ABSTRACT
The high acquisition rate of drug resistance by Mycobacterium tuberculosis necessitates the ongoing search for new drugs to be incorporated in the tuberculosis (TB) regimen. Compounds used for the treatment of other diseases have the potential to be repurposed for the treatment of TB. In this study, a high-throughput screening of compounds against thiol-deficient Mycobacterium smegmatis strains and subsequent validation with thiol-deficient M. tuberculosis strains revealed that ΔegtA and ΔmshA mutants had increased susceptibility to azaguanine (Aza) and sulfaguanidine (Su); ΔegtB and ΔegtE mutants had increased susceptibility to bacitracin (Ba); and ΔegtA, ΔmshA, and ΔegtB mutants had increased susceptibility to fusaric acid (Fu). Further analyses revealed that some of these compounds were able to modulate the levels of thiols and oxidative stress in M. tuberculosis. This study reports the activities of Aza, Su, Fu, and Ba against M. tuberculosis and provides a rationale for further investigations.
KEYWORDS: thiols, antimicrobial agents, oxidative stress, susceptibility testing
INTRODUCTION
In spite of the improved tuberculosis (TB) regimen, TB remains the second leading cause of death due to an infectious disease (after HIV infection) (1, 2). The treatment of TB caused by drug-susceptible Mycobacterium tuberculosis requires treatment for at least 6 months, while the treatment of TB caused by multidrug-resistant M. tuberculosis and extensively drug-resistant M. tuberculosis entails second-line drugs (which are usually toxic) for a longer period (3–6). Due to the length of the treatment and the toxicity of the second-line drugs, patients often fail to complete their treatment or do not adhere strictly to the regimen (7, 8). Regimens that speed up the cure of TB are therefore required. Enzymes or molecules that enable M. tuberculosis to thrive within the host have been investigated, with the purpose of validating them as potential drug targets.
Ergothioneine (ERG) (9, 10), mycothiol (MSH) (11, 12), and gamma-glutamylcysteine (GGC) (13) are low-molecular-weight thiols that protect M. tuberculosis against cellular stress. MSH is synthesized by a multistep process in mycobacteria, and several compounds targeted against enzymes involved in this process have been investigated. UDP-(5F)-GlcNAc was identified as the competitive inhibitor of the glycosyl transferase MshA of Corynebacterium glutamicum (14). MshA is essential for MSH biosynthesis (15); however, an MSH-deficient M. tuberculosis ΔmshA mutant had no in vivo growth defect (15), suggesting that inhibitors of MshA may not be bactericidal. MSH-deficient mutants, however, do display increased sensitivity to a number of antibiotics (10, 16), suggesting that MshA inhibitors may be able to enhance the activity of other drugs.
Deletion of the mshC gene (essential for MSH biosynthesis) was successfully achieved in Mycobacterium smegmatis (17) and Mycobacterium bovis (18) but only in the presence of a second copy in M. tuberculosis, indicating that it is essential for the in vitro/in vivo growth of M. tuberculosis (19). This suggests that the enzyme MshC has additional functions and prompted further investigations into inhibitors of MshC that can be developed as anti-TB drugs (20–22). A coupled spectrophotometric assay for the screening of MshC inhibitors identified NTF1836 (22), while a high-throughput screen using a coupled luminescent assay revealed dequalinium chloride as another potent inhibitor of MshC (21). Structurally related analogues of NTF1836 were subsequently investigated and found to inhibit MshC and to kill both replicating and nonreplicating M. tuberculosis (20).
Among the enzymes involved in ERG biosynthesis, EgtD and EgtB were found to be essential for ERG biosynthesis, while EgtA is required for ERG biosynthesis and essential for GGC biosynthesis (13). Inhibitors of EgtD or EgtB have not yet been investigated, but buthionine sulfoximine (BSO) was shown to inhibit the activity of EgtA (23). Since BSO inhibits the biosynthesis of glutathione (GSH) in eukaryotes (24) and GSH is required to control M. tuberculosis growth within the host (25–27), an alternative inhibitor of EgtA is required. Differences in the cysteine binding site of GGC synthases from different species (28) may allow the development of a mycobacterium-specific EgtA inhibitor. The enzymes involved in ERG biosynthesis are not essential for the in vitro growth of M. tuberculosis; however, ERG-deficient mutants have an ex vivo and in vivo growth defect and are sensitive to current first-line TB drugs (10). This suggests that inhibitors of thiol production may enhance the bactericidal activity of other compounds. In this study, we screened thiol-deficient mutants against a library of compounds in order to identify compounds that have increased activity against the mutants.
RESULTS AND DISCUSSION
M. smegmatis (29) and M. tuberculosis (10) ΔegtD mutants are ERG deficient, while the M. tuberculosis ΔegtB mutant is ERG deficient and the M. tuberculosis ΔegtA mutant is GGC and ERG deficient (13). The MshA enzyme is essential for MSH biosynthesis in both M. tuberculosis (15) and M. smegmatis (30). In order to identify compounds that differentially inhibit ERG- or MSH-deficient strains, the M. smegmatis ΔmshA and ΔegtD mutants and the wild-type (WT) strain were screened against the Prestwick (Illkirch, France) chemical and phytochemical libraries of 1,600 compounds at 80 μM (Table 1). To validate the hits and select the most efficient compounds, a growth curve analysis of the mutants affected by the compounds was performed at a lower concentration (60 μM) using a plate reader. 8-Azaguanine (Aza) and sulfaguanidine (Su) showed increased inhibition of the ΔmshA mutant, while bacitracin (Ba) was more active against the M. smegmatis ΔegtD mutant. This was further validated by dose-response curve analyses of the mutants relative to the wild type. The ΔmshA mutant showed differential sensitivity to Aza and Su from 12.5 μM and 25 μM, respectively, while the ΔegtD mutant showed differential sensitivity to Ba from 50 μM (see Fig. S1 to S3 in the supplemental material). Various M. tuberculosis strains harboring mutations in genes involved in ERG and MSH biosynthesis, namely, the ΔegtD and ΔegtB mutants, which are ERG deficient; the ΔegtA mutant, which is ERG and GGC deficient; and the ΔmshA mutant, which is MSH deficient (13) were then selected for comparison. Growth of these strains was evaluated at the average MIC for the wild type (the mean of the concentrations in the well with complete growth inhibition [GI] and the adjacent wells with no visible growth inhibition [see Table S1 in the supplemental material]) and compared to that of the untreated controls (see Fig. S4 to S7 in the supplemental material). The percent GI at the end of the growth curves (about 2 weeks) was calculated as indicated in Table 2. The ability of each compound to generate oxidative stress and to modulate thiol levels was investigated by measuring the levels of ERG, MSH, GGC, and H2O2 in the WT strain when cultured to stationary phase (for about 2 weeks) in the presence or absence of the compound (Table 3).
TABLE 1.
M. smegmatis hits at 80 μM from the Prestwick chemical and phytochemical libraries
| Compound | Effecta |
||
|---|---|---|---|
| Wild type | ΔegtD | ΔmshA | |
| Azaguanine-8 | + | + | − |
| Sulfaguanidine | + | + | − |
| Apomorphine hydrochloride | + | − | + |
| Trifluoperazine dihydrochloride | + | + | − |
| Beta-Escin | + | − | + |
| Ibutilide fumarate | + | + | − |
| Bacitracin | + | − | + |
| 4-Aminosalycylic acid | + | + | − |
| Ketorolac tromethamine | + | + | − |
| Usnic acid | + | − | + |
| Fusaric acid | ± | − | ± |
−, growth inhibition; +, no growth inhibition; ±, partial inhibition.
TABLE 2.
Growth inhibition of M. tuberculosis strains by tested compounds
| Strain | Growth inhibition (%) by: |
|||
|---|---|---|---|---|
| Ba | Su | Aza | Fu | |
| Wild type | 46.2 ± 3.7 | 39.5 ± 4.4 | 60 ± 3.7 | 14.9 ± 5.6 |
| ΔmshA | 35.5 ± 13 | 51.7 ± 7 | 87.7 ± 4.8 | 40.5 ± 14 |
| ΔegtA | 42 ± 6 | 62 ± 1.7 | 68.4 ± 0.5 | 30.16 ± 8 |
| ΔegtB | 76.5 ± 6.5 | 46 ± 11 | 54.3 ± 5 | 46.8 ± 9 |
| ΔegtD | 45 ± 6.3 | 32.4 ± 1.2 | 33.5 ± 19 | 9.5 ± 2.7 |
| ΔmshAc | 74.5 ± 5 | 70 ± 8 | 63 ± 7.4 | 76.5 ± 1.4 |
| ΔegtAc | 48 ± 3.5 | 63 ± 7 | 81 ± 13 | 29 ± 14 |
| ΔegtBc | 74.6 ± 1.5 | 43 ± 6 | 33 ± 4.3 | 48.5 ± 7 |
| ΔegtDc | 36 ± 5 | 35 ± 2.3 | 57 ± 2 | 32 ± 8 |
| Wild typea | 49.8 ± 4 | |||
| ΔegtE | 90 ± 1.7 | NDb | ND | ND |
Data from a different set of independent experiments.
ND, not done.
TABLE 3.
Levels of thiols and ROS in treated and untreated wild-type samples
| Drug treatment | Level (per 105 CFU) of: |
|||||
|---|---|---|---|---|---|---|
| IE (pg) | EE (pg) | IM (area) | EM (area) | GGC (pg) | ROS (FIb) | |
| UTa | 310.2 ± 49.8 | 67.2 ± 28.5 | 8,101.0 ± 2,819 | 18.0 ± 12.9 | 18.4 ± 10.8 | 101.6 ± 43 |
| Ba | 583.7 ± 12.5 | 758.9 ± 47 | 7,763.2 ± 483 | 118.5 ± 5.6 | 3.5 ± 0.16 | 122.3 ± 8.4 |
| Fu | 121.5 ± 15.7 | 43.4 ± 18.4 | 2,519.6 ± 412 | 12.5 ± 3.3 | 3.2 ± 0.8 | 33.4 ± 3.6 |
| Aza | 314.6 ± 29.3 | 232.0 ± 20.8 | 3,350.3 ± 161 | 0 ± 0 | 70.4 ± 11.6 | 105.3 ± 11.7 |
| Su | 8,995.5 ± 7,200 | 2,045 ± 1,532 | 90,239 ± 64,519 | 413 ± 103 | 260.1 ± 43.6 | 1,723 ± 1,089.5 |
UT, untreated.
FI, fluorescence intensity (in fluorescence units).
Ba is a cyclic polypeptide (produced by Bacillus licheniformis and Bacillus subtilis) that is included in livestock feed (31) and used as an ointment for localized skin infections (32). In bacteria, Ba binds undecaprenyl pyrophosphate (C55-PP) (33), which functions as a lipid carrier and transports peptidoglycan components across the cell membrane (34). In the presence of Ba, this transport is not complete because Ba prevents the cleavage of the pyrophosphate and consequently inhibits cell wall biosynthesis (33, 35–37). Increased growth inhibition was observed for the ERG-deficient M. smegmatis ΔegtD mutant (Table 1; see Fig. S3 in the supplemental material) and the ERG-deficient M. tuberculosis ΔegtB mutant (Table 2; see Fig. S4 in the supplemental material), but not for the ERG-deficient M. tuberculosis ΔegtD mutant (Table 2). This indicates that the increased sensitivity of these strains to Ba is not associated with the lack of ERG.
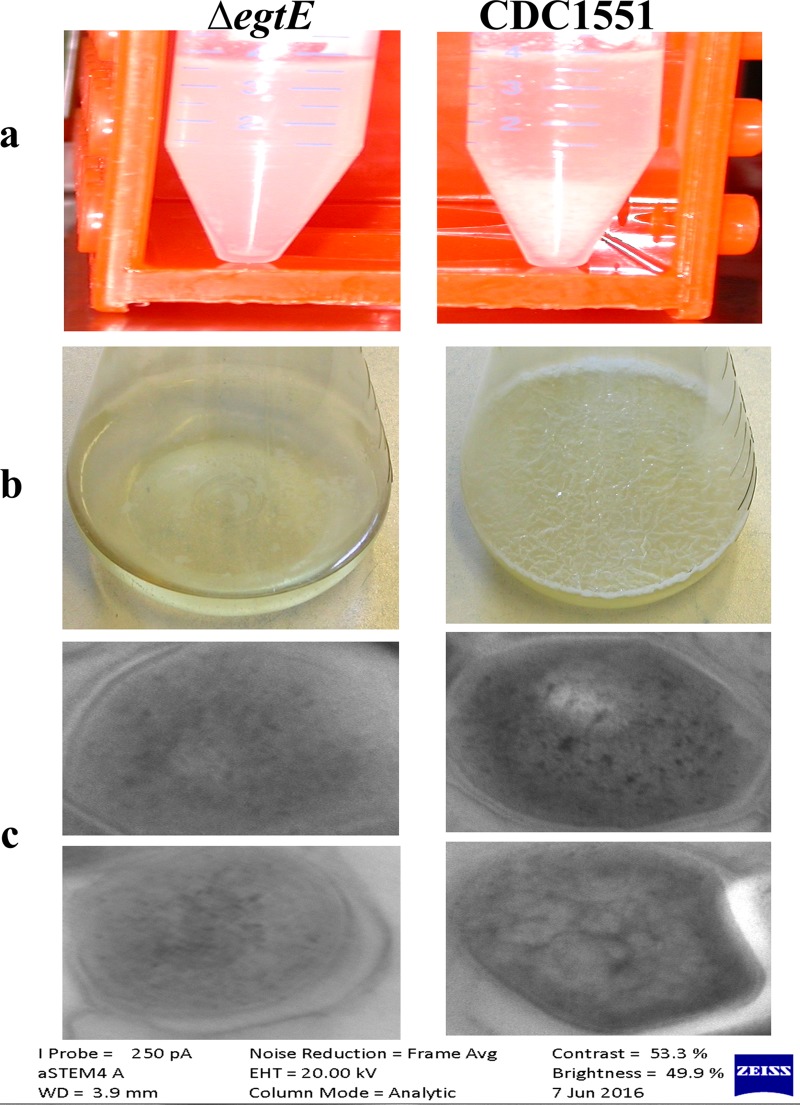
EgtE is involved in the final step of ERG biosynthesis; however, it is not essential for ERG biosynthesis, since the ΔegtE M. tuberculosis mutant still produces a significant amount of ERG (13). Interestingly, the M. tuberculosis ΔegtE mutant was more sensitive to Ba than the wild type (Table 2; see Fig. S4 in the supplemental material). The enzyme EgtE shares homology with cysteine desulfurases, and these enzymes have been implicated in the modulation of the lipid content of mycobacterial membranes (13, 38). We observed that the ΔegtE mutant did not visibly aggregate during stationary phase (Fig. 1a), indicating that EgtE may modulate the lipid constitution of M. tuberculosis. To confirm this, we further investigated the ability of the ΔegtE mutant to form a biofilm layer (39) and to be stained by osmium tetroxide (40). The ΔegtE mutant was unable to form a biofilm layer (Fig. 1b) and appeared brighter than the wild type when stained with osmium tetroxide, indicating depletion of the lipid constituents of the membrane (Fig. 1c). Slow-growing mycobacteria are less susceptible to Ba due to permeability factors, since they have a thicker lipid membrane layer (41–43) and Ba is a large molecule (1,422.9 g/mol). The increased susceptibility of the ΔegtE mutant to Ba may therefore be due to the altered membrane of the mutant.
FIG 1.
Investigation of alterations in lipid reconstitution of the ΔegtE mutant of CDC1551. (a) Effect of EgtE deficiency on CDC1551 liquid culture granularity during the stationary phase of growth. The ΔegtE mutant did not display visible aggregates, while the wild type displayed aggregates that sank to the bottom of a 50-ml tube. (b) Effect of EgtE deficiency on the ability of CDC1551 to form a biofilm layer. The CDC1551 ΔegtE mutant lost its ability to form a biofilm. (c) Transmission electron microscopy revealing that the CDC1551 wild-type strain retained darker coloration from osmium tetroxide treatment than the CDC1551 ΔegtE mutant, indicating depletion of the lipid content in the mutant.
It was previously shown that extracellular glutathione served as a defense against extracellular toxins in Saccharomyces cerevisiae cells (44). Extracellular thiols may also protect mycobacteria against extracellular toxins, such as high-molecular-weight antibiotics like Ba. Analysis of the extracellular fraction of thiols from our previously generated data (13) revealed that ∼33% of ERG and ∼7% of MSH was extracellular in the wild-type strain (see Tables S2 and S3 in the supplemental material) during exponential phase. During stationary phase, ∼23% of ERG was extracellular in the wild type, while the amount of extracellular MSH (EM) was negligible (Table 3; see Table S4 in the supplemental material). Previous reports indicated a high proportion of extracellular ERG (EE) in the wild-type M. smegmatis strain (29). This inconsistency could be strain and/or extraction method related. Quantification of ERG in M. smegmatis using the current method revealed about 40% of EE (intracellular ergothioneine [IE], ∼29.5 ± 9.8 pg/105 CFU; EE, ∼17.6 ± 1.1 pg/105 CFU) and a negligible amount of EM present during early log phase. The M. smegmatis (29) and M. tuberculosis ΔmshA mutants, as well as the M. tuberculosis ΔegtD mutant, are not sensitive to Ba (Tables 1 and 2), while the M. smegmatis ΔegtD mutant (29) (Table 1) and the M. tuberculosis ΔegtE and ΔegtB mutants showed increased sensitivity to Ba. The strains displaying increased susceptibility also produced negligible extracellular thiol (see Tables S2 to S4 in the supplemental material) (29). Specifically, EM was elevated in the M. tuberculosis ΔegtA and ΔegtD mutants, but not in the ΔegtB mutant, during the exponential and stationary phases of growth (see Tables S2 and S4 in the supplemental material), while EE was elevated in the ΔmshA mutant during the exponential and stationary phases (see Tables S3 and S4 in the supplemental material). In view of previous evidence on the general detoxification abilities of ERG (45–47) and MSH (48), it is possible that extracellular thiols protect M. tuberculosis, as well, against compounds with low membrane permeability, such as Ba. Flow cytometry analyses of the ΔegtA mutant (producing a high level of EM) and ΔmshA mutant (producing a high level of EE) revealed that the damaged membranes of these mutants were comparable to that of the wild type (Table 4). Since ∼8% of cells in the wild-type culture had damaged membranes, we cannot rule out the possibility that the thiols in the culture media could have originated from these cells. However, the observation that elevated levels of thiols were present extracellularly in the ΔegtA and ΔmshA mutants while the percentage of intact cells remained the same suggests that secretion may occur in these strains (see Tables S2 to S4 in the supplemental material). A similar phenotype was observed in S. cerevisiae, as it was shown that the wild-type strain secretes very little glutathione while specific mutants could secrete a significant amount (49). Hence, secreted thiols in the ΔegtA, ΔegtD, and ΔmshA mutants may have protected them against Ba, as previously shown with glutathione (44) (see Fig. S8 in the supplemental material). The MIC of Ba is very high (see Table S1 in the supplemental material), which necessitates a high therapeutic dose, but due to its high toxicity in mammals (50, 51), it may not be an ideal TB drug. However, to overcome these limitations, structural analogues of Ba with enhanced activities and less toxicity have been investigated (36, 52). It was previously indicated that the high MIC of Ba is associated with its low permeability, particularly in the case of Gram-positive mycobacteria (such as M. tuberculosis) that have a more complex membrane lipid constitution (41–43). The susceptibility of the ΔegtE mutant, which displays an altered membrane lipid profile (Fig. 1 and Table 3; see Fig. S3 in the supplemental material), suggests that the combination of Ba with compounds that can alter the lipid component of the mycobacterial membrane could decrease the therapeutic dose required. Isoniazid (INH) was shown to inhibit mycolic acid biosynthesis (required for the constitution of the mycobacterial cell wall) (53), and in this study, the MIC of Ba was unaltered in combination with rifampin (RIF); however, it dropped from 8,300 μg/ml to 16 μg/ml in the presence of INH, while that of INH was unaltered. This supports the hypothesis that compounds such as INH that can alter the membrane or that the cell wall of mycobacteria can enhance the activity of Ba. Ba was unable to modulate the level of oxidative stress (Table 3), suggesting that the increase in the production of extracellular thiols caused by Ba (Table 3) is not a result of an alteration in the redox state of mycobacteria and supports a role in extracellular detoxification.
TABLE 4.
Flow cytometry analyses of M. tuberculosis cultures (OD600 ≥ 1)
| Straina | No. (±SD) of: |
||
|---|---|---|---|
| DCb | LCDMc | LCIMd | |
| Wild type | 2.6 ± 0.2 | 5.3 ± 0.2 | 92 ± 0 |
| ΔegtA | 2 ± 0.4 | 4.2 ± 0.2 | 94 ± 0.2 |
| ΔmshA | 2.3 ± 0.1 | 7 ± 0.6 | 91 ± 0.5 |
| HT | 96 ± 0.1 | 0.5 ± 0 | 3.1 ± 0.1 |
| SDS | 4 ± 0.1 | 10 ± 0.1 | 86 ± 0.1 |
HT, heat-treated wild-type cells; SDS, wild-type cells treated with 0.05% SDS for ∼1 h.
DC, dead cells.
LCDM, live cells with damaged membranes.
LCIM, live cells with intact membranes.
