ABSTRACT
Johne's disease, caused by Mycobacterium avium subsp. paratuberculosis, is a bovine chronic infection that is endemic in Japan and many other countries. The expression of immunoinhibitory molecules is upregulated in cattle with Johne's disease, but the mechanism of immunosuppression is poorly understood. Prostaglandin E2 (PGE2) is immunosuppressive in humans, but few veterinary data are available. In this study, functional and kinetic analyses of PGE2 were performed to investigate the immunosuppressive effect of PGE2 during Johne's disease. In vitro PGE2 treatment decreased T-cell proliferation and Th1 cytokine production and upregulated the expression of immunoinhibitory molecules such as interleukin-10 and programmed death ligand 1 (PD-L1) in peripheral blood mononuclear cells (PBMCs) from healthy cattle. PGE2 was upregulated in sera and intestinal lesions of cattle with Johne's disease. In vitro stimulation with Johnin purified protein derivative (J-PPD) induced cyclooxygenase-2 (COX-2) transcription, PGE2 production, and upregulation of PD-L1 and immunoinhibitory receptors in PBMCs from cattle infected with M. avium subsp. paratuberculosis. Therefore, Johnin-specific Th1 responses could be limited by the PGE2 pathway in cattle. In contrast, downregulation of PGE2 with a COX-2 inhibitor promoted J-PPD-stimulated CD8+ T-cell proliferation and Th1 cytokine production in PBMCs from the experimentally infected cattle. PD-L1 blockade induced J-PPD-stimulated CD8+ T-cell proliferation and interferon gamma production in vitro. Combined treatment with a COX-2 inhibitor and anti-PD-L1 antibodies enhanced J-PPD-stimulated CD8+ T-cell proliferation in vitro, suggesting that the blockade of both pathways is a potential therapeutic strategy to control Johne's disease. The effects of COX-2 inhibition warrant further study as a novel treatment of Johne's disease.
KEYWORDS: PGE2, immunoinhibitory molecules, PD-1, PD-L1, T-cell exhaustion, immunotherapy, COX-2 inhibitor, Johne's disease, cattle
INTRODUCTION
Prostaglandin E2 (PGE2) is an inflammatory mediator (1) derived from arachidonic acid by cyclooxygenase isoenzymes (COX-1 and -2) and PGE synthases (2). COX-1 is a widely expressed constitutive enzyme in many tissues, including the kidneys, stomach, and vascular endothelium (3). COX-2 is an inducible enzyme, and its expression is regulated by inflammatory cytokines and growth factors via activation of nuclear factor-kappa B (NF-κB) (4). PGE2 has suppressive activity in T cells, natural killer (NK) cells, dendritic cells (DCs), and macrophages via specific EP2 and EP4 receptors. The interaction of PGE2 and EP2 or EP4 activates the cyclic AMP (cAMP)/protein kinase A (PKA)/cAMP response element binding protein (CREB) pathway to induce the expression of anti-inflammatory and immunosuppressive genes (5, 6). Previous studies in humans have demonstrated that PGE2 inhibits Th1 responses and the differentiation of DCs and induces several subsets of immunosuppressive cells, including M2 macrophages, myeloid-derived suppressor cells (MDSCs), and regulatory T (Treg) cells (7–9). Other studies have found an association of PGE2 with progression of chronic infections. PGE2 upregulates the human immunodeficiency virus type 1 (HIV-1) long terminal repeat-driven gene in T cells (10, 11). COX-2 expression is upregulated in hepatitis C virus (HCV) infection, and PGE2 induces RNA replication of HCV (12). Because of the involvement of PGE2 in the immunopathogenesis of chronic infections, COX-2 inhibitors have been considered candidate immunotherapy drugs. COX-2 inhibitors activate immune responses in vitro and in vivo (13, 14). COX-2 inhibition results in the activation of interleukin-12 (IL-12) production from antigen-presenting cells and the suppression of IL-10 production in splenocytes of tumor-bearing mice (13). In patients with chronic HIV-1 infection, COX-2 inhibitors were found to improve T-cell-dependent functions and vaccine responses (14). Thus, the COX-2–PGE2 pathway limits Th1 responses during chronic diseases.
Mycobacterium avium subsp. paratuberculosis is a facultative intracellular pathogen causing Johne's disease (paratuberculosis) in ruminants (15). The clinical signs of Johne's disease include chronic diarrhea, severe weight loss, reduction in milk production, and mortality (15). Johne's disease is endemic worldwide; no country or region has been found to be free of this disease (16). In early infections, M. avium subsp. paratuberculosis induces strong Th1 responses characterized by interferon gamma (IFN-γ), and macrophages activated by IFN-γ kill intracellular mycobacteria (17–19). The Th1 response declines during the late subclinical stage, which allows bacterial growth and progression to clinical disease (20–22). The Th1 response is the key in the control of progression of Johne's disease.
Programmed death 1 (PD-1) and lymphocyte activation gene 3 (LAG-3) are immunoinhibitory receptors that act in a negative-feedback system to inhibit excessive immune responses via interactions with their ligands, programmed death ligand 1 (PD-L1) and major histocompatibility complex class II (MHC II) (23, 24). In chronic infections, these immunoinhibitory molecules are involved in the exhaustion of antigen-specific T cells (25, 26). PD-1 and LAG-3 are upregulated on CD4+ and/or CD8+ T cells during subclinical Johne's disease in cattle, and an immunoinhibitory ligand, PD-L1, is expressed on M. avium subsp. paratuberculosis-infected macrophages of the ileum (27). In addition, antibody blockade of the PD-1 and LAG-3 pathways reactivates M. avium subsp. paratuberculosis-specific Th1 cytokine production in vitro (27). The dysfunction of the Th1 response during Johne's disease is mediated by immunoinhibitory molecules on T cells, but it is not known how these immunoinhibitory molecules are upregulated during the course of the disease.
The association of PGE2 and immunoinhibitory molecules has been investigated in mouse models and in human patients (28–30). In a murine tumor model, PGE2 regulated PD-L1 expression in tumor-associated macrophages and MDSCs (28). Another study reported a positive correlation between COX-2 and PD-L1 expression in human melanoma cells (29). Additionally, in a mouse model of chronic infection, EP2 and EP4 were upregulated on CD8+ cytotoxic T cells (CTLs) and impaired CTL function and survival via PGE2 signaling (30). Concurrent blockade of the PGE2 and PD-1/PD-L1 pathways was shown to restore CTL function and improve viral control (30).
Few veterinary studies are available on the immunosuppressive effect of PGE2, the association of PGE2 and immunoinhibitory pathways, and the contribution to T-cell dysfunction or chronic disease progression. This study investigated the immunosuppressive function and kinetics of PGE2 to investigate immunopathogenesis in Johne's disease in cattle.
RESULTS
Immunosuppressive effects of PGE2.
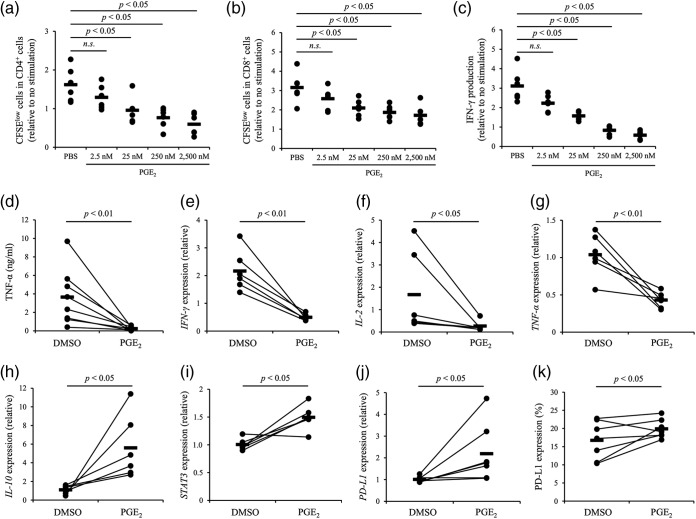
To evaluate immunosuppression induced by PGE2, T-cell proliferation, cytokine secretion, and gene expression (cytokine and STAT3 genes) were analyzed by cultivation assay of peripheral blood mononuclear cells (PBMCs) from uninfected cattle under PGE2 treatment. PGE2 inhibited proliferation of CD4+ and CD8+ T cells (Fig. 1a and b) and IFN-γ and TNF-α production from PBMCs (Fig. 1c and d). PGE2 downregulated the mRNA expression of IFN-γ, IL-2, and tumor necrosis factor alpha (TNF-α) (Fig. 1e to g) and upregulated IL-10 and STAT3 mRNA expression (Fig. 1h and i). The results indicate that PGE2 promotes IL-10 signaling and inhibits Th1 responses in cattle. Since PGE2 is known to regulate PD-L1 expression in humans (28), PGE2 regulation of PD-L1 expression was investigated in PBMCs of the healthy cattle. As shown in Fig. 1j and k, PGE2 upregulated PD-L1 expression in PBMCs. Overall, these results indicate that PGE2 has immunosuppressive activity against bovine PBMCs.
FIG 1.
