ABSTRACT
We performed a multicenter, prospective, randomized study to investigate the efficacy and safety of clofazimine (CLO) for treatment of extensively drug-resistant tuberculosis (XDR-TB) in China. Forty-nine patients infected with XDR-TB were randomly assigned to either the control group or the CLO group, both of which received 36 months of individually customized treatment. The primary endpoint was the time to sputum culture conversion on solid medium. Clinical outcomes of patients were evaluated at the time of treatment completion. Of the 22 patients in the experimental group, 7 (31.8%) met the treatment criterion of “cure” and 1 (4.5%) “complete treatment,” for a total of 8 (36.4%) exhibiting successful treatment outcomes without relapse. In the control group, 6 patients (22.2%) were cured and 6 (22.2%) completed treatment by the end of the study. Statistical analysis revealed no significant difference in successful outcome rates between the CLO group and the control group. The average sputum culture conversion time for the experimental group was 19.7 months, which was not statistically different from that for the control group (20.3 months; P = 0.57). Of the 22 patients in the CLO group, 12 (54.5%) experienced adverse events after starting CLO treatment. The most frequently observed adverse event was liver damage, with 31.8% of patients (7/22 patients) in the CLO group versus 11.1% (3/27 patients) in the control group exhibiting this adverse event. Our study demonstrates that inclusion of CLO in background treatment regimens for XDR-TB is of limited benefit, especially since hepatic disorders arise as major adverse events with CLO treatment. (This study is registered with the Chinese Clinical Trial Registry [ChiCTR, www.chictr.org.cn] under identifier ChiCTR1800014800.)
KEYWORDS: clofazimine, extensively drug-resistant tuberculosis, clinical outcome, efficacy, safety
INTRODUCTION
Despite recent progress in reducing the global incidence and mortality rates of tuberculosis (TB), increasing prevalence of multidrug-resistant TB (MDR-TB), with resistance to rifampin (RIF) and isoniazid (INH), poses a serious obstacle to TB control (1, 2). Of even greater concern, MDR-TB isolates with additional resistance to a fluoroquinolone (FQ) and at least one injectable drug (kanamycin, amikacin, or capreomycin) have been reported and are referred to as extensively drug-resistant TB (XDR-TB) (3, 4). In fact, the World Health Organization (WHO) estimated that almost 10% of 480,000 MDR-TB cases in 2013 were actually XDR-TB (5). Treatment of MDR-TB and XDR-TB cases requires burdensome, ineffective, and poorly tolerated second-line anti-TB agents, with recommended treatment courses lasting up to 24 months (6). Unfortunately, even patients who complete treatment often exhibit unsatisfactory treatment outcomes, with a success rate of only ∼50% among MDR-TB patients by the end of treatment (7). Response rates among XDR-TB patients are even worse, due to their extended drug resistance to even the most potent second-line anti-TB drugs, thus highlighting the urgent need for improved regimens against XDR-TB.
Clofazimine (CLO), a member of the riminophenazine antibiotic class, was initially developed for the treatment of TB (8, 9). Unfortunately, preliminary experiments showed that CLO exhibited poor in vivo efficacy against Mycobacterium tuberculosis in guinea pig and simian models, despite its potent activity against Mycobacterium leprae (10, 11). Therefore, CLO was subsequently used widely only to treat M. leprae rather than M. tuberculosis infections. In recent years, a series of studies have been carried out to evaluate the efficacy, safety, and tolerability of CLO-containing regimens for the treatment of drug-resistant TB, especially MDR-TB and XDR-TB cases (12–15). A meta-analysis found that a greater percentage of patients infected with MDR-TB experienced favorable treatment outcomes after treatment with CLO, compared with patients who received no CLO (8). In addition, contradictory evidence with regard to the efficacy of CLO-containing regimens for treatment of XDR-TB across numerous clinical trials has been attributed mainly to the limited number of XDR-TB patients enrolled in those studies (16). Thus, in order to broaden the evidence base, we carried out a randomized clinical prospective study to evaluate the efficacy of CLO when added to the recommended regimen for treatment of XDR-TB patients. Safety and tolerability were also assessed in this cohort study.
RESULTS
Study patients.
