ABSTRACT
Botulinum neurotoxins (BoNTs), the most poisonous substances known in nature, pose significant concern to health authorities. The only approved therapeutic for botulism is antitoxin. While administered to patients only after symptom onset, antitoxin efficacy is evaluated in animals mostly in relation to time postintoxication regardless of symptoms. This is most likely due to the difficulty in measuring early symptoms of botulism in animals. In this study, a rabbit spirometry model was developed to quantify early respiratory symptoms of type E botulism that were further used as a trigger for treatment. Impaired respiration, in the form of a reduced minute volume, was detected as early as 18.1 ± 2.9 h after intramuscular exposure to 2 rabbit 50% lethal doses (LD50) of BoNT serotype E (BoNT/E), preceding any visible symptoms. All rabbits treated with antitoxin immediately following symptom onset survived. Postsymptom antitoxin efficacy was further evaluated in relation to toxin and antitoxin dosages as well as delayed antitoxin administration. Our system enabled us to demonstrate, for the first time, full antitoxin protection of animals treated with antitoxin after the onset of objective and quantitative type E botulism symptoms. This model may be utilized to evaluate the efficacy of antitoxins for additional serotypes of BoNT as well as that of next-generation anti-BoNT drugs that enter affected cells and act when antitoxin is no longer effective.
KEYWORDS: botulinum, postsymptom, therapy
INTRODUCTION
Botulinum neurotoxins (BoNTs) are produced by the anaerobic bacterium Clostridium botulinum and are the most potent toxins known in nature, with an estimated human 50% lethal dose (HLD50) of 1 ng/kg of body weight (1). Among the different serotypes of the toxin, the A, B, E, and, rarely, F serotypes have been documented as being toxic for humans (2). Following entry into the circulation, BoNTs block acetylcholine transmission across neuromuscular junctions at presynaptic motor neuron terminals and cause bilateral flaccid paralysis that eventually ends in respiratory failure (3, 4). Widespread outbreaks of foodborne botulism might involve dozens of infected people who, without adequate treatment, may die (5–7), and BoNTs are thus of significant concern to health authorities. In addition, due to their extreme potency, BoNTs are classified as category A biothreat agents (8).
Standard therapy for botulism includes the administration of botulinum antitoxin and, in severe cases, intensive supportive care by means of mechanical ventilation. Antitoxin preparations are derived from equine plasma for adult patients (9) or from human plasma in cases of infant botulism (10). The development of “second-generation” antitoxins is now under consideration. These preparations are based on combinations of human-origin monoclonal antibodies that are intended to facilitate better toxin clearance and reduce potential side effects associated with the injection of the heterologous horse antibodies (11–15).
Botulinum antitoxin is expected to be useful mainly for neutralizing circulating BoNT molecules that are not yet bound to nerve endings (16). Hence, prompt antitoxin treatment should slow the disease course and reduce pulmonary distress by preventing toxin from binding its target (17). Indeed, data collected from observations of human clinical cases and from animal studies support the notion that there is a critical “therapeutic time window” for effective antitoxin treatment following botulinum intoxication. In type A botulism cases in the 1970s in the United States, reviewed by Tacket et al., patients who received antitoxin in the first 24 h after symptom onset (early treatment) or even later (late treatment) had lower fatality rates (10% and 15%, respectively) than did those who did not receive antitoxin at all (46%). Moreover, patients who received antitoxin early had a short disease course and did not need intubation, compared to patients who received late or no antitoxin treatment (17). Additionally, in a large type A botulism outbreak, two groups of intubated patients who received antitoxin either 4 days (early) or 6 days (late) after exposure were compared, and it was shown that patients treated early needed mechanical ventilation for a shorter period (5). Successful treatment with antitoxin was demonstrated for type E botulism as well. The fatality rate of botulism was ∼30% without antitoxin therapy but dropped to ∼4 to 8% with the use of antitoxin therapy (18, 19). The advantage of early antitoxin administration has also been demonstrated for heptavalent botulism antitoxin (HBAT) (14). It should be noted that in addition to antitoxin treatment, patient management through mechanical ventilation and other supportive measures has also contributed to the reduction in fatality rates.
While antitoxin is administered to patients only after symptom onset, its use in animal studies has been related mostly to time postintoxication regardless of symptoms (20–32). To evaluate antitoxin efficacy in a more clinically relevant timeline of treatment, we recently developed a mouse model for postsymptom, antibotulinum therapy (33). In that study, mice intoxicated with either BoNT serotype A (BoNT/A) or BoNT/B were fully protected when treated with a specific antitoxin after the manifestation of waist contraction, i.e., the “wasp waist” symptom. However, in mice exposed to BoNT/E, the time to death (TTD) was significantly shorter than those with BoNT/A and BoNT/B intoxications. Moreover, in BoNT/E-intoxicated mice, symptoms were ambiguous and could not be observed in all animals. Consequently, antitoxin therapy was ineffective in the time window between symptom onset and death.
Type E botulism is more abundant in Canada, Japan, and northern countries than are types A and B due to optimal environmental conditions favoring the proliferation of C. botulinum type E in food of oceanic origin (34). BoNT/E exerts its toxicity significantly earlier and faster than do BoNT/A and BoNT/B, mainly by entering neurons more rapidly and acting faster to inhibit neurotransmission (35). Indeed, individuals who were exposed to BoNT/E in food were admitted to the hospital as early as 24 h after the ingestion of contaminated food, whereas in cases of type A botulism, the average time to hospitalization may vary up to 7 days postexposure (36). Interestingly, recovery from type E botulism is faster than is recovery from type A botulism, apparently owing to the larger deletion of SNAP-25 (37) and to the enhanced susceptibility of the BoNT/E catalytic domain to ubiquitin-dependent degradation (38). Thus, although considered less dramatic, with a higher probability for recovery and a shorter duration of mechanical ventilation, type E botulism is the earliest to appear and therefore demands earlier diagnostic and medical responses.
In this study, a novel rabbit model for the evaluation of postsymptom antitoxin efficacy for type E botulism is described. The model relies on the early detection of deviations in physiological respiration parameters as measured by spirometry.
Preexposure respiratory data were individually collected and statistically analyzed to determine confidence limits. Rabbits exposed to BoNT/E showed a significantly reduced respiratory minute volume (MV). A positive and statistically significant correlation was obtained between time to symptoms (TTS) and TTD. The administration of antitoxin immediately after symptom onset was fully protective, and complete respiratory recovery was observed usually within 2 to 3 days of exposure. The effect of toxin and antitoxin dosages, as well as that of expanding the therapeutic window, is presented.
RESULTS
Experimental design.
The main purpose of this study was to evaluate antitoxin efficacy in a clinically relevant timeline, i.e., after the manifestation of botulism symptoms. In an attempt to define a quantitative symptom that will enable the evaluation of antibotulinum therapy in symptomatic animals, we monitored physiological respiratory parameters in naive (preexposed) unanesthetized rabbits using a flowmeter.
Data for respiration physiology parameters, including minute volume, rate, and tidal volume, were collected and analyzed. At least 12 independent measurements of each parameter were carried out for each individual animal over a period of 7 days in order to calculate the means and standard deviations (SD) (Fig. 1). Upper and lower confidence limits were determined as the means ± 2 SD, for which the probability (P) of measuring values above or below these limits in two successive measurements was 0.0025. Accordingly, values below the lower limit in BoNT-exposed rabbits were expected to be attributed to breathing distress due to intoxication and thus were considered clinical symptoms of botulism.
FIG 1.
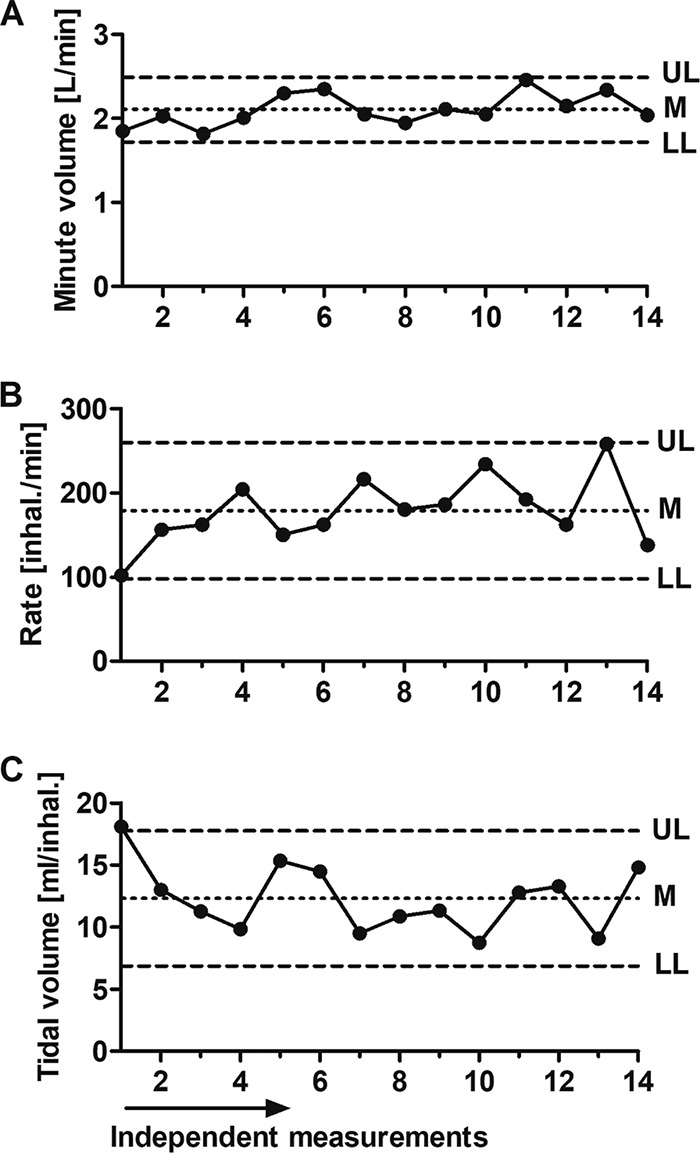
Rabbit respiratory parameters. Shown is a representative depiction of individual data collected from 14 independent measurements of a preexposed, unanesthetized rabbit. (A) Minute volume (MV) is the air volume consumption per minute. (B) Rate is the number of inhalations per minute. (C) Tidal volume (TV) is the air volume per inhalation. UL, upper limit; LL, lower limit; M, mean (UL = M + 2 × SD; LL = M − 2 × SD).
Characterization of respiration physiology in intoxicated rabbits.
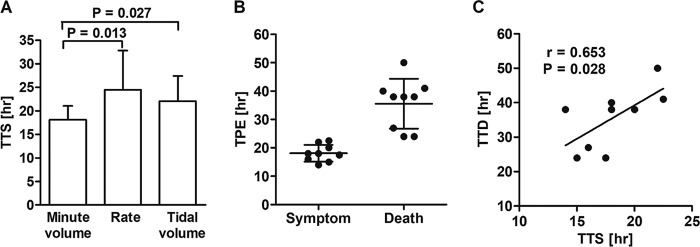
BoNT/E toxicity in rabbits was first studied by the determination of LD50 values. One rabbit LD50 via the intramuscular (i.m.) route (1 RbIMLD50) was found to be equal to 200 mouse intraperitoneal LD50 (MsIPLD50)/kg. Rabbits were then exposed to 2 RbIMLD50 (400 MsIPLD50/kg), and spirometry was monitored. To determine the earliest clinical symptom, the three spirometry parameters for all tested rabbits were scored according to the TTS, i.e., the time postexposure at which a statistically significant deviation from the predefined limit (before intoxication) occurred. The earliest deviation was observed for the MV parameter that presented the lowest relative standard deviation as well. Thus, MV was chosen to become the leading clinical symptom (Fig. 2A). The correlation between TTD and TTS was tested for the MV parameter and found to be positive and statistically significant (r = 0.65; P = 0.028) (Fig. 2C), practically meaning that rabbits that presented the symptom earlier also succumbed to the toxin earlier. This result implies that TTD can be predicted by TTS and that the deviation from normal MV values could serve as a clinical symptom of botulism. Additionally, when rabbits were exposed to 2 RbIMLD50, an aberrant MV could be observed at approximately the halfway point of the disease time course, as the mean TTS was 18.1 ± 2.9 h, and the mean TTD was 35.6 ± 8.8 h (Fig. 2B).
