ABSTRACT
Increasing antimicrobial resistance among uropathogens limits treatment options for patients with complicated urinary tract infection (cUTI). Plazomicin, a new aminoglycoside, has in vitro activity against multidrug-resistant Enterobacteriaceae, including isolates resistant to currently available aminoglycosides, as well as extended-spectrum β-lactamase-producing and carbapenem-resistant Enterobacteriaceae. We evaluated the efficacy and safety of plazomicin in a double-blind, comparator-controlled, phase 2 study in adults with cUTI or acute pyelonephritis. Patients were randomized 1:1:1 to receive intravenous plazomicin (10 or 15 mg/kg of body weight) or intravenous levofloxacin (750 mg) once daily for 5 days. Coprimary efficacy endpoints were microbiological eradication at the test of cure (TOC; 5 to 12 days after the last dose) in the modified intent-to-treat (MITT) and microbiologically evaluable (ME) populations. Overall, 145 patients were randomized to treatment. In the groups receiving plazomicin at 10 mg/kg, plazomicin at 15 mg/kg, and levofloxacin, microbiological eradication rates were, respectively, 50.0% (6 patients with microbiological eradication at TOC/12 patients treated [95% confidence interval {CI}, 21.1 to 78.9%]), 60.8% (31/51 [95% CI, 46.1 to 74.2%]), and 58.6% (17/29 [95% CI, 38.9 to 76.5%]) in the MITT population and 85.7% (6/7 [95% CI, 42.1 to 99.6%]), 88.6% (31/35 [95% CI, 73.3 to 96.8%]), and 81.0% (17/21 [95% CI, 58.1 to 94.6%]) in the ME population. In the MITT population, 66.7% (95% CI, 34.9 to 90.1%), 70.6% (95% CI, 56.2 to 82.5%), and 65.5% (95% CI, 45.7 to 82.1%) of the patients in the three groups, respectively, were assessed by the investigator to be clinically cured at TOC. Adverse events were reported in 31.8%, 35.1%, and 47.7% of the patients in the three groups, respectively. Serum creatinine values were generally stable over the course of the study. No plazomicin-treated patients with evaluable audiometry data had postbaseline sensorineural, conductive, or mixed hearing loss. In summary, plazomicin demonstrated microbiological and clinical success and an overall safety profile supportive of further clinical development. (This study has been registered at ClinicalTrials.gov under identifier NCT01096849.)
KEYWORDS: aminoglycosides, antibacterial therapy, clinical trials, complicated urinary tract infection, plazomicin, pyelonephritis
INTRODUCTION
Bacterial urinary tract infections (UTIs) are common in both community and hospital settings and pose a substantial burden to patients and health care systems (1, 2). Complicated UTIs (cUTIs) and acute pyelonephritis (AP) can be challenging to treat and often require parenteral therapy (3).
The majority of cUTIs are caused by Enterobacteriaceae, most commonly, Escherichia coli (4). Treatment of these infections is increasingly challenging due to the global rise of antibacterial resistance (4–9). Multidrug-resistant (MDR) Enterobacteriaceae, including extended-spectrum β-lactamase (ESBL)- and carbapenemase-producing organisms, limit treatment options for patients with cUTIs (7, 8, 10–13). Antibiotic-resistant Enterobacteriaceae causing cUTIs are associated with lengthy hospital stays, increased health care expenditures, and elevated rates of mortality (1, 14–19). New antibiotics for the treatment of infections due to MDR Enterobacteriaceae are urgently needed (20, 21).
Aminoglycosides are a well-established class of antibiotics that are especially useful in the treatment of serious infections caused by Gram-negative bacteria due to their rapid, concentration-dependent bactericidal action and ability to act synergistically with other antibiotics (22). Although the use of aminoglycosides has declined over the years due to concerns about toxicity, they have recently reemerged as a practical approach to treating patients with cUTIs caused by MDR Gram-negative bacteria (23–25). Ideally, aminoglycosides are dosed once daily, a treatment strategy that has been shown to reduce toxicity while maintaining efficacy compared with multiple daily doses (26–29). However, data from large, prospective randomized controlled trials evaluating the safety and efficacy of once-daily aminoglycosides in cUTIs are limited (30).
Plazomicin is a new aminoglycoside derived from sisomicin with structural modifications that protect it from aminoglycoside-modifying enzymes, which are the most common mechanism of resistance to older aminoglycosides (e.g., amikacin, gentamicin, tobramycin) in Enterobacteriaceae (31). In vitro studies have shown that plazomicin is rapidly bactericidal and has potent activity against MDR Enterobacteriaceae, including aminoglycoside-resistant (31–33), ESBL-producing (34–36), and carbapenem-resistant (37–40) Enterobacteriaceae.