Immunosuppressive effects of PGE2. (a to d) PBMCs from uninfected cattle (n = 6 [a to c] or 8 [d]) were cultured with PGE2 in the presence of anti-CD3 and anti-CD28 MAbs for 72 h. The proliferation of CD4+ cells (a) and CD8+ cells (b) was assayed by flow cytometry. IFN-γ (c) and TNF-α (d) production was determined by ELISA. (e to k) PBMCs from uninfected cattle (n = 6 [e to i] or 7 [j and k]) were cultured with PGE2 for 24 h. Real-time PCR was performed in duplicate to quantitate the mRNA expression of IFN-γ (e), IL-2 (f), TNF-α (g), IL-10 (h), STAT3 (i), and PD-L1 (j). The expression of PD-L1 protein was measured by flow cytometry (k). Statistical significance was determined by the Steel-Dwass test (a to c) or the Wilcoxon signed-rank test (d to k).
Activation of immune responses by COX-2 inhibition.
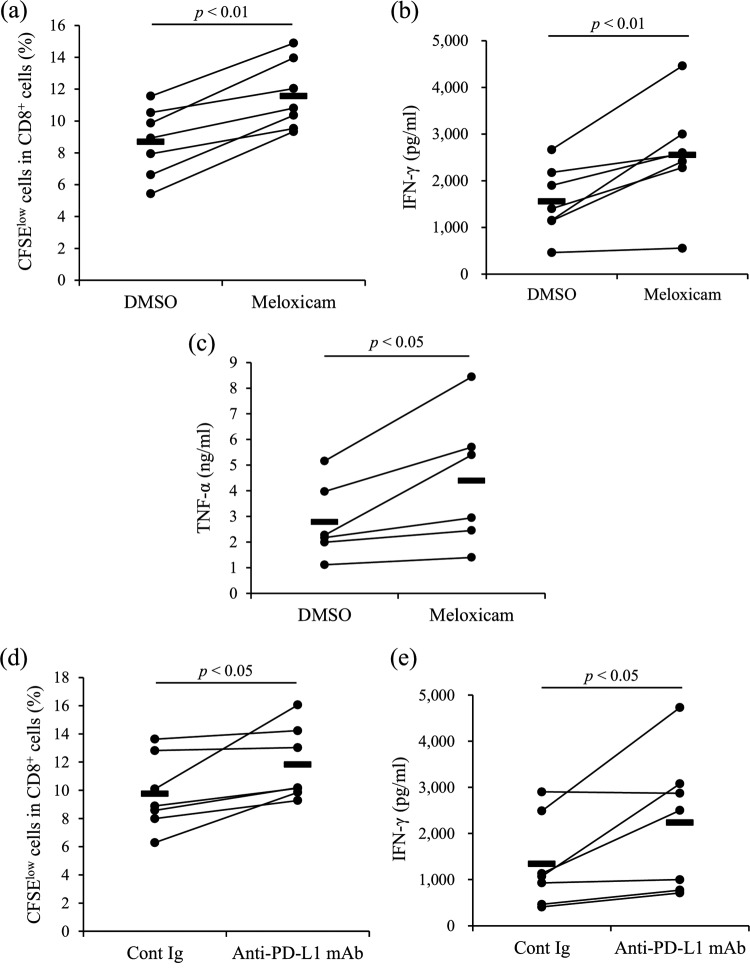
To demonstrate the effects of COX-2 inhibition on T-cell function, production of IFN-γ and TNF-α and T-cell proliferation were evaluated by the 3-day culture assay using PBMCs from uninfected animals in the presence of meloxicam. Meloxicam treatment significantly increased both IFN-γ and TNF-α production in PBMCs and the proliferation of CD8+ T cells (Fig. 2a to c). This result indicates that meloxicam activates the T-cell response in cattle.
FIG 2.
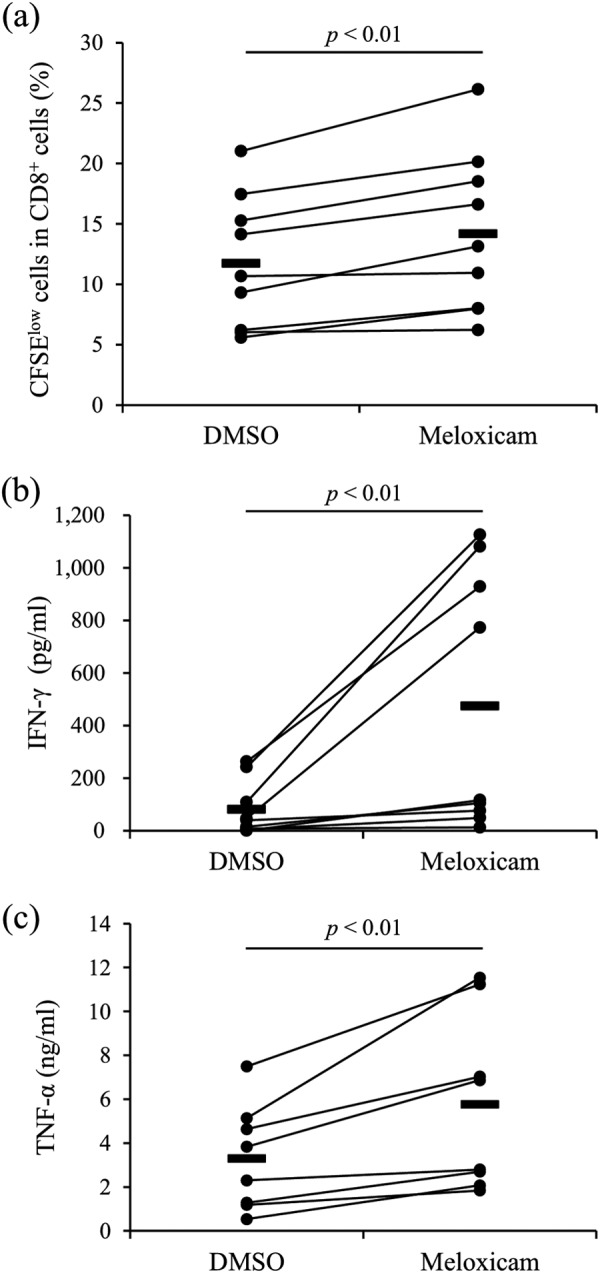
Functional analysis of the COX-2 inhibitor. PBMCs from uninfected cattle (n = 9 [a and b] or 8 [c]) were cultured with meloxicam in the presence of anti-CD3 and anti-CD28 MAbs. CD8+ cell proliferation was assayed by flow cytometry (a). IFN-γ (b) and TNF-α (c) production was determined by ELISA in duplicate. Statistical significance was determined by the Wilcoxon signed-rank test.
Kinetic analysis of PGE2 in cattle infected with M. avium subsp. paratuberculosis.
In humans, PGE2 is associated with the progression of some chronic infections (10–12). Compared with that in uninfected cattle, the serum PGE2 concentration was significantly increased in cattle with natural infection of M. avium subsp. paratuberculosis (Fig. 3a). Johnin purified protein derivative (J-PPD) antigen stimulation increased PGE2 production in PBMCs from the infected animals but not in PBMCs from the uninfected animals (Fig. 3c), and meloxicam inhibited PGE2 production under the J-PPD stimulation (Fig. 3c). COX-2 expression in PBMCs from the infected animals was induced by the stimulation of J-PPD (Fig. 3b). The results indicate that the COX-2–PGE2 pathway was activated by Johnin antigen stimulation in PBMCs of cattle infected with M. avium subsp. paratuberculosis.
FIG 3.
