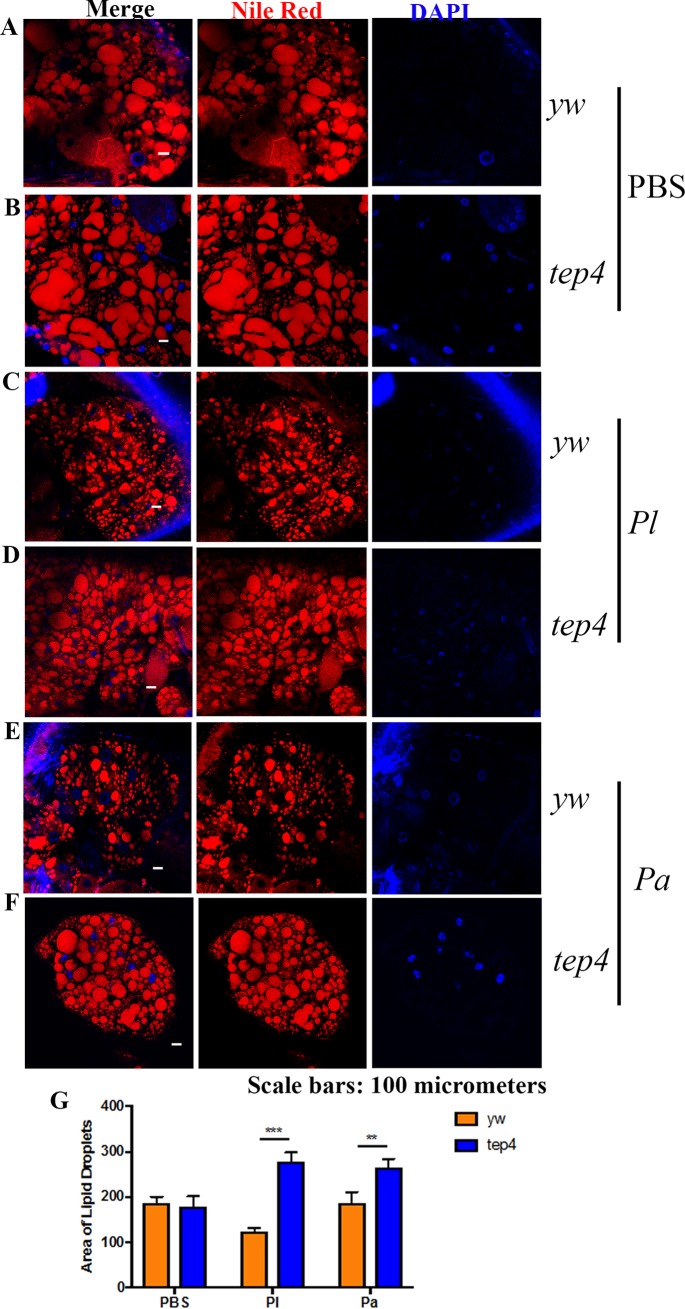
Drosophila tep2 and tep4 mutants contain large lipid droplets in the fat body after Photorhabdus infection.
Previous studies of Drosophila identified the participation of lipid droplets (LDs) in the antimicrobial immune response (40, 41). Here, we evaluated the status of LDs localized in the fat body of tep mutant flies and background controls in response to pathogenic and nonpathogenic bacterial infections. We observed that uninfected tep mutants and their controls displayed similar-sized LDs (Fig. 5A, B, and G and 6A, B, and G). In agreement with the results for triglycerides, we noticed larger LDs in tep2 and tep4 mutants after Photorhabdus infection than in control flies (Fig. 5C to G and 6C to G). These results indicate that the inactivation of Tep2 or Tep4 regulates the size of LDs in the fat body of flies in response to Photorhabdus infection.
FIG 5.
Drosophila mutants for Tep2 display large lipid droplets after Photorhabdus infection. (A to F) Fat body tissues were stained with Nile Red-O as well as DAPI (4′,6-diamidino-2-phenylindole) and observed under a confocal microscope (Olympus) at a ×20 magnification. Lipid droplets (red) and nuclei (blue) are shown for flies of the background control strain (w1118) (A, C, and E) and the tep2 mutant strain (B, D, and F) 18 h after infection with Photorhabdus luminescens (Pl) or P. asymbiotica (Pa) or injection with 1× PBS (negative control). (G) Areas of lipid droplets in fat body cells of the background control strain (w1118) as well as tep2 mutants were quantified by using ImageJ. The means from at least three independent fat body samples are shown, and error bars represent standard deviations. Significant differences are shown with asterisks (***, P < 0.001).
FIG 6.
Drosophila mutants for Tep4 display large lipid droplets after Photorhabdus infection. (A to F) Fat body tissues were stained with Nile Red-O as well as DAPI and observed under a confocal microscope (Olympus) at a ×20 magnification. Lipid droplets (red) and nuclei (blue) are shown for flies of the background control strain (yw) (A, C, and E) and the tep4 strain (B, D, and F) 18 h after infection with Photorhabdus luminescens (Pl) or P. asymbiotica (Pa) or injection with 1× PBS (negative control). (G) Areas of lipid droplets in fat body cells of the background control strain (yw) as well as tep4 mutants were quantified by using ImageJ. The means from three independent fat body samples are shown, and error bars represent standard deviations. Significant differences are shown with asterisks (**, P < 0.01; ***, P < 0.001).
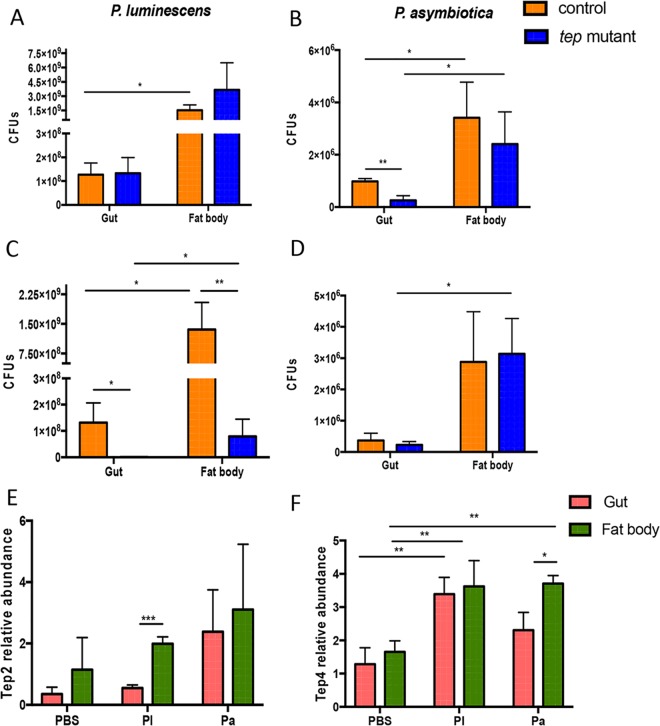
Drosophila tep2 and tep4 mutants contain fewer Photorhabdus cells in gut and fat body.