A total of 49 XDR-TB-infected patients who met the inclusion criteria were enrolled in the study. On the basis of randomized assignment, 22 patients were assigned to the experimental group, while the other 27 were assigned to the control group (Fig. 1). Of the 49 XDR-TB patients, 43 had received anti-TB treatment for more than 1 year before their recruitment into the study. The mean number of drugs to which patient isolates were resistant was 6.0 for both groups. No significant differences in demographic, clinical, or drug susceptibility features between the two groups were observed (Table 1).
FIG 1.
Enrollment and outcomes.
TABLE 1.
Demographic and clinical characteristics of XDR-TB patients enrolled in this study
| Characteristic | Experimental group (n = 22) | Control group (n = 27) | P |
|---|---|---|---|
| Age (mean [range]) (yr) | 42.40 (25–65) | 42.46 (20–62) | 0.987 |
| Male (no. [%]) | 17 (77.3) | 19 (70.4) | 0.587 |
| Body mass index (mean [range]) (kg/m2) | 18.91 (12.91–25.35) | 21.15 (12.49–26.75) | 0.191 |
| Treatment history (no. [%]) | |||
| New case | 0 (0.0) | 3 (11.1) | 0.310 |
| Previously treated case | 22 (100.0) | 24 (88.9) | |
| Treatment duration for previously treated patients (mean [range]) (mo) | 36.14 (9–96) | 24.96 (1–66) | 0.194 |
| Comorbidity (no. [%]) | |||
| Diabetes mellitus | 1 (4.5) | 1 (3.7) | 1.000 |
| COPDa | 1 (4.5) | 2 (7.4) | |
| Cardiopathy | 0 (0.0) | 1 (3.7) | |
| Course of disease (no. [%]) | |||
| ≤1 y before randomization | 0 (0.0) | 4 (14.8) | 0.221 |
| >1 y to 3 y before randomization | 6 (27.3) | 7 (25.9) | |
| >3 y before randomization | 16 (72.7) | 16 (59.3) | |
| Previous treatment (no. [%]) | |||
| ≤1 y before randomization | 1 (4.5) | 5 (18.5) | 0.265 |
| >1 y to 3 y before randomization | 10 (45.5) | 13 (48.1) | |
| >3 y before randomization | 11 (50.0) | 9 (33.3) | |
| Susceptibility test result of resistance (no. [%]) | |||
| Isoniazid | 22 (100.0) | 27 (100.0) | |
| Rifampin | 22 (100.0) | 27 (100.0) | |
| Ethambutol | 13 (59.1) | 16 (59.3) | 0.990 |
| Ofloxacin | 22 (100.0) | 27 (100.0) | |
| Amikacin | 22 (100.0) | 27 (100.0) | |
| Capreomycin | 20 (90.9) | 24 (88.9) | 1.000 |
| No. of drugs with resistance (median [range]) | 6.0 (4–8) | 6.0 (4–8) | |
| Background regimen (no. [%]) | |||
| Prothionamide | 22 (100.0) | 27 (100.0) | |
| Pyrazinamide | 22 (100.0) | 27 (100.0) | |
| Moxifloxacin or levofloxacin | 22 (100.0) | 27 (100.0) | |
| p-Aminosalicylic acid | 20 (90.9) | 22 (81.5) | 0.598 |
| Capreomycin or amikacin | 19 (86.4) | 23 (85.2) | 1.000 |
| Ethambutol | 22 (100.0) | 27 (100.0) |
COPD, chronic obstructive pulmonary disease.
Patients were monitored during 36 months of XDR-TB treatment. Of 22 patients in the experimental group, therapy was discontinued for 5 patients who were lost to follow-up monitoring (n = 2), suffered an adverse event (n = 2), or withdrew consent (n = 1). In the control group, four patients discontinued therapy due to lack of follow-up monitoring (n = 2) or adverse events (n = 2) (Fig. 1).
Treatment outcomes.