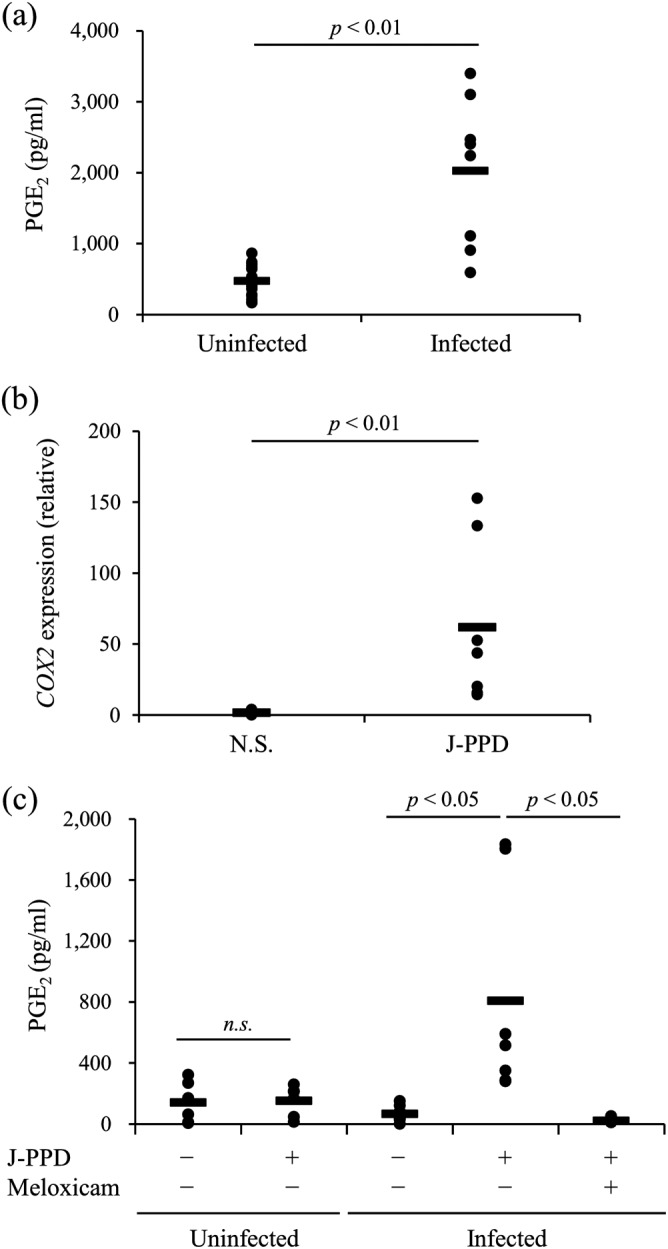
Kinetic analysis of PGE2 in cattle infected with M. avium subsp. paratuberculosis. (a) Serum PGE2 concentrations in cattle uninfected (n = 16) or naturally infected with M. avium subsp. paratuberculosis (n = 8) were determined by ELISA. (b) The expression of COX-2 mRNA in PBMCs from cattle experimentally infected with M. avium subsp. paratuberculosis (n = 7) cultured with J-PPD was assayed by real-time PCR. (c) PBMCs of the uninfected (n = 6) and experimentally infected (n = 7) cattle were incubated with J-PPD. The concentration of PGE2 in culture supernatants was determined by ELISA in duplicate. Statistical significance was determined by the Wilcoxon signed-rank test (a and b) or the Steel-Dwass test (c). N.S., no stimulation.
Upregulation of PD-L1, PD-1, and LAG-3 expression by J-PPD stimulation.
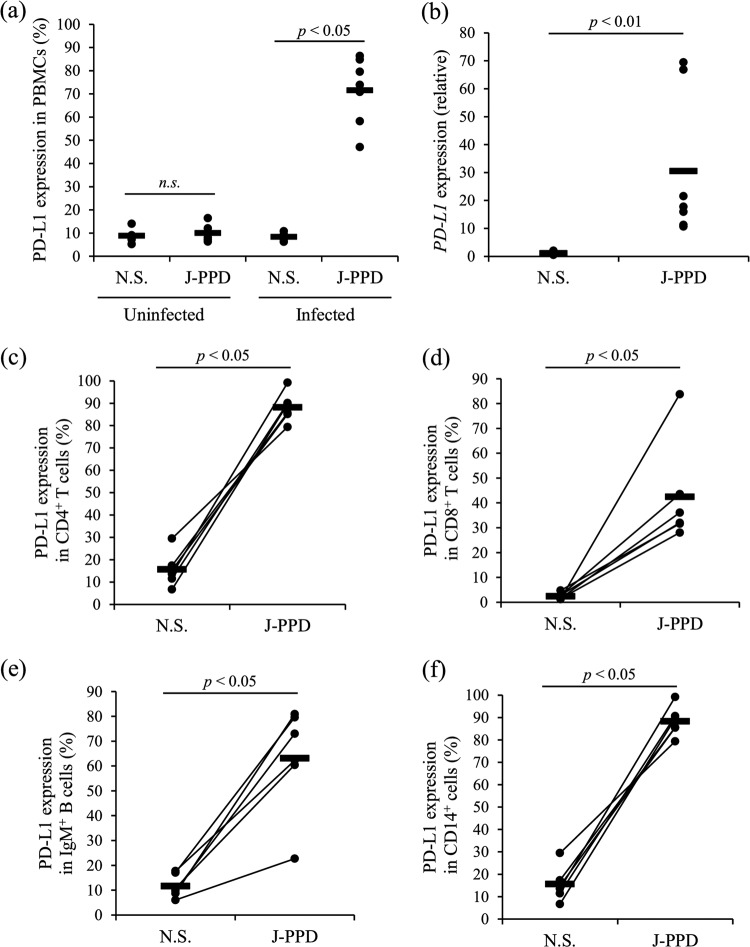
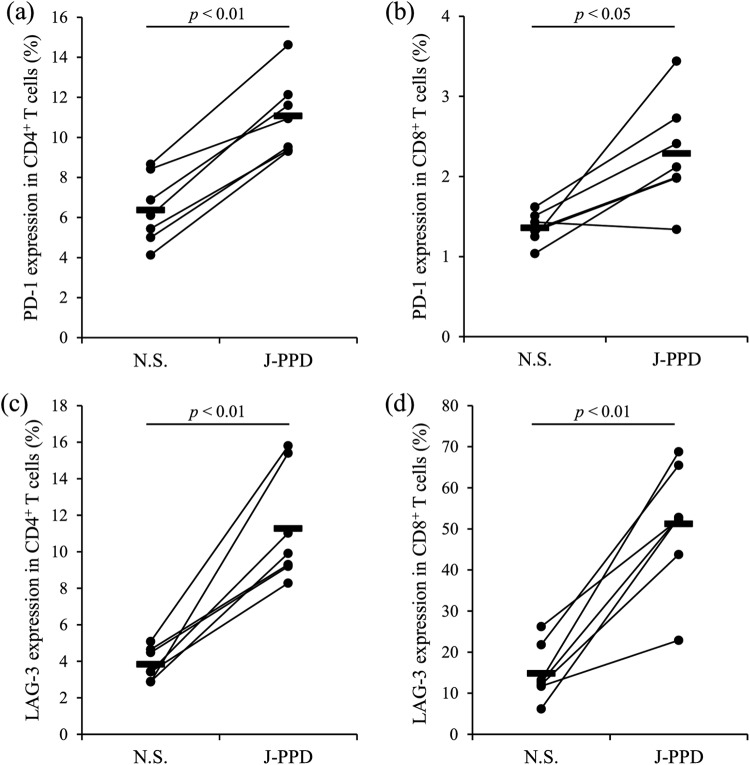
The expression of the immunoinhibitory molecules PD-L1, PD-1, and LAG-3 in PBMCs from cattle experimentally infected with M. avium subsp. paratuberculosis was analyzed following stimulation by J-PPD for 24 h. Compared with that in the uninfected cattle, J-PPD significantly increased PD-L1 expression at the protein and mRNA levels in PBMCs of the experimentally infected animals (Fig. 4a and b). Flow cytometric analysis indicated that the expression of PD-L1 was significantly upregulated in CD4+ T cells, CD8+ T cells, IgM+ B cells, and CD14+ cells of the infected animals by J-PPD stimulation (Fig. 4c to f). The expression of PD-1 and LAG-3 was also upregulated in CD4+ T cells and CD8+ T cells of the infected animals by J-PPD stimulation (Fig. 5a to d). In vitro stimulation by Johnin antigen thus strongly induced the expression of immunoinhibitory molecules in PBMCs of cattle infected with M. avium subsp. paratuberculosis.
FIG 4.
Upregulation of PD-L1 expression by J-PPD. PBMCs from cattle experimentally infected with M. avium subsp. paratuberculosis (n = 6) were incubated with J-PPD, and the expression of PD-L1 in PBMCs (a), CD4+ T cells (c), CD8+ T cells (d), IgM+ B cells (e), and CD14+ cells (f) was assayed by flow cytometry. PBMCs of uninfected cattle (n = 5) were incubated with J-PPD, and PD-L1 expression in PBMCs was assayed by flow cytometry (a). Expression of PD-L1 mRNA in PBMCs of the experimentally infected cattle (n = 7) was assayed by real-time PCR in duplicate (b). Statistical significance was determined by the Wilcoxon signed-rank test. N.S., no stimulation.
FIG 5.
Upregulation of PD-1 and LAG-3 expression by J-PPD. PBMCs from cattle experimentally infected with M. avium subsp. paratuberculosis (n = 7) were incubated with J-PPD, and the expression of PD-1 (a and b) and LAG-3 (c and d) on CD4+ T cells (a and c) and CD8+ T cells (b and d) was assayed by flow cytometry. Statistical significance was determined by the Wilcoxon signed-rank test. N.S., no stimulation.
Expression of PGE2, EP2, and PD-L1 on M. avium subsp. paratuberculosis-infected cells of the ileum.
