ABSTRACT
Loss-of-function mutations in the signal transducer and activator of transcription 3 gene (stat3) result in autosomal dominant hyper-IgE syndrome (AD-HIES), a condition in which patients have recurrent debilitating infections, including frequent pneumococcal and staphylococcal pneumonias. stat3 mutations cause defective adaptive TH17 cellular responses, an immune mechanism believed to be critical for clearance of pneumococcal colonization and diminished antibody responses. Here we wished to evaluate the role of stat3 in the clearance of pneumococcal carriage and immunity using mice with a stat3 mutation recapitulating AD-HIES. We show here that naive AD-HIES mice have prolonged nasal carriage of pneumococcus compared to WT mice. Mutant and wild-type mice were then immunized with a pneumococcal whole-cell vaccine (WCV) that provides TH17-mediated protection against pneumococcal colonization and antibody-mediated protection against pneumonia and sepsis. WCV-immunized AD-HIES mice made significantly less pneumococcus-specific interleukin-17A (IL-17A) and antibody than WT mice. The WCV-elicited protection against colonization was abrogated in AD-HIES mice, but immunization with WCV still protected AD-HIES mice against aspiration pneumonia/sepsis. Taken together, our results suggest that impaired clearance of nasopharyngeal carriage due to poor adaptive IL-17A responses may contribute to the increased rates of pneumococcal respiratory infection in AD-HIES patients.
KEYWORDS: autosomal dominant hyper-IgE syndrome, IL-17A, Job's syndrome, Streptococcus pneumoniae, pneumococcal vaccine
INTRODUCTION
While pneumococcal conjugate vaccines (PCVs) have led to a dramatic reduction in the rates of invasive pneumococcal infections, such as meningitis and bacteremia, mucosal infections, such as otitis media, sinusitis, and community-acquired pneumonia, which represent a significant burden of morbidity and even mortality, have been less impacted (1, 2). Additionally, the phenomenon of serotype replacement following PCV introduction and the complexity of the manufacture of expanded-valence PCVs together provide further rationales for the development of serotype-independent pneumococcal vaccines. Critical to these endeavors is the development of a deeper understanding of the mechanisms of immunity to pneumococcal infections.
Through the PCV experience and the development of other investigational pneumococcal vaccines, an understanding of which immune effectors are relevant in preventing the various types of pneumococcal infection is emerging (3). Some clues have been obtained by the characterization of specific immune defects in patients with primary or secondary immune deficiencies that are at increased risk of infection with Streptococcus pneumoniae. The increased rates of invasive pneumococcal disease in patients with agammaglobulinemia or asplenia (4, 5) suggest a protective role of antibody, while similar findings in both pediatric and adult HIV-positive patients with low CD4+ cell counts (6) suggest the importance of cell-mediated immunity. Recently, further characterization of the clinical features of patients with autosomal dominant hyper-IgE syndrome (AD-HIES), also referred to as Job's syndrome, has revealed that, in addition to the known predisposition of these patients to frequent staphylococcal and candidal skin infections, this condition is associated with an increased risk of recurrent pneumococcal pneumonia (7). The genetic defects identified in AD-HIES patients are generally heterozygous dominant negative mutations in the signal transducer and activator of transcription 3 gene (stat3) (8, 9). While STAT3 has been implicated in many signaling and cytokine pathways, the predominant immune defect identified in AD-HIES patients is impaired differentiation of CD4+ TH17 cells (10–12). Impaired antibody durability and responses to infection and immunization have also been demonstrated in AD-HIES patients (13, 14); studies suggest that this may be due to a combination of B-cell-specific and T helper cell dependence on STAT3 activation (15, 16). In addition, the site specificity of the role of TH17 cells and the associated phenotype of recurrent skin and pulmonary infections in AD-HIES patients may be partly explained by the finding that human keratinocytes and bronchial epithelial cells have stronger dependence on TH17-axis cytokines than other cell types (17).
TH17 cell-mediated immunity plays an important role in the defense against many pathogens, particularly those that cause skin and pulmonary infections (18, 19). TH17 cells secrete effector cytokines, such as interleukin-17A (IL-17A), IL-17F, and IL-22, that induce monocyte and neutrophil recruitment and antimicrobial peptide production. The role of TH17 cells in providing protection against pneumococcal infection was derived from the preclinical evaluation of an unencapsulated killed pneumococcal whole-cell vaccine (WCV). In mice, protection against invasive pneumococcal disease can be demonstrated by the passive transfer of serum from animals previously immunized with the WCV. The WCV provides multiserotype protection against colonization in an antibody-independent and IL-17A-dependent manner (20, 21). The critical role of IL-17A in protection against colonization was subsequently confirmed using different immunogens or natural exposure to pneumococci (22–24). More recent work also demonstrates that resident TH17 cells may also contribute to protection against pulmonary infection (25).
To date, however, there has been no direct demonstration of the role of the TH17 cells in the prevention of pneumococcal disease (or, more broadly, other infectious diseases) in humans. The pneumococcal WCV is currently in phase 2 clinical trials in toddlers, with plans to evaluate its impact on pneumococcal carriage in infants in the near future. Genocea Biosciences performed a phase 2 intentional pneumococcal colonization challenge clinical trial of a candidate pneumococcal vaccine that aimed to generate TH17 responses to three proteins identified to be potent inducers of TH17 responses in mice and humans (24, 26, 27). While there was a consistent reduction in the frequency of colonization in vaccine-immunized individuals compared to placebo recipients, the results did not meet statistical significance and no TH17 responses were reported (28).
