ABSTRACT
The objective of this study was to investigate predisposing factors and outcomes of infective endocarditis (IE) caused by non-HACEK Gram-negative bacilli (GNB) in a contemporary multicenter cohort. Patients with IE due to GNB, prospectively observed in 26 Italian centers from 2004 to 2011, were analyzed. Using a case-control design, each case was compared to three age- and sex-matched controls with IE due to other etiologies. Logistic regression was performed to identify risk factors for IE due to GNB. Factors associated with early and late mortality were assessed by Cox regression analysis. The study group comprised 58 patients with IE due to GNB. We found that Escherichia coli was the most common pathogen, followed by Pseudomonas aeruginosa and Klebsiella pneumoniae. The genitourinary tract as a source of infection (odds ratio [OR], 13.59; 95% confidence interval [CI], 4.63 to 39.93; P < 0.001), immunosuppression (OR, 5.16; 95% CI, 1.60 to 16.24; P = 0.006), and the presence of a cardiac implantable electronic device (CIED) (OR, 3.57; 95% CI, 1.55 to 8.20; P = 0.003) were factors independently associated with IE due to GNB. In-hospital mortality was 13.8%, and mortality rose to 30.6% at 1 year. A multidrug-resistant (MDR) etiology was associated with in-hospital mortality (hazard ratio [HR], 21.849; 95% CI, 2.672 to 178.683; P = 0.004) and 1-year mortality (HR, 4.408; 95% CI, 1.581 to 12.287; P = 0.005). We conclude that the presence of a genitourinary focus, immunosuppressive therapy, and an indwelling CIED are factors associated with IE due to GNB. MDR etiology is the major determinant of in-hospital and long-term mortality.
KEYWORDS: Gram-negative bacteria, infective endocarditis, multidrug resistance
INTRODUCTION
In recent years, infections due to Gram-negative bacilli (GNB) have raised growing concerns because of their increasing spread, high mortality, and high health care costs. Non-HACEK (species other than Haemophilus species, Aggregatibacter actinomycetemcomitans [previously known as Actinobacillus actinomycetemcomitans], Cardiobacterium hominis, Eikenella corrodens, and Kingella species) GNB are uncommon causes of infective endocarditis (IE) (1). The International Collaboration on Endocarditis (ICE) study, a multinational IE registry, reported an incidence of endocarditis due to non-HACEK GNB of approximately 2% (2). Available reports suggest that IE due to GNB is associated with high in-hospital mortality (1, 3, 4). As with other bloodstream infections, the frequency of IE caused by GNB from central catheter infection or in immunocompromised or elderly patients is on the rise (5). Moreover, the spread of multidrug-resistant (MDR) strains is a growing concern worldwide (6–9). At present, however, there are no specific clinical clues to the presence of IE in patients presenting with GNB bacteremia. Furthermore, when empirical antimicrobials should cover possible GNB in patients presenting with IE remains unknown.
Hence, the aim of this study was to identify factors associated with endocarditis due to non-HACEK GNB and to describe the outcomes for these patients in a large contemporary multicenter cohort of patients.
RESULTS
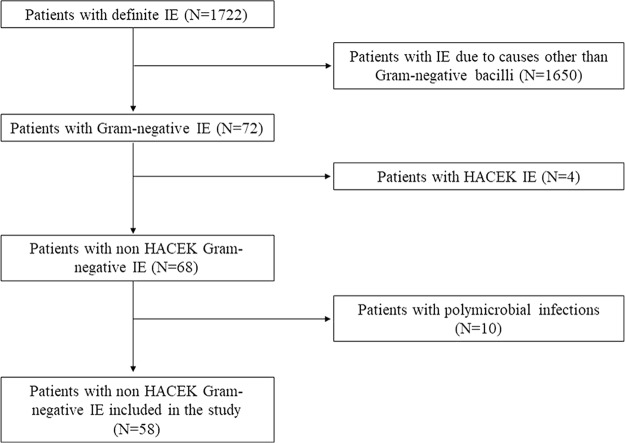
Of the 1,722 patients included in the Italian Endocarditis Study (SEI) database, 72 had IE due to GNB. Four patients had a HACEK group etiology and were subsequently excluded. Among the remaining 68 patients (3.9% of all episodes), 10 had polymicrobial infections (9 cases of IE due to mixed Gram-negative and Gram-positive bacteria and 1 case with mixed GNB and Candida spp.) and were also excluded from the analysis. Thus, the final cohort comprised 58 patients. A flow chart of the study is shown in Fig. 1.
FIG 1.
Flow diagram of the study.
Table 1 and Fig. S1 in the supplemental material show the species distribution of the causative pathogens of IE due to non-HACEK GNB. The most common etiologies were Escherichia coli (18 episodes [31%]), Pseudomonas aeruginosa (11 episodes [19%]), and Klebsiella pneumoniae (6 episodes [10.3%]). Three patients (5.2%) had polymicrobial IE due to GNB (more than 1 GNB isolated). The percentage of MDR strains was 27.6%. The median time between the start of symptoms and the diagnosis of IE due to GNB was 22 days (interquartile range [IQR], 8 to 39 days).
TABLE 1.
Etiologies of IE due to non-HACEK GNB
| Etiology | No. of patients (% of totala) |
|---|---|
| Enterobacteriaceae | |
| Escherichia coli | 18 (31) |
| Klebsiella pneumoniae | 6 (10.3) |
| Citrobacter koseri | 3 (5.2) |
| Proteus mirabilis | 2 (3.5) |
| Serratia marcescens | 2 (3.5) |
| Enterobacter spp. | 2 (3.5) |
| Yersinia enterocolitica | 1 (1.7) |
| Total | 34 (58.6) |
| Nonfermenting bacteria | |
| Pseudomonas spp. | 11 (19) |
| Acinetobacter baumannii | 1 (1.7) |
| Other bacteria | |
| Campylobacter fetus | 2 (3.5) |
| Fusobacterium spp. | 2 (3.5) |
| Achromobacter xylosoxidans | 1 (1.7) |
| Brucella spp. | 1 (1.7) |
| Prevotella buccae | 1 (1.7) |
| Raoultella spp. | 1 (1.7) |
| Sphingomonas paucimobilis | 1 (1.7) |
| GNB coinfectionsb | 3 (5.2) |
| MDR etiology | 16 (27.6) |
A total of 58 patients with IE due to non-HACEK GNB were studied.