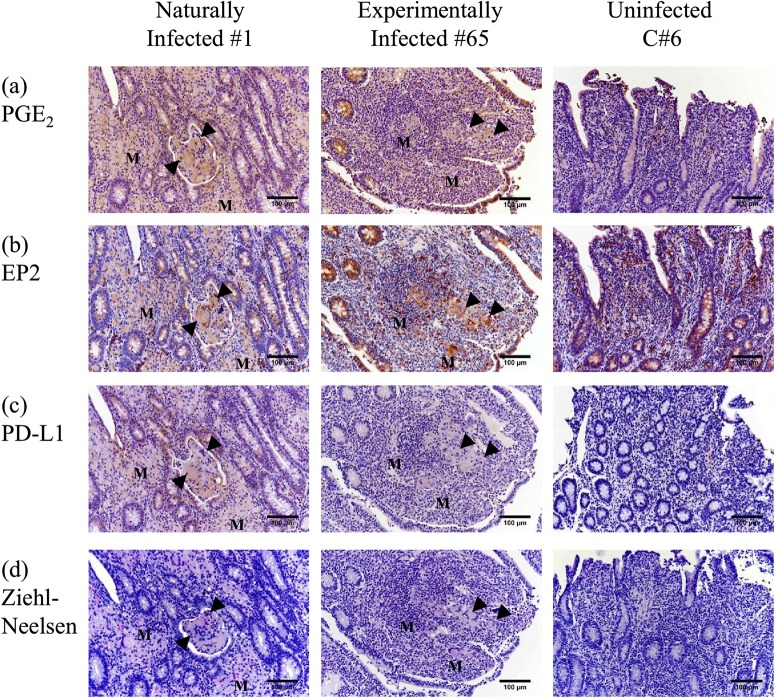
A previous study confirmed PD-L1 expression in macrophages infected with M. avium subsp. paratuberculosis in the ileum, with possible association with the clinical disease (27). This study investigated the expression of PGE2 and EP2 in the intestinal macrophages of cattle with Johne's disease. PGE2 expression was detected in cattle naturally and experimentally infected with M. avium subsp. paratuberculosis (1 and 65) but not in the uninfected cattle (C6) (Fig. 6a). Expression of EP2, a PGE2 receptor, was detected in both the infected and uninfected animals (Fig. 6b), and both PD-L1+ and M. avium subsp. paratuberculosis-infected cells were observed in the same lesion (Fig. 6c and d). The results demonstrated coexpression of PGE2 and PD-L1 in the ilea of cattle with Johne's disease.
FIG 6.
PGE2, EP2, and PD-L1 expression and localization of M. avium subsp. paratuberculosis in the ileal mucosa of cattle with or without Johne's disease. (a to c) Immunohistochemical staining of PGE2 (a), EP2 (b), and PD-L1 (c) in ileal tissue of cattle naturally or experimentally infected with M. avium subsp. paratuberculosis (1 and 65) and uninfected cattle (C6) was performed using anti-human PGE2 antibody (rabbit polyclonal), anti-human EP2 MAb [EPR8030(B)], and anti-bovine PD-L1 MAb (6C11-3A11). (d) Ziehl-Neelsen staining for acid-fast bacilli in ileal tissue of the infected and uninfected cattle was also performed. M, accumulations of M. avium subsp. paratuberculosis-infected macrophages. Arrowheads, M. avium subsp. paratuberculosis-infected Langhans giant cells.
Activation of J-PPD-specific T-cell responses by a COX-2 inhibitor.
J-PPD can induce IFN-γ production from T cells in cattle with M. avium subsp. paratuberculosis infection (31). This study investigated whether the COX-2 inhibitor meloxicam activates cytokine production and T-cell proliferation in response to J-PPD. As shown in Fig. 7, J-PPD upregulated IFN-γ and TNF-α production in meloxicam-treated cultures of PBMCs from infected cattle (Fig. 7b and c). The proliferation of CD8+ cells was also induced by meloxicam treatment (Fig. 7a). Meloxicam thus activated J-PPD-specific immune responses.
FIG 7.
Activation of T-cell responses by the COX-2 inhibitor or anti-PD-L1 MAb. (a to c) PBMCs from cattle experimentally infected with M. avium subsp. paratuberculosis (n = 7 [a and b] or 6 [c]) were cultured with meloxicam in the presence of J-PPD. CD8+ cell proliferation (a) was assayed by flow cytometry. IFN-γ (b) and TNF-α (c) production was determined by ELISA in duplicate. (d and e) PBMCs from the infected cattle (n = 7) were cultured with anti-PD-L1 MAb in the presence of J-PPD. CD8+ cell proliferation (d) was assayed by flow cytometry. IFN-γ production (e) was determined by ELISA in duplicate. Statistical significance was determined by the Wilcoxon signed-rank test. Cont Ig, rat control IgG.
Activation of J-PPD-specific T-cell responses by PD-L1 blockade.
Blockade of the PD-1/PD-L1 pathway can activate IFN-γ production from PBMCs in bovine chronic infections, such as bovine leukemia virus (BLV) infection and bovine mycoplasmosis (32, 33). To demonstrate the effects of PD-L1 blockade on cattle infected with M. avium subsp. paratuberculosis, PBMCs from the experimentally infected cattle were incubated with an anti-PD-L1 monoclonal antibody (MAb) in the presence of J-PPD. As expected, J-PPD-specific proliferation of CD8+ cells and IFN-γ production were activated by the anti-PD-L1 MAb (Fig. 7d and e).
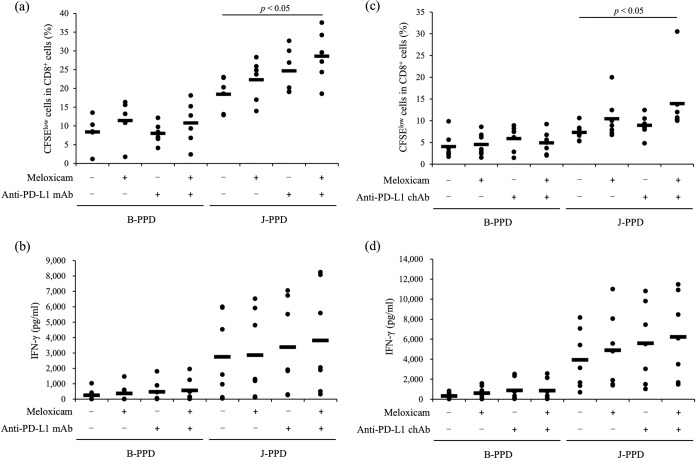
Combined effects of meloxicam and anti-PD-L1 antibody on activation of J-PPD-specific T-cell response.
Compared with that in negative controls, the proliferation of CD8+ cells was significantly increased by the combination of meloxicam and anti-PD-L1 MAb (Fig. 8a). Stimulation by B-PPD, a negative-control antigen, had no effect in either group (Fig. 8a). IFN-γ production was increased by meloxicam and anti-PD-L1 MAb, but the difference from the results with negative controls did not reach significance (Fig. 8b). A previous study found that an anti-PD-L1 chimeric antibody (ChAb) activated immune responses in BLV infection in vitro and in vivo (34). In this study, combined treatment with the COX-2 inhibitor and anti-PD-L1 ChAb significantly increased CD8+ cell proliferation compared with that for the negative controls (Fig. 8c). The results obtained by combining a COX-2 inhibitor and an anti-PD-L1 antibody support their potential as a novel treatment of Johne's disease.
FIG 8.
Activation of T-cell responses by the combination of meloxicam and anti-PD-L1 antibody. (a and b) PBMCs from cattle experimentally infected with M. avium subsp. paratuberculosis (n = 6 [a] or 7 [b]) were incubated with meloxicam and anti-PD-L1 MAb in the presence of J-PPD or B-PPD. CD8+ cell proliferation was assayed by flow cytometry (a). IFN-γ production was determined by ELISA in duplicate (b). (c and d) PBMCs from the infected cattle (n = 7) were incubated with meloxicam and anti-PD-L1 ChAb in the presence of J-PPD or B-PPD. CD8+ cell proliferation was assayed by flow cytometry (c). IFN-γ production was determined by ELISA in duplicate (d). Statistical significance was determined by the Steel-Dwass test.
DISCUSSION
During the early phase of Johne's disease in cattle, strong Th1 responses are elicited by T cells specific to M. avium subsp. paratuberculosis that are activated by M. avium subsp. paratuberculosis-infected macrophages following antigen presentation (17–19). At the late subclinical stage, a decline in the Th1 response contributes to bacterial growth and progression to clinical disease (20–22). The loss of functional Th1 responses during subclinical Johne's disease is mediated by immunoinhibitory molecules (27), but the mechanism of the upregulation of these molecules during the disease course is not clear. This study investigated the immunosuppressive effect of PGE2 because its involvement in the regulation of immune responses in chronic human infections is well documented (10–12). PGE2 inhibited the proliferation of CD4+ and CD8+ T cells and the production of the Th1 cytokines IFN-γ and TNF-α in cultures of PBMCs isolated from cattle. PGE2 treatment downregulated the expression of IFN-γ, IL-2, and TNF-α mRNAs and upregulated the expression of IL-10 and STAT3 mRNAs in cultured PBMCs. PGE2 production was increased in the blood of animals infected with M. avium subsp. paratuberculosis. The Th1 response plays a critical role in the control of M. avium subsp. paratuberculosis infection and disease progression, but this infection induces IL-10, which activates STAT3 via IL-10 receptor signaling, which in turn results in immunosuppression and bacterial persistence (35, 36). Our results show that PGE2 contributed to the progression of Johne's disease by the suppression of Th1 responses and the activation of IL-10 production. Previous studies have associated PGE2 with mycobacterial infections (37, 38), but the role of PGE2 was not described. The results of this study contribute to the understanding of PGE2 activity during infection with mycobacteria.
