ABSTRACT
This study was performed to investigate the intrapulmonary penetration of lascufloxacin in humans. Thirty healthy adult male Japanese subjects, allocated into five groups, received lascufloxacin in a single oral dose of 75 mg. Bronchoalveolar lavage and blood sampling were performed simultaneously in each subject at 1, 2, 4, 6, or 24 h after administration, and lascufloxacin concentrations in plasma, epithelial lining fluid, and alveolar macrophages were determined. Lascufloxacin was rapidly distributed to the epithelial lining fluid with a time to maximum drug concentration (Tmax) of 1 h, which was identical to that in plasma. The maximum concentration of drug (Cmax) values in plasma, epithelial lining fluid, and alveolar macrophages were 0.576, 12.3, and 21.8 μg/ml, respectively. The corresponding area under the concentration-time curve from 0 to 24 h (AUC0–24) values were 7.67, 123, and 325 μg · h/ml. The mean drug concentrations in the epithelial lining fluid and alveolar macrophages were much higher than those in plasma at all time points examined, and the average site-to-free plasma concentration ratios fell within the ranges of 57.5 to 86.4 and 71.0 to 217, respectively. Drug levels in epithelial lining fluid and alveolar macrophages exceeded the MIC90 values for common respiratory pathogens. (This study was registered at JAPIC under registration number JapicCTI-142547.)
KEYWORDS: drug penetration, lascufloxacin, quinolones
INTRODUCTION
Lascufloxacin (AM-1977) is a novel 8-methoxy fluoroquinolone antibacterial agent with unique pharmacophores at the 1st and 7th positions of the quinoline nucleus (1). Its oral and parenteral formulations are being developed for the treatment of community-acquired pneumonia and other respiratory tract infections in Japan. Lascufloxacin has potent in vitro activity against various respiratory pathogens, such as Staphylococcus aureus, Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, and Mycoplasma pneumoniae (1). Data from microbiological studies suggested incomplete cross-resistance to lascufloxacin in strains resistant to existing quinolones and potent antibacterial activities against sequentially selected quinolone-resistant mutant Gram-positive bacteria (1).
A preclinical pharmacodynamic study using a mouse thigh infection model indicated that the ratios of the free area under the curve (fAUC) to MIC in plasma required for bacteriostasis, or 1-log or 2-log CFU killing against S. pneumoniae isolates, were 10, 16, and 28, respectively. Following single and repeated doses in phase I studies, it was suggested that lascufloxacin should achieve these pharmacodynamic targets at a dose of ≤100 mg per day, which is about 5 times lower than those of existing quinolones, such as levofloxacin (2). Murine pulmonary experimental results supported this dose setting: lascufloxacin showed significant bacterial killing in the mouse model when we emulated the area under the concentration-time curve (AUC) in plasma in the clinical dose (lascufloxacin, 75 mg per day [q.d.]); (levofloxacin, 500 mg q.d.) (3).
Phase I studies of lascufloxacin exhibited favorable pharmacokinetic profiles with a complete gastrointestinal absorption, an adequate elimination half-life, 15.6 to 18.2 h, suitable for once-daily dosing, and an approximately dose-proportional increase in AUC as well as in maximum concentration in plasma (Cmax): total body clearance and volume of distribution were 8.07 liters/h and 188 liters after 100 mg oral administration (2); the respective values were 7.62 liters/h and 172 liters after a 100-mg intravenous administration (4), and human plasma protein binding was 74.0% (2). These pharmacokinetic profiles and antibacterial activities suggest that lascufloxacin has potential as an efficient treatment for respiratory infections.
It is widely believed that effective antibacterial therapy can be achieved by the use of an agent with reliable antibacterial activity delivered to the site of infection at adequate concentrations. For pneumonia, epithelial lining fluid (ELF) is considered to represent the environment of the site of infection for extracellular pathogens, and the alveolar cellular space is thought to be the site of infection for intracellular pathogens, where drug concentration measurement can provide information on the penetration into alveolar macrophages (AM) (5–7). As demonstrated in studies of other antibacterial agents, it is possible to obtain accurate measurements of the drug concentrations in these sites using urea as a marker, by performing bronchoalveolar lavage (BAL) and using a highly sensitive assay (5, 7). Some researchers investigated the pulmonary penetration of antibacterials using this method and reported some differences in the penetration of these agents into the ELF and AM (6, 7) and also among quinolones (8–15).
This study was performed to evaluate the plasma and intrapulmonary (ELF and AM) pharmacokinetic profiles of lascufloxacin in healthy adult volunteers after a single oral administration of the clinical optimal dose, 75 mg.
(These data were presented in part at ASM Microbe, Boston, MA, 16 to 20 June, 2016.)
RESULTS
Subjects.
Thirty-one healthy male Japanese subjects were enrolled and completed the study. Of the 31 subjects, 30 were included in the evaluable population for pharmacokinetics, as both BAL and plasma samples were collected appropriately from them. One subject was excluded from the pharmacokinetic population because there was insufficient recovery of cells from the specimen for determination of drug concentration in AM. There were no differences in background characteristics among groups or subjects allocated to each BAL time point. The total number of cells recovered, the cellular fraction of AM in BAL fluid, and the plasma-to-BAL fluid urea concentration ratio were 1.42 × 106 to 4.28 × 106 cells, 93.2% to 94.7%, and 69.4 to 78.2, respectively (Table 1).
TABLE 1.
Characteristics of the 30 subjects in the studya
| Time (h) of sampling | No. of subjects | Age (yr) | Height (cm) | Wt (kg) | Body mass index (kg/cm2) | Total no. of cells in BAL fluid | % cellular fraction of AM in BAL fluid | Ureaplasma/ureaBALb |
|---|---|---|---|---|---|---|---|---|
| 1 | 6 | 25.3 ± 4.7 | 172.20 ± 4.77 | 62.48 ± 6.62 | 21.03 ± 1.35 | 3.39 × 106 ± 1.42 × 106 | 93.2 ± 3.3 | 77.9 ± 25.1 |
| 2 | 6 | 22.2 ± 2.9 | 172.28 ± 7.93 | 61.92 ± 7.84 | 20.79 ± 1.19 | 4.28 × 106 ± 1.90 × 106 | 93.2 ± 2.6 | 78.2 ± 31.6 |
| 4 | 6 | 21.8 ± 2.1 | 170.72 ± 5.16 | 62.48 ± 5.17 | 21.47 ± 2.00 | 4.13 × 106 ± 1.69 × 106 | 94.2 ± 1.8 | 73.7 ± 26.1 |
| 6 | 6 | 22.3 ± 1.6 | 170.72 ± 3.40 | 60.25 ± 4.41 | 20.66 ± 1.24 | 1.42 × 106 ± 0.870 × 106 | 94.7 ± 3.6 | 69.4 ± 16.7 |
| 24 | 6 | 23.0 ± 2.2 | 169.52 ± 6.44 | 60.32 ± 7.09 | 20.97 ± 1.60 | 2.22 × 106 ± 2.16 × 106 | 94.0 ± 3.6 | 72.4 ± 18.5 |
| Total | 30 | 22.9 ± 3.0 | 171.09 ± 5.44 | 61.49 ± 5.99 | 20.99 ± 1.43 |
Data are means and SD for each group.
Ureaplasma/ureaBAL, plasma-to-BAL fluid urea concentration ratio.
Pharmacokinetic assessments.
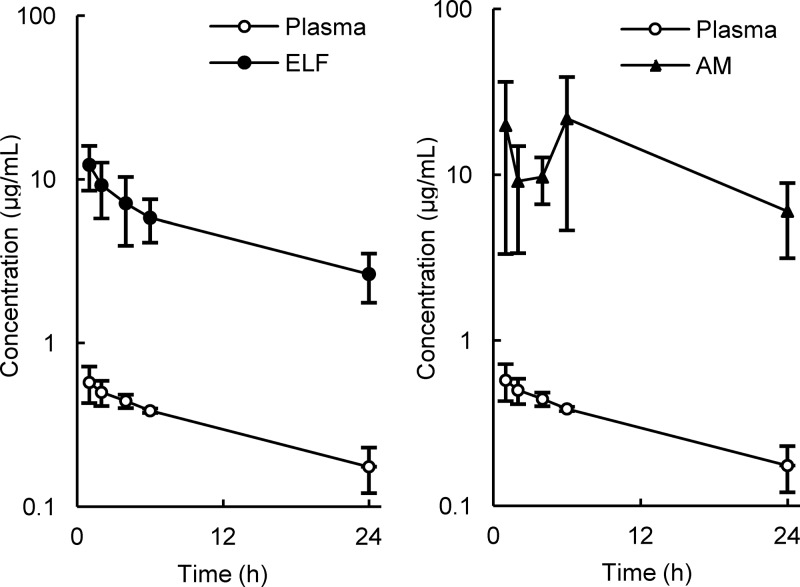
The mean concentrations of lascufloxacin in plasma, ELF, and AM are listed in Table 2 and illustrated in Fig. 1 and 2. The mean drug concentrations in ELF and AM were much higher than those in plasma at all sampling time points.
TABLE 2.
