ABSTRACT
The new 2-aminomethylphenol, JPC-3210, has potent in vitro antimalarial activity against multidrug-resistant Plasmodium falciparum lines, low cytotoxicity, and high in vivo efficacy against murine malaria. Here we report on the pharmacokinetics of JPC-3210 in mice and monkeys and the results of in vitro screening assays, including the inhibition of cytochrome P450 (CYP450) isozymes. In mice, JPC-3210 was rapidly absorbed and had an extensive tissue distribution, with a brain tissue-to-plasma concentration ratio of about 5.4. JPC-3210 had a lengthy plasma elimination half-life of about 4.5 days in mice and 11.8 days in monkeys. JPC-3210 exhibited linear single-oral-dose pharmacokinetics across the dose range of 5 to 40 mg/kg of body weight with high oral bioavailability (∼86%) in mice. Systemic blood exposure of JPC-3210 was 16.6% higher in P. berghei-infected mice than in healthy mice. In vitro studies with mice and human hepatocytes revealed little metabolism and the high metabolic stability of JPC-3210. The abundance of human metabolites from oxidation and glucuronidation was 2.0% and 2.5%, respectively. CYP450 studies in human liver microsomes showed JPC-3210 to be an inhibitor of CYP2D6 and, to a lesser extent, CYP3A4 isozymes, suggesting the possibility of a metabolic drug-drug interaction with drugs that are metabolized by these isozymes. In vitro studies showed that JPC-3210 is highly protein bound to human plasma (97%). These desirable pharmacological findings of a lengthy blood elimination half-life, high oral bioavailability, and low metabolism as well as high in vivo potency have led the Medicines for Malaria Venture to select JPC-3210 (MMV892646) for further advanced preclinical development.
KEYWORDS: 2-aminomethylphenol, antimalarial drug discovery, pharmacokinetics, protein binding, metabolic stability and metabolism, cytochrome P450 inhibition
INTRODUCTION
Although antimalarial drugs continue to reduce malaria-related morbidity and mortality, there were still 212 million malaria cases and 429,000 deaths from malaria in 2015 (1, 2). Currently, the most effective antimalarials are artemisinin-based combination therapies (ACTs), such as artesunate-mefloquine and dihydroartemisinin-piperaquine. The ACTs are recommended for first-line treatment of uncomplicated malaria, with the artemisinin derivative rapidly reducing the parasite biomass and the slower-acting partner drug preventing recrudescence (3). However, the development and spread of resistance (2) to both partner drugs in ACTs in Southeast Asia (4–6), particularly in the Greater Mekong subregions, are of immense concern, with health authorities trying to instigate ACT resistance containment and elimination measures (7). For example, in western Cambodia (2012 to 2014) and southern Vietnam (2014 and 2015), dihydroartemisinin-piperaquine failure rates were reported to be as high as 54% (8) and 26% (9), respectively. Along the Thailand-Myanmar border, the efficacy of artesunate-mefloquine also continues to decline, from 100% in 2003 to 81% in 2013, and those patients with both Plasmodium falciparum K13 (PfK13) propeller mutations (a molecular marker for artemisinin resistance) and multiple copies of P. falciparum mdr1 (a molecular marker for mefloquine resistance) are 14 times more likely to fail treatment (10).
The demise of ACTs with resistance to both partner drugs highlights the need to develop new antimalarial drugs. In addition to developing fast-acting nonartemisinin derivatives, newer longer-acting partner drugs are urgently required. We recently reported on the preclinical development of a 2-aminomethylphenol antimalarial compound, JPC-2997 (11), that resulted from comprehensive structure-activity relationship (SAR) studies of this class of molecule. These SAR studies have subsequently identified a superior trifluoromethyl pyridine analog of 2-aminomethylphenol, JPC-3210 (12), with improved in vitro and in vivo activity and a longer elimination half-life in mice (13). Thus, JPC-3210 has replaced JPC-2997 as the lead compound and is currently in preclinical development by the Medicines for Malaria Venture (MMV) and given the number MMV892646.
To aid the preclinical development of JPC-3210, we have assessed the pharmacokinetics (PK) of JPC-3210 in healthy mice, infected mice, and healthy monkeys. Moreover, we also determined the oral bioavailability, red blood cell partitioning, and plasma protein binding of JPC-3210 and its concentrations in whole blood and mouse brain tissue. Finally, we determined the in vitro metabolism and the potential influence of JPC-3210 on the activity of cytochrome P450 (CYP450) isoforms.
RESULTS AND DISCUSSION
Pharmacokinetics of JPC-3210 in healthy and infected mice.
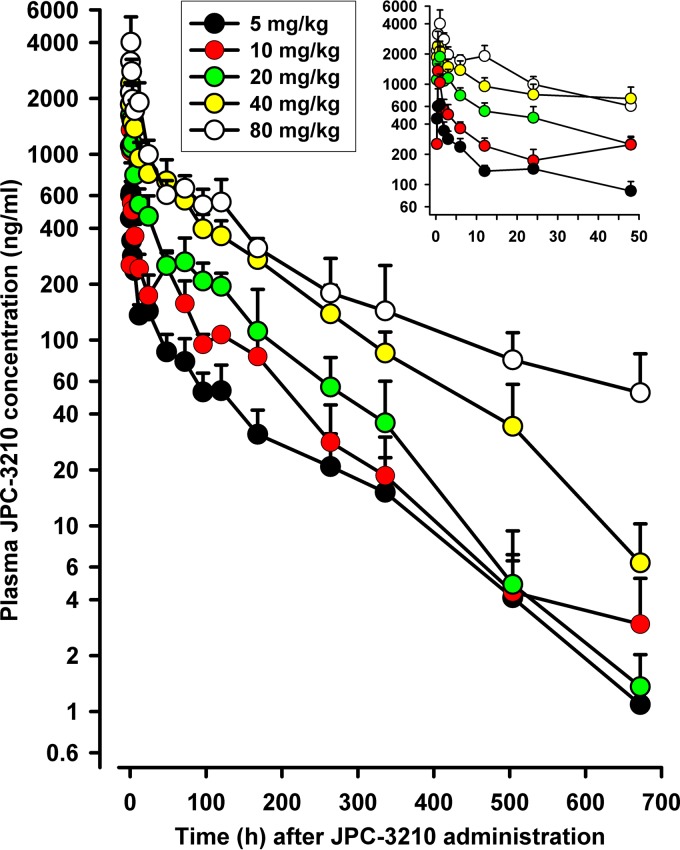
The mean plasma concentration-versus-time profiles of JPC-3210 in mice administered single escalating oral doses of 5 to 80 mg/kg of body weight of JPC-3210 are shown in Fig. 1. JPC-3210 was rapidly absorbed, with the maximum concentration (Cmax) occurring within 0.5 to 1 h after dosing, and thereafter, plasma concentrations declined in a biphasic fashion, with a terminal elimination half-life (t1/2) of about 108 h across the five doses evaluated. Although the Cmax of JPC-3210 increased less than dose proportionally, linear PK were seen across the range of 5 to 40 mg/kg, with the values of the area under the concentration-time curve (AUC) and apparent oral clearance (CL/F) being proportional to the dose (Table 1). For example, following 40 mg/kg of JPC-3210, the plasma Cmax was 2,397 ng/ml, the time to Cmax (Tmax) was 0.5 h, and t1/2 was 109 h. The blood concentration-versus-time curves of JPC-3210 paralleled the plasma profiles, with the blood-to-plasma AUC ratios being 0.63 for 5 mg/kg, 0.66 for 10 mg/kg, 0.62 for 20 mg/kg, 0.67 for 40 mg/kg, and 1.12 for 80 mg/kg, suggesting that JPC-3210 does not associate with red blood cells (data not shown). This is contrary to the findings for other quinoline antimalarials, such as chloroquine (14), desethylamodiaquine (15), piperaquine (16), and pyronaridine (17), which concentrate in red blood cells.