This phase 2 study evaluated the efficacy and safety of once-daily plazomicin versus once-daily levofloxacin for the treatment of adult patients with cUTIs or AP.
(Part of this research was previously presented at the 52nd Interscience Conference on Antimicrobial Agents and Chemotherapy, 9 to 12 September 2012, San Francisco, CA [41], and the 25th Annual European Congress of Clinical Microbiology and Infectious Diseases, 25 to 28 April 2015, Copenhagen, Denmark.)
RESULTS
Patient disposition and baseline characteristics.
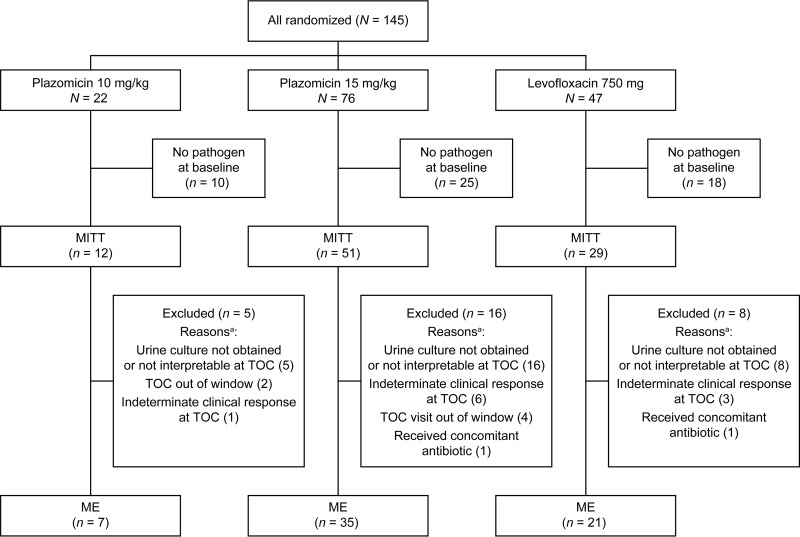
A total of 145 patients were randomized to treatment (22, 76, and 47 to treatment with plazomicin at 10 mg/kg, plazomicin at 15 mg/kg, and levofloxacin, respectively); 125 (86.2%) completed the study; 5 did not receive study drug. Of the randomized patients, 92 (63.4%) qualified for inclusion in the modified intent-to-treat (MITT) population and 63 (43.4%) qualified for inclusion in the microbiologically evaluable (ME) population (Fig. 1). The safety population included 140 patients who received at least one dose of study drug.
FIG 1.
Patient disposition and analysis populations. a, patients could have more than one reason for exclusion.
Twenty patients were prematurely withdrawn from the study for the following reasons: consent was withdrawn (n = 7), loss to follow-up (n = 5), administrative issues (n = 3), a lack of efficacy (n = 2), adverse events (AEs) (n = 2), and investigator decision (n = 1). The two patients who were prematurely withdrawn from the study due to a lack of efficacy (both of whom were in the group receiving plazomicin at 15 mg/kg) were included in the safety and MITT populations but not the ME population; both were counted as clinical failures at the test of cure (TOC), at which point neither had an interpretable urine culture result. The two patients who were prematurely withdrawn from the study due to AEs (one in the levofloxacin group, who required additional antibiotics for bacteremia, and one in the group receiving plazomicin at 15 mg/kg) were included in the safety population; both patients were clinical failures at TOC.
The demographic and baseline characteristics for the MITT population were similar across treatment groups (Table 1). The patients had a mean age of 42.4 years (range, 18 to 82 years), 83.7% were women, and 48.9% had a diagnosis of AP.
TABLE 1.
Patient baseline characteristics (MITT population)
| Characteristic | Values for patients receiving: |
||
|---|---|---|---|
| Plazomicin at 10 mg/kg (n = 12) | Plazomicin at 15 mg/kg (n = 51) | Levofloxacin at 750 mg (n = 29) | |
| Mean ± SD age (yr) | 41.5 ± 20.02 | 39.5 ± 15.2 | 47.9 ± 15.1 |
| No. (%) of female patients | 10 (83.3) | 42 (82.4) | 25 (86.2) |
| No. (%) of patients from the following region: | |||
| North America | 4 (33.3) | 27 (52.9) | 15 (51.7) |
| India | 5 (41.7) | 12 (23.5) | 6 (20.7) |
| Latin America | 3 (25.0) | 12 (23.5) | 8 (27.6) |
| Mean ± SD body wt (kg) | 66.4 ± 15.9 | 68.6 ± 14.5 | 72.4 ± 13.9 |
| No. (%) of patients with ≥1 prior/ongoing medical disorder | 11 (91.7) | 44 (86.3) | 20 (69.0) |
| No. (%) of patients with the following primary diagnosis: | |||
| AP | 4 (33.3) | 24 (47.1) | 17 (58.6) |
| cUTI | 8 (66.7) | 27 (52.9) | 12 (41.4) |
| No. of patients with cUTI with indwelling catheter/total no. of patients with cUTI (%) | 1/8 (12.5) | 4/27 (14.8) | 1/12 (8.3) |
| Mean ± SD estimated CLCR (ml/min)a | 109.1 ± 27.8 | 115.4 ± 54.3 | 108.3 ± 39.4 |
CLCR was estimated for the safety population using the Cockcroft-Gault formula, as follows: CLCR = {([140 − age] × weight)/(72 × serum creatinine concentration)} × 0.85 (if female), where weight is in kilograms and the serum creatinine concentration is in milligrams per deciliter.
Pathogens at baseline.
In the MITT population, the most commonly isolated organisms were Enterobacteriaceae (n = 84), of which 69 were E. coli and 7 were Klebsiella pneumoniae. Only one patient (randomized to the group receiving plazomicin at 15 mg/kg) had more than one Gram-negative organism (Morganella morganii and Proteus mirabilis), quantified at ≥105 CFU/ml at the baseline. Gram-positive bacteria were isolated from six patients.
Among 68 Enterobacteriaceae isolates recovered at the baseline for which MIC data were available, plazomicin MICs ranged from ≤0.12 to 8 μg/ml and levofloxacin MICs ranged from ≤0.12 to >4 μg/ml. Of the two Gram-negative bacterial isolates with plazomicin MICs of >4 μg/ml, one was E. coli and the other was Pseudomonas aeruginosa. Baseline susceptibility testing of Enterobacteriaceae using Clinical and Laboratory Standards Institute (CLSI) (42) and European Committee on Antimicrobial Susceptibility Testing (EUCAST) (43) criteria showed that 27.9% (n = 19), including 6 in the levofloxacin treatment group, were resistant to levofloxacin (MIC > 4 μg/ml); 17.6% (n = 12) were resistant to ceftazidime by both CLSI and EUCAST criteria; 39.7% (n = 27) were resistant to trimethoprim-sulfamethoxazole by CLSI criteria and nonsusceptible per EUCAST criteria; 14.7% (n = 10) were resistant to gentamicin by CLSI criteria (MIC ≥ 16 μg/ml) and 17.6% (n = 12) were nonsusceptible to gentamicin by EUCAST criteria (MIC ≥ 4 μg/ml); and although none of the isolates were resistant to amikacin by CLSI criteria (MIC ≥ 64 μg/ml), 2 were nonsusceptible by EUCAST criteria (MIC ≥ 16 μg/ml). Overall, 16.2% (n = 11) of these Enterobacteriaceae were classified as MDR, defined as nonsusceptible to at least one agent in three or more antimicrobial categories (44).
Microbiological outcomes.
The microbiological eradication rates at TOC were similar across treatment groups for both the MITT and ME populations (Table 2). The treatment differences between the groups receiving plazomicin at 15 mg/kg and levofloxacin were 2.2% (95% confidence interval [CI], −22.9 to 27.2%) and 7.6% (95% CI, −16.0 to 31.3%) in the MITT and ME populations, respectively. For patients in the ME population, microbiological recurrence at long-term follow-up (LFU) occurred in 6.5% (2/31) and 23.5% (4/17) of the patients in the groups receiving treatment with plazomicin at 15 mg/kg and levofloxacin, respectively.
TABLE 2.
Microbiological outcome at TOC (primary efficacy endpoint)a
| Population | Treatment | No. of patients | No. (%) of patients with eradication | 95% CI for eradication (%) | No. (%) of patients with noneradication | No. (%) of patients with indeterminate outcomeb |
|---|---|---|---|---|---|---|
| MITT | Plazomicin at 10 mg/kg | 12 | 6 (50.0) | 21.1–78.9 | 1 (8.3) | 5 (41.7) |
| Plazomicin at 15 mg/kg | 51 | