ABSTRACT
Interleukin-10 (IL-10)-producing regulatory B (Breg) cells were found to be induced in a variety of infectious diseases. However, its importance in the regulation of immune response to malaria is still unclear. Here, we investigated the dynamics, phenotype, and function of Breg cells using Plasmodium chabaudi chabaudi AS-infected C57BL/6 and BALB/c mice. BALB/c mice were more susceptible to infection and had a stronger IL-10 response in spleen than C57BL/6 mice. Analysis of the surface markers of IL-10-producing cells with flow cytometry showed that CD19+ B cells were one of the primary IL-10-producing populations in P. c. chabaudi AS-infected C57BL/6 and BALB/c mice, especially in the latter one. The Breg cells had a heterogeneous phenotype which shifted during infection. The well-established Breg subset, CD19+ CD5+ CD1dhi cells, accounted for less than 20% of IL-10-producing B cells in both strains during the course of infection. Most Breg cells were IgG+ and CD138− from day 0 to day 8 postinfection. Adoptive transfer of Breg cells to C57BL/6 mice infected with P. c. chabaudi AS led to a transient increase of parasitemia without an impact on survival rate. Our finding reveals that B cells play an active and important regulatory role in addition to mediating humoral immunity in immune response against malaria, which should be paid more attention in developing therapeutic or vaccine strategies against malaria involving stimulation of B cells.
KEYWORDS: malaria, IL-10, regulatory B cells, P. c. chabaudi AS, B10
INTRODUCTION
Malaria, an infectious disease caused by parasites of the genus Plasmodium, remains a major public health problem in tropical areas, threatening the lives of hundreds of thousands of people (1). Malaria parasites have a complicated life cycle and induce immune responses with particular features. Once injected into human blood via mosquito bites, the parasites multiply first in the liver and then enter the circulation, where they invade and damage red blood cells, causing clinical symptoms. The blood stage of infection leads to production of both proinflammatory and anti-inflammatory mediators by the host immune system (2). The proinflammatory response characterized by increased interferon gamma (IFN-γ) production plays a critical role in limiting parasite replication while it sometimes contributes to malaria pathology (3, 4). The anti-inflammatory cytokines, although having a counter effect on the protective immunity (5, 6), are regarded as necessary for suppressing severe pathology (7, 8). The presentation and outcome of the malaria infection depends, at least in part, on the fine balance of the proinflammatory and anti-inflammatory responses.
Interleukin-10 (IL-10), an important anti-inflammatory cytokine, has been extensively studied in human malaria as well as in mouse models. IL-10 is effectively induced in Plasmodium infection, and there is evidence that the serum level of IL-10 is related to clinical manifestation of the patients (3, 9–12). A number of clinical investigations showed that plasma IL-10 concentration was positively correlated with parasitemia (13–16). Various studies in different populations revealed that patients with severe malaria-associated anemia had relatively less circulating IL-10 (16–18), and patients with cerebral malaria had a higher level of IL-10 (12, 17, 19), although there were reports with contradicting data (20, 21). The association between IL-10 level and clinical presentation was further supported by recent findings that single-nucleotide polymorphisms of the IL-10 gene were significantly linked to malaria susceptibility or symptoms (22–25). In addition, animal experiments with IL-10 knockout mouse or neutralizing anti-IL-10 antibodies proved the importance of IL-10 in controlling immunopathology in malaria (26, 27). Thus, IL-10 is one of the key players in immunoregulation in malaria; however, its induction pathways and functional mechanisms have not been fully elucidated in the context of Plasmodium infection.
IL-10 has multiple cellular sources, including lymphocytes and monocyte/macrophages (28). CD4+ T cells were thought to be the major source of IL-10 in humans and mice infected with Plasmodium parasites (29, 30), and other cells were also involved in the production of IL-10 during malaria infection (31, 32). Regulatory T (Treg) cells, one of the subpopulations of CD4+ T cells, were intensively studied in mouse models of malaria and were shown to modulate the inflammatory response via IL-10 production (33–35). Nevertheless, a non-Treg cell source of interleukin-10 was reported to be critical in preventing experimental cerebral malaria (36). Moreover, Wang et al. found that depletion of regulatory T cells enhanced the proinflammatory responses in P. c. chabaudi AS-infected DBA/2 mice and prolonged their survival time, whereas blocking IL-10 signal caused excessive proinflammation responses and earlier death of mice (37). These findings suggest that IL-10 signal as a whole has more profound regulatory effects than Tregs on their own, and non-Treg sources of IL-10 play a crucial role in maintaining an appropriate immune response against malaria.
It has been more than a decade since Mizoguchi et al. found that a subset of B cells was able to suppress inflammatory reactions by producing cytokines such as IL-10 (38). These IL-10-producing B cells are called regulatory B (Breg) cells, and it has become evident that Breg cells play a critical role in the immunoregulation of a variety of diseases. Breg cells suppress immunopathology in autoimmune diseases (39, 40) and dampen anti-tumor immunity and host defense in cancer and bacterial and viral infections (41–43). The function of Breg cells in parasitic diseases is complex and seems to depend on parasite species and their pathogenic mechanisms (44). So far, studies investigating the immunomodulatory role of B cells in Plasmodium infection are rare (44). It was noticed a long time ago that B cells were required for the switch from Th1- to Th2-regulated immune responses in malaria (45), but only recently was the importance of B cell-mediated immunoregulation in malaria confirmed. Two independent studies demonstrated that IL-10-producing B cells confer protection against experimental cerebral malaria through IL-10-mediated inhibition of inflammatory responses (46, 47). However, the role of Breg cells in uncomplicated malaria remains unknown. Here, using Plasmodium chabaudi-infected mice, we investigated the cellular sources of IL-10 and analyzed the phenotype and function of IL-10-producing B cells in common malaria (48). We showed that Breg cells were one of the primary IL-10-producing cells during P. c. chabaudi AS infection. Our findings suggested that more importance should be attached to the immunoregulatory role of B cells in the context of Plasmodium infection.
RESULTS
BALB/c mice are more susceptible to P. c. chabaudi AS infection and have a stronger splenic IL-10 response than C57BL/6 mice.
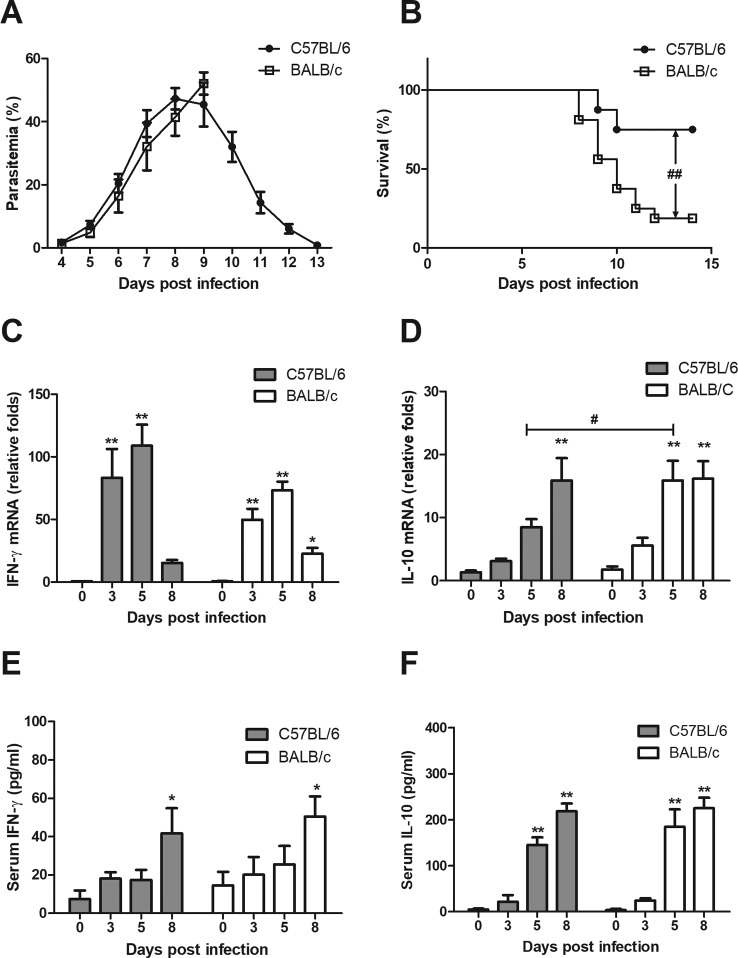
BALB/c and C57BL/6 strains are known to have distinct predispositions to different types of immune response. BALB/c mice tend to mount potent Th2 responses, whereas C57BL/6 mice are inclined to organize strong Th1 responses. We used these two strains to explore the IL-10 response pattern and its connection with disease outcome in P. c. chabaudi AS infection. In both strains, parasitized erythrocytes emerged from peripheral blood from day 4 postinfection (p.i.) and kept increasing at a similar rate during the first week of infection. After reaching the peak on day 8 p.i., parasitemia in C57BL/6 mice stopped rising and started to drop rapidly. By day 13 p.i., almost all parasites were cleared from peripheral blood of C57BL/6 mice. However, the parasitemia in BALB/c mice was not controlled and led to death of mice from day 8 p.i. (Fig. 1A). Thus, most BALB/c mice succumbed to infection within 2 weeks postinfection, whereas the majority of C57BL/6 mice survived (P value of <0.01) (Fig. 1B).
FIG 1.
Parasitemia, survival, and cytokine responses of BALB/c and C57BL/6 mice during P. c. chabaudi AS infection. BALB/c and C57BL/6 mice were administered with 1 × 106 pRBC, and the parasitemia (A) and survival rate (B) were monitored daily. For evaluation of cytokine production, mice were sacrificed at the indicated time. Serum samples were prepared and total RNA was isolated from spleen. The mRNA level of IFN-γ (C) and IL-10 (D) were measured by real-time PCR. The serum concentration of IFN-γ (E) and IL-10 (F) was determined with ELISA. All data except survival rate are presented as arithmetic means ± SE. Single and double asterisks indicate P values of <0.05 and <0.01, respectively, compared with uninfected mice of the same strain. A pound sign means a P value of <0.05 compared with the other strain at the same time point. The P value for the difference in survival between the two strains was less than 0.01.
We then looked into the changes of cytokine profiles of the two strains during P. c. chabaudi AS infection. The mRNA levels of proinflammatory cytokine IFN-γ and anti-inflammatory cytokine IL-10 in spleen from both strains were determined with real-time PCR. As shown in Fig. 1C, the mRNA expression of IFN-γ was dramatically induced in both C57BL/6 and BALB/c mice. The highest mRNA level appeared on day 5 p.i. in both strains. C57BL/6 mice seemed to have a higher level of IFN-γ mRNA than BALB/c mice, but the difference had no statistical significance. The change of the mRNA level of IL-10 was different between the two strains. C57BL/6 mice showed a slow and gradual increase of IL-10 mRNA after infection and reached the peak on day 8 p.i. In comparison, BALB/c mice exhibited a more rapid increase of IL-10 mRNA and remained at a high level from day 5 to day 8 p.i. The IL-10 mRNA level in spleen from BALB/c mice was significantly higher than that from C57BL/6 mice on day 5 p.i. (Fig. 1D). We also detected the serum concentrations of IFN-γ and IL-10 but did not find significant difference between C57BL/6 and BALB/c mice before and after infection (Fig. 1E and F). Our result suggested that BALB/c mice had an early and long-lasting IL-10 response in spleen during P. c. chabaudi AS infection, which might contribute to their sensitivity, since IL-10 response is associated with disease exacerbation during Plasmodium infection (5, 6).
CD19+ B cells are the major IL-10-producing population in spleen from P. c. chabaudi AS-infected BALB/c mice.
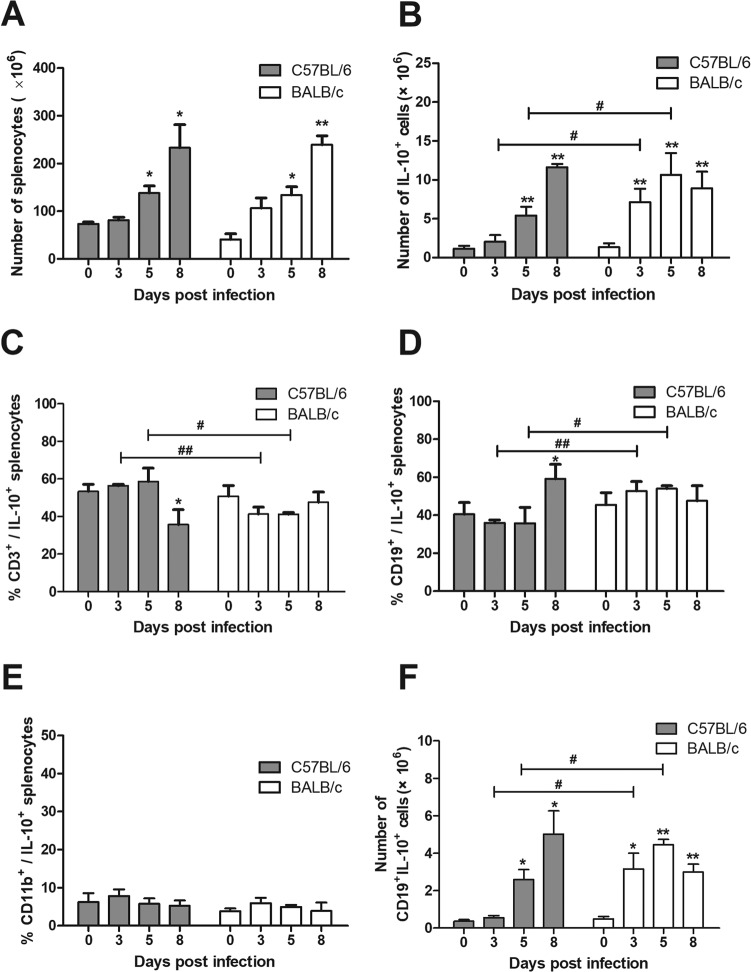
During P. c. chabaudi AS infection, the number of spleen cells as well as total IL-10-producing splenocytes increased apparently in both BALB/c and C57BL/6 mice (Fig. 2A and B). The absolute numbers of IL-10-producing splenocytes grew faster in BALB/c mice and turned out to be higher than that in C57BL/6 mice on days 3 and 5 p.i., which was in keeping with the changes of IL-10 mRNA in spleen from the two strains (Fig. 1D). As IL-10 could be produced by T cells, B cells, and monocytes/macrophages, we investigated the cellular sources of IL-10 using fluorescent antibodies against CD3, CD19, and CD11b (Mac-1), which are the surface markers of T cells, B cells, and macrophages, respectively. CD3+ cells accounted for more than 50% of IL-10-producing cells from day 0 to day 5 p.i. in C57BL/6 mice, while they were less than 50% in BALB/c mice after infection. The frequency of CD3+ cells in IL-10-producing cells from C57BL/6 mice was significantly higher than that from BALB/c mice on days 3 and 5 p.i. (Fig. 2C). It is notable that CD19+ cells constituted more than 50% of total IL-10-producing cells in spleen from BALB/c mice on days 3 and 5 p.i., and their proportion also exceeded 30% in C57BL/6 mice during the course of infection (Fig. 2D). BALB/c mice had more CD19+ IL-10+ cells than C57BL/6 mice on days 3 and 5 p.i. both in percentage (Fig. 2D) and absolute number (Fig. 2F). The proportion of CD11b+ cells in IL-10-producing cells was less than 10% in both strains before and after infection (Fig. 2E). These data indicated that CD19+ B cells were an important source of IL-10, comparable to T cells, in spleen during P. c. chabaudi AS infection. BALB/c mice had an earlier and stronger CD19+ IL-10+ cell response than C57BL/6 mice, which may account, in large part, for the higher IL-10 production in BALB/c mice.
FIG 2.