FIG 1.
Mean ± SD plasma concentration-versus-time profiles of JPC-3210 in mice administered a single oral dose of 5, 10, 20, 40, or 80 mg/kg of JPC-3210. (Inset) Mean ± SD plasma concentration-time profiles of JPC-3210 over the first 48 h after dosing with 5, 10, 20, 40, or 80 mg/kg of JPC-3210. Each data point represents the mean JPC-3210 concentration from 5 mice.
TABLE 1.
Pharmacokinetic properties of JPC-3210 in healthy mice administered a single oral dose of JPC-3210 of between 5 mg/kg and 80 mg/kga
| Dose (mg/kg) | Cmax (ng/ml) | Tmax (h) | t1/2 (h) | AUC (μg · h/liter) | V/F (liters/kg) | CL/F (ml/h/kg) |
|---|---|---|---|---|---|---|
| 5 | 622 | 1 | 112 | 20,691 | 39 | 242 |
| 10 | 1,357 | 0.5 | 90 | 37,893 | 34 | 264 |
| 20 | 2,117 | 1 | 94 | 69,134 | 39 | 289 |
| 40 | 2,397 | 0.5 | 109 | 138,509 | 45 | 289 |
| 80 | 4,015 | 1 | 137 | 199,951 | 79 | 400 |
Data were derived from plasma concentration-versus-time profiles.
JPC-3210 is extensively distributed to tissues with a mean apparent volume of distribution (V/F) of 40 ± 5 liters/kg and has a low CL/F of 271 ± 23 ml/h/kg across the dose range of 5 to 40 mg/kg. The lengthy t1/2 and high V/F of JPC-3210 are in accord with the compound being lipophilic, with a relatively high calculated partition coefficient between n-octanol and water (clogP) of 5.63 (unpublished data). The administered doses of JPC-3210 were well tolerated in mice, with no adverse events being observed.
A comparison of the PK of JPC-3210 in healthy and P. berghei-infected mice after a single oral dose of 80 mg/kg of JPC-3210 is presented in Table 2. In the present study, the mean ± standard deviation (SD) parasitemia was 6.9% ± 2.2% before treatment. As previously reported, JPC-3210 is not a rapidly acting antimalarial drug (13). By day 4 posttreatment, 4 of 5 mice were still parasitemic, but by day 5, all mice were blood film negative after the single oral dose of 80 mg/kg of JPC-3210, with no recurrence of malaria over a 28-day follow-up period.
TABLE 2.
Pharmacokinetic properties of JPC-3210 in healthy and Plasmodium berghei (ANKA strain)-infected mice following a single oral dose of 80 mg/kg of JPC-3210
| Compartment and animal status | Cmax (ng/ml) | Tmax (h) | t1/2 (h) | AUC (μg · h/liter) | V/F (liters/kg) | CL/F (ml/h/kg) |
|---|---|---|---|---|---|---|
| Plasma | ||||||
| Healthy | 4,015 | 1 | 137 | 199,951 | 79 | 400 |
| Infected | 2,652 | 1 | 125 | 219,018 | 66 | 365 |
| Blood | ||||||
| Healthy | 2,929 | 1 | 152 | 224,244 | 78 | 357 |
| Infected | 3,407 | 0.5 | 106 | 268,886 | 46 | 298 |
The blood Cmax and AUC of JPC-3210 were 1.16-fold and 1.20-fold higher, respectively, in mice infected with P. berghei than in healthy mice. This reflects the fact that the rate of absorption (Cmax) and the extent of exposure (AUC) of JPC-3210 were higher in infected mice than in healthy mice. Although it is quite possible that parasitized red blood cells accumulate JPC-3210, a contraction in the volume of distribution (i.e., a reduction in the tissue/organ concentrations of JPC-3210) could also contribute to the increase in the blood concentrations of JPC-3210 due to infection (V/F, 46 liters/kg versus 78 liters/kg). The Tmax of JPC-3210, about 1 h, was comparable between healthy and infected mice. The t1/2 of JPC-3210 in blood was estimated to be 106 h in healthy mice and 152 h in infected mice. The corresponding t1/2 values in plasma were 137 h and 125 h.
High oral bioavailability of JPC-3210.
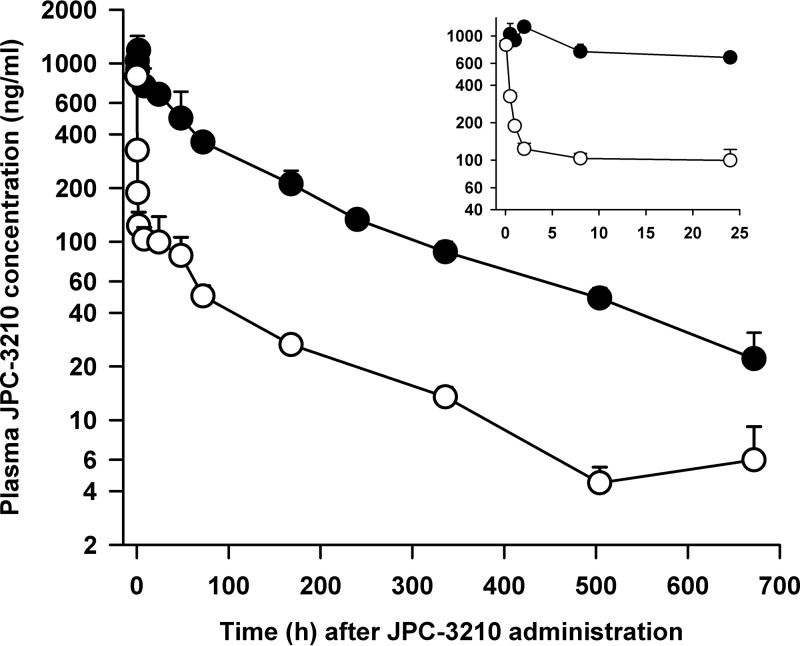
The plasma concentration-versus-time profiles of JPC-3210 following oral (16 mg/kg) or intravenous (2 mg/kg) administration of JPC-3210 to healthy mice are shown in Fig. 2, and the PK data are summarized in the supplemental material. After intravenous administration, the plasma concentrations of JPC-3210 declined in a monophasic fashion, with the mean values of the PK parameters being as follows: Cmax, 847 ng/ml; Tmax, 0.083 h, and t1/2,139 h. The corresponding PK values after oral dosing were 1,180 ng/ml, 2 h, and 169 h. The area under the concentration-time curve from 0 h to the last data point (AUC0→last) after oral dosing was 111,000 μg · h/liter, whereas AUC0→last after intravenous dosing was 16,100 μg · h/liter. The oral bioavailability of JPC-3210 was high at 86.2% (AUC0→last, 13,875 μg · h/liters/16,100 μg · h/liters).
FIG 2.