Pending further results from clinical trials, further examination of the role of TH17 in the prevention of pneumococcal carriage can be achieved through the study of mice that carry the same mutation as AD-HIES patients. To this end, we first compared the kinetics of clearance of pneumococcal carriage in naive wild-type (WT) and AD-HIES mice. We then examined the cellular and humoral immunogenicity of the WCV in AD-HIES mice. Once findings in WCV-immunized AD-HIES mice recapitulated the defective adaptive T-cell and antibody responses described in AD-HIES patients, we evaluated the efficacy of immunization with the WCV in preventing pneumococcal colonization and invasive disease in these mice. Taken together, findings from these studies may provide additional insights to explain the susceptibility of AD-HIES and other immunocompromised patients to pneumococcal respiratory infections.
RESULTS
Generation of transgenic AD-HIES mice.
Transgenic AD-HIES mice were originally generated by transfection of embryonic stem cells with a ROSA26 vector carrying a Cre-inducible (29) R382Q mutation in the stat3 DNA binding domain; this mutation has been reported in a majority of patients with AD-HIES (9). Affected animals constitutively express the R382Q mutant STAT3 in all tissues, and offspring with the heterozygous stat3 genotype (AD-HIES mice) have no obvious physical, growth, or survival differences from WT littermate controls under normal housing and feeding conditions, as described previously (30).
Clearance of carriage is prolonged in naive AD-HIES mice.
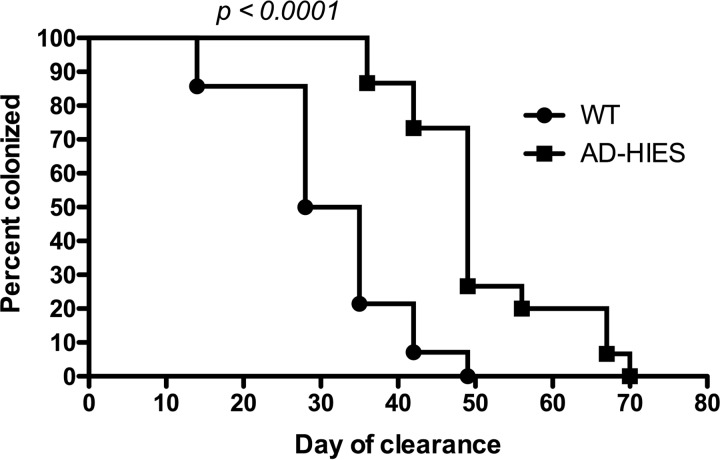
Based on the evidence obtained in mice supporting a role of IL-17A in the clearance of pneumococcal carriage (20, 22, 24), we hypothesized that naive AD-HIES mice would carry pneumococcus longer after a colonization challenge than WT mice. No animals showed signs of illness after challenge, suggesting that the immune defect in these mice did not make them more susceptible to pneumonia or invasive disease following a single colonization event. WT mice cleared carriage after a median of 32 days, whereas AD-HIES mice cleared carriage after a median of 49 days (P < 0.0001) (Fig. 1). While minimal systemic IL-17A is typically measurable following a single colonization challenge, WCA-stimulated whole blood from WT animals that had cleared colonization demonstrated low IL-17A levels, but these levels were nevertheless significantly higher than those measured in AD-HIES mice (median IL-17A concentration, 100 pg/ml versus 25 pg/ml for WT and AD-HIES mice, respectively; P = 0.0052; data not shown).
FIG 1.
Nasal carriage in AD-HIES mice is prolonged compared to that in WT mice. Naive WT (n = 14) and AD-HIES (n = 15) mice were nasally challenged with 2 × 107 CFU of a strain of type 6B pneumococcus in 20 μl given intranasally. The nares of restrained mice were rinsed weekly with 20 μl of PBS 3 times. The rinses were plated for pneumococcal enumeration. The day of clearance was defined as the 1st day of a negative rinse if subsequent rinses were also negative. The data represent combined results from two independent experiments. P values were calculated by the log-rank (Mantel-Cox) test.
Both IL-17A and antibody responses to WCV immunization are impaired in AD-HIES mice.
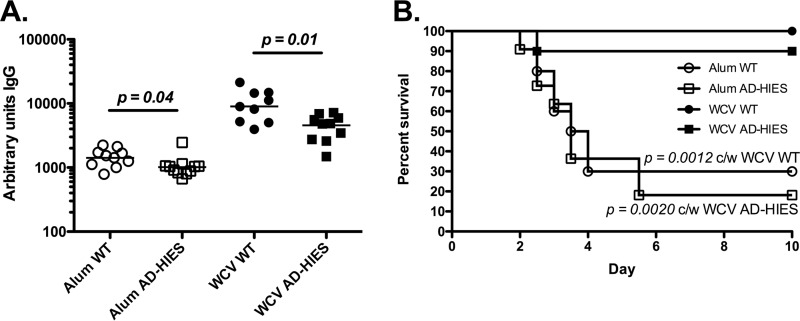
Intact STAT3 signaling plays a critical role in the differentiation of TH17 cells and in elicitation of appropriate antibody responses to immunization (11, 12, 14, 15). Therefore, we hypothesized that the AD-HIES mice would demonstrate attenuated IL-17A and antibody responses following immunization with WCV. Indeed, little to no IL-17A was measured from the supernatant of WCA-stimulated whole blood of AD-HIES mice following two immunizations with WCV; the median IL-17A concentration was 27 pg/ml in whole blood from AD-HIES mice and 956 pg/ml in whole blood from WT animals (P = 0.0001) (Fig. 2A). Immunization with WCV did elicit a pneumococcus-specific IgG response in AD-HIES mice, but this was significantly lower than the IgG response in immunized WT mice (Fig. 2B; median number of units of IgG in AD-HIES and WT mice, 12,530 and 58,600, respectively; P = 0.0005).