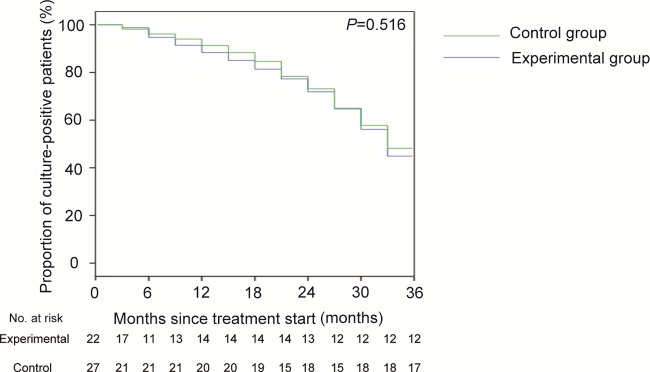
Of the 22 patients in the experimental group, 7 patients (31.8%) met the definition of “cure” and 1 (4.5%) met the definition of “complete treatment,” for a total of 8/22 patients (36.4%) who had successful treatment outcomes without relapse (Table 2). In contrast, 7/22 patients (31.8%) failed to clear the bacteria and 2/22 died of XDR-TB during treatment. Of 27 patients in the control group, 6/27 patients (22.2%) were cured and 6/27 (22.2%) completed treatment, while 8/27 patients (29.6%) failed treatment and 3/27 (11.1%) died by study completion. Statistical analysis revealed that there was no significant difference in the rates of successful outcomes between the CLO group and the control group. We analyzed the time to sputum culture conversion using data collected every 3 months during the follow-up period. The average time to sputum culture conversion for the experimental group was 19.7 months, which was not statistically significantly different from that for the control group (20.3 months; P = 0.57) (Fig. 2).
TABLE 2.
Clinical outcomes of patients enrolled in this study
| Clinical outcome | No. of patients (%) |
P | |
|---|---|---|---|
| Experimental group | Control group | ||
| Favorable outcome | 0.493 | ||
| Cure | 7 (31.8) | 6 (22.2) | 0.449 |
| Treatment completion | 1 (4.5) | 6 (22.2) | 0.178 |
| Adverse outcome | 0.493 | ||
| Failure | 7 (31.8) | 8 (29.6) | 0.869 |
| Death | 2 (9.1) | 3 (11.1) | 1.000 |
| Default | 5 (22.7) | 4 (14.8) | 0.733 |
FIG 2.
Times to sputum culture conversion in the control and experimental groups.
Safety.
Of the 22 patients in the CLO group, 12 experienced adverse events after starting CLO treatment. The most frequently observed adverse event was liver damage, with 31.8% of patients in the CLO group versus 11.1% in the control group exhibiting this adverse event; however, the incidence rate of hepatic damage in the CLO group was not significantly higher than that for the control group (P = 0.09). The median time to onset of hepatic damage was 9 months for the CLO group (range, 4 to 24 months) and 6 months for the control group (range, 2 to 11 months). Importantly, among the patients experiencing hepatic damage, the median time to symptom improvement was only 3 weeks for both groups. Skin discoloration occurred in only 5 patients from the CLO group. In addition, the rates of other adverse events for patients treated with CLO were similar to those for patients not treated with CLO (P > 0.05) (Table 3).
TABLE 3.
Adverse events during 36 months of treatment among patients enrolled in this study
| Adverse event | No. of patients (%) |
P | |
|---|---|---|---|
| Experimental group | Control group | ||
| Skin discoloration | 5 (22.7) | 0 (0.0) | 0.014 |
| Hepatic damage | 7 (31.8) | 3 (11.1) | 0.090 |
| Renal damage | 2 (9.1) | 3 (11.1) | 1.000 |
| Other | 1 (4.6) | 3 (11.1) | 0.617 |
DISCUSSION
In this study, we found that the inclusion of CLO as part of an individualized regimen for the treatment of XDR-TB did not increase the rate of favorable outcomes or shorten the time to culture conversion, compared with patients who received no CLO. Indeed, a large variance in the success rates for patients receiving CLO-containing drug combinations has been observed in other studies (8). In line with our observations, reports from Brazil and Sri Lanka revealed that the success rates for drug-resistant TB patients treated with CLO-containing regimens were not significantly different from those for patients treated with non-CLO-containing regimens (17, 18). In contrast, a series of other studies revealed that the presence of CLO was an independent indicator of culture conversion among drug-resistant TB patients (13, 19). There are several potential explanations for these inconsistent findings. On one hand, the limited benefit of CLO may reflect the favorable efficacy of the background regimens used in the present study. In a retrospective multicenter study from China, the treatment success rate in the XDR-TB group was only 13% (20). Similar poor clinical outcomes were reported for XDR-TB patients from South Korea (18%) and the United States (20%) (21, 22), with results lower than the ∼50% culture conversion rate in our study. Compared with the 24-month regimens endorsed by the WHO, personalized regimens using six drugs and 36-month treatment duration might produce more favorable clinical outcomes in the treatment of XDR-TB. In view of our data from a limited sample of XDR-TB cases, the poor efficacy achieved after inclusion of CLO in potent drug treatment regimens should be verified in a controlled clinical trial incorporating more patients. Our data indicate that the addition of CLO may not improve primary and final clinical outcomes in the treatment of XDR-TB. On the other hand, there is no doubt that TB patients infected with organisms with broader-spectrum drug resistance patterns exhibit poorer treatment outcomes. The patients enrolled in this study were all XDR-TB patients with prolonged previous treatment durations, while most participants in previous studies were MDR-TB patients and thus were more susceptible to second-line anti-TB drugs than were patients with XDR-TB. As noted by Velayati and colleagues (23), the cell walls of XDR-TB isolates are significantly thicker than those of both MDR-TB and susceptible isolates, thus potentially reducing the permeability of the bacteria to drugs and increasing overall drug resistance, including resistance to CLO.