Mean ± SD plasma concentration-versus-time profiles of JPC-3210 in mice administered a single oral dose (16 mg/kg; closed circles) and an intravenous dose (2 mg/kg; open circles) of JPC-3210. (Inset) Mean ± SD plasma concentration-time profiles of JPC-3210 over the first 24 h after dosing with a single oral dose (16 mg/kg; closed circles) and an intravenous dose (2 mg/kg; open circles) of JPC-3210. Each data point represents the mean JPC-3210 concentration from 3 mice.
JPC-3210 distributes to mouse brain tissue.
The extent of drug partitioning into brain tissue may affect the efficacy and/or toxicity of drugs, including antimalarials. In the present study, JPC-3210 was found to concentrate in the brain, with a mean ± SD brain tissue-to-plasma ratio (Kp) of 4.6 ± 1.4 at 2 h, 5.0 ± 0.7 at 24 h, and 6.7 ± 1.3 at 72 h after oral administration of JPC-3210 in mice, which are in accord with its relatively high apparent volume of distribution and lipophilic properties. In comparison to JPC-3210, mefloquine, a drug that can cause neurological disturbances in some individuals due to its highly lipophilic properties and extensive distribution to tissues, has a Kp of 3.9 at 24 h after intravenous administration of the drug in mice (18). However, Kp is based on a crude homogenization of brain tissue and as such ignores the compartmentalization of the brain. A high Kp may favor nonspecific binding to brain lipids rather than unbound brain concentrations at the requisite site of action (19). Further in vitro, in vivo, and in silico studies are required to assess JPC-3210's potential neurotoxicity and permeability across the blood-brain barrier, including distinguishing the total JPC-3210 concentration from the unbound brain concentration.
Pharmacokinetics of JPC-3210 in monkeys.
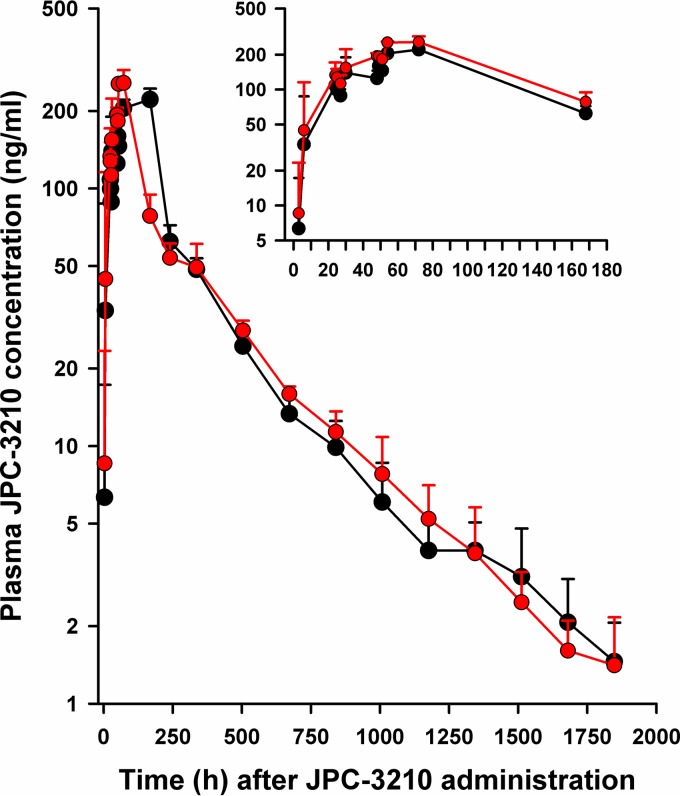
JPC-3210 was well tolerated in cynomolgus macaque monkeys after administration of 10 mg/kg given once daily for 3 days, with no serious adverse events being observed (see the supplemental material). Hematology and biochemical indices were comparable before and after dosing (see the supplemental material). The mean blood and plasma concentration-versus-time profiles of JPC-3210 in the monkeys are shown in Fig. 3. The enterically coated, extended-release tablets of JPC-3210 were designed to prevent the dissolution or disintegration of the dosage form in the stomach of the monkeys. The slow release of JPC-3210 is expected to reduce the peak blood concentrations of the compound, which should assist in minimizing toxicity compared with that of noncoated tablets of JPC-3210.
FIG 3.
Mean ± SD blood and plasma concentration-versus-time profiles of JPC-3210 in cynomolgus macaque monkeys administered 10 mg/kg of JPC-3210 daily for 3 days (red circles, blood; black circles, plasma). (Inset) Mean ± SD blood and plasma concentration-time profiles of JPC-3210 over the first 168 h after dosing with 10 mg/kg of JPC-3210 daily for 3 days. Each data point represents the mean JPC-3210 concentration from 3 monkeys.
The slow absorption of JPC-3210 was evident, with plasma JPC-3210 concentrations being below the limit of quantification (0.5 ng/ml) at 1 h after the first dose in all 3 monkeys and only one monkey having measureable JPC-3210 concentrations at 3 h after dosing. By 6 h after the first dose, all monkeys had measureable JPC-3210 concentrations, with a mean plasma value of 34 ± 54 ng/ml (median, 3.8 ng/ml; range, 1.2 to 96 ng/ml). Before the next dose at 24 h and 48 h, the mean plasma JPC-3210 concentrations were 108 ± 28 ng/ml and 125 ± 20 ng/ml, respectively. With the sampling schedule used, the Cmax of JPC-3210 was 221 ± 23 ng/ml, which was achieved at 72 h after starting the 3-day regimen. Following achievement of the Cmax, plasma JPC-3210 concentrations declined biphasically, with a mean area under the concentration-time curve from the last data point to infinity (AUC0→∞) of 44,901 ± 4,581 μg · h/liter and a lengthy t1/2 of 284 ± 42 h. Blood JPC-3210 concentrations paralleled the plasma profile for each monkey, with a mean AUC0→∞ of 54,914 ± 2,678 μg · h/liters and a t1/2 of 286 ± 42 h. The mean blood-to-plasma JPC-3210 concentration ratio was 1.23 ± 0.06.
JPC-3210 has an association with human red blood cells.
Human red blood cell partitioning in vitro revealed a mean ± SD blood cell-to-plasma concentration ratio of 1.16 ± 0.01, suggesting uptake by blood cells. This value was in accord with the blood-to-plasma JPC-3210 concentration ratio of 1.23 measured in the cynomolgus macaque monkey study. In contrast, in mice there was a limited association of JPC-3210 with red blood cells, with a blood-to-plasma JPC-3210 concentration ratio of approximately 0.65 across the dose range of 5 to 40 mg/kg, which suggests differences in JPC-3210 uptake in blood cells between monkeys and mice in vivo.
JPC-3210 is highly plasma protein bound.
In undiluted human plasma, the protein binding of JPC-3210 was very high, with no evidence of concentration dependence being detected at concentrations between 500 nM (97.62% ± 0.04%) and 5,000 nM (97.66% ± 0.29%). The corresponding mean ± SD values for desethylamodiaquine were 82.26% ± 1.87% and 84.14% ± 1.80%, and those for tafenoquine were >99.5% at both 500 nM and 5,000 nM. The protein binding values obtained in the present study for desethylamodiaquine and tafenoquine are in accord with the findings by others of 86% for desethylamodiaquine (20) and ≥99.5% for tafenoquine (21), using equilibrium dialysis.
JPC-3210 is metabolically stable with low metabolism.