FIG 2.
Characterization of respiration physiology in intoxicated rabbits. Following exposure to 2 RbIMLD50, respiration parameters were analyzed and tested for suitability to serve as clinical symptoms. (A) Time from intoxication to a statistically significant deviation from the mean value of each of the three parameters. For each pair of parameters, means were compared by a t test. (B) The time to symptoms (TTS) was determined for each rabbit (n = 9) based on the MV parameter. TPE, time postexposure. (C) Correlation between time to death (TTD) and TTS for the MV symptom.
Administration of antitoxin after the onset of respiratory symptoms fully protects BoNT/E-intoxicated rabbits.
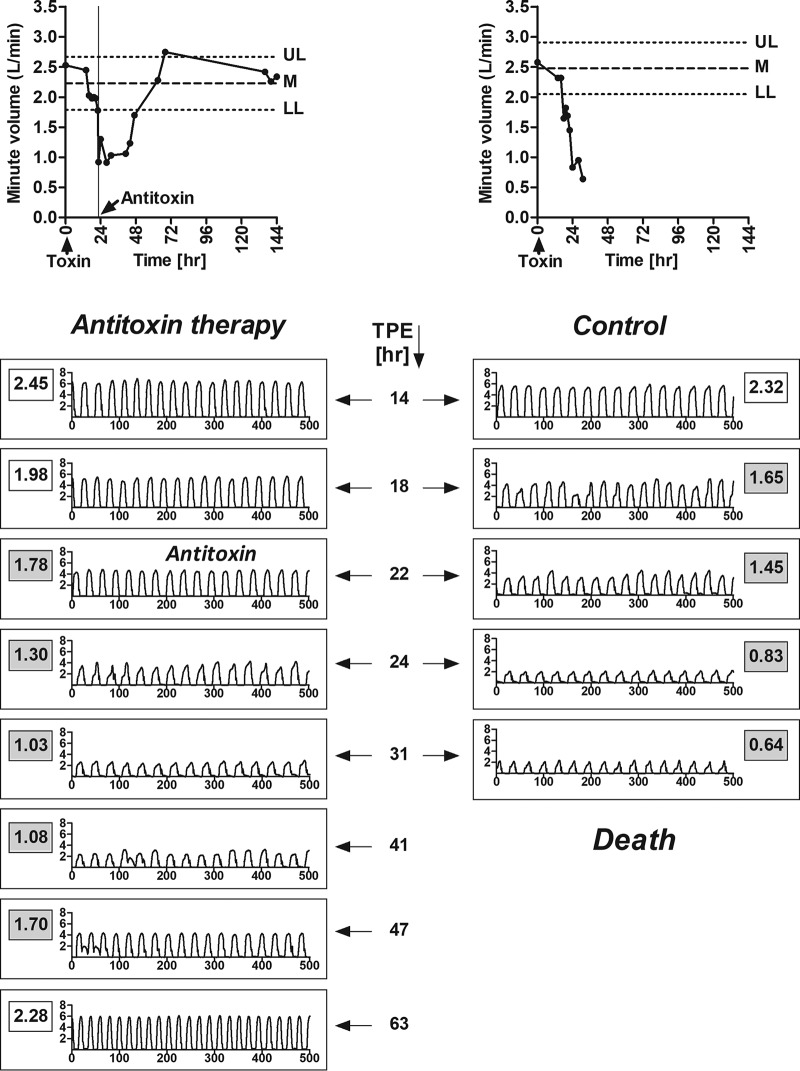
To test the efficacy of antitoxin in a clinically relevant timeline, the MV was monitored before and after the administration of 2 RbIMLD50 of BoNT/E. Rabbits were treated with 30 IU/kg of anti-BoNT/E antitoxin (antitoxin E) immediately after the onset of the MV symptom (Fig. 3).
FIG 3.
Antitoxin is fully protective when administered immediately after symptom onset. (Top) MV values were monitored before and after exposure to 2 RbIMLD50 of BoNT/E with 30 IU/kg of antitoxin (left) or without antitoxin (control) (right). UL, upper limit; LL, lower limit; M, mean. (Bottom) Inhalation profile during the disease course, expressed as MV (liters per minute) by time (seconds). For each time point, the MV value is indicated in a rectangle with a white background (normal) or a gray background (symptomatic). TPE, time postexposure. Data are representative of results from at least 4 treated and 9 untreated control rabbits, collected in 4 independent experiments.
Following antitoxin treatment, the MV continued to decline until pulmonary distress could be observed even without spirometry. Nevertheless, the MV gradually declined to normal values, and full respiration recovery was retained (Fig. 3). Antitoxin therapy given immediately after MV symptom onset had an efficacy of 100%, as all treated rabbits survived. To the best of our knowledge, this is the first demonstration of complete protection of symptomatic animals intoxicated with BoNT/E.
To define the time window for treatment after symptom onset, antitoxin was administered 2, 5, or 8 h after the manifestation of symptoms, and survival was monitored (Fig. 4). When antitoxin treatment was given 2 h after MV symptom onset, three out of four rabbits survived intoxication by 2 RbIMLD50 BoNT/E (Fig. 4). Interestingly, the one rabbit that succumbed to the toxin became symptomatic only 27 h after toxin exposure while still presenting a normal TTD at 35 h, creating a significant narrow therapeutic time window compared to those of other rabbits that showed symptoms at 18 h postexposure. Two out of three rabbits survived when antitoxin treatment was further delayed and administered 5 h after the appearance of MV symptoms. However, when treatment was administered 8 h after symptom onset, none of the exposed animals survived.
FIG 4.
