ABSTRACT
In seeking substitutions for the current Chagas disease treatment, which has several relevant side effects, new therapeutic candidates have been extensively investigated. In this context, a balanced interaction between mediators of the host immune response seems to be a key element for therapeutic success, as a proinflammatory microenvironment modulated by interleukin-10 (IL-10) is shown to be relevant to potentiate anti-Trypanosoma cruzi drug activity. This study aimed to identify the potential immunomodulatory activities of the anti-T. cruzi K777, pyronaridine (PYR), and furazolidone (FUR) compounds in peripheral blood mononuclear cells (PBMC) from noninfected (NI) subjects and chronic Chagas disease (CD) patients. Our results showed low cytotoxicity to PBMC populations, with 50% cytotoxic concentrations (CC50) of 71.0 μM (K777), 9.0 μM (PYR), and greater than 20 μM (FUR). In addition, K777 showed no impact on the exposure index (EI) of phytohemagglutinin-stimulated leukocytes (PHA), while PYR and FUR treatments induced increased EI of monocytes and T lymphocytes at late stages of apoptosis in NI subjects. Moreover, K777 induced a more prominent proinflammatory response (tumor necrosis factor alpha-positive [TNF-α+] CD8+/CD4+, gamma interferon-positive [IFN-γ+] CD4+/CD8+ modulated by interleukin-10-positive [IL-10+] CD4+ T/CD8+ T) than did PYR (TNF-α+ CD8+, IL-10+ CD8+) and FUR (TNF-α+ CD8+, IL-10+ CD8+). Signature analysis of intracytoplasmic cytokines corroborated the proinflammatory/modulated (K777) and proinflammatory (PYR and FUR) profiles previously found. In conclusion, the lead compound K777 may induce beneficial changes in the immunological profile of patients presenting the chronic phase of Chagas disease and may contribute to a more effective therapy against the disease.
KEYWORDS: Chagas disease, screening compounds, Trypanosoma cruzi, immunomodulation
INTRODUCTION
Chagas disease (CD) is an important public health problem not only in Latin America, where it is endemic, but also in other areas such as Europe, North America, Japan, and Australia, where it is increasingly spreading. Around 7 million people are affected worldwide, and approximately 10,000 deaths occur annually, making CD the major cause of death from a parasitic disease in Latin America (1). In Brazil, the most current epidemiological data on the disease estimate its prevalence at 2 million people infected with the parasite (2).
Current treatment options for CD are limited to two nitroheterocyclic drugs, nifurtimox (NFX; Lampit [Bayer]) (3) and benznidazole (BZ) (Rochagan [LAFEPE] and Abarax [ELEA]) (4). These compounds are active in the acute phase of the disease, with up to 80% efficacy (5). However, such drugs have limited effect against the CD-advanced chronic phase and side effects are well documented, which have restricted its use and led to discontinuation of treatment, especially in adult populations (6, 7).
Given the aspects mentioned above, new approaches have been explored in the last 2 decades, consisting of the search for future candidates for the specific chemotherapy of CD. A detailed update on the research and development of new drugs is available in a comprehensive review published by the Drugs for Neglected Diseases Initiative (DNDi) (8). Among the currently investigated compounds, K777, pyronaridine (PYR), and furazolidone (FUR) were promising candidates for anti-Trypanosoma cruzi therapy. The efficacy of K777 was documented in vitro (9), in immunodeficient mice (10), and in dogs (11). Recently, PYR and FUR demonstrated in vivo efficacy against T. cruzi, reducing 85.2% and 100%, respectively, of the parasite burden after 4 days treatment (12).
Besides the great relevance of the discovery of new bioactive and selective compounds against T. cruzi for progress in the treatment of infection by this parasite, host immunity is a determinant factor in the disease pathology and a fundamental complementary element during CD therapy, representing an important resistance component. Despite the large number of studies on the immune response following infection by T. cruzi (13–15), there are relatively few studies on the impact of treatment on this response.
Previous studies from our group have demonstrated that a proinflammatory profile mediated by gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) is relevant in potentiating the activity of the anti-T. cruzi drug and that interleukin-10 (IL-10) may be a key element in the modulation of the immune response (16–18). Thus, the aim of this study was to analyze the putative immunomodulatory activity of the anti-T. cruzi lead compounds K777, PYR, and FUR using peripheral blood mononuclear cells (PBMC). To the best of our knowledge, this is the first report describing the impact of these trypanocidal agents on the functional profile of PBMC populations.
RESULTS
K777 and FUR have little cytotoxicity in PBMC in vitro.
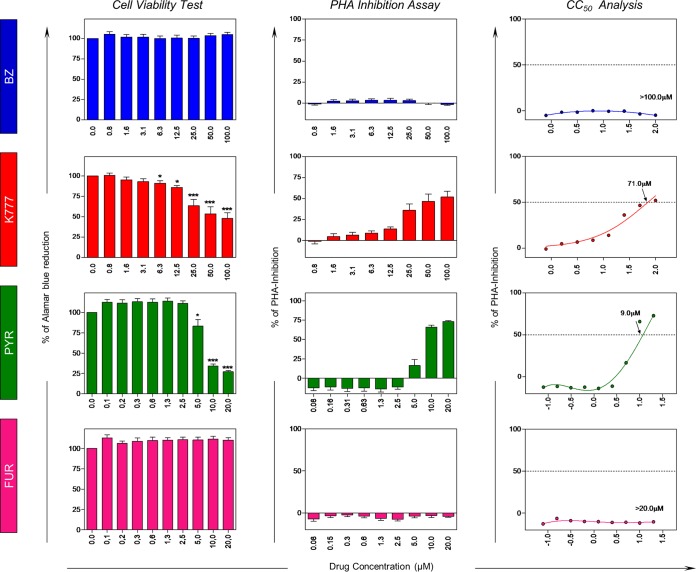
Table 1 shows the selectivity indexes (SI) of the lead anti-Trypanosoma cruzi compounds tested. Our data showed that FUR and K777 presented higher SI than PYR. In addition, Fig. 1 shows the cell viability (represented by the percentage of reduction) and inhibition of cell proliferation, as well as dose-response curves of the evaluated compounds. Our data showed that in the presence of the reference drug BZ, cell viability remained close to 100% without significant inhibition in PBMC proliferation. On the other hand, K777 and PYR promoted cellular inhibitory effects in the highest concentrations tested, with 50% cytotoxic concentrations (CC50) of 71.0 μM and 9.0 μM, respectively. Additionally, FUR did not demonstrate cytotoxic activity on the viability and proliferation of the PBMC populations until 20 μM.
TABLE 1.
Selectivity indexes of anti-Trypanosoma cruzi lead compounds
| Anti-T. cruzi compounda | EC50 (μM)b | CC50 (μM)c | SId |
|---|---|---|---|
| BZ | 3.81 | 2,381.0 | 625.0 |
| K777 | 3.60 | 19.0 | 5.3 |
| PYR | 4.60 | <11.0 | <2.4 |
| FUR | 0.89 | 355.3 | 399.0 |
BZ, benznidazole; PYR, pyronaridine; FUR, furazolidone.
Minimum effective concentration for inhibiting 50% of the Trypanosoma cruzi strain Tulahuen.
Cytotoxic concentration of the drug reference BZ and lead compounds causing death of 50% of viable L929 cells.
SI (selectivity index) = CC50/EC50.
FIG 1.