Because hepatocytes are metabolically competent with respect to a wide range of phase I and II pathways, they provide very useful mechanistic, metabolic, and potential toxicity information for new chemical entities (22). JPC-3210 was metabolically stable at 10 μM in hepatocytes from mice, dogs, monkeys, and humans (<5% conversion at 10 μM after 4 h of incubation). At 1 μM JPC-3210, <5% was converted in mouse and dog hepatocytes, whereas <11% was converted in monkey and human hepatocytes. In contrast, rat hepatocytes converted over 50% and 20% of JPC-3210 at 1 μM and 10 μM of the compound, respectively.
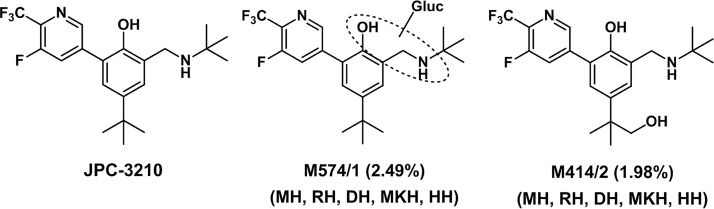
Investigations of JPC-3210 metabolites using liquid chromatography-tandem mass spectrometry (LC-MS/MS) of hepatocyte extracts resulted in the tentative identification of 10 metabolites, with oxidation (metabolite M414/1-2) and glucuronidation (metabolite M574/1) being the major routes of JPC-3210 metabolism. Consistent with the metabolic stability data, JPC-3210 was the predominant component in mouse, dog, monkey, and human hepatocytes. M414/1-2, M574/1, and M590/3 (oxidation and glucuronidation) were identified as minor metabolites in mouse and dog hepatocytes, accounting for ≤1.6% of the total drug-related components. In contrast, ≤12.1% of the total drug-related components were metabolites in monkey hepatocytes, with M414/2 being the most abundant metabolite, accounting for 10% and 7.4% in hepatocyte incubations with 1 μM and 10 μM JPC-3210, respectively. In human hepatocytes, ≤7.1% of JPC-3210 was metabolized, with M574/1 being the most abundant metabolite (4.5% at 1 μM and 2.5% at 10 μM), followed by M414/2 (2.4% at 1 μM and 2.0% at 10 μM) (Fig. 4), and with other metabolites (M414/1, M428/1, M430/2, and M590/1-4) each accounting for <0.2% of the total drug-related components.
FIG 4.
Structures of JPC-3210 and the two main metabolites identified in human hepatocytes in vitro after 4 h of incubation. Percentages of original JPC-3210 are shown. MH, mouse hepatocytes; RH, rat hepatocytes; DH, dog hepatocytes; MKH, monkey hepatocytes; HH, human hepatocytes.
Cytochrome P450 inhibition profile for JPC-3210.
In expectation that JPC-3210 will be developed as a partner drug for malaria treatment, there is the possibility that PK drug-drug interactions (DDI) will lead to the reduced efficacy or toxicity of the coadministered drugs. Because the cytochrome P450 (CYP450) enzymes play a major role in the metabolism of drugs, inhibition of CYP450 enzymes is one of the most common causes of DDI and is screened as early as possible in drug development (23). To predict a potential clinical DDI, we evaluated the time-dependent inhibition of human cytochrome P450 enzymes by JPC-3210, which showed the compound to be a reversible potent inhibitor of CYP2D6 with a 50% inhibitory concentration (IC50) of 1.1 μM, whereas the IC50 of paroxetine is 1.2 μM. No evidence for mechanism-based inhibition of CYP2D6 was observed. JPC-3210 was a moderate inhibitor of CYP3A4 (testosterone) with potential mechanism-based inhibition (IC50 preincubation, 16.5 μM without NADPH versus 4.9 μM with NADPH; IC50 fold shift, 3.4). The compound was not an inhibitor of CYP1A2, CYP2B6, CYP2C8, CYP2C9, or CYP2C19, with IC50s being >40 μM. These findings suggest that a DDI could occur between JPC-3210 and other antimalarial drugs that are metabolized significantly by either CYP2D6 or CYP3A4, such as primaquine (24), piperaquine (16), pyronaridine (25, 26), and mefloquine (27). Of note, pyronaridine, the latest ACT long-acting partner drug marketed as Pyramax, has the same inhibitory effect (IC50, 1.1 μM) on CYP2D6 as JPC-3210. The clinical importance of an antimalarial DDI has recently been reported with the coadministration of pyronaridine and primaquine, leading to a decrease in the oral clearance of primaquine, presumably due to pyronaridine's inhibition of CYP2D6 and/or CYP3A4 (28). Thus, the clinical relevance of JPC-3210's inhibitory effect on CPY2D6 does require in vivo investigations and will be dependent on its blood exposure and protein binding.
Conclusions.
MMV (www.mmv.org) has defined ideal and minimally acceptable characteristics of clinical candidate molecules which are needed to treat and prevent malaria (29). Of the four target candidate profiles (TCPs) defined, JPC-3210 appears to meet the criteria for both TCP 2 (a long-duration partner to complete the clearance of the blood-stage parasites) and TCP 4 (chemoprotection). The long elimination half-life of JPC-3210, together with its lengthy protective effect, suggests that it may have potential as a chemoprophylactic agent. MMV has listed JPC-3210 as part of its pipeline of new antimalarial compounds for further preclinical evaluation (30).
JPC-3210 is a potent new antimalarial compound with linear PK across the dose range of 5 to 40 mg/kg; it has a high oral bioavailability of 86% and a long elimination half-life of about 4.5 days in mice. In cynomolgus monkeys, the plasma elimination half-life was also lengthy, at about 11.8 days. JPC-3210 has a low level of hepatic metabolism but has some CYP2D6- and CYP3A4-inhibitory activity. The favorable pharmacological properties make JPC-3210 suitable for further development as an ACT partner drug in a two- or three-drug combination and/or as a chemoprophylactic agent. Toxicological studies of JPC-3210 in rats and targeted drug metabolism PK studies are now being planned by MMV.
MATERIALS AND METHODS
Drugs.
4-(tert-Butyl)-2-[(tert-butylamino)methyl]-6-[5-fluoro-6-(trifluoromethyl)pyridin-3-yl]phenol (JPC-3210; chemical formula, C21H26F4N2O; molecular weight, 398.44) and its stable deuterated analog (internal standard for LC-MS/MS), 4-(1,1-di(methyl-d3)ethyl-2,2,2-d3)-2-((tert-butylamino)methyl)-6-(5-fluoro-6-(trifluoromethyl)pyridin-3-yl)phen-3,5-d2-ol hydrochloride (JPC-3302; chemical formula, C21H16D11ClF4N2O; molecular weight, 445.97), were provided by Jacobus Pharmaceutical Company. The synthesis will be described in detail elsewhere. Desethylamodiaquine (the active metabolite of amodiaquine) was obtained from the WorldWide Antimalarial Resistance Network Reference Standard Programme (Bangkok, Thailand), and tafenoquine was obtained from Sigma-Aldrich (St. Louis, MO).
Pharmacokinetics of JPC-3210 in healthy and infected mice.
For the PK studies, Swiss outbred ARC (Animal Resource Centre, Murdoch, Western Australia) female mice (age, 6 to 8 weeks; weight range, 22 g to 39 g) were used. For the single-escalating-dose study of JPC-3210, groups (n = 5) of healthy mice were given 5, 10, 20, 40, or 80 mg/kg of JPC-3210 by oral gavage. For the infection study, groups (n = 5) of healthy mice were inoculated intraperitoneally with 2 × 106 erythrocytes infected with Plasmodium berghei of the chloroquine-sensitive ANKA strain. For the determination of parasitemia, blood (∼0.020 ml) was collected from the tail vein of mice on day 4 after parasite inoculation for producing thin blood films, with an expected parasitemia of 6 to 9%. The mice were then treated with a single oral dose of 80 mg/kg of JPC-3210 by oral gavage. This dose was selected as it was expected to clear an established infection of P. berghei, without recrudescence. In contrast, a lower dose of 40 mg/kg of JPC-3210 does not prevent the reappearance of infection (unpublished data) and, thus, would not provide the opportunity to characterize the disposition of JPC-3210 over the 28-day follow-up period. Blood films were collected on days 4 and 5 after JPC-3210 administration and then intermittently every 3 to 4 days until day 28 of follow-up after commencement of treatment. Blood films were Giemsa stained at 10% for 5 min and read by a WHO-certified level 1 microscopist.
JPC-3210 was prepared in ethanol-Tween 80-distilled water (10:10:80 [vol/vol/vol]). For the PK studies in healthy and infected mice, groups of 5 mice were used at each of the 18 PK sampling time points. The mice, anesthetized with carbon dioxide, were bled by cardiac puncture at 0 h (before dosing), at 0.25, 0.5, 1, 2, 3, 6, 12, and 24 h, and then at days 2, 3, 4, 5, 7, 11, 14, 21, and 28 after JPC-3210 administration. Blood samples (0.7 ml) were collected using EDTA as the anticoagulant. After aliquoting 0.2 ml of blood, the remaining blood was centrifuged at 16,000 × g for 5 min at 4°C and the plasma was collected. Blood and plasma samples were then stored at −80°C until analyzed by LC-MS/MS.
Oral bioavailability.
The oral bioavailability of JPC-3210 in groups of 3 CD-1 male mice (age, 7 to 8 weeks; weight range, 28 g to 35 g; Charles River Laboratories, Wilmington, MA) was performed by PharmOptima (Portage, MI) as described by Sietsema (31). Thirty-six mice were administered an intravenous dose of 2 mg/kg of JPC-3210, and 36 mice were administered an oral dose of 16 mg/kg of JPC-3210. The intravenous dose of JPC-3210 was prepared in ethanol–5% Tween 80 in phosphate-buffered saline solution (1:49 [vol/vol]) and administered by bolus (slow-push) injection in the tail vein. The oral dose of JPC-3210 was prepared as a uniform suspension in hydroxyethylcellulose-Tween 80-distilled water (0.5:0.1:99.4 [vol/vol/vol]) and administered by oral gavage. At the time points 0, 0.5, 1, 2, 8, 24, 48, 72 h and then days 7, 10, 14, 21, and 28 for oral dosing and 0, 0.083, 0.5, 1, 2, 8, 24, 48, 72 h and then days 7, 14, 21, and 28 for intravenous administration, the mice were anesthetized with carbon dioxide and blood was collected by cardiac puncture using EDTA as the anticoagulant. Blood samples were centrifuged at 1,500 × g for 10 min at 4°C, and the plasma was separated. The plasma samples were stored at −20°C until analyzed by LC-MS/MS.
Mouse brain tissue concentration of JPC-3210.
Because the chlorophenylphenol, WR 194,965, which was used to derive the synthesis of aminomethylphenols, such as JPC-3210, produced dose-limiting central nervous system effects (e.g., light-headedness and nausea) in humans (32), it was considered pertinent to determine the brain penetration of JPC-3210. The mouse brain tissue concentrations were determined by PharmOptima (Portage, MI) in 3 groups of 4 CD-1 male mice (age, 7 weeks; weight range, 28 g to 31 g) administered 16 mg/kg of JPC-3210 by oral gavage. At the time points 2, 24, and 72 h, mice (n = 12) were anesthetized with carbon dioxide, blood was collected by cardiac puncture using EDTA as the anticoagulant and centrifuged, and plasma was collected and stored at −20°C until analyzed. Following blood collection, the mice were perfused with phosphate-buffered saline to remove residual blood from the brain vasculature and the brains were removed and stored at −80°C. Each brain was placed in a Precellys 24 Dual tube (containing mixed ceramic beads), and a consistent aliquot of 50:50 methanol-water per milligram of total brain weight was added to each tube. Brain samples were homogenized (at a Precellys temperature of between −10°C to 0°C) at 5,500 rpm for 3 cycles of 30 s each with 20-s pauses between cycles. The concentrations of JPC-3210 in brain and plasma samples were analyzed by LC-MS/MS, and the brain tissue homogenate (in nanograms per gram)-to-plasma (in nanograms per milliliter) ratio (the most commonly used parameter for measuring brain penetration) was calculated.
Pharmacokinetics of JPC-3210 in monkeys.
Three male cynomolgus macaque (Macaca fascicularis) monkeys (mean age, 3.4 ± 0.3 years; mean body weight, 5.3 ± 0.5 kg; body weight range, 5.0 kg to 5.9 kg) were used for the PK study. The three monkeys were administered enterically coated, extended-release tablets containing 50 mg of JPC-3210 as the free base (equivalent to ∼10 mg/kg of JPC-3210; total dose, 30 mg/kg). The preparation of the enterically coated, extended-release tablets will be reported elsewhere. The monkeys received one tablet per day for three consecutive days at 24-h intervals. The tablet was placed at the tip (precut) of the Kendall 18 Fr feeding tube (Covidien, Norwalk, CT). An Argyle catheter (Covidien, Norwalk, CT) (going through the Kendall catheter) was used as a stylet to push the tablet into the animal's stomach.
Blood samples (0.5 ml on each occasion) for PK analysis were collected from the femoral and saphenous veins of the monkey at 0 (before dosing), 1, 3, 6, 24, 25, 27, 30, 48, 49, 51, and 54 h and then at 3, 7, 10, 14, 21, 25, 28, 35, 42, 48, 49, 56, 63, 70, and 77 days after commencement of JPC-3210 administration. Blood samples were collected in BD Microtainer NaFl-EDTA tubes (Becton, Dickinson and Company, Franklin Lakes, NJ). The blood samples were transferred to Eppendorf LoBind tubes (Sigma-Aldrich, St. Louis, MO) and centrifuged at 1,350 × g at 4°C for 10 min. The separated plasma samples were transferred to clean LoBind tubes and stored at −80°C. The samples were shipped to the Australian Army Malaria Institute (AMI; Brisbane, Australia) on dry ice for LC-MS/MS analysis. Additional blood samples were collected on day −14 before treatment with JPC-3210 and 54 h and day +77 after starting treatment to establish values for complete blood counts and blood chemistries. These blood collections were prior to drug dosing, at 6 h after the last dose of the 10 mg/kg for 3 days JPC-3210 regimen, and at the conclusion of the study (see the supplemental material). For tolerability assessment, the monkey's appetite, attitude, and body weight were recorded during the study, and the data are shown in the supplemental material.
Red blood cell partitioning of JPC-3210.
The in vitro blood partitioning of JPC-3210 was determined using an LC-MS/MS-based depletion assay that measures the concentration of JPC-3210 in plasma that has been equilibrating with red blood cells as previously described (33). Briefly, JPC-3210 was spiked in duplicate into fresh human whole blood and plasma (single donor) to a targeted concentration of 500 ng/ml. Both the spiked whole blood and plasma control were mixed gently and then incubated at 37°C for 60 min. The whole-blood samples were then centrifuged at 3,000 × g for 5 min to yield the plasma. The plasma samples from both incubations were analyzed by LC-MS/MS. The red blood cell partitioning ratio was calculated as the measurement of JPC-3210 in the plasma control against the measurement of JPC-3210 in the plasma equilibrated with the red blood cells.