Our data also demonstrated that 12 (55%) of 22 patients in this study experienced adverse events after starting CLO treatment. This rate is similar to results reported from Bangladesh (48%) but lower than rates in Shanghai (89%) and Norway (70%), while higher than rates in the United States (12%) and in the U.S. state of Georgia (11%) (8, 12, 24). There are several potential factors that determine the incidence of adverse events. First, adverse events are thought be related to the administered dose, because decreasing the dose of CLO in one study brought benefits to patients suffering from skin discoloration and gastrointestinal side effects (12). Second, an extended duration of treatment could also contribute to the high incidence of adverse effects.
Despite the lack of significant differences due to the small sample size, our results indicate that the inclusion of CLO in the background regimen may yield an increased incidence of hepatic disorders, compared with the control group. In contrast, a recent clinical trial by Tang et al. demonstrated that there was no significant increase in the incidence of hepatic disorders when CLO was added to the treatment regimen (13). This discrepancy may stem from the application of a less aggressive drug treatment regimen against MDR-TB in that study, compared to the regimen used against XDR-TB in this study. Although hepatic disorders are not a common adverse event associated with CLO administration, several agents in the background regimen, such as protionamide, pyrazinamide, p-aminosalicylic acid, and ethambutol, could contribute to hepatic disorders (25). Given that the major in vivo drug metabolism of CLO relies on liver cytochrome P450 activity for detoxification (26), we hypothesize that the addition of CLO to the background regimen may increase the metabolic burden of liver cells, thereby leading to a high rate of hepatic damage when CLO is used in combination with other drugs. Hence, our data raise the question of whether routine liver function tests should be performed to monitor XDR-TB patients receiving treatments containing CLO.
We also acknowledge several obvious limitations of the present study. First, despite the enrollment of all patients who met the criteria throughout the study period, the small number of patients, associated with a low prevalence of XDR-TB, limits the overall significance of our study conclusions. Second, the correlation between in vitro susceptibility to CLO and clinical outcomes was not analyzed, since a standardized in vitro drug susceptibility test method for CLO was not employed during the study period. Third, the treatment outcomes for XDR-TB patients were assessed only at the end of treatment; therefore, the posttreatment relapse rate was not analyzed. Despite these limitations, our study provides important insights into the role of CLO for the treatment of XDR-TB.
In conclusion, our randomized controlled trial has demonstrated that the inclusion of CLO in the background treatment regimen is of limited benefit for patients with XDR-TB. In addition, hepatic disorders emerge as major adverse events for patients receiving complicated regimens containing CLO. In view of the limitations of this study, a controlled clinical trial with more patients, to determine the efficacy of CLO in the treatment of patients with XDR-TB, is warranted.
MATERIALS AND METHODS
Participants.
Patients were recruited from 11 specialized TB hospitals in China, including Beijing Chest Hospital, Shanghai Pulmonary Hospital, The First Affiliated Hospital of Chongqing Medical University, The Third People's Hospital of Shenzhen, The Sixth People's Hospital of Nantong, Shenyang Chest Hospital, Chongqing Public Health Medical Center, Jiamusi Tuberculosis Control Hospital, The First Affiliated Hospital of Xiamen University, Hangzhou Red Cross Hospital, and Qingdao Chest Hospital. From 2009 to 2010, we enrolled pulmonary patients with positive sputum smears, 18 to 65 years of age, who were harboring XDR-TB, on the basis of in vitro phenotypic drug susceptibility testing. Susceptibility testing was performed for M. tuberculosis isolates using rifampin, isoniazid, ethambutol, ofloxacin, amikacin, and capreomycin (except pyrazinamide), according to the proportion method, on Lowenstein-Jenson (L-J) medium. Exclusion criteria included (i) allergy to CLO or any other antimicrobial agents used in this study; (ii) previous treatment with CLO; (iii) a positive result for human immunodeficiency virus (HIV); (iv) pregnant or breastfeeding; (v) severe cardiovascular, liver, kidney, or other comorbidities; and (vi) a history of psychiatric illness.