Viability of peripheral blood mononuclear cells (PBMC) from healthy subjects (NI, n = 12) after 72-h in vitro treatment with reference drug BZ and K777, PYR, and FUR anti-T. cruzi lead compounds by alamarBlue. Data are presented as means ± standard errors of the means (SEM). Significance is indicated by asterisks: P < 0.05 (*), P < 0.01 (**), and P < 0.001 (***).
K777 treatment promotes apoptotic and cytokine+ cell profiles similar to those induced by the reference drug in NI subjects.
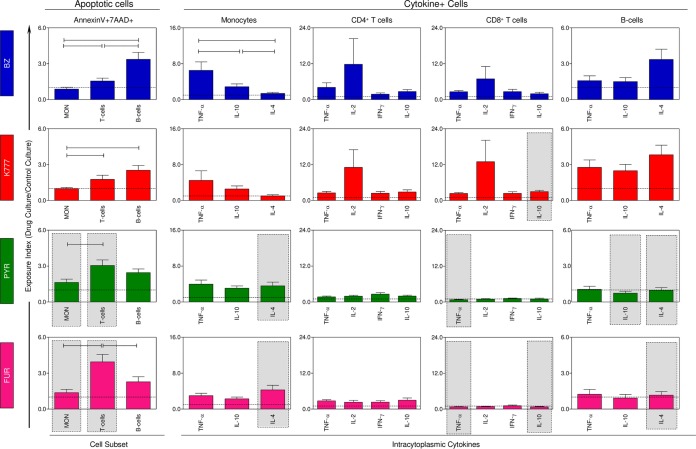
Figure 2 shows the impact of in vitro treatment with compounds K777, PYR, and FUR on PBMC from noninfected (NI) subjects through late-apoptotic-cell and cytokine-positive (cytokine+)-cell profiles. The data are shown as exposure index (EI = value for compound-treated cultures/value for untreated cultures). According to our results, the EI of apoptotic B cells was predominant in BZ and K777 treatments, whereas cultures treated with PYR and FUR showed a late apoptosis dominance of T cells. In comparative analysis with the reference drug, K777 maintained an apoptosis induction profile similar to that of BZ, whereas PYR and FUR treatments showed a significant increase of EI to apoptotic monocytes and T cells. In addition, analysis of leukocyte subsets and their cytokine production demonstrated that K777 treatment did not induce significant changes in the PBMC, except for the increase in IL-10+ CD8+ T cells. In contrast, PYR and FUR have been shown to induce several changes in the profile of intracytoplasmic cytokines in NI, enhancing IL-4 production by monocytes while reducing TNF-α, IL-10, and IL-4 production by CD8+ T cells and/or B-cells.
FIG 2.
Drug-induced changes on in vitro apoptotic cell death and intracytoplasmic cytokine profile of peripheral blood mononuclear cells (PBMC) from healthy subjects. The results are presented as the means ± SEM of exposure index (EI = value for treated cultures/value for control cultures) of PBMC measured from cultures treated with BZ, K777, PYR, and FUR from NI subjects (n = 18). Statistically significant differences between populations undergoing the same treatment are represented by a solid black line. Significant differences for the same population between different treatments tested compared to BZ are represented by a gray rectangle.
K777 treatment promotes an apoptotic cell profile similar to that of the reference drug and induces a modulated proinflammatory profile, while PYR and FUR promote the reduction of cytokine+ T cells in CD patients.
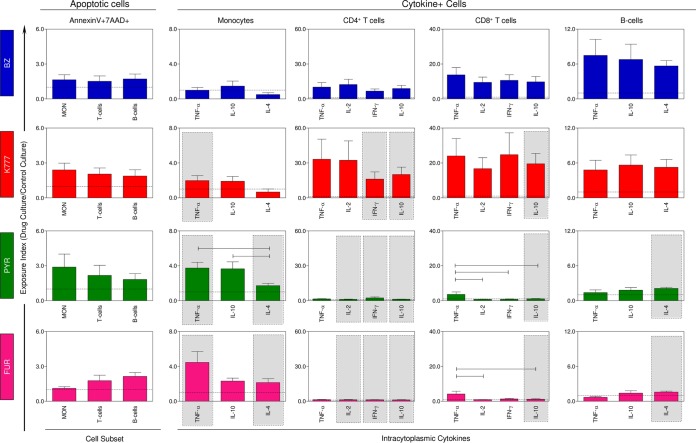
Figure 3 shows the impact of in vitro treatment with compounds K777, PYR, and FUR on PBMC from CD patients through late apoptotic cell and cytokine+ cell profiles. Our data showed that CD groups treated with the different anti-T. cruzi lead compounds presented an apoptotic pattern similar to that obtained with the reference drug. However, the compounds have been shown to induce different cytokine profiles in leukocyte subsets. K777 appears to induce a proinflammatory response (TNF-α+ CD14+, IFN-γ+ CD4+) modulated by IL-10 (IL-10+ CD4+, IL-10+ CD8+), while PYR and FUR showed a tendency to increase monocyte activation and, conversely, reduce production of cytokines considered essential for disease control and therapeutic success in adaptive immunity, such as IFN-γ and IL-10, from lymphocyte subpopulations.
FIG 3.
Drug-induced changes on in vitro apoptotic cell death and intracytoplasmic cytokine profile of peripheral blood mononuclear cells (PBMC) from Chagas disease patients. The results are presented as the means ± SEM of exposure index (EI = value for treated cultures/value for control cultures) of PBMC from CD patients (n = 20) measured from cultures treated with BZ, K777, PYR, and FUR. Statistically significant differences between populations undergoing the same treatment are represented by a solid black line (segment). Significant differences for the same population subjected to different treatments tested compared to BZ are represented by a gray rectangle.
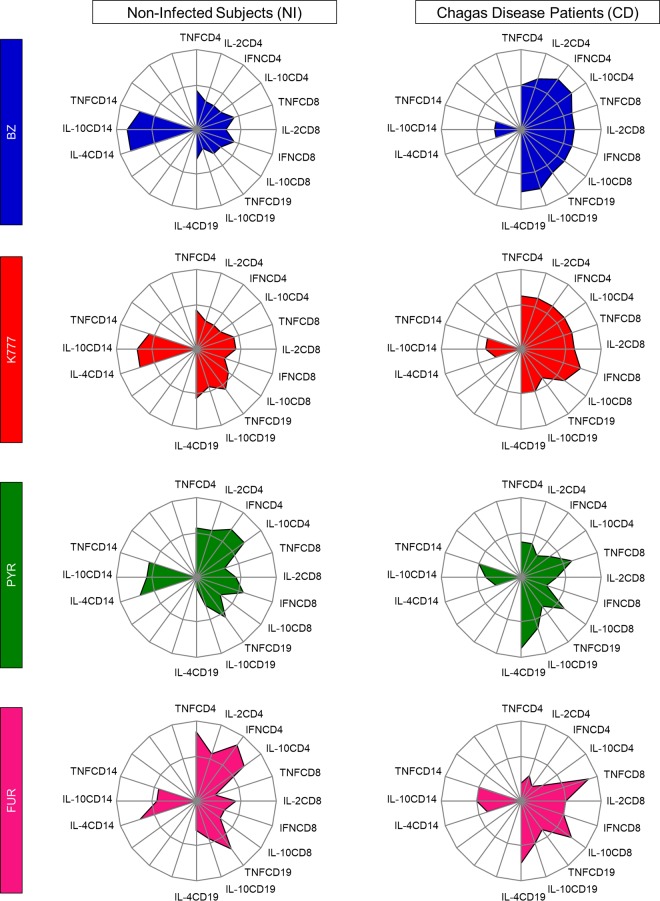
K777, PYR, and FUR induce distinct changes in the signature of intracytoplasmic cytokines of healthy subjects and Chagas disease patients.