Previously, we reported a lower level of persistence of Photorhabdus in tep2 and tep4 mutant flies (11, 35). Here, we estimated the number of bacteria in the gut and fat body of tep mutants 18 h after infection with E. coli and Photorhabdus. We found higher numbers of Photorhabdus CFU in the fat body than in the gut in all four fly strains (Fig. 7). However, there were no changes in P. luminescens burdens between tep2 and control flies in either the fat body or gut (Fig. 7A). Instead, there were significantly fewer P. asymbiotica CFU in the gut of tep2 mutants than in control flies (Fig. 7B). Similar to our previously reported results (11), we observed significantly fewer CFU of P. luminescens in both tissues of tep4 mutants than in the tissues of control flies, whereas there was no difference in P. asymbiotica CFU between the two strains (Fig. 7C and D). We were unable to detect the presence of E. coli in either tissue of tep mutants and their background controls by quantitative PCR (qPCR) at 18 hpi. These results suggest that the inactivation of Tep2 or Tep4 regulates Photorhabdus replication in the fly gut and fat body during early and late hours of infection.
FIG 7.
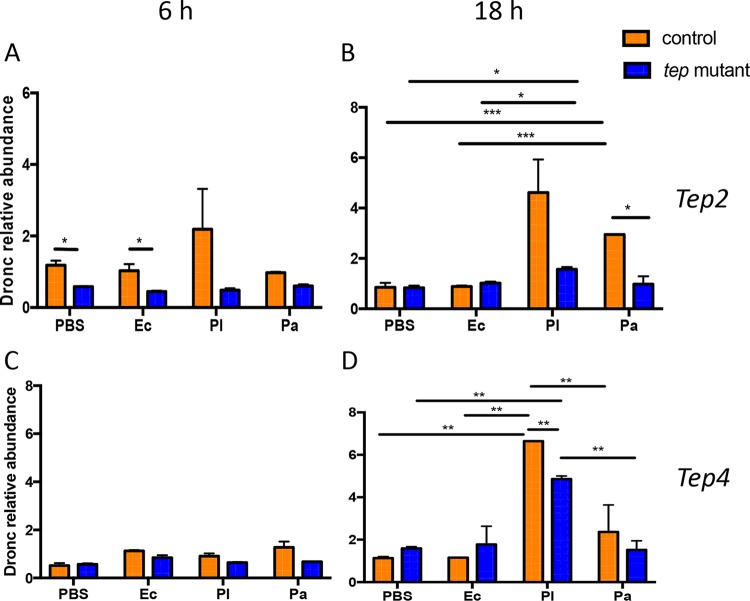
Pathogen burden and Tep gene transcript levels are altered in the gut and fat body of Drosophila flies in response to Photorhabdus infection. (A to D) CFU of P. luminescens (A and C) and P. asymbiotica (B and D) in the gut and fat body of tep2 and tep4 mutant flies and background control flies (w1118 and yw, respectively) (n = 5 per experimental condition) at 18 h postinfection. CFU were estimated by quantitative PCR of Photorhabdus 16S rRNA levels. (E and F) Transcript levels of Tep2 (E) and Tep4 (F) in the gut and fat body tissues of w1118 flies (n = 5) 18 h after infection with E. coli (Ec), P. luminescens (Pl), or P. asymbiotica (Pa) or injection with 1× PBS (negative control). Gene transcript levels are shown as relative abundances of transcripts normalized to the value for the ribosomal protein L32 gene (RpL32) and expressed as a ratio compared to values for uninfected flies. The means from three independent experiments are shown, and error bars represent standard deviations. Significant differences are indicated with asterisks (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
Photorhabdus infection increases Tep2 and Tep4 transcript levels in the fly gut and fat body.
Tep genes are induced in the larval fat body and in the abdominal epithelium of the gut in the adult fly (31). We recently found increased transcript levels of Tep2 and Tep4 in yw and w1118 background fly strains upon E. coli or Photorhabdus infection (11, 35). Therefore, we investigated whether Tep2 and Tep4 are expressed mainly in the gut and fat body of background control flies 18 h after infection with these bacteria. We found that Tep2 was upregulated in the fly fat body upon infection with P. luminescens only, whereas infection with this pathogen induced Tep4 in both tissues (Fig. 7F). In addition, Tep4 was upregulated in the fat body of P. asymbiotica-infected flies (Fig. 7F). These results indicate that the Drosophila gut is a source of Tep2 expression, whereas both gut and fat body tissues are involved in the induction of Tep4 expression in adult flies in response to Photorhabdus infection.
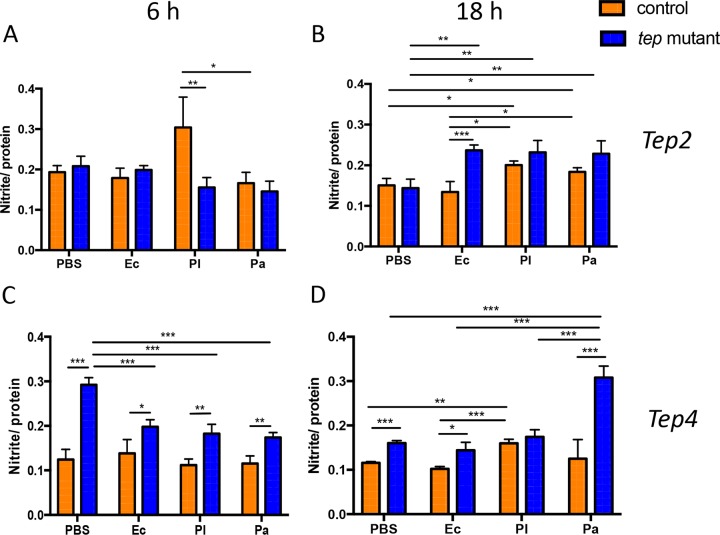
Drosophila tep2 and tep4 mutants have high nitric oxide activity in response to bacterial infection.
Because tep mutants contain larger amounts of sugars and triglycerides but lower bacterial burdens in the fat body and gut, we measured stress levels in these flies upon Photorhabdus or E. coli infection. For this, we estimated the levels of nitrite, a by-product of nitric oxide production that is used as a measure of stress in insects (42). We found that tep2 mutants had significantly lower nitrite levels than those of w1118 flies 6 h after infection with P. luminescens (Fig. 8A) but significantly higher nitrite levels 18 h after infection with E. coli or Photorhabdus than those of their counterparts injected with PBS (Fig. 8B). Similarly, w1118 flies had higher nitrite levels 18 h after infection with Photorhabdus than those of E. coli-infected or PBS-injected individuals (Fig. 8B). In tep4 mutants, there was a decrease in the nitrite quantity 6 h after infection with Photorhabdus or E. coli compared to that in PBS-injected flies (Fig. 8C). However, yw flies injected with P. luminescens had significantly increased nitrite levels compared to those in yw flies injected with E. coli or PBS at 18 hpi (Fig. 8D). We also found higher nitrite levels in tep4 mutant flies injected with P. asymbiotica than in those injected with PBS, E. coli, or P. luminescens at 18 hpi (Fig. 8D). Finally, tep4 mutants contained large amounts of nitrite after injection with PBS, E. coli, or P. luminescens at 6 and 18 hpi compared to those in yw background flies (Fig. 8C and D). These results indicate that flies with inactivated Tep4 have increased stress levels in response to Photorhabdus or E. coli infection.