Study design.
This was a multicenter, prospective, randomized study conducted in 11 hospitals in China. After enrollment, participants were randomly assigned to either the control group or the CLO group by using a computer-generated random-number table, as administered by the staff of Beijing Chest Hospital. Participants and clinical staff members were not blinded throughout the study period.
The WHO-endorsed four-drug regimen included capreomycin, moxifloxacin, p-aminosalicylic acid, and ethambutol (pyrazinamide) for both groups (27). Drug susceptibility testing was used to determine two other effective drugs from drug groups 4 and 5 to add to the WHO-endorsed regimen, for an individualized treatment regimen for each patient. Finally, patients enrolled in the CLO group also received 100 mg CLO once daily in addition to their individualized background regimens. Detailed administration of overall drug regimens for each treatment phase are summarized in Fig. 3. Drugs were administered by means of directly observed therapy (DOT) during hospitalization. A trained family member administered DOT during the outpatient period, and health workers monitored therapeutic compliance by means of weekly telephone interviews and monthly checks of pill counts. Medical expenditures were covered through health care insurance and financial support through the National Science and Technology Major Program of China. The study was approved by the ethics committees of Beijing Chest Hospital, Capital Medical University. Each participant provided written informed consent prior to enrollment in this study. This study is registered with the Chinese Clinical Trial Registry (ChiCTR, www.chictr.org.cn) under identifier ChiCTR1800014800.
FIG 3.
Treatment regimens for the control and experimental groups in this study. Cm, capreomycin; Mfx, moxifloxacin; Gfx, gatifloxacin; Z, pyrazinamide; E, ethambutol; PAS, p-aminosalicylic acid; Pto, protionamide.
Efficacy assessment.
After enrollment, clinical visits were scheduled to establish baseline pretreatment patient information. All patients were then monitored for 3 years through clinical assessments every 3 months. Routine examinations during each clinical visit included a physical examination, radiological examination, complete blood count, and biochemical examination of blood. In addition, sputum samples were collected for culture of M. tuberculosis on L-J medium. Drug susceptibility testing for first- and second-line drugs was performed using the proportion method, on L-J medium (27).
The primary endpoint was used to determine the percentage of patients with overall successful outcomes by the end of treatment. Patient treatment outcomes were classified based on criteria outlined by the WHO and presented in previous publications, as described by Laserson et al. (28). Briefly, patients were assigned the treatment outcome of cure if they completed treatment with no evidence of failure and provided three or more consecutive negative cultures, taken at least 30 days apart, after the intensive phase. Patients with treatment outcomes designated complete treatment finished the treatment regimen according to the study design but did not meet the definition of cure due to a lack of three or more consecutive negative cultures, taken at least 30 days apart, after the intensive phase. Patients with treatment outcomes designated treatment failure included patients who terminated treatment or required a change in their permanent treatment regimen involving at least two anti-TB drugs. Treatment failure included lack of conversion by the end of the intensive phase, bacterial reversion to positive status during the continuation phase after conversion to negative status, or adverse drug reactions. The outcome designated death included patients who died for any reason during the course of treatment. The outcome designated default included patients whose treatment was interrupted for 2 or more consecutive months. Patients with successful outcomes were patients who either were cured or completed treatment.
The safety and tolerability of treatment were monitored through blood counts, alanine aminotransferase levels, aspartate aminotransferase levels, uric acid examinations, and patient self-reporting. Hepatic damage was defined as elevation of serum transaminase levels to at least 3 times the upper limits of normal levels in the presence of gastrointestinal symptoms or elevation of serum transaminase levels to at least 5 times the upper limits of normal levels without symptoms. Renal damage was defined as elevation of creatinine levels to at least 1.3 times the upper limit of normal levels. Skin discoloration was defined as the visible presence of reddish discoloration/pigmentation and ichthyotic changes of the skin. Adverse events were recorded daily. A patient safety committee supervised the monitoring of patient safety throughout the study and stopped treatment in the event of the occurrence of anti-TB-drug-related adverse events defined as grade 4 or higher.