Dynamics of IL-10-producing cells and the frequency of IL-10-producing CD3+, CD19+, and CD11b+ cells in P. c. chabaudi AS-infected C57BL/6 and BALB/c mice. Mice were infected with P. c. chabaudi AS and sacrificed at the indicated time. (A) Spleen cells were isolated and counted. The percentages of IL-10-producing cells in spleen cells and their phenotypes were analyzed with flow cytometry. (B) The absolute numbers of total IL-10-producing splenocytes were calculated. The percentages of CD3+ (C), CD19+ (D), and CD11b+ (E) cells in IL-10-producing cells as well as the absolute number of CD19+ IL-10+ cells (F) in spleen from C57BL/6 and BALB/c mice are shown. Data are presented as arithmetic means ± SE. Single and double asterisks indicate P values of <0.05 and <0.01, respectively, compared with uninfected mice of the same strain. Single and double pound signs mean P values of <0.05 and <0.01, respectively, compared with the other strain at the same time point.
CD19+ CD5+ CD1dhi B10 cells represent only a small part of Breg cells in P. c. chabaudi AS-infected mice.
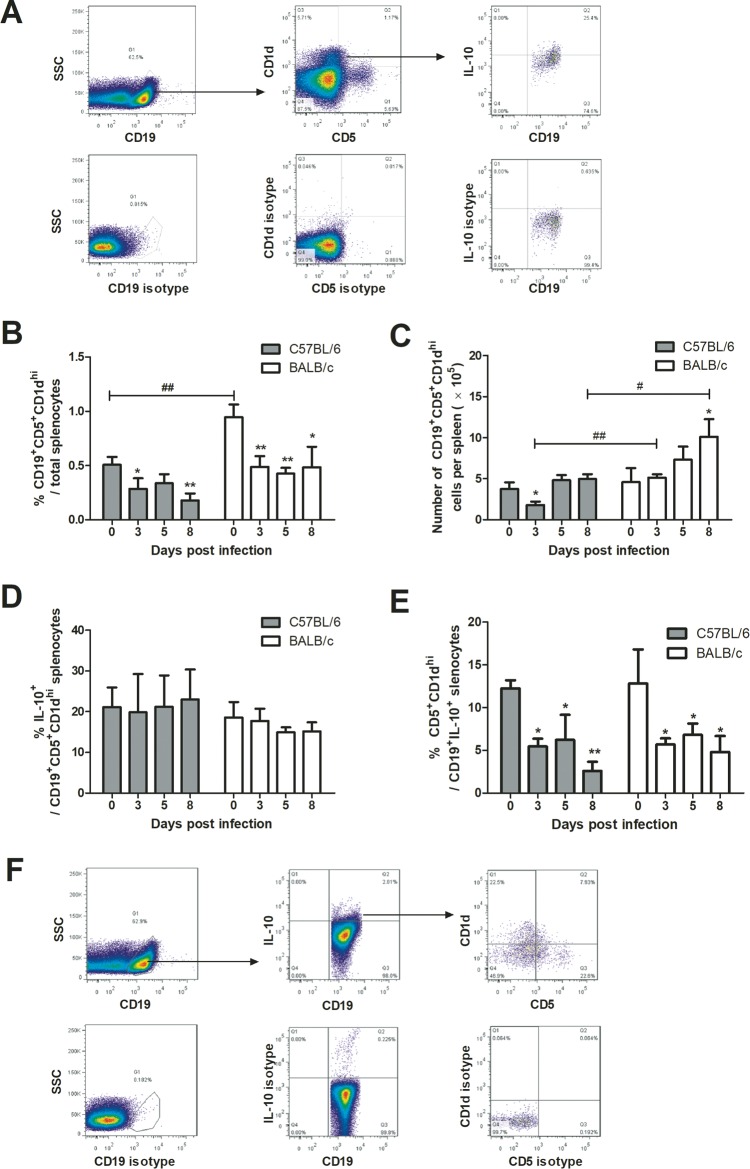
The CD19+ CD5+ CD1dhi population, also called B10 cells, is a well-defined regulatory B cell subset in mouse spleen (49, 50). B10-like cells have been found in a mouse model of cerebral malaria caused by Plasmodium berghei infection (46). To see if B10 cells are the predominant population of Breg cells in P. c. chabaudi AS-infected mice, we examined the dynamic change of the frequency and the number of CD19+ CD5+ CD1dhi cells in splenic Breg cells during P. c. chabaudi AS infection by flow cytometry. CD19+ CD5+ CD1dhi cells, gated as shown in Fig. 3A, constitute 0.5% and 1% of splenocytes from uninfected C57BL/6 and BALB/c mice, respectively (Fig. 3B). The frequency of these cells dropped significantly after P. c. chabaudi AS infection in both strains; however, the absolute number did not change in the same way. The number of CD19+ CD5+ CD1dhi cells in spleen from C57BL/6 mice was kept stable after infection except for a transient drop on day 3 p.i., while that from BALB/c mice increased gradually from 5 × 105 to 10 × 105 with the progression of infection (Fig. 3C). About 20% of CD19+ CD5+ CD1dhi cells were detected to produce IL-10 in uninfected C57BL/6 and BALB/c mice, and the frequency was not changed much by infection (Fig. 3D). As the dynamic of CD19+ CD5+ CD1dhi cells was not consistent with that of CD19+ IL-10+ cells, we checked the frequency of CD5+ CD1dhi cells in CD19+ IL-10+ cells. The highest frequency of these cells, varying between 10% and 20%, appeared before infection in both strains and dropped rapidly after infection. The CD5+ CD1dhi cells accounted for less than 10% of CD19+ IL-10+ B cells from day 3 p.i. in both C57BL/6 and BALB/c mice (Fig. 3E). These data indicated that CD19+ CD5+ CD1dhi B10 cells represented only a small portion of IL-10-producing B cells in P. c. chabaudi AS-infected mice. Therefore, B cell populations other than D19+ CD5+ CD1dhi B10 cells are likely to contribute more to IL-10 production induced by P. c. chabaudi AS infection.
FIG 3.
Dynamics of CD19+ CD5+ CD1dhi cells and their contribution to IL-10 production during the course of P. c. chabaudi AS infection in C57BL/6 and BALB/c mice. (A) Spleen cells were isolated from mice and stained for membrane CD19, CD5, and CD1d as well as intracellular IL-10. CD19+ CD5+ CD1dhi cells were gated as shown in the left and middle panels, and the subgating of IL-10+ cells in the CD19+ CD5+ CD1dhi population is displayed on the right. (B and C) Change of frequency and absolute number of CD19+ CD5+ CD1dhi cells in spleen from C57BL/6 and BALB/c mice. The percentage of IL-10-producing cells in CD19+ CD5+ CD1dhi cells (D) and the proportion of CD5+ CD1dhi B cells in CD19+ IL-10+ cells (E) were also analyzed. (F) Gating strategy used for panel E. Data are presented as arithmetic means ± SE. Single and double asterisks indicate P values of <0.05 and <0.01, respectively, compared with uninfected mice of the same strain. Single and double pound signs mean P values of <0.05 and <0.01, respectively, compared with the other strain at the same time point.
The phenotypes of most Breg cells are IgG+ and CD138− during the course of P. c. chabaudi AS infection.
As various phenotypes of Breg cells have been reported in different diseases or disease models (51, 52), we tried to identify the common surface markers on Breg cells in P. c. chabaudi AS-infected mice. We checked the expression of membrane immunoglobulins and some previously reported surface molecules on CD19+ IL-10+ Breg cells from C57BL/6 and BALB/c mice on days 0 and 8 p.i. Membrane IgM and IgD were expressed on about one-third and more than half of CD19+ IL-10+ cells, respectively, in both strains on day 0 p.i. With infection progressing, the proportion of IgM+ or IgD+ Breg cells dropped significantly. IgG+ cells were the prevalent population on both days 0 and 8 p.i. and accounted for over 80% of Breg cells. The alternative B cell marker B220 was also expressed on a large proportion of Breg cells. Differential expression of CD5 on Breg cells was found between C57BL/6 and BALB/c mice on day 0 p.i.; however, by day 8 p.i., the CD5+ Breg cells in C57BL/6 mice increased to a level similar to that in BALB/c mice. Previously, Allan et al. found that CD1d on human B cells was downregulated by activation (53). We also observed a significant decrease in the frequency of CD1d+ cells in CD19+ IL-10+ populations on day 8 p.i. in both strains (Table 1).
TABLE 1.