Aza is a guanine analogue used for the treatment of acute leukemia (54). The possibility of using Aza in TB therapy has never been explored. In spite of its high toxicity, we sought to investigate its potential use in a TB regimen and to provide a rationale for the design of a less toxic analogue. Aza is one of the few compounds in the tested libraries that had increased activity on the MSH-deficient M. smegmatis ΔmshA mutant (Table 1) and on the MSH-deficient M. tuberculosisΔmshA mutant relative to the wild type (Tables 1 and 2; see Fig. S1 and S5 in the supplemental material). This suggests that the susceptibility phenotype is associated with loss of the enzyme MshA and/or depletion of MSH. Previous studies revealed that Aza is incorporated into the RNA and consequently affects protein synthesis (55, 56). More recently, Aza was identified as a competitive inhibitor of methionine adenosyltransferase, which catalyzes the formation of S-adenosylmethionine (SAM) from methionine and ATP (57). The first enzyme involved in ERG biosynthesis, EgtD, is a SAM-dependent enzyme (58), and the production of ERG (in contrast to that of MSH) increased in the Aza-treated samples (Table 3). Therefore, it is possible that Aza is able to modulate ERG production (Table 3) through the step catalyzed by EgtD, which may explain the apparent resistance of the ΔegtD mutant to Aza while its complemented strain is more susceptible (Table 2). Chloroquine is able to bind to the allosteric site (SAM-binding pocket) of SAM-dependent enzymes (59), and in this study, it was able to inhibit the activity of EgtD (see Fig. S9 and Text S1 in the supplemental material). Therefore, if Aza affects the synthesis of methionine adenosyltransferase (57) and consequently the production of SAM, the activity of EgtD may have been modulated, thus affecting the production of ERG (Table 3). However, it remains unclear why it would result in an increase (Table 3) in the production of ERG instead of a decrease. Nevertheless, this suggests that the possible modulation of ERG production by Aza through EgtD is not a straightforward mechanism and therefore warrants further investigation. It is worth noting that the production of MSH decreased, in contrast to the increase in the production of ERG (Table 3). This suggests that although Aza may be able to modulate the levels of thiols (Table 3), that of total thiols is tightly regulated at a specific level to maintain the redox state in mycobacteria. This may explain the unaltered level of oxidative stress in the Aza-treated wild-type strain (Table 3). On the other hand, the MSH-deficient ΔmshA mutant is sensitive to Aza and has been shown to produce elevated levels of ERG (13, 29, 60); therefore, modulation of ERG production in this mutant by Aza through EgtD may have altered the redox state of the mutant and caused its sensitivity. The production of MSH and ERG was restored to approximately wild-type levels in the complemented ΔmshA strain (see Tables S2 and S3 in the supplemental material) (13), which is less sensitive to Aza (Table 3), further supporting the detrimental effect of the modulation of ERG production in the ΔmshA mutant.
Su is used in veterinary medicine to treat bacillary dysentery and other enteric infections (61). Su displayed increased growth inhibition of the M. smegmatis ΔmshA mutant (Table 1) and the M. tuberculosis ΔmshA (∼52%) and ΔegtA (∼62%) mutants (Table 2; see Fig. S6 in the supplemental material). In spite of the high variation between replicates, comparison of individual replicates indicated that Su was able to increase the levels of oxidative stress and thiols simultaneously (Table 3), suggesting that Su is a pro-oxidant and that the increase in thiol levels could be to counteract the increased levels of reactive oxygen species (ROS) caused by Su. This hypothesis is supported by the increased sensitivity of the ΔegtA mutant (lacking both GGC and ERG) and the ΔmshA mutant (lacking MSH). GGC is required to detoxify ROS (13, 62, 63), while the ability of MSH to detoxify ROS is also well documented (15, 48). Su is a sulfonamide like sulfamethoxazole (SMX), which blocks folate synthesis (64). Sulfonamides are analogues of para-aminobenzoic acid (PABA), which is an intermediate in folate biosynthesis (65). Folate deficiency is associated with oxidative stress (66), which could explain the elevated level of oxidative stress generated by Su (Table 3). In this study, we were able to show that the elevated level of oxidative stress generated by Su could be alleviated by PABA (see Fig. S10 in the supplemental material). This was achieved at a concentration that was 4 times that of Su (see Fig. S10). This further demonstrates that Su is a competitive inhibitor of folate biosynthesis, as previously demonstrated with sulfanilamide, another sulfonamide (67). In addition, the ability of PABA to alleviate the oxidative stress generated by Su is associated with the ability of PABA to counteract the ability of Su to inhibit the biosynthesis of folate (causing folate deficiency and consequently oxidative stress [66]) and/or the ability of PABA to scavenge ROS, as previously shown (68). This is further supported by previous studies that demonstrated that Acetobacter xylinum subsp. sucrofermentans BPR3001E, a Su-resistant mutant, produced a high intracellular level of PABA relative to its wild-type strain, BPR2001 (69).
It was previously shown by a CFU-based method that the ΔegtA mutant is sensitive to the first-line drug RIF (10). This was confirmed in this study, as the RIF MIC for the ΔegtA mutant was less than that for the wild type (0.04 μg/ml versus 0.16 μg/ml by the broth microdilution method). The MIC was halved in the presence of Su, while that of Su was also halved in the presence of RIF, indicating an additive interaction between Su and RIF (∑ fractional inhibitory concentration [FIC], ∼1).
Su and RIF were also found to have an additive effect on the wild-type strain (∑FIC ≤ 1). However, their MICs in combination in the ΔegtA mutant (RIF, 0.02 μg/ml; Su, 1.6 μg/ml) are less than their MICs in combination in the wild-type strain (RIF 0.08 μg/ml; Su, 3.1 μg/ml). Therefore, a combination of RIF, Su, and a potential EgtA inhibitor would be more efficient than a combination of RIF and Su alone. As a proof of concept, methionine sulfoximine (MSO) (shown to partially inhibit the activity of EgtA [23]) was found to decrease the MIC of Su for the wild type in this study.
Fusaric acid (Fu) is an extract of Fusarium sp. strain DZ-27 (70). At a concentration of 80 μM, Fu was able to partially inhibit the growth of the M. smegmatis wild type and the ΔmshA mutant but completely inhibited the growth of the ΔegtD mutant based on the color of resazurin (Table 1). Further growth curve analysis of the wild type relative to the M. smegmatis ΔegtD mutant at a lower concentration, 60 μM, revealed that the growth of the wild type was inhibited to the same extent as that of the mutant at this concentration. Attempts to obtain a concentration that displayed significant growth inhibition of the M. smegmatis ΔegtD mutant relative to the wild type was unsuccessful. However, in view of previous studies indicating the potential activity of Fu against mycobacteria (70), we sought to determine if the activity of Fu is enhanced in mutant strains of M. tuberculosis, thereby providing a means for investigating a low and less toxic therapeutic dose of Fu. The M. tuberculosis ΔegtD mutant was not more sensitive to Fu, while all the other M. tuberculosis mutants tested showed increased susceptibility to Fu (Table 2; see Fig. S7 in the supplemental material). The ability of Fu to chelate metals is well documented (70, 71), and iron sequestration accounts for at least some of its activity against Pseudomonas protegens (71). Consistent with this idea, treatment of wild-type M. tuberculosis resulted in lower levels of ROS (Table 3), presumably due to a decrease in the levels of iron driving the Fenton-Weisser reaction (12) (Table 3). In addition, Fu was able to decrease the production of thiols (Table 3). Consistent with this finding, it was shown that Fu was able to decrease the production of antioxidants in human cancerous esophageal SNO cells (72). The decrease in thiol levels (Table 3) in the Fu-treated wild-type strain may be in response to a decrease in ROS; however, the reverse was not previously observed (73). Fu has several modes of action; it is able to induce lipid peroxidation and to damage the cell membrane due to its lipophilic structure (an n-butyl aliphatic side chain), is involved in metal chelation, induces the expression of genes coding for the efflux pumps (fuaABC operon), etc. (70–72, 74). Therefore, the reason for the increased susceptibility of the tested mutants to Fu remains unclear. Due to its ability to inhibit dopamine beta-monooxygenase, Fu is the major component of bupicomide, which is used in the treatment of hypertension (75, 76). Therefore, its therapeutic use has been explored in humans, but not yet as an anti-TB compound. We did not observe any interaction between Fu and the first-line drug INH, as their MICs were not altered in combination; however, the MIC of Fu decreased drastically (from 20 μg/ml to 0.16 μg/ml) in the presence of RIF, while that of RIF remained the same, indicating that RIF may enhance the activity of Fu. Further investigations are required to confirm its suitability in a TB regimen.
Conclusion.
In conclusion, this study shows for the first time the potential activities of Aza and Su against M. tuberculosis and shows that the activities of Aza, Su, and Fu are enhanced in specific M. tuberculosis mutants. In addition, though the activity of Ba was previously investigated in mycobacteria, this study suggests that the activity of the compound against M. tuberculosis can be enhanced in combination with compounds that can alter the mycobacterial membrane.
MATERIALS AND METHODS
Chemicals.
Sodium chloride, glucose, dimethyl sulfoxide (DMSO), Tween 80, tyloxapol, bacitracin, azaguanine, and sulfaguanidine were purchased from Sigma-Aldrich (St. Louis, MO, USA). Albumin was purchased from Roche Diagnostic GmbH (Mannheim, Germany) and Difco Middlebrook 7H9 from Becton Dickinson (Franklin Lakes, NJ, USA). Azaguanine was successfully dissolved in sterile double-distilled water (10 mg/ml), bacitracin was dissolved in either water or DMSO (100 mg/ml), sulfaguanidine was successfully dissolved in 50% HCl (10 mg/ml or 50 mg/ml), and fusaric acid was successfully dissolved in N,N-dimethylformamide (20 mg/ml or 5 mg/ml) to constitute stocks that were subsequently diluted in water or medium to the desired concentrations during experiments.
The phytochemical (320 compounds) and chemical (1,280 compounds) libraries were purchased from Prestwick (Illkirch, France).
Growth conditions.
M. smegmatis strains were grown to exponential phase in Difco Middlebrook 7H9 broth supplemented with 1% glucose salt (GS) solution (5% Tween 80, 8.5% NaCl, 20% glucose) and diluted to an optical density at 600 nm (OD600) of ∼0.02 to perform the screen.
M. tuberculosis wild type and mutants were grown in Middlebrook 7H9 supplemented with albumin (5 g/liter), dextrose (2 g/liter), sodium chloride (0.85 g/liter) and 0.05% tyloxapol or Tween 80.
High-throughput screening.
High-throughput screening (HTS) was performed by a Tecan Freedom EVO 200 robot equipped with a Te-Mo 363 96-channel liquid handler dispenser and a 384-pin stainless steel pin tool (V&P Scientific) with a 200-μl capillary capacity. It was set up to dispense a final volume of 150 μl of 7H9 in the first and last rows and columns of dark 96-well plates, which were used as negative controls. In addition, previous optimizations revealed that evaporation occurred in surrounding wells (the first and last rows and columns) during incubation. Therefore, to avoid spurious interpretation of results, test samples were not added to the first or last rows or columns. A volume of 50 μl of culture diluted to an OD600 of ∼0.02 was added to wells containing 50 μl of prealiquoted compounds to make up a final concentration of 80 μM. A previous optimization revealed that the compound known as Chicago blue from the chemical library interfered with resazurin during cell viability analyses. Therefore, Chicago blue was replaced with 50 μl 7H9 medium for every strain, and this was used as the positive control. Growth inhibition was evaluated with the aid of resazurin, which was added to each well to a final concentration of 0.006%. Growth inhibition was scored when the well remained blue even after 24 h. Because of the interference of the fluorescence of resazurin and colorful compounds, it is possible that some hits were missed, or the change in color of resazurin may have been misinterpreted.
Further validations were performed by analyzing the growth curves of the specific M. smegmatis mutants as follows. Mycobacteria prepared in dark 96-well plates with the specific compounds were incubated in a plate reader (Infinite 200) with the following specifications: application, Tecan i-control; target temperature, 37°C; shaking (orbital) duration, 300 s; shaking (orbital) amplitude, 2.5 mm; mode, absorbance; wavelength, 600 nm; bandwidth, 9 nm; number of flashes, 25; settle time, 0 ms; interval time, 4 h.
Drug sensitivity testing of M. tuberculosis mutants.
The MICs for the CDC1551 wild-type strain of identified hits were investigated using the broth microdilution method at an OD600 of ∼0.002, as previously described (31). The average MIC (the mean of the concentrations in the well with complete growth inhibition [GI] and the adjacent well with no visible growth inhibition) was used for downstream investigations. Liquid cultures containing various drugs at the predetermined average MIC were inoculated to an initial OD600 of 0.05 with different strains and incubated for 14 days at 37°C without shaking. The OD600 was measured every other day to obtain a growth profile of each strain in the presence of the tested compounds. In addition, the growth inhibition percentage was evaluated relative to the untreated cultures on the 15th day of growth. At that stage, the wild-type cultures were serially diluted and plated for CFU counting to normalize data for downstream experiments. This experiment was repeated at least twice for each drug for every strain tested.
Quantification of total ROS.
A volume of 1 ml of treated and untreated wild-type cultures from the experiment described above was treated with a final concentration of 10 μM DCFDA (2′,7′-dichlorofluorescein diacetate) (77) and aliquoted in a black 96-well plate with an optical bottom (100 μl per well). The plate was incubated for 70 min at 37°C in a plate reader. The fluorescence intensity at an excitation wavelength of 488 nm and an emission wavelength of 520 nm was recorded and standardized to the CFU.
Quantification of thiols.
Volumes of 5 ml of the untreated and treated wild-type cultures were pelleted, and 1 ml of the supernatants was filtered twice and frozen. The pellet was washed once in 5 ml of double-distilled sterile water and frozen. The supernatants were lyophilized overnight. The lyophilized supernatants and the frozen pellets were resuspended in 500 μl of the lysis buffer (50% acetonitrile, 20 mM HEPES, pH ∼8, 2 mM monobromobimane) and sonicated at 60°C in a water bath sonicator for 30 min protected from light. Lysed samples were centrifuged, and the supernatants were filtered and acidified to a final concentration of ∼1 mM acetic acid. The acidified samples were processed for ultraperformance liquid chromatography-mass spectrometry (UPLC-MS) analyses for the quantification of MSH, ERG, and GGC as previously described (29).
Flow cytometry analysis of the mycobacterial membrane.
Analysis of M. tuberculosis membrane integrity was performed with a LIVE/DEAD Baclight bacterial viability and counting kit (Molecular Probes Life Technologies), as previously described (29). Briefly, 1 ml of exponential-phase M. tuberculosis culture was pelleted, washed, and resuspended in 150 mM NaCl, 22 μM propidium iodide (PI), and 5 μM Syto9. One hundred microliters of the cell suspensions was added to BD Falcon tubes containing 977.4 μl of the running buffer. Stained M. tuberculosis cells were analyzed using a BD FACSJazz cell sorter (Becton Dickinson Biosciences, Belgium) with linear amplifiers to measure forward light scatter (FSC) and logarithmic amplifiers for side scatter and all fluorescence measurements.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge Novartis (Basel, Switzerland), the DST/NRF Centre of Excellence in Biomedical Tuberculosis Research (South Africa), and the SAMRC Centre for Tuberculosis Research for funding this project.
We also acknowledge Miriam Alloza Trabado and Silvia Speroni for their technical assistance during the HTS; Malcolm Taylor for performing the liquid chromatography (LC)-MS; and John Michie, Andrea Gutschmidt, and Ronald Dreyer for their technical assistance during flow cytometry analyses. We thank Mohamed Jaffer and the Electron Microscope Unit, Centre for Imaging and Analysis of the University of Cape Town, and Dumisile Lumkwana and the Imaging Unit of the Central Analytical Facility of Stellenbosch University for processing the samples for electron microscopy.
C.S.E., M.J.W., I.W., C.C., and B.B. wrote and revised the manuscript. C.S.E., M.J.W., I.W., C.C., and B.B. conceived the experiments. C.S.E. and C.C. designed the experiments. C.S.E. performed experiments and analyzed the data. Acquisition of funding to perform this study was done by C.S.E., M.J.W., I.W., C.C., and B.B.
We declare no competing financial interests.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02236-17.
REFERENCES
- 1.WHO. 2017. Global tuberculosis report 2017. WHO, Geneva, Switzerland: http://apps.who.int/iris/bitstream/10665/259366/1/9789241565516-eng.pdf. [Google Scholar]
- 2.Panteleev AM, Ivanov AK, Vinogradova EN, Fomenkova NV, Suprun TI. 2005. Analysis of death rates in patients with tuberculosis and HIV infection. Probl Tuberk Bolezn Legk 10:46–48. (In Russian.) [PubMed] [Google Scholar]
- 3.CDC. 2009. Plan to combat extensively drug-resistant tuberculosis: recommendations of the Federal Tuberculosis Task Force. MMWR Recomm Rep 58:1–43. [PubMed] [Google Scholar]
- 4.CDC. 2006. Emergence of Mycobacterium tuberculosis with extensive resistance to second-line drugs—worldwide, 2000-2004. MMWR Morb Mortal Wkly Rep 55:301–305. [PubMed] [Google Scholar]
- 5.WHO. 2014. Global tuberculosis report 2014. WHO, Geneva, Switzerland: http://apps.who.int/iris/bitstream/10665/137094/1/9789241564809_eng.pdf. [Google Scholar]
- 6.WHO ST Partnership. 2012. Annual meeting of childhood TB subgroup, 11 November 2012. http://www.stoptb.org.
- 7.Naing NN, D'Este C, Isa AR, Salleh R, Bakar N, Mahmod MR. 2001. Factors contributing to poor compliance with anti-TB treatment among tuberculosis patients. Southeast Asian J Trop Med Public Health 32:369–382. [PubMed] [Google Scholar]
- 8.Lertmaharit S, Kamol-Ratankul P, Sawert H, Jittimanee S, Wangmanee S. 2005. Factors associated with compliance among tuberculosis patients in Thailand. J Med Assoc Thai 88(Suppl 4):S149–S156. [PubMed] [Google Scholar]
- 9.Richard-Greenblatt M, Bach H, Adamson J, Pena-Diaz S, Wu L, Steyn AJC, Av-Gay Y. 2015. Regulation of ergothioneine biosynthesis and its effect on Mycobacterium tuberculosis growth and infectivity. J Biol Chem 290:23064–23076. doi: 10.1074/jbc.M115.648642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saini V, Cumming BM, Guidry L, Lamprecht DA, Adamson JH, Reddy VP, Chinta KC, Mazorodze JH, Glasgow JN, Richard-Greenblatt M, Gomez-Velasco A, Bach H, Av-Gay Y, Eoh H, Rhee K, Steyn AJC. 2016. Ergothioneine maintains redox and bioenergetic homeostasis essential for drug susceptibility and virulence of Mycobacterium tuberculosis. Cell Rep 14:572–585. doi: 10.1016/j.celrep.2015.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buchmeier NA, Newton GL, Koledin T, Fahey RC. 2003. Association of mycothiol with protection of Mycobacterium tuberculosis from toxic oxidants and antibiotics. Mol Microbiol 47:1723–1732. doi: 10.1046/j.1365-2958.2003.03416.x. [DOI] [PubMed] [Google Scholar]
- 12.Vilchèze C, Hartman T, Weinrick B, Jacobs WRJ. 2013. Mycobacterium tuberculosis is extraordinarily sensitive to killing by a vitamin C-induced Fenton reaction. Nat Commun 4:1881. doi: 10.1038/ncomms2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sao Emani C, Williams MJ, Van Helden PD, Taylor MJC, Wiid IJ, Baker B. 2018. Gamma-glutamylcysteine protects ergothioneine-deficient Mycobacterium tuberculosis mutants against oxidative and nitrosative stress. Biochem Biophys Res Commun 495:174–178. doi: 10.1016/j.bbrc.2017.10.163. [DOI] [PubMed] [Google Scholar]
- 14.Frantom PA, Coward JK, Blanchard JS. 2010. UDP-(5F)-GlcNAc acts as a slow-binding inhibitor of MshA, a retaining glycosyltransferase. J Am Chem Soc 132:6626–6627. doi: 10.1021/ja101231a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vilchèze C, Av-Gay Y, Attarian R, Liu Z, Hazbón MH, Colangeli R, Chen B, Liu W, Alland D, Sacchettini JC, Jacobs WRJ. 2008. Mycothiol biosynthesis is essential for ethionamide susceptibility in Mycobacterium tuberculosis. Mol Microbiol 69:1316–1329. doi: 10.1111/j.1365-2958.2008.06365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rawat M, Newton GL, Ko M, Martinez GJ, Fahey RC, Av-Gay Y. 2002. Mycothiol-deficient Mycobacterium smegmatis mutants are hypersensitive to alkylating agents, free radicals, and antibiotics. Antimicrob Agents Chemother 46:3348–3355. doi: 10.1128/AAC.46.11.3348-3355.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rawat M, Johnson C, Cadiz V, Av-Gay Y. 2007. Comparative analysis of mutants in the mycothiol biosynthesis pathway in Mycobacterium smegmatis. Biochem Biophys Res Commun 363:71–76. doi: 10.1016/j.bbrc.2007.08.142. [DOI] [PubMed] [Google Scholar]
- 18.Vilchèze C, Av-Gay Y, Barnes SW, Larsen MH, Walker JR, Glynne RJ, Jacobs WR. 2011. Coresistance to isoniazid and ethionamide maps to mycothiol biosynthetic genes in Mycobacterium bovis. Antimicrob Agents Chemother 55:4422–4423. doi: 10.1128/AAC.00564-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sareen D, Newton GL, Fahey RC, Buchmeier NA. 2003. Mycothiol is essential for growth of Mycobacterium tuberculosis Erdman. J Bacteriol 185:6736–6740. doi: 10.1128/JB.185.22.6736-6740.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newton GL, Buchmeier N, La Clair JJ, Fahey RC. 2011. Evaluation of NTF1836 as an inhibitor of the mycothiol biosynthetic enzyme MshC in growing and non-replicating Mycobacterium tuberculosis. Bioorg Med Chem 19:3956–3964. doi: 10.1016/j.bmc.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gutierrez-Lugo M-T, Baker H, Shiloach J, Boshoff H, Bewley CA. 2009. Dequalinium, a new inhibitor of Mycobacterium tuberculosis mycothiol ligase identified by high-throughput screening. J Biomol Screen 14:643–652. doi: 10.1177/1087057109335743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newton GL, Ta P, Sareen D, Fahey RC. 2006. A coupled spectrophotometric assay for l-cysteine:1-d-myo-inosityl 2-amino-2-deoxy-alpha-d-glucopyranoside ligase and its application for inhibitor screening. Anal Biochem 353:167–173. doi: 10.1016/j.ab.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 23.Harth G, Maslesa-Galić S, Tullius MV, Horwitz MA. 2005. All four Mycobacterium tuberculosis glnA genes encode glutamine synthetase activities but only GlnA1 is abundantly expressed and essential for bacterial homeostasis. Mol Microbiol 58:1157–1172. doi: 10.1111/j.1365-2958.2005.04899.x. [DOI] [PubMed] [Google Scholar]
- 24.Griffith OW, Meister A. 1979. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine). J Biol Chem 254:7558–7560. [PubMed] [Google Scholar]