Statistical analysis.
The sample size calculation was based on an assumption of favorable outcome rates of 25% and 65% for the control and experimental groups, respectively (29). To achieve 80% power using α set to 0.05, 22 patients were needed for each experimental condition. Given that 20% of the patients in each study group might have outcomes that could not be evaluated, we determined that a sample size of 28 patients per group would suffice.
Statistical analysis was performed using SPSS software, version 22 (SPSS, Chicago, IL, USA). The chi-square test was used to evaluate the distribution patterns of categorical variables. If the smallest expected frequency was less than 5, then Fisher's exact test was used to compare categorical variables. Kaplan-Meier analysis was used to compare the times to smear and culture conversion between patients in the control group and those in the CLO group. Statistical significance was declared for P values of <0.05.
ACKNOWLEDGMENTS
We express our thanks to Yongai Luo and Xichen Huang (The First Affiliated Hospital of Chongqing Medical University), Yuan Gao (Shenyang Chest Hospital), Qiangzhong Sun (Chongqing Infectious Disease Medical Center), Junwei Shi (The Sixth People's Hospital of Nantong City Jiangsu Province), Wenjuan Lv (Jiamusi Tuberculosis Control Hospital), and Jian Lu (The Third People's Hospital of Shenzhen) for their time and effort in data collection and patient follow-up monitoring.
H. Huang, X. Chen, and N. Chu designed the study. Y. Pang, W. Jing, and T. Xu participated in data analysis. Q. Wang, Y. Pang, and W. Jing wrote the manuscript. Y. Liu, N. Wang, H. Yin, Q. Zhang, Z. Ye, M. Zhu, F. Li, P. Liu, T. Wu, W. Chen, W. Wu, Z. Qin, C. Qiu, Q. Deng, Jing Wang, R. Guo, Y. Du, and Jun Wang participated in data collection and patient follow-up monitoring. All authors approved the final version of the article.
This work was supported by the National Science and Technology Major Program of China (grant 2017ZX09304009).
We declare no conflicts of interest.
REFERENCES
- 1.Gandhi NR, Nunn P, Dheda K, Schaaf HS, Zignol M, van Soolingen D, Jensen P, Bayona J. 2010. Multidrug-resistant and extensively drug-resistant tuberculosis: a threat to global control of tuberculosis. Lancet 375:1830–1843. doi: 10.1016/S0140-6736(10)60410-2. [DOI] [PubMed] [Google Scholar]
- 2.Lange C, Abubakar I, Alffenaar JW, Bothamley G, Caminero JA, Carvalho AC, Chang KC, Codecasa L, Correia A, Crudu V, Davies P, Dedicoat M, Drobniewski F, Duarte R, Ehlers C, Erkens C, Goletti D, Gunther G, Ibraim E, Kampmann B, Kuksa L, de Lange W, van Leth F, van Lunzen J, Matteelli A, Menzies D, Monedero I, Richter E, Rusch-Gerdes S, Sandgren A, Scardigli A, Skrahina A, Tortoli E, Volchenkov G, Wagner D, van der Werf MJ, Williams B, Yew WW, Zellweger JP, Cirillo DM. 2014. Management of patients with multidrug-resistant/extensively drug-resistant tuberculosis in Europe: a TBNET consensus statement. Eur Respir J 44:23–63. doi: 10.1183/09031936.00188313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang L, Pang Y, Yu X, Wang Y, Gao M, Huang H, Zhao Y. 2014. Linezolid in the treatment of extensively drug-resistant tuberculosis. Infection 42:705–711. doi: 10.1007/s15010-014-0632-2. [DOI] [PubMed] [Google Scholar]
- 4.Pym AS, Diacon AH, Tang SJ, Conradie F, Danilovits M, Chuchottaworn C, Vasilyeva I, Andries K, Bakare N, De Marez T, Haxaire-Theeuwes M, Lounis N, Meyvisch P, Van Baelen B, van Heeswijk RP, Dannemann B. 2016. Bedaquiline in the treatment of multidrug- and extensively drug-resistant tuberculosis. Eur Respir J 47:564–574. doi: 10.1183/13993003.00724-2015. [DOI] [PubMed] [Google Scholar]