Surface molecule expression by CD19+ IL-10+ splenocytes on days 0 and 8 after P. c. chabaudi AS infection in C57BL/6 and BALB/c mice
| Surface molecule | Mean (±SD) % positive cellsa |
|||
|---|---|---|---|---|
| C57BL/6 |
BALB/c |
|||
| Day 0 | Day 8 | Day 0 | Day 8 | |
| IgM+ | 31.6 ± 2.62 | 23.14 ± 3.60* | 33.5 ± 5.85 | 21.54 ± 9.75* |
| IgD+ | 72.13 ± 4.98 | 29.44 ± 1.91** | 52.83 ± 8.75# | 24.96 ± 12.25* |
| IgG+ | 94.38 ± 3.77 | 88.67 ± 3.20 | 91.32 ± 6.66 | 85.67 ± 8.49 |
| B220+ | 75.68 ± 11.35 | 87.37 ± 3.68 | 72.11 ± 7.93 | 80.90 ± 7.37 |
| CD5+ | 13.61 ± 2.30 | 35.84 ± 2.03** | 29.41 ± 9.37# | 27.14 ± 13.76 |
| CD1d+ | 61.43 ± 2.66 | 17.52 ± 3.06** | 65.57 ± 5.69 | 17.67 ± 5.88** |
| CD138+ | 1.79 ± 0.36 | 1.06 ± 0.30 | 1.53 ± 0.39 | 1.60 ± 0.29 |
| Tim-1+ | 1.41 ± 0.92 | 7.45 ± 2.39* | 2.20 ± 0.57 | 13.49 ± 4.27* |
Data shown are for each surface molecule in IL-19+ IL-10+ spleen cells (3 to 6 mice/group). * and **, P values of <0.05 and <0.01, respectively, compared with data from the same strain on day 0 p.i. #, P < 0.05 compared to the other strain at the same time point.
Recently, some CD138+ plasmacytoblasts were reported to produce IL-10 (54). We also checked the expression of CD138 on Breg cells and found that the majority of Breg cells are CD138− before and after infection. Tim-1 (T cell immunoglobulin and mucin domain-containing molecule-1) was reported to define certain Breg cell populations (55); however, it was only expressed by less than 5% of Breg cells from C57BL/6 and BALB/c mice before infection. Nevertheless, the frequency of Tim-1+ Breg cells increased significantly on day 8 p.i. in both strains, although this still amounted to a very small fraction of Breg cells. These observations indicated that the phenotype of Breg populations changed during P. c. chabaudi AS infection, and the isotype-switched IgG+ B cells constituted most of the Breg cells before and after the infection (Table 1).
Adoptive transfer of Breg cells to C57BL/6 mice with P. c. chabaudi AS infection leads to transient increase of parasitemia.
As Breg cells are one of the important producers of IL-10 during P. c. chabaudi AS infection, we tried to evaluate the impact of Breg cell-mediated immunoregulation on the course or outcomes of disease via adoptive transfer experiments. Taking into account that Breg cells may inhibit protective immune responses, we chose a resistant strain to carry out the experiment. Breg cells were isolated from P. c. chabaudi AS-infected C57BL/6 mice on day 8 p.i. and adoptively transferred (106/mouse) into C57BL/6 mice on day 5 p.i. This timing was to mimic the dynamics of Breg cells in BALB/c mice during P. c. chabaudi AS infection. IL-10− B cells harvested from the Breg isolation process were used as a control. Recipient mice transferred with Breg cells showed an earlier and higher peak of parasitemia than mice receiving IL-10− B cells (Fig. 4A). These mice also have a higher splenic IL-10 level than the control group of mice 2 days after the adoptive transfer (Fig. 4B). The IFN-γ level from mice receiving Breg cells was lower than that from the control mice without statistical significance. (Fig. 4C). Nevertheless, the suppressive effects of Breg cells were transient, and all mice transferred with IL-10+ and IL-10− B cells survived the infection. These data confirmed that Breg cells did have a regulatory effect in the mouse model of uncomplicated malaria, but their role may not be decisive in the survival from infection on the C57BL/6 background.
FIG 4.
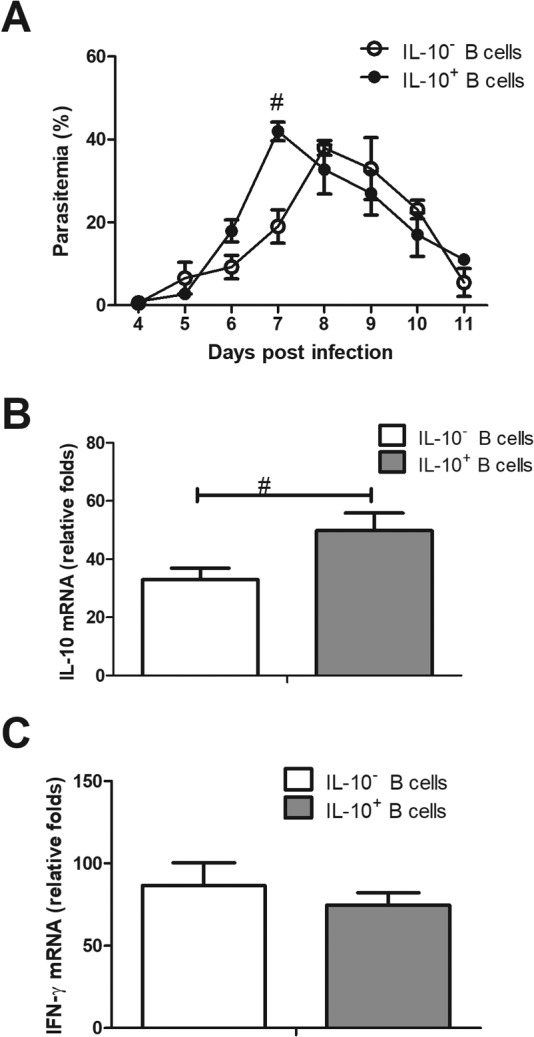
Effects of adoptive transfer of Breg cells to P. c. chabaudi AS-infected C57BL/6 mice. Breg cells (1 × 106) and IL-10− B cells were isolated from C57BL/6 mice 8 days p.i. and transferred intravenously to C57BL/6 mice on the fifth day of infection. (A) Parasitemia was monitored daily. The recipient mice were sacrificed on day 7 p.i. and the mRNA levels of IL-10 (B) and IFN-γ (C) of spleen were measured. Data are presented as arithmetic means ± SE. A pound sign means a P value of <0.05 compared with the other strain at the same time point. One data set out of two independent experiments with similar results is shown.
DISCUSSION
We showed here that IL-10-producing regulatory B cells were substantially induced by Plasmodium chabaudi infection. These Breg cells were heterogeneous in phenotype and exerted suppressive effects on host defense against malaria parasites. This finding reveals the essential immunoregulatory function of B cells in addition to producing antibodies in the context of Plasmodium infection.
To address the possible impact of IL-10 response on the course of Plasmodium infection in a mouse model, we took advantage of the prototypical Th1- and Th2-type mouse strains, i.e., C57BL/6 and BALB/c mice, which may have different IFN-γ and IL-10 responses to P. c. chabaudi AS infection. As it turned out, Th2-prone BALB/c mice displayed significantly higher sensitivity to infection than Th1-prone C57BL/6 mice. Splenic cytokine examination confirmed that susceptible BALB/c mice had an early and stronger IL-10 response than the resistant C57BL/6 mice. This observation was similar to the finding with Plasmodium yoelii-infected DBA2 and BALB/c mice, in which susceptible BALB/c mice had higher IL-10 and lower IFN-γ production than resistant DBA2 mice (56). These results stressed again the importance of proper balance between proinflammatory and anti-inflammatory responses for survival from malaria. Moreover, it implies that BALB/c mice can serve as a good model for the study of the cellular source of IL-10 induced by Plasmodium infection.
It is known that Breg cells are induced in some infectious diseases, and they have been reported to be the primary producer of IL-10 in some cases. An elegant experiment performed by Lee and Kung showed that the marginal-zone B cell was the most dominant and relevant IL-10 source in the context of Listeria susceptibility (57). A study on patients with chronic hepatitis B indicated that increased Breg cells are a major source of elevated IL-10 in the culture supernatant of peripheral blood mononuclear cells (58). However, the extent to which B cells contribute to IL-10 production in malaria is still unclear. We showed here that B cells were a chief producer of IL-10 other than T cells in Plasmodium chabaudi-infected C57BL/6 and BALB/c mice, especially for the latter ones. Our result has some similarities with a previous report by Kobayashi et al. (31). They found that a strong IL-10 response early in infection is associated with the lethal outcome of infection caused by a Plasmodium yoelii 17X lethal variant compared with the infection caused by the nonlethal variant. Both CD4+ T cells and non-T cells were involved in the production of IL-10 in mice infected with the lethal variant (31). Our result provided a possible explanation for the cellular source of the non-T cell-produced IL-10 in their experiment and highlights the importance of B cell as one of the major IL-10 producers in malaria immune response.
Various Breg subsets have been described in different disease models or inflammatory environments. Liu et al. showed that Breg cells from mice with experimental cerebral malaria expressed high levels of CD1d and CD5, which defined the population of B10 cells (46). Therefore, we checked the dynamic changes of B10 cells and their frequency in IL-10-producing B cells in our experiment. We found that B10 cells accounted for less than 20% of IL-10-producing B cells in spleen before and throughout the process of infection, which means that the majority of Breg cells were not B10 cells. The activation and expansion of non-B10 Breg cells during Plasmodium parasite infection were also observed in another study on experimental cerebral malaria. Bao et al. found that the Breg cells from C57BL/6 mice that underwent three cycles of Plasmodium berghei infection and radical treatment were CD5 negative (47). This observation and our data suggested that multiple populations of B cells were activated to produce IL-10 in Plasmodium infection. This notion is in line with the view that the activation of Breg cells is not lineage specific (50).
Several other Breg populations, such as transitional 2 B cells (CD19+ CD21hi CD23hi), marginal-zone B cells (CD19+ CD21hi CD23−), CD138+ plasma cells and plasmablasts, Tim-1+ B cells, etc., have been described to be involved in immune response to infection or immune-mediated pathologies (55, 57, 59, 60). Therefore, we were curious about the phenotype of non-B10 Breg cells activated during P. c. chabaudi infection. We found that most Breg cells were neither plasma/plasmablasts nor Tim-1+ B cells. They also did not seem to be transitional 2 B cells, because they were CD23− (data not shown). About half the Breg cells expressed CD21, which matched the phenotype of marginal-zone B cells. The only common surface molecule that most of the Breg cells shared was membrane IgG, which meant that these Breg cells were isotype-switched B cells. IgG+ or isotype-switched Breg cells were also reported in other studies (61, 62). It is not clear if the dominance of IgG+ Breg cells observed in our experiment is unique to malaria infection and, if so, why they were selectively activated to produce IL-10. More studies on the activation and differentiation of Breg cells in malaria models may help to elucidate these questions.
Liu et al. reported that adoptive transfer of IL-10+ Breg cells in the mouse model of cerebral malaria significantly reduced pathological inflammation in brain tissue and improved the survival of mice (46). Therefore, it is worth knowing what role Breg cells played in malaria without severe immune-mediated pathology. We adoptively transferred Breg cells to P. c. chabaudi AS-infected C57BL/6 mice at early stages of infection and found that this transfer resulted in a transient increase of parasitemia and suppression of Th1 response. Our result was similar to the report of Jeong et al. showing that adoptive transfer of IL-10-producing B cells induced by infection of Babesia microti, an intraerythrocytic protozoan, led to greater parasitemia in recipient mice (63). In both experiments, transfer of Breg cells did not exhibit impact on survival of infected mice, although our previous data implied a relationship between the activation of Breg cells and the succumbing of BALB/c mice to P. c. chabaudi AS infection. We suppose that this is due to the resistant genetic background of recipient mice, and the effects of Breg cells might be more detrimental on a sensitive background. Further investigation of the role of Breg cells in BALB/c mice will help to clarify the influence of genetic background on Breg's function. Despite the minor effects on survival of a resistant strain, our results confirmed that Breg cells induced by P. c. chabaudi AS infection had suppressive activity on host defense. This effect might be utilized by the parasites to build a favorable microenvironment for their growth without causing death of the hosts.
As IL-10 is reported to be essential for the generation of anti-Plasmodium humoral immunity (64), Breg cells may be involved in the regulation of antibody responses to plasmodium infection. Recently, a number of studies revealed that Breg cells controlled the differentiation of follicular helper T cells, which were crucial for germinal center antibody responses and the differentiation of memory B cells (65, 66). There is also evidence that activation of Breg cells was negatively associated with the magnitude of secondary antibody response (67). Therefore, the massive activation of Breg cells in Plasmodium infection might have consequences other than suppression of Th1 response.
Taken together, our results suggest that B cells have important regulatory functions in immune response against malaria which might have been undervalued before. There are more questions that need to be addressed in future studies. For example, what is the mechanism of Breg cells' activation during Plasmodium infection? Do plasmodium parasites have specific components that cause massive activation of Breg cells? Does the activation of Breg cells impair the development of immune memory and the long-term protection against malaria? Solving these questions may help to deepen the understanding of immune evasion mechanisms of malaria parasites and provide new insight into the therapeutic and preventive strategies against malaria.
MATERIALS AND METHODS
Animals, parasites, and infection.
Female C57BL/6 and BALB/c mice, 6 to 8 weeks old, were purchased from the Center of Zoology, Chinese Academy of Sciences (Shanghai branch), and kept under specific-pathogen-free conditions in the animal facility at China Medical University. All experimental procedures were approved by the China Medical University Animal Care and Use Committee. The parasite strain of Plasmodium chabaudi chabaudi AS was maintained in our department by cryopreservation with periodic passage in mice. For experimental infection, each mouse was inoculated intraperitoneally (i.p.) with 1 × 106 P. c. chabaudi AS parasitized red blood cells (pRBCs). From day 3 after P. c. chabaudi AS infection, a Giemsa-stained thin blood smear was prepared daily from tail blood, and the level of parasitemia was determined by calculating the proportion of parasite-infected red blood cells. The deaths of mice were monitored once a day for 2 weeks for survival rate study.
Real-time PCR.
Mice were euthanized on specific time points and spleens were harvested. Total spleen RNA was isolated with TRIzol reagent and reverse transcribed with PrimeScript RT master mix (TAKALA, Dalian, China). The levels of IL-10 mRNA were determined by real-time PCR on a Corbett Rotor-Gene 6000 instrument using SYBR Premix Ex Taq II. The ribosomal protein S17 (Rps17) was used as a loading control. The sequences of primers used were the following: RPS17 F, 5-TGTCGGGATCCACCTCAATG-3; R, 5-CGCCATTACCCCAGCAAG-3; IFN-γ F, AAAGACAATCAGGCCATCAG; R, TGGGTTGTTGACCTCAAACT; IL-10 F, 5-CAGTACGCCGGGAAGACA-3; R, 5-GCATTAAGGAGTCGGTTAGCA-3.
ELISA.
The concentrations of IFN-γ and IL-10 in serum samples from P. c. chabaudi AS-infected mice were determined with a sandwich enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer's protocol (R&D Systems, Minneapolis, MN, USA). The optical density values were measured using a microplate reader (iMark Microplate reader; Bio-Rad, USA) at 450 nm. The serum cytokine concentrations (in picograms per milliliter) were determined using a standard curve from recombinant cytokines provided by the kit.
Isolation of splenocytes.
Spleens were disaggregated by being pressed through a sterile fine-wire metal mesh, and spleen cells were collected by centrifugation at 350 × g for 10 min. Red blood cells were removed by hypotonic lysis with 0.17 M NH4Cl. Trypan blue exclusion assay was used for the evaluation of viability of harvested splenocytes, and only samples with viability greater than 95% were used for downstream procedures.
Intracellular IL-10 staining and flow cytometry.
Splenocytes were prepared and cultured in duplicate at 2 × 106 cells/ml in 50-ml conical tubes and stimulated with 50 ng/ml of phorbol myristate acetate (PMA; Sigma-Aldrich, St. Louis, MO, USA), 1 μg/ml of ionomycin (Sigma-Aldrich), and 10 μg/ml lipopolysaccharide (LPS; Sigma-Aldrich) in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) and antibiotics for 1 h at 37°C in a 5% CO2 chamber and then exposed to 2 μM monensin (GolgiPlug; BD Biosciences, San Jose, CA, USA) for an additional 4 h. After that, Fc receptors on cell surfaces were blocked with anti-CD16/CD32 (FcIII/II receptor) antibody (BioLegend, San Diego, CA, USA), and the cells were subjected to surface and intracellular staining. For intracellular staining, splenocytes were fixed and permeabilized with BD Cytofix/Cytoperm (BD Biosciences, San Diego, California) and washed with Perm/Wash buffer (BD Biosciences, San Diego, California) according to the manufacture's instructions.
The following antibodies and their isotype controls were used for surface staining: fluorescein isothiocyanate (FITC)-conjugated anti-CD11b (M1/70), peridinin chlorophyll protein (PerCP)/cy5.5-conjugated anti-CD19 (6D5), allophycocyanin (APC)-conjugated anti-CD3 (281-2), FITC-conjugated anti-CD1d (1B1), APC-conjugated anti-CD5 (53-7.3), FITC-conjugated anti-IgM (RMM-1), APC-conjugated anti-IgD (11-26c.2a), Alexa Fluor 647-conjugated anti-IgG (Poly4053), FITC-conjugated anti-CD45R/B220 (RA3-6B2), APC-conjugated anti-CD138 (281-1), and phycoerythrin (PE)-anti-Tim-1 (RMT1-4). PE-conjugated anti-IL-10 (JES5-16E3), APC-conjugated anti-IL-10(JES5-16E3), and their isotype controls were used for intracellular staining. All antibodies were purchased from BioLegend, San Diego, CA. Stained cells were kept in 1% paraformaldehyde solution before being analyzed on a FACSCalibur (BD Biosciences) machine. Totals of 50,000 to 500,000 cells were collected from each sample, and data were analyzed with FlowJo software.
Isolation and adoptive transfer of Breg cells.
IL-10-producing Breg cells were isolated using the regulatory B cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's protocol. Briefly, splenocytes were prepared from P. c. chabaudi AS-infected C57BL/6 mice on day 8 p.i. and were incubated with regulatory B cell biotin-antibody cocktail for 10 min at 2 to ∼8°C. B cells were enriched by negative selection using magnetic bead sorting. The harvested B cells were then stimulated with LPS (10 μg/ml) for 48 h in RPMI 1640 containing 10% FBS and 5 × 10−5 M 2-mercaptoethanol. PMA (50 ng/ml) and ionomycin (500 ng/ml) were added to the culture in the last 5 h. The cells were then incubated with an IL-10-catching reagent for 45 min at 37°C to allow the binding of the reagent to the surface of IL-10-secreting B cells. The IL-10+ and IL-10− B cells were separated with anti-IL-10 magnetic microbeads. For adoptive transfer, 1 × 106 IL-10+ B cells were injected intravenously into C57BL/6 mice on the fifth day of infection. The purity of IL-10+ B cells was over 80%, and IL-10− B cells were used as a control.
Statistical analysis.
All data, unless otherwise noted, are presented as means with standard errors (SE). Significance of differences between two groups was analyzed by a t test, and the comparisons among multiple groups were conducted using a one-way analysis of variance. The statistical difference between survival was assessed with the log-rank (Mantel-Cox) test and Gehan-Breslow-Wilcoxon test. Data were analyzed using Prism 5 (GraphPad), and P values equal to or less than 0.05 were considered significant.
ACKNOWLEDGMENTS
This work was sponsored by the Science and Technology Programs of Shenyang City (17-231-1-47), Higher Education Scientific Research Programs of Liaoning Province (L2014304), and Science and Technology Programs of Liaoning Province (2013225303) of China.
REFERENCES
- 1.World Health Organization. 2016. World malaria report 2016. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Stevenson MM, Riley EM. 2004. Innate immunity to malaria. Nat Rev Immunol 4:169–180. doi: 10.1038/nri1311. [DOI] [PubMed] [Google Scholar]
- 3.Jason J, Archibald LK, Nwanyanwu OC, Bell M, Buchanan I, Larned J, Kazembe PN, Dobbie H, Parekh B, Byrd MG, Eick A, Han A, Jarvis WR. 2001. Cytokines and malaria parasitemia. Clin Immunol 100:208–218. doi: 10.1006/clim.2001.5057. [DOI] [PubMed] [Google Scholar]
- 4.Yazdani SS, Mukherjee P, Chauhan VS, Chitnis CE. 2006. Immune responses to asexual blood-stages of malaria parasites. Curr Mol Med 6:187–203. doi: 10.2174/156652406776055212. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi F, Ishida H, Matsui T, Tsuji M. 2000. Effects of in vivo administration of anti-IL-10 or anti-IFN-gamma monoclonal antibody on the host defense mechanism against Plasmodium yoelii yoelii infection. J Vet Med Sci 62:583–587. doi: 10.1292/jvms.62.583. [DOI] [PubMed] [Google Scholar]
- 6.Hugosson E, Montgomery SM, Premji Z, Troye-Blomberg M, Bjorkman A. 2004. Higher IL-10 levels are associated with less effective clearance of Plasmodium falciparum parasites. Parasite Immunol 26:111–117. doi: 10.1111/j.0141-9838.2004.00678.x. [DOI] [PubMed] [Google Scholar]
- 7.Li C, Sanni LA, Omer F, Riley E, Langhorne J. 2003. Pathology of Plasmodium chabaudi chabaudi infection and mortality in interleukin-10-deficient mice are ameliorated by anti-tumor necrosis factor alpha and exacerbated by anti-transforming growth factor beta antibodies. Infect Immun 71:4850–4856. doi: 10.1128/IAI.71.9.4850-4856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahanta A, Kar SK, Kakati S, Baruah S. 2015. Heightened inflammation in severe malaria is associated with decreased IL-10 expression levels and neutrophils. Innate Immun 21:546–552. doi: 10.1177/1753425914561277. [DOI] [PubMed] [Google Scholar]
- 9.Jain V, Singh PP, Silawat N, Patel R, Saxena A, Bharti PK, Shukla M, Biswas S, Singh N. 2010. A preliminary study on pro- and anti-inflammatory cytokine profiles in Plasmodium vivax malaria patients from central zone of India. Acta Trop 113:263–268. doi: 10.1016/j.actatropica.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 10.Iriemenam NC, Okafor CM, Balogun HA, Ayede I, Omosun Y, Persson JO, Hagstedt M, Anumudu CI, Nwuba RI, Troye-Blomberg M, Berzins K. 2009. Cytokine profiles and antibody responses to Plasmodium falciparum malaria infection in individuals living in Ibadan, southwest Nigeria. Afr Health Sci 9:66–74. [PMC free article] [PubMed] [Google Scholar]
- 11.Ageely HM, Dawoud HA, Heiba AA. 2008. Anemia, interleukin-10, tumor necrosis factor alpha, and erythropoietin levels in children with acute, complicated and uncomplicated malignant malaria in Jazan, Saudi Arabia. J Egypt Soc Parasitol 38:359–370. [PubMed] [Google Scholar]
- 12.Peyron F, Burdin N, Ringwald P, Vuillez JP, Rousset F, Banchereau J. 1994. High levels of circulating IL-10 in human malaria. Clin Exp Immunol 95:300–303. doi: 10.1111/j.1365-2249.1994.tb06527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodrigues-da-Silva RN, Lima-Junior Jda C, Fonseca Bde P, Antas PR, Baldez A, Storer FL, Santos F, Banic DM, Oliveira-Ferreira J. 2014. Alterations in cytokines and haematological parameters during the acute and convalescent phases of Plasmodium falciparum and Plasmodium vivax infections. Mem Inst Oswaldo Cruz 109:154–162. doi: 10.1590/0074-0276140275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guiyedi V, Becavin C, Herbert F, Gray J, Cazenave PA, Kombila M, Crisanti A, Fesel C, Pied S. 2015. Asymptomatic Plasmodium falciparum infection in children is associated with increased auto-antibody production, high IL-10 plasma levels and antibodies to merozoite surface protein 3. Malar J 14:162. doi: 10.1186/s12936-015-0658-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noone C, Parkinson M, Dowling DJ, Aldridge A, Kirwan P, Molloy SF, Asaolu SO, Holland C, O'Neill SM. 2013. Plasma cytokines, chemokines and cellular immune responses in pre-school Nigerian children infected with Plasmodium falciparum. Malar J 12:5. doi: 10.1186/1475-2875-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thuma PE, van Dijk J, Bucala R, Debebe Z, Nekhai S, Kuddo T, Nouraie M, Weiss G, Gordeuk VR. 2011. Distinct clinical and immunologic profiles in severe malarial anemia and cerebral malaria in Zambia. J Infect Dis 203:211–219. doi: 10.1093/infdis/jiq041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lyke KE, Burges R, Cissoko Y, Sangare L, Dao M, Diarra I, Kone A, Harley R, Plowe CV, Doumbo OK, Sztein MB. 2004. Serum levels of the proinflammatory cytokines interleukin-1 beta (IL-1beta), IL-6, IL-8, IL-10, tumor necrosis factor alpha, and IL-12(p70) in Malian children with severe Plasmodium falciparum malaria and matched uncomplicated malaria or healthy controls. Infect Immun 72:5630–5637. doi: 10.1128/IAI.72.10.5630-5637.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Awandare GA, Goka B, Boeuf P, Tetteh JK, Kurtzhals JA, Behr C, Akanmori BD. 2006. Increased levels of inflammatory mediators in children with severe Plasmodium falciparum malaria with respiratory distress. J Infect Dis 194:1438–1446. doi: 10.1086/508547. [DOI] [PubMed] [Google Scholar]
- 19.Baptista JL, Vanham G, Wery M, Van Marck E. 1997. Cytokine levels during mild and cerebral falciparum malaria in children living in a mesoendemic area. Trop Med Int Health 2:673–679. doi: 10.1046/j.1365-3156.1997.d01-355.x. [DOI] [PubMed] [Google Scholar]
- 20.Fernandes AA, Carvalho LJ, Zanini GM, Ventura AM, Souza JM, Cotias PM, Silva-Filho IL, Daniel-Ribeiro CT. 2008. Similar cytokine responses and degrees of anemia in patients with Plasmodium falciparum and Plasmodium vivax infections in the Brazilian Amazon region. Clin Vaccine Immunol 15:650–658. doi: 10.1128/CVI.00475-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ong'echa JM, Remo AM, Kristoff J, Hittner JB, Were T, Ouma C, Otieno RO, Vulule JM, Keller CC, Awandare GA, Perkins DJ. 2008. Increased circulating interleukin (IL)-23 in children with malarial anemia: in vivo and in vitro relationship with co-regulatory cytokines IL-12 and IL-10. Clin Immunol 126:211–221. doi: 10.1016/j.clim.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pereira VA, Sanchez-Arcila JC, Teva A, Perce-da-Silva DS, Vasconcelos MP, Lima CA, Aprigio CJ, Rodrigues-da-Silva RN, Santos DO, Banic DM, Bonecini-Almeida MG, Lima-Junior JC, Oliveira-Ferreira J. 2015. IL10A genotypic association with decreased IL-10 circulating levels in malaria infected individuals from endemic area of the Brazilian Amazon. Malar J 14:30. doi: 10.1186/s12936-015-0548-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Domingues W, Kanunfre KA, Rodrigues JC, Teixeira LE, Yamamoto L, Okay TS. 2016. Preliminary report on the putative association of Il10-3575 T/a genetic polymorphism with malaria symptoms. Rev Inst Med Trop Sao Paulo 58:30. doi: 10.1590/s1678-9946201658030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang G, Manaca MN, McNamara-Smith M, Mayor A, Nhabomba A, Berthoud TK, Khoo SK, Wiertsema S, Aguilar R, Barbosa A, Quinto L, Candelaria P, Schultz EN, Hayden CM, Goldblatt J, Guinovart C, Alonso PL, Lesouef PN, Dobano C. 2012. Interleukin-10 (IL-10) polymorphisms are associated with IL-10 production and clinical malaria in young children. Infect Immun 80:2316–2322. doi: 10.1128/IAI.00261-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ouma C, Davenport GC, Were T, Otieno MF, Hittner JB, Vulule JM, Martinson J, Ong'echa JM, Ferrell RE, Perkins DJ. 2008. Haplotypes of IL-10 promoter variants are associated with susceptibility to severe malarial anemia and functional changes in IL-10 production. Hum Genet 124:515–524. doi: 10.1007/s00439-008-0578-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li C, Corraliza I, Langhorne J. 1999. A defect in interleukin-10 leads to enhanced malarial disease in Plasmodium chabaudi chabaudi infection in mice. Infect Immun 67:4435–4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanni LA, Jarra W, Li C, Langhorne J. 2004. Cerebral edema and cerebral hemorrhages in interleukin-10-deficient mice infected with Plasmodium chabaudi. Infect Immun 72:3054–3058. doi: 10.1128/IAI.72.5.3054-3058.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deshpande P, Shastry P. 2004. Modulation of cytokine profiles by malaria pigment–hemozoin: role of IL-10 in suppression of proliferative responses of mitogen stimulated human PBMC. Cytokine 28:205–213. doi: 10.1016/j.cyto.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Niikura M, Inoue S, Kobayashi F. 2011. Role of interleukin-10 in malaria: focusing on coinfection with lethal and nonlethal murine malaria parasites. J Biomed Biotechnol 2011:383962. doi: 10.1155/2011/383962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tiboni O, Di Pasquale G, Ciferri O. 1978. Purification of the elongation factors present in spinach chloroplasts. Eur J Biochem 92:471–477. doi: 10.1111/j.1432-1033.1978.tb12769.x. [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi F, Morii T, Matsui T, Fujino T, Watanabe Y, Weidanz WP, Tsuji M. 1996. Production of interleukin 10 during malaria caused by lethal and nonlethal variants of Plasmodium yoelii yoelii. Parasitol Res 82:385–391. doi: 10.1007/s004360050133. [DOI] [PubMed] [Google Scholar]
- 32.Winkler S, Willheim M, Baier K, Graninger W, Kremsner PG. 1999. Frequency of cytokine-producing CD4-CD8- peripheral blood mononuclear cells in patients with Plasmodium falciparum malaria. Eur Cytokine Netw 10:155–160. [PubMed] [Google Scholar]
- 33.Couper KN, Blount DG, Wilson MS, Hafalla JC, Belkaid Y, Kamanaka M, Flavell RA, de Souza JB, Riley EM. 2008. IL-10 from CD4CD25Foxp3CD127 adaptive regulatory T cells modulates parasite clearance and pathology during malaria infection. PLoS Pathog 4:e1000004. doi: 10.1371/journal.ppat.1000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bisseye C, van der Sande M, Morgan WD, Holder AA, Pinder M, Ismaili J. 2009. Plasmodium falciparum infection of the placenta impacts on the T helper type 1 (Th1)/Th2 balance of neonatal T cells through CD4(+)CD25(+) forkhead box P3(+) regulatory T cells and interleukin-10. Clin Exp Immunol 158:287–293. doi: 10.1111/j.1365-2249.2009.04014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walther M, Jeffries D, Finney OC, Njie M, Ebonyi A, Deininger S, Lawrence E, Ngwa-Amambua A, Jayasooriya S, Cheeseman IH, Gomez-Escobar N, Okebe J, Conway DJ, Riley EM. 2009. Distinct roles for FOXP3 and FOXP3 CD4 T cells in regulating cellular immunity to uncomplicated and severe Plasmodium falciparum malaria. PLoS Pathog 5:e1000364. doi: 10.1371/journal.ppat.1000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amante FH, Stanley AC, Randall LM, Zhou Y, Haque A, McSweeney K, Waters AP, Janse CJ, Good MF, Hill GR, Engwerda CR. 2007. A role for natural regulatory T cells in the pathogenesis of experimental cerebral malaria. Am J Pathol 171:548–559. doi: 10.2353/ajpath.2007.061033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang GG, Chen G, Feng H, Liu J, Jiang YJ, Shang H, Cao YM. 2013. Plasmodium chabaudi AS: distinct CD4(+)CD25(+)Foxp3(+) regulatory T cell responses during infection in DBA/2 and BALB/c mice. Parasitol Int 62:24–31. doi: 10.1016/j.parint.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 38.Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. 2002. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity 16:219–230. doi: 10.1016/S1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 39.Ireland SJ, Blazek M, Harp CT, Greenberg B, Frohman EM, Davis LS, Monson NL. 2012. Antibody-independent B cell effector functions in relapsing remitting multiple sclerosis: clues to increased inflammatory and reduced regulatory B cell capacity. Autoimmunity 45:400–414. doi: 10.3109/08916934.2012.665529. [DOI] [PubMed] [Google Scholar]