Comprising one case with E. coli and Proteus mirabilis, one with Pseudomonas aeruginosa and Pseudomonas fluorescens, and one with Pseudomonas aeruginosa and Stenotrophomonas maltophilia.
Predisposing factors for infective endocarditis due to non-HACEK GNB.
As shown in Table 2, patients with IE due to GNB were compared to 174 control patients. The causative pathogens among controls were Staphylococcus aureus (48 cases [27.6%]), coagulase-negative staphylococci (31 cases [17.8%]), enterococci (31 cases [17.8%], 24 with Enterococcus faecalis, 6 with Enterococcus faecium, and 1 with Enterococcus durans), Streptococcus gallolyticus (29 cases [16.7%]), viridans group streptococci (26 cases [14.9%]), Streptococcus agalactiae (4 cases [2.3%]), Candida spp. (2 cases [1.2%]), and others (1.7%). Compared to matched controls, patients with IE due to GNB had a higher prevalence of cardiac implantable electronic device (CIED)-related endocarditis, more frequently had an indwelling central intravascular access device or a CIED at the time of IE onset, showed a higher rate of diabetes mellitus, and were more likely to receive concomitant immunosuppressive therapy. The genitourinary tract was the most frequent source of infection in IE due to GNB. The appearance of new valve regurgitation was significantly more common in controls than in patients with IE due to GNB (P = 0.007). Moreover, vegetations were larger in patients with IE due to GNB (14 mm [IQR, 6.5 to 20 mm]) than in controls (10 mm [IQR, 8 to 15 mm]) (P = 0.027).
TABLE 2.
Comparison between patients with IE due to GNB (cases) and patients with IE due to other etiologies
| Variablea | Valueb for patients with IE due to: |
P | |
|---|---|---|---|
| GNB (n = 58) | Other etiologies (n = 174) | ||
| Demographic data | |||
| Males | 39 (67.2) | 117 (67.6) | 1.0 |
| Median (range) age (yr) | 69.5 (57.75–77) | 69.5 (60–76) | 1.0 |
| Type of endocarditis | |||
| NVE | 34 (58.6) | 118 (67.8) | 0.202 |
| PVE | 16 (27.6) | 48 (27.6) | 1.0 |
| Device-related IE | 8 (13.8) | 8 (4.6) | 0.017 |
| Comorbidities | |||
| Diabetes | 19 (32.8) | 30 (17.2) | 0.012 |
| Hemodialysis | 1 (1.7) | 5 (2.9) | 0.633 |
| Malignancies | 10 (17.2) | 16 (9.2) | 0.093 |
| HIV infection | 2 (3.4) | 4 (2.3) | 0.633 |
| Risk factors | |||
| IVDU | 5 (8.6) | 15 (8.6) | 1.0 |
| Dental procedures | 3 (5.2) | 13 (7.5) | 0.537 |
| CVC | 12 (20.7) | 16 (10) | 0.021 |
| CIED | 15 (25.9) | 19 (10.9) | 0.005 |
| Congenital heart disease | 4 (6.9) | 10 (5.7) | 0.758 |
| Immunosuppression | 9 (15.5) | 7 (4) | 0.003 |
| Pre-existing valvular disease | 18 (31) | 57 (32.8) | 0.808 |
| Invasive procedures within 180 days | |||
| Dental procedures | 3 (5.2) | 13 (7.5) | 0.537 |
| Esophagogastroscopy | 1 (1.7) | 5 (2.9) | 0.633 |
| Colonoscopy | 4 (6.9) | 6 (3.4) | 0.263 |
| Cystoscopy | 3 (5.2) | 3 (1.7) | 0.152 |
| Route of acquisition | |||
| Community acquired | 32 (55.2) | 116 (66.7) | 0.115 |
| Healthcare associated | 24 (41.4) | 49 (28.2) | 0.060 |
| Nosocomially acquired | 2 (3.4) | 9 (5.2) | 0.593 |
| Complications | |||
| Stroke | 7 (12.1) | 27 (15.5) | 0.520 |
| Embolism | 14 (24.1) | 64 (36.8) | 0.078 |
| Heart failure | 15 (25.9) | 72 (41.4) | 0.030 |
| Septic shock | 2 (3.4) | 3 (1.7) | 0.434 |
| Arrhythmias | 5 (8.6) | 27 (15.5) | 0.187 |
| Echocardiographic findings | |||
| Evidence of vegetation | 50 (86.2) | 155 (89.1) | 0.555 |
| Median (range) size of vegetation (mm) | 14 (6.5–20) | 10 (8–15) | 0.027 |
| Cardiac abscess | 3 (5.2) | 17 (9.8) | 0.280 |
| Perforation | 4 (6.9) | 8 (4.6) | 0.494 |
| Dehiscence | 6 (10.3) | 9 (5.2) | 0.165 |
| New regurgitation | 10 (17.2) | 63 (36.2) | 0.007 |
| Fistula | 0 (0) | 4 (2.3) | 0.244 |
| Presumed source of infection | |||
| Genitourinary tract | 16 (27.6) | 6 (3.4) | <0.001 |
| Skin | 8 (13.8) | 28 (16.1) | 0.675 |
| Nonoral gastrointestinal tract | 9 (15.5) | 37 (21.3) | 0.342 |
| Oral | 3 (5.2) | 18 (10.3) | 0.234 |
| Other or unknown | 24 (41.4) | 81 (46.6) | 0.493 |
| Treatment (surgery plus antibiotic therapy) | 25 (43.1) | 79 (45.4) | 0.760 |
| Outcomes | |||
| All-cause in-hospital mortality | 8 (13.8) | 23 (13.2) | 0.911 |
| IE-related in-hospital mortality | 8 (13.8) | 21 (12.1) | 0.731 |
| Cumulative all-cause 1-yr mortalityc | 15 (30.6) | NA | |
| Cumulative IE-related 1-yr mortalityc | 11 (22.4) | NA | |
NVE, native valve endocarditis; PVE, prosthetic valve endocarditis; IVDU, intravenous drug user; CVC, central venous catheter (including any of the following: arteriovenous fistula, chronic intravenous catheter, or acute central catheter); CIED, cardiac implantable electronic device (12 permanent pacemakers and 3 cardioverter defibrillators in patients with endocarditis due to GNB; 17 permanent pacemakers and 2 cardioverter defibrillators in patients with other causes of endocarditis).
