ABSTRACT
We report our clinical experience treating a 2-month-old infant with congenital diaphragmatic hernia who experienced prolonged bacteremia with Burkholderia cepacia complex (Bcc) despite conventional antibiotic therapy and appropriate source control measures. The infection resolved after initiation of ceftazidime-avibactam. Whole-genome sequencing revealed that the isolate most closely resembled B. contaminans and identified the mechanism of resistance that likely contributed to clinical cure with this agent. Ceftazidime-avibactam should be considered salvage therapy for Bcc infections if other treatment options have been exhausted.
KEYWORDS: Burkholderia cepacia complex, whole-genome sequencing, bacteremia, ceftazidime-avibactam
INTRODUCTION
The Burkholderia cepacia complex (Bcc) consists of Gram-negative non-glucose-fermenting organisms frequently found in the environment (1). They are opportunistic pathogens generally confined to patients with cystic fibrosis, chronic granulomatous disease, or indwelling hardware. With the recent national outbreak related to contaminated oral liquid docusate sodium, the population at risk of infectious syndromes from Bcc has expanded (2).
Infections due to Bcc can be challenging to manage, as Bcc is intrinsically resistant to a number of commonly used antibiotics. Trimethoprim-sulfamethoxazole (TMP-SMX) and ceftazidime are considered first-line options for Bcc infections (1). However, in vitro resistance to TMP-SMX and ceftazidime in Bcc isolates has been reported at approximately 10 to 40% (1, 3) and 30 to 40% (1, 3, 4), respectively. Along with reduced susceptibilities to first-line options, drug intolerance, particularly to TMP-SMX, can further limit therapeutic options. We report our experience with the use of ceftazidime-avibactam for the treatment of persistent Bcc bacteremia and the use of whole-genome sequencing (WGS) to explore why this therapy may have been successful.
CASE PRESENTATION
A full-term female infant was born with a prenatally diagnosed congenital diaphragmatic hernia that was surgically repaired at 2 weeks of age. She gradually recovered in the pediatric intensive care unit and during this time remained infection free, off antibiotic therapy. At 2 months of age, she developed fevers and hypotension, requiring vasopressors (defined as day 1). Blood cultures were obtained, and cefepime and vancomycin were empirically initiated. Twenty hours after culture obtainment, Gram-negative rods were recovered and determined to the species level as Bcc, at which time cefepime and vancomycin were discontinued and trimethoprim-sulfamethoxazole (TMP-SMX) was prescribed (5 mg/kg of body weight/dose intravenously [i.v.] every 6 h). By day 6, because of persistent bacteremia, ceftazidime (50 mg/kg/dose i.v. every 8 h) was added to TMP-SMX. The ceftazidime MIC was 8 μg/ml (5) and remained at 8 μg/ml (the Clinical and Laboratory Standards Institute [CLSI] breakpoint) for the duration of bacteremia. All central lines were removed by day 9. Extensive full-body imaging was repeated to evaluate for deep-seated sources of infection and endocarditis or endovascular sources, but none were identified. By day 12, the same daily dose of ceftazidime was continued, but it was administered as a continuous infusion. On day 16, ceftazidime was changed to extended-infusion meropenem (40 mg/kg/dose i.v. infused over 3 h given every 8 h), with the continuation of TMP-SMX. The meropenem MIC until this time was 2 μg/ml and, within 2 days of initiation of meropenem therapy, increased and persisted at 4 μg/ml. Because of persistent bacteremia, on day 32 of positive blood cultures, her antibiotic regimen was changed to continuous-infusion ceftazidime-avibactam (50 mg/kg/dose i.v. infused over 8 h given every 8 h). She had no further positive blood cultures within 24 h of receiving this antibiotic. She received a total of 6 weeks of ceftazidime-avibactam for a presumed endovascular infection and achieved clinical cure. She remains free of Bcc infections 10 months later.
Daily blood cultures were obtained from the infant and incubated on the BD Bactec FX blood culture system (Becton, Dickinson, Sparks, MD, USA). Positive blood cultures were Gram stained and inoculated onto tryptic soy agar with 5% sheep's blood and MacConkey agar for recovery of Gram-negative organisms. Isolates were identified by matrix-assisted laser desorption–ionization time of flight mass spectrometry (MALDI-TOF MS; Bruker Daltonics Inc., Billerica, MA). Antimicrobial susceptibility testing (AST) was performed using the BD Phoenix automated instrument. Antibiotics recommended for testing against Bcc by the CLSI were evaluated (5). The organism was highly susceptible to ceftazidime-avibactam, with a zone of inhibition of 33 mm (susceptibility is defined as a zone diameter of ≥18 mm, with the Pseudomonas aeruginosa Food and Drug Administration zone diameter breakpoints applied). Additionally, using the ceftazidime-avibactam Etest (bioMérieux), the ceftazidime-avibactam MIC was 2 μg/ml. WGS was used to understand the mechanism of resistance that contributed to successful treatment with ceftazidime-avibactam.
CHALLENGE QUESTION
What is the underlying mechanism of resistance that may explain the lack of clinical improvement with ceftazidime and clinical success with ceftazidime-avibactam?
A. The production of a KPC-3 carbapenemase.
B. The production of an NDM-1 metallo-β-lactamase.
C. The loss of OprD porin expression.
D. The production of a Pen-like β-lactamase.
E. Upregulation of MexAB-OprM efflux pumps.
TREATMENT AND OUTCOME
WGS was performed using both Illumina MiSeq (Illumina, San Diego, California) and Oxford Nanopore MinION (Oxford, England) sequencing technologies on the isolate obtained on day 32. Genomic DNA was extracted from pure cultures using the DNeasy PowerBiofilm kit (Qiagen, Hilden, Germany). A sequencing library was prepared from 1 ng of DNA using the Nextera XT kit. The Illumina library was sequenced on the MiSeq using v2 Micro 2 × 150-bp-read-length reagents and generated roughly 5 million paired-end reads. These reads were used to correct the error-prone Nanopore assembly using the Pilon v1.22 software package in conjunction with the short-read aligner Bowtie2 v2.2.6. The mean coverage was approximately 125. The Illumina data were also independently assembled using SPAdes Genome Assembler v3.10.1.
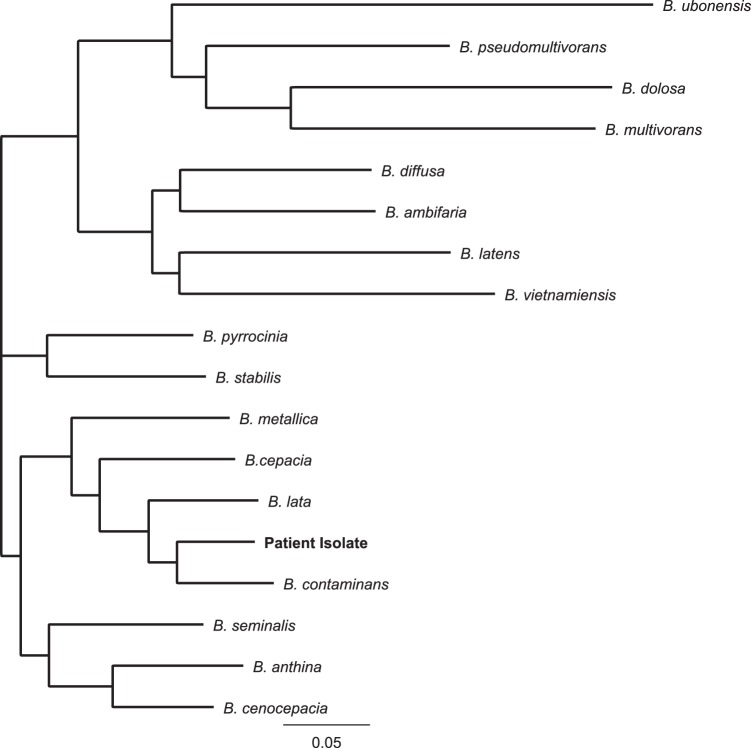
A nanopore sequencing library was prepared using 5 μg of genomic DNA with the one-dimensional ligation kit (SQK-LSK108) from Oxford Nanopore Technologies. The isolate was sequenced on an R9.4 flow cell (FLO-MIN106) with a MinION MkIb sequencer. The MinKNOW acquisition software was used to collect raw data, and Albacore v1.2.6 was used to base-call. The run generated around 11.4 million reads, with a median read length of 4.8 kb, and was assembled using Canu v1.6. The resulting assembly closed the genome, which contained no ambiguous nucleotides or contig orientations. Genomes were annotated using Prokka 7 and were deposited in NCBI (accession no. PQVP00000000). A custom Bcc database was created from Bcc annotated isolates found in GenBank. A dendrogram was generated by comparing the clinical Bcc isolate to sequences in the custom database, and our patient's isolate most closely resembled B. contaminans (Fig. 1).
FIG 1.
Dendrogram comparing the patient's Burkholderia cepacia complex isolate to other Burkholderia species within the Burkholderia cepacia complex. The isolate is most closely related to B. contaminans.
A blapen-like sequence was located on the chromosome and identified in both the Illumina and Nanopore assemblies using an alignment search (BLAST). The blapen-like gene from the patient isolate was closely related to the blapen-like gene from B. contaminans (96% identity). The blapen-like gene appears to be highly varied among Bcc species, ranging from 79% to 96% identity (Fig. 2). Additional findings included a chromosomal blaAmpC gene (identical to sequences present in B. contaminans) and a class D β-lactamase. The Comprehensive Antibiotic Resistance Database (CARD) was searched for other resistance genes using the Resistance Gene Identifier (RGI). Additionally, ResFinder, CARD, ARG-ANNOT, NCBI BARRGD, NCBI β-lactams, EcOH, PlasmidFinder, and VFDB were queried using the ABRicate software package. Of note, the modified carbapenem inactivation method was performed on the isolate, and it was negative for carbapenemase production.
FIG 2.
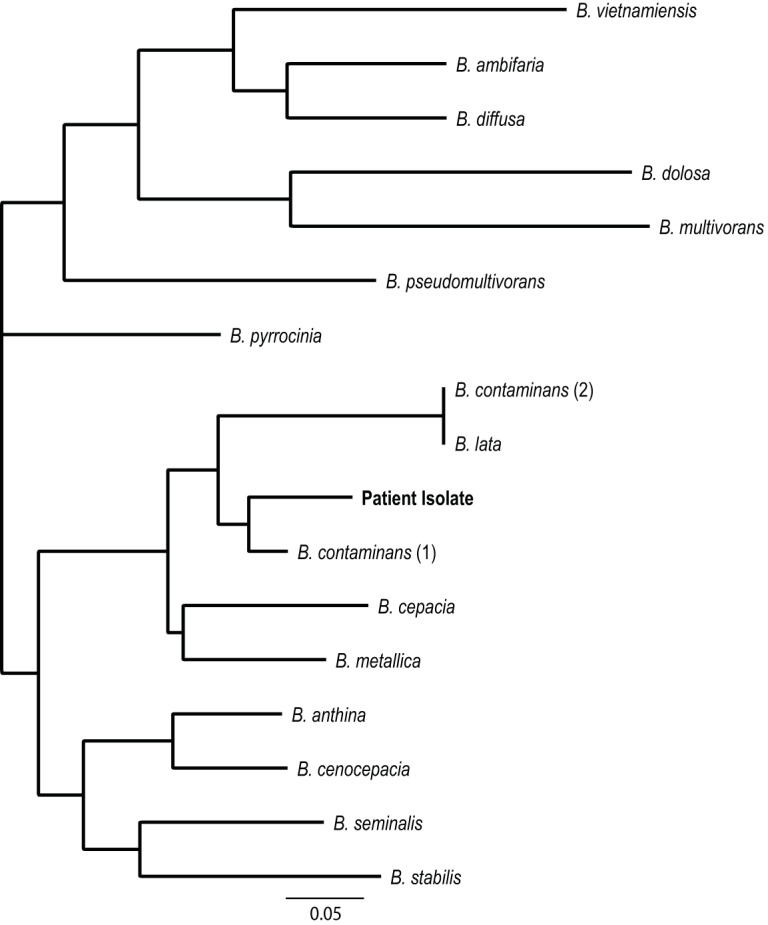
Dendrogram and alignment of the mutational profile of the pen-like gene from the patient isolate to the pen-like genes found in other Burkholderia cepacia complex species. The percentages of identity of the blapen-like genes between species ranged from <79 to 96%, with the patient isolate's gene most closely resembling the blapen-like gene from B. contaminans (96% identity).