- 5.Guglielmetti L, Le Du D, Jachym M, Henry B, Martin D, Caumes E, Veziris N, Metivier N, Robert J. 2015. Compassionate use of bedaquiline for the treatment of multidrug-resistant and extensively drug-resistant tuberculosis: interim analysis of a French cohort. Clin Infect Dis 60:188–194. doi: 10.1093/cid/ciu786. [DOI] [PubMed] [Google Scholar]
- 6.Falzon D, Schunemann HJ, Harausz E, Gonzalez-Angulo L, Lienhardt C, Jaramillo E, Weyer K. 2017. World Health Organization treatment guidelines for drug-resistant tuberculosis, 2016 update. Eur Respir J 49:1602308. doi: 10.1183/13993003.02308-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orenstein EW, Basu S, Shah NS, Andrews JR, Friedland GH, Moll AP, Gandhi NR, Galvani AP. 2009. Treatment outcomes among patients with multidrug-resistant tuberculosis: systematic review and meta-analysis. Lancet Infect Dis 9:153–161. doi: 10.1016/S1473-3099(09)70041-6. [DOI] [PubMed] [Google Scholar]
- 8.Dey T, Brigden G, Cox H, Shubber Z, Cooke G, Ford N. 2013. Outcomes of clofazimine for the treatment of drug-resistant tuberculosis: a systematic review and meta-analysis. J Antimicrob Chemother 68:284–293. doi: 10.1093/jac/dks389. [DOI] [PubMed] [Google Scholar]
- 9.Barry VC, Belton JG, Conalty ML, Denneny JM, Edward DW, O'Sullivan JF, Twomey D, Winder F. 1957. A new series of phenazines (rimino-compounds) with high antituberculosis activity. Nature 179:1013–1015. doi: 10.1038/1791013a0. [DOI] [PubMed] [Google Scholar]
- 10.Cholo MC, Steel HC, Fourie PB, Germishuizen WA, Anderson R. 2012. Clofazimine: current status and future prospects. J Antimicrob Chemother 67:290–298. doi: 10.1093/jac/dkr444. [DOI] [PubMed] [Google Scholar]
- 11.Gangadharam PR, Perumal VK, Podapati NR, Kesavalu L, Iseman MD. 1988. In vivo activity of amikacin alone or in combination with clofazimine or rifabutin or both against acute experimental Mycobacterium avium complex infections in beige mice. Antimicrob Agents Chemother 32:1400–1403. doi: 10.1128/AAC.32.9.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu HB, Jiang RH, Xiao HP. 2012. Clofazimine in the treatment of multidrug-resistant tuberculosis. Clin Microbiol Infect 18:1104–1110. doi: 10.1111/j.1469-0691.2011.03716.x. [DOI] [PubMed] [Google Scholar]
- 13.Tang S, Yao L, Hao X, Liu Y, Zeng L, Liu G, Li M, Li F, Wu M, Zhu Y, Sun H, Gu J, Wang X, Zhang Z. 2015. Clofazimine for the treatment of multidrug-resistant tuberculosis: prospective, multicenter, randomized controlled study in China. Clin Infect Dis 60:1361–1367. doi: 10.1093/cid/ciu843. [DOI] [PubMed] [Google Scholar]
- 14.Verma RK, Germishuizen WA, Motheo MP, Agrawal AK, Singh AK, Mohan M, Gupta P, Gupta UD, Cholo M, Anderson R, Fourie PB, Misra A. 2013. Inhaled microparticles containing clofazimine are efficacious in treatment of experimental tuberculosis in mice. Antimicrob Agents Chemother 57:1050–1052. doi: 10.1128/AAC.01897-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Donnell MR, Padayatchi N, Metcalfe JZ. 2016. Elucidating the role of clofazimine for the treatment of tuberculosis. Int J Tuberc Lung Dis 20:52–57. doi: 10.5588/ijtld.16.0073. [DOI] [PubMed] [Google Scholar]
- 16.Chang KC, Yew WW, Tam CM, Leung CC. 2013. WHO group 5 drugs and difficult multidrug-resistant tuberculosis: a systematic review with cohort analysis and meta-analysis. Antimicrob Agents Chemother 57:4097–4104. doi: 10.1128/AAC.00120-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Senaratne WV. 2004. Outcome of treatment of multidrug resistant tuberculosis. Ceylon Med J 49:86–87. doi: 10.4038/cmj.v49i3.3246. [DOI] [PubMed] [Google Scholar]