JPC-3210 plasma protein binding.
Plasma protein binding was investigated using an ultrafiltration method (34). This procedure uses centrifugation through a semipermeable membrane to separate small molecules, such as antimalarial drugs, from plasma proteins and protein-drug complexes. Briefly, 500 μl of fresh human plasma was spiked with JPC-3210 in duplicate at both 500 nM (199.2 ng/ml) and 5,000 nM (1,992 ng/ml) and incubated at 37°C for 1 h to allow drug-protein binding to occur. The control drugs, desethylamodiaquine (500 nM [308.6 ng/ml] and 5,000 nM [3,086 ng/ml]) and tafenoquine (500 nM [231.7 ng/ml] and 5,000 nM [2,317 ng/ml]) were set up in parallel. Centrifugal filter devices (Microcon Ultracel 10K; Merck Millipore, Bayswater, VIC, Australia) were prepared by centrifuging 400 μl of the nonionic surfactant, 5% Tween 80, at 2,000 × g for 30 min to reduce nonspecific binding of drug to the device (35). After the 1 h of incubation, duplicate 50-μl samples were taken and transferred to microcentrifuge tubes, labeled as prefiltration samples, and stored at −80°C. Four hundred microliters of each plasma-drug mix was then loaded into a prepared ultrafiltration device and spun at 2,000 × g for 30 min. Fifty-microliter samples of eluate were collected in duplicate from each device and assayed by LC-MS/MS together with the prefiltration samples. The percent drug binding to plasma protein was determined by calculating the fraction of drug that passed through the filter membrane.
Drug and pharmacokinetic analysis.
Blood and plasma concentrations of JPC-3210 were measured by LC-MS/MS. The lower limit of quantification of JPC-3210 was 0.5 ng/ml in both blood and plasma, with an inaccuracy of <7.8%, using 50 μl of sample, as previously described (13), with the modification that JPC-3302 (m/z 410.3/337.4) was used as the internal standard. The interassay precision of the analysis (percent coefficient of variation [CV]) for JPC-3210 in blood over the concentration range of 0.5 ng/ml to 1,000 ng/ml was <7.4% (n = 10), and that in plasma over the concentration range of 0.5 ng/ml to 2,000 ng/ml was <6.6% (n = 10).
PK parameters were the maximum concentration (Cmax), the time to reach the maximum concentration (Tmax), area under the concentration-time curve from 0 h to the last data point (AUC0→last) and from the last data point to infinity (AUClast→∞), terminal elimination half-life (t1/2), apparent oral clearance (CL/F), and apparent volume of distribution (V/F). These parameters were determined from the blood and plasma concentration-time data by noncompartmental analysis (36). The blood-to-plasma concentration ratio was calculated using the ratio of AUC0→∞ for blood to AUC0→∞ for plasma. For the determination of oral bioavailability (F [in percent]), the AUC0→last value after oral administration was dose normalized for comparison with the AUC0→last value after intravenous administration, and the ratio of the AUC after oral administration/AUC after intravenous administration, expressed as a percentage, was calculated. Blood and plasma concentrations of JPC-3210, including PK data, are summarized as means ± SDs.
In vitro metabolic studies of JPC-3210 in hepatocytes.
The in vitro hepatocyte studies were performed using established methods (37, 38). JPC-3210 and JPC-3302 were prepared in methanol (final methanol concentration, <1%) in hepatocyte cultures from cryopreserved stocks (Celsis In-Vitro Technologies, Inc., Baltimore, MD). The cryopreserved hepatocytes were from mice (CD-1), rats (Sprague-Dawley), dogs (beagle), monkeys (cynomolgus), and humans (mixed gender, pooled from ≥3 individuals). Hepatocyte concentrations were adjusted to 1 × 106 viable cells/ml in hepatocyte incubation medium in 48-well cell culture plates. JPC-3210 at 1 μM and 10 μM was separately incubated in triplicate with mouse, rat, dog, monkey, and human hepatocytes at 37°C in a climate-controlled incubator (5% CO2, 95% air and 60 to 80% humidity) for 0, 0.25, 0.5, 1, 2, and 4 h. The incubations were terminated by the addition of ice-cold methanol containing 0.5 μM JPC-3302 as an internal standard. The extracts were subjected to LC-MS/MS analysis. The metabolic stability of JPC-3210 in hepatocyte incubation extracts at each incubation time point and for each species was determined by LC-MS/MS. The amounts of unchanged JPC-3210 at each time point were compared to that at 0 h of incubation for the calculation of JPC-3210 metabolic stability. After an additional 4 h of incubation, samples were prepared with 50 μM JPC-3210 and 50 μM JPC-3302 for metabolite identification analysis. Semiquantitation of metabolite abundance was based on comparison of the area under the curve of chromatographic peaks putatively identified to be the specific metabolites. Hepatocyte cell viability was assessed at 0, 2, and 4 h of incubation for JPC-3210 using trypan blue exclusion. Positive controls, 7-ethoxycoumarin (Sigma-Aldrich, St. Louis, MO) and 7-hydroxycoumarin (Sigma-Aldrich, St. Louis, MO) at 100 μM, were run concurrently to assess phase I and phase II metabolic activities in hepatocytes. JPC-3210 in the incubation medium without hepatocytes was used as a negative control to determine the stability of JPC-3210.
Metabolite characterization and identification were carried out by LC-MS using a Thermo LTQ-Orbitrap mass spectrometer (Waltham, MA). The metabolites were tentatively identified by interpretation of MS fragmentation pathways. A metabolite code (i.e., Mxxx/x, where M represents metabolite, xxx represents the monoisotopic mass of the compound, and/x is a sequential compound number) was used for compound identification. Semiquantitation of metabolite abundance was based on comparison of the areas under the curve of the chromatographic peaks putatively identified to be the specific metabolites.
In vitro CYP450 activities by JPC-3210.
Studies of cytochrome P450 (CYP450) inhibition of JPC-3210 were conducted in a time-dependent manner as previously described (39). Briefly, JPC-3210 at seven increasing concentrations (0.01, 0.1, 0.4, 1, 4, 10, and 40 μM) was preincubated in triplicate for 30 min with human liver microsomes (HLM) with and without the presence of 2 mM NADPH in 100 mM potassium phosphate (pH 7.4) containing 5 mM magnesium chloride. After 30 min of preincubation, probe substrate, including tacrine at 25 μM for CYP1A2, amodiaquine at 5 μM for CYP2C8, tolbutamide at 100 μM for CYP2C9, mephenytoin at 100 μM for CYP2C19, dextromethorphan at 5 μM for CYP2D6, midazolam at 2.5 μM and testosterone at 50 μM for CYP3A4/5, and bupropion at 100 μM for CYP2B6, was added to the preincubation reaction mixture and 2 mM NADPH was added to the preincubation reaction mixture without NADPH. Selective CYP inhibitors at various concentrations, furafylline for CYP1A2, gemfibrozil glucuronide for CYP2C8, mifepristone for CYP3A4/5, paroxetine for CYP2D6, tienilic acid for CYP2C9, and ticlopidine for CYP2C19 and CYP2B6, were run in parallel as positive controls. After optimal incubation at 37°C (for 5 to 60 min, based on the substrate), the reactions were terminated by addition of methanol containing an internal standard (propranolol) for analytical quantification. The quenched samples were incubated at 4°C for 10 min and then centrifuged at 1,500 × g for 10 min at 4°C. The supernatant was removed and analyzed by LC-MS/MS for the probe metabolite. A decrease in the formation of the metabolite compared to the vehicle control was used to calculate an IC50 (the test concentration which produces 50% inhibition) with and without NADPH preincubation and to determine the fold shift in the IC50 with NADPH preincubation versus the IC50 without NADPH preincubation.