PD-L1 is upregulated in the macrophages in the peripheral blood and in the ilea of cattle with Johne's disease (27), but the mechanism underlying PD-L1 upregulation has been unknown. In this study, PGE2 upregulated in vitro PD-L1 expression in the PBMCs of healthy cattle. Additionally, J-PPD stimulation induced PGE2 production by activating COX-2 and promoting PD-L1 expression in the PBMCs of cattle infected with M. avium subsp. paratuberculosis but not in uninfected cattle. In PBMC cultures of infected animals, J-PPD-specific CD4+ T cells secrete mainly IFN-γ and TNF-α by J-PPD stimulation (17–19). COX-2 transcription is known to be induced by inflammatory cytokines (TNF-α, IL-1, etc.), Toll-like receptor (TLR) signaling, and oxidative stress via the NF-κB pathway in human cells (39, 40). In addition, a previous paper has shown that IFN-γ-induced TNF activates NF-κB, which is required for full COX-2 expression in murine macrophages (41). Therefore, TNF-α produced from J-PPD-specific T cells and/or IFN-γ-stimulated macrophages might activate COX-2 transcription via the TNF–NF-κB pathway and result in increased PGE2 production in the PBMC cultivation assay with J-PPD stimulation.
In this study, in vitro treatment of PGE2 upregulated transcription of the IL-10 and STAT3 genes in the PBMCs of healthy cattle. The inhibition of STAT3 activity has been shown to downregulate PD-L1 expression in non-small-cell lung cancer (42). The results indicated that PGE2 may regulate PD-L1 expression in bovine PBMCs by activating IL-10–STAT3 signaling. PD-L1 expression in M. avium subsp. paratuberculosis-infected macrophages has previously been associated with diseases involving the ileum (27). In this study, PGE2 was expressed in M. avium subsp. paratuberculosis-infected macrophages and Langhans giant cells in the ilea of animals with clinical Johne's disease. The overall findings suggest that the PGE2–IL-10–STAT3–PD-L1 axis is involved in the downregulation of the Th1 response and the formation of pathological lesions in the intestine during Johne's disease. It is still unclear what types of cell subsets produce PGE2 and what types of prostanoid receptors, EP1, EP2, EP3, or EP4, mediate immunosuppression by PGE2 on T cells during Johne's disease. Further investigation is required to determine the mechanism involved.
Macrophages and CD8+ cytotoxic T cells activated by Th1 cytokines are involved in the killing of intracellular bacteria during Johne's disease (17–19). COX-2 inhibition induced the proliferation of CD8+ T cells and Th1 cytokine production in PBMCs from M. avium subsp. paratuberculosis-infected cattle following J-PPD stimulation. Combined blockade of PGE2 and PD-1/PD-L1 strongly stimulated the proliferation of CD8+ T cells in the infected animals. IFN-γ production tended to be activated by dual blockade of PGE2 and PD-1/PD-L1, although significance was not observed because of the limited number of samples that were evaluated. The combination of COX-2 inhibition and PD-1/PD-L1 blockade has therapeutic potential for the control of Johne's disease. Selective COX-2 inhibitors, including meloxicam, are launched and widely used as anti-inflammatory compounds in the dairy and beef industries. Previous studies have investigated the in vivo effect of meloxicam on T-cell subsets in healthy or vaccinated cattle (43, 44). However, the in vivo effect of meloxicam on the Th1 response during Johne's disease is still unclear and should be determined in further experiments in infected cattle. In addition, we are developing a method for large-scale production of bovinized chimeric antibody using a mammalian expression system (34, 45), which would enable antibody therapy even in cattle. Further study is warranted to investigate the clinical effectiveness and cost-effectiveness of these inhibitors against Johne's disease. Previous studies have demonstrated that immunoinhibitory molecules mediate T-cell exhaustion in cattle with other infectious diseases, including bovine leukosis, mycoplasmosis, and anaplasmosis (33, 46–51), but the involvement of PGE2 in the immunopathogenesis of those infections has not been investigated.
This is the first study of the immunosuppressive effects of PGE2 in cattle and the immunomodulatory effect of COX-2 inhibition during Johne's disease in cattle. The results suggest cotargeting PGE2 and PD-L1 as a novel immunotherapy for Johne's disease. Pilot clinical trials have shown that antibodies blocking the PD-1/PD-L1 pathway have immunomodulatory and antiviral effects (34, 45). Cost-effectiveness is an obstacle to this approach because large amounts of the therapeutic antibodies are required. If the antibody dose can be reduced by combination with a COX-2 inhibitor, this approach could have a significant impact. Further study will open up new avenues for the treatment of bovine chronic infections, including Johne's disease.
MATERIALS AND METHODS
Animals.
Blood from Holstein cattle not infected with M. avium subsp. paratuberculosis was obtained from dairy farms in Hokkaido, Japan, which have no history of Johne's disease. Seven male Holstein calves (animals 80 to 86) between 3 and 4 weeks of age were orally inoculated with intestinal tissue homogenate from an infected cow containing M. avium subsp. paratuberculosis (6.8 × 106 CFU) once daily for 20 days, as described previously with slight modifications (27, 52). All infected calves were kept in a biosafety level 2 animal facility at the National Institute of Animal Health (Tsukuba, Ibaraki, Japan). These cattle did not show clinical symptoms. All animal experiments were approved by the National Institute of Animal Health Ethics Committee.
Cell preparation and culture.
Peripheral blood samples were collected from cattle that were uninfected or experimentally infected with M. avium subsp. paratuberculosis. Peripheral blood mononuclear cells (PBMCs) were isolated from blood samples by density gradient centrifugation on Percoll (GE Healthcare, Buckinghamshire, England, UK). Cells were cultured in 200 μl RPMI 1640 medium (Sigma-Aldrich, St. Louis, MO, USA) including 10% heat-inactivated fetal bovine serum (Thermo Fisher Scientific, Waltham, MA, USA), 200 IU/ml penicillin, 200 μg/ml streptomycin, and 0.01% l-glutamine (Thermo Fisher Scientific) in 96-well culture plates (Corning Inc., Corning, NY, USA) at 37°C in a 5% CO2 atmosphere. The culture assays of PBMCs from the experimentally infected animals were carried out using samples at various time points between 82 and 120 weeks after the first inoculation.
Functional analysis of PGE2.
To evaluate immunosuppressive functions of PGE2, PBMCs (4 × 105 cells/well) from uninfected cattle were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) (Sigma-Aldrich) and incubated with 2.5 to 2,500 nM PGE2 (Cayman Chemical, Ann Arbor, MI, USA) for 72 h. Cultures were stimulated by adding 1 μg/ml of anti-CD3 monoclonal antibody (MAb) (MM1A; Washington State University Monoclonal Antibody Center, Pullman, WA, USA) and 1 μg/ml of anti-CD28 MAb (CC220; Bio-Rad, Hercules, CA, USA) to each well.
To demonstrate the effects of PGE2 on IFN-γ and TNF-α production, 72-h culture supernatants of CFSE-labeled PBMCs were collected, and IFN-γ and TNF-α were determined by enzyme-linked immunosorbent assay (ELISA) for bovine IFN-γ (Mabtech, Nacka Strand, Sweden) and bovine TNF-α (Kingfisher Biotech, St. Paul, MN, USA), following the manufacturer's instructions.
To demonstrate the effects of PGE2 on cell proliferation, CFSE-labeled PBMCs were harvested after 72 h and incubated in phosphate-buffered saline (PBS) with 10% goat serum (Thermo Fisher Scientific) for 15 min at room temperature to prevent nonspecific reactions. Cells were washed with PBS containing 1% bovine serum albumin (Sigma-Aldrich) (1% BSA-PBS) and stained with Alexa Fluor 647-conjugated anti-CD4 MAb (CC30; Bio-Rad), peridinin-chlorophyll-protein complex/cyanin 5.5 (PerCP/Cy 5.5)-conjugated anti-CD8 MAb (CC63; Bio-Rad), and phycoerythrin/cyanin 7 (PE/Cy7)-conjugated anti-IgM MAb (IL-A30; Bio-Rad) for 25 min at room temperature. CC30 was prelabeled with a Zenon Alexa Fluor 647 mouse IgG1 labeling kit (Thermo Fisher Scientific). CC63 and IL-A30 were conjugated with PerCP/Cy5.5 and PE/Cy7, respectively, by using Lightning-Link antibody labeling kits (Innova Biosciences, Cambridge, England, UK). The stained cells were then washed with 1% BSA-PBS and analyzed immediately by FACS Verse (BD Biosciences, San Jose, CA, USA) and FCS Express 4 (De Novo Software, Glendale, CA, USA). The primary antibodies used in this experiment are shown in Table S1 in the supplemental material.
To investigate the effect of PGE2 on the transcriptional profile of genes involved in the T-cell response, PBMCs (4 × 105 cells/well) from uninfected cattle were incubated with 2,500 nM PGE2 (Cayman Chemical) for 24 h. Total RNA was extracted from the cultivated cells in TRI Reagent (Molecular Research Center, Cincinnati, OH, USA) following the manufacturer's instructions. cDNA was synthesized from the obtained total RNA using PrimeScript reverse transcriptase (TaKaRa Bio, Otsu, Japan) following the manufacturer's instructions. The mRNA expression of COX-2, PD-L1, STAT3, and some cytokines in PBMCs was assayed by real-time PCR performed with a LightCycler 480 system II (Roche Diagnostics, Mannheim, Germany) using SYBR Premix DimerEraser (TaKaRa Bio) following the manufacturer's instructions. The GAPDH and ACTB genes were used as internal control genes. All assays were performed in duplicate. The primer sequences used in this study are shown in Table S2 in the supplemental material.
Kinetic analysis of PGE2.
The PGE2 concentrations in sera of uninfected animals and animals naturally infected with M. avium subsp. paratuberculosis were measured using the Prostaglandin E2 Express enzyme-linked immunosorbent assay (ELISA) kit (Cayman Chemical) following the manufacturer's instructions with slight modifications. To indicate whether PGE2 production was promoted by Johnin antigen stimulation, PBMCs (4 × 105 cells/well) from the infected cattle were incubated with 1 μg/ml of Johnin purified protein derivative (J-PPD) for 5 days. J-PPD is the inactivated crude protein fraction prepared from the culture supernatant of M. avium subsp. paratuberculosis, which is an immunogenic antigen widely used to evaluate T-cell responses against the bacterial infection ex vivo. Total RNA then was extracted from the cultivated cells, and cDNA was synthesized from the obtained total RNA as described above. The mRNA expression of COX-2 in PBMCs was then assayed by real-time PCR as described above (Table S2). Culture supernatants were collected, and the PGE2 concentration was determined by ELISA as described above.
Flow cytometry assay of PD-L1, PD-1, and LAG-3 expression.
PBMCs (1 × 106 cells/well) from cattle uninfected or experimentally infected with M. avium subsp. paratuberculosis were incubated with 1 μg/ml of J-PPD for 24 h. After 24 h, cells were harvested and incubated in PBS with 10% goat serum (Thermo Fisher Scientific) for 15 min at room temperature.
To analyze PD-L1 expression, the treated cells were washed with 1% BSA-PBS and stained with anti-bovine PD-L1 MAb (4G12, rat IgG2a) (32) or a rat IgG2a isotype control (R35-95; BD Biosciences) for 25 min at room temperature. To detect PD-L1 on T cells and B cells, cells were washed with 1% BSA-PBS after the reaction with primary antibody and then stained with phycoerythrin (PE)-conjugated anti-CD3 MAb (MM1A; Washington State University Monoclonal Antibody Center), fluorescein isothiocyanate (FITC)-conjugated anti-CD4 MAb (CC8; Bio-Rad), PerCP/Cy5.5-conjugated anti-CD8 MAb (CC63; Bio-Rad), PE/Cy7-conjugated anti-IgM MAb (IL-A30; Bio-Rad), and allophycocyanin (APC)-conjugated anti-rat immunoglobulin antibody (Southern Biotech, Birmingham, AL, USA) for 25 min at room temperature. MM1A was prelabeled with the Zenon R-PE mouse IgG1 labeling kit (Thermo Fisher Scientific). CC63 and IL-A30 were conjugated with PerCP/Cy5.5 and PE/Cy7, respectively, by using Lightning-Link antibody labeling kits (Innova Biosciences). To detect PD-L1 on CD14+ cells, cells were washed with 1% BSA-PBS after the reaction with primary antibody and then stained with PerCP/Cy5.5-conjugated anti-CD14 MAb (CAM36A; Washington State University Monoclonal Antibody Center) and APC-conjugated anti-rat immunoglobulin antibody (Southern Biotech) for 25 min at room temperature. CAM36A was prelabeled using Lightning-Link PerCP/Cy5.5 antibody labeling kit (Innova Biosciences).
To analyze PD-1 expression, the treated cells were washed with 1% BSA-PBS and then stained with anti-bovine PD-1 MAb (5D2, rat IgG2a) (46) or a rat IgG2a isotype control (R35-95; BD Biosciences) for 25 min at room temperature. To analyze LAG-3 expression, the treated cells were washed with 1% BSA-PBS and then stained with anti-bovine LAG-3 MAb (71-2D8, rat IgG1) (27) or a rat IgG1 isotype control (R3-34; BD Biosciences) for 25 min at room temperature. Cells were washed with 1% BSA-PBS after the reaction with primary antibody and then stained with PerCP/Cy5.5-conjugated anti-CD3 MAb (MM1A; Washington State University Monoclonal Antibody Center), FITC-conjugated anti-CD4 MAb (CC8; Bio-Rad), PE-conjugated anti-CD8 MAb (CC63; Bio-Rad), PE/Cy7-conjugated anti-IgM MAb (IL-A30; Bio-Rad), and APC-conjugated anti-rat immunoglobulin antibody (Southern Biotech) for 25 min at room temperature. MM1A and IL-A30 were conjugated with PerCP/Cy5.5 and PE/Cy7, respectively, by using Lightning-Link antibody labeling kits (Innova Biosciences). The stained cells were washed with 1% BSA-PBS and analyzed immediately by FACS Verse (BD Biosciences) and FCS Express 4 (De Novo Software). The primary antibodies used in this experiment are shown in Table S1 in the supplemental material.
Immunohistochemical assay of PD-L1, EP2, and PGE2 and Ziehl-Neelsen staining.
Immunohistochemical assays were performed as previously described with slight modifications (27). Sections of ileum tissue from cattle experimentally or naturally infected with M. avium subsp. paratuberculosis were evaluated. A naturally infected animal (naturally infected animal 1) had clinical symptoms of Johne's disease. Tissue samples from experimentally infected cattle were obtained from an animal (animal 65) with bacterial shedding in feces and clinical symptoms such as diarrhea (Table 1) (27). Anti-prostaglandin E2 antibody (Abcam, Cambridge, England, UK), anti-prostaglandin E receptor EP2 antibody [EPR8030(B); Abcam], or anti-PD-L1 MAb (6C11-3A11, rat IgG2a) (see Fig. S1 in the supplemental material) (32) was used for the primary antibody for immunohistochemistry. Ziehl-Neelsen staining was performed as previously described (27).
TABLE 1.
Bacterial loads in fecal and tissue samples at necropsy
| Animal | Status |
M. avium subsp. paratuberculosis DNA (pg) ina: |
|
|---|---|---|---|
| Feces | Ileum tissue | ||
| C6 | Uninfected control | ND | ND |
| 65b | Experimentally infected | 0.589 | 26.8 |
| 1 | Naturally infected | NA | NA |
ND, not detected; NA, not available.
The data for animal 65 have been published previously (27).
Functional assay of COX-2 inhibition.
To evaluate the immunostimulatory effects of COX-2 inhibition, PBMCs (4 × 105 cells/well) from cattle uninfected or experimentally infected with M. avium subsp. paratuberculosis were labeled with CFSE (Sigma-Aldrich) and incubated with 1 μM meloxicam (Sigma-Aldrich) or dimethyl sulfoxide (DMSO) (Nacalai Tesque, Kyoto, Japan) for 3 days (uninfected cattle) or 5 days (infected cattle). For stimulation of PBMCs from the uninfected cattle, 1 μg/ml of anti-CD3 MAb (MM1A; Washington State University Monoclonal Antibody Center) and 1 μg/ml of anti-CD28 MAb (CC220; Bio-Rad) were added to cultures; 1 μg/ml of J-PPD was added to cultures of PBMCs from the infected cattle. After cultivation, IFN-γ and TNF-α concentrations were measured by ELISA, and cell proliferation was measured by flow cytometry as described above.
Functional analysis of combined COX-2 inhibition and PD-L1 blockade.
To investigate the immunostimulatory effects of combined treatment with COX-2 inhibitor and anti-PD-L1 blocking antibodies, PBMCs (4 × 105 cells/well) from cattle experimentally infected with M. avium subsp. paratuberculosis were labeled with CFSE (Sigma-Aldrich) and incubated with 1 μM meloxicam and 10 μg/ml of anti-PD-L1 antibody in the presence of 1 μg/ml J-PPD for 5 days. DMSO (Nacalai Tesque) and rat IgG (10 μg/ml; Sigma-Aldrich) were used as negative controls. PPD purified from M. bovis BCG strain (B-PPD) (1 μg/ml) was used as a negative-control antigen. In this assay, two types of anti-PD-L1 blocking antibodies, anti-PD-L1 rat MAb (4G12) (32) and anti-PD-L1 rat-bovine chimeric antibody (ChAb) (Boch4G12) (34), were used to evaluate the combination effect. After 5 days of culture, the IFN-γ concentration was determined by ELISA, and cell proliferation was measured by flow cytometry as described above.
Statistical analysis.
Differences were evaluated by the Wilcoxon signed-rank test and the Steel-Dwass test. A P value of <0.05 was considered statistically significant.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Hideyuki Takahashi and Tomio Ibayashi for their valuable advice and discussions. We thank Enago (http://www.enago.jp) for English language review.
