ABSTRACT
Nitroimidazoles (metronidazole [MTZ] and tinidazole [TNZ]) are the only drugs recommended for treatment of Trichomonas vaginalis infections. MTZ resistance occurs in 4% to 10% of cases of vaginal trichomoniasis (R. D. Kirkcaldy et al., Emerg Infect Dis 18:939–943, 2012; J. R. Schwebke and F. J. Barrientes, Antimicrob Agents Chemother 50:4209–4210, 2006) and TNZ resistance in 1% of cases (J. R. Schwebke and F. J. Barrientes, Antimicrob Agents Chemother 50:4209–4210, 2006). Emerging nitroimidazole-resistant trichomoniasis is concerning, because few alternatives to standard therapy exist. We assessed the prevalence of in vitro aerobic MTZ and secnidazole resistance among T. vaginalis isolates collected in 2015 to 2016 from 100 women in Birmingham, Alabama, with positive cultures. Archived specimens were treated with secnidazole or MTZ (0.2 to 400 μg/ml) for 48 h, according to U.S. Centers for Disease Control and Prevention protocols. Ninety-six (96%) of the 100 clinical Trichomonas isolates tested demonstrated lower minimum lethal concentrations for secnidazole than for MTZ, suggesting that secnidazole has better in vitro activity than MTZ.
KEYWORDS: Trichomonas vaginalis, metronidazole, nitroimidazoles, secnidazole
INTRODUCTION
Trichomonas vaginalis infection is the most common nonviral, sexually transmitted infection in the world. Although trichomoniasis is not a reportable disease, 248 million incident (new) cases occur worldwide and ∼7.4 million cases occur in the United States (1). T. vaginalis is a flagellated parasitic protozoan with an anaerobic lifestyle (2). The prevalence of T. vaginalis infections in the reproductive age group (14 to 49 years of age) is ∼3.1%, with a significantly higher prevalence rate of 13.3% among African-American women in the same age group (3). Trichomoniasis in male sexual partners is not well recognized; T. vaginalis was detected in 71.7% of male partners of women with T. vaginalis infections, with ∼77% of the male partners being asymptomatic (4). Symptomatic women present with vaginal discharge, dysuria, itching, vulvar irritation, and abdominal pain. Studies show associations between T. vaginalis and vaginitis, cervicitis, urethritis, bacterial vaginosis, candidiasis, herpes simplex virus 1 and 2, chlamydia, gonorrhea, and syphilis (5), while several cross-sectional and cohort studies have indicated a higher risk of HIV acquisition among T. vaginalis-positive women, compared with T. vaginalis-negative women (6).
Metronidazole (MTZ) (α-hydroxyethyl-2-methyl-5-nitroimidazole) has historically been the drug of choice for the treatment of T. vaginalis infections and, in randomized clinical trials, recommended MTZ regimens have resulted in cure rates of approximately 84% to 98% (7–9). The World Health Organization and U.S. Centers for Disease Control and Prevention (CDC) guidelines for the treatment of T. vaginalis include 2 g of MTZ or tinidazole (TNZ) in a single dose as the recommended treatment regimens and 500 mg of MTZ twice a day for 7 days as the alternative treatment regimen (10). If patients fail single-dose MTZ therapy, then they can be given single-dose TNZ or 7-day-dose MTZ therapy. If those regimens fail, then 2 g of MTZ twice a day for 7 days can be administered. If that treatment fails and there is no history of sexual reexposure, then a consultation for medication resistance testing should follow. For T. vaginalis that remains persistent or for patients who are allergic to those medications, other intravaginal treatments have been studied or are under investigation for the treatment of T. vaginalis, including acetarsol (11), boric acid (12), furazolidone (13), and paromomycin (14). Nitazoxanide was examined as an alternative oral agent for MTZ-resistant T. vaginalis but was not found to be very effective (15).
MTZ is a 5-nitroimidazole that is metabolized primarily in the liver by side chain oxidation and glucuronidation. Hydroxymetronidazole is the main product produced when MTZ is metabolized. The half-life of MTZ is ∼8.7 h, compared with ∼12 h for the hydroxy metabolite. MTZ is a small molecule that enters T. vaginalis via passive diffusion. MTZ is reduced in the hydrogenosomes of T. vaginalis by the enzyme pyruvate:ferredoxin oxidoreductase (16). Single-dose MTZ is generally well tolerated, with patients suffering few or no side effects in response to standard regimens. Common adverse reactions include nausea, vomiting, headache, insomnia, dizziness, drowsiness, rash, dry mouth, and metallic taste (mainly from oral MTZ). More serious adverse effects are rare but include eosinophilia, leukopenia, palpitations, confusion, and peripheral neuropathy (17). Adverse reactions to MTZ are of greater concern for patients with refractory infections who receive much higher doses, leading to patient intolerance, incomplete treatment courses, and treatment failure (18, 19). A number of nitroimidazole derivatives beside MTZ and TNZ have been investigated for the treatment of T. vaginalis infections. The modes of action of the derivatives are similar, but their pharmacokinetics, tissue distributions, levels in serum, trichomonacidal activity, and toxicity vary.