Concentrations of lascufloxacin in plasma, epithelial lining fluid, and alveolar macrophages, and site-to-plasma concentration ratiosa
| Time (h) of sampling | Concn (μg/ml) |
Site-to-plasma concn ratio |
|||||
|---|---|---|---|---|---|---|---|
| Cp | CELF | CAM | CELF/Cp | CELF/Cfpb | CAM/Cp | CAM/Cfpb | |
| 1 | 0.576 ± 0.145 | 12.3 ± 3.74 | 19.9 ± 16.5 | 22.4 ± 9.05 | 86.4 ± 35.0 | 31.9 ± 20.3 | 123 ± 77.8 |
| 2 | 0.501 ± 0.0876 | 9.22 ± 3.45 | 9.13 ± 5.76 | 18.4 ± 5.68 | 70.9 ± 21.9 | 18.5 ± 12.0 | 71.0 ± 46.0 |
| 4 | 0.443 ± 0.0419 | 7.15 ± 3.21 | 9.68 ± 3.04 | 16.5 ± 7.56 | 63.6 ± 29.2 | 21.9 ± 6.75 | 84.3 ± 25.9 |
| 6 | 0.387 ± 0.0119 | 5.84 ± 1.72 | 21.8 ± 17.2 | 15.2 ± 4.74 | 58.3 ± 18.3 | 56.4 ± 43.7 | 217 ± 167 |
| 24 | 0.176 ± 0.0545 | 2.65 ± 0.880 | 6.03 ± 2.88 | 15.0 ± 2.38 | 57.5 ± 9.30 | 38.0 ± 22.6 | 146 ± 87.2 |
Abbreviations: Cp, CELF, and CAM, concentrations of lascufloxacin in plasma, epithelial lining fluid (ELF), and alveolar macrophages (AM), respectively; Cfp, free concentration of lascufloxacin in plasma. Data are means and SD for six subjects.
The free fraction of lascufloxacin in plasma is assumed to be 0.26.
FIG 1.
Mean concentrations of lascufloxacin in plasma, epithelial lining fluid (ELF; left panel), and alveolar macrophages (AM; right panel). The data are means and SD for six subjects in each group.
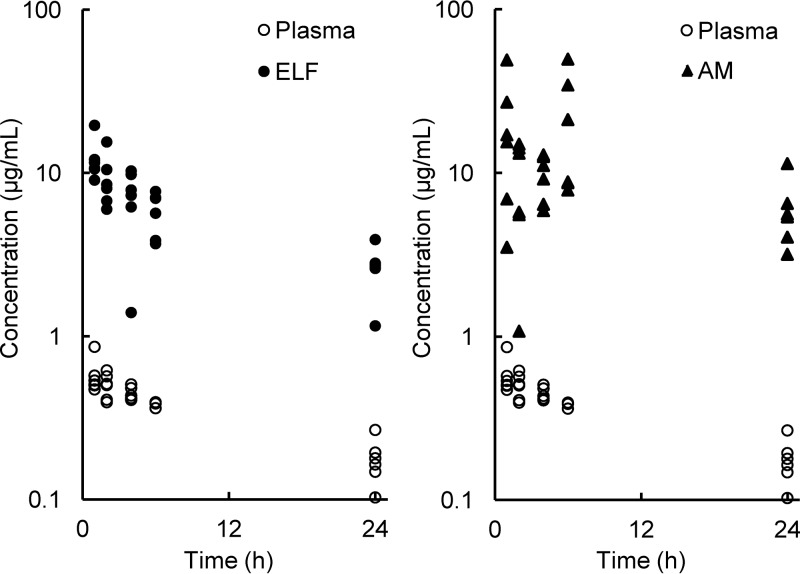
FIG 2.
Individual concentrations of lascufloxacin in plasma, epithelial lining fluid (ELF; left panel), and alveolar macrophages (AM; right panel).
The concentrations of lascufloxacin in ELF and plasma achieved a peak at 1 h, the first sampling point, and both concentration curves decreased gradually over 24 h. The mean ratios of ELF-to-free plasma concentrations of lascufloxacin ranged from 57.5 to 86.4.
The concentrations of lascufloxacin in AM fluctuated within the range of 9.13 to 21.8 μg/ml until 6 h after dosing and decreased to 6.03 μg/ml at 24 h. Large interindividual differences were observed in lascufloxacin concentrations in AM in comparison to those in ELF or plasma at all sampling time points (Fig. 2). The mean ratios of AM-to-free plasma concentration of lascufloxacin ranged from 71.0 to 217.
The plasma and pulmonary pharmacokinetic parameters are listed in Table 3. The concentrations of lascufloxacin in plasma and ELF achieved mean Cmax of 0.576 and 12.3 μg/ml with time to Cmax (Tmax) of 1 h, mean AUC0–24 of 7.67 and 123 μg · h/ml, and the ELF-to-free plasma Cmax and AUC0–24 ratios were 82.1 and 61.7, respectively. The concentrations of lascufloxacin in AM showed a mean Cmax of 21.8 μg/ml with Tmax of 6 h, mean AUC0–24 of 325 μg · h/ml, and AM-to-free plasma Cmax and AUC0–24 ratios were 146 and 163, respectively.
TABLE 3.