- 25.Venketaraman V, Rodgers T, Linares R, Reilly N, Swaminathan S, Hom D, Millman AC, Wallis R, Connell ND. 2006. Glutathione and growth inhibition of Mycobacterium tuberculosis in healthy and HIV infected subjects. AIDS Res Ther 3:5. doi: 10.1186/1742-6405-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dayaram YK, Talaue MT, Connell ND, Venketaraman V. 2006. Characterization of a glutathione metabolic mutant of Mycobacterium tuberculosis and its resistance to glutathione and nitrosoglutathione. J Bacteriol 188:1364–1372. doi: 10.1128/JB.188.4.1364-1372.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Venketaraman V, Dayaram YK, Amin AG, Ngo R, Green RM, Talaue MT, Mann J, Connell ND. 2003. Role of glutathione in macrophage control of mycobacteria. Infect Immun 71:1864–1871. doi: 10.1128/IAI.71.4.1864-1871.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hibi T, Nii H, Nakatsu T, Kimura A, Kato H, Hiratake J, Oda J. 2004. Crystal structure of γ-glutamylcysteine synthetase: insights into the mechanism of catalysis by a key enzyme for glutathione homeostasis. Proc Natl Acad Sci U S A 101:15052–15057. doi: 10.1073/pnas.0403277101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sao Emani C, Williams MJ, Wiid IJ, Hiten NF, Viljoen AJ, Pietersen R-DD, van Helden PD, Baker B. 2013. Ergothioneine is a secreted antioxidant in Mycobacterium smegmatis. Antimicrob Agents Chemother 57:3202–3207. doi: 10.1128/AAC.02572-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newton GL, Koledin T, Gorovitz B, Rawat M, Fahey RC, Av-Gay Y. 2003. The glycosyltransferase gene encoding the enzyme catalyzing the first step of mycothiol biosynthesis (mshA). J Bacteriol 185:3476–3479. doi: 10.1128/JB.185.11.3476-3479.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarmah AK, Meyer MT, Boxall ABA. 2006. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 65:725–759. doi: 10.1016/j.chemosphere.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 32.Drucker CR. 2012. Update on topical antibiotics in dermatology. Dermatol Ther 25:6–11. doi: 10.1111/j.1529-8019.2012.01493.x. [DOI] [PubMed] [Google Scholar]
- 33.Stone KJ, Strominger JL. 1971. Mechanism of action of bacitracin: complexation with metal ion and C55-isoprenyl pyrophosphate. Proc Natl Acad Sci U S A 68:3223–3227. http://www.pnas.org/content/68/12/3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valvano MA. 2008. Undecaprenyl phosphate recycling comes of age. Mol Microbiol 67:232–235. doi: 10.1111/j.1365-2958.2007.06052.x. [DOI] [PubMed] [Google Scholar]
- 35.Ming L-J, Epperson JD. 2002. Metal binding and structure-activity relationship of the metalloantibiotic peptide bacitracin. J Inorg Biochem 91:46–58. doi: 10.1016/S0162-0134(02)00464-6. [DOI] [PubMed] [Google Scholar]
- 36.Economou NJ, Cocklin S, Loll PJ. 2013. High-resolution crystal structure reveals molecular details of target recognition by bacitracin. Proc Natl Acad Sci U S A 110:14207–14212. doi: 10.1073/pnas.1308268110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kingston AW, Zhao H, Cook GM, Helmann JD. 2014. Accumulation of heptaprenyl diphosphate sensitizes Bacillus subtilis to bacitracin: implications for the mechanism of resistance mediated by the BceAB transporter. Mol Microbiol 93:37–49. doi: 10.1111/mmi.12637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rybniker J, Pojer F, Marienhagen J, Kolly GS, Chen JM, van Gumpel E, Hartmann P, Cole ST. 2014. The cysteine desulfurase IscS of Mycobacterium tuberculosis is involved in iron-sulfur cluster biogenesis and oxidative stress defence. Biochem J 459:467–478. doi: 10.1042/BJ20130732. [DOI] [PubMed] [Google Scholar]
- 39.Ojha AK, Baughn AD, Sambandan D, Hsu T, Trivelli X, Guerardel Y, Alahari A, Kremer L, Jacobs WR, Hatfull GF. 2008. Growth of Mycobacterium tuberculosis biofilms containing free mycolic acids and harbouring drug-tolerant bacteria. Mol Microbiol 69:164–174. doi: 10.1111/j.1365-2958.2008.06274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scheller EL, Troiano N, VanHoutan JN, Bouxsein MA, Fretz JA, Xi Y, Nelson T, Katz G, Berry R, Church CD, Doucette CR, Rodeheffer MS, MacDougald OA, Rosen CJ, Horowitz MC. 2014. Use of osmium tetroxide staining with microcomputerized tomography to visualize and quantify bone marrow adipose tissue in vivo. Methods Enzymol 537:123–139. doi: 10.1016/B978-0-12-411619-1.00007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rieber M, Imaeda T, Cesari IM. 1969. Bacitracin action on membranes of mycobacteria. J Gen Microbiol 55:155–159. doi: 10.1099/00221287-55-1-155. [DOI] [PubMed] [Google Scholar]
- 42.Imaeda T, Kanetsuna F, Galindo B. 1968. Ultrastructure of cell walls of genus Mycobacterium. J Ultrastruct Res 25:46–63. doi: 10.1016/S0022-5320(68)80059-0. [DOI] [PubMed] [Google Scholar]
- 43.Imaeda T, Ogura M. 1963. Formation of intracytoplasmic membrane system of mycobacteria related to cell division. J Bacteriol 85:150–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thorsen M, Jacobson T, Vooijs R, Navarrete C, Bliek T, Schat H, Tamás MJ. 2012. Glutathione serves an extracellular defence function to decrease arsenite accumulation and toxicity in yeast. Mol Microbiol 84:1177–1188. doi: 10.1111/j.1365-2958.2012.08085.x. [DOI] [PubMed] [Google Scholar]
- 45.Akanmu D, Cecchini R, Aruoma OI, Halliwell B. 1991. The antioxidant action of ergothioneine. Arch Biochem Biophys 288:10–16. doi: 10.1016/0003-9861(91)90158-F. [DOI] [PubMed] [Google Scholar]
- 46.Obayashi K, Kurihara K, Okano Y, Masaki H, Yarosh DB. 2005. l-Ergothioneine scavenges superoxide and singlet oxygen and suppresses TNF-alpha and MMP-1 expression in UV-irradiated human dermal fibroblasts. J Cosmet Sci 56:17–27. [PubMed] [Google Scholar]
- 47.Hanlon DP. 1971. Interaction of ergothioneine with metal ions and metalloenzymes. J Med Chem 14:1084–1087. doi: 10.1021/jm00293a017. [DOI] [PubMed] [Google Scholar]
- 48.Liu Y-B, Long M-X, Yin Y-J, Si M-R, Zhang L, Lu Z-Q, Wang Y, Shen X-H. 2013. Physiological roles of mycothiol in detoxification and tolerance to multiple poisonous chemicals in Corynebacterium glutamicum. Arch Microbiol 195:419–429. doi: 10.1007/s00203-013-0889-3. [DOI] [PubMed] [Google Scholar]
- 49.Perrone GG, Grant CM, Dawes IW. 2005. Genetic and environmental factors influencing glutathione homeostasis in Saccharomyces cerevisiae. Mol Biol Cell 16:218–230. doi: 10.1091/mbc.E04-07-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Michie AJ, Zintel HA. 1949. The nephrotoxicity of bacitracin in man. Surgery 26:626–632. [PubMed] [Google Scholar]
- 51.Scudi JV, Coret IA, Antopol W. 1947. Some pharmacological characteristics of bacitracin; chronic toxicity studies of commercial bacitracin in the dog and monkey. Proc Soc Exp Biol Med 66:558–561. doi: 10.3181/00379727-66-16156. [DOI] [PubMed] [Google Scholar]