FIG 8.
Drosophila mutants for Tep2 and Tep4 exhibit elevated levels of nitric oxide after infection with Photorhabdus. Nitrite levels in tep loss-of-function mutants and background controls (n = 5) injected with E. coli (Ec), P. luminescens (Pl), P. asymbiotica (Pa), or 1× PBS (negative control) were estimated. The concentration of nitrite (micromolar) is normalized to the protein content (micrograms per milliliter) and represented as a ratio of total nitrite levels to total protein levels in tep2 mutants (A and B) and tep4 mutants (C and D) with the corresponding background control strains (w1118 and yw, respectively) at 6 and 18 h postinjection. The means from three independent experiments are shown, and error bars represent standard deviations. Significant differences are shown with asterisks (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
Drosophila tep2 and tep4 mutants undergo reduced cell death upon infection with Photorhabdus.
To investigate whether the prolonged-survival phenotype of tep mutants in response to Photorhabdus infection is due to reduced apoptotic death (11), we examined the transcript levels of Dronc, an ortholog of mammalian caspase-9 (43). The initiator (apical) caspase DRONC is required for the induction of apoptosis in flies (44). We found that w1118 flies had increased Dronc transcript levels 6 h after infection with E. coli or injection with PBS compared to those in tep2 mutants (Fig. 9A). Eighteen hours after infection with P. asymbiotica, w1118 flies displayed increased transcript levels of Dronc compared to those in tep2 mutants and w1118 flies infected with E. coli or injected with PBS (Fig. 9B). Moreover, tep2 mutants had significantly higher Dronc transcript levels after P. luminescens infection than did those injected with E. coli or PBS (Fig. 9B). yw flies had increased transcript levels of Dronc 18 h after infection with P. luminescens compared to those in flies injected with P. asymbiotica, E. coli, or PBS (Fig. 9C and D). For tep4 mutants, we observed lower Dronc transcript levels than those in yw flies 18 h after infection with P. luminescens (Fig. 9C and D). In addition, Dronc transcript levels in tep4 mutants 18 h after infection with P. luminescens were high in tep4 mutants compared to those in flies infected with P. asymbiotica or injected with PBS (Fig. 9D). These findings suggest that the inactivation of Tep2 or Tep4 is linked to reduced cell death in Photorhabdus-infected flies, which is probably due to a lower level of persistence of the pathogens.
FIG 9.
Drosophila mutants for Tep2 and Tep4 have reduced apoptosis upon Photorhabdus infection. Shown are transcript levels of Dronc in tep2 (A and B) and tep4 (C and D) mutant flies compared to the corresponding background controls (w1118 and yw, respectively; n = 3 to 5) 6 and 18 h after infection with E. coli (Ec), P. luminescens (Pl), or P. asymbiotica (Pa) or injection with 1× PBS (negative control). Gene transcript levels are shown as relative abundances of transcripts normalized to the value for the ribosomal protein L32 housekeeping gene (RpL32) and expressed as a ratio compared to the values for uninfected flies. The means from three independent experiments are shown, and error bars represent standard deviations. Significant differences are indicated with asterisks (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
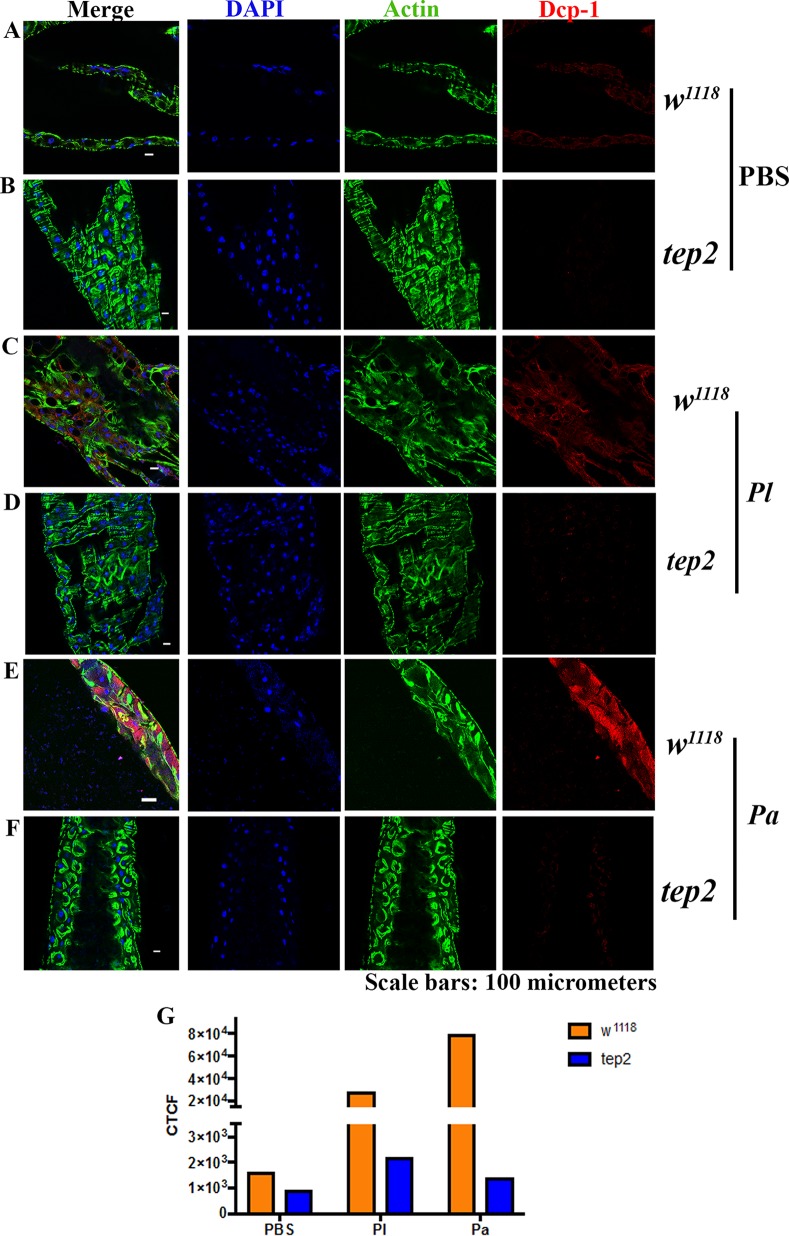
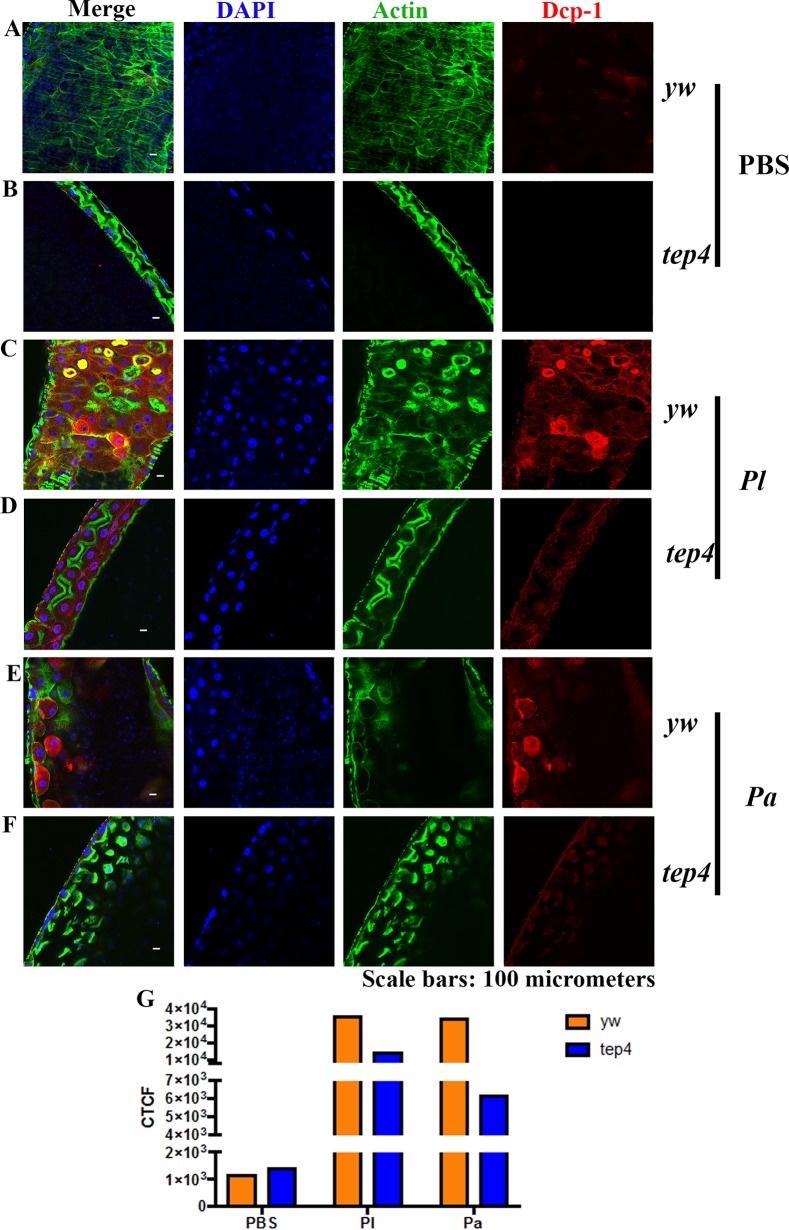
To investigate cell death at the tissue level, we also estimated the expression level of the death caspase-1 (DCP-1) protein in the midgut of tep2 and tep4 mutants as well as in the corresponding background control flies. We chose the midgut for these experiments because Photorhabdus damages this tissue by inducing cell death (17). We were not able to identify changes in DCP-1 expression between tep2 mutants and control flies injected with PBS (Fig. 10A and B). However, we found lower DCP-1 expression levels in tep2 mutants than in w1118 flies infected with Photorhabdus bacteria at 18 hpi (Fig. 10C to G). Similarly, there were no changes in DCP-1 expression levels between tep4 mutants and yw flies injected with PBS (Fig. 11A and B). Finally, we found that tep4 mutants had lower expression levels of DCP-1 than did yw flies 18 h after infection with Photorhabdus (Fig. 11C to G). These results indicate that the inactivation of Tep2 or Tep4 results in decreased expression levels of caspases in flies infected with Photorhabdus pathogens.
FIG 10.
Drosophila mutants for Tep2 show low DCP-1 expression levels in the midgut following infection with P. luminescens. (A to F) Guts from 7- to 10-day-old tep2 mutants and background control flies (w1118) were stained with DCP-1 (red), DAPI (blue), and phalloidin (green). The dissected, stained tissues of flies injected with 1× PBS (A and B), P. luminescens (Pl) (C and D), and P. asymbiotica (Pa) (E and F) were observed at a ×40 magnification under a confocal microscope. (G) Corrected total cell fluorescence (CTCF) was measured to quantify the expression level of DCP-1 in both background control flies (w1118) and tep2 mutant flies by using ImageJ.
FIG 11.
Drosophila mutants for Tep4 display reduced DCP-1 expression in the midgut upon infection with Photorhabdus. (A to F) Guts from 7- to 10-day-old tep4 mutants and background control flies (yw) were stained with DCP-1 (red), DAPI (blue), and phalloidin (green). The dissected, stained tissues of flies injected with 1× PBS (A and B), P. luminescens (Pl) (C and D), and P. asymbiotica (Pa) (E and F) were viewed at a ×40 magnification by using confocal microscopy. (G) Corrected total cell fluorescence (CTCF) was measured to quantify the expression levels of DCP-1 in both background control flies (yw) and tep4 mutant flies by using ImageJ.
DISCUSSION
In this study, we investigated the pathological defects in tep loss-of-function mutant flies in response to Photorhabdus infection. We evaluated the amounts of different metabolites, such as carbohydrates and lipids, to monitor metabolic activity in the presence or absence Photorhabdus infection. We also examined the levels of stress, cell death, and pathogen burden in tep mutant flies as indicators of inflammation upon infection with these pathogens. We report that the inactivation of Tep2 or Tep4 results in increased physiological responses and reduced inflammation in flies infected with Photorhabdus bacteria.
Metabolic changes in the whole animal reflect changes that take place at the physiological or immunological level (45). Hence, by examining physiological activities in infected tep mutants, we aimed to understand the cause(s) for their altered survival response to Photorhabdus (11). Previously, we showed that TEP2 and TEP4 are involved in regulating the activation of immune signaling pathways in Photorhabdus-infected flies (11, 35). Several studies have linked immune signaling pathway activity to metabolic status in Drosophila in the context of infection (7). For example, insulin signaling and triglyceride synthesis were attenuated in Toll gain-of-function mutants, but not in Imd mutants, in the absence of infection (46). The induction of the Toll pathway by bacterial infection results in a reduction of insulin signaling. In addition, the Imd pathway negatively modulates certain metabolic genes in response to Gram-negative bacterial infection in fruit flies (47). Therefore, we propose that the increased levels of carbohydrates and triglycerides in tep mutants compared to those in the background control flies could be the result of an indirect attenuation of insulin signaling due to differential regulation of immune signaling in the absence of functional TEP molecules. Moreover, the decreased levels of trehalose and glycogen in tep2 and tep4 mutants, respectively, during the late stages of Photorhabdus infection may be the result of using up the stored energy in these flies. Once these energy reservoirs are exhausted, the synthesis of various metabolites, such as proteins, lipids, and carbohydrates, in response to bacterial infection might cease in tep mutant flies (48).
Lipid droplets are multifunctional organs present in most organisms ranging from bacteria to eukaryotes. They are abundantly present in fat-storing tissues, such as insect fat body cells. Lipid droplets perform immune activities in mammals, mosquitoes, and Drosophila (40, 49, 50). Interestingly, lipid droplets have been shown to accumulate in neutrophils and macrophages during infection in mammals (50). Moreover, the constitutive activation of Toll and Imd pathways in Aedes mosquitoes leads to the accumulation of lipid droplets in the midgut (40, 49). Therefore, our present findings indicate that the induction of Toll and Imd signaling in flies with inactivated tep genes could be linked to changes in the numbers of lipid droplets in the context of Photorhabdus infection. The exact mechanism of this physiological alteration requires further investigation and will form the basis of our future studies.
The fat body and gut tissues of Drosophila are the sites of systemic and local antimicrobial peptide synthesis, respectively (51, 52). Tep genes are upregulated in the abdominal epithelium and larval fat body upon septic injury (31), and our results are in accordance, as we report the induction of Tep2 and Tep4 in the fat body as well as the gut after Photorhabdus infection. During Photorhabdus infection, the bacteria first grow excessively in the insect hemolymph and gut and subsequently proliferate in the fat body (14, 18). The increased colonization of Photorhabdus in the fat body at late time points suggests that this tissue might form the main target for these pathogens, which could lead to the suppression of the humoral immune response (53). In addition, the presence of Photorhabdus in the gut could also form an evasion strategy for direct interference with the gut epithelial immune response (17). Interestingly, the level of persistence of Photorhabdus in tep mutants is consistently low, which implies that there is a potential interaction of TEP molecules with the pathogens. We propose that this interaction might promote Photorhabdus pathogenicity, which could lead to the depletion of energy stores and, ultimately, the death of the fly.
Nitric oxide signaling is known to activate the innate immune response in Drosophila upon infection with Gram-negative bacteria (6, 54). Nitric oxide synthase provides protection against Photorhabdus infection in Manduca sexta and Drosophila and can increase melanization and clot formation (55, 56). Increased nitric oxide levels in uninfected and infected tep mutants could potentially activate humoral immune responses early in an infection by Photorhabdus. Therefore, we propose that the inactivation of Tep2 or Tep4 interferes with nitric oxide activity that could stimulate immune signaling pathways in the fly (35, 36). Consequently, this would result in increased host survival, lower pathogen burdens, and higher melanization activity in tep mutants during the course of Photorhabdus infection (11, 35, 54).
In addition to apoptosis, caspases participate in immunity and inflammation (6, 57), and interestingly, sterile inflammation in Drosophila can be induced in the absence of pathogens (58). Therefore, Dronc upregulation in w1118 flies injected with buffer or nonpathogenic E. coli bacteria could be the result of wounding. Photorhabdus pathogens secrete virulence factors that cause the apoptosis of insect hemocytes and cells in the gut and fat body (17, 59). We showed previously that the inactivation of Tep2 or Tep4 increases hemocyte viability in Photorhabdus-infected flies (35, 36). Reduced transcript levels of Dronc, encoding an apical caspase protein containing a caspase recruitment domain (60), in tep mutants signify that these flies undergo less inflammation and cell death during the course of Photorhabdus infection. Similarly, reduced expression levels of DCP-1 in the tep mutants indicate that these flies undergo less inflammation upon Photorhabdus infection. Moreover, as tep2 and tep4 mutants contain fewer hemocytes when infected by P. asymbiotica (35, 36), they can probably sustain lower levels of inflammation caused by the bacteria. Nitric oxide is capable of acting as both an inducer and an inhibitor of apoptosis by targeting preapoptotic and antiapoptotic molecules, respectively, in mammals as well as in Drosophila (61–63). Therefore, an alternative explanation for the increased transcript levels of Dronc in the background control flies could be that the inactivation of Tep2 or Tep4 may result in elevated levels of nitric oxide that could lead to the inhibition of apoptosis in Photorhabdus-infected flies. However, the protective effect of reduced apoptosis probably ceases once nitric oxide levels misbalance the expression of antiapoptotic versus preapoptotic genes, which could result in increased expression levels of proapoptotic genes during the late stages of Photorhabdus infection (after 18 hpi) that could consequently increase the survival of tep mutant flies.
In conclusion, here, we present evidence that the inactivation of Tep2 or Tep4 in Drosophila increases the metabolic energy stores of the fly in response to Photorhabdus infection. We show that Tep gene inactivation reduces numbers of Photorhabdus bacteria in the fat body and gut and increases Dronc and DCP-1 expression levels as well as nitric oxide production in infected mutants. Our results suggest a novel function of TEP molecules in the interaction of Drosophila with the virulent pathogen Photorhabdus. Similar research will contribute to a better understanding of the exact function of insect TEP molecules in the host antibacterial immune response and will allow a comprehensive functional comparison with mammalian complement factors.
MATERIALS AND METHODS
Fly strains and bacterial stocks.
Loss-of-function tep2 (f02756, Harvard, and piggyBac) and tep4 (15936, Bloomington, and p-element) mutants and their background strains (w1118 and yw) were used in all experiments. All fly strains were fed on instant Drosophila medium (Carolina Biological Supply) in deionized water and maintained at 25°C with a 12-h-light/12-h-dark photoperiod.
Photorhabdus luminescens subsp. laumondii (strain TT01), P. asymbiotica subsp. asymbiotica (strain ATCC 43949), and Escherichia coli (strain K-12) were used for infections. Bacteria were grown in sterile Luria-Bertani (LB) broth for approximately 18 to 22 h at 30°C on a rotary shaker at 220 rpm. The cultures were pelleted, washed, and resuspended in 1× sterile PBS (Sigma-Aldrich). Bacteria were diluted in 1× PBS to optical densities (ODs) (at 600 nm) of 0.1 for P. luminescens, 0.25 for P. asymbiotica, and 0.015 for E. coli by using a spectrophotometer (NanoDrop 2000c; Thermo Fisher Scientific) (11).
Infection assays.
Seven- to ten-day-old adult Drosophila melanogaster flies were anesthetized with carbon dioxide and injected intrathoracically with 100 to 300 CFU of each bacterial preparation (P. luminescens, P. asymbiotica, or E. coli) or sterile 1× PBS (septic injury control) by using a Nanoject II apparatus (Drummond Scientific) equipped with glass capillaries prepared with a micropipette puller (Sutter Instruments). Whole flies or gut or fat body tissues were subsequently collected at 6 and 18 hpi and stored at −80°C until further use.
Quantification of trehalose, glycogen, glucose, and triglyceride levels.
Adult flies (n = 5) were injected with P. luminescens, P. asymbiotica, E. coli, or 1× PBS and collected at 6 and 18 hpi. Groups of flies were washed, and samples were prepared for colorimetric assays of trehalose, glycogen, glucose, and triglyceride, as previously described (25, 64). All samples and standards were run in duplicates, and at least three independent experiments were carried out for each assay. The levels of metabolites were normalized to the total protein content present in the sample.
Lipid droplet staining and quantification.
Fat body tissues were dissected in 1× PBS and fixed in 4% paraformaldehyde prepared in 1× PBS for 30 min at room temperature. The tissues were then washed twice in 1× PBS and stained with a 1:1,000 dilution of 0.05% Nile Red-O in 1 mg/ml methanol for 30 min in the dark. Tissues were mounted in Vectashield (catalog number H1200; Vector Laboratories), and images were taken by using an Olympus confocal microscope. To quantify lipid droplet sizes, the area of the 15 largest lipid droplets per fat body was measured by using Image J. This was repeated for at least three independent samples for each fly strain.
RNA isolation, gene transcript levels, and bacterial loads.
RNA was isolated from frozen flies (n = 5) or gut or fat body tissues by using the PrepEase RNA spin kit (Affymetrix USB) or TRIzol reagent (Ambion, Thermo Fisher Scientific) according to the manufacturers' protocols. RNA samples were adjusted to 350 ng for cDNA synthesis (Applied Biosystems, Thermo Fisher Scientific). Quantitative reverse transcription-PCR (qRT-PCR) was performed by using the CFX96 Touch real-time PCR detection system (Bio-Rad), and the ΔΔCT method was used for analysis of results (11). Data are presented as the ratio of injected flies to untreated flies (baseline controls), as previously described (11). A list of primers used for the qRT-PCR assays is shown in Table 1.
TABLE 1.
List of primers used in this studya
| Gene | GenBank accession no. | Primer direction | Primer sequence (5′–3′) | Tm (°C) |
|---|---|---|---|---|
| RpL32 | CG7939 | Forward | GATGACCATCCGCCCAGCA | 61 |
| Reverse | CGGACCGACAGCTGCTTGGC | |||
| Pl16s | KC237383 | Forward | ACAGAGTTGGATCTTGACGTTACCC | 61 |
| Reverse | AATCTTGTTTGCTCCCCACGCTT | |||
| Dronc | CG8091 | Forward | AGCTTGCTAACGCAGGGTC | 61 |
| Reverse | CCTTTATCTCGCTAAACGAACGG | |||
| Tep2 | CG7052 | Forward | CGTTCTGCTGGCTTTCTTC | 52.5 |
| Reverse | ATACTGGTCGTCCGTCTTGTC | |||
| Tep4 | CG10363 | Forward | GCTGCAGAACCAGATCGAAATC | 61 |
| Reverse | ATGACTTTGGCGACGTCTTGAT |
Tm, melting temperature.
To estimate bacterial loads, standard curves were generated for each bacterial strain by using primers to amplify their 16S rRNA genes, as described previously (12). qPCR was performed on bacterial cDNA samples, as described above. Numbers of CFU were determined from the standard curves. All experiments were performed at least three times.
Nitric oxide estimation.
Equal numbers (n = 4) of male and female adult flies were homogenized in 1× PBS at 6 and 18 hpi. Following centrifugation at 10,000 × g for 10 min at 4°C, the supernatants were collected and mixed with Griess reagent at a 1:1 ratio (Sigma-Aldrich). Following incubation for 15 min at room temperature, the absorbance (595 nm) was measured by using a spectrophotometer (NanoDrop 2000c). A silver nitrite standard curve was constructed to estimate the concentration of nitrite in the samples. Nitric oxide levels are represented as the concentration of nitrite normalized to the total protein content, and the experiments were performed at least three times.
Gut staining and fluorescence quantification.
Guts from infected or uninfected 7- to 10-day-old adult flies were dissected in 1× PBS, as mentioned above. Gut tissues were fixed for 30 min in 4% paraformaldehyde in PBST (PBS plus 0.3% Triton). After two washes in PBST, guts were treated with a 1:100 dilution of Dcp-1 primary antibody (Cell Signaling Technology) in PBST at 4°C overnight. Following two washes, the tissues were blocked for 2 h in PBSTB (PBST plus 0.1% bovine serum albumin). Rinsing was done twice in PBST, and tissues were incubated with a 1:500 dilution of Alexa Fluor 544 anti-rabbit secondary antibody for 2 h at room temperature. After two washes, tissues were finally incubated with phalloidin-actin for 20 min before being mounted in Vectashield mounting medium (catalog number H1200; Vector Laboratories). Images were taken by using an Olympus confocal microscope.
Images were first converted into 16-bit gray scale images, and three random areas of DCP-1 expression were used for quantification. Relative amounts of fluorescence were measured with ImageJ software by using Shanbhag thresholding on images and calculating the resulting area, integrated density, and mean fluorescence of the background. The following equation was used: corrected total fluorescence = integrated density − (area × mean fluorescence of background).
Statistical analysis.
All statistical analyses were performed by using GraphPad Prism7 software. For gene transcript levels, nitric oxide quantification, and metabolic activity measurements, data were analyzed by using two-way analysis of variance (ANOVA) with a Tukey post hoc test for multiple comparisons. For bacterial load estimations, samples were analyzed by using a two-tailed t test. P values of <0.05 were considered statistically significant.
ACKNOWLEDGMENTS
We thank members of the Department of Biological Sciences at GWU for critical reading of the manuscript.
This research was supported by a start-up fund from the Columbian College of Arts and Sciences at GWU to I.E. and by Harlan summer fellowships from the Department of Biological Sciences at GWU and a scholarship from the Cosmos Club Foundation (Washington, DC) to U.S. The I.E. laboratory is funded by the National Institutes of Health (grant 1R01AI110675-01A1).
REFERENCES
- 1.Shirasu-Hiza MM, Schneider DS. 2007. Confronting physiology: how do infected flies die? Cell Microbiol 9:2775–2783. doi: 10.1111/j.1462-5822.2007.01042.x. [DOI] [PubMed] [Google Scholar]
- 2.Apidianakis Y, Rahme LG. 2009. Drosophila melanogaster as a model host for studying Pseudomonas aeruginosa infection. Nat Protoc 4:1285–1294. doi: 10.1038/nprot.2009.124. [DOI] [PubMed] [Google Scholar]
- 3.Razzell W, Wood W, Martin P. 2011. Swatting flies: modelling wound healing and inflammation in Drosophila. Dis Model Mech 4:569–574. doi: 10.1242/dmm.006825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nehme NT, Liegeois S, Kele B, Giammarinaro P, Pradel E, Hoffmann JA, Ewbank JJ, Ferrandon D. 2007. A model of bacterial intestinal infections in Drosophila melanogaster. PLoS Pathog 3:e173. doi: 10.1371/journal.ppat.0030173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnold PA, Johnson KN, White CR. 2013. Physiological and metabolic consequences of viral infection in Drosophila melanogaster. J Exp Biol 216:3350–3357. doi: 10.1242/jeb.088138. [DOI] [PubMed] [Google Scholar]
- 6.Lemaitre B, Hoffmann J. 2007. The host defense of Drosophila melanogaster. Annu Rev Immunol 25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- 7.Dionne M. 2014. Immune-metabolic interaction in Drosophila. Fly (Austin) 8:75–79. doi: 10.4161/fly.28113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ayres JS, Schneider DS. 2009. The role of anorexia in resistance and tolerance to infections in Drosophila. PLoS Biol 7:e1000150. doi: 10.1371/journal.pbio.1000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chambers MC, Song KH, Schneider DS. 2012. Listeria monocytogenes infection causes metabolic shifts in Drosophila melanogaster. PLoS One 7:e50679. doi: 10.1371/journal.pone.0050679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castillo JC, Shokal U, Eleftherianos I. 2013. Immune gene transcription in Drosophila adult flies infected by entomopathogenic nematodes and their mutualistic bacteria. J Insect Physiol 59:179–185. doi: 10.1016/j.jinsphys.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Shokal U, Eleftherianos I. 2017. Thioester-containing protein-4 regulates the Drosophila immune signaling and function against the pathogen Photorhabdus. J Innate Immun 9:83–93. doi: 10.1159/000450610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shokal U, Yadav S, Atri J, Accetta J, Kenney E, Banks K, Katakam A, Jaenike J, Eleftherianos I. 2016. Effects of co-occurring Wolbachia and Spiroplasma endosymbionts on the Drosophila immune response against insect pathogenic and non-pathogenic bacteria. BMC Microbiol 16:16. doi: 10.1186/s12866-016-0634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castillo JC, Creasy T, Kumari P, Shetty A, Shokal U, Tallon LJ, Eleftherianos I. 2015. Drosophila anti-nematode and antibacterial immune regulators revealed by RNA-Seq. BMC Genomics 16:519. doi: 10.1186/s12864-015-1690-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waterfield NR, Ciche T, Clarke D. 2009. Photorhabdus and a host of hosts. Annu Rev Microbiol 63:557–574. doi: 10.1146/annurev.micro.091208.073507. [DOI] [PubMed] [Google Scholar]