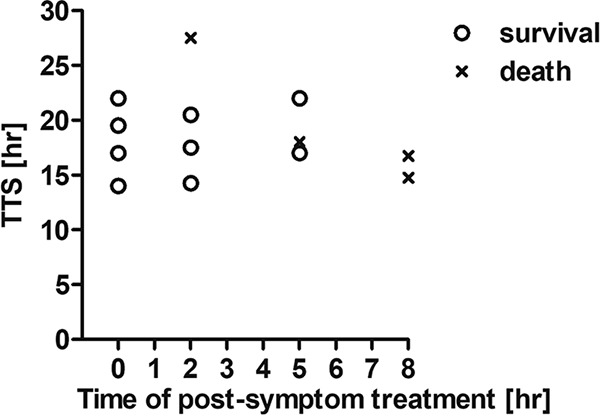
Efficacy of delayed antitoxin treatment following symptom onset. Rabbits were intoxicated with 2 RbIMLD50 of BoNT/E and treated with 30 IU/kg of antitoxin at different time points following the onset of the MV clinical symptom: immediately (0 h) and 2, 5, and 8 h after symptom onset. Each plotted symbol refers to an individual animal.
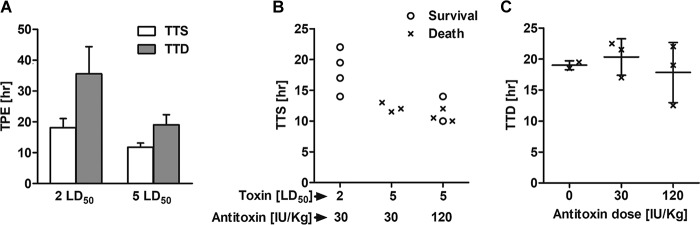
After elucidating the time window for antitoxin treatment in rabbits exposed to BoNT/E, we sought to explore how the protective dose of antitoxin is affected by the toxin dose given at exposure. For this purpose, rabbits were injected with 5 RbIMLD50 and treated with either 30 IU/kg or 120 IU/kg of antitoxin immediately after MV symptom onset (Fig. 5). As expected, TTS and TTD values for rabbits exposed to 5 RbIMLD50 were significantly lower than those for rabbits intoxicated with 2 RbIMLD50 (Fig. 5A). However, while clinical symptoms appeared after ∼50% of the disease course in rabbits exposed to 2 RbIMLD50 (TTS = 18.1 ± 2.9 h; TTD = 35.6 ± 8.8 h), with exposure to 5 RbIMLD50, clinical symptoms appeared only after >60% of the disease course (TTS = 11.8 ± 1.3 h; TTD = 19 ± 3.3 h). Indeed, when antitoxin was administered immediately after symptom onset and in a dosage similar to that in the former experiments (30 IU/kg), no survival was observed for rabbits exposed to 5 RbIMLD50. However, increasing the antitoxin dose 4-fold to 120 IU/kg led to 40% survival, as two out of five rabbits survived (Fig. 5B). The effect of antitoxin treatment on rabbits that did not survive was analyzed, and the results showed that the TTD was not delayed for nonsurviving rabbits treated with either 30 IU/kg or 120 IU/kg (Fig. 5C).
FIG 5.
Antitoxin treatment efficacy is dependent on toxin and antitoxin doses. (A) Rabbits were exposed to either 2 or 5 RbIMLD50 of BoNT/E without antitoxin treatment, and the TTS and TTD were then monitored and compared. (B) Rabbits were exposed to either 2 or 5 RbIMLD50 of BoNT/E and treated with either 30 or 120 IU/kg of antitoxin immediately after symptom onset. Protection levels among the different toxin and antitoxin combinations were compared. (C) Influence of antitoxin dose on TTD. Each plotted symbol refers to data for an individual animal.
DISCUSSION
Antitoxin administration is the only approved therapy available to treat botulism. However, due to the concern of potential adverse effects associated with its equine source and immunogenic nature, antitoxin is given only to symptomatic patients.
To fully study and appreciate the pharmacodynamics and pharmacokinetics of botulinum antitoxin and to evaluate the time window for treatment after the onset of botulism symptoms, it is mandatory to use symptomatic animal models. However, identifying and quantitating relevant systemic clinical symptoms for which the therapeutic time window could be referred to are major challenges in developing such models. While human patients report early botulism symptoms such as blurred vision, dry mouth, and diplopia or just odd feelings long before the appearance of observed signs such as ptosis and difficulty speaking (19), animals, especially rodents, do not present such facial symptoms and obviously cannot report their situation. As a consequence, the use of antitoxin in animal studies has been related mostly to time postintoxication, whereas data regarding treatment after the onset of symptoms have been collected only post factum (20–32). To the best of our knowledge, only a few attempts to directly measure postsymptom antitoxin efficacy were reported, mostly for BoNT/A-intoxicated nonhuman primates (14, 39–42). In those studies, as in all other animal models reported thus far, symptoms were based on subjective observations, and therefore, their onset might not be witnessed precisely and may vary substantially among different studies. Subjective impressions and elusive early symptoms make it difficult to establish a meaningful model for symptomatic animals. For these reasons, reliable data to establish postsymptom antitoxin efficacy, such as dosage and timing, still need further elucidation (14, 17, 43); hence, the prognosis for successful antitoxin treatment for symptomatic patients with botulism is unclear.
In this study, we used spirometry as an accurate, unambiguous, quantitative, and objective means to detect early symptoms of botulism in rabbits. Deviation from the normal MV following BoNT/E intoxication was the earliest symptom to be measured among other spirometry-related parameters, and individual preexposure respiration data showed high repeatability with a relative standard deviation (RSD) as low as 10%. Thus, the postintoxication MV decline was selected to serve as a clinical symptom, i.e., the MV symptom. The ability to accurately quantify it by a spirometer, together with the positive and statistically significant correlation (Fig. 2C) between the TTS and TTD and its systemic nature, qualified the MV symptom to become a reliable clinical symptom of botulism. In this regard, it is important to note that impaired respiration is a fundamental symptom of human botulism regardless of the exposure route.
The MV symptom in our rabbit model allowed us to expand the time window for treatment by detecting a statistically significant deviation from normal respiration long before (8 to 10 h on average) any visible signs could be observed. The use of an impaired MV as an early symptom of rabbit botulism may seem surprising, as respiratory distress in human botulism cases is considered a late clinical symptom. However, in our rabbit model, the MV symptom preceded all other signs of botulism. One explanation for this phenomenon could involve the high-resolution monitoring of intoxicated rabbits using MV measurements, which allows the uncovering of subtle changes in air consumption while pulmonary distress is yet to be noticed, as opposed to human cases, for which spirometry is not used prior to the progression of dyspnea. One can speculate that if the mean MV value of a healthy human individual was recorded in advance, it might have been possible to notice decrease in the MV much earlier after exposure to the toxin, but this is not a realistic scenario.
The ability to demonstrate postsymptom protection from BoNT/E intoxication is especially important, taking into account the significantly earlier and faster toxicity that it presents compared to the two other serotypes that are common in human botulism, i.e., serotypes A and B (36). Indeed, recent results obtained by using the spirometry system in BoNT/A-intoxicated rabbits confirmed that even at 4 RbIMLD50, the TTD and TTS appeared far later than after exposure to 2 RbIMLD50 of BoNT/E (A. Torgeman, A. Schwartz, E. Diamant, Z. Baruchi, E. Dor, A. Ben David, A. Pass, A. Barnea, A. Tal, A. Rosner, O. Rosen, and R. Zichel, unpublished data). Thus, the demonstration of efficient postsymptom antitoxin protection in rabbits exposed to BoNT/E may be considered more challenging and serve as proof of the validity of the spirometry model.
In our model, rabbits that were treated with antitoxin following MV symptom onset continued to deteriorate for another ∼20 h until the MV declined to less than 50% of normal values. Fully normal respiration performance could be measured as early as 20 to 120 h from the time of treatment. As with the kinetics of symptom onset, respiration recovery preceded complete physical fitness of the animals, suggesting that in rabbits exposed to BoNT/E, spirometry is an adequate tool to detect early symptoms that are also the earliest to resolve. The relatively fast recovery of rabbits in the present study is in line with other works. In an antitoxin protection mouse model, Cheng et al. reported that animals that had been treated with antitoxin following exposure to hundreds of LD50 of BoNT/A and that survived the challenge recovered in a few days (28). In human cases where large amounts of toxin are consumed, recovery from botulism may take weeks or even months. In such cases, artificial respiration is expected to keep the patient alive, and slow recovery results from the significant amount of toxin that has already penetrated the neurons. In contrast to human botulism, most animal models cannot involve mechanical ventilation. Antitoxin neutralizes some of the toxin in the bloodstream, thereby converting it into a sublethal amount. Consequently, animals treated with antitoxin can recover faster than human patients.
When the toxin exposure dose was increased from 2 RbIMLD50 to 5 RbIMLD50, postsymptom antitoxin efficacy dropped from 100% to 0% protection. A similar phenomenon was described previously by Ono and colleagues, who compared the efficacies of antitoxin administered at various time points after oral exposure to BoNT/E (25). In that study, rabbits exposed to 3.6 RbIMLD50 did not survive treatment with 840 IU antitoxin administered at 2 h postintoxication, whereas rabbits exposed to 1.8 RbIMLD50 and treated at the same time fully survived. It should be noted that in that study, TTDs were shorter than those in the present work. However, such differences could be attributed to the different route of toxin administration, especially as BoNT/E has been shown to go through further activation when administered orally (44–46). Increasing the antitoxin dose administered to rabbits exposed to 5 RbIMLD50 in our study from 30 IU/kg to 120 IU/kg led to improved efficacy (0/3 versus 2/5 surviving animals, respectively). Enhanced protection by increased antitoxin doses was also documented in animal studies where treatment was evaluated by time postexposure. Ono et al. demonstrated that a 10-fold increment of the antitoxin dose administered 5 h after exposure to rabbits intoxicated orally with BoNT/E (1.8 RbIMLD50) postponed the time to death in some of the animals. In those same studies, attempts to protect rabbits (and monkeys) after the onset of symptoms were generally unsuccessful. In a recent publication presenting a countermeasure against inhalational botulism, a controlled antitoxin efficacy evaluation was described. Rhesus monkeys were treated with increasing doses of antitoxin either 24 h after inhalational exposure to BoNT/A (before symptoms could be observed) or at the time of first symptoms. Almost all animals treated at 24 h postexposure (14 IU/kg antitoxin or higher) survived, whereas even a 30-fold-higher antitoxin dose failed to confer any protection when administered at the first signs of disease (39). However, in another recent rhesus macaque study, different results were obtained, as treatment with HBAT after the onset of clinical signs following intravenous exposure to BoNT/A (1.7 LD50) conferred partial protection (46% survival) (42).
Interestingly, in our study, an increased antitoxin dose did not lead to a delayed TTD in nonsurviving rabbits. This implies that a lethal amount of toxin had already penetrated target cells at the time of antitoxin treatment, and thus, neutralization was not possible. Alternatively, it could also imply that the antitoxin dose injected into these rabbits was insufficient to block the entire amount of circulating toxin and, hence, could not prevent or slow the critical lethal toxin dose from entering neural cells. Both explanations highlight the importance of intensive respiratory care in addition to antitoxin treatment and emphasize the need for new drugs that block the toxin intracellularly and/or restore neurotransmission.
The spirometry model described here is the first animal model shown to simulate successful antitoxin treatment of botulism after the onset of objective and quantitative clinical symptoms. Hence, our rabbit model can be used to evaluate the efficacy of antitoxin preparations in a relevant therapeutic timeline. Moreover, the rabbit spirometry model can also be used to test the potency of new-generation anti-BoNT drugs, including those that aim to act intracellularly at stages where the toxin is no longer available for antitoxin. The therapeutic effect of such new-generation drugs can be evaluated either alone at a time when antitoxin is not effective or in combination with antitoxin for its potential effect of increasing antitoxin efficacy.
MATERIALS AND METHODS
Ethics statement.
All animal experiments were performed in accordance with Israeli law and were approved by the Ethics Committee for animal experiments at the Israel Institute for Biological Research (protocol number RB-26-2010). All efforts were made to minimize suffering.
Bacteria and toxins.
The Clostridium botulinum E strain was obtained from the IIBR collection (E450). Sequence analysis revealed the compliance of the neurotoxin gene with the serotype of NCTC11219 (GenBank accession number X62683) for Clostridium botulinum subtype E botulinum (47).
The Clostridium botulinum E toxin complex (consisting of the neurotoxin and the nontoxic nonhemagglutinin fractions) was prepared from the concentrated supernatant of cultures grown for 6 days in anaerobic culture tubes. Botulinum E was used in its activated form throughout the study. Activation was performed by treatment with 0.1% (wt/vol) trypsin (37°C for 1 h). Toxin activity was calibrated by a standard mouse bioassay. All work was done in a biosafety level 2 (BSL2) laboratory under appropriate biosafety procedures.