Values are numbers (percentages) of patients except where otherwise indicated.
Cumulative 1-year mortality was calculated for 49 patients (8 patients who died during the hospital stay and 41 patients with available follow-up after 1 year). NA, not available.
Upon multivariate analysis (Table 3), the genitourinary tract as a source of infection (odds ratio [OR], 13.59; 95% confidence interval [CI], 4.63 to 39.93; P < 0.001), immunosuppression (OR, 5.16; 95% CI, 1.60 to 16.62; P = 0.006), and the presence of a CIED (OR, 3.57; 95% CI, 1.55 to 8.20; P = 0.003) were factors independently associated with IE caused by GNB, while the development of new valve regurgitation (OR, 0.39; 95% CI, 0.17 to 0.90; P = 0.027) was a factor independently associated with IE due to other etiologies.
TABLE 3.
Multivariate analysis of factors independently associated with Gram-negative IE
| Factora | Univariate analysis |
Multivariate analysis |
||||||
|---|---|---|---|---|---|---|---|---|
| OR | 95.0% CI |
P | OR | 95.0% CI |
P | |||
| Lower | Upper | Lower | Upper | |||||
| Genitourinary tract | 10.67 | 3.93 | 28.92 | <0.001 | 13.60 | 4.63 | 39.93 | <0.001 |
| Immunosuppression | 4.38 | 1.55 | 12.37 | 0.003 | 5.16 | 1.6 | 16.62 | 0.006 |
| CIED | 2.85 | 1.33 | 6.06 | 0.005 | 3.60 | 1.55 | 8.20 | 0.003 |
| New regurgitation | 0.37 | 0.17 | 0.77 | 0.007 | 0.39 | 0.17 | 0.90 | 0.027 |
| Device-related endocarditis | 3.32 | 1.19 | 9.30 | 0.017 | ||||
| CVC | 2.56 | 1.13 | 5.80 | 0.021 | ||||
| Heart failure | 0.48 | 0.25 | 0.94 | 0.003 | ||||
CIED, cardiac implantable electronic device; CVC: central venous catheter (including any of the following: arteriovenous fistula, chronic intravenous catheter, or acute central catheter).
Treatment and outcomes of patients with endocarditis due to non-HACEK GNB.
Table 4 describes the antibiotic regimens received by 58 patients with IE due to GNB. Most patients received an antibiotic regimen (50%) containing penicillin or cephalosporin (penicillin–penicillinase inhibitor or a third-generation cephalosporin, such as ceftriaxone or ceftazidime), variably combined with an aminoglycoside or a fluoroquinolone. The second most frequently used regimen (22.4%) was the association of a penicillin or cephalosporin and a carbapenem, with or without aminoglycosides or fluoroquinolones. A substantial 27.6% of cases were treated with a carbapenem with or without aminoglycosides, or a fluoroquinolone, or other antibiotics (including colistin).
TABLE 4.
Treatment strategies for IE due to non-HACEK GNB
| Antibiotic therapya | No. (% of totalb) |
|---|---|
| Noncarbapenem β-lactamc ± aminoglycosides ± fluoroquinolones | 29 (50) |
| Penicillin or cephalosporin only | 5 |
| Non-carbapenem β-lactam + aminoglycosides | 14 |
| Non-carbapenem β-lactam + fluoroquinolones | 8 |
| Non-carbapenem β-lactam + aminoglycosides + fluoroquinolones | 2 |
| Carbapenem ± aminoglycosides ± fluoroquinolones | 8 (13.8) |
| Carbapenem only | 3 |
| Carbapenem + aminoglycosides | 1 |
| Carbapenem + fluoroquinolones | 3 |
| Carbapenem + aminoglycosides + fluoroquinolones | 1 |
| Noncarbapenem β-lactam +carbapenem ± aminoglycosides ± fluoroquinolones | 13 (22.4) |
| Non-carbapenem β-lactam + carbapenem | 4 |
| Non-carbapenem β-lactam + carbapenem + aminoglycosides | 3 |
| Non-carbapenem β-lactam + carbapenem + fluoroquinolones | 4 |
| Non-carbapenem β-lactam + carbapenem + aminoglycosides + fluoroquinolones | 2 |
| Colistin-containing regimens | 3 (5.2) |
| Colistin + carbapenem + rifampin | 2 |
| Colistin + penicillin or cephalosporin + carbapenem + rifampin | 1 |
| Other regimens | 5 (8.6) |
| Non-carbapenem β-lactam + rifampin | 1 |
| Non-carbapenem β-lactam + tobramycin + clindamycin | 1 |
| Aminoglycosides + rifampin | 1 |
| Aminoglycosides + fluoroquinolones + rifampin | 2 |
±, with or without.
A total of 58 patients with IE due to non-HACEK GNB were studied.
Includes penicillin alone, penicillin–penicillinase inhibitor combinations, and third-generation cephalosporins.