- 15.Ciche TA, Ensign JC. 2003. For the insect pathogen Photorhabdus luminescens, which end of a nematode is out? Appl Environ Microbiol 69:1890–1897. doi: 10.1128/AEM.69.4.1890-1897.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ffrench-Constant RH, Dowling A, Waterfield NR. 2007. Insecticidal toxins from Photorhabdus bacteria and their potential use in agriculture. Toxicon 49:436–451. doi: 10.1016/j.toxicon.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 17.Eleftherianos I, ffrench-Constant RH, Clarke DJ, Dowling AJ, Reynolds SE. 2010. Dissecting the immune response to the entomopathogen Photorhabdus. Trends Microbiol 18:552–560. doi: 10.1016/j.tim.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Silva CP, Waterfield NR, Daborn PJ, Dean P, Chilver T, Au CP, Sharma S, Potter U, Reynolds SE, ffrench-Constant RH. 2002. Bacterial infection of a model insect: Photorhabdus luminescens and Manduca sexta. Cell Microbiol 4:329–339. doi: 10.1046/j.1462-5822.2002.00194.x. [DOI] [PubMed] [Google Scholar]
- 19.Daborn PJ, Waterfield N, Silva CP, Au CP, Sharma S, ffrench-Constant RH. 2002. A single Photorhabdus gene, makes caterpillars floppy (mcf), allows Escherichia coli to persist within and kill insects. Proc Natl Acad Sci U S A 99:10742–10747. doi: 10.1073/pnas.102068099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lang AE, Schmidt G, Schlosser A, Hey TD, Larrinua IM, Sheets JJ, Mannherz HG, Aktories K. 2010. Photorhabdus luminescens toxins ADP-ribosylate actin and RhoA to force actin clustering. Science 327:1139–1142. doi: 10.1126/science.1184557. [DOI] [PubMed] [Google Scholar]
- 21.Blackburn M, Golubeva E, Bowen D, ffrench-Constant RH. 1998. A novel insecticidal toxin from Photorhabdus luminescens, toxin complex a (Tca), and its histopathological effects on the midgut of Manduca sexta. Appl Environ Microbiol 64:3036–3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eleftherianos I, Boundy S, Joyce SA, Aslam S, Marshall JW, Cox RJ, Simpson TJ, Clarke DJ, ffrench-Constant RH, Reynolds SE. 2007. An antibiotic produced by an insect-pathogenic bacterium suppresses host defenses through phenoloxidase inhibition. Proc Natl Acad Sci U S A 104:2419–2424. doi: 10.1073/pnas.0610525104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eleftherianos I, Waterfield NR, Bone P, Boundy S, ffrench-Constant RH, Reynolds SE. 2009. A single locus from the entomopathogenic bacterium Photorhabdus luminescens inhibits activated Manduca sexta phenoloxidase. FEMS Microbiol Lett 293:170–176. doi: 10.1111/j.1574-6968.2009.01523.x. [DOI] [PubMed] [Google Scholar]
- 24.Hu K, Webster JM. 2000. Antibiotic production in relation to bacterial growth and nematode development in Photorhabdus-Heterorhabditis infected Galleria mellonella larvae. FEMS Microbiol Lett 189:219–223. doi: 10.1111/j.1574-6968.2000.tb09234.x. [DOI] [PubMed] [Google Scholar]
- 25.McCormack S, Yadav S, Shokal U, Kenney E, Cooper D, Eleftherianos I. 2016. The insulin receptor substrate Chico regulates antibacterial immune function in Drosophila. Immun Ageing 13:15. doi: 10.1186/s12979-016-0072-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christophides GK, Vlachou D, Kafatos FC. 2004. Comparative and functional genomics of the innate immune system in the malaria vector Anopheles gambiae. Immunol Rev 198:127–148. doi: 10.1111/j.0105-2896.2004.0127.x. [DOI] [PubMed] [Google Scholar]
- 27.Markiewski MM, Lambris JD. 2007. The role of complement in inflammatory diseases from behind the scenes into the spotlight. Am J Pathol 171:715–727. doi: 10.2353/ajpath.2007.070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shokal U, Eleftherianos I. 2017. Evolution and function of thioester-containing proteins and the complement system in the innate immune response. Front Immunol 8:759. doi: 10.3389/fimmu.2017.00759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blandin SA, Marois E, Levashina EA. 2008. Antimalarial responses in Anopheles gambiae: from a complement-like protein to a complement-like pathway. Cell Host Microbe 3:364–374. doi: 10.1016/j.chom.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 30.Fraiture M, Baxter RH, Steinert S, Chelliah Y, Frolet C, Quispe-Tintaya W, Hoffmann JA, Blandin SA, Levashina EA. 2009. Two mosquito LRR proteins function as complement control factors in the TEP1-mediated killing of Plasmodium. Cell Host Microbe 5:273–284. doi: 10.1016/j.chom.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 31.Bou Aoun R, Hetru C, Troxler L, Doucet D, Ferrandon D, Matt N. 2011. Analysis of thioester-containing proteins during the innate immune response of Drosophila melanogaster. J Innate Immun 3:52–64. doi: 10.1159/000321554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lagueux M, Perrodou E, Levashina EA, Capovilla M, Hoffmann JA. 2000. Constitutive expression of a complement-like protein in Toll and JAK gain-of-function mutants of Drosophila. Proc Natl Acad Sci U S A 97:11427–11432. doi: 10.1073/pnas.97.21.11427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stroschein-Stevenson SL, Foley E, O'Farrell PH, Johnson AD. 2006. Identification of Drosophila gene products required for phagocytosis of Candida albicans. PLoS Biol 4:e4. doi: 10.1371/journal.pbio.0040004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dostalova A, Rommelaere S, Poidevin M, Lemaitre B. 2017. Thioester-containing proteins regulate the Toll pathway and play a role in Drosophila defence against microbial pathogens and parasitoid wasps. BMC Biol 15:79. doi: 10.1186/s12915-017-0408-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shokal U, Kopydlowski H, Eleftherianos I. 2 June 2017. The distinct function of Tep2 and Tep6 in the immune defense of Drosophila melanogaster against the pathogen Photorhabdus. Virulence doi: 10.1080/21505594.2017.1330240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shokal U, Eleftherianos I. 2017. The Drosophila thioester containing protein-4 participates in the induction of the cellular immune response to the pathogen Photorhabdus. Dev Comp Immunol 76:200–208. doi: 10.1016/j.dci.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 37.Dionne MS, Pham LN, Shirasu-Hiza M, Schneider DS. 2006. Akt and FOXO dysregulation contribute to infection-induced wasting in Drosophila. Curr Biol 16:1977–1985. doi: 10.1016/j.cub.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 38.Yasugi T, Yamada T, Nishimura T. 2017. Adaptation to dietary conditions by trehalose metabolism in Drosophila. Sci Rep 7:1619. doi: 10.1038/s41598-017-01754-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chtarbanova S, Lamiable O, Lee KZ, Galiana D, Troxler L, Meignin C, Hetru C, Hoffmann JA, Daeffler L, Imler JL. 2014. Drosophila C virus systemic infection leads to intestinal obstruction. J Virol 88:14057–14069. doi: 10.1128/JVI.02320-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anand P, Cermelli S, Li Z, Kassan A, Bosch M, Sigua R, Huang L, Ouellette AJ, Pol A, Welte MA, Gross SP. 2012. A novel role for lipid droplets in the organismal antibacterial response. eLife 1:e00003. doi: 10.7554/eLife.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rynes J, Donohoe CD, Frommolt P, Brodesser S, Jindra M, Uhlirova M. 2012. Activating transcription factor 3 regulates immune and metabolic homeostasis. Mol Cell Biol 32:3949–3962. doi: 10.1128/MCB.00429-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ajjuri RR, O'Donnell JM. 4 December 2013. Novel whole-tissue quantitative assay of nitric oxide levels in Drosophila neuroinflammatory response. J Vis Exp doi: 10.3791/50892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meier P, Silke J, Leevers SJ, Evan GI. 2000. The Drosophila caspase DRONC is regulated by DIAP1. EMBO J 19:598–611. doi: 10.1093/emboj/19.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kondo S, Senoo-Matsuda N, Hiromi Y, Miura M. 2006. DRONC coordinates cell death and compensatory proliferation. Mol Cell Biol 26:7258–7268. doi: 10.1128/MCB.00183-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ganeshan K, Chawla A. 2014. Metabolic regulation of immune responses. Annu Rev Immunol 32:609–634. doi: 10.1146/annurev-immunol-032713-120236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DiAngelo JR, Bland ML, Bambina S, Cherry S, Birnbaum MJ. 2009. The immune response attenuates growth and nutrient storage in Drosophila by reducing insulin signaling. Proc Natl Acad Sci U S A 106:20853–20858. doi: 10.1073/pnas.0906749106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Erkosar B, Defaye A, Bozonnet N, Puthier D, Royet J, Leulier F. 2014. Drosophila microbiota modulates host metabolic gene expression via IMD/NF-kappaB signaling. PLoS One 9:e94729. doi: 10.1371/journal.pone.0094729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hand SC, Hardewig I. 1996. Downregulation of cellular metabolism during environmental stress: mechanisms and implications. Annu Rev Physiol 58:539–563. doi: 10.1146/annurev.ph.58.030196.002543. [DOI] [PubMed] [Google Scholar]
- 49.Barletta AB, Alves LR, Silva MC, Sim S, Dimopoulos G, Liechocki S, Maya-Monteiro CM, Sorgine MH. 2016. Emerging role of lipid droplets in Aedes aegypti immune response against bacteria and dengue virus. Sci Rep 6:19928. doi: 10.1038/srep19928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bozza PT, Magalhaes KG, Weller PF. 2009. Leukocyte lipid bodies—biogenesis and functions in inflammation. Biochim Biophys Acta 1791:540–551. doi: 10.1016/j.bbalip.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferrandon D, Imler JL, Hetru C, Hoffmann JA. 2007. The Drosophila systemic immune response: sensing and signalling during bacterial and fungal infections. Nat Rev Immunol 7:862–874. doi: 10.1038/nri2194. [DOI] [PubMed] [Google Scholar]
- 52.Capo F, Charroux B, Royet J. 2016. Bacteria sensing mechanisms in Drosophila gut: local and systemic consequences. Dev Comp Immunol 64:11–21. doi: 10.1016/j.dci.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 53.Hallem EA, Rengarajan M, Ciche TA, Sternberg PW. 2007. Nematodes, bacteria, and flies: a tripartite model for nematode parasitism. Curr Biol 17:898–904. doi: 10.1016/j.cub.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 54.Foley E, O'Farrell PH. 2003. Nitric oxide contributes to induction of innate immune responses to gram-negative bacteria in Drosophila. Genes Dev 17:115–125. doi: 10.1101/gad.1018503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arefin B, Kucerova L, Krautz R, Kranenburg H, Parvin F, Theopold U. 2015. Apoptosis in hemocytes induces a shift in effector mechanisms in the Drosophila immune system and leads to a pro-inflammatory state. PLoS One 10:e0136593. doi: 10.1371/journal.pone.0136593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kraaijeveld AR, Elrayes NP, Schuppe H, Newland PL. 2011. l-Arginine enhances immunity to parasitoids in Drosophila melanogaster and increases NO production in lamellocytes. Dev Comp Immunol 35:857–864. doi: 10.1016/j.dci.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 57.Shalini S, Dorstyn L, Dawar S, Kumar S. 2015. Old, new and emerging functions of caspases. Cell Death Differ 22:526–539. doi: 10.1038/cdd.2014.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shaukat Z, Liu D, Gregory S. 2015. Sterile inflammation in Drosophila. Mediators Inflamm 2015:369286. doi: 10.1155/2015/369286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Costa SC, Girard PA, Brehelin M, Zumbihl R. 2009. The emerging human pathogen Photorhabdus asymbiotica is a facultative intracellular bacterium and induces apoptosis of macrophage-like cells. Infect Immun 77:1022–1030. doi: 10.1128/IAI.01064-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Daish TJ, Mills K, Kumar S. 2004. Drosophila caspase DRONC is required for specific developmental cell death pathways and stress-induced apoptosis. Dev Cell 7:909–915. doi: 10.1016/j.devcel.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 61.Brune B, von Knethen A, Sandau KB. 1999. Nitric oxide (NO): an effector of apoptosis. Cell Death Differ 6:969–975. doi: 10.1038/sj.cdd.4400582. [DOI] [PubMed] [Google Scholar]
- 62.Dzhansugurova LB. 2011. Effect of nitric oxide on expression of apoptotic genes and HSP70 in Drosophila. Ontogenez 42:425–438. (In Russian.) [PubMed] [Google Scholar]
- 63.Tejedo JR, Tapia-Limonchi R, Mora-Castilla S, Cahuana GM, Hmadcha A, Martin F, Bedoya FJ, Soria B. 2010. Low concentrations of nitric oxide delay the differentiation of embryonic stem cells and promote their survival. Cell Death Dis 1:e80. doi: 10.1038/cddis.2010.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tennessen JM, Barry WE, Cox J, Thummel CS. 2014. Methods for studying metabolism in Drosophila. Methods 68:105–115. doi: 10.1016/j.ymeth.2014.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]