Antitoxin.
Horse anti-BoNT/E plasma was collected from hyperimmune animals immunized with toxoid prepared by dialyzing the toxin complex against 0.14% formalin at 35°C for 2 weeks (48). The Fc fragment was removed from horse IgG by pepsin digestion. Purified F(ab)′2 antitoxin neutralizing activity was determined according to the European Pharmacopoeia (49).
Spirometry system.
Inhalation parameters were recorded by a computer-monitored spirometry system. The system consisted of a snout-only mask connected to a T-type nonrebreathing two-way valve. A model 4100 thermal mass flowmeter (TSI Inc., Shoreview, MN, USA), with low flow resistance, was connected to the inhalation port of the valve. During measurements, nonanesthetized rabbits respired freely. The data collected from naive animals were in line with previously reported values for normal rabbit respiration physiology (50).
Inhalation data were collected at 20-ms intervals (500 data points in 10 s). Spirometry parameters, including minute volume, rate, and tidal volume, were measured, and data were analyzed with Microsoft Excel (2013). At least 12 independent measurements collected over 7 days were used to calculate the individual means and SD for each of the three spirometry parameters (Fig. 1).
Antitoxin efficacy studies.
Naive rabbits, previously monitored for individual respiration performance, were injected i.m. in the quadriceps musculature of the hind limb with 2 RbIMLD50 of BoNT/E in 0.5 ml of gelatin-phosphate buffer (50 mM Na-phosphate, 0.2% gelatin [pH 6.5]). Rabbit spirometry was monitored starting at 12 h postintoxication and then at 2-h intervals. An animal that presented a significant deviation from its preintoxication respiration performance (lower than the mean value minus 2 standard deviations) was considered suspect. A rabbit was considered symptomatic if another deviation was measured within 30 min. Antitoxin was intravenously administered to symptomatic rabbits at the indicated times after symptom onset. Two antitoxin doses were tested, 30 IU/kg and 120 IU/kg, as indicated.
ACKNOWLEDGMENTS
This work was supported by the Israel Institute for Biological Research (grant number SB/196/5122).
The funder had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
We declare no conflicts of interest.
Eran Diamant, Avi Pass, and Ran Zichel conceived and designed the work. Eran Diamant, Avi Pass, Osnat Rosen, Alon Ben David, Amram Torgeman, Ada Barnea, Arnon Tal, Amir Rosner, and Ran Zichel performed the experiments and analyzed or interpreted the data. Avi Pass and Alon Ben David implemented supporting algorithms. Eran Diamant and Ran Zichel drafted the manuscript, and Avi Pass, Osnat Rosen, Alon Ben David, Amram Torgeman, Ada Barnea, Arnon Tal, and Amir Rosner critically revised the manuscript.
REFERENCES
- 1.Arnon SS, Schechter R, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Fine AD, Hauer J, Layton M, Lillibridge S, Osterholm MT, O'Toole T, Parker G, Perl TM, Russell PK, Swerdlow DL, Tonat K. 2001. Botulinum toxin as a biological weapon: medical and public health management. JAMA 285:1059–1070. doi: 10.1001/jama.285.8.1059. [DOI] [PubMed] [Google Scholar]
- 2.Pirazzini M, Rossetto O, Eleopra R, Montecucco C. 2017. Botulinum neurotoxins: biology, pharmacology, and toxicology. Pharmacol Rev 69:200–235. doi: 10.1124/pr.116.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dembek ZF, Smith LA, Rusnak JM. 2007. Botulism: cause, effects, diagnosis, clinical and laboratory identification, and treatment modalities. Disaster Med Public Health Prep 1:122–134. doi: 10.1097/DMP.0b013e318158c5fd. [DOI] [PubMed] [Google Scholar]
- 4.Sugiyama H. 1980. Clostridium botulinum neurotoxin. Microbiol Rev 44:419–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kongsaengdao S, Samintarapanya K, Rusmeechan S, Wongsa A, Pothirat C, Permpikul C, Pongpakdee S, Puavilai W, Kateruttanakul P, Phengtham U, Panjapornpon K, Janma J, Piyavechviratama K, Sithinamsuwan P, Deesomchok A, Tongyoo S, Vilaichone W, Boonyapisit K, Mayotarn S, Piya-Isragul B, Rattanaphon A, Intalapaporn P, Dusitanond P, Harnsomburana P, Laowittawas W, Chairangsaris P, Suwantamee J, Wongmek W, Ratanarat R, Poompichate A, Panyadilok H, Sutcharitchan N, Chuesuwan A, Oranrigsupau P, Sutthapas C, Tanprawate S, Lorsuwansiri J, Phattana N. 2006. An outbreak of botulism in Thailand: clinical manifestations and management of severe respiratory failure. Clin Infect Dis 43:1247–1256. doi: 10.1086/508176. [DOI] [PubMed] [Google Scholar]
- 6.Weber JT, Hibbs RG Jr, Darwish A, Mishu B, Corwin AL, Rakha M, Hatheway CL, El Sharkawy S, El-Rahim SA, Al-Hamd MFS, Sarn JE, Blake PA, Tauxe RV. 1993. A massive outbreak of type E botulism associated with traditional salted fish in Cairo. J Infect Dis 167:451–454. doi: 10.1093/infdis/167.2.451. [DOI] [PubMed] [Google Scholar]
- 7.McCarty CL, Angelo K, Beer KD, Cibulskas-White K, Quinn K, de Fijter S, Bokanyi R, St Germain E, Baransi K, Barlow K, Shafer G, Hanna L, Spindler K, Walz E, DiOrio M, Jackson BR, Luquez C, Mahon BE, Basler C, Curran K, Matanock A, Walsh K, Slifka KJ, Rao AK. 2015. Large outbreak of botulism associated with a church potluck meal—Ohio, 2015. MMWR Morb Mortal Wkly Rep 64:802–803. doi: 10.15585/mmwr.mm6429a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. 2013. Bioterrorism agents/diseases. Centers for Disease Control and Prevention, Atlanta, GA: https://emergency.cdc.gov/agent/agentlist-category.asp. [Google Scholar]
- 9.Centers for Disease Control and Prevention. 2010. Investigational heptavalent botulinum antitoxin (HBAT) to replace licensed botulinum antitoxin AB and investigational botulinum antitoxin E. MMWR Morb Mortal Wkly Rep 59:299. [PubMed] [Google Scholar]
- 10.Division of Communicable Disease Control, California Department of Public Health. Infant botulism treatment and prevention program. California Department of Public Health, Sacramento, CA: http://www.infantbotulism.org/. [Google Scholar]
- 11.Meng Q, Garcia-Rodriguez C, Manzanarez G, Silberg MA, Conrad F, Bettencourt J, Pan X, Breece T, To R, Li M, Lee D, Thorner L, Tomic MT, Marks JD. 2012. Engineered domain-based assays to identify individual antibodies in oligoclonal combinations targeting the same protein. Anal Biochem 430:141–150. doi: 10.1016/j.ab.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meng Q, Li M, Silberg MA, Conrad F, Bettencourt J, To R, Huang C, Ma J, Meyer K, Shimizu R, Cao L, Tomic MT, Marks JD. 2012. Domain-based assays of individual antibody concentrations in an oligoclonal combination targeting a single protein. Anal Biochem 421:351–361. doi: 10.1016/j.ab.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torgeman A, Ozeri E, Ben David A, Diamant E, Rosen O, Schwartz A, Barnea A, Makovitzki A, Mimran A, Zichel R. 2017. Role of homologous Fc fragment in the potency and efficacy of anti-botulinum antibody preparations. Toxins (Basel) 9:E180. doi: 10.3390/toxins9060180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.US Food and Drug Administration. 2017. Prescribing information for BAT. US Food and Drug Administration, Washington, DC: https://www.fda.gov/downloads/.../UCM345147.pdf. [Google Scholar]
- 15.Rasetti-Escargueil C, Avril A, Miethe S, Mazuet C, Derman Y, Selby K, Thullier P, Pelat T, Urbain R, Fontayne A, Korkeala H, Sesardic D, Hust M, Popoff MR. 2017. The European AntibotABE Framework Program and its update: development of innovative botulinum antibodies. Toxins (Basel) 9:E309. doi: 10.3390/toxins9100309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sobel J. 2005. Botulism. Clin Infect Dis 41:1167–1173. doi: 10.1086/444507. [DOI] [PubMed] [Google Scholar]
- 17.Tacket CO, Shandera WX, Mann JM, Hargrett NT, Blake PA. 1984. Equine antitoxin use and other factors that predict outcome in type A foodborne botulism. Am J Med 76:794–798. doi: 10.1016/0002-9343(84)90988-4. [DOI] [PubMed] [Google Scholar]
- 18.Iida H. 1970. Epidemiological and clinical observations of botulism outbreaks in Japan, p 357–359. In Herzberg M. (ed), Proceedings of the First U.S.-Japan Conference on Toxic Microorganisms. UJNR Joint Panels on Toxic Micro-organisms and US Department of the Interior, Washington, DC. [Google Scholar]
- 19.Mottate K, Yokote H, Mori S, Horita A, Miyatsu Y, Torii Y, Kozaki S, Iwaki M, Takahashi M, Ginnaga A. 2016. Retrospective survey to evaluate the safety and efficacy of Japanese botulinum antitoxin therapy in Japan. Toxicon 110:12–18. doi: 10.1016/j.toxicon.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 20.Franz DR, Pitt LM, Clayton MA, Hanes MA, Rose KJ. 1993. Efficacy of prophylactic and therapeutic administration of antitoxin for inhalation botulism, p 473–476. In Das-Gupta BR. (ed), Botulinum and tetanus neurotoxins: neurotransmission and biomedical aspects. Plenum Press, New York, NY. [Google Scholar]
- 21.Habermann E, Bernath S. 1975. Preparation, measurement and possible use of human antitoxin against Cl. botulinum A, B, and E toxins. Med Microbiol Immunol 161:203–210. doi: 10.1007/BF02121011. [DOI] [PubMed] [Google Scholar]
- 22.Iida H, Ono T, Karashimada T. 1970. Experimental studies on the serum therapy of type E botulism: the relationship between the amount of toxin in the blood and the effect of antitoxic serum. Jpn J Med Sci Biol 23:344–347. [PubMed] [Google Scholar]
- 23.Iida H, Ono T, Karashimada T, Ando Y. 1970. Studies on the serum therapy of type E botulism: absorption of toxin from the gastrointestinal tract. Jpn J Med Sci Biol 23:282–285. [PubMed] [Google Scholar]
- 24.Ono T, Karashimada T, Iida H. 1969. Studies on the serum therapy of type E botulism (part II). J Infect Dis 120:534–538. doi: 10.1093/infdis/120.5.534. [DOI] [PubMed] [Google Scholar]
- 25.Ono T, Karashimada T, Iida H. 1970. Studies of the serum therapy of type E botulism. 3. Jpn J Med Sci Biol 23:177–191. doi: 10.7883/yoken1952.23.177. [DOI] [PubMed] [Google Scholar]
- 26.Lewis GE Jr, Metzger JF. 1979. Studies on the prophylaxis and treatment of botulism. Toxicon 17:102. doi: 10.1016/0041-0101(79)90103-X. [DOI] [Google Scholar]
- 27.Cheng LW, Henderson TD II, Lam TI, Stanker LH. 2015. Use of monoclonal antibodies in the sensitive detection and neutralization of botulinum neurotoxin serotype B. Toxins (Basel) 7:5068–5078. doi: 10.3390/toxins7124863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng LW, Stanker LH, Henderson TD II, Lou J, Marks JD. 2009. Antibody protection against botulinum neurotoxin intoxication in mice. Infect Immun 77:4305–4313. doi: 10.1128/IAI.00405-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mukherjee J, McCann C, Ofori K, Hill J, Baldwin K, Shoemaker CB, Harrison P, Tzipori S. 2012. Sheep monoclonal antibodies prevent systemic effects of botulinum neurotoxin A1. Toxins (Basel) 4:1565–1581. doi: 10.3390/toxins4121565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sepulveda J, Mukherjee J, Tzipori S, Simpson LL, Shoemaker CB. 2010. Efficient serum clearance of botulinum neurotoxin achieved using a pool of small antitoxin binding agents. Infect Immun 78:756–763. doi: 10.1128/IAI.01084-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu YZ, Zhang SM, Ma Y, Zhu HQ, Wang WB, Du Y, Zhou XW, Wang RL, Wang S, Yu WY, Huang PT, Sun ZW. 2010. Development and evaluation of candidate vaccine and antitoxin against botulinum neurotoxin serotype F. Clin Immunol 137:271–280. doi: 10.1016/j.clim.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Yu YZ, Zhang SM, Wang WB, Du Y, Zhu HQ, Wang RL, Zhou XW, Lin JB, Wang S, Yu WY, Huang PT, Sun ZW. 2010. Development and preclinical evaluation of a new F(ab′)(2) antitoxin against botulinum neurotoxin serotype A. Biochimie 92:1315–1320. doi: 10.1016/j.biochi.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 33.Mimran A, Chay Y, Barnea A, Halperin G, Reuveny S, Zichel R. 2012. Evaluating the efficacy of post-clinical symptom anti-toxin therapy in a mouse model of botulism A and B. Proc 49th Interagency Botulism Res Coord Committee (IBRCC), Baltimore, MD. [Google Scholar]
- 34.Horowitz BZ. 2010. Type E botulism. Clin Toxicol (Phila) 48:880–895. doi: 10.3109/15563650.2010.526943. [DOI] [PubMed] [Google Scholar]
- 35.Wang J, Meng J, Lawrence GW, Zurawski TH, Sasse A, Bodeker MO, Gilmore MA, Fernandez-Salas E, Francis J, Steward LE, Aoki KR, Dolly JO. 2008. Novel chimeras of botulinum neurotoxins A and E unveil contributions from the binding, translocation, and protease domains to their functional characteristics. J Biol Chem 283:16993–17002. doi: 10.1074/jbc.M710442200. [DOI] [PubMed] [Google Scholar]
- 36.Woodruff BA, Griffin PM, McCroskey LM, Smart JF, Wainwright RB, Bryant RG, Hutwagner LC, Hatheway CL. 1992. Clinical and laboratory comparison of botulism from toxin types A, B, and E in the United States, 1975-1988. J Infect Dis 166:1281–1286. doi: 10.1093/infdis/166.6.1281. [DOI] [PubMed] [Google Scholar]
- 37.Eleopra R, Tugnoli V, Rossetto O, De Grandis D, Montecucco C. 1998. Different time courses of recovery after poisoning with botulinum neurotoxin serotypes A and E in humans. Neurosci Lett 256:135–138. doi: 10.1016/S0304-3940(98)00775-7. [DOI] [PubMed] [Google Scholar]
- 38.Tsai YC, Maditz R, Kuo CL, Fishman PS, Shoemaker CB, Oyler GA, Weissman AM. 2010. Targeting botulinum neurotoxin persistence by the ubiquitin-proteasome system. Proc Natl Acad Sci U S A 107:16554–16559. doi: 10.1073/pnas.1008302107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adler M, Franz DR. 2016. Toxicity of botulinum neurotoxin by inhalation: implications in bioterrorism, p 167–185. In Salem H, Katz SA (ed), Aerobiology: the toxicology of airborne pathogens and toxins. Royal Society of Chemistry, Cambridge, United Kingdom. [Google Scholar]
- 40.Dack GM, Wood WL. 1928. Serum therapy of botulism in monkeys. J Infect Dis 42:209–212. doi: 10.1093/infdis/42.3.209. [DOI] [Google Scholar]
- 41.Oberst FW, Crook JW, Cresthull P, House MJ. 1968. Evaluation of botulinum antitoxin, supportive therapy, and artificial respiration in monkeys with experimental botulism. Clin Pharmacol Ther 9:209–214. doi: 10.1002/cpt196892209. [DOI] [PubMed] [Google Scholar]
- 42.Kodihalli S, Emanuel A, Takla T, Hua Y, Hobbs C, LeClaire R, O'Donnell DC. 2017. Therapeutic efficacy of equine botulism antitoxin in rhesus macaques. PLoS One 12:e0186892. doi: 10.1371/journal.pone.0186892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adler M, Gul N, Eitzen E, Oyler G, Molles B. 2014. Prevention and treatment of botulism, p 291–342. In Foster KA. (ed), Molecular aspects of botulinum neurotoxin. Springer, New York, NY. [Google Scholar]
- 44.Das Gupta BR, Sugiyama H. 1972. Role of a protease in natural activation of Clostridium botulinum neurotoxin. Infect Immun 6:587–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duff JT, Wright GG, Yarinsky A. 1956. Activation of Clostridium botulinum type E toxin by trypsin. J Bacteriol 72:455–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dolman CE. 1957. Type E (fish-borne) botulism: a review. Jpn J Med Sci Biol 10:383–395. doi: 10.7883/yoken1952.10.383. [DOI] [PubMed] [Google Scholar]
- 47.Whelan SM, Elmore MJ, Bodsworth NJ, Atkinson T, Minton NP. 1992. The complete amino acid sequence of the Clostridium botulinum type-E neurotoxin, derived by nucleotide-sequence analysis of the encoding gene. Eur J Biochem 204:657–667. doi: 10.1111/j.1432-1033.1992.tb16679.x. [DOI] [PubMed] [Google Scholar]
- 48.Diamant E, Lachmi BE, Keren A, Barnea A, Marcus H, Cohen S, David AB, Zichel R. 2014. Evaluating the synergistic neutralizing effect of anti-botulinum oligoclonal antibody preparations. PLoS One 9:e87089. doi: 10.1371/journal.pone.0087089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.European Directorate for the Quality of Medicines and Healthcare. 2014. European Pharmacopoeia, 8th ed, vol 1, p 1029 EDQM Council of Europe, Strasbourg, France. [Google Scholar]
- 50.Bide RW, Armour SJ, Yee E. 2000. Allometric respiration/body mass data for animals to be used for estimates of inhalation toxicity to young adult humans. J Appl Toxicol 20:273–290. doi:. [DOI] [PubMed] [Google Scholar]