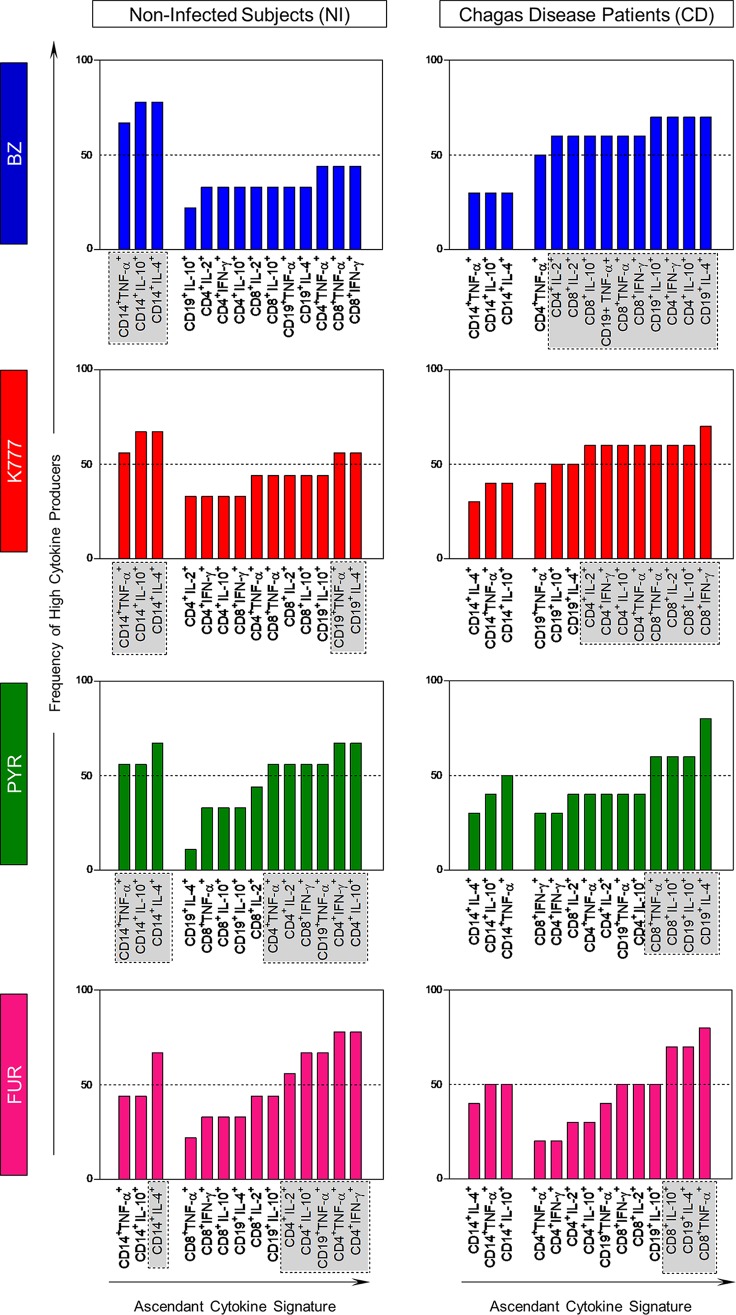
The global median level of intracellular cytokines (data not shown) was used to perform an evaluation of the cytokine signature produced by leukocyte from the innate (monocytes) and adaptive (CD4+ T cells, CD8+ T cells, and B cells) immune response, as illustrated in Fig. 4 and 5. Collectively, our data showed that NI subjects treated with lead compounds exhibited increased adaptive immunity, with high production of the biomarkers of interest. Among CD patients, our data showed that K777 maintained a signature profile very similar to that of the reference drug, promoting an increase in the majority of leukocytes, with high production of both inflammatory and regulatory cytokines. On the other hand, PYR and FUR were able to promote changes in the profiles of only CD8+ T cells and B cells.
FIG 4.
Drug-induced changes on in vitro overall signature of high cytokine producers. Signatures of high frequency of cytokine-producing cells from innate (CD14) and adaptive (CD4, CD8, CD19) immune compartments by noninfected subjects (NI, n = 18) and Chagas disease patients (CD, n = 20) after treatment with BZ (blue bars), K777 (red bars), PYR (green bars), and FUR (pink bars) lead compounds. The ascendant frequency of cytokine-producing cells from innate and adaptive immune compartments in each group is represented by bars. Dotted lines represent the 50th percentile that was used as a cutoff to identify relevant differences. The results are presented as exposure index (EI = value for treated cultures/value for control cultures).
FIG 5.
Drug-induced changes on in vitro intracytoplasmic cytokine signatures of innate and adaptive immunity. Radar graphs represent the balance of subjects with a high frequency of inflammatory (IL-2, TNF, IFN) or regulatory (IL-10, IL-4) cytokine-producing cells of innate (CD14) and adaptive (CD4, CD8, CD19) immunity. Graphs were constructed with each axis displaying the proportion of subjects with a high frequency of cytokine-producing cells within a given leukocyte subset. The values of each axis can be joined to form the central polygon area that represents the general inflammatory/regulatory cytokine balance. Increasing or decreasing central polygon areas reflect either higher or lower contributions, respectively, of the inflammatory versus regulatory cytokine balance in each group. Analysis of the radar chart axes highlights the contribution of a distinct leukocyte subset for the overall cytokine balance after treatment with BZ (blue), K777 (red), PYR (green), and FUR (pink) lead compounds. Groups were categorized as noninfected subjects (NI, n = 18) and Chagas disease patients (CD, n = 20).
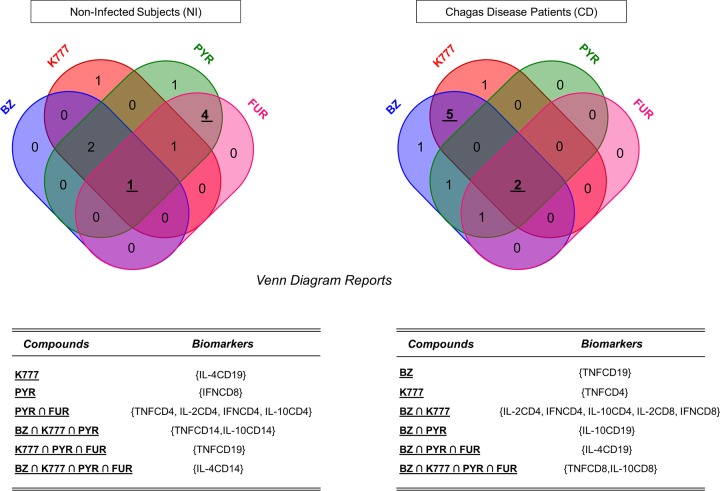
A Venn diagram analysis for the lead compounds is shown in Fig. 6 and shows one biomarker (IL-4+ CD14+) in common from NI subjects among all anti-T. cruzi lead compounds. PYR and FUR induced a similar immune response, with four common biomarkers (TNF-α+ CD4+, IL-2+ CD4+, IFN-γ+ CD4+, IL-10+ CD4+) in the NI group. Among CD patients, TNF-α+ CD8+ and IL-10+ CD8+ were the common biomarkers among the four treatments employed. Also, BZ and K777 induced similar immune responses, with five common biomarkers (IL-2+ CD4+, IFN-γ+ CD4+, IL-10+ CD4+, IL-2+ CD8+, IFN-γ+ CD8+). Thus, our findings confirm that the lead compound K777 demonstrated great similarity to the reference drug BZ, inducing a comparable immune response profile (proinflammatory cytokines modulated by regulatory cytokines) in CD patients.