FIG 2.
Evaluation of IL-17A and IgG responses and protection against carriage in WCV-immunized AD-HIES mice. WT and AD-HIES mice (10 or 11 animals of each genotype per immunization group) were immunized s.c. at days 0 and 14 with alum alone or WCV-alum. (A) At day 28 (14 days after the last immunization), whole blood was stimulated with WCA (without alum) and the concentration of IL-17A in the supernatants of stimulated peripheral blood was measured by ELISA. (B) Pneumococcus-specific IgG levels were measured in serum. AU, arbitrary units. (C) At day 42 (28 days after the last immunization), mice were colonized with 2 × 107 CFU of type 6B pneumococcus in 20 μl given intranasally. Pneumococci were enumerated from nasal washes 10 days later. The bars show the median values. P values were calculated by the Mann-Whitney test. NS, not significant.
WCV-induced protection against nasopharyngeal colonization is abrogated in AD-HIES mice.
Prior work using WCV-immunized WT and IL-17A receptor knockout mice demonstrated a critical role of IL-17A in the clearance of pneumococcal carriage (20); here we investigated the effect of stat3 mutation on WCV-mediated protection against colonization. WCV-immunized WT mice were protected against colonization challenge, as expected (P = 0.009 when the numbers of CFU in nasal washes from alum- and WCV-immunized WT mice were compared; Fig. 2C). In contrast, there was no significant difference in the nasopharyngeal pneumococcal burden recovered from alum-immunized AD-HIES mice and that recovered from WCV-immunized AD-HIES mice; WCV-immunized WT mice were significantly protected compared to immunized AD-HIES mice (P = 0.009) (Fig. 2C). Therefore, the protection afforded by this vaccine is abrogated in the AD-HIES background with a loss-of-function mutation in stat3.
AD-HIES mice are protected against fatal aspiration pneumonia/sepsis by WCV immunization.
In previous studies, we showed that WCV-mediated protection against fatal pneumonia in WT mice is not abrogated by depletion of CD4+ T cells and can be adoptively transferred by administration to mice of serum from WCV-immunized rabbits (21), suggesting that antibody alone is sufficient for WCV-elicited protection against pneumonia. We examined whether the diminished antibody responses generated by WCV immunization of AD-HIES mice would nevertheless be sufficient to confer protection in a fatal aspiration pneumonia challenge model. To minimize the possibility that multiple doses of WCV could overcome the antibody deficiency seen in AD-HIES mice, we challenged mice in an aspiration pneumonia model following a single immunization with WCV. WCV-immunized WT mice were significantly protected compared to alum-immunized WT mice (100% of WCV-immunized WT mice but only 30% of alum-immunized WT mice survived; P = 0.0012) (Fig. 3B). WT mice made significantly larger amounts of antipneumococcal IgG than AD-HIES mice (median number of units of IgG, 8,927 in WT mice versus 4,827 in AD-HIES mice; P = 0.01) (Fig. 3A). Despite this lower response, WCV-immunized AD-HIES mice were significantly protected against fatal pneumonia compared to their alum-immunized AD-HIES controls (90% of WCV-immunized AD-HIES but only 18% of alum-immunized AD-HIES mice survived; P = 0.002) (Fig. 3B). Therefore, WCV is able to provide protection against sepsis following pneumococcal aspiration in AD-HIES mice.
FIG 3.
A single dose of WCV protects AD-HIES mice against death following aspiration pneumonia/sepsis. (A) WT and AD-HIES mice (10 or 11 animals of each genotype per immunization group) were immunized s.c. at day 0 with alum alone or WCV. At day 14, the pneumococcus-specific IgG level in serum was measured. (B) At day 28, mice were infected with 6 × 106 CFU of type 3 pneumococcus in 100 μl, given while the mice were under anesthesia. Mice were monitored daily and euthanized at the earliest sign of illness by observers blind to the genotype. P values were calculated by the log-rank (Mantel-Cox) test. c/w, compared with.
DISCUSSION
Colonization with pneumococcus is considered the sentinel event in pneumococcal infection (31, 32). The majority of healthy individuals are only transiently colonized and clear the carriage without subsequent manifestations of infection. In patients who progress to pneumococcal infection prior to the clearance of carriage, the majority of their infections are of the respiratory epithelial and mucosal surfaces, with manifestations of sinusitis, otitis media, or lower respiratory tract infection. One study of the clinical disease spectrum in 60 AD-HIES patients found that, over time, about 90% of patients experienced pneumonia, with the leading bacterial causes being equally split between Staphylococcus aureus (31%) and Streptococcus pneumoniae (30%) (7). The finding of prolonged pneumococcal carriage in AD-HIES mice may implicate this as a factor leading to the increased susceptibility of AD-HIES patients to pneumococcal pneumonia. Further evaluation of the rates and durations of pneumococcal carriage in AD-HIES patients would be required to support this hypothesis.