Animal ethics.
The mouse studies for characterizing the PK of JPC-3210 following single oral escalating doses and murine malaria infection were approved by the Army Malaria Institute Animal Ethics Committee (AMIAEC 04-2013 and AMIAEC 12-2013) and the Defense Animal Ethics Committee (DAEC 03-2016) in accord with the Australian Code for the Care and Use of Animals for Scientific Purposes. The mouse studies for oral bioavailability and brain tissue concentrations of JPC-3210 were approved by the PharmOptima Institutional Animal Care and Use Committee (IACUC 13-02-04). The monkey studies for characterizing the PK of JPC-3210 were approved by the Mannheimer Foundation, Inc., Institutional Animal Care and Use Committee (IACUC 2015-02).
Supplementary Material
ACKNOWLEDGMENTS
We thank Stephen McLeod-Robertson and Thomas Travers for technical excellence with the rodent studies. Metabolic stability and metabolism studies were performed by XenoBiotic Laboratories Inc. (Plainsboro, NJ). Cytochrome studies were performed by Cyprotex (Watertown, MA). Oral bioavailability and brain tissue-to-plasma concentration ratio studies were performed by PharmOptima (Portage, MI). Pharmacokinetic sampling of cynomolgus macaque monkeys was performed by Mannheimer Foundation, Inc., (Homestead, FL).
The opinions expressed are those of the authors and do not necessarily reflect those of the Australian Defense Organization or any extant policy.
This research was funded by the Australian Defense Organization and Jacobus Pharmaceutical Company Inc.
With the exception of G.D.H., G.A.S., J.A., L.R.J., and D.P.J., who work for Jacobus Pharmaceutical Company Inc., we have no conflicts of interest to declare.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01335-17.
REFERENCES
- 1.World Health Organization. 2016. World malaria report 2016. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, Battle KE, Moyes CL, Henry A, Eckhoff PA, Wenger EA, Briët O, Penny MA, Smith TA, Bennett A, Yukich J, Eisele TP, Griffin JT, Fergus CA, Lynch M, Lindgren F, Cohen JM, Murray CL, Smith DL, Hay SI, Cibulskis RE, Gething PW. 2015. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526:207–211. doi: 10.1038/nature15535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. 2015. Guidelines for the treatment of malaria, 3rd ed World Health Organization, Geneva, Switzerland. [Google Scholar]
- 4.Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P, Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S, Singhasivanon P, Day NP, Lindegardh N, Socheat D, White NJ. 2009. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 361:455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rogers WO, Sem R, Tero T, Chim P, Lim P, Muth S, Socheat D, Ariey F, Wongsrichanalai C. 2009. Failure of artesunate-mefloquine combination therapy for uncomplicated Plasmodium falciparum malaria in southern Cambodia. Malar J 8:10. doi: 10.1186/1475-2875-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saunders DL, Vanachayangkul P, Lon C. 2014. Dihydroartemisinin-piperaquine failure in Cambodia. N Engl J Med 371:484–485. doi: 10.1056/NEJMc1403007. [DOI] [PubMed] [Google Scholar]
- 7.Woodrow CJ, White NJ. 2017. The clinical impact of artemisinin resistance in Southeast Asia and the potential for future spread. FEMS Microbiol Rev 41:34–48. doi: 10.1093/femsre/fuw037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spring MD, Lin JT, Manning JE, Vanachayangkul P, Somethy S, Bun R, Se Y, Chann S, Ittiverakul M, Sia-ngam P, Kuntawunginn W, Arsanok M, Buathong N, Chaorattanakawee S, Gosi P, Ta-aksorn W, Chanarat N, Sundrakes S, Kong N, Heng TK, Nou S, Teja-isavadharm P, Pichyangkul S, Phann ST, Balasubramanian S, Juliano JJ, Meshnick SR, Chour CM, Prom S, Lanteri CA, Lon C, Saunders DL. 2015. Dihydroartemisinin-piperaquine failure associated with a triple mutant including kelch13 C580Y in Cambodia: an observational cohort study. Lancet Infect Dis 15:683–691. doi: 10.1016/S1473-3099(15)70049-6. [DOI] [PubMed] [Google Scholar]
- 9.Thanh NV, Thuy-Nhien N, Tuyen NTK, Tong NT, Nha-Ca NT, Dong LT, Quang HH, Farrar J, Thwaites G, White NJ, Wolbers M, Hien TT. 2017. Rapid decline in the susceptibility of Plasmodium falciparum to dihydroartemisinin-piperaquine in the south of Vietnam. Malar J 16:27. doi: 10.1186/s12936-017-1680-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phyo AP, Ashley EA, Anderson TJ, Bozdech Z, Carrara VI, Sriprawat K, Nair S, White MM, Dziekan J, Ling C, Proux S, Konghahong K, Jeeyapant A, Woodrow CJ, Imwong M, McGready R, Lwin KM, Day NP, White NJ, Nosten F. 2016. Declining efficacy of artemisinin combination therapy against P. falciparum malaria on the Thai-Myanmar border (2003–2013): the role of parasite genetic factors. Clin Infect Dis 63:784–791. doi: 10.1093/cid/ciw388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Birrell GW, Chavchich M, Ager AL, Shieh HM, Heffernan GD, Zhao W, Krasucki PE, Saionz KW, Terpinski J, Schiehser GA, Jacobus LR, Shanks GD, Jacobus DP, Edstein MD. 2015. JPC-2997, a new aminomethylphenol with high in vitro and in vivo antimalarial activities against blood stages of Plasmodium. Antimicrob Agents Chemother 59:170–177. doi: 10.1128/AAC.03762-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heffernan GD, Jacobus DP, Krasucki PE, Saionz KW, Schiehser GA, Shieh HM, Terpinski J, Zhao W, Ager AL, Chavchich M, Birrell GW, Shanks GD, Edstein MD. 2015. Identification of 2-aminomethylphenol antimalarials with potent in vitro and in vivo activity against Plasmodium blood stages, abstr MEDI 409. Abstr 250th Am Chem Soc Natl Meet Exposition, Boston, MA. [Google Scholar]
- 13.Chavchich M, Birrell GW, Ager AL, MacKenzie DO, Heffernan GD, Schiehser GA, Jacobus LR, Shanks GD, Jacobus DP, Edstein MD. 2016. Lead selection of a new aminomethylphenol, JPC-3210, for malaria treatment and prevention. Antimicrob Agents Chemother 60:3115–3118. doi: 10.1128/AAC.03066-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frisk-Holmberg M, Bergqvist Y, Termond E, Domeij-Nyberg B. 1984. The single dose kinetics of chloroquine and its major metabolite desethylchloroquine in healthy subjects. Eur J Clin Pharmacol 26:521–530. doi: 10.1007/BF00542151. [DOI] [PubMed] [Google Scholar]