FIG 6.
Functional biomarkers upregulated by anti-T. cruzi lead compounds shown in Venn diagrams. Venn diagrams were built for noninfected subjects and Chagas disease patients after treatment with BZ (blue), K777 (red), PYR (green), and FUR (pink) lead compounds in order to determine the induction levels of common biomarkers. The internal values in the diagram represent the number of single or common biomarkers among the lead compounds.
DISCUSSION
The lack of an effective drug for the chronic phase of Chagas disease has been the major concern for the treatment of the disease. The Benznidazole Evaluation for Interrupting Trypanosomiasis (BENEFIT) clinical trial (Clinical Trials registration number NCT00123916), initiated in 2005, was conducted in Argentina, Brazil, Bolivia, Colombia, and El Salvador. The program focused on the potential benefit of BZ treatment in patients with Chagas cardiomyopathy, attracting great expectations (19, 20). However, the drug was not able to reduce the progression of heart disease within 5 years of follow-up of the patients. In addition, negative PCR results showed significant variation depending on geographic location (21). In this perspective, several studies have been conducted towards the discovery of bioactive and selective agents against T. cruzi, including compounds such as fexinidazole (22), posaconazole (23), and ravuconazole (24). Indeed, new compounds have been rediscovered for CD treatment and have attracted great scientific interest recently, highlighting the lead compounds evaluated in present study.
The anti-T. cruzi activity of K777, a vinyl sulfone inhibitor, was identified by James McKerrow (University of California, San Francisco, USA) by screening for cysteine protease inhibitors against T. cruzi-infected macrophages. The compound was reported to block the proliferation of extracellular epimastigotes/intracellular amastigotes and prevent metacyclogenesis in vitro (25, 26). PYR, on the other hand, is a derivative of the benzonapthyridines and was originally discovered as an antimalarial compound (27–29), acting to inhibit the formation of hemozoin (β-hematin) in vitro (30). It has also been shown to be active against Babesia spp. and Theileria spp. (31) and was able to inhibit the proliferation of a variety of tumor cells (32). FUR is a synthetic nitrofuran with potent antibacterial and antiprotozoal activity (33–35). In addition, it has demonstrated potent antiproliferative properties and induced apoptosis in cell lines in acute myeloid leukemia (36), and some studies have shown its effectiveness in the treatment of leishmaniasis in humans (37).
A cytotoxicity assay for each compound was performed, since the identification and definition of safety limits for all chemotherapeutic potentials are critical (38). According to our observations, the lead compounds K777, PYR, and FUR exhibited CC50 of 19.0 μM, <11.0 μM, and >355.3 μM, respectively, on L929 cells as well as CC50 of 71.0 μM, 9.0 μM, and >20 μM on PBMC. There are no previous studies of cytotoxicity for these compounds on PBMC populations. However, the toxic effects of compounds K777 and FUR were investigated on bovine embryo skeletal muscle (BESM) cells, and according to image-based assays these compounds exhibited CC50 of >20.0 μM and >50.0 μM, respectively (39). Additionally, Ekins et al. previously reported that a mouse myoblast cell line (C2C12) treated with PYR and FUR demonstrated CC50 of 3.0 μM and >10.0 μM, respectively (12). Thus, although the toxicity of the compounds varies according to the cell types employed, the available data are in line with our results demonstrating that K777 and FUR presented low cytotoxicity.
Another important point on the evaluation of new drugs includes the induction of cell death signaling responses during chemotherapy. Our results also describe the induction of PBMC apoptosis caused by the lead compounds. In this context, K777 did not promote changes in the apoptosis levels compared to BZ. On the other hand, there was an increase in cell death of monocytes and T cells from NI subjects treated with PYR and FUR. Previous studies have shown that T. cruzi infection can induce apoptosis in T cells and neutrophils (40), causing several deleterious consequences to the host immune system. However, our data showed no statistical difference in apoptosis of cells from CD patients. Thus, our results confirm that the concentrations of the compounds used in this study are safe for PBMC populations and do not exacerbate the apoptotic process throughout the disease.
The interactions between macrophages and lymphocytes, through cytokines, are of extreme relevance for the establishment of an efficient immune response and able to control the replication of pathogens in infectious processes. In addition, an efficient immunomodulatory activity is essential to minimize pathology by different agents, including the parasites (41–43). Based on that, we evaluated the hypothetical immunomodulatory potential of K777, PYR, and FUR in the expression of cytokines by monocyte and lymphocyte subsets. Our results showed similar microenvironments for the lead compound K777 and the reference drug BZ in the cytokine profiles produced by PBMC from NI subjects.
Among CD patients, K777 favored a modulated proinflammatory response, given the premise that the blood of infected patients should be characterized by potentially immunoprotective properties when treated with the drug of interest. The importance of a modulated proinflammatory profile in CD is well documented in PBMC samples. Although IFN-γ has been implicated in the regulation of the adequate development of the inflammatory response (44, 45), the literature indicates that PBMC in CD patients with cardiac involvement produce more IFN-γ and less IL-10 than in asymptomatic patients during the chronic phase (46, 47). These findings are supported by previous studies on trypanocidal therapy, showing that BZ treatment induced NK cells and CD8+ T cells to produce IFN-γ and that this cytokine and IL-10, produced by CD4+ T cells and B cells, were key elements for the control of tissue damage induced by the proinflammatory response (16–18). Particularly in the advanced chronic phase of T. cruzi infection, there is no evidence that BZ is able to sustain its immunomodulatory effect by IL-10. For this reason, the use of combination therapy in the treatment of chronic CD has been shown to be an important alternative that may provide a pronounced immunomodulatory activity with consequent maximization of the desired effects (48).
In conclusion, our results demonstrated that the anti-T. cruzi lead compounds promote different effects on the functional capacity of PBMC in healthy individuals and patients with chronic Chagas disease. Thus, the present work demonstrates that the compound K777 induces changes in the immunological profile that may be beneficial in the treatment of patients in the chronic phase of Chagas disease.
MATERIALS AND METHODS
Human subjects and ethics statement.
Noninfected (NI) subjects were selected from the blood bank at Felício Rocho Hospital, Minas Gerais (MG), Brazil, and ranged in age from 18 to 65 years, whereas CD patients who agreed to participate in this study were identified and selected in the outpatient clinic at Instituto René Rachou/Fiocruz, Minas Gerais, Brazil, with ages ranging from 19 to 69 years. Serology for Chagas disease was determined by two or more tests (indirect immunofluorescence, enzyme-linked immunosorbent assay [ELISA], or indirect hemagglutination), and patients were considered infected when at least two different tests were positive (49). The exclusion criteria for both populations were significant anemia, evidence of hypothyroidism or hyperthyroidism, leukopenia, ischemic heart disease, and other significant chronic or acute systemic diseases. Patients treated within the past 10 years were also excluded.
Written informed consent was obtained from all individuals prior to their inclusion in the study. Independent of their participation in this study, all individuals enrolled were submitted to a standard screening protocol, follow-up, and clinical treatment. This study was carried out in full accordance with resolution number 466/2012 of the Brazilian National Health Council for research involving humans and was approved by the Ethics Committee at Instituto René Rachou—FIOCRUZ (CEPSH/IRR number 1.136.140/2015).
Anti-T. cruzi lead compounds and reference drug.