blapen-like genes, major resistance determinants in Bcc, encode inducible, inhibitor-resistant chromosomal class A carbapenemases, rendering Bcc nonsusceptible to a number of β-lactams (6). This enzyme structurally resembles Klebsiella pneumoniae carbapenemases (i.e., KPCs) (4, 7) but functions more like an SHV-type extended-spectrum β-lactamase (3). Avibactam forms a reversible acyl-enzyme complex with class A and class C β-lactamases and certain class D β-lactamases, all of which were produced by this infant's isolate (8). In vitro results have shown that the addition of avibactam to ceftazidime can restore susceptibility to strains for which ceftazidime MICs are elevated, rendering these isolates susceptible to ceftazidime-avibactam. In a collection of 50 Bcc isolates from cystic fibrosis patients, 68% of isolates were susceptible to ceftazidime (3). The addition of avibactam to ceftazidime restored activity against all tested isolates (3). In another collection of 49 ceftazidime-resistant Bcc isolates, 63% were susceptible to ceftazidime-avibactam (9). Furthermore, the combination of ceftazidime-avibactam was shown to significantly improve the outcomes of Galleria mellonella larvae infected with a Bcc strain for which ceftazidime MICs were 32 μg/ml compared to outcomes after treatment with ceftazidime alone (3).
The potential for ceftazidime-avibactam to be a treatment option for recalcitrant Bcc infections is of particular importance, as Bcc has the ability to evade the activities of multiple classes of antibiotics. Distorted dihydrofolate reductase and DNA gyrase targets can limit the effectiveness of trimethoprim-sulfamethoxazole and levofloxacin, respectively (10). Unique lipopolysaccharides with reduced net negative charges limit the binding of polymyxins, and Bcc organisms are considered intrinsically resistant to these agents (11). Furthermore, a number of β-lactamases, porin mutations, and efflux pumps intrinsic to Bcc limit the activities of other commonly prescribed antibiotics (10). The incremental benefits of avibactam to ceftazidime are varied across the Burkholderia species (12). Although not the case with this patient's isolate, some Bcc genomes harbor metallo-β-lactamase genes which are not inactivated by avibactam (www.burkholderia.com). If treatment with ceftazidime-avibactam is being considered for Bcc infections, prior susceptibility testing is recommended to confirm activity.
It is not entirely clear why other agents that were reported as susceptible in vitro did not successfully eradicate Bcc from the bloodstream of this patient. There are few to no data indicating that the in vitro activities of commonly used antibiotics for Bcc infections translate into clinical success. Reasons for the inactivity of TMP-SMX are unclear. One possible explanation for the failure of ceftazidime and meropenem in this patient's case was that the MICs were simply too high to reach adequate target attainment for effective killing. For the isolate from the infant, the ceftazidime MIC was 8 μg/ml and the meropenem MIC was 4 μg/ml, which are the current CLSI susceptibility breakpoints for Bcc (5). In contrast, the breakpoints for Enterobacteriaceae against ceftazidime and meropenem are 4 μg/ml and 1 μg/ml, respectively. Using these breakpoints, this patient's isolate would have been nonsusceptible to these agents. The breakpoints of Bcc and other nonfermenters have not been lowered to harmonize with the Enterobacteriaceae breakpoints because there has been a paucity of data to allow for a data-driven review of the breakpoints.
In summary, avibactam appears to be a potent inhibitor of Pen-like β-lactamases that can be produced by Bcc (the correct answer to the challenge question is “D”). Although this case represents a single clinical experience, when combined with available experimental data, it suggests that ceftazidime-avibactam should be considered potential salvage therapy for Bcc infections if other treatment options have been exhausted.
COMMENTARY
Although ubiquitous in the environment, Burkholderia cepacia complex (Bcc) organisms remain a relatively uncommon cause of human disease compared to other Gram-negative pathogens. However, when infections do occur, they are typically implicated in health care-related exposures or infections in immunocompromised hosts, specifically patients with cystic fibrosis, and often cause significant morbidity and mortality. A major reason for this is due to their intrinsic level of antibiotic resistance to a variety of agents. In the above-described challenging clinical case, Tamma et al. highlight the difficulties in treating these infections, as they present the first case report of successful therapy of Bcc bacteremia with ceftazidime-avibactam.