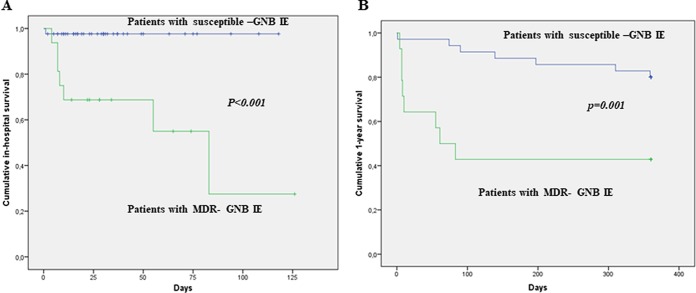
Overall in-hospital mortality was 13.8% (all deaths were attributable to IE), while 1-year cumulative mortality was 30.6% (with a cumulative IE-related 1-year mortality of 22.4%). A comprehensive comparison between survivors and nonsurvivors is provided in Table S1 in the supplemental material. Nonsurvivors had a higher median age, were more likely to be affected by non-nosocomial health care-associated (HCA) IE and IE due to MDR pathogens, and had a higher incidence of heart failure or septic shock. Regarding antibiotic therapy, survivors were more frequently treated with a penicillin- or cephalosporin-containing regimen (penicillin or cephalosporin with or without an aminoglycoside), while nonsurvivors were more frequently treated with a regimen containing a carbapenem (with or without an aminoglycoside). No significant mortality differences between patients receiving antibiotic therapy alone and those undergoing cardiac surgery were detected. However, among 21 patients with complications (including the development of a cardiac abscess, fistula, dehiscence, valve perforation, or de novo heart failure), those receiving medical therapy alone (n = 10) showed higher mortality rates than those undergoing surgery (n = 11) (60% versus 18.2%, respectively; P = 0.049 [data not shown]). Upon Cox regression analysis (Table 5), an MDR etiology was the only factor independently associated with in-hospital mortality (hazard ratio [HR], 21.849; 95% CI, 2.672 to 178.683; P = 0.004). Of 50 patients who survived the initial hospitalization, 41 (82%) were followed for as long as 1 year after hospitalization; of these, 7 (17%) patients died during the 1-year follow-up. Thus, the cumulative 1-year mortality amounted to 15 (30.6%) patients. As shown in Table 5, MDR etiology was confirmed to be an independent factor associated with 1-year mortality (HR, 4.408; 95% CI, 1.581 to 12.287; P = 0.005). Figure 2 shows the Kaplan-Meier in-hospital (Fig. 2A) and 1-year (Fig. 2B) survival curves of patients with IE due to MDR GNB and those with IE due to susceptible GNB.
TABLE 5.
Cox regression analysis investigating the effect of MDR etiology on in-hospital and 1-year mortality among patients with IE due to non-HACEK GNB
| MDR etiology on: | Cox regression analysis |
|||
|---|---|---|---|---|
| HR | 95.0% CI |
P | ||
| Lower | Upper | |||
| In-hospital mortality | 21.849 | 2.672 | 178.683 | 0.004 |
| 1-yr mortality | 4.408 | 1.581 | 12.287 | 0.005 |
FIG 2.
Kaplan-Meier plots showing in-hospital (A) and 1-year (B) survival of patients with IE caused by GNB according to multidrug-resistant etiology.
DISCUSSION
This is one of the largest series describing the risk factors and outcomes of patients affected by endocarditis due to non-HACEK GNB. We found that the genitourinary tract as a source of infection, the presence of a CIED, and the receipt of immunosuppressive agents are distinctive factors of endocarditis due to GNB. We also found that an MDR etiology is the major determinant of in-hospital and long-term mortality among patients with IE due to GNB.
The first reports of endocarditis due to non-HACEK GNB date back to some outbreaks among injection drug users in large urban areas in the 1970s and 1980s (10–12). Subsequently, an increasing incidence of IE due to GNB and a changing pattern of risk factors were described among patients with health care contacts (4, 5). Most of the knowledge about this clinical entity derived from some case series, single-center experiences, and the ICE study, including 49 patients with IE due to non-HACEK GNB (1, 4, 13–17). Thus, our investigation represents the largest contemporary cohort of patients with IE due to non-HACEK GNB for which data have been collected to date.
In our study, the incidence of IE due to non-HACEK GNB was 3.9%, slightly higher than that previously observed in the ICE study (1.8%) (1). The higher percentage probably reflects the upward trend of infections caused by GNB in recent years (18). We identified the presence of a CIED (pacemaker or cardioverter defibrillator) and the receipt of immunosuppressive therapy as factors independently associated with the development of IE due to GNB. Over recent years, the growing use of CIEDs has led to an increase in the incidence of IE due to difficult-to-treat organisms, such as methicillin-resistant Staphylococcus aureus, Candida spp., and GNB (19–22). It has been documented that a significant percentage of device-related IE cases (from 16% to 23%) are caused by non-Staphylococcus species (23, 24), represented mostly by GNB, with a predominance of Pseudomonas aeruginosa (24). It has also been demonstrated that, in contrast to the pattern for infections with Gram-positive bacteria, among patients with CIED-related IE, the GNB isolated from the pocket or infected skin tissue is highly concordant with the strain isolated from the bloodstream (23). These findings indicate that a clinical suspicion of IE due to non-HACEK GNB should promptly arise for patients with CIEDs presenting with fever and evidence of pocket or lead GNB infection.
GNB remain uncommon causes of IE due to their high sensitivity to serum bactericidal activity and their inability to adhere to the endocardium (25). However, the receipt of immunosuppressive therapy constituted a further risk factor for IE due to GNB, suggesting that a failure in the immune system could favor valve colonization (26, 27). As expected, the genitourinary tract was the most common source of infection in patients with endocarditis due to non-HACEK GNB, playing a role as a portal of entry of GNB into the bloodstream (28, 29).
In our series, mortality due to IE caused by GNB was not significantly higher than that observed among control patients with other etiologies, and these findings are consistent with those of previous studies (reporting in-hospital and 1-year fatality rates of 16% and 29%, respectively) (30). Of interest, MDR etiology was the factor independently associated with short- and long-term mortality among patients affected by IE due to non-HACEK GNB. Similarly, we observed that the administration of a noncarbapenem β-lactam (a third-generation cephalosporin or penicillin–penicillinase inhibitor) was associated with a better outcome, with a significant reduction of in-hospital mortality. Thus, the possibility of using traditional β-lactams appears to be associated with improved survival. Of importance, the greatest mortality risk occurred during the first weeks after diagnosis; this seems to suggest a crucial role for the highly active antibiotic therapy provided in hospital after IE diagnosis. Current guidelines of the American Heart Association/Infectious Diseases Society of America recommend the use of a combination antibiotic therapy with a β-lactam (penicillins, cephalosporins, or carbapenems) and either an aminoglycoside or fluoroquinolone for 6 weeks for the treatment of endocarditis caused by non-HACEK GNB (31). However, indications for the treatment of IE due to MDR GNB are still debated, because of the high risk of clinical failure. In these cases, antibiotic therapy should be individualized, combination therapy should be provided if possible, and a consultation for the evaluation of a prompt surgical removal of infected valves should be performed.
The major strength of our study is that it represents the largest contemporary study describing IE due to GNB. Moreover, the SEI is a multicenter prospective study, characteristics that increase the value of the results obtained. In contrast to the ICE study, which compared the limited cohort of patients with IE due to GNB to all other patients with IE (more than 2,000), we applied a matched design to reduce differences between the two groups and, consequently, risks of bias. While previous reports merely described isolated species in IE due to GNB, we were able to illustrate the susceptibility patterns of isolated organisms as well, providing updated microbiology data for IE caused by GNB. Finally, this is the first study that provides data on 1-year survival in this cohort of patients.
Our study has some limitations. First of all, because of the rarity of infection, we were able to investigate only a small number of patients overall. Moreover, a certain number of patients, though only a few, were lost to 1-year follow-up. Finally, this study has not been designed to show the superiority of a specific antibiotic regimen, and data regarding antibiotic treatment should be confirmed in controlled randomized trials. However, since IE due to non-HACEK GNB is a rare clinical entity, it is unlikely that such studies will be performed. Thus, our observations could represent a valuable experience for the selection of antibiotic therapy for IE due to GNB. Moreover, we provided the potential explanation of this finding, which seems to be strictly related to the MDR etiology.
In conclusion, we evaluated clinical features and outcomes of IE due to non-HACEK GNB in a contemporary multicenter cohort. In the context of patients with IE, peculiar features of GNB etiology include the genitourinary tract as a source of infection, the presence of an implanted cardiac device, and receipt of immunosuppressive agents. De novo heart failure is less typical of these patients, and sepsis signs may be more prominent. Infection caused by an MDR strain is associated with increased mortality rates. The use of an antibiotic regimen containing a penicillin or cephalosporin could be suggested if a susceptible strain was isolated, but new β-lactam agents with broad activity against resistant strains could represent a therapeutic option for patients with endocarditis due to MDR GNB strains.
MATERIALS AND METHODS
Study design and patient population.
All cases of IE due to non-HACEK GNB enrolled in the Italian Endocarditis Study (SEI) were included in this study. The SEI is a national multicenter collaboration involving investigators from 26 centers located throughout Italy who collected all consecutive episodes of IE identified between January 2004 and December 2011 in the participating sites (32). These included Infectious Diseases or Internal Medicine centers located in facilities with on-site cardiac surgery. Patients were identified prospectively using predefined, homogeneous inclusion and exclusion criteria to ensure consecutive enrollment (32). All patients provided\informed consent to participate in the study, whose protocol was approved by local Ethics Committees.
The diagnosis of IE was performed in accordance with the modified Duke criteria (33). Only patients fulfilling criteria for definite IE were included in the study. Of all patients included in the SEI, those with blood or heart valve cultures positive for a GNB were identified. To ensure that the diagnosis of endocarditis due to GNB was accurate, the following additional criteria were applied in interpreting the blood culture results: (i) the patient's bacteremia had to meet the definition for persistently positive blood cultures when the modified Duke criteria were applied; (ii) a single blood culture positive for a GNB was not considered a minor microbiological criterion when the modified Duke criteria were applied. All cases of IE due to HACEK organisms and all polymicrobial IE cases (IE due to GNB plus Gram-positive bacteria or due to GNB plus Candida spp.) were excluded from the study analysis. All patients with IE due to non-HACEK GNB were included in the study analysis representing the case group. For each case of IE due to GNB, we randomly selected 3 patients included in the SEI cohort with definite IE due to other infective causes (control group). The three controls were sex and age matched (±2 years) with each case of IE due to GNB.
Data collection and study definition.
An ad hoc case report form was used at all sites to collect the data (32). On admission to the hospital, all patients underwent clinical history taking, physical examination, blood tests, including multiple blood cultures, transthoracic echocardiography (TTE) and, when needed, transesophageal echocardiography (TEE), as well as a cardiac surgery consultation. Demographic data, comorbidities, and risk factors for endocarditis (intravenous drug use, invasive procedures in the last 180 days, the presence of a prosthetic valve, and congenital heart disease) were collected. Concomitant immunosuppression therapy was defined as concomitant use of steroids (prednisolone at >0.5 mg/kg/day or the equivalent for >1 month), chemotherapy or anti-tumor necrosis factor, cyclophosphamide, azathioprine, methotrexate, mycophenolate mofetil, or calcineurin inhibitor therapy. Information about the presence of a central intravascular access device (including arteriovenous fistulas or long-term or short-term central catheters) and cardiac implantable electronic devices (CIEDs) (both permanent pacemakers and implantable cardioverter defibrillators) was also recorded.