BZ and K777 were kindly provided by James McKerrow (University of California, San Diego). The compounds PYR and FUR were purchased from Sigma Chemical Co. (St. Louis, MO, USA). All compounds were diluted in dimethyl sulfoxide (DMSO) according to the manufacturer's instructions. The final concentration never exceeded 0.5% (vol/vol), which avoided toxicity to the host cells.
In vitro anti-T. cruzi activity, cellular toxicity, and selectivity.
The in vitro anti-T. cruzi activity was evaluated on murine L929 fibroblast cells infected with the Tulahuen strain of the parasite, according to the method described previously (50). The compounds were tested at concentrations ranging from 0.35 μM to 3,843.2 μM during a 96-h period. Controls with uninfected cells, untreated infected cells, and infected cells treated with benznidazole (reference drug) at the concentration of 15.2 μM or DMSO (1%, vol/vol) were used. The results were expressed as the percentage of T. cruzi growth inhibition in infected cells tested with the compounds to the inhibition in untreated infected cells. The compounds were evaluated for cytotoxicity and selectivity on uninfected fibroblasts (50). Experiments were performed in quadruplicates for each compound, and data are representative of at least two independent experiments. The results obtained were expressed as 50% inhibitory concentrations (IC50s), calculated by linear interpolation, and the selectivity index (SI) was determined based on the ratio between the CC50 for the fibroblast cells and the IC50 for T. cruzi.
Isolation of PBMC.
Peripheral blood mononuclear cells (PBMC) from NI subjects and CD patients were isolated from heparinized blood by density gradient centrifugation on Histopaque-1077 (Sigma Chemical Co., USA) according to Gazzinelli et al. (51). Cells were resuspended in RPMI 1640 (Gibco, Paisley, UK) medium at a final concentration of 1 × 106 cells/ml. The PBMC counting was performed using the Automatic Count Counter (Countess Automated Cell Counter; Invitrogen).
Cytotoxicity assay.
The measurement of cytotoxicity of the compounds of interest by the viability and proliferation of PBMC was performed from NI subject cells by the alamarBlue colorimetric test (Bio-Rad Laboratories, Hercules, CA, USA). Briefly, PBMC were cultured in 96-well plates (Falcon, USA) at a concentration of 2 × 106 cells/ml and in 2-fold dilutions covering a range from 100.0 μM to 0.8 μM for BZ and K777 and 20.0 μM to 0.08 μM for PYR and FUR. Plates were incubated at 37°C in humidified 5% CO2 atmosphere for 72 h, and 22 μl of alamarBlue was added for an additional 21 h. Spectrophotometer SpectraMax M5 (Molecular Devices, Sunnyvale, CA, USA) was used to quantify the absorbance in wavelengths of 570 nm (oxidized state) and 600 nm (reduced state) after exposure to alamarBlue. Results were reported as the percentage of reduction and inhibition of PBMC proliferation of the test samples compared to phytohemagglutinin (PHA)-stimulated controls. Reduction in cell viability of more than 30% was considered cytotoxic, as recommended by the International Organization for Standardization (ISO) (38).
PBMC cultures.
PBMC from NI subjects and CD patients were used at a concentration of 1 × 106 cells/ml. Briefly, cells were cultured in 48-well cell culture plates (Falcon, BD) in CMBlast culture medium (RPMI 1640 supplemented with 1.6% l-glutamine, 3% antibiotic/antimycotic, and 10% normal AB+ human serum; Gibco, USA) and in the presence of reference drug BZ (final concentration, 10.0 μM) and lead compounds K777 (1.0 μM), PYR (1.25 μM), and FUR (10.0 μM). These concentrations were chosen after cytotoxicity assays. The cultures were stimulated with 2.5 μg/ml of PHA (Sigma Chemical Co., USA) and incubated for 72 h at 37°C in a humidified atmosphere containing 5% CO2. During the last 4 h of culture, brefeldin A (BFA; Sigma Chemical Co., USA) at 10 μg/ml was added, impairing protein secretion by the Golgi complex (52) for cytokine intracellular staining.
Analysis of apoptosis profiles.
Long-term stimulation PBMC cultures from NI and DC groups were analyzed in order to characterize the apoptosis profiles from different cell populations. For the annexin V analysis, aliquots of 50 μl were transferred to polystyrene tubes and incubated for 30 min at room temperature (RT) with anti-CD14 APC (MφP9), anti-CD3 PE (UCHT1), and CD19 APC (HIB19) (BD Pharmingen, San Jose, CA, USA) monoclonal antibodies (MAbs). Following incubation, cells were washed with 2 ml of 1× phosphate-buffered saline (PBS) by centrifugation at 400 × g for 7 min at 4°C. The cells were resuspended in annexin V binding buffer (0.1 M HEPES-NaOH [pH 7.4], 1.4 M NaCl, 25 mM CaCl2; Biosciences, San Jose, CA), for the working solution (1×) and then incubated for 15 min at RT (25°C) in the dark with annexin V-fluorescein isothiocyanate (FITC) and 7-amino-actinomycin (7-AAD; BD Pharmingen, USA) staining, according to the manufacturer's instructions. The reaction was stopped by the addition of 100 μl of 1× binding buffer for each tube. Flow cytometric acquisition was performed within a maximum of 1 h. Phenotypic analyses were performed by flow cytometry using a Becton Dickinson FACScalibur flow cytometer, collecting data on 30,000 events/sample for lymphocytes (gated by forward scatter [FSC] and side scatter [SSC] properties) and 5,000 events/sample for monocytes, using FlowJo software (TreeStar Inc., Ashland, OR, USA).
Gating strategy to identify apoptotic cells.
Apoptotic monocyte populations were determined by FL4-CD14 APC versus SSC graphs followed by FL1-annexin V-FITC versus FL3-7-AAD plots. Apoptotic lymphocytes subpopulations were first selected by the parameters FSC versus SSC corresponding to the total lymphocytes followed by FL1-annexin V-FITC versus FL3-7-AAD for CD3 PE (T lymphocytes) and CD19 APC (B lymphocytes), considering always the double-positive (annexin-V+ and 7-AAD+ cells) cells to be the population of interest (corresponding to late apoptosis).
Intracellular cytokines profile.
After an additional 4 h of culture in the presence of 10 μg/ml brefeldin A (Sigma Chemical Co., USA) (53), the cultures from NI and CD were treated with 2 mm EDTA (Sigma Chemical Co., USA) and washed once with 2 ml of fluorescence-activated cell sorter (FACS) buffer prepared as PBS, 0.5% of bovine serum albumin, and 0.1% sodium azide (Sigma Chemical Co., USA). Aliquots of 200 μl cells from cultures were transferred to polystyrene tubes and stained with CD14 FITC (MφP9), CD4 APC (RPA-T4), CD8 PerCP (SK1), and CD19 APC (HIB19) MAbs (BD Pharmingen, USA) for 30 min at RT in the dark. The cells were then fixed in formaldehyde (4%) and permeabilized with saponin buffer (0.5%) (Sigma Chemical Co., USA) for 15 min. Finally, the cells were incubated with 20 μl of phycoerythrin (PE)-labeled anti-cytokine MAbs to IL-2 (MQ1-17H12), IL-4 (8D4-8), IL-10 (JES3-19F1), IFN-γ (4S.B3), and TNF-α (6401.1111) from BD Pharmingen for 30 min at RT in the dark. After intracytoplasmic cytokine staining, the cells were washed and then fixed in MAX FACS FIX solution (MFF) and stored at 4°C prior to flow cytometry acquisition and analysis. Phenotypic analyses were performed by flow cytometry using a Becton Dickinson FACScalibur flow cytometer, collecting data on 30,000 events/sample for lymphocytes (gate by forward and side scatter properties) and 5,000 events/sample for monocytes and using FlowJo software (TreeStar Inc., USA).