Pharmacokinetic parameters of lascufloxacin in plasma, ELF, and AMa
| Sample | Tmax (h) | Cmax (μg/ml) | AUC0–24 (μg · h/ml) | Site-to-plasma Cmax ratio | Site-to-free plasma Cmax ratio | Site-to-plasma AUC0–24 ratio | Site-to-free plasma AUC0–24 ratio |
|---|---|---|---|---|---|---|---|
| Plasma | 1 | 0.576 | 7.67 | ||||
| ELF | 1 | 12.3 | 123 | 21.4 | 82.1 | 16.0 | 61.7 |
| AM | 6 | 21.8 | 325 | 37.8 | 146 | 42.4 | 163 |
Pharmacokinetic parameters were calculated from the mean concentration-time profile of lascufloxacin. ELF, epithelial lining fluid; AM, alveolar macrophages.
Safety.
Lascufloxacin was well tolerated by all subjects, without serious adverse events (AEs) and with no serious abnormal changes in vital signs or in the results of 12-lead electrocardiogram (ECG) or clinical laboratory tests. Of the 31 subjects enrolled in this study, 17 showed a total of 25 nonserious AEs. The most commonly reported AEs were increases in C-reactive protein in nine subjects, fever after the BAL procedure in six subjects, leukocytosis and headache in three subjects each, and feeling of body heat in two subjects. All of these AEs were considered to be related to the BAL procedure, and a causal relationship to the study drug was excluded.
DISCUSSION
In this study, we examined the pulmonary distribution and pharmacokinetics of lascufloxacin after a single oral administration in healthy adult volunteers. The concentration-time curve of lascufloxacin in plasma (Tables 2 and 3) was almost the same as that in another phase I study, in which 75 mg of lascufloxacin was administered to 24 healthy male volunteers, and serial plasma samples were taken from all subjects over 24 h: the mean AUC0–24 was 6.95 μg · h/ml (in-house data of Kyorin Pharmaceutical Co., Ltd.).
The mean observed peak concentration of lascufloxacin in ELF at Tmax of 1 h was 12.3 μg/ml, with no apparent delay of peak compared to that in plasma, and the concentration in ELF was maintained at more than 58 times higher than the free concentrations in plasma for 24 h after oral administration.
The observed concentrations of lascufloxacin in AM were also much higher than those in plasma for 24 h, but those in ELF showed a different behavior. The mean concentration of lascufloxacin in ELF decreased in parallel with that in plasma, and the mean ELF-to-free plasma ratios were relatively constant throughout the sampling period. In contrast, the mean AM-to-plasma ratios fluctuated over time. In this study, BAL and blood samples were taken only once from each subject at one of the scheduled time points because repeated BAL sampling from each subject was difficult for safety reasons. Therefore, the possible influence of interindividual variation of drug concentration could not be excluded. While large standard deviations (SD) in drug concentrations were observed in AM, the standard deviations were smaller in plasma or ELF. These findings do not explain the possibility that the bimodal lascufloxacin concentrations in AM depended mainly on the interindividual variation of drug exposure; some unknown transport mechanisms differing between individuals are suspected to control the drug distribution into the AM.
In general, some technical problems in the urea method should also be considered when interpreting the experimental results: the lysis of AM in ELF and overestimation of the diffusion of urea into the BAL fluid recovered because of the prolonged dwell time during the lavage procedure (6, 16). The ranges of standard deviations for ELF were wider than those for plasma, and the ranges of SD for AM were wider than those for ELF, as shown in Table 2. Therefore, there may have been some technical errors in the experiments, although these errors would have been small enough to not affect the conclusions because of the small variation of experiment markers, as shown in Table 1.
Lascufloxacin binds to proteins, mainly albumin, in plasma: protein binding of lascufloxacin was 74.0% (2). In contrast, the fraction of lascufloxacin bound in ELF may be negligible, because the concentration of albumin in ELF was shown to be less than 1/10 that in plasma (16). Therefore, the site-to-free plasma concentrations should be used to estimate the penetration of lascufloxacin in ELF and AM instead of the site-to-total plasma concentrations. The ELF- and AM-to-free plasma concentration ratios of lascufloxacin observed in humans were much higher than those for other commercially available quinolones. Documented ELF-to-free plasma AUC ratios in humans for ciprofloxacin, levofloxacin, and garenoxacin are approximately 1 to 5, and the ratio for moxifloxacin, which is known to have higher pulmonary penetration, is approximately 9 or 10 (6). Reported AM-to-free plasma AUC ratios in humans for ciprofloxacin, levofloxacin, and garenoxacin are approximately 8 to 56, while the ratio for moxifloxacin is 25 or 51 (6). On the other hand, lascufloxacin showed remarkable pulmonary penetration, with ELF-to-free plasma and AM-to-free plasma AUC0–24 ratios of 61.7 and 163, respectively, in this study. Several quinolones are known to be highly distributed to ELF and AM, mediated by passive diffusion (17) or active transport by macrophages or other cells (18, 19). The augmented penetration of lascufloxacin into the ELF and AM may be mediated by the same mechanisms to a greater extent or by additional as-yet-undefined mechanisms. Further studies are necessary to elucidate the intrapulmonary penetration of quinolones, including lascufloxacin, in humans.