In this case, the patient, a presumably immunocompetent full-term infant, developed Bcc bacteremia approximately 1.5 months after a diaphragmatic hernia repair. The patient had minimal antibiotic exposure earlier in her hospital course, and potential niduses of infection were removed. Susceptibility testing revealed somewhat high (though technically susceptible) MICs of ceftazidime and meropenem for the isolate per CLSI breakpoints (5). However, the bacteremia persisted (>30 days) despite various therapeutic attempts with combinations of trimethoprim-sulfamethoxazole (TMP-SMX), ceftazidime, and meropenem. Ultimately, she was started on ceftazidime-avibactam, resulting in rapid clearance of the pathogen from the blood. While the majority of studies done on Bcc isolates and their susceptibilities involve non-blood specimens in patients with cystic fibrosis who have had significant antibiotic exposure, Bcc strains have been shown to often be intrinsically resistant to aminoglycosides, first- and second-generation cephalosporins, polymyxins, and traditional antipseudomonal β-lactams, including piperacillin and ticarcillin (13, 14). As such, recommended first-line therapy includes ceftazidime, TMP-SMX, minocycline, chloramphenicol, and meropenem or a combination of two or more of these agents in light of increasing resistance (15). Given the increasing reports of resistance to TMP-SMX and ceftazidime, as well as the promising activities of newer agents, specifically ceftazidime-avibactam, as was demonstrated in this case, it is perhaps time to rethink which drugs should be used for treatment of Bcc infections.
One of the most common resistance mechanisms seen in Burkholderia species is production of β-lactamases, including PenA and PenI, class A β-lactamases that use a serine as the nucleophile and hydrolyze β-lactams, ultimately resulting in β-lactam hydrolysis (7). These particular enzymes are located chromosomally and are usually inducible. Recent studies have shown that avibactam, a non-β-lactam β-lactamase inhibitor, is able to inhibit class A, class C, and class D β-lactamases (5, 16). While various Bcc isolates may harbor multiple mechanisms of resistance, with the presence of β-lactamases being one, avibactam has been shown to allow for restoration of effective ceftazidime activity in vitro in isolates that were otherwise ceftazidime resistant (1, 9, 10). Whole-genome sequencing of the patient's isolate in this case confirmed the presence of class A, C, and D β-lactamases and the absence of metallo-β-lactamases, further supporting the likelihood that ceftazidime-avibactam would effectively resolve her bacteremia.
As the authors point out, while it is unclear as to why TMP-SMX was not successful in sterilizing the patient's blood, the MICs of meropenem (increasing up to 4 μg/ml during the hospital course) and ceftazidime (8 μg/ml), while sensitive, were right on the edge of the established CLSI breakpoints and in the setting of inducible resistance, may explain the clinical failure of these first-line agents.
Challenges to utilizing ceftazidime-avibactam as first-line therapy remain and include the possibility that Bcc strains can contain metallo-β-lactamases (which, if present, would render ceftazidime-avibactam ineffective), the drug combination's limited availability, its high cost, and the fact that susceptibility testing for ceftazidime-avibactam is not widely available at present. Despite these potential limitations, this first reported case of successful treatment of persistent Bcc bacteremia treated with ceftazidime-avibactam by Tamma and colleagues suggests that ceftazidime-avibactam should be considered for early use, particularly if first-line therapies prove ineffective or susceptibility testing indicates isolates with borderline MIC values.
ACKNOWLEDGMENTS
The case study was supported by funding from the National Institutes of Health (grant K23-AI127935 awarded to P.D.T. and grant R21-AI130608 awarded to P.J.S.). None of the case authors report any conflicts of interest.
This Journal section presents a real, challenging case involving a multidrug-resistant organism. The case authors present the rationale for their therapeutic strategy and discuss the impact of mechanisms of resistance on clinical outcome. Expert clinicians then provide a commentary on the case.
REFERENCES
- 1.El Chakhtoura NG, Saade E, Wilson BM, Perez F, Papp-Wallace KM, Bonomo RA. 2017. A 17-year nationwide study of Burkholderia cepacia complex bloodstream infections among patients in the United States Veterans Health Administration. Clin Infect Dis 65:1253–1259. doi: 10.1093/cid/cix548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 25 August 2017 Multistate outbreak of Burkholderia cepacia infections associated with oral liquid docusate sodium. Centers for Disease Control and Prevention, Atlanta, Ga: https://www.cdc.gov/hai/outbreaks/b-cepacia/index.html Accessed 18 October 2017. [Google Scholar]
- 3.Papp-Wallace KM, Becka SA, Zeiser ET, Ohuchi N, Mojica MF, Gatta JA, Falleni M, Tosi D, Borghi E, Winkler ML, Wilson BM, LiPuma JJ, Nukaga M, Bonomo RA. 2017. Overcoming an extremely drug resistant (XDR) pathogen: avibactam restores susceptibility to ceftazidime for Burkholderia cepacia complex isolates from cystic fibrosis patients. ACS Infect Dis 3:502–511. doi: 10.1021/acsinfecdis.7b00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papp-Wallace KM, Becka SA, Taracila MA, Zeiser ET, Gatta JA, LiPuma JJ, Bonomo RA. 2017. Exploring the role of the Ω-loop in the evolution of ceftazidime resistance in the PenA β-lactamase from Burkholderia multivorans, an important cystic fibrosis pathogen. Antimicrob Agents Chemother 61:e01941-16. doi: 10.1128/AAC.01941-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clinical and Laboratory Standards Institute. 2017. Performance standards for antimicrobial susceptibility testing. CLSI, Wayne, PA. [Google Scholar]
- 6.Poirel L, Rodriguez-Martinez JM, Plesiat P, Nordmann P. 2009. Naturally occurring class A β-lactamases from the Burkholderia cepacia complex. Antimicrob Agents Chemother 53:876–882. doi: 10.1128/AAC.00946-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papp-Wallace KM, Taracila MA, Gatta JA, Ohuchi N, Bonomo RA, Nukaga M. 2013. Insights into beta-lactamases from Burkholderia species, two phylogenetically related yet distinct resistance determinants. J Biol Chem 288:19090–19102. doi: 10.1074/jbc.M113.458315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ehmann DE, Jahic H, Ross PL, Gu RF, Hu J, Durand-Reville TF, Lahiri S, Thresher J, Livchak S, Gao N, Palmer T, Walkup GK, Fisher SL. 2013. Kinetics of avibactam inhibition against class A, C, and D beta-lactamases. J Biol Chem 288:27960–27971. doi: 10.1074/jbc.M113.485979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mushtaq S, Warner M, Livermore DM. 2010. In vitro activity of ceftazidime+NXL104 against Pseudomonas aeruginosa and other non-fermenters. J Antimicrob Chemother 65:2376–2381. doi: 10.1093/jac/dkq306. [DOI] [PubMed] [Google Scholar]
- 10.Rhodes KA, Schweizer HP. 2016. Antibiotic resistance in Burkholderia species. Drug Resist Updat 28:82–90. doi: 10.1016/j.drup.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loutet SA, Valvano MA. 2011. Extreme antimicrobial peptide and polymyxin B resistance in the genus Burkholderia. Front Microbiol 2:159. doi: 10.3389/fmicb.2011.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Everaert A, Coenye T. 2016. Effect of β-lactamase inhibitors on in vitro activity of β-lactam antibiotics against Burkholderia cepacia complex species. Antimicrob Resist Infect Control 5:44. doi: 10.1186/s13756-016-0142-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou J, Chen Y, Tabibi S, Alba L, Garber E, Saiman L. 2007. Antimicrobial susceptibility and synergy studies of Burkholderia cepacia complex isolated from patients with cystic fibrosis. Antimicrob Agents Chemother 51:1085–1088. doi: 10.1128/AAC.00954-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu D, Chang S, Chen Y, Luh K, Hsieh W. 1997. In vitro activities of antimicrobial agents, alone and in combinations, against Burkholderia cepacia isolated from blood. Diagn Microbiol Infect Dis 28:187–191. doi: 10.1016/S0732-8893(97)00069-2. [DOI] [PubMed] [Google Scholar]
- 15.Mandell G, Bennett J, Dolin R. 2014. Mandell, Douglas, and Bennett's principles and practice of infectious diseases, 8th ed Saunders, Philadelphia, PA. [Google Scholar]
- 16.Lagace-Wiens P, Walkty A, Karlowsky J. 2014. Ceftazidime-avibactam: an evidence-based review of its pharmacology and potential use in the treatment of Gram-negative bacterial infections. Core Evid 9:13–25. doi: 10.2147/CE.S40698. [DOI] [PMC free article] [PubMed] [Google Scholar]