- 18.Dalcolmo M, Gayoso R, Sotgiu G, D'Ambrosio L, Rocha JL, Borga L, Fandinho F, Braga JU, Galesi VM, Barreira D, Sanchez DA, Dockhorn F, Centis R, Caminero JA, Migliori GB. 2017. Effectiveness and safety of clofazimine in multidrug-resistant tuberculosis: a nationwide report from Brazil. Eur Respir J 49:1602445. doi: 10.1183/13993003.02445-2016. [DOI] [PubMed] [Google Scholar]
- 19.Padayatchi N, Gopal M, Naidoo R, Werner L, Naidoo K, Master I, O'Donnell MR. 2014. Clofazimine in the treatment of extensively drug-resistant tuberculosis with HIV coinfection in South Africa: a retrospective cohort study. J Antimicrob Chemother 69:3103–3107. doi: 10.1093/jac/dku235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang S, Tan S, Yao L, Li F, Li L, Guo X, Liu Y, Hao X, Li Y, Ding X, Zhang Z, Tong L, Huang J. 2013. Risk factors for poor treatment outcomes in patients with MDR-TB and XDR-TB in China: retrospective multi-center investigation. PLoS One 8:e82943. doi: 10.1371/journal.pone.0082943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan ED, Strand MJ, Iseman MD. 2009. Multidrug-resistant tuberculosis (TB) resistant to fluoroquinolones and streptomycin but susceptible to second-line injection therapy has a better prognosis than extensively drug-resistant TB. Clin Infect Dis 48:e50–e52. doi: 10.1086/597010. [DOI] [PubMed] [Google Scholar]
- 22.Jeon DS, Kim DH, Kang HS, Hwang SH, Min JH, Kim JH, Sung NM, Carroll MW, Park SK. 2009. Survival and predictors of outcomes in non-HIV-infected patients with extensively drug-resistant tuberculosis. Int J Tuberc Lung Dis 13:594–600. [PubMed] [Google Scholar]
- 23.Velayati AA, Farnia P, Ibrahim TA, Haroun RZ, Kuan HO, Ghanavi J, Kabarei AN, Tabarsi P, Omar AR, Varahram M, Masjedi MR. 2009. Differences in cell wall thickness between resistant and nonresistant strains of Mycobacterium tuberculosis: using transmission electron microscopy. Chemotherapy 55:303–307. doi: 10.1159/000226425. [DOI] [PubMed] [Google Scholar]
- 24.Goble M, Iseman MD, Madsen LA, Waite D, Ackerson L, Horsburgh CR Jr. 1993. Treatment of 171 patients with pulmonary tuberculosis resistant to isoniazid and rifampin. N Engl J Med 328:527–532. doi: 10.1056/NEJM199302253280802. [DOI] [PubMed] [Google Scholar]
- 25.Tostmann A, Boeree MJ, Aarnoutse RE, de Lange WC, van der Ven AJ, Dekhuijzen R. 2008. Antituberculosis drug-induced hepatotoxicity: concise up-to-date review. J Gastroenterol Hepatol 23:192–202. doi: 10.1111/j.1440-1746.2007.05207.x. [DOI] [PubMed] [Google Scholar]
- 26.Venkatesan K. 1997. Pharmacokinetics and drug interactions of newer anti-leprosy drugs. Indian J Dermatol Venereol Leprol 63:148–152. [PubMed] [Google Scholar]
- 27.Pang Y, Zhou Y, Zhao B, Liu G, Jiang G, Xia H, Song Y, Shang Y, Wang S, Zhao YL. 2012. Spoligotyping and drug resistance analysis of Mycobacterium tuberculosis strains from national survey in China. PLoS One 7:e32976. doi: 10.1371/journal.pone.0032976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laserson KF, Thorpe LE, Leimane V, Weyer K, Mitnick CD, Riekstina V, Zarovska E, Rich ML, Fraser HS, Alarcon E, Cegielski JP, Grzemska M, Gupta R, Espinal M. 2005. Speaking the same language: treatment outcome definitions for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 9:640–645. [PubMed] [Google Scholar]
- 29.Gopal M, Padayatchi N, Metcalfe JZ, O'Donnell MR. 2013. Systematic review of clofazimine for the treatment of drug-resistant tuberculosis. Int J Tuberc Lung Dis 17:1001–1007. doi: 10.5588/ijtld.12.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]