- 15.Winstanley P, Edwards G, Orme M, Breckenridge A. 1987. The disposition of amodiaquine in man after oral administration. Br J Clin Pharmacol 23:1–7. doi: 10.1111/j.1365-2125.1987.tb03002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.European Medicines Agency. Eurartesim, INN-piperaquine & INN-artenimol. Summary of product characteristics. European Medicines Agency, London, United Kingdom: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001199/WC500118113.pdf. [Google Scholar]
- 17.Chen YC, Fleckenstein L. 2001. Improved assay method for the determination of pyronaridine in plasma and whole blood by high-performance liquid chromatography for application to clinical pharmacokinetic studies. J Chromatogr B Analyt Technol Biomed Life Sci 752:39–46. doi: 10.1016/S0378-4347(00)00512-0. [DOI] [PubMed] [Google Scholar]
- 18.Dow GS, Milner E, Bathurst I, Bhonsle J, Caridha D, Gardner S, Gerena L, Kozar M, Lanteri C, Mannila A, McCalmont W, Moon J, Read KD, Norval S, Roncal N, Shackleford DM, Sousa J, Steuten J, White KL, Zeng Q, Charman SA. 2011. Central nervous system exposure of next generation quinoline methanols is reduced relative to mefloquine after intravenous dosing in mice. Malar J 10:150. doi: 10.1186/1475-2875-10-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reichel A. 2009. Addressing central nervous system (CNS) penetration in drug discovery: basics and implications of the evolving new concept. Chem Biodivers 6:2030–2049. doi: 10.1002/cbdv.200900103. [DOI] [PubMed] [Google Scholar]
- 20.O'Neill PM, Park BK, Shone AE, Maggs JL, Roberts P, Stocks PA, Biagini GA, Bray PG, Gibbons P, Berry N, Winstanley PA, Mukhtar A, Bonar-Law R, Hindley S, Bambal RB, Davis CB, Bates M, Hart TK, Gresham SL, Lawrence RM, Brigandi RA, Gomez-delas Heras FM, Gargallo DV, Ward SA. 2009. Candidate selection and preclinical evaluation of N-tert-butyl isoquine (GSK369796), an affordable and effective 4-aminoquinoline antimalarial for the 21st century. J Med Chem 52:1408–1415. doi: 10.1021/jm8012618. [DOI] [PubMed] [Google Scholar]
- 21.GlaxoSmithKline. 2002. Tafenoquine (SB-252263/WR 238605) investigator brochure. GlaxoSmithKline, London, United Kingdom. [Google Scholar]
- 22.Sahi J, Grepper S, Smith C. 2010. Hepatocytes as a tool in drug metabolism, transport and safety evaluations in drug discovery. Curr Drug Discov Technol 7:188–198. doi: 10.2174/157016310793180576. [DOI] [PubMed] [Google Scholar]
- 23.Shearer TW, Smith KS, Diaz D, Asher C, Ramirez J. 2005. The role of in vitro ADME assays in antimalarial drug discovery and development. Comb Chem High Throughput Screen 8:89–98. doi: 10.2174/1386207053328129. [DOI] [PubMed] [Google Scholar]
- 24.Marcsisin SR, Reichard G, Pybus BS. 2016. Primaquine pharmacology in the context of CYP 2D6 pharmacogenomics: current state of the art. Pharmacol Ther 161:1–10. doi: 10.1016/j.pharmthera.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 25.European Medicines Agency. 2012. Pyramax: public assessment report. Report EMA/CHMP/61768/2012 Committee for Medicinal Products for Human Use (CHMP), European Medicines Agency, London, United Kingdom. [Google Scholar]
- 26.Croft SL, Duparc S, Arbe-Barnes SJ, Craft JC, Shin CS, Fleckenstein L, Borghini-Fuhrer I, Rim HJ. 2012. Review of pyronaridine anti-malarial properties and product characteristics. Malar J 11:270. doi: 10.1186/1475-2875-11-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fontaine F, de Sousa G, Burcham PC, Duchêne P, Rahmani R. 2000. Role of cytochrome P450 3A in the metabolism of mefloquine in human and animal hepatocytes. Life Sci 66:2193–2212. doi: 10.1016/S0024-3205(00)00546-4. [DOI] [PubMed] [Google Scholar]
- 28.Jittamala P, Pukrittayakamee S, Ashley EA, Nosten F, Hanboonkunupakarn B, Lee SJ, Thana P, Chairat K, Blessborn D, Panapipat S, White NJ, Day NP, Tarning J. 2015. Pharmacokinetic interactions between primaquine and pyronaridine-artesunate in healthy adult Thai subjects. Antimicrob Agent Chemother 59:505–513. doi: 10.1128/AAC.03829-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burrows JN, van Huijsduijnen RH, Mohrle JJ, Oeuvray C, Wells TN. 2013. Designing the next generation of medicines for malaria control and eradication. Malar J 12:187. doi: 10.1186/1475-2875-12-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Medicines for Malaria Venture. 2017. Research and development. Global portfolio of antimalarial medicines. Medicines for Malaria Venture, Geneva, Switzerland: https://www.mmv.org/research-development Accessed 7 June 2017. [Google Scholar]
- 31.Sietsema WK. 1989. The absolute oral bioavailability of selected drugs. Int J Clin Pharmacol Ther Toxicol 27:179–211. [PubMed] [Google Scholar]
- 32.Schmidt LH, Crosby R. 1978. Antimalarial activities of WR-194,965, an alpha-amino-o-cresol derivative. Antimicrob Agents Chemother 14:672–679. doi: 10.1128/AAC.14.5.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu S, Li S, Yang H, Lee F, Wu J-T, Qian MG. 2005. A novel liquid chromatography/tandem mass spectrometry based depletion method for measuring red blood cell partitioning of pharmaceutical compounds in drug discovery. Rapid Commun Mass Spectrom 19:250–254. doi: 10.1002/rcm.1777. [DOI] [PubMed] [Google Scholar]
- 34.Zhang F, Xue J, Shao J, Jia L. 2012. Compilation of 222 drugs' plasma protein binding data and guidance for study designs. Drug Discov Today 17:475–485. doi: 10.1016/j.drudis.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 35.Lee KJ, Mower R, Hollenbeck T, Castelo J, Johnson N, Gordon P, Sinko PJ, Holme K, Lee YH. 2003. Modulation of nonspecific binding in ultrafiltration protein binding studies. Pharm Res 20:1015–1021. doi: 10.1023/A:1024406221962. [DOI] [PubMed] [Google Scholar]
- 36.Gibaldi M, Perrier D. 1982. Pharmacokinetics, 2nd ed Marcel Dekker Inc, New York, NY. [Google Scholar]
- 37.Li AP. 1997. Primary hepatocyte cultures as an in vitro experimental model for the evaluation of pharmacokinetic drug-drug interactions. Adv Pharmacol 43:103–130. doi: 10.1016/S1054-3589(08)60203-3. [DOI] [PubMed] [Google Scholar]
- 38.Jia L, Liu X. 2007. The conduct of drug metabolism studies considered good practice (II): in vitro experiments. Curr Drug Metab 8:822–829. doi: 10.2174/138920007782798207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Obach RS, Walsky RL, Venkatakrishnan K. 2007. Mechanism-based inactivation of human cytochrome P450 enzymes and the prediction of drug-drug interactions. Drug Metab Dispos 35:246–255. doi: 10.1124/dmd.106.012633. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.