- 40.Li X, Braun J, Wei B. 2011. Regulatory B cells in autoimmune diseases and mucosal immune homeostasis. Autoimmunity 44:58–68. doi: 10.3109/08916931003782189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sarvaria A, Madrigal JA, Saudemont A. 2017. B cell regulation in cancer and anti-tumor immunity. Cell Mol Immunol 14:662–674. doi: 10.1038/cmi.2017.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dai YC, Zhong J, Xu JF. 2017. Regulatory B cells in infectious disease (review). Mol Med Rep 16:3–10. doi: 10.3892/mmr.2017.6605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fillatreau S. 2016. Regulatory roles of B cells in infectious diseases. Clin Exp Rheumatol 34:1–5. [PubMed] [Google Scholar]
- 44.Soares RR, Antinarelli LMR, Abramo C, Macedo GC, Coimbra ES, Scopel KKG. 2017. What do we know about the role of regulatory B cells (Breg) during the course of infection of two major parasitic diseases, malaria and leishmaniasis? Pathog Glob Health 111:107–115. doi: 10.1080/20477724.2017.1308902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taylor-Robinson AW, Phillips RS. 1994. B cells are required for the switch from Th1- to Th2-regulated immune responses to Plasmodium chabaudi chabaudi infection. Infect Immun 62:2490–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu Y, Chen Y, Li Z, Han Y, Sun Y, Wang Q, Liu B, Su Z. 2013. Role of IL-10-producing regulatory B cells in control of cerebral malaria in Plasmodium berghei infected mice. Eur J Immunol 43:2907–2918. doi: 10.1002/eji.201343512. [DOI] [PubMed] [Google Scholar]
- 47.Bao LQ, Huy NT, Kikuchi M, Yanagi T, Senba M, Shuaibu MN, Honma K, Yui K, Hirayama K. 2013. CD19(+) B cells confer protection against experimental cerebral malaria in semi-immune rodent model. PLoS One 8:e64836. doi: 10.1371/journal.pone.0064836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stephens R, Culleton RL, Lamb TJ. 2012. The contribution of Plasmodium chabaudi to our understanding of malaria. Trends Parasitol 28:73–82. doi: 10.1016/j.pt.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. 2008. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity 28:639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 50.Rosser EC, Mauri C. 2015. Regulatory B cells: origin, phenotype, and function. Immunity 42:607–612. doi: 10.1016/j.immuni.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 51.Mauri C, Menon M. 2015. The expanding family of regulatory B cells. Int Immunol 27:479–486. doi: 10.1093/intimm/dxv038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Noh J, Noh G. 2012. Allergen-specific responses of CD19(high) and CD19(low) B cells in non-IgE-mediated food allergy of late eczematous reactions in atopic dermatitis: presence of IL-17- and IL-32-producing regulatory B cells (Br17 & Br32). Inflamm Allergy Drug Targets 11:320–329. doi: 10.2174/187152812800959022. [DOI] [PubMed] [Google Scholar]
- 53.Allan LL, Stax AM, Zheng DJ, Chung BK, Kozak FK, Tan R, van den Elzen P. 2011. CD1d and CD1c expression in human B cells is regulated by activation and retinoic acid receptor signaling. J Immunol 186:5261–5272. doi: 10.4049/jimmunol.1003615. [DOI] [PubMed] [Google Scholar]
- 54.Fillatreau S. 2015. Regulatory plasma cells. Curr Opin Pharmacol 23:1–5. doi: 10.1016/j.coph.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 55.Xiao S, Brooks CR, Sobel RA, Kuchroo VK. 2015. Tim-1 is essential for induction and maintenance of IL-10 in regulatory B cells and their regulation of tissue inflammation. J Immunol 194:1602–1608. doi: 10.4049/jimmunol.1402632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu Y, Wang QH, Zheng L, Feng H, Liu J, Ma SH, Cao YM. 2007. Plasmodium yoelii: distinct CD4(+)CD25(+) regulatory T cell responses during the early stages of infection in susceptible and resistant mice. Exp Parasitol 115:301–304. doi: 10.1016/j.exppara.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 57.Lee CC, Kung JT. 2012. Marginal zone B cell is a major source of Il-10 in Listeria monocytogenes susceptibility. J Immunol 189:3319–3327. doi: 10.4049/jimmunol.1201247. [DOI] [PubMed] [Google Scholar]
- 58.Gong Y, Zhao C, Zhao P, Wang M, Zhou G, Han F, Cui Y, Qian J, Zhang H, Xiong H, Sheng J, Jiang T. 2015. Role of IL-10-producing regulatory B cells in chronic hepatitis B virus infection. Dig Dis Sci 60:1308–1314. doi: 10.1007/s10620-014-3358-1. [DOI] [PubMed] [Google Scholar]
- 59.Evans JG, Chavez-Rueda KA, Eddaoudi A, Meyer-Bahlburg A, Rawlings DJ, Ehrenstein MR, Mauri C. 2007. Novel suppressive function of transitional 2 B cells in experimental arthritis. J Immunol 178:7868–7878. doi: 10.4049/jimmunol.178.12.7868. [DOI] [PubMed] [Google Scholar]
- 60.Matsumoto M, Baba A, Yokota T, Nishikawa H, Ohkawa Y, Kayama H, Kallies A, Nutt SL, Sakaguchi S, Takeda K, Kurosaki T, Baba Y. 2014. Interleukin-10-producing plasmablasts exert regulatory function in autoimmune inflammation. Immunity 41:1040–1051. doi: 10.1016/j.immuni.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 61.Maseda D, Smith SH, DiLillo DJ, Bryant JM, Candando KM, Weaver CT, Tedder TF. 2012. Regulatory B10 cells differentiate into antibody-secreting cells after transient IL-10 production in vivo. J Immunol 188:1036–1048. doi: 10.4049/jimmunol.1102500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wei X, Jin Y, Tian Y, Zhang H, Wu J, Lu W, Lu X. 2016. Regulatory B cells contribute to the impaired antitumor immunity in ovarian cancer patients. Tumour Biol 37:6581–6588. doi: 10.1007/s13277-015-4538-0. [DOI] [PubMed] [Google Scholar]
- 63.Jeong YI, Hong SH, Cho SH, Lee WJ, Lee SE. 2012. Induction of IL-10-producing CD1dhighCD5+ regulatory B cells following Babesia microti-infection. PLoS One 7:e46553. doi: 10.1371/journal.pone.0046553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guthmiller JJ, Graham AC, Zander RA, Pope RL, Butler NS. 2017. Cutting edge: IL-10 is essential for the generation of germinal center B cell responses and anti-Plasmodium humoral immunity. J Immunol 198:617–622. doi: 10.4049/jimmunol.1601762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lal G, Kulkarni N, Nakayama Y, Singh AK, Sethi A, Burrell BE, Brinkman CC, Iwami D, Zhang T, Hehlgans T, Bromberg JS. 2016. IL-10 from marginal zone precursor B cells controls the differentiation of Th17, Tfh and Tfr cells in transplantation tolerance. Immunol Lett 170:52–63. doi: 10.1016/j.imlet.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Achour A, Simon Q, Mohr A, Seite JF, Youinou P, Bendaoud B, Ghedira I, Pers JO, Jamin C. 2017. Human regulatory B cells control the TFH cell response. J Allergy Clin Immunol 140:215–222. doi: 10.1016/j.jaci.2016.09.042. [DOI] [PubMed] [Google Scholar]
- 67.Vomhof-DeKrey EE, Yates J, Hagglof T, Lanthier P, Amiel E, Veerapen N, Besra GS, Karlsson MC, Leadbetter EA. 2015. Cognate interaction with iNKT cells expands IL-10-producing B regulatory cells. Proc Natl Acad Sci U S A 112:12474–12479. doi: 10.1073/pnas.1504790112. [DOI] [PMC free article] [PubMed] [Google Scholar]