ABSTRACT
Cefotaxime is the first-line treatment for meningitis in neonates and young infants. However, limited data on cefotaxime cerebrospinal fluid (CSF) concentrations in neonates and young infants were available. The aim of the present study was to evaluate the penetration of cefotaxime into CSF in neonates and young infants. Blood and CSF samples were collected from neonates and young infants treated with cefotaxime using an opportunistic pharmacokinetic sampling strategy, and concentrations were quantified by high-performance liquid chromatography-tandem mass spectrometry. The analysis was performed using NONMEM and R software. Thirty neonates and young infants (postmenstrual age range, 25.4 to 47.4 weeks) were included. A total of 67 plasma samples and 30 CSF samples were available for analysis. Cefotaxime plasma and CSF concentrations ranged from 2.30 to 175.42 mg/liter and from 0.39 to 25.38 mg/liter, respectively. The median ratio of the CSF concentration to the plasma concentration was 0.28 (range, 0.06 to 0.76). Monte Carlo simulation demonstrated that 88.4% and 63.9% of hypothetical neonates treated with 50 mg/kg of body weight three times a day (TID) would reach the pharmacodynamic target (the percentage of the dosing interval that the free antimicrobial drug concentration remains above the MIC, 70%) using the standard EUCAST MIC susceptibility breakpoints of 2 mg/liter and 4 mg/liter, respectively. The penetration of cefotaxime into the CSF of neonates and young infants was evaluated using an opportunistic sampling approach. A dosage regimen of 50 mg/kg TID could cover the most causative pathogens with MICs of <2 mg/liter. Individual dosage adaptation was required for more resistant bacterial strains, such as Staphylococcus aureus.
KEYWORDS: cefotaxime, cerebrospinal fluid, infants, neonates
INTRODUCTION
Neonatal bacterial meningitis has significant morbidity and mortality (1). It has case fatality rates of 26% in the first week of life and 18% at between 7 and 59 days of age (2). Cefotaxime is recommended as a first-line treatment for meningitis in neonates (3). It exhibits time-dependent killing, and bactericidal efficacy relates to the percentage of the dosing interval that the drug concentration remains above the MIC (TMIC) (4).
The concentration of antimicrobial agents in cerebrospinal fluid (CSF) is crucial for meningitis treatment. In order to reach the central nervous system, the antimicrobial agents have to pass the blood-brain barrier. Consequently, biochemical characteristics, such as the degree of protein binding, lipophilicity, and diffusibility, as well as the disease state, influence the penetration of antimicrobial agents into CSF. Although several studies have reported the cefotaxime CSF concentrations (5–10) in children and adults, limited data were available for neonates and young infants. Given the potential impact of the developmental physiology of the brain in the neonatal period, the aim of the present study was to evaluate the penetration of cefotaxime into CSF in neonates and young infants using an opportunistic sampling-based population pharmacokinetics (PK) approach.
RESULTS
Study population.
Sixty-seven blood samples and 30 CSF samples were obtained from 30 neonates and young infants. The median gestational age (GA), postnatal age (PNA), postmenstrual age (PMA), and weight were 31.0 weeks (range, 23.0 to 40.4 weeks), 20 days (range, 3 to 88 days), 33.3 weeks (range, 25.4 to 47.4 weeks), and 1,705 g (range, 720 to 4,715 g), respectively. The median dose and serum creatinine concentration were 49.7 mg/kg of body weight three times a day (TID) (range, 30.0 to 61.5 mg/kg TID) and 41.5 mg/dl (range, 13 to 120 mg/dl), respectively.
Model evaluation.
The predictive performance of the model was evaluated, and the results of calculation of the mean prediction error (MPE) and the mean absolute prediction error (MAE) were 9.3% ± 39.6% and 25.8% ± 31.3%, respectively. In addition, the percentages of the population prediction error within ±20% and ±30% were 52.2% and 76.1%, respectively.
Cefotaxime penetration into cerebrospinal fluid and dosing regimen evaluation.
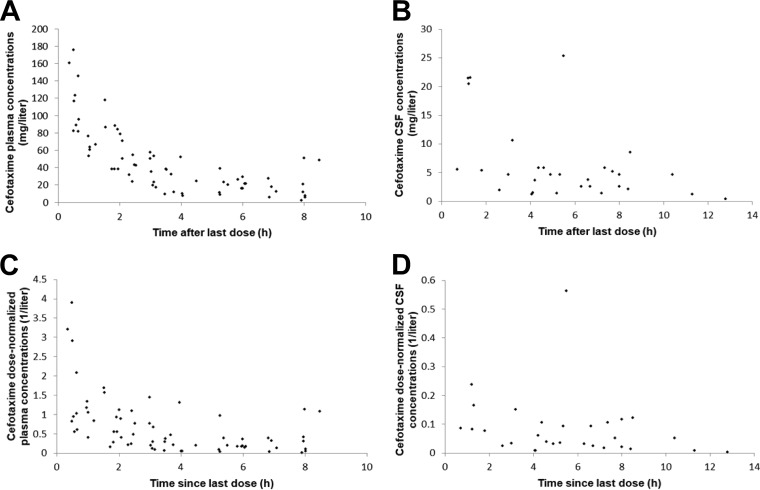
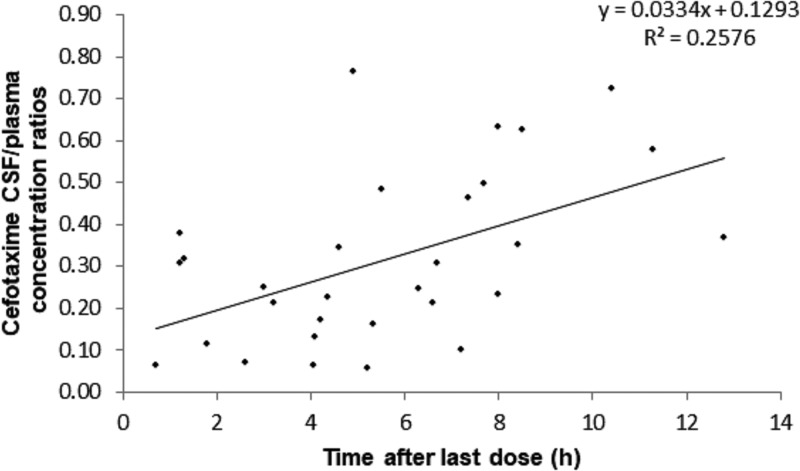
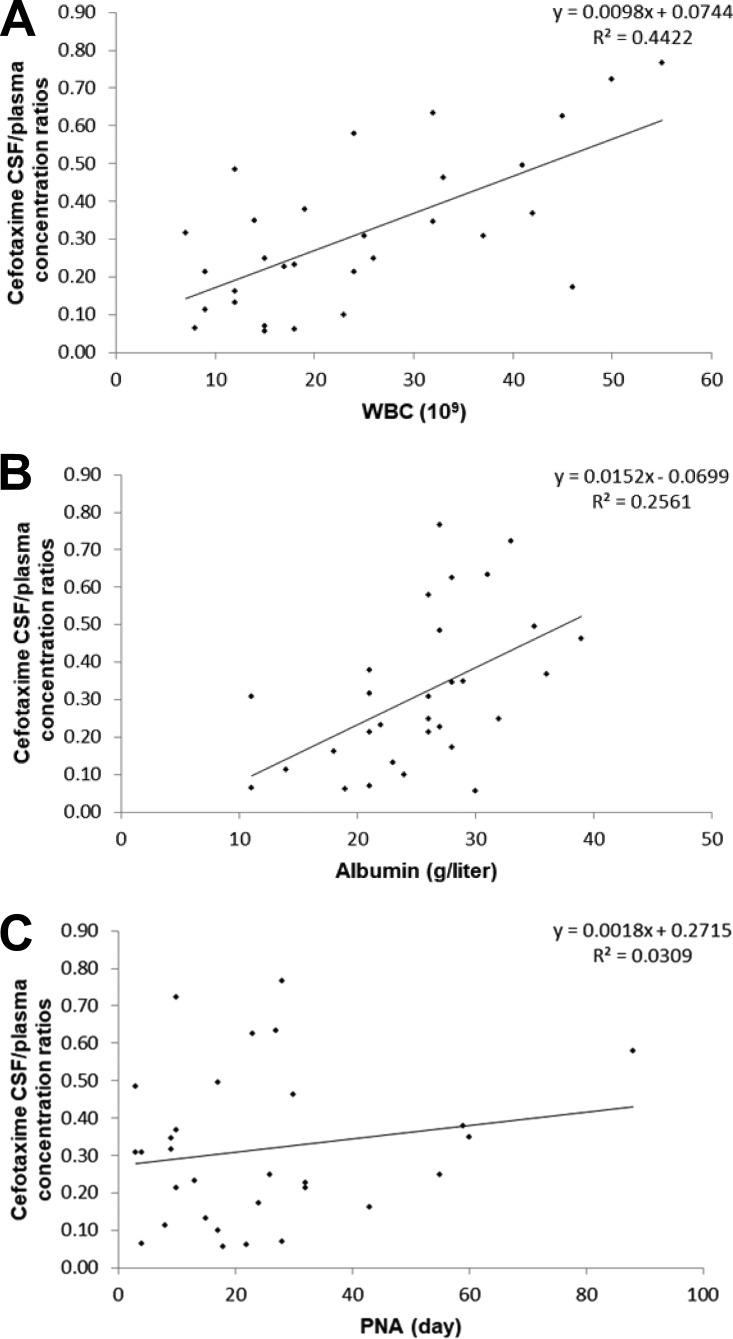
Cefotaxime plasma and CSF concentrations ranged from 2.30 to 175.42 mg/liter and from 0.39 to 25.38 mg/liter, respectively. The profiles of the plasma concentrations, CSF concentrations, dose-normalized plasma concentrations, and dose-normalized CSF concentrations versus time are shown in Fig. 1A to D. The median CSF/plasma concentration ratio was 0.28 (range, 0.06 to 0.76). A trend for a correlation between the CSF collection time and the CSF/plasma concentration ratio was demonstrated, suggesting less elimination from (or diffusion into) the CSF than from (or diffusion into) the systemic circulation (Fig. 2). The profiles of the white blood cell (WBC) count, albumin concentration, and PNA versus cefotaxime CSF/plasma concentration ratios are shown in Fig. 3A to C. The WBC count and albumin concentration had a statistically significant correlation with the CSF/plasma concentration ratio (Pearson's correlation coefficient < 0.01). The correlation between PNA and the CSF/plasma concentration ratio was not significant (Pearson's correlation coefficient = 0.349). Monte Carlo simulation demonstrated that 88.4% and 63.9% of hypothetical neonates treated with 50 mg/kg TID would reach the pharmacodynamic (PD) target (percentage of the dosing interval that the free antimicrobial drug concentration remains above the MIC [TMIC], 70%) using the standard EUCAST MIC susceptibility breakpoints of 2 mg/liter and 4 mg/liter, respectively.
FIG 1.
(A) Cefotaxime plasma concentrations versus time; (B) cefotaxime cerebrospinal fluid (CSF) concentrations versus time; (C) cefotaxime dose-normalized plasma concentrations versus time; (D) cefotaxime dose-normalized CSF concentrations versus time.
FIG 2.
Cefotaxime CSF/plasma concentration ratios versus time.
FIG 3.
(A) White blood cell (WBC) count versus cefotaxime CSF/plasma concentration ratio; (B) albumin concentration versus cefotaxime CSF/plasma concentration ratio; (C) postnatal age (PNA) versus cefotaxime CSF/plasma concentration ratio.
DISCUSSION
Quantitative research on the penetration of drugs into CSF is indispensable to optimize antimicrobial therapy. Our study for the first time evaluated cefotaxime penetration into the CSF in neonates and young infants.
As shown in Results, the median cefotaxime penetration into the CSF was 0.28, which was similar to the findings of a study in children with meningitis (8). However, a large interindividual variability of the penetration was demonstrated. In addition to the already mentioned general factors (the protein binding, lipophilicity, and diffusibility of the drug) influencing the penetration capacity of antibiotic drugs through the blood-brain barrier, it has been postulated that brain development, disease severity, and transporters (11) also influence passage through this barrier in this vulnerable population. Further exploration of the impact of these covariates on penetration within the population will be meaningful to individual cefotaxime therapy. From a pharmacokinetic (PK)-pharmacodynamic (PD) point of view, for cephalosporins a target of a TMIC of 50% is generally accepted in adults (4). To ensure efficacy in neonates with their peculiar immunocompromised status (12) and to avoid the induction of antibiotic resistance (13), we selected a target of a TMIC of 70%. In this study, 88.4% of the simulated patients achieved the target (TMIC, 70%) in CSF for the MIC breakpoint of 2 mg/liter, which can cover the most common pathogens causing neonatal sepsis (Escherichia coli, group B Streptococcus, coagulase-negative staphylococci, and Enterobacter and Klebsiella species). The results were similar to the observed CSF concentrations, which showed that 76.7% of samples had concentrations higher than 2 mg/liter. However, for the MIC breakpoint of 4 mg/liter, only 63.9% of simulated patients achieved the pharmacodynamic target, indicating the need for individual dosage adaptation for more resistant bacterial strains, such as Staphylococcus aureus.
Studies of the pharmacokinetics of antimicrobials in CSF are rarely conducted in neonates due to the complexity of pharmacokinetic sampling designs. The traditional design needs repeated samples collected at fixed times and concurrent sampling from both blood and CSF. Obviously, this design presents big challenges in neonates, including frequent restrictions on vascular access and puncture operations, difficulty with the precise timing of sample collection, and the provision of consent for nontherapeutic operations. The use of population pharmacokinetic approaches coupled with the use of opportunistic sampling paradigms could dramatically decrease the barriers to the conduct of such a study in neonates. This approach can be enriched through the use of residual blood samples that are obtained as part of standard medical care. As we have demonstrated for the study of the pharmacokinetics of ciprofloxacin in neonates, an appropriately constructed pharmacokinetic model using opportunistic samples can reliably optimize antimicrobial therapy in neonates (14). This approach has been well used in several studies of the pharmacokinetics of antimicrobials in neonates (15–17). However, the generalizability of a population pharmacokinetic study using an opportunistic sampling approach depends on the density and quality of sampling, as well as the stability of the drug under evaluation (14).
Our study had some limitations. A physiological model describing the process of the penetration of cefotaxime into CSF was not successfully modeled due to the small sample size. The evaluation of the dosing regimen was based on the median ratio of the CSF to the plasma concentration. However, our dosing simulation results are coincident with the observed CSF concentrations. The factors influencing the variability of penetration should be further evaluated. In addition, the concentration of the metabolite of cefotaxime, desacetylcefotaxime, was not quantified. It has been reported that the contribution of desacetylcefotaxime to the bactericidal activity of cefotaxime therapy is limited (18). It is not expected to have a significant impact on meningitis treatment.
Conclusion.
The penetration of cefotaxime into CSF was evaluated in neonates and young infants. A population pharmacokinetic approach coupled with an opportunistic sampling paradigm is promising in studies of the pharmacokinetics of antimicrobials in the CSF of neonates. A model-based dosing regimen was developed to optimize cefotaxime treatment of meningitis in neonates.
MATERIALS AND METHODS
Clinical characteristics and drug administration.
This open-label population pharmacokinetic study of cefotaxime was conducted in our neonatal intensive care units (Shandong Provincial Qianfoshan Hospital and Robert Debre Hospital) between 2016 and 2017. Neonates and young infants (postmenstrual age [PMA], ≤44 weeks) receiving cefotaxime as part of their routine clinical care (50 mg/kg TID) for suspected or proven bacterial meningitis were enrolled in this noninterventional study, which was designed in accordance with the legal requirements and the Declaration of Helsinki and was approved by the institutional ethics committees of Shandong Provincial Qianfoshan Hospital and Robert Debre Hospital.
The pharmacokinetic data were obtained according to the local clinical practice using an opportunistic pharmacokinetic sampling approach (14): samples were exclusively collected from blood and CSF remaining after routine biochemical and microbiological tests performed as part of patient clinical care. To exclude the possibility of contamination of CSF with blood, the samples were collected by doctors who were very skilled at lumbar puncture (spinal tap) and operated according to guiding principles (19, 20). A from bench-to-beside standard operation procedure was set up to record the exact timings of drug administration, pharmacokinetic sampling, and different operations. Samples were stored at −80°C in the laboratory until analysis.
The following clinical data were collected: gestational age (GA), postnatal age (PNA), postmenstrual age (PMA) (21), birth weight, current weight, white blood cell (WBC) count, albumin and creatinine concentrations, and whether there was a need for ventilation.
Method of analysis of cefotaxime in plasma and cerebrospinal fluid.
The analytical method used to determine cefotaxime concentrations has been reported in our previous study (22). Briefly, the cefotaxime concentration was determined using multiplex high-performance liquid chromatography-tandem mass spectrometry with a microvolume of 50 μl. The calibration curve ranged from 0.05 mg/liter to 150 mg/liter. The lower limit of quantification was 0.05 mg/liter. The intraday and interday coefficients of variation for the controls were 7.7% and 7.3%, respectively, for plasma and 5.2% and 4.9%, respectively, for CSF. The short-term stability (at ambient temperature) and long-term stability (at −80°C) of cefotaxime in blood and CSF were documented for at least 48 h and 12 months, respectively.
Cefotaxime penetration into cerebrospinal fluid.
Assessment of the level of cefotaxime penetration into CSF was evaluated by use of the ratio of the cefotaxime CSF concentration to plasma concentration. Because plasma cefotaxime concentrations were not concurrently obtained with CSF sample collection, plasma concentrations at the time of CSF sample collection were calculated via Bayesian estimation using our previously developed model (22).
To ensure the accuracy and precise of prediction, the predictive performance of the developed model was first evaluated using the mean prediction error (MPE) and mean absolute prediction error (MAE) (23) (equations 1 and 2). In addition, the percentages of the population prediction error within ±20% and ±30% were calculated.
| (1) |
| (2) |
where PREDi is the predicted concentration in individual i, OBSi is the observed concentration in individual i, and N is the number of neonates. The correlation between the WBC count, the albumin concentration, PNA, and the cefotaxime CSF/plasma concentration ratio was investigated.
Dosing regimen evaluation.
Regarding microbiological aspects, Escherichia coli, group B Streptococcus, coagulase-negative staphylococci, Staphylococcus aureus, and Enterobacter and Klebsiella species are the organisms most commonly isolated in neonatal sepsis (24). The susceptibility breakpoints of EUCAST were 2 mg/liter for most of these pathogens, except Staphylococcus aureus, for which a MIC of 4 mg/liter was required (25). The pharmacokinetic (PK)-pharmacodynamic (PD) relationship for cefotaxime is the percentage of the time that the free antimicrobial drug concentration remains above the MIC (TMIC). One hundred simulations were performed using the original data set, the plasma concentrations during the times of the dosing interval when the TMIC was 70% were calculated for each simulated patient, and CSF concentrations were obtained using the following equation:
| (3) |
The relationships of the cefotaxime concentrations in CSF to the breakpoints were examined.