Previous work using a WCV that expresses the OVA peptide to immunize mice that lack mature B and T cells except for OVA-specific CD4+ T cells demonstrated that the WCV-elicited protection against colonization is antigen specific both during induction and during recall of immunity (33). Thus, our findings of low pneumococcus-specific IL-17A responses following WCV immunization in AD-HIES mice corroborate the phenotype of defective stat3-dependent antigen-specific TH17 cell differentiation that has been shown from ex vivo stimulation of T cells from AD-HIES patients with streptococcal, staphylococcal, and candidal antigens (12). Furthermore, the lack of efficacy of the WCV in protecting AD-HIES animals against pneumococcal colonization is consistent with the findings of our previous work demonstrating that WCV-elicited protection against colonization is CD4+ T cell and IL-17A dependent (20, 21). At the same time, despite significantly lower antibody responses to WCV immunization, a single immunization with WCV was sufficient to protect AD-HIES mice against fatal pneumococcal respiratory infection and sepsis, a mechanism the we have previously shown to be antibody dependent (21).
Our findings have important implications in the field of infection prevention in immunocompromised hosts, in particular, those with HIV infection. It is well established that, despite effective antiretroviral therapy (ART), HIV-infected individuals have higher rates of pneumococcal colonization (34), as well as higher rates of pneumococcal pneumonia and invasive disease (35, 36), than HIV-uninfected controls. While pneumococcal conjugate vaccines are safe and immunogenic in HIV-infected individuals, the generated antibody responses vary in functionality with ART and virologic control, tend to be less durable, and have lower efficacy in preventing invasive disease (37). Moreover, nonvaccine and nontypeable pneumococcal serotypes predominate in surveillance studies of pneumococcal carriage in HIV-infected individuals in areas of high PCV coverage (38, 39), suggesting that the efficacy of PCV immunization in HIV-infected individuals may be further limited. Prior work from our group has demonstrated broad reactivity and serotype coverage of the antibody response elicited by WCV immunization in animals (40). Thus, the WCV may represent an important approach to providing serotype-independent protection against pneumococcal disease in immunocompromised patients at increased risk of these infections. In addition to AD-HIES and HIV-infected individuals, patients with Toll-like receptor signaling defects, such as MyD88/IRAK4 deficiency, that are susceptible to invasive pneumococcal disease but that can generate low to normal antibody responses to other protein and polysaccharide antigens (41) may well benefit from the immunity to invasive disease conferred by WCV.
It is likely that multiple host factors, such as the nature of the different stat3 mutations, combine with microbe-specific determinants of pathogenicity and immunity to contribute to the susceptibility of AD-HIES patients to different infections. However, the findings described herein strongly suggest that deficient IL-17A responses in an AD-HIES mouse background make these hosts susceptible to prolonged carriage of pneumococcus, which may explain the increased rate of pneumococcal pneumonia in these patients. Further studies with individuals identified from registries of AD-HIES patients to longitudinally characterize their pneumococcal carriage status and clinical infection histories combined with evaluation of their antigen-specific antibody and cellular immune responses would inform our understanding of which host and pathogen factors are most critical to explain the increased susceptibility in these patients and thus offer novel therapeutic options.
MATERIALS AND METHODS
Mice.
To generate transgenic mice, we targeted a STAT3 cDNA carrying the homologous R382Q mutation previously reported in patients with AD-HIES (9) into a STOP-eGFP-ROSA26TV vector (42) into murine ES cells. Targeted clones were used to generate chimeras, which were bred with C57BL/6 mice for 6 generations. Expression of mutant STAT3 in all tissues was achieved by crossing mice with a germ line targeting stopper STAT3 R382Q with EIIA-Cre mice purchased from The Jackson Laboratory (Bar Harbor, ME). WT and AD-HIES mice were bred in the C57BL/6 background. WT dams (Taconic Biosciences, Hudson, NY) were bred with males carrying a heterozygous mutation in stat3, yielding heterozygous AD-HIES. Littermate WT control mice were used in all experiments. Genotyping of infant mice was performed by PCR screening using primers specific for the mutated stat3 DNA. Animals were identified by toe clipping or ear punching and were caged with same-sex littermates and by immunization group, when indicated, for the length of the experiment. Researchers remained blind to the genotype until the finalization of all experimental data. Similar numbers of male and female animals between 6 and 10 weeks of age were used in immunization or infection studies. At least 10 WT and AD-HIES animals each were used in all experimental groups. All animal studies were approved by the Animal Care and Use Committee of Boston Children's Hospital.
WCV immunization and immunogenicity studies.
The pneumococcal WCV was derived from capsule- and autolysin-negative, pneumolysoid-expressing, pneumococcal strain RM200 as described previously (43). For immunization, the killed pneumococcal whole-cell antigen (WCA) was adsorbed to aluminum (alum as aluminum hydroxide; Allhydrogel; Brenntag). Mice were immunized with 1 to 2 doses of WCV containing 100 μg WCA and 250 μg alum per dose. Immunizations were administered subcutaneously (s.c.) in the flank in 200-μl volumes. When two doses were used, immunizations were administered 2 weeks apart. Two weeks after the last immunization, animals were bled retroorbitally while they were under anesthesia. Whole blood was stimulated with WCA (without alum) for 6 days, and IL-17A levels in supernatants from the stimulations (mouse IL-17A enzyme-linked immunosorbent assay [ELISA]; R&D Systems) were measured as previously described (20). Serum was separated from whole blood, and pneumococcus-specific IgG was measured by ELISA as previously described (21). Similar whole-blood stimulation procedures were followed to evaluate the IL-17A responses in nonimmunized mice following primary colonization and subsequent clearance of carriage.