The reported MIC90 values against methicillin-sensitive S. aureus, methicillin-resistant S. aureus, S. pneumoniae, H. influenzae, M. catarrhalis, and M. pneumoniae are 0.015, 2, 0.06, 0.06, 0.06, and 0.25 μg/ml, respectively (1). The present study demonstrated that the concentrations of lascufloxacin in both ELF (2.65 to 12.3 μg/ml) and AM (6.03 to 21.8 μg/ml) markedly exceed the MIC90 values of the target pathogens causing respiratory tract infections. Based on the results of pharmacokinetic/pharmacodynamic (PK/PD) analyses for phase II and III studies in patients with respiratory tract infections, more than 90% of clinical and microbiological efficacy could be produced when the fAUC 0–24/MIC values exceeded 4. Such a pharmacodynamic target was achieved even after oral administration at a dose of 75 mg once a day, which is the lowest dose level among the existing quinolones (in-house data of Kyorin Pharmaceutical Co., Ltd.). The resultant plasma AUC0–24 and site-to-free plasma ratios of lascufloxacin in ELF and AM in this study would support these PK/PD scenarios in clinical studies.
Despite the development and wide use of antibacterials, pneumonia is still the leading cause of infection-related mortality worldwide, and the emergence of strains resistant to existing antibacterial drugs has become a serious clinical concern. Japanese nationwide surveillance revealed that approximately one-half of S. pneumoniae clinical strains were penicillin intermediate resistant or penicillin resistant, and 80% of isolated strains were resistant to clarithromycin and azithromycin (20). Most clinical isolates of M. pneumoniae were resistant to macrolides (21). Thus, there is an urgent need for new treatment options against these pathogens involved in respiratory tract infections.
At the time of contribution of this paper, application for approval of the oral formulation of lascufloxacin for clinical use has been submitted, and its intravenous formulation is under phase III clinical trials in Japan. Based on the excellent pulmonary distribution and potent antibacterial activity against typical and atypical pathogens, lascufloxacin is a promising new agent for the treatment of community-acquired pneumonia and lower respiratory tract infections.
MATERIALS AND METHODS
Study design and subjects.
The study had a prospective, open-label single-dose design to evaluate the plasma and intrapulmonary pharmacokinetics of lascufloxacin after oral administration to healthy adult male volunteers. Subjects were allocated into five groups (six per group) so that each underwent bronchoscopy only once at time points of 1, 2, 4, 6, or 24 h after administration of the study drug. All subjects were given a single oral dose of 75 mg of lascufloxacin tablets (Kyorin Pharmaceutical Co., Ltd., Tokyo, Japan), under fasting conditions.
Healthy Japanese male subjects aged 20 to 40 years were recruited for this study; principal eligibility criteria included a body mass index of 18.5 to 24.9 kg/m2, no history of smoking, and no clinically significant abnormalities in vital signs, ECG, or clinical laboratory tests according to diagnosis during the screening period. Exclusion criteria included mainly a history of hypersensitivity to lidocaine, atropine, local anesthetics, or other medications, medical histories of food allergy or atopic disease, the presence of serious functional disorders or complications that would represent an obstacle to the investigation, a history of excessive alcohol or caffeine consumption, and a positive HIV or hepatitis B or C virus status. All subjects were provided written informed consent prior to enrollment in the study. The protocol was approved by the Institutional Review Board of the study site. The study was conducted at Osaka Pharmacology Clinical Research Hospital (Osaka, Japan) in accordance with the Declaration of Helsinki and the guidelines on Good Clinical Practice. This study was registered at JAPIC under registration number JapicCTI-142547.
BAL.
Each subject underwent bronchoscopy with the BAL procedure by a skillful pulmonologist at one of the protocol-specified time points, i.e., 1, 2, 4, 6, or 24 h after administration of the study drug. Subjects were pretreated with anesthesia consisting of 0.5% lidocaine nebulizer and atropine sulfate injection 15 min before bronchoscopy. Topical lidocaine anesthesia on the pharynx was then performed using a Jackson-type spray.
The BAL and successive procedures were conducted according to the method described previously (22, 23). Briefly, a fiber-optic bronchoscope was inserted up to the right middle lobe and wedged in place. BAL was carried out by instillation of four 50-ml aliquots of sterile saline into the subsegmental bronchus of the right middle lobe, and each specimen was immediately aspirated. Instillation of 50 ml of saline and its aspiration were performed within 1 min. The first aliquot of the aspirate was discarded, and the remaining three aliquots were pooled and immediately placed on ice.