Secnidazole is widely employed outside the United States for the treatment of intestinal and extraintestinal parasites (20–22). However, secnidazole has recently been approved by the FDA for the treatment of bacterial vaginosis. Secnidazole is a nitroimidazole with a half-life of ∼20 h, which is longer than that of other nitroimidazoles, thus permitting its use as a single dose. Secnidazole administered by the oral or intravenous route is rapidly distributed throughout the body, reaching target organs at excellent concentrations, and the drug has low (virtually nonexistent) toxicity. The adverse effects of secnidazole are similar to those of all nitroimidazoles and include gastrointestinal disorders, headache, neurological disorders, allergic reactions, and leukopenia. However, the incidence of these effects is relatively low, and the effects are usually mild, especially with short-term treatment regimens, such as those involving a single dose (23). Previous studies indicated that a single dose of secnidazole cured 97% of patients with trichomoniasis (24), in addition to having a longer half-life and being better tolerated than MTZ (25). In the current study, we compared the in vitro efficacy of secnidazole with that of MTZ in clinical isolates of T. vaginalis.
RESULTS
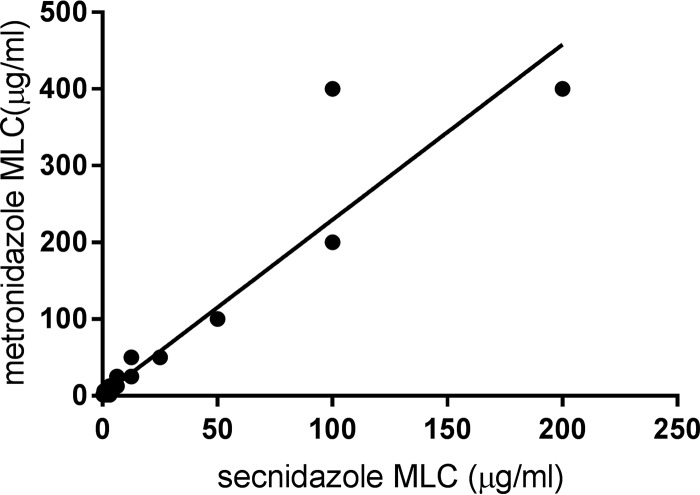
Under aerobic conditions, the isolates evaluated in this study were much more sensitive to secnidazole than to MTZ. The minimum lethal concentration (MLC) (mean ± standard deviation) for secnidazole was 5.9 ± 13.2 μg/ml, while that for MTZ was 13.5 ± 26.9 μg/ml (Fig. 1). The median MLC for MTZ (6.3 μg/ml) was significantly higher than the median MLC for secnidazole (1.6 μg/ml) (Fig. 1). Ninety-six (96%) of the 100 clinical Trichomonas isolates tested demonstrated a lower MLC for secnidazole than for MTZ, while 3 of the isolates had similar MLCs for MTZ and secnidazole (P < 0.0001, Wilcoxon signed-rank test) (Fig. 2). One isolate demonstrated a higher MLC for secnidazole (3.1 μg/ml) than for MTZ (1.6 μg/ml). The prevalence of low-level MTZ resistance was 7%, while 1% of the isolates exhibited moderate MTZ resistance. Four percent of the isolates exhibited low-level resistance to secnidazole, while none of the isolates demonstrated moderate- or high-level secnidazole resistance. Correlation analysis revealed a strong relationship between the MLC for MTZ and the MLC for secnidazole (r = 0.9496; P < 0.0001), indicating that the isolates with increased resistance to MTZ also demonstrated decreased sensitivity to secnidazole (Fig. 3). This finding is not surprising, as both compounds are 5-nitroimidazoles.
FIG 1.
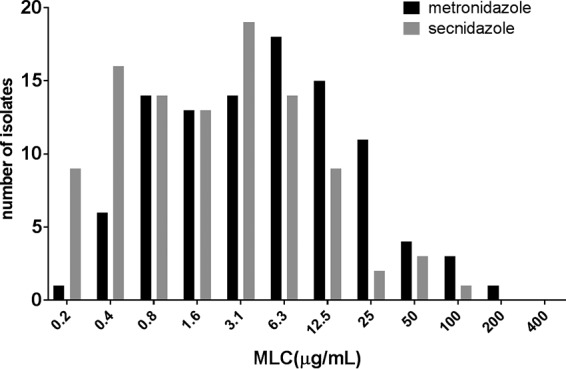
Distribution of secnidazole and MTZ MLCs for 100 clinical isolates. Susceptibility to MTZ and secnidazole was defined as MLCs of <25 μg/ml, low-level resistance as MLCs of 50 to 100 μg/ml, moderate-level resistance as MLCs of 200 μg/ml, and high-level resistance as MLCs of >400 μg/ml.
FIG 2.
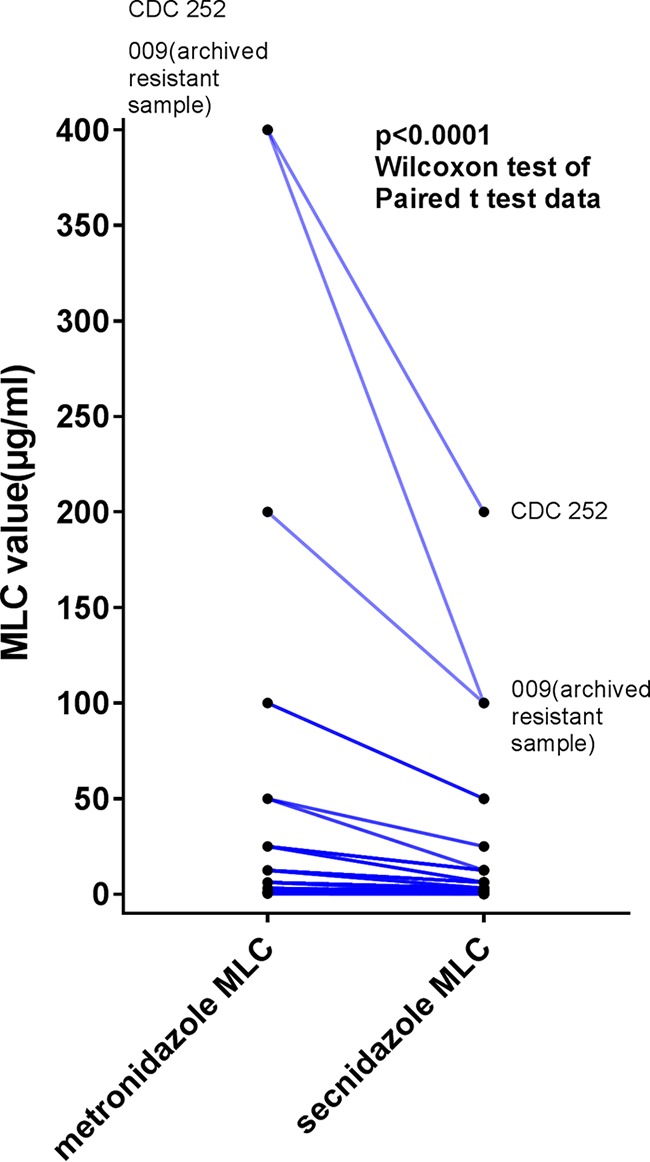
Comparison of MTZ and secnidazole activities in 100 Trichomonas vaginalis isolates. The MLCs for each drug were determined as described in the text. The MLCs for MTZ were consistently higher than for those for secnidazole (P < 0.0001, Wilcoxon signed-rank test).
FIG 3.
Regression analyses of MLCs for MTZ and secnidazole. The diagonal line represents the line of identity, indicating equal concentrations of the two drugs. The MLCs for MTZ were strongly correlated with the MLCs for secnidazole (r = 0.9496; P < 0.0001).
DISCUSSION
Our data suggest that secnidazole has superior efficacy in vitro, in comparison with MTZ, against T. vaginalis infections. Our data also suggest that the concentration of secnidazole needed for therapeutic efficacy may be lower than that of MTZ, and further clinical studies are warranted. Our studies are consistent with previous studies that compared the efficacy of other 5-nitroimidazoles to that of MTZ. In most studies, TNZ performed better than MTZ in the treatment of T. vaginalis infections. Our study is the first in vitro study to compare the efficacy of secnidazole in treating T. vaginalis infections to that of MTZ. While secnidazole belongs to the same class of drugs as MTZ, its pharmacokinetics are significantly different (26, 27), and the drugs may thus have different in vivo efficacies. Secnidazole has a longer half-life and relatively fewer adverse effects, in comparison with MTZ. In addition, it can be administered as a single dose, ensuring better patient compliance. Our data suggest that T. vaginalis may respond to lower concentrations of secnidazole than MTZ, potentially making secnidazole a viable, single-dose alternative to MTZ. Our data set did not identify T. vaginalis isolates with high-level MTZ resistance. We identified 5 clinical isolates that exhibited low-level MTZ resistance, and all of those isolates responded to significantly lower concentrations of secnidazole. We also identified 1 clinical isolate with moderate-level MTZ resistance, which had a significantly lower MLC for secnidazole. Interestingly, our control isolates with established high-level resistance to MTZ responded to significantly lower levels of secnidazole. In view of the increasing incidence of MTZ-resistant T. vaginalis infections and the sole reliance on 5-nitroimidazoles to treat T. vaginalis, a therapeutic alternative to MTZ is needed. While secnidazole may not be effective for all patients with MTZ-resistant Trichomonas infections, the increased sensitivity of MTZ-resistant trichomonads to secnidazole suggests that patients with infections that are refractory to MTZ treatment may still respond to secnidazole, as indicated by previous studies with patients with T. vaginalis infections who failed treatment with MTZ and responded to TNZ (28).
A major limitation of our study is the reported inconsistency between in vitro susceptibility results and clinical outcomes of treatment, particularly for infections with low-level in vitro resistance. Clinical resistance and treatment failure have been reported for T. vaginalis isolates for which the MLCs of nitroimidazoles were as low as 12.5 μg/ml, and treatment success has occurred for infections with T. vaginalis isolates for which the MLCs of nitroimidazoles were 100 to 200 μg/ml (29). Thus, the clinical efficacy of secnidazole in treating patients with infections that are refractory to MTZ needs to be demonstrated. A recent evaluation of the utility of susceptibility testing for women for whom clinical treatment failed found that treatment recommendations based on susceptibility results might have a beneficial role in guiding the clinical management of some women, particularly those with persistent infections (30). Although TNZ and MTZ are the only 5-nitroimidazoles approved for treatment of T. vaginalis infections in the United States, other nitroimidazoles, such as ornidazole, tenonitrazole, and nimorazole, are available in Europe and could be alternatives to MTZ. Secnidazole belongs to the same class of drugs as MTZ, and the emergence of clinically notable nitroimidazole resistance would also be expected to adversely influence the effectiveness of secnidazole for the treatment of trichomoniasis.
The emerging resistance of T. vaginalis to MTZ and the reliance on a single class of antimicrobial drugs warrants the evaluation of existing compounds and the development of novel systemic treatment options. Secnidazole administered as a single dose, with a longer half-life and relatively fewer adverse effects, may be useful as an adjunct or alternative to MTZ, especially for patients with MTZ-resistant T. vaginalis infections.
MATERIALS AND METHODS
The study was approved by the University of Alabama at Birmingham Institutional Review Board. Archived, deidentified, clinical isolates of T. vaginalis that had been banked in liquid nitrogen were used for this study. The samples had been collected between July 2015 and June 2017, in accordance with institutional review board guidelines, and all patients had consented to the storage and use of their samples for subsequent testing. Isolates were assayed for MTZ and secnidazole susceptibility under aerobic conditions, according to the method described previously (31), using serial drug concentrations from 400 μg/ml to 0.2 μg/ml. In brief, trichomonads were grown in Diamond's medium 1025 (containing trypticase soy broth, yeast extract, maltose, and calf serum) adjusted to pH 5.9 and supplemented with streptomycin at 1 g/liter and penicillin G at 100 U/ml. A hemocytometer was used to count the trichomonad population and to adjust it to a concentration of 66,666 cells/ml. Isolates were tested in microtiter plates under aerobic conditions. MLCs for MTZ and secnidazole were tested in triplicate. Plates were then incubated at 37°C for 46 to 50 h and read with a Zeiss Axiovert inverted microscope. The concentration of drug in the microplate wells in which no motile trichomonads were observed was reported as the MLC. Control strains included CDC252 (resistant) and CDC520 (sensitive) and an archived sample (isolate 009) that had previously exhibited in vitro resistance to both MTZ and TNZ (32). Low-level resistance was defined as an aerobic MLC of 50 to 100 μg/ml, moderate-level resistance as an aerobic MLC of 200 μg/ml, and high-level resistance as an aerobic MLC of >400 μg/ml (29). The MLCs for MTZ and secnidazole were analyzed using the Wilcoxon signed-rank test to evaluate the differences between treatments.