Y. Sajiki, S. Konnai, S. Murata, Y. Mori, and K. Ohashi were responsible for the conception and design of the study. Y. Sajiki, S. Konnai, T. Okagawa, A. Nishimori, N. Maekawa, S. Goto, R. Ikebuchi, R. Nagata, S. Kawaji, Y. Kagawa, S. Yamada, and Y. Kato performed the experiments. Y. Sajiki, S. Konnai, T. Okagawa, Y. Suzuki, S. Murata, Y. Mori, and K. Ohashi analyzed the data. R. Nagata, S. Kawaji, S. Yamada, Y. Kato, and Y. Mori provided intellectual input, laboratory materials, reagents, and/or analytic tools. Y. Sajiki wrote the manuscript. S. Konnai, T. Okagawa, Y. Suzuki, S. Murata, Y. Mori, and K. Ohashi contributed to the revision of the manuscript. All authors reviewed and approved the final manuscript.
This work was supported by JSPS KAKENHI, Research Project for Improving Animal Disease Prevention Technologies to Combat Antimicrobial Resistance 2017-2021 FY of the Ministry of Agriculture, Forestry and Fisheries of Japan and by grants from the Science and Technology Research Promotion Program for Agriculture, Forestry, Fisheries, and Food Industry, Japan (number 26058B, to S. Konnai) and the NARO, Bio-oriented Technology Research Advancement Institution (the special scheme project on regional developing strategy; grant 16817557 to S. Konnai). This research was partially supported by the Platform Project for Supporting Drug Discovery and Life Science Research (Basis for Supporting Innovative Drug Discovery and Life Science Research [BINDS]) and a grant from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT), Japan, for the Joint Research Program of the Research Center for Zoonosis Control, Hokkaido University, to Y. Suzuki.
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
S. Konnai, T. Okagawa, A. Nishimori, N. Maekawa, S. Goto, Y. Suzuki, S. Murata, and K. Ohashi have a patent pending for materials and techniques described in this paper (Japanese patent application number 2017-140891).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00910-17.
REFERENCES
- 1.Weller LC, Collington JS, Hartnell A, Conroy MD, Kaise T, Barker EJ, Wilson SM, Taylor WG, Jose JP, Williams JT. 2007. Chemotactic action of prostaglandin E2 on mouse mast cells acting via the PGE2 receptor 3. Proc Natl Acad Sci U S A 104:11712–11717. doi: 10.1073/pnas.0701700104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phipps RP, Stein SH, Roper RL. 1991. A new view of prostaglandin E regulation of the immune response. Immunol Today 12:349–352. doi: 10.1016/0167-5699(91)90064-Z. [DOI] [PubMed] [Google Scholar]
- 3.Morita I. 2002. Distinct functions of COX-1 and COX-2. Prostaglandins Other Lipid Mediat 68-69:165–175. [DOI] [PubMed] [Google Scholar]
- 4.Subbaramaiah K, Telang N, Ramonetti TJ, Araki R, DeVito B, Weksler BB, Dannenberg JA. 1996. Transcription of cyclooxygenase-2 is enhanced in transformed mammary epithelial cells. Cancer Res 56:4424–4429. [PubMed] [Google Scholar]
- 5.Honda A, Sugimoto Y, Namba T, Watabe A, Irie A, Negishi M, Narumiya S, Ichikawa A. 1993. Cloning and expression of cDNA for mouse prostaglandin E receptor EP2 subtype. J Biol Chem 268:7759–7762. [PubMed] [Google Scholar]
- 6.Yao C, Hirata T, Soontrapa K, Ma X, Takemori H, Narumiya S. 2013. Prostaglandin E2 promotes Th1 differentiation via synergistic amplification of IL-12 signaling by cAMP and PI3-kinase. Nat Commun 4:1685. doi: 10.1038/ncomms2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Betz M, Fox SB. 1991. Prostaglandin E2 inhibits production of Th1 lymphokines but not of Th2 lymphokines. J Immunol 146:108–113. [PubMed] [Google Scholar]
- 8.Wang D, DuBois RN. 2013. An inflammatory mediator, prostaglandin E2, in colorectal cancer. Cancer J 19:502–510. doi: 10.1097/PPO.0000000000000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang D, DuBois RN. 2016. The role of prostaglandin E2 in tumor associated immunosuppression. Trends Mol Med 22:1–3. doi: 10.1016/j.molmed.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dumais N, Barbeau B, Olivier M, Tremblay MJ. 1998. Prostaglandin E2 up-regulates HIV-1 long terminal repeat-driven gene activity in T cells via NF-kappaB-dependent and -independent signaling pathways. J Biol Chem 273:27306–27314. doi: 10.1074/jbc.273.42.27306. [DOI] [PubMed] [Google Scholar]
- 11.Dumais N, Bounou S, Olivier M, Tremblay MJ. 2002. Prostaglandin E2-mediated activation of HIV-1 long terminal repeat transcription in human T cells necessitates CCAAT/enhancer binding protein (C/EBP) binding sites in addition to cooperative interactions between C/EBPβ and cyclic adenosine 5′-monophosphate response element binding protein. J Immunol 168:274–282. doi: 10.4049/jimmunol.168.1.274. [DOI] [PubMed] [Google Scholar]
- 12.Waris D, Siddiqui A. 2005. Hepatitis C virus stimulates the expression of cyclooxygenase-2 via oxidative stress: role of prostaglandin E2 in RNA replication. J Virol 79:9725–9734. doi: 10.1128/JVI.79.15.9725-9734.2005. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Stolina M, Sharma S, Lin Y, Dohadwala M, Gardner B, Luo J, Zhu L, Kronenberg M, Miller PW, Portanova J, Lee JC, Dubinett SM. 2000. Specific inhibition of cyclooxygenase 2 restores antitumor reactivity by altering the balance of IL-10 and IL-12 synthesis. J Immunol 164:361–370. doi: 10.4049/jimmunol.164.1.361. [DOI] [PubMed] [Google Scholar]
- 14.Pettersen FO, Torheim EA, Dahm AE, Aaberge IS, Lind A, Holm M, Aandahl EM, Sandset PM, Taskén K, Kvale D. 2011. An exploratory trial of cyclooxygenase type 2 inhibitor in HIV-1 infection: downregulated immune activation and improved T cell-dependent vaccine responses. J Virol 85:6557–6566. doi: 10.1128/JVI.00073-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rathnaiah G, Zinniel DK, Bannantine JP, Stabel JR, Gröhn YT, Collins MT, Barletta RG. 2017. Pathogenesis, molecular genetics, and genomics of Mycobacterium avium subsp. paratuberculosis, the etiologic agent of Johne's disease. Front Vet Sci 4:187. doi: 10.3389/fvets.2017.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li L, Katani R, Schilling M, Kapur V. 2016. Molecular epidemiology of Mycobacterium avium subsp. paratuberculosis on dairy farms. Annu Rev Anim Biosci 4:155–176. doi: 10.1146/annurev-animal-021815-111304. [DOI] [PubMed] [Google Scholar]
- 17.Stabel JR. 2006. Host responses to Mycobacterium avium subsp. paratuberculosis: a complex arsenal. Anim Heal Res Rev 7:61–70. doi: 10.1017/S1466252307001168. [DOI] [PubMed] [Google Scholar]
- 18.Coussens PM. 2004. Model for immune responses to Mycobacterium avium subspecies paratuberculosis in cattle. Infect Immun 72:3089–3096. doi: 10.1128/IAI.72.6.3089-3096.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sohal JS, Singh SV, Tyagi P, Subhodh S, Singh PK, Singh AV, Narayanasamy K, Sheoran N, Singh Sandhu K. 2008. Immunology of mycobacterial infections: with special reference to Mycobacterium avium subspecies paratuberculosis. Immunobiology 231:585–598. doi: 10.1016/j.imbio.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Bassey EO, Collins MT. 1997. Study of T-lymphocyte subsets of healthy and Mycobacterium avium subsp. paratuberculosis-infected cattle. Infect Immun 65:4869–4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burrells C, Clarke CJ, Colston A, Kay JM, Porter J, Little D, Sharp JM. 1999. Interferon-gamma and interleukin-2 release by lymphocytes derived from the blood, mesenteric lymph nodes and intestines of normal sheep and those affected with paratuberculosis (Johne's disease). Vet Immunol Immunopathol 68:139–148. doi: 10.1016/S0165-2427(99)00022-7. [DOI] [PubMed] [Google Scholar]
- 22.Weiss DJ, Evanson OA, Souza CD. 2006. Mucosal immune response in cattle with subclinical Johne's disease. Vet Pathol 43:127–135. doi: 10.1354/vp.43-2-127. [DOI] [PubMed] [Google Scholar]
- 23.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. 1999. Development of lupus like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif carrying immunoreceptor. Immunity 11:141–151. doi: 10.1016/S1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 24.Sierro S, Romero P, Speiser DE. 2011. The CD4-like molecule LAG-3, biology and therapeutic applications. Expert Opin Ther Targets 15:91–101. doi: 10.1517/14712598.2011.540563. [DOI] [PubMed] [Google Scholar]
- 25.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. 2008. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wherry EJ. 2011. T cell exhaustion. Nat Immunol 12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 27.Okagawa T, Konnai S, Nishimori A, Ikebuchi R, Mizorogi S, Nagata R, Kawaji S, Tanaka S, Kagawa Y, Murata S, Mori Y, Ohashi K. 2016. Bovine immunoinhibitory receptors contribute to suppression of Mycobacterium avium subsp. paratuberculosis-specific T-cell responses. Infect Immun 84:77–89. doi: 10.1128/IAI.01014-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prima V, Kaliberova LN, Kaliberov S, Curiel DT, Kusmartsev S. 2016. COX2/mPGES1/PGE2 pathway regulates PD-L1 expression in tumor-associated macrophages and myeloid-derived suppressor cells. Proc Natl Acad Sci U S A 144:1117–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Botti G, Fratangelo F, Cerrone M, Liguori G, Cantile M, Anniciello AM, Scala S, D'Alterio C, Trimarco C, Ianaro A, Cirino G, Caracò C, Colombino M, Palmieri G, Pepe S, Ascierto PA, Sabbatino F, Scognamiglio G. 2017. COX-2 expression positively correlates with PD-L1 expression in human melanoma cells. J Transl Med 15:46. doi: 10.1186/s12967-017-1150-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen JH, Perry CJ, Tsui YC, Staron MM, Parish IA, Dominguez CX, Rosenberg DW, Kaech SM. 2015. Prostaglandin E2 and programmed cell death 1 signaling coordinately impair CTL function and survival during chronic viral infection. Nat Med 21:327–334. doi: 10.1038/nm.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plattner BL, Chiang YW, Roth JA, Platt R, Huffman E, Zylstra J, Hostetter JM. 2011. Direct inoculation of Mycobacterium avium subspecies paratuberculosis into ileocecal Peyer's patches results in colonization of the intestine in a calf model. Vet Pathol 48:584–592. doi: 10.1177/0300985810383874. [DOI] [PubMed] [Google Scholar]
- 32.Ikebuchi R, Konnai S, Okagawa T, Yokoyama K, Nakajima C, Suzuki Y, Murata S, Ohashi K. 2014. Influence of PD-L1 cross-linking on cell death in PD-L1-expressing cell lines and bovine lymphocytes. Immunology 142:551–561. doi: 10.1111/imm.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goto S, Konnai S, Okagawa T, Nishimori A, Maekawa N, Gondaira S, Higuchi H, Koiwa M, Tajima M, Kohara J, Ogasawara S, Kato Y, Suzuki Y, Murata S, Ohashi K. 2017. Increase of cells expressing PD-1 and PD-L1 and enhancement of IFN-γ production via PD-1/PD-L1 blockade in bovine mycoplasmosis. Immun Inflamm Dis 5:355–363. doi: 10.1002/iid3.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishimori A, Konnai S, Okagawa T, Maekawa N, Ikebuchi R, Goto S, Sajiki Y, Suzuki Y, Kohara J, Ogasawara S, Kato Y, Murata S, Ohashi K. 2017. In vitro and in vivo antivirus activity of an anti-programmed death-ligand 1 (PD-L1) rat-bovine chimeric antibody against bovine leukemia virus infection. PLoS One 12:e0174916. doi: 10.1371/journal.pone.0174916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weiss DJ, Evanson OA, de Souza C, Abrahamsen MS. 2005. A critical role of interleukin-10 in the response of bovine macrophages to infection by Mycobacterium avium subsp.paratuberculosis. Am J Vet Res 66:721–726. doi: 10.2460/ajvr.2005.66.721. [DOI] [PubMed] [Google Scholar]
- 36.Hussain T, Shah SZ, Zhao D, Sreevatsan S, Zhou X. 2016. The role of IL-10 in Mycobacterium avium subsp. paratuberculosis infection. Cell Commun Signal 14:29. doi: 10.1186/s12964-016-0152-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edwards CK 3rd, Hedegaard HB, Zlotnik A, Gangadharam PR, Johnston RB Jr, Pabst MJ. 1986. Chronic infection due to Mycobacterium intracellulare in mice: association with macrophage release of prostaglandin E2 and reversal by injection of indomethacin, muramyl dipeptide, or interferon-gamma. J Immunol 136:1820–1827. [PubMed] [Google Scholar]
- 38.Chen M, Divangahi M, Gan H, Shin DS, Hong S, Lee DM, Serhan CN, Behar SM, Remold HG. 2008. Lipid mediators in innate immunity against tuberculosis: opposing roles of PGE2 and LXA4 in the induction of macrophage death. J Exp Med 205:2791–2801. doi: 10.1084/jem.20080767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmedtje JF, Ji Y-S, Liu W-L, DuBois RN, Runge MS. 1997. Hypoxia induces cyclooxygenase-2 via the NF-κB p65 transcription factor in human vascular endothelial cells. J Biol Chem 272:601–608. [DOI] [PubMed] [Google Scholar]
- 40.Jobin C, Morteau O, Han DS, Balfour Sartor R. 1998. Specific NF-kappaB blockade selectively inhibits tumour necrosis factor-alpha-induced COX-2 but not constitutive COX-1 gene expression in HT-29 cells. Immunology 95:537–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vila-del Sol V, Fresno M. 2005. Involvement of TNF and NF-κB in the transcriptional control of cyclooxygenase-2 expression by IFN-γ in macrophages. J Immunol 174:2825–2833. doi: 10.4049/jimmunol.174.5.2825. [DOI] [PubMed] [Google Scholar]
- 42.Abdelhamed S, Ogura K, Yokoyama S, Saiki I, Hayakawa Y. 2016. AKT-STAT3 pathway as a downstream target of EGFR signaling to regulate PD-L1 expression on NSCLC cells. J Cancer 7:1579–1586. doi: 10.7150/jca.14713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maślanka T. 2014. Effect of dexamethasone and meloxicam on counts of selected T lymphocyte subpopulations and NK cells in cattle—in vivo investigations. Res Vet Sci 96:338–346. doi: 10.1016/j.rvsc.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 44.Van Engen NK, Platt R, Roth JA, Stock ML, Engelken T, Vann RC, Wulf LW, Busby WD, Wang C, Kalkwarf EM, Coetzee JF. 2016. Impact of oral meloxicam and long-distance transport on cell-mediated and humoral immune responses in feedlot steers receiving modified live BVDV booster vaccination on arrival. Vet Immunol Immunopathol 175:42–50. doi: 10.1016/j.vetimm.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 45.Okagawa T, Konnai S, Nishimori A, Maekawa N, Ikebuchi R, Goto S, Nakajima C, Kohara J, Ogasawara S, Kato Y, Suzuki Y, Murata S, Ohashi K. 2017. Anti-bovine programmed death-1 rat-bovine chimeric antibody for immunotherapy of bovine leukemia virus infection in cattle. Front Immunol 8:650. doi: 10.3389/fimmu.2017.00650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ikebuchi R, Konnai S, Okagawa T, Yokoyama K, Nakajima C, Suzuki Y, Murata S, Ohashi K. 2013. Blockade of bovine PD-1 increases T cell function and inhibits bovine leukemia virus expression in B cells in vitro. Vet Res 44:59. doi: 10.1186/1297-9716-44-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ikebuchi R, Konnai S, Shirai T, Sunden Y, Murata S, Onuma M, Ohashi K. 2011. Increase of cells expressing PD-L1 in bovine leukemia virus infection and enhancement of anti-viral immune responses in vitro via PD-L1 blockade. Vet Res 42:103. doi: 10.1186/1297-9716-42-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shirai T, Konnai S, Ikebuchi R, Okagawa T, Suzuki S, Sunden Y, Onuma M, Murata S, Ohashi K. 2011. Molecular cloning of bovine lymphocyte activation gene-3 and its expression characteristics in bovine leukemia virus-infected cattle. Vet Immunol Immunopathol 144:462–467. doi: 10.1016/j.vetimm.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 49.Okagawa T, Konnai S, Ikebuchi R, Suzuki S, Shirai T, Sunden Y, Onuma M, Murata S, Ohashi K. 2012. Increased bovine Tim-3 and its ligand expressions during bovine leukemia virus infection. Vet Res 43:45. doi: 10.1186/1297-9716-43-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Okagawa T, Konnai S, Deringer JR, Ueti MW, Scoles GA, Murata S, Ohashi K, Brown WC. 2016. Cooperation of PD-1 and LAG-3 contributes to T-cell exhaustion in Anaplasma marginale-infected cattle. Infect Immun 84:2779–2790. doi: 10.1128/IAI.00278-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suzuki S, Konnai S, Okagawa T, Ikebuchi R, Nishimori A, Kohara J, Mingala CN, Murata S, Ohashi K. 2015. Increased expression of the regulatory T cell-associated marker CTLA-4 in bovine leukemia virus infection. Vet Immunol Immunopathol 163:115–124. doi: 10.1016/j.vetimm.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 52.Nagata R, Kawaji S, Minakawa Y, Wang X, Yanaka T, Mori Y. 2010. A specific induction of interleukin-10 by the Map41 recombinant PPE antigen of Mycobacterium avium subsp. paratuberculosis. Vet Immunol Immunopathol 135:71–78. doi: 10.1016/j.vetimm.2009.11.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.