- 52.Wagner B, Schumann D, Linne U, Koert U, Marahiel MA. 2006. Rational design of bacitracin A derivatives by incorporating natural product derived heterocycles. J Am Chem Soc 128:10513–10520. doi: 10.1021/ja062906w. [DOI] [PubMed] [Google Scholar]
- 53.Quémard A, Lacave C, Lanéelle G. 1991. Isoniazid inhibition of mycolic acid synthesis by cell extracts of sensitive and resistant strains of Mycobacterium aurum. Antimicrob Agents Chemother 35:1035–1039. doi: 10.1128/AAC.35.6.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dourado M, Sarmento AB, Pereira SV, Alves V, Silva T, Pinto AM, Rosa MS. 2007. CD26/DPPIV expression and 8-azaguanine response in T-acute lymphoblastic leukaemia cell lines in culture. Pathophysiology 14:3–10. doi: 10.1016/j.pathophys.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 55.Lasnitzki I, Matthews RE, Smith JD. 1954. Incorporation of 8-azaguanine into nucleic acids. Nature 173:346–348. doi: 10.1038/173346a0. [DOI] [PubMed] [Google Scholar]
- 56.Mandel HG, Markham R. 1958. The effects of 8-azaguanine on the biosynthesis of ribonucleic acid in Bacillus cereus. Biochem J 69:297–306. doi: 10.1042/bj0690297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berger BJ, Knodel MH. 2003. Characterisation of methionine adenosyltransferase from Mycobacterium smegmatis and M. tuberculosis. BMC Microbiol 3:12. doi: 10.1186/1471-2180-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seebeck FP. 2010. In vitro reconstitution of Mycobacterial ergothioneine biosynthesis. J Am Chem Soc 132:6632–6633. doi: 10.1021/ja101721e. [DOI] [PubMed] [Google Scholar]
- 59.Pacifici GM, Donatelli P, Giuliani L. 1992. Histamine N-methyl transferase: inhibition by drugs. Br J Clin Pharmacol 34:322–327. doi: 10.1111/j.1365-2125.1992.tb05637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ta P, Buchmeier N, Newton GL, Rawat M, Fahey RC. 2011. Organic hydroperoxide resistance protein and ergothioneine compensate for loss of mycothiol in Mycobacterium smegmatis mutants. J Bacteriol 193:1981–1990. doi: 10.1128/JB.01402-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Black S, Overman RS, Elvehjem CA, Link KP. 1942. The effect of sulfaguanidine on rat growth and plasma prothrombin. J Biol Chem 145:137–143. [Google Scholar]
- 62.Quintana-Cabrera R, Fernandez-Fernandez S, Bobo-Jimenez V, Escobar J, Sastre J, Almeida A, Bolaños JP. 2012. γ-Glutamylcysteine detoxifies reactive oxygen species by acting as glutathione peroxidase-1 cofactor. Nat Commun 3:718. doi: 10.1038/ncomms1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mukherjee A, Roy G, Guimond C, Ouellette M. 2009. The gamma-glutamylcysteine synthetase gene of Leishmania is essential and involved in response to oxidants. Mol Microbiol 74:914–927. doi: 10.1111/j.1365-2958.2009.06907.x. [DOI] [PubMed] [Google Scholar]
- 64.Chakraborty S, Gruber T, Barry CE, Boshoff HI, Rhee KY. 2013. Para-aminosalicylic acid acts as an alternative substrate of folate metabolism in Mycobacterium tuberculosis. Science 339:88–91. doi: 10.1126/science.1228980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Minato Y, Thiede JM, Kordus SL, McKlveen EJ, Turman BJ, Baughn AD. 2015. Mycobacterium tuberculosis folate metabolism and the mechanistic basis for para-aminosalicylic acid susceptibility and resistance. Antimicrob Agents Chemother 59:5097–5106. doi: 10.1128/AAC.00647-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pravenec M, Kožich V, Krijt J, Sokolová J, Zídek V, Landa V, Sǐmáková M, Mlejnek P, Sǐlhavý J, Oliyarnyk O, Kazdová L, Kurtz TW. 2013. Folate deficiency is associated with oxidative stress, increased blood pressure, and insulin resistance in spontaneously hypertensive rats. Am J Hypertens 26:135–140. doi: 10.1093/ajh/hps015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Woods DD. 1940. The relation of p-aminobenzoic acid to the mechanism of the action of sulphanilamide. Br J Exp Pathol 21:74–90. [Google Scholar]
- 68.Hu M-L, Chen Y-K, Chen L-C, Sano M. 1995. Para-aminobenzoic acid scavenges reactive oxygen species and protects DNA against UV and free radical damage. J Nutr Biochem 6:504–508. doi: 10.1016/0955-2863(95)00082-B. [DOI] [Google Scholar]
- 69.Ishikawa A, Tsuchida T, Yoshinaga F. 1998. Relationship between sulfaguanidine resistance and increased cellulose production in Acetobacter xylinum BPR3001E. Biosci Biotechnol Biochem 62:1234–1236. doi: 10.1271/bbb.62.1234. [DOI] [PubMed] [Google Scholar]
- 70.Pan J-H, Chen Y, Huang Y-H, Tao Y-W, Wang J, Li Y, Peng Y, Dong T, Lai X-M, Lin Y-C. 2011. Antimycobacterial activity of fusaric acid from a mangrove endophyte and its metal complexes. Arch Pharm Res 34:1177–1181. doi: 10.1007/s12272-011-0716-9. [DOI] [PubMed] [Google Scholar]
- 71.Ruiz JA, Bernar EM, Jung K. 2015. Production of siderophores increases resistance to fusaric acid in Pseudomonas protegens Pf-5. PLoS One 10:e0117040. doi: 10.1371/journal.pone.0117040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Devnarain N, Tiloke C, Nagiah S, Chuturgoon AA. 2017. Fusaric acid induces oxidative stress and apoptosis in human cancerous oesophageal SNO cells. Toxicon 126:4–11. doi: 10.1016/j.toxicon.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 73.Ung KSE, Av-Gay Y. 2006. Mycothiol-dependent mycobacterial response to oxidative stress. FEBS Lett 580:2712–2716. doi: 10.1016/j.febslet.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 74.Hu R-M, Liao S-T, Huang C-C, Huang Y-W, Yang T-C. 2012. An inducible fusaric acid tripartite efflux pump contributes to the fusaric acid resistance in Stenotrophomonas maltophilia. PLoS One 7:e51053. doi: 10.1371/journal.pone.0051053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chrysant SG, Adamopoulos P, Tsuchiya M, Frohlich ED. 1976. Systemic and renal hemodynamic effects of bupicomide: a new vasodilator. Am Heart J 92:335–339. doi: 10.1016/S0002-8703(76)80114-7. [DOI] [PubMed] [Google Scholar]
- 76.Velasco M, Gilbert CA, Rutledge CO, McNay JL. 1975. Antihypertensive effect of a dopamine beta hydroxylase inhibitor, bupicomide: a comparison with hydralazine. Clin Pharmacol Ther 18:145–153. doi: 10.1002/cpt1975182145. [DOI] [PubMed] [Google Scholar]
- 77.Hsieh C-Y, Chen C-L, Yang K-C, Ma C-T, Choi P-C, Lin C-F. 2015. Detection of reactive oxygen species during the cell cycle under normal culture conditions using a modified fixed-sample staining method. J Immunoassay Immunochem 36:149–161. doi: 10.1080/15321819.2014.910806. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.