Colonization of naive mice.
For colonization challenge, strain 0603 (serotype 6B; this strain is resistant to trimethoprim-sulfamethoxazole and has been described elsewhere [44]) was pelleted and washed, and 2 × 107 CFU was delivered intranasally in 20 μl to each animal while it was gently restrained. This strain was isolated from a patient with invasive pneumococcal disease and represents one of the prevalent serotypes causing invasive disease in the pre-PCV era. To evaluate the kinetics of clearance in naive animals, live sampling, which provides semiquantitative assessments of the density of carriage, was performed as follows. With the animal under gentle restraint in a 50-ml conical tube with the bottom of the tube removed (exposing only the animal's nose), 20 μl sterile phosphate-buffered saline (PBS) was dropped onto the nares and recollected from each nostril via aspiration. Simultaneously, the animals were held over a sterile petri dish into which droplets expelled from the nares were collected. This was repeated twice, with each nasal wash sample being combined into an Eppendorf tube containing 50 μl sterile PBS. The petri dish was rinsed with 200 μl sterile PBS, and the recovered fluid was combined into the Eppendorf tube containing the nasal wash samples. Nasal wash samples were plated neat (200 μl) and at 10-fold dilutions (100 μl) onto blood agar plates containing trimethoprim-sulfamethoxazole and gentamicin. Sampling was repeated weekly. These live-sampling methods provide qualitative but not quantitative data about an animal's colonization status; thus, they are used to determine the time to clearance following a colonization challenge. An animal was considered to have cleared colonization on the first day of no growth in the neat and diluted samples if the nasal wash samples remained negative at all subsequent time points.
Colonization and aspiration pneumonia challenge of immunized mice.
For colonization challenge, 14 days after IL-17A and antibody assessment and 28 days after the last immunization, WCV-immunized animals were colonized as described above (in which 2 × 107 CFU of strain 0603 in 20 μl was dropped onto the nares). Here no live sampling was performed; rather, at 10 days after infection, animals were euthanized by CO2 inhalation, and the density of pneumococcal colonization was enumerated from plated tracheal washes as described previously, which provides quantitative assessments of the density of carriage (44). For aspiration pneumonia challenge, at 14 days after antibody assessment and 28 days after the last immunization, immunized animals were infected intranasally while they were under isoflurane anesthesia with 6 × 106 CFU of strain WU2 (serotype 3) (45) in 100 μl. This strain was also originally isolated from a patient with invasive pneumococcal disease and is one of only a few serotypes capable of causing pathogenicity in the fatal aspiration/sepsis model in mice. After infection, the animals were monitored twice daily for signs of illness (including slow movement, ruffled fur, and decreased responsiveness, among other signs) and were euthanized at the earliest sign of illness. As described above, the researchers remained blind to the genotype until finalization of all experimental data.
Statistics.
Statistical analyses were performed using Prism software (v5.0; GraphPad Software, Inc.). When indicated, significance was determined using the Mann-Whitney test or the log-rank (Mantel-Cox) test, with a P value of <0.05 being considered significant. For nasopharyngeal colonization experiments, we included at least 10 mice per immunization group, which, on the basis of our experience with WCV and colonization challenge, achieves an 80% power to detect a 1-log difference in the density of colonization between immunized and control animals with an α value of 0.05. Similarly, for the fatal aspiration/sepsis experiments, 10 mice per immunization group achieved an 80% power to detect a significant difference in survival at 7 days postinfection with an α value of 0.05.
ACKNOWLEDGMENTS
K.M. acknowledges the support provided by NIH grant K08AI09532 and the Charles H. Hood Foundation, Inc., Boston, MA. K.M. and R.M. gratefully acknowledge the support from the Translational Research Program at Boston Children's Hospital. This work was also supported by PATH.
REFERENCES
- 1.Eskola J, Kilpi T, Palmu A, Jokinen J, Haapakoski J, Herva E, Takala A, Kayhty H, Karma P, Kohberger R, Siber G, Makela PH. 2001. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Engl J Med 344:403–409. doi: 10.1056/NEJM200102083440602. [DOI] [PubMed] [Google Scholar]
- 2.Lucero MG, Dulalia VE, Nillos LT, Williams G, Parreno RA, Nohynek H, Riley ID, Makela H. 2009. Pneumococcal conjugate vaccines for preventing vaccine-type invasive pneumococcal disease and X-ray defined pneumonia in children less than two years of age. Cochrane Database Syst Rev 2009:CD004977. doi: 10.1002/14651858.CD004977.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moffitt K, Malley R. 2016. Rationale and prospects for novel pneumococcal vaccines. Hum Vaccin Immunother 12:383–392. doi: 10.1080/21645515.2015.1087625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Austrian R. 1984. Pneumococcal infections, p 257–288. In Germanier R. (ed), Bacterial vaccines. Academic Press, Orlando, FL. [Google Scholar]
- 5.Gaschignard J, Levy C, Chrabieh M, Boisson B, Bost-Bru C, Dauger S, Dubos F, Durand P, Gaudelus J, Gendrel D, Gras Le Guen C, Grimprel E, Guyon G, Jeudy C, Jeziorski E, Leclerc F, Leger PL, Lesage F, Lorrot M, Pellier I, Pinquier D, de Pontual L, Sachs P, Thomas C, Tissieres P, Valla FV, Desprez P, Fremeaux-Bacchi V, Varon E, Bossuyt X, Cohen R, Abel L, Casanova JL, Puel A, Picard C. 2014. Invasive pneumococcal disease in children can reveal a primary immunodeficiency. Clin Infect Dis 59:244–251. doi: 10.1093/cid/ciu274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dworkin MS, Ward JW, Hanson DL, Jones JL, Kaplan JE. 2001. Pneumococcal disease among human immunodeficiency virus-infected persons: incidence, risk factors, and impact of vaccination. Clin Infect Dis 32:794–800. doi: 10.1086/319218. [DOI] [PubMed] [Google Scholar]
- 7.Chandesris MO, Melki I, Natividad A, Puel A, Fieschi C, Yun L, Thumerelle C, Oksenhendler E, Boutboul D, Thomas C, Hoarau C, Lebranchu Y, Stephan JL, Cazorla C, Aladjidi N, Micheau M, Tron F, Baruchel A, Barlogis V, Palenzuela G, Mathey C, Dominique S, Body G, Munzer M, Fouyssac F, Jaussaud R, Bader-Meunier B, Mahlaoui N, Blanche S, Debre M, Le Bourgeois M, Gandemer V, Lambert N, Grandin V, Ndaga S, Jacques C, Harre C, Forveille M, Alyanakian MA, Durandy A, Bodemer C, Suarez F, Hermine O, Lortholary O, Casanova JL, Fischer A, Picard C. 2012. Autosomal dominant STAT3 deficiency and hyper-IgE syndrome: molecular, cellular, and clinical features from a French national survey. Medicine 91:e1–e19. doi: 10.1097/MD.0b013e31825f95b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holland SM, DeLeo FR, Elloumi HZ, Hsu AP, Uzel G, Brodsky N, Freeman AF, Demidowich A, Davis J, Turner ML, Anderson VL, Darnell DN, Welch PA, Kuhns DB, Frucht DM, Malech HL, Gallin JI, Kobayashi SD, Whitney AR, Voyich JM, Musser JM, Woellner C, Schaffer AA, Puck JM, Grimbacher B. 2007. STAT3 mutations in the hyper-IgE syndrome. N Engl J Med 357:1608–1619. doi: 10.1056/NEJMoa073687. [DOI] [PubMed] [Google Scholar]
- 9.Minegishi Y, Saito M, Tsuchiya S, Tsuge I, Takada H, Hara T, Kawamura N, Ariga T, Pasic S, Stojkovic O, Metin A, Karasuyama H. 2007. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature 448:1058–1062. doi: 10.1038/nature06096. [DOI] [PubMed] [Google Scholar]
- 10.de Beaucoudrey L, Puel A, Filipe-Santos O, Cobat A, Ghandil P, Chrabieh M, Feinberg J, von Bernuth H, Samarina A, Janniere L, Fieschi C, Stephan JL, Boileau C, Lyonnet S, Jondeau G, Cormier-Daire V, Le Merrer M, Hoarau C, Lebranchu Y, Lortholary O, Chandesris MO, Tron F, Gambineri E, Bianchi L, Rodriguez-Gallego C, Zitnik SE, Vasconcelos J, Guedes M, Vitor AB, Marodi L, Chapel H, Reid B, Roifman C, Nadal D, Reichenbach J, Caragol I, Garty BZ, Dogu F, Camcioglu Y, Gulle S, Sanal O, Fischer A, Abel L, Stockinger B, Picard C, Casanova JL. 2008. Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J Exp Med 205:1543–1550. doi: 10.1084/jem.20080321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma CS, Chew GY, Simpson N, Priyadarshi A, Wong M, Grimbacher B, Fulcher DA, Tangye SG, Cook MC. 2008. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J Exp Med 205:1551–1557. doi: 10.1084/jem.20080218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, Kanno Y, Spalding C, Elloumi HZ, Paulson ML, Davis J, Hsu A, Asher AI, O'Shea J, Holland SM, Paul WE, Douek DC. 2008. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature 452:773–776. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leung DY, Ambrosino DM, Arbeit RD, Newton JL, Geha RS. 1988. Impaired antibody responses in the hyperimmunoglobulin E syndrome. J Allergy Clin Immunol 81:1082–1087. doi: 10.1016/0091-6749(88)90873-1. [DOI] [PubMed] [Google Scholar]