Gating strategy to identify cell subsets and intracellular cytokines.
Cytokine profiling of CD14+ cells, CD4+, and CD8+ T-cell subsets was performed by initially gating the monocytes on FL1-CD14 FITC versus SSC and lymphocytes on FSC versus SSC dot plot distribution, followed by quantification of cytokine-expressing cells on FL1 FITC versus FL2 PE for monocytes, FL4 APC versus FL2 PE for CD4+ T-cells, and FL3 PerCP versus FL2 PE for CD8+ T-cell dot plots combinations.
Cytokine signature analysis.
The signatures of the intracellular cytokine profiles were determined according to the method previously proposed by Luiza-Silva et al. (54). For this, the exposure index (EI) of all analyzed biomarkers was used. Initially, we calculated the global median level for each cell population and/or cytokine considering the EI of noninfected subjects and patients with Chagas disease (NI+CD). Thus, the global median was used as a cutoff point to segregate individuals in high (greater than the global median) and low (less than or equal to the global median) cytokine producers.
Ascendant cytokine profile.
The percentage of high-producer individuals was used to obtain the ascendant cytokine profile induced by each anti-T. cruzi lead compound. For this, bar charts were constructed by GraphPad Prism 5.0 software (GraphPad Software, Inc., San Diego, CA, USA), and results above the 50th percentile were considered relevant, highlighted by gray rectangles in Fig. 2 and 3.
Radar charts.
For the establishment of overall cytokine signatures, the percentage of high producers calculated for each cytokine+ leukocyte subset was compiled on radar charts using Microsoft Excel Software (Microsoft Corp., USA). An increase or decrease of the polygonal area reflects a greater or lesser participation, respectively, of cells of innate and adaptive immunity in the production of proinflammatory and/or modulatory cytokines in the treatment with each trypanocidal compound.
Venn diagram.
Common and unique biomarkers upregulated by four anti-T cruzi compounds were represented in a Venn diagram. Bioinformatics & Evolutionary Genomics software (Ghent, Belgium) was used for analysis of the intersections (∩) in the NI and DC groups among the different in vitro treatments.
Statistical analysis.
The CC50 of the lead compounds of interest was calculated by nonlinear regression. Multiple analyses between groups were done by one-way analysis of variance (ANOVA) followed by Tukey's posttest. A comparison of each lead compound with the reference drug was performed with the paired t test. Independent analyses between two groups of cytokines were performed with the unpaired t test. The confidence interval was defined as 95% (α = 0.05), and differences were considered statistically significant at P values of <0.05. All statistical analyses were performed using GraphPad Prism 5.0 software (GraphPad Software, Inc., San Diego, CA, USA).
ACKNOWLEDGMENTS
This work was supported by the European Community's Seventh Framework Programme through collaborative project grant number 602773 (FP7-Health-2013-Innovation-602773) under the project KINDReD (Kinetoplastid Drug Development: Strengthening the preclinical pipeline). O.A.M.F. and A.T.C. are thankful to CNPq for the PQ fellowship program. F.F.A. thanks the PNPD/CAPES fellowship program.
We thank our collaborator James McKerrow for kindly providing the compounds benznidazole and K777. We thank the Program for Technological Development in Tools for Health—PDTIS-FIOCRUZ for use of its facilities.
REFERENCES
- 1.WHO. 2015. Chagas disease (American trypanosomiasis). World Health Organization, Geneva, Switerland: http://www.who.int/mediacentre/factsheets/fs340/en/ Accessed 12 November 2015. [Google Scholar]
- 2.Ministério da Saúde, Brasília, DF, Brazil. 2014. Doença de Chagas. Ministério da Saúde, Brasília, DF, Brazil: http://portalsaude.saude.gov.br/index.php/o-ministerio/principal/secretarias/svs/doenca-de-chagas. [Google Scholar]
- 3.Bock M, Gonnert R, Haberkorn A. 1969. Studies with Bay 2502 on animals. Bol Chil Parasitol 24:13–19. [PubMed] [Google Scholar]
- 4.Richle R. 1973. Chemotherapy of experimental acute Chagas disease in mice: beneficial effect of Ro-71051 on parasitemia and tissue parasitism. Le Progres Medical 101:282. [Google Scholar]
- 5.Sosa Estani S, Segura EL, Ruiz AM, Velazquez E, Porcel BM, Yampotis C. 1998. Efficacy of chemotherapy with benznidazole in children in the indeterminate phase of Chagas' disease. Am J Trop Med Hyg 59:526–529. doi: 10.4269/ajtmh.1998.59.526. [DOI] [PubMed] [Google Scholar]
- 6.Castro JA, de Mecca MM, Bartel LC. 2006. Toxic side effects of drugs used to treat Chagas' disease (American trypanosomiasis). Hum Exp Toxicol 25:471–479. doi: 10.1191/0960327106het653oa. [DOI] [PubMed] [Google Scholar]
- 7.Viotti R, Vigliano C, Lococo B, Alvarez MG, Petti M, Bertocchi G, Armenti A. 2009. Side effects of benznidazole as treatment in chronic Chagas disease: fears and realities. Expert Rev Anti Infect Ther 7:157–163. doi: 10.1586/14787210.7.2.157. [DOI] [PubMed] [Google Scholar]
- 8.Chatelain E. 2015. Chagas disease drug discovery: toward a new era. J Biomol Screen 20:22–35. doi: 10.1177/1087057114550585. [DOI] [PubMed] [Google Scholar]
- 9.McKerrow JH, Doyle PS, Engel JC, Podust LM, Robertson SA, Ferreira R, Saxton T, Arkin M, Kerr ID, Brinen LS, Craik CS. 2009. Two approaches to discovering and developing new drugs for Chagas disease. Mem Inst Oswaldo Cruz 104(Suppl 1):S263–S269. doi: 10.1590/S0074-02762009000900034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doyle PS, Zhou YM, Engel JC, McKerrow JH. 2007. A cysteine protease inhibitor cures Chagas' disease in an immunodeficient-mouse model of infection. Antimicrob Agents Chemother 51:3932–3939. doi: 10.1128/AAC.00436-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barr SC, Warner KL, Kornreic BG, Piscitelli J, Wolfe A, Benet L, McKerrow JH. 2005. A cysteine protease inhibitor protects dogs from cardiac damage during infection by Trypanosoma cruzi. Antimicrob Agents Chemother 49:5160–5161. doi: 10.1128/AAC.49.12.5160-5161.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ekins S, de Siqueira-Neto JL, McCall LI, Sarker M, Yadav M, Ponder EL, Kallel EA, Kellar D, Chen S, Arkin M, Bunin BA, McKerrow JH, Talcott C. 2015. Machine learning models and pathway genome data base for Trypanosoma cruzi drug discovery. PLoS Negl Trop Dis 9:e0003878. doi: 10.1371/journal.pntd.0003878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Golgher D, Gazzinelli RT. 2004. Innate and acquired immunity in the pathogenesis of Chagas disease. Autoimmunity 37:399–409. doi: 10.1080/08916930410001713115. [DOI] [PubMed] [Google Scholar]
- 14.Junqueira C, Caetano B, Bartholomeu DC, Melo MB, Ropert C, Rodrigues MM, Gazzinelli RT. 2010. The endless race between Trypanosoma cruzi and host immunity: lessons for and beyond Chagas disease. Expert Rev Mol Med 12:e29. doi: 10.1017/S1462399410001560. [DOI] [PubMed] [Google Scholar]
- 15.Tarleton RL. 2007. Immune system recognition of Trypanosoma cruzi. Curr Opin Immunol 19:430–434. doi: 10.1016/j.coi.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Sathler-Avelar R, Vitelli-Avelar DM, Massara RL, Borges JD, Lana M, Teixeira-Carvalho A, Dias JC, Eloi-Santos SM, Martins-Filho OA. 2006. Benznidazole treatment during early-indeterminate Chagas' disease shifted the cytokine expression by innate and adaptive immunity cells toward a type 1-modulated immune profile. Scand J Immunol 64:554–563. doi: 10.1111/j.1365-3083.2006.01843.x. [DOI] [PubMed] [Google Scholar]
- 17.Sathler-Avelar R, Vitelli-Avelar DM, Massara RL, de Lana M, Pinto Dias JC, Teixeira-Carvalho A, Eloi-Santos SM, Martins-Filho OA. 2008. Etiological treatment during early chronic indeterminate Chagas disease incites an activated status on innate and adaptive immunity associated with a type 1-modulated cytokine pattern. Microbes Infect 10:103–113. doi: 10.1016/j.micinf.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Sathler-Avelar R, Vitelli-Avelar DM, Eloi-Santos SM, Gontijo ED, Teixeira-Carvalho A, Martins-Filho OA. 2012. Blood leukocytes from benznidazole-treated indeterminate Chagas disease patients display an overall type-1-modulated cytokine profile upon short-term in vitro stimulation with Trypanosoma cruzi antigens. BMC Infect Dis 12:123. doi: 10.1186/1471-2334-12-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marin-Neto JA, Rassi A Jr, Morillo CA, Avezum A, Connolly SJ, Sosa-Estani S, Rosas F, Yusuf S, BENEFIT Investigators. 2008. Rationale and design of a randomized placebo-controlled trial assessing the effects of etiologic treatment in Chagas' cardiomyopathy: the BENznidazole Evaluation For Interrupting Trypanosomiasis (BENEFIT). Am Heart J 156:37–43. doi: 10.1016/j.ahj.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Marin-Neto JA, Rassi A Jr, Avezum A Jr, Mattos AC, Rassi A, Morillo CA, Sosa-Estani S, Yusuf S, BENEFIT Investigators. 2009. The BENEFIT trial: testing the hypothesis that trypanocidal therapy is beneficial for patients with chronic Chagas heart disease. Mem Inst Oswaldo Cruz 104(Suppl 1):S319–S324. doi: 10.1590/S0074-02762009000900042. [DOI] [PubMed] [Google Scholar]
- 21.Morillo CA, Marin-Neto JA, Avezum A, Sosa-Estani S, Rassi A Jr, Rosas F, Villena E, Quiroz R, Bonilla R, Britto C, Guhl F, Velazquez E, Bonilla L, Meeks B, Rao-Melacini P, Pogue J, Mattos A, Lazdins J, Rassi A, Connolly SJ, Yusuf S, Investigators B. 2015. Randomized trial of benznidazole for chronic Chagas' cardiomyopathy. N Engl J Med 373:1295–1306. doi: 10.1056/NEJMoa1507574. [DOI] [PubMed] [Google Scholar]
- 22.Bahia MT, de Andrade IM, Martins TA, do Nascimento AF, Diniz Lde F, Caldas IS, Talvani A, Trunz BB, Torreele E, Ribeiro I. 2012. Fexinidazole: a potential new drug candidate for Chagas disease. PLoS Negl Trop Dis 6:e1870. doi: 10.1371/journal.pntd.0001870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Molina J, Martins-Filho O, Brener Z, Romanha AJ, Loebenberg D, Urbina JA. 2000. Activities of the triazole derivative SCH 56592 (posaconazole) against drug-resistant strains of the protozoan parasite Trypanosoma (Schizotrypanum) cruzi in immunocompetent and immunosuppressed murine hosts. Antimicrob Agents Chemother 44:150–155. doi: 10.1128/AAC.44.1.150-155.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diniz Lde F, Caldas IS, Guedes PM, Crepalde G, de Lana M, Carneiro CM, Talvani A, Urbina JA, Bahia MT. 2010. Effects of ravuconazole treatment on parasite load and immune response in dogs experimentally infected with Trypanosoma cruzi. Antimicrob Agents Chemother 54:2979–2986. doi: 10.1128/AAC.01742-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engel JC, Doyle PS, Hsieh I, McKerrow JH. 1998. Cysteine protease inhibitors cure an experimental Trypanosoma cruzi infection. J Exp Med 188:725–734. doi: 10.1084/jem.188.4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engel JC, Doyle PS, Palmer J, Hsieh I, Bainton DF, McKerrow JH. 1998. Cysteine protease inhibitors alter Golgi complex ultrastructure and function in Trypanosoma cruzi. J Cell Sci 111(Part 5):597–606. [DOI] [PubMed] [Google Scholar]
- 27.Childs GE, Hausler B, Milhous W, Chen C, Wimonwattrawatee T, Pooyindee N, Boudreau EF. 1988. In vitro activity of pyronaridine against field isolates and reference clones of Plasmodium falciparum. Am J Trop Med Hyg 38:24–29. doi: 10.4269/ajtmh.1988.38.24. [DOI] [PubMed] [Google Scholar]
- 28.Pradines B, Mabika Mamfoumbi M, Parzy D, Owono Medang M, Lebeau C, Mourou Mbina JR, Doury JC, Kombila M. 1999. In vitro susceptibility of African isolates of Plasmodium falciparum from Gabon to pyronaridine. Am J Trop Med Hyg 60:105–108. doi: 10.4269/ajtmh.1999.60.105. [DOI] [PubMed] [Google Scholar]
- 29.Vivas L, Rattray L, Stewart L, Bongard E, Robinson BL, Peters W, Croft SL. 2008. Anti-malarial efficacy of pyronaridine and artesunate in combination in vitro and in vivo. Acta Trop 105:222–228. doi: 10.1016/j.actatropica.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 30.Auparakkitanon S, Chapoomram S, Kuaha K, Chirachariyavej T, Wilairat P. 2006. Targeting of hematin by the antimalarial pyronaridine. Antimicrob Agents Chemother 50:2197–2200. doi: 10.1128/AAC.00119-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rizk MA, El-Sayed SA, Terkawi MA, Youssef MA, El Said el Sel S, Elsayed G, El-Khodery S, El-Ashker M, Elsify A, Omar M, Salama A, Yokoyama N, Igarashi I. 2015. Optimization of a fluorescence-based assay for large-scale drug screening against Babesia and Theileria parasites. PLoS One 10:e0125276. doi: 10.1371/journal.pone.0125276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qi J, Wang S, Liu G, Peng H, Wang J, Zhu Z, Yang C. 2004. Pyronaridine, a novel modulator of P-glycoprotein-mediated multidrug resistance in tumor cells in vitro and in vivo. Biochem Biophys Res Commun 319:1124–1131. doi: 10.1016/j.bbrc.2004.05.099. [DOI] [PubMed] [Google Scholar]