ACKNOWLEDGMENTS
This work is supported by the National Science and Technology Major Projects for Major New Drugs Innovation and Development (2017ZX09304029-002), the Young Taishan Scholars Program, and the Young Scholars Program of Shandong University.
We have no conflicts of interest to declare.
REFERENCES
- 1.Hristeva L, Booy R, Bowler I, Wilkinson AR. 1993. Prospective surveillance of neonatal meningitis. Arch Dis Child 69:14–18. doi: 10.1136/adc.69.1_Spec_No.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mwaniki MK, Talbert AW, Njuguna P, English M, Were E, Lowe BS, Newton CR, Berkley JA. 2011. Clinical indicators of bacterial meningitis among neonates and young infants in rural Kenya. BMC Infect Dis 11:301. doi: 10.1186/1471-2334-11-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. 2005. Pocket book of hospital care for children: guidelines for the management of common illness with limited resources. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 4.Craig WA. 1995. Interrelationship between pharmacokinetics and pharmacodynamics in determining dosage regimens for broad-spectrum cephalosporins. Diagn Microbiol Infect Dis 22:89–96. doi: 10.1016/0732-8893(95)00053-D. [DOI] [PubMed] [Google Scholar]
- 5.Sáez-Llorens X, Castaño E, García R, Báez C, Pérez M, Tejeira F, McCracken GH Jr. 1995. Prospective randomized comparison of cefepime and cefotaxime for treatment of bacterial meningitis in infants and children. Antimicrob Agents Chemother 39:937–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Humbert G, Leroy A, Nair SR, Cherubin CE. 1984. Concentrations of cefotaxime and the desacetyl metabolite in serum and CSF of patients with meningitis. J Antimicrob Chemother 13:487–494. doi: 10.1093/jac/13.5.487. [DOI] [PubMed] [Google Scholar]
- 7.Trang JM, Jacobs RF, Kearns GL, Brown AL, Wells TG, Underwood FL, Kluza RB. 1985. Cefotaxime and desacetylcefotaxime pharmacokinetics in infants and children with meningitis. Antimicrob Agents Chemother 28:791–795. doi: 10.1128/AAC.28.6.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asmar BI, Thirumoorthi MC, Buckley JA, Kobos DM, Dajani AS. 1985. Cefotaxime diffusion into cerebrospinal fluid of children with meningitis. Antimicrob Agents Chemother 28:138–140. doi: 10.1128/AAC.28.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peretti P, Sueri L, Tosi M, Ciammarughi R, Cadeo GP, Milanesi B. 1984. Cefotaxime in the cerebrospinal fluid and serum in patients with purulent meningitis. J Antimicrob Chemother 14(Suppl B):117–123. [DOI] [PubMed] [Google Scholar]
- 10.Nau R, Prange HW, Muth P, Mahr G, Menck S, Kolenda H, Sörgel F. 1993. Passage of cefotaxime and ceftriaxone into cerebrospinal fluid of patients with uninflamed meninges. Antimicrob Agents Chemother 37:1518–1524. doi: 10.1128/AAC.37.7.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sugiyama Y, Kusuhara H, Suzuki H. 1999. Kinetic and biochemical analysis of carrier-mediated efflux of drugs through the blood-brain and blood-cerebrospinal fluid barriers: importance in the drug delivery to the brain. J Control Release 62:179–186. doi: 10.1016/S0168-3659(99)00036-X. [DOI] [PubMed] [Google Scholar]
- 12.Wynn JL, Wong HR. 2010. Pathophysiology and treatment of septic shock in neonates. Clin Perinatol 37:439–479. doi: 10.1016/j.clp.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacGowan AP. 2004. Elements of design: the knowledge on which we build. Clin Microbiol Infect 10(Suppl 2):S6–S11. doi: 10.1111/j.1470-9465.2004.00863.x. [DOI] [PubMed] [Google Scholar]