The BAL fluid was filtered through sterile gauze and centrifuged at 400 × g for 5 min at 4°C to separate the cells from the supernatant. The cells sedimented from the BAL fluid were retained for cell counting and fractionation. The BAL cells and supernatant samples were refrigerated at −85°C until shipping to the laboratories for urea nitrogen and lascufloxacin concentration analyses.
Blood samples.
Blood sampling was conducted once in each subject to analyze concentrations of urea and lascufloxacin in plasma within 5 min of completion of the corresponding BAL procedure. Blood samples were collected into heparinized tubes and centrifuged. Plasma samples were transferred into storage tubes and stored at −85°C until shipping to the laboratories for urea nitrogen and lascufloxacin concentration analyses.
Determination of lascufloxacin concentrations in plasma, BAL fluid, and AM.
The concentrations of lascufloxacin in plasma were determined by solid-phase extraction and separation by high-performance liquid chromatography (HPLC) with fluorescence detection at JCL Bioassay Corporation (Hyogo, Japan). Briefly, an internal standard (AM-1954) (24) was added to 200 μl of plasma, and the mixture was applied to a solid-phase extraction cartridge (Oasis HLB, 10 mg/1 ml; Waters Corp., Milford, MA), which was washed twice with 1 ml of 5% methanol and then eluted with 1 ml of methanol. The eluate was evaporated to dryness under a stream of nitrogen gas. The resultant residue was dissolved with a mixture of 0.01 mol/liter phosphoric acid and acetonitrile (70:30 [vol/vol]). The mobile phase for HPLC consisted of a mixture of 0.01 mol/liter phosphoric acid and acetonitrile (70:30[vol/vol]) containing 0.02% 1-octanesulfonic acid sodium salt. A part of the sample prepared as described above was pumped through an Inertsil ODS-3 column (5-μm particle size, 4.6 by 150 mm; GL Sciences, Tokyo, Japan) at a flow rate of 1.0 ml/min. Fluorescence detection was performed at wavelengths of 289 nm (excitation) and 498 nm (emission). The lower limit of quantification (LLOQ) for the plasma assay was 5 ng/ml, and the calibration range was 5 to 5,000 ng/ml. The intra- and interday precisions ranged from 3.4% to 9.1% and from 1.3% to 3.8%, respectively.
The concentrations of lascufloxacin in BAL fluid and AM were determined by solid-phase extraction and separation by liquid chromatography-tandem mass spectrometry in positive ionization mode at Sumika Chemical Analysis Service, Ltd. (Osaka, Japan). The solid-phase extraction procedure was almost the same as that for plasma except with use of Oasis HLB (30 mg/1 ml; Waters Corp.) as the solid-phase extraction cartridge. The mobile phase for liquid chromatography was a mixture of 0.1% formic acid (A) and acetonitrile containing 0.1% formic acid (B) (A/B = 90:10 [vol/vol] in 0 to 7 min, 80:20 in 7 to 9 min, 10:90 in 9 to 12.5 min, and 90:10 in 12.5 to 15 min). A part of the sample prepared as described above was pumped through a Sumipax ODS Z-CLUE column (3-μm particle size, 2.0 by 50 mm; Sumika Chemical Analysis Service, Ltd., Tokyo, Japan) at a flow rate of 0.3 ml/min. Lascufloxacin and the internal standard were ionized prior to detection in multiple-reaction monitoring mode while monitoring the following transitions: m/z 440 to 317 for lascufloxacin and m/z 438 to 303 for the internal standard. The LLOQ for the BAL fluid and AM assay was 0.1 ng/ml, and the calibration range was 0.1 to 100 ng/ml. The intra- and interday precision ranges were from 2.4% to 8.3% and from 5.7% to 9.9%, respectively.
Determination of urea in plasma and BAL fluid.
The concentrations of urea in plasma and BAL fluid were analyzed according to the urease-indophenol method with a commercially available kit (Urea N B; Wako Pure Chemical Industries, Ltd., Osaka, Japan) at Shin Nippon Biomedical Laboratories, Ltd. (Kagoshima, Japan). Plasma was diluted 200-fold with physiological saline before analysis. After incubation with reagents, the absorbance values of standards, samples, and quality control samples at 570 nm were measured against the absorbance of the blank. The LLOQ for plasma and BAL fluid assay was 5 mg/dl, and the calibration range was 5 to 50 mg/dl. The intra- and interday precision ranges were from 0.8% to 2.0% and from 2.7% to 4.7%, respectively.
Calculation of lascufloxacin concentrations in ELF and AM.
Lascufloxacin concentrations in ELF (CELF) and AM (CAM) were calculated by the urea nitrogen dilution method, as described previously (22, 23).
CELF was determined as follows: CELF = CBAL × (ureaplasma/ureaBAL), where CBAL is the concentration of lascufloxacin in the supernatant of BAL fluid, and ureaplasma and ureaBAL are the concentrations of urea in plasma and BAL fluid, respectively.
CAM was determined as follows: CAM = Csuspension/VAM, where Csuspension and VAM are the concentration of lascufloxacin and the volume of AM in 1 ml of cell suspension, respectively. The reported volume of 2.42 μl/106 cells was used to calculate the volume of AM in the suspension (16). A differential cell count of the BAL fluid was performed to determine the number of AM.
Pharmacokinetic analyses.
Plasma and pulmonary pharmacokinetic parameters were calculated using Microsoft Excel 2007 (Microsoft Corporation, Redmond, WA). The maximal drug concentration (Cmax), time needed to reach the maximum drug concentration (Tmax), and area under the concentration-time curve from 0 to 24 h (AUC0–24) were determined with the mean lascufloxacin concentrations in plasma, ELF, and AM at each sampling time point. The AUC0–24 values were calculated by the linear trapezoidal rule. Drug pulmonary penetration was indicated by the ratio of the mean Cmax and AUC0–24 for ELF or AM to those for plasma.
Safety.
Safety assessment of AEs was performed for subjective and objective symptoms, vital signs, ECG, and clinical laboratory tests. In this study, chest X rays were also performed twice at the time of screening and 1 day after the BAL procedure. Investigators evaluated all clinical AEs in terms of severity (mild, moderate, or severe), duration, outcome, and causal relationship to the study drug.
ACKNOWLEDGMENTS
This study was sponsored by Kyorin Pharmaceutical Co., Ltd.
We are grateful for the technical assistance of Masaki Mukae, Saiko Yamada, Kayo Matsumoto, Yukio Hori, Noriko Goto, Shinichi Miyaji, Saeko Fukushima, Mineko Idate, Ryoko Yokota, and Yumie Aoki.
Hidetoshi Furuie is the principal investigator, Masaharu Nishimura is a medical expert, and the other authors are employees of Kyorin Pharmaceutical Co., Ltd.
REFERENCES
- 1.Kishii R, Yamaguchi Y, Takei M. 2017. In vitro activities and spectrum of the novel fluoroquinolone lascufloxacin (KRP-AM1977). Antimicrob Agents Chemother 61:e00120–17. doi: 10.1128/AAC.00120-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Totsuka K, Odajima M, Nakauchi M, Sesoko S, Nakashima M. 2016. Phase I study to determine the safety and pharmacokinetics (PK) of single and multiple oral doses of lascufloxacin (AM-1977) in healthy subjects, abstr SUNDAY-467. Abstr ASM Microbe, 16 to 20 June 2016, Boston, MA. [Google Scholar]
- 3.Kishii R, Yamamuro N, Yamaguchi Y, Abukawa H, Takei M. 2016. In vivo bactericidal activity of lascufloxacin (AM-1977), a newly developed fluoroquinolone, against mouse pulmonary infection model caused by Streptococcus pneumoniae, abstr SUNDAY-471. Abstr ASM Microbe, 16 to 20 June 2016, Boston, MA. [Google Scholar]
- 4.Totsuka K, Odajima M, Nakauchi M, Sesoko S, Nakashima M. 2016. Phase I study to determine the safety and pharmacokinetics (PK) of single and multiple intravenous infusion of lascufloxacin (AM-1977) in healthy subjects, abstr SUNDAY-470. Abstr ASM Microbe, 16 to 20 June 2016, Boston, MA. [Google Scholar]
- 5.Baldwin DR, Honeybourne D, Wise R. 1992. Pulmonary disposition of antimicrobial agents: methodological considerations. Antimicrob Agents Chemother 36:1171–1175. doi: 10.1128/AAC.36.6.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiem S, Schentag JJ. 2008. Interpretation of antibiotic concentration ratios measured in epithelial lining fluid. Antimicrob Agents Chemother 52:24–36. doi: 10.1128/AAC.00133-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodvold KA, George JM, Yoo L. 2011. Penetration of anti-infective agents into pulmonary epithelial lining fluid. Clin Pharmacokinet 50:637–664. doi: 10.2165/11594090-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 8.Baldwin DR, Wise R, Andrews JM, Gill M, Honeybourne D. 1993. Comparative bronchoalveolar concentrations of ciprofloxacin and lomefloxacin following oral administration. Respir Med 87:595–601. doi: 10.1016/S0954-6111(05)80262-8. [DOI] [PubMed] [Google Scholar]
- 9.Schüler P, Zemper K, Borner K, Koeppe P, Schaberg T, Lode H. 1997. Penetration of sparfloxacin and ciprofloxacin into alveolar macrophages, epithelial lining fluid, and polymorphonuclear leucocytes. Eur Respir J 10:1130–1136. doi: 10.1183/09031936.97.10051130. [DOI] [PubMed] [Google Scholar]
- 10.Andrews JM, Honeybourne D, Jevons G, Brenwald NP, Cunningham B, Wise R. 1997. Concentrations of levofloxacin (HR355) in the respiratory tract following a single oral dose in patients undergoing fibre-optic bronchoscopy. J Antimicrob Chemother 40:573–577. doi: 10.1093/jac/40.4.573. [DOI] [PubMed] [Google Scholar]
- 11.Soman A, Honeybourne D, Andrews J, Jevons G, Wise R. 1999. Concentrations of moxifloxacin in serum and pulmonary compartments following a single 400 mg oral dose in patients undergoing fibre-optic bronchoscopy. J Antimicrob Chemother 44:835–838. doi: 10.1093/jac/44.6.835. [DOI] [PubMed] [Google Scholar]
- 12.Gotfried MH, Danziger LH, Rodvold KA. 2001. Steady-state plasma and intrapulmonary concentrations of levofloxacin and ciprofloxacin in healthy adult subjects. Chest 119:1114–1122. doi: 10.1378/chest.119.4.1114. [DOI] [PubMed] [Google Scholar]
- 13.Andrews J, Honeybourne D, Jevons G, Boyce M, Wise R, Bello A, Gajjar D. 2003. Concentrations of garenoxacin in plasma, bronchial mucosa, alveolar macrophages and epithelial lining fluid following a single oral 600 mg dose in healthy adult subjects. J Antimicrob Chemother 51:727–730. doi: 10.1093/jac/dkg110. [DOI] [PubMed] [Google Scholar]
- 14.Capitano B, Mattoes HM, Shore E, O'Brien A, Braman S, Sutherland C, Nicolau DP. 2004. Steady-state intrapulmonary concentrations of moxifloxacin, levofloxacin, and azithromycin in older adults. Chest 125:965–973. doi: 10.1378/chest.125.3.965. [DOI] [PubMed] [Google Scholar]
- 15.Conte JE Jr, Golden JA, Mclver M, Zurlinden E. 2006. Intrapulmonary pharmacokinetics and pharmacodynamics of high-dose levofloxacin in healthy volunteer subjects. Int J Antimicrob Agents 28:114–121. doi: 10.1016/j.ijantimicag.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 16.Rennard SI, Basset G, Lecossier D, O'Donnell KM, Pinkston P, Martin PG, Crystal RG. 1986. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J Appl Physiol 60:532–538. doi: 10.1152/jappl.1986.60.2.532. [DOI] [PubMed] [Google Scholar]
- 17.Sun J, Deguchi Y, Chen JM, Zhang RH, Morimoto K. 2002. Interactions between quinolone antibiotics and phospholipid membrane for prediction of alveolar macrophage uptake in vitro. Acta Pharmacol Sin 23:430–438. [PubMed] [Google Scholar]
- 18.Michot JM, Seral C, Van Bambeke F, Mingeot-Leclercq MP, Tulkens PM. 2005. Influence of efflux transporters on the accumulation and efflux of four quinolones (ciprofloxacin, levofloxacin, garenoxacin, and moxifloxacin) in J774 macrophages. Antimicrob Agents Chemother 49:2429–2437. doi: 10.1128/AAC.49.6.2429-2437.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brillault J, De Castro WV, Harnois T, Kitzis A, Olivier JC, Couet W. 2009. P-glycoprotein-mediated transport of moxifloxacin in a Cacu-3 lung epithelial cell model. Antimicrob Agents Chemother 53:1457–1462. doi: 10.1128/AAC.01253-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamaguchi K, Tateda K, Ohno A, Ishii Y, Murakami H. 2016. Surveillance of in vitro susceptibilities to levofloxacin and various antibacterial agents for 11,762 clinical isolates obtained from 69 centers in 2013. Jpn J Antibiot 69:1–25. doi: 10.1038/ja.2015.113. [DOI] [PubMed] [Google Scholar]
- 21.Yamazaki T, Kenri T. 2016. Epidemiology of Mycoplasma pneumoniae infections in Japan and therapeutic strategies for macrolide-resistant M. pneumoniae. Front Microbiol 7:693. doi: 10.3389/fmicb.2016.00693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furuie H, Saisho Y, Yoshikawa T, Shimada J. 2010. Intrapulmonary pharmacokinetics of S-013420, a novel bicyclolide antibacterial, in healthy Japanese subjects. Antimicrob Agents Chemother 54:866–870. doi: 10.1128/AAC.00567-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishizuka H, Toyama K, Yoshiba S, Okabe H, Furuie H. 2012. Intrapulmonary distribution and pharmacokinetics of laninamivir, a neuraminidase inhibitor, after a single inhaled administration of its prodrug, laninamivir octanoate, in healthy volunteers. Antimicrob Agents Chemother 56:3873–3878. doi: 10.1128/AAC.06456-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fukuda Y. 2009. New approaches to overcoming bacterial resistance. Drugs Future 34:127–136. doi: 10.1358/dof.2009.034.02.1313642. [DOI] [Google Scholar]