- 33.Eisig JN, Silva FM, Rodriguez TN, Hashimoto CL, Barbuti RC. 2005. A furazolidone-based quadruple therapy for Helicobacter pylori retreatment in patients with peptic ulcer disease. Clinics (Sao Paulo) 60:485–488. doi: 10.1590/S1807-59322005000600010. [DOI] [PubMed] [Google Scholar]
- 34.Hausen MA, Freitas JC Jr, Monteiro-Leal LH. 2006. The effects of metronidazole and furazolidone during Giardia differentiation into cysts. Exp Parasitol 113:135–141. doi: 10.1016/j.exppara.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 35.Narcisi EM, Secor WE. 1996. In vitro effect of tinidazole and furazolidone on metronidazole-resistant Trichomonas vaginalis. Antimicrob Agents Chemother 40:1121–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang X, Sun L, Qiu JJ, Sun X, Li S, Wang X, So CW, Dong S. 2013. A novel application of furazolidone: anti-leukemic activity in acute myeloid leukemia. PLoS One 8:e72335. doi: 10.1371/journal.pone.0072335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reimao JQ, Taniwaki NN, Tempone AG. 2010. Furazolidone is a selective in vitro candidate against Leishmania (L.) chagasi: an ultrastructural study. Parasitol Res 106:1465–1469. doi: 10.1007/s00436-010-1826-x. [DOI] [PubMed] [Google Scholar]
- 38.International Organization for Standardization. 2009. International standard: biological evaluation of medical devices part 5: tests for cytotoxicity: in vitro methods. ISO10993-5. International Organization for Standardization, Geneva, Switzerland. [Google Scholar]
- 39.Engel JC, Ang KK, Chen S, Arkin MR, McKerrow JH, Doyle PS. 2010. Image-based high-throughput drug screening targeting the intracellular stage of Trypanosoma cruzi, the agent of Chagas' disease. Antimicrob Agents Chemother 54:3326–3334. doi: 10.1128/AAC.01777-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Souza EM, Araujo-Jorge TC, Bailly C, Lansiaux A, Batista MM, Oliveira GM, Soeiro MN. 2003. Host and parasite apoptosis following Trypanosoma cruzi infection in in vitro and in vivo models. Cell Tissue Res 314:223–235. doi: 10.1007/s00441-003-0782-5. [DOI] [PubMed] [Google Scholar]
- 41.Antonelli LR, Dutra WO, Almeida RP, Bacellar O, Carvalho EM, Gollob KJ. 2005. Activated inflammatory T cells correlate with lesion size in human cutaneous leishmaniasis. Immunol Lett 101:226–230. doi: 10.1016/j.imlet.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 42.de Souza PE, Gomez RS, Xavier GM, dos Santos JS, Gollob KJ, Dutra WO. 2005. Systemic leukocyte activation in patients with central giant cell lesions. J Oral Pathol Med 34:312–317. doi: 10.1111/j.1600-0714.2004.00276.x. [DOI] [PubMed] [Google Scholar]
- 43.Gaze ST, Dutra WO, Lessa M, Lessa H, Guimaraes LH, Jesus AR, Carvalho LP, Machado P, Carvalho EM, Gollob KJ. 2006. Mucosal leishmaniasis patients display an activated inflammatory T-cell phenotype associated with a nonbalanced monocyte population. Scand J Immunol 63:70–78. doi: 10.1111/j.1365-3083.2005.01707.x. [DOI] [PubMed] [Google Scholar]
- 44.Talvani A, Ribeiro CS, Aliberti JC, Michailowsky V, Santos PV, Murta SM, Romanha AJ, Almeida IC, Farber J, Lannes-Vieira J, Silva JS, Gazzinelli RT. 2000. Kinetics of cytokine gene expression in experimental chagasic cardiomyopathy: tissue parasitism and endogenous IFN-gamma as important determinants of chemokine mRNA expression during infection with Trypanosoma cruzi. Microbes Infect 2:851–866. doi: 10.1016/S1286-4579(00)00388-9. [DOI] [PubMed] [Google Scholar]
- 45.Teixeira MM, Gazzinelli RT, Silva JS. 2002. Chemokines, inflammation and Trypanosoma cruzi infection. Trends Parasitol 18:262–265. doi: 10.1016/S1471-4922(02)02283-3. [DOI] [PubMed] [Google Scholar]
- 46.Gomes JA, Bahia-Oliveira LM, Rocha MO, Martins-Filho OA, Gazzinelli G, Correa-Oliveira R. 2003. Evidence that development of severe cardiomyopathy in human Chagas' disease is due to a Th1-specific immune response. Infect Immun 71:1185–1193. doi: 10.1128/IAI.71.3.1185-1193.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Souza PE, Rocha MO, Rocha-Vieira E, Menezes CA, Chaves AC, Gollob KJ, Dutra WO. 2004. Monocytes from patients with indeterminate and cardiac forms of Chagas' disease display distinct phenotypic and functional characteristics associated with morbidity. Infect Immun 72:5283–5291. doi: 10.1128/IAI.72.9.5283-5291.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leite ALJ, Paula Costa G, Lopes LR, Reis Mota LWD, Vieira PMA, Talvani A. 2017. The immunomodulatory effects of the Enalapril in combination with Benznidazole during acute and chronic phases of the experimental infection with Trypanosoma cruzi. Acta Trop 174:136–145. doi: 10.1016/j.actatropica.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 49.WHO. 2002. Control of Chagas disease. WHO Technical Report Series. World Health Organization, Geneva, Switzerland. [PubMed] [Google Scholar]
- 50.Romanha AJ, Castro SL, Soeiro MN, Lannes-Vieira J, Ribeiro I, Talvani A, Bourdin B, Blum B, Olivieri B, Zani C, Spadafora C, Chiari E, Chatelain E, Chaves G, Calzada JE, Bustamante JM, Freitas-Junior LH, Romero LI, Bahia MT, Lotrowska M, Soares M, Andrade SG, Armstrong T, Degrave W, Andrade ZA. 2010. In vitro and in vivo experimental models for drug screening and development for Chagas disease. Mem Inst Oswaldo Cruz 105:233–238. doi: 10.1590/S0074-02762010000200022. [DOI] [PubMed] [Google Scholar]
- 51.Gazzinelli G, Katz N, Rocha RS, Colley DG. 1983. Immune responses during human schistosomiasis mansoni. X. Production and standardization of an antigen-induced mitogenic activity by peripheral blood mononuclear cells from treated, but not active cases of schistosomiasis. J Immunol 130:2891–2895. [PubMed] [Google Scholar]
- 52.Dutra WO, Colley DG, Pinto-Dias JC, Gazzinelli G, Brener Z, Pereira ME, Coffman RL, Correa-Oliveira R, Carvalho-Parra JF. 2000. Self and nonself stimulatory molecules induce preferential expansion of CD5+ B cells or activated T cells of chagasic patients, respectively. Scand J Immunol 51:91–97. doi: 10.1046/j.1365-3083.2000.00648.x. [DOI] [PubMed] [Google Scholar]
- 53.Jung T, Schauer U, Heusser C, Neumann C, Rieger C. 1993. Detection of intracellular cytokines by flow cytometry. J Immunol Methods 159:197–207. doi: 10.1016/0022-1759(93)90158-4. [DOI] [PubMed] [Google Scholar]
- 54.Silva ML, Martins MA, Espirito-Santo LR, Campi-Azevedo AC, Silveira-Lemos D, Ribeiro JG, Homma A, Kroon EG, Teixeira-Carvalho A, Eloi-Santos SM, Martins-Filho OA. 2011. Characterization of main cytokine sources from the innate and adaptive immune responses following primary 17DD yellow fever vaccination in adults. Vaccine 29:583–592. doi: 10.1016/j.vaccine.2010.08.046. [DOI] [PubMed] [Google Scholar]