IE was classified as native valve endocarditis (NVE), prosthetic valve endocarditis (PVE), or CIED-related IE (6). The latter included pacemaker and implantable cardioverter defibrillator lead infections.
With regard to the pattern of IE acquisition, clinical cases were categorized as nosocomially acquired (NA), nonnosocomial health care-associated (HCA), and community acquired (CA). NA-IE was defined as IE developing in a patient hospitalized for more than 48 h before the onset of signs or symptoms consistent with IE. HCA was defined as IE diagnosed on the basis of signs and symptoms appearing within 48 h of admission in a patient with prior health care contacts. The latter include (i) receipt of intravenous therapy, wound care, or specialized nursing care at home within the 30 days before the onset of IE, (ii) attending of a hospital or hemodialysis clinic within the 30 days before the onset of IE, (iii) hospitalization in an acute-care hospital for 2 or more days in the 90 days before the onset of IE, or (iv) hospitalization in a nursing home or a long-term-care facility. Finally, CA IE was defined as IE diagnosed at the time of admission (or within 48 h of admission) in a patient not fulfilling the criteria for health care-associated infection (32).
Data about the potential complications of IE documented by the baseline echocardiogram, including cardiac abscesses, perforation, dehiscence, new regurgitation or fistula, were recorded. Moreover, data about other possible complications of IE, such as stroke, embolism, heart failure, septic shock, or arrhythmias, were also collected. Data about surgical therapy, antimicrobial treatment, and the outcome of hospitalization were also recorded.
Antibiotic treatments were classified as follows: (i) a noncarbapenem β-lactam with or without aminoglycosides and with or without fluoroquinolone, (ii) carbapenem with or without aminoglycosides and with or without fluoroquinolones, (iii) a noncarbapenem β-lactam plus carbapenem with or without aminoglycosides and with or without fluoroquinolones, (iv) a colistin-containing regimen, or (v) other regimens.
Data about overall and IE-attributable mortality were recorded. Mortality was attributed to IE if one of the following criteria were satisfied: (i) blood cultures were positive for the causative pathogen at the time of death; (ii) death occurred before the resolution of signs or symptoms of IE; (iii) death occurred after the diagnosis of endocarditis without another explanation; (iv) death was caused by a documented complication of infective endocarditis (refractory pulmonary edema or cardiogenic shock due to acute regurgitation or valve obstruction; major arrhythmia; acute coronary syndrome due to vegetation embolism or coronary obstruction); (v) autopsy findings or a death certificate indicated IE as a cause of death, (vi) relapse of IE was documented (34).
For all patients with IE due to non-HACEK GNB, a 1-year follow-up evaluation was performed, through a clinical visit or phone interview. During the follow-up period, a new TTE was performed for selected patients according to patient history and physical examination.
Identification and susceptibility testing were performed using automated methods such as BacT/Alert (bioMérieux; Marcy-L'Étiole, France) and Vitek 2 broth microdilution (bioMérieux). MDR bacteria were defined as microorganisms nonsusceptible to at least one agent in three or more antimicrobial categories (35).
Study outcomes.
The first objective of this study was to identify risk factors associated with the development of endocarditis due to non-HACEK GNB. To achieve this goal, a comparison between cases and controls was performed. The second objective was to identify factors associated with in-hospital mortality. To this end, we compared survivors and nonsurvivors with IE due to GNB, and we performed Cox regression analysis on factors independently associated with death.
Statistical analysis.
Statistical analysis was performed using commercially available statistical software packages (SPSS, version 20.0 [SPSS, Inc., Chicago, IL] and R, version 3.0.2 [R development Core Team, Vienna, Austria]). The chi-square test or Fisher's exact test was used for categorical variables and the two-tailed t test or Mann-Whitney U test for continuous variables, as appropriate. Values for continuous and categorical variables are expressed as means ± standard deviations (SD) or medians (interquartile ranges [IQR]) and as the number and percentage of the group from which they are derived, respectively.
Multivariate analysis to identify risk factors for IE due to GNB was performed using a logistic regression model. In the model, predictors were selected via a stepwise procedure optimizing the Akaike Information Criterion. Odds ratios with 95% confidence intervals were calculated to evaluate the strength of any association.
The Cox regression model was used to determine the effects of different variables on in-hospital survival among patients with IE due to GNB. Hazard ratios with 95% CIs were calculated to evaluate the strength of each association. The proportionality-of-hazards assumption for the Cox model has been checked using plots of Schoenfeld residuals. Statistical significance was established at a P value of ≤0.05. All reported P values are two-tailed.
Supplementary Material
ACKNOWLEDGMENT
We declare no conflict of interests.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02208-17.