- 14.Speckmann C, Enders A, Woellner C, Thiel D, Rensing-Ehl A, Schlesier M, Rohr J, Jakob T, Oswald E, Kopp MV, Sanal O, Litzman J, Plebani A, Pietrogrande MC, Franco JL, Espanol T, Grimbacher B, Ehl S. 2008. Reduced memory B cells in patients with hyper IgE syndrome. Clin Immunol 129:448–454. doi: 10.1016/j.clim.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Avery DT, Deenick EK, Ma CS, Suryani S, Simpson N, Chew GY, Chan TD, Palendira U, Bustamante J, Boisson-Dupuis S, Choo S, Bleasel KE, Peake J, King C, French MA, Engelhard D, Al-Hajjar S, Al-Muhsen S, Magdorf K, Roesler J, Arkwright PD, Hissaria P, Riminton DS, Wong M, Brink R, Fulcher DA, Casanova JL, Cook MC, Tangye SG. 2010. B cell-intrinsic signaling through IL-21 receptor and STAT3 is required for establishing long-lived antibody responses in humans. J Exp Med 207:155–171. doi: 10.1084/jem.20091706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deenick EK, Avery DT, Chan A, Berglund LJ, Ives ML, Moens L, Stoddard JL, Bustamante J, Boisson-Dupuis S, Tsumura M, Kobayashi M, Arkwright PD, Averbuch D, Engelhard D, Roesler J, Peake J, Wong M, Adelstein S, Choo S, Smart JM, French MA, Fulcher DA, Cook MC, Picard C, Durandy A, Klein C, Holland SM, Uzel G, Casanova JL, Ma CS, Tangye SG. 2013. Naive and memory human B cells have distinct requirements for STAT3 activation to differentiate into antibody-secreting plasma cells. J Exp Med 210:2739–2753. doi: 10.1084/jem.20130323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Minegishi Y, Saito M, Nagasawa M, Takada H, Hara T, Tsuchiya S, Agematsu K, Yamada M, Kawamura N, Ariga T, Tsuge I, Karasuyama H. 2009. Molecular explanation for the contradiction between systemic Th17 defect and localized bacterial infection in hyper-IgE syndrome. J Exp Med 206:1291–1301. doi: 10.1084/jem.20082767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khader SA, Gaffen SL, Kolls JK. 2009. Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa. Mucosal Immunol 2:403–411. doi: 10.1038/mi.2009.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolls JK, Khader SA. 2010. The role of Th17 cytokines in primary mucosal immunity. Cytokine Growth Factor Rev 21:443–448. doi: 10.1016/j.cytogfr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu YJ, Gross J, Bogaert D, Finn A, Bagrade L, Zhang Q, Kolls JK, Srivastava A, Lundgren A, Forte S, Thompson CM, Harney KF, Anderson PW, Lipsitch M, Malley R. 2008. Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS Pathog 4:e1000159. doi: 10.1371/journal.ppat.1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu YJ, Leite L, Goncalves VM, Dias WDO, Liberman C, Fratelli F, Alderson M, Tate A, Maisonneuve JF, Robertson G, Graca R, Sayeed S, Thompson CM, Anderson P, Malley R. 2010. GMP-grade pneumococcal whole-cell vaccine injected subcutaneously protects mice from nasopharyngeal colonization and fatal aspiration-sepsis. Vaccine 28:7468–7475. doi: 10.1016/j.vaccine.2010.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Z, Clarke TB, Weiser JN. 2009. Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. J Clin Invest 119:1899–1909. doi: 10.1172/JCI36731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malley R, Srivastava A, Lipsitch M, Thompson CM, Watkins C, Tzianabos A, Anderson PW. 2006. Antibody-independent, interleukin-17A-mediated, cross-serotype immunity to pneumococci in mice immunized intranasally with the cell wall polysaccharide. Infect Immun 74:2187–2195. doi: 10.1128/IAI.74.4.2187-2195.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moffitt KL, Gierahn TM, Lu YJ, Gouveia P, Alderson M, Flechtner JB, Higgins DE, Malley R. 2011. T(H)17-based vaccine design for prevention of Streptococcus pneumoniae colonization. Cell Host Microbe 9:158–165. doi: 10.1016/j.chom.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith NM, Wasserman GA, Coleman FT, Hilliard KL, Yamamoto K, Lipsitz E, Malley R, Dooms H, Jones MR, Quinton LJ, Mizgerd JP. 2017. Regionally compartmentalized resident memory T cells mediate naturally acquired protection against pneumococcal pneumonia. Mucosal Immunol 11:220–235. doi: 10.1038/mi.2017.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, Gierahn T, Thompson CM, Trzcinski K, Ford CB, Croucher N, Gouveia P, Flechtner JB, Malley R, Lipsitch M. 2012. Distinct effects on diversifying selection by two mechanisms of immunity against Streptococcus pneumoniae. PLoS Pathog 8:e1002989. doi: 10.1371/journal.ppat.1002989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skoberne M, Morris MMA, Hopson K, Chan J, O'Toole M, Velez D, Siddall N, Hart M, Cooney M, Flechtner JB, Hetherington S. 2014. Safety and immunogenicity of a novel lipidated protein subunit Streptococcus pneumoniae vaccine. Abstr 54th Intersci Conf Antimicrob Agents Chemother, Washington, DC. [Google Scholar]
- 28.Skoberne M, Gritzfeld FDJF, Wright A, Collins A, Siddall N, Larson S, Yu D, Le B, Mitsi E, Reine J, German EL, Flechtner JB, Oliphant TH, Morris A, Hetherington S, Fitzgerald R, Gordon SB. 2016. GEN-004 vaccine is safe, immunogenic, and reduces acquisition of colonization in experimental human pneumococcal challenge model. Abstr Int Symp Pneumococcus Pneumococcal Dis, Glasgow, Scotland. [Google Scholar]
- 29.Lakso M, Pichel JG, Gorman JR, Sauer B, Okamoto Y, Lee E, Alt FW, Westphal H. 1996. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc Natl Acad Sci U S A 93:5860–5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rucci F, Volpi S, Moffitt K, Ching K, Blasi S, Divij M, Fujiwara Y, Malley R, Notarangelo L, Manis J. 2014. Altered B cell responses are key to the hyper IgE phenotype in Job's syndrome. Abstr 16th Biennial Meet Eur Soc Immunodeficiencies, Prague, Czech Republic. [Google Scholar]
- 31.Simell B, Auranen K, Kayhty H, Goldblatt D, Dagan R, O'Brien KL, Pneumococcal Carriage Group. 2012. The fundamental link between pneumococcal carriage and disease. Expert Rev Vaccines 11:841–855. doi: 10.1586/erv.12.53. [DOI] [PubMed] [Google Scholar]
- 32.Gray BM, Converse GM III, Dillon HC Jr. 1980. Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J Infect Dis 142:923–933. doi: 10.1093/infdis/142.6.923. [DOI] [PubMed] [Google Scholar]
- 33.Trzcinski K, Thompson CM, Srivastava A, Basset A, Malley R, Lipsitch M. 2008. Protection against nasopharyngeal colonization by Streptococcus pneumoniae is mediated by antigen-specific CD4+ T cells. Infect Immun 76:2678–2684. doi: 10.1128/IAI.00141-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heinsbroek E, Tafatatha T, Phiri A, Ngwira B, Crampin AC, Read JM, French N. 2015. Persisting high prevalence of pneumococcal carriage among HIV-infected adults receiving antiretroviral therapy in Malawi: a cohort study. AIDS 29:1837–1844. doi: 10.1097/QAD.0000000000000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Madeddu G, Fiori ML, Mura MS. 2010. Bacterial community-acquired pneumonia in HIV-infected patients. Curr Opin Pulm Med 16:201–207. doi: 10.1097/MCP.0b013e3283375825. [DOI] [PubMed] [Google Scholar]
- 36.Jones N, Huebner R, Khoosal M, Crewe-Brown H, Klugman K. 1998. The impact of HIV on Streptococcus pneumoniae bacteraemia in a South African population. AIDS 12:2177–2184. doi: 10.1097/00002030-199816000-00013. [DOI] [PubMed] [Google Scholar]
- 37.Nunes MC, Madhi SA. 2012. Safety, immunogenicity and efficacy of pneumococcal conjugate vaccine in HIV-infected individuals. Hum Vaccin Immunother 8:161–173. doi: 10.4161/hv.18432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feola TD, Bonville CA, Cibula DA, Jose S, Nattanmai G, Domachowske JB, Suryadevara M. 2016. Nasopharyngeal pneumococcal carriage rates among HIV-infected adults following widespread pediatric use of conjugate pneumococcal vaccine-13. Hum Vaccin Immunother 12:2441–2446. doi: 10.1080/21645515.2016.1172758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Donkor ES, Annan JA, Badoe EV, Dayie NT, Labi AK, Slotved HC. 2017. Pneumococcal carriage among HIV infected children in Accra, Ghana. BMC Infect Dis 17:133. doi: 10.1186/s12879-017-2224-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moffitt KL, Yadav P, Weinberger DM, Anderson PW, Malley R. 2012. Broad antibody and T cell reactivity induced by a pneumococcal whole-cell vaccine. Vaccine 30:4316–4322. doi: 10.1016/j.vaccine.2012.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Picard C, von Bernuth H, Ghandil P, Chrabieh M, Levy O, Arkwright PD, McDonald D, Geha RS, Takada H, Krause JC, Creech CB, Ku CL, Ehl S, Marodi L, Al-Muhsen S, Al-Hajjar S, Al-Ghonaium A, Day-Good NK, Holland SM, Gallin JI, Chapel H, Speert DP, Rodriguez-Gallego C, Colino E, Garty BZ, Roifman C, Hara T, Yoshikawa H, Nonoyama S, Domachowske J, Issekutz AC, Tang M, Smart J, Zitnik SE, Hoarau C, Kumararatne DS, Thrasher AJ, Davies EG, Bethune C, Sirvent N, de Ricaud D, Camcioglu Y, Vasconcelos J, Guedes M, Vitor AB, Rodrigo C, Almazan F, Mendez M, Arostegui JI, Alsina L, et al. . 2010. Clinical features and outcome of patients with IRAK-4 and MyD88 deficiency. Medicine 89:403–425. doi: 10.1097/MD.0b013e3181fd8ec3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sasaki Y, Derudder E, Hobeika E, Pelanda R, Reth M, Rajewsky K, Schmidt-Supprian M. 2006. Canonical NF-kappaB activity, dispensable for B cell development, replaces BAFF-receptor signals and promotes B cell proliferation upon activation. Immunity 24:729–739. doi: 10.1016/j.immuni.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 43.Lu YJ, Yadav P, Clements JD, Forte S, Srivastava A, Thompson CM, Seid R, Look J, Alderson M, Tate A, Maisonneuve JF, Robertson G, Anderson PW, Malley R. 2010. Options for inactivation, adjuvant, and route of topical administration of a killed, unencapsulated pneumococcal whole-cell vaccine. Clin Vaccine Immunol 17:1005–1012. doi: 10.1128/CVI.00036-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malley R, Lipsitch M, Stack A, Saladino R, Fleisher G, Pelton S, Thompson C, Briles D, Anderson P. 2001. Intranasal immunization with killed unencapsulated whole cells prevents colonization and invasive disease by capsulated pneumococci. Infect Immun 69:4870–4873. doi: 10.1128/IAI.69.8.4870-4873.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Briles DE, Nahm M, Schroer K, Davie J, Baker P, Kearney J, Barletta R. 1981. Antiphosphocholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 streptococcus pneumoniae. J Exp Med 153:694–705. doi: 10.1084/jem.153.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]