ACKNOWLEDGMENTS
Support for this work was provided by Symbiomix Therapeutics, LLC (Newark, NJ).
J.R.S. acts as a consultant for Symbiomix Therapeutics.
REFERENCES
- 1.Kissinger P. 2015. Trichomonas vaginalis: a review of epidemiologic, clinical and treatment issues. BMC Infect Dis 15:307. doi: 10.1186/s12879-015-1055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harp DF, Chowdhury I. 2011. Trichomoniasis: evaluation to execution. Eur J Obstet Gynecol Reprod Biol 157:3–9. doi: 10.1016/j.ejogrb.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sutton M, Sternberg M, Koumans EH, McQuillan G, Berman S, Markowitz L. 2007. The prevalence of Trichomonas vaginalis infection among reproductive-age women in the United States, 2001–2004. Clin Infect Dis 45:1319–1326. doi: 10.1086/522532. [DOI] [PubMed] [Google Scholar]
- 4.Sena AC, Miller WC, Hobbs MM, Schwebke JR, Leone PA, Swygard H, Atashili J, Cohen MS. 2007. Trichomonas vaginalis infection in male sexual partners: implications for diagnosis, treatment, and prevention. Clin Infect Dis 44:13–22. doi: 10.1086/511144. [DOI] [PubMed] [Google Scholar]
- 5.Allsworth JE, Ratner JA, Peipert JF. 2009. Trichomoniasis and other sexually transmitted infections: results from the 2001–2004 National Health and Nutrition Examination Surveys. Sex Transm Dis 36:738–744. doi: 10.1097/OLQ.0b013e3181b38a4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kissinger P, Adamski A. 2013. Trichomoniasis and HIV interactions: a review. Sex Transm Infect 89:426–433. doi: 10.1136/sextrans-2012-051005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thin RN, Symonds MA, Booker R, Cook S, Langlet F. 1979. Double-blind comparison of a single dose and a five-day course of metronidazole in the treatment of trichomoniasis. Br J Vener Dis 55:354–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gabriel G, Robertson E, Thin RN. 1982. Single dose treatment of trichomoniasis. J Int Med Res 10:129–130. doi: 10.1177/030006058201000212. [DOI] [PubMed] [Google Scholar]
- 9.Spence MR, Harwell TS, Davies MC, Smith JL. 1997. The minimum single oral metronidazole dose for treating trichomoniasis: a randomized, blinded study. Obstet Gynecol 89:699–703. doi: 10.1016/S0029-7844(97)81437-8. [DOI] [PubMed] [Google Scholar]
- 10.Workowski KA, Bolan GA. 2015. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 64(RR-3):1–137. [PMC free article] [PubMed] [Google Scholar]
- 11.Chen MY, Smith NA, Fox EF, Bingham JS, Barlow D. 1999. Acetarsol pessaries in the treatment of metronidazole resistant Trichomonas vaginalis. Int J STD AIDS 10:277–280. doi: 10.1258/0956462991913943. [DOI] [PubMed] [Google Scholar]
- 12.Muzny C, Barnes A, Mena L. 2012. Symptomatic Trichomonas vaginalis infection in the setting of severe nitroimidazole allergy: successful treatment with boric acid. Sex Health 9:389–391. doi: 10.1071/SH11114. [DOI] [PubMed] [Google Scholar]
- 13.Goldman LM, Upcroft JA, Workowski K, Rapkin A. 2009. Treatment of metronidazole-resistant Trichomonas vaginalis. Sex Health 6:345–347. doi: 10.1071/SH09064. [DOI] [PubMed] [Google Scholar]
- 14.Nyirjesy P, Gilbert J, Mulcahy LJ. 2011. Resistant trichomoniasis: successful treatment with combination therapy. Sex Transm Dis 38:962–963. doi: 10.1097/OLQ.0b013e31822037e4. [DOI] [PubMed] [Google Scholar]
- 15.Dan M, Sobel JD. 2007. Failure of nitazoxanide to cure trichomoniasis in three women. Sex Transm Dis 34:813–814. [DOI] [PubMed] [Google Scholar]
- 16.Chapman A, Cammack R, Linstead D, Lloyd D. 1985. The generation of metronidazole radicals in hydrogenosomes isolated from Trichomonas vaginalis. J Gen Microbiol 131:2141–2144. [DOI] [PubMed] [Google Scholar]
- 17.Lossick JG. 1990. Epidemiology of urogenital trichomoniasis. Springer-Verlag, New York, NY. [Google Scholar]
- 18.Ti TY, Lee HS, Khoo YM. 1996. Disposition of intravenous metronidazole in Asian surgical patients. Antimicrob Agents Chemother 40:2248–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis DA, Habgood L, White R, Barker KF, Murphy SM. 1997. Managing vaginal trichomoniasis resistant to high-dose metronidazole therapy. Int J STD AIDS 8:780–784. doi: 10.1258/0956462971919110. [DOI] [PubMed] [Google Scholar]
- 20.Escobedo AA, Canete R, Gonzalez ME, Pareja A, Cimerman S, Almirall P. 2003. A randomized trial comparing mebendazole and secnidazole for the treatment of giardiasis. Ann Trop Med Parasitol 97:499–504. doi: 10.1179/000349803235002380. [DOI] [PubMed] [Google Scholar]
- 21.Ahuja V, Dhar A, Bal C, Sharma MP. 1998. Lansoprazole and secnidazole with clarithromycin, amoxycillin or pefloxacin in the eradication of Helicobacter pylori in a developing country. Aliment Pharmacol Ther 12:551–555. doi: 10.1046/j.1365-2036.1998.00338.x. [DOI] [PubMed] [Google Scholar]
- 22.Nunez JT, Gomez G. 2005. Low-dose secnidazole in the treatment of bacterial vaginosis. Int J Gynaecol Obstet 88:281–285. doi: 10.1016/j.ijgo.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 23.Meingassner JG, Heyworth PG. 1981. Intestinal and urogenital flagellates. Antibiot Chemother (1971) 30:163–202. doi: 10.1159/000398097. [DOI] [PubMed] [Google Scholar]
- 24.Videau D, Niel G, Siboulet A, Catalan F. 1978. Secnidazole: a 5-nitroimidazole derivative with a long half-life. Br J Vener Dis 54:77–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cudmore SL, Delgaty KL, Hayward-McClelland SF, Petrin DP, Garber GE. 2004. Treatment of infections caused by metronidazole-resistant Trichomonas vaginalis. Clin Microbiol Rev 17:783–793. doi: 10.1128/CMR.17.4.783-793.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Upcroft JA, Dunn LA, Wright JM, Benakli K, Upcroft P, Vanelle P. 2006. 5-Nitroimidazole drugs effective against metronidazole-resistant Trichomonas vaginalis and Giardia duodenalis. Antimicrob Agents Chemother 50:344–347. doi: 10.1128/AAC.50.1.344-347.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Upcroft JA, Campbell RW, Benakli K, Upcroft P, Vanelle P. 1999. Efficacy of new 5-nitroimidazoles against metronidazole-susceptible and -resistant Giardia, Trichomonas, and Entamoeba spp. Antimicrob Agents Chemother 43:73–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sobel JD, Nyirjesy P, Brown W. 2001. Tinidazole therapy for metronidazole-resistant vaginal trichomoniasis. Clin Infect Dis 33:1341–1346. doi: 10.1086/323034. [DOI] [PubMed] [Google Scholar]
- 29.Lossick JG, Muller M, Gorrell TE. 1986. In vitro drug susceptibility and doses of metronidazole required for cure in cases of refractory vaginal trichomoniasis. J Infect Dis 153:948–955. doi: 10.1093/infdis/153.5.948. [DOI] [PubMed] [Google Scholar]
- 30.Bosserman EA, Helms DJ, Mosure DJ, Secor WE, Workowski KA. 2011. Utility of antimicrobial susceptibility testing in Trichomonas vaginalis-infected women with clinical treatment failure. Sex Transm Dis 38:983–987. doi: 10.1097/OLQ.0b013e318224db39. [DOI] [PubMed] [Google Scholar]
- 31.Meingassner JG, Thurner J. 1979. Strain of Trichomonas vaginalis resistant to metronidazole and other 5-nitroimidazoles. Antimicrob Agents Chemother 15:254–257. doi: 10.1128/AAC.15.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwebke JR, Barrientes FJ. 2006. Prevalence of Trichomonas vaginalis isolates with resistance to metronidazole and tinidazole. Antimicrob Agents Chemother 50:4209–4210. doi: 10.1128/AAC.00814-06. [DOI] [PMC free article] [PubMed] [Google Scholar]