- 14.Leroux S, Turner MA, Barin-Le Guellec C, Hill H, van den Anker JN, Kearns GL, Jacqz-Aigrain E, Zhao W. 2015. Pharmacokinetic studies in neonates: the utility of an opportunistic sampling design. Clin Pharmacokinet 54:1273–1285. doi: 10.1007/s40262-015-0291-1. [DOI] [PubMed] [Google Scholar]
- 15.Zhao W, Hill H, Le Guellec C, Neal T, Mahoney S, Paulus S, Castellan C, Kassai B, van den Anker JN, Kearns GL, Turner MA, Jacqz-Aigrain E, TINN Consortium. 2014. Population pharmacokinetics of ciprofloxacin in neonates and young infants less than three months of age. Antimicrob Agents Chemother 58:6572–6580. doi: 10.1128/AAC.03568-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen-Wolkowiez M, Ouellet D, Smith PB, James LP, Ross A, Sullivan JE, Walsh MC, Zadell A, Newman N, White NR, Kashuba AD, Benjamin DK Jr. 2012. Population pharmacokinetics of metronidazole evaluated using scavenged samples from preterm infants. Antimicrob Agents Chemother 56:1828–1837. doi: 10.1128/AAC.06071-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wade KC, Wu D, Kaufman DA, Ward RM, Benjamin DK Jr, Sullivan JE, Ramey N, Jayaraman B, Hoppu K, Adamson PC, Gastonguay MR, Barrett JS, National Institute of Child Health and Development Pediatric Pharmacology Research Unit Network. 2008. Population pharmacokinetics of fluconazole in young infants. Antimicrob Agents Chemother 52:4043–4049. doi: 10.1128/AAC.00569-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vallée F, LeBel M. 1991. Comparative study of pharmacokinetics and serum bactericidal activity of ceftizoxime and cefotaxime. Antimicrob Agents Chemother 35:2057–2064. doi: 10.1128/AAC.35.10.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldman L, Schafer AI. 2016. Goldman-Cecil medicine, 25th ed Elsevier, New York, NY. [Google Scholar]
- 20.Daroff RB, Fenichel GM, Jankovic J, Mazziotta JC. 2012. Bradley's neurology in clinical practice, 6th ed Elsevier Saunders, Philadelphia, PA. [Google Scholar]
- 21.Engle WA, American Academy of Pediatrics Committee on Fetus and Newborn. 2004. Age terminology during the perinatal period. Pediatrics 114:1362–1364. doi: 10.1542/peds.2004-1915. [DOI] [PubMed] [Google Scholar]
- 22.Leroux S, Roué JM, Gouyon JB, Biran V, Zheng H, Zhao W, Jacqz-Aigrain E. 2016. A population and developmental pharmacokinetic analysis to evaluate and optimize cefotaxime dosing regimen in neonates and young infants. Antimicrob Agents Chemother 60:6626–6634. doi: 10.1128/AAC.01045-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van der Meer AF, Marcus MA, Touw DJ, Proost JH, Neef C. 2011. Optimal sampling strategy development methodology using maximum a posteriori Bayesian estimation. Ther Drug Monit 33:133–146. doi: 10.1097/FTD.0b013e31820f40f8. [DOI] [PubMed] [Google Scholar]
- 24.Stockmann C, Spigarelli MG, Campbell SC, Constance JE, Courter JD, Thorell EA, Olson J, Sherwin CM. 2014. Considerations in the pharmacologic treatment and prevention of neonatal sepsis. Paediatr Drugs 16:67–81. doi: 10.1007/s40272-013-0057-x. [DOI] [PubMed] [Google Scholar]
- 25.European Committee on Antimicrobial Susceptibility Testing. 2016. Breakpoint tables for interpretation of MICs and zone diameters. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_6.0_Breakpoint_table.pdf. [Google Scholar]