REFERENCES
- 1.Morpeth S, Murdoch D, Cabell CH, Karchmer AW, Pappas P, Levine D, Nacinovich F, Tattevin P, Fernández-Hidalgo N, Dickerman S, Bouza E, del Río A, Lejko-Zupanc T, de Oliveira Ramos A, Iarussi D, Klein J, Chirouze C, Bedimo R, Corey GR, Fowler VG Jr; International Collaboration on Endocarditis Prospective Cohort Study (ICE-PCS) Investigators. 2007. Non-HACEK gram-negative bacillus endocarditis. Ann Intern Med 147:829–835. doi: 10.7326/0003-4819-147-12-200712180-00002. [DOI] [PubMed] [Google Scholar]
- 2.Murdoch DR, Corey GR, Hoen B, Miró JM, Fowler VG Jr, Bayer AS, Karchmer AW, Olaison L, Pappas PA, Moreillon P, Chambers ST, Chu VH, Falcó V, Holland DJ, Jones P, Klein JL, Raymond NJ, Read KM, Tripodi MF, Utili R, Wang A, Woods CW, Cabell CH; International Collaboration on Endocarditis-Prospective Cohort Study (ICE-PCS) Investigators. 2009. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med 169:463–473. doi: 10.1001/archinternmed.2008.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Falcone M, Russo A, Iacovelli A, Restuccia G, Ceccarelli G, Giordano A, Farcomeni A, Morelli A, Venditti M. 2016. Predictors of outcome in ICU patients with septic shock caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae. Clin Microbiol Infect 22:444–450. doi: 10.1016/j.cmi.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 4.Reyes MP, Reyes KC. 2008. Gram-negative endocarditis. Curr Infect Dis Rep 10:267–274. doi: 10.1007/s11908-008-0044-5. [DOI] [PubMed] [Google Scholar]
- 5.Prendergast BD. 2006. The changing face of infective endocarditis. Heart 92:879–885. doi: 10.1136/hrt.2005.067256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tacconelli E, Cataldo MA, Dancer SJ, De Angelis G, Falcone M, Frank U, Kahlmeter G, Pan A, Petrosillo N, Rodríguez-Baño J, Singh N, Venditti M, Yokoe DS, Cookson B; European Society of Clinical Microbiology. 2014. ESCMID guidelines for the management of the infection control measures to reduce transmission of multidrug-resistant Gram-negative bacteria in hospitalized patients. Clin Microbiol Infect 20(Suppl 1):S1–S55. doi: 10.1111/1469-0691.12427. [DOI] [PubMed] [Google Scholar]
- 7.Ceccarelli G, Falcone M, Giordano A, Mezzatesta ML, Caio C, Stefani S. 2013. Successful ertapenem-doripenem combination treatment of bacteremic ventilator-associated pneumonia due to colistin-resistant KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 57:2900–2901. doi: 10.1128/AAC.00188-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orsi GB, Falcone M, Venditti M. 2011. Surveillance and management of multidrug-resistant microorganisms. Expert Rev Anti Infect Ther 9:653–679. doi: 10.1586/eri.11.77. [DOI] [PubMed] [Google Scholar]
- 9.Gutiérrez-Gutiérrez B, Salamanca E, de Cueto M, Hsueh PR, Viale P, Paño-Pardo JR, Venditti M, Tumbarello M, Daikos G, Cantón R, Doi Y, Tuon FF, Karaiskos I, Pérez-Nadales E, Schwaber MJ, Azap ÖK, Souli M, Roilides E, Pournaras S, Akova M, Pérez F, Bermejo J, Oliver A, Almela M, Lowman W, Almirante B, Bonomo RA, Carmeli Y, Paterson DL, Pascual A, Rodríguez-Baño J; REIPI/ESGBIS/INCREMENT Investigators. 2017. Effect of appropriate combination therapy on mortality of patients with bloodstream infections due to carbapenemase-producing Enterobacteriaceae (INCREMENT): a retrospective cohort study. Lancet Infect Dis 17:726–734. doi: 10.1016/S1473-3099(17)30228-1. [DOI] [PubMed] [Google Scholar]
- 10.Komshian SV, Tablan OC, Palutke W, Reyes MP. 1990. Characteristics of left-sided endocarditis due to Pseudomonas aeruginosa in the Detroit Medical Center. Rev Infect Dis 12:693–702. doi: 10.1093/clinids/12.4.693. [DOI] [PubMed] [Google Scholar]
- 11.Wieland M, Lederman MM, Kline-King C, Keys TF, Lerner PI, Bass SN, Chmielewski R, Banks VD, Ellner JJ. 1986. Left-sided endocarditis due to Pseudomonas aeruginosa. A report of 10 cases and review of the literature. Medicine (Baltimore) 65:180–189. [DOI] [PubMed] [Google Scholar]
- 12.Mills J, Drew D. 1976. Serratia marcescens endocarditis: a regional illness associated with intravenous drug abuse. Ann Intern Med 84:29–35. doi: 10.7326/0003-4819-84-1-29. [DOI] [PubMed] [Google Scholar]
- 13.Branger S, Casalta JP, Habib G, Collard F, Raoult D. 2005. Escherichia coli endocarditis: seven new cases in adults and review of the literature. Eur J Clin Microbiol Infect Dis 24:537–541. doi: 10.1007/s10096-005-1379-6. [DOI] [PubMed] [Google Scholar]
- 14.Durante-Mangoni E, Tripodi MF, Albisinni R, Utili R. 2010. Management of Gram-negative and fungal endocarditis. Int J Antimicrob Agents 36(Suppl 2):S40–S45. doi: 10.1016/j.ijantimicag.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 15.Raza SS, Sultan OW, Sohail MR. 2010. Gram-negative bacterial endocarditis in adults: state-of-the-heart. Expert Rev Anti Infect Ther 8:879–885. doi: 10.1586/eri.10.76. [DOI] [PubMed] [Google Scholar]
- 16.Kalra A, Cooley C, Tsigrelis C. 2011. Treatment of endocarditis due to Proteus species: a literature review. Int J Infect Dis 15:e222–e225. doi: 10.1016/j.ijid.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Loubet P, Lescure FX, Lepage L, Kirsch M, Armand-Lefevre L, Bouadma L, Lariven S, Duval X, Yazdanpanah Y, Joly V. 2015. Endocarditis due to gram-negative bacilli at a French teaching hospital over a 6-year period: clinical characteristics and outcome. Infect Dis (Lond) 47:889–895. doi: 10.3109/23744235.2015.1075660. [DOI] [PubMed] [Google Scholar]
- 18.Ani C, Farshidpanah S, Bellinghausen Stewart A, Nguyen HB. 2015. Variations in organism-specific severe sepsis mortality in the United States: 1999–2008. Crit Care Med 43:65–77. doi: 10.1097/CCM.0000000000000555. [DOI] [PubMed] [Google Scholar]
- 19.Greenspon AJ, Patel JD, Lau E, Ochoa JA, Frisch DR, Ho RT, Pavri BB, Kurtz SM. 2011. 16-year trends in the infection burden for pacemakers and implantable cardioverter-defibrillators in the United States 1993 to 2008. J Am Coll Cardiol 58:1001–1006. doi: 10.1016/j.jacc.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 20.Athan E, Chu VH, Tattevin P, Selton-Suty C, Jones P, Naber C, Miró JM, Ninot S, Fernández-Hidalgo N, Durante-Mangoni E, Spelman D, Hoen B, Lejko-Zupanc T, Cecchi E, Thuny F, Hannan MM, Pappas P, Henry M, Fowler VG Jr, Crowley AL, Wang A; ICE-PCS Investigators. 2012. Clinical characteristics and outcome of infective endocarditis involving implantable cardiac devices. JAMA 307:1727–1735. doi: 10.1001/jama.2012.497. [DOI] [PubMed] [Google Scholar]
- 21.Glavis-Bloom J, Vasher S, Marmor M, Fine AB, Chan PA, Tashima KT, Lonks JR, Kojic EM. 2015. Candida and cardiovascular implantable electronic devices: a case of lead and native aortic valve endocarditis and literature review. Mycoses 58:637–641. doi: 10.1111/myc.12391. [DOI] [PubMed] [Google Scholar]
- 22.Durante-Mangoni E, Andini R, Agrusta F, Iossa D, Mattucci I, Bernardo M, Utili R. 2014. Infective endocarditis due to multidrug resistant gram-negative bacilli: single centre experience over 5 years. Eur J Intern Med 25:657–661. doi: 10.1016/j.ejim.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 23.Fukunaga M, Goya M, Nagashima M, Hiroshima K, Yamada T, An Y, Hayashi K, Makihara Y, Ohe M, Ichihashi K, Ohtsuka M, Miyazaki H, Ando K. 2017. Identification of causative organism in cardiac implantable electronic device infections. J Cardiol 70:411–415. doi: 10.1016/j.jjcc.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Viola GM, Awan LL, Ostrosky-Zeichner L, Chan W, Darouiche RO. 2012. Infections of cardiac implantable electronic devices: a retrospective multicenter observational study. Medicine (Baltimore) 91:123–130. doi: 10.1097/MD.0b013e31825592a7. [DOI] [PubMed] [Google Scholar]
- 25.Aubron C, Charpentier J, Trouillet JL, Offenstadt G, Mercat A, Bernardin G, Hyvernat H, Wolff M. 2006. Native-valve infective endocarditis caused by Enterobacteriaceae: report on 9 cases and literature review. Scand J Infect Dis 38:873–881. doi: 10.1080/00365540600740488. [DOI] [PubMed] [Google Scholar]
- 26.Gould K, Ramirez-Ronda CH, Holmes RK, Sanford JP. 1975. Adherence of bacteria to heart valves in vitro. J Clin Invest 56:1364–1370. doi: 10.1172/JCI108216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moreillon P, Que YA. 2004. Infective endocarditis. Lancet 363:139–149. doi: 10.1016/S0140-6736(03)15266-X. [DOI] [PubMed] [Google Scholar]
- 28.Meyers BR, Sherman E, Mendelson MH, Velasquez G, Srulevitch-Chin E, Hubbard M, Hirschman SZ. 1989. Bloodstream infections in the elderly. Am J Med 86:379–384. doi: 10.1016/0002-9343(89)90333-1. [DOI] [PubMed] [Google Scholar]
- 29.Micol R, Lortholary O, Jaureguy F, Bonacorsi S, Bingen E, Lefort A, Mémain N, Bouchaud O, Larroche C. 2006. Escherichia coli native valve endocarditis. Clin Microbiol Infect 12:401–403. doi: 10.1111/j.1469-0691.2006.01375.x. [DOI] [PubMed] [Google Scholar]
- 30.Bishara J, Leibovici L, Gartman-Israel D, Sagie A, Kazakov A, Miroshnik E, Ashkenazi S, Pitlik S. 2001. Long-term outcome of infective endocarditis: the impact of early surgical intervention. Clin Infect Dis 33:1636–1643. doi: 10.1086/323785. [DOI] [PubMed] [Google Scholar]
- 31.Baddour LM, Wilson WR, Bayer AS, Fowler VG Jr, Tleyjeh IM, Rybak MJ, Barsic B, Lockhart PB, Gewitz MH, Levison ME, Bolger AF, Steckelberg JM, Baltimore RS, Fink AM, O'Gara P, Taubert KA; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. 2015. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 132:1435–1486. doi: 10.1161/CIR.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 32.Leone S, Ravasio V, Durante-Mangoni E, Crapis M, Carosi G, Scotton PG, Barzaghi N, Falcone M, Chinello P, Pasticci MB, Grossi P, Utili R, Viale P, Rizzi M, Suter F. 2012. Epidemiology, characteristics, and outcome of infective endocarditis in Italy: the Italian Study on Endocarditis. Infection 40:527–535. doi: 10.1007/s15010-012-0285-y. [DOI] [PubMed] [Google Scholar]
- 33.Rizzi M, Ravasio V, Carobbio A, Mattucci I, Crapis M, Stellini R, Pasticci MB, Chinello P, Falcone M, Grossi P, Barbaro F, Pan A, Viale P, Durante-Mangoni E; Investigators of the Italian Study on Endocarditis. 2014. Predicting the occurrence of embolic events: an analysis of 1456 episodes of infective endocarditis from the Italian Study on Endocarditis (SEI). BMC Infect Dis 14:230. doi: 10.1186/1471-2334-14-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Casapao AM, Lodise TP, Davis SL, Claeys KC, Kullar R, Levine DP, Rybak MJ. 2015. Association between vancomycin day 1 exposure profile and outcomes among patients with methicillin-resistant Staphylococcus aureus infective endocarditis. Antimicrob Agents Chemother 59:2978–2985. doi: 10.1128/AAC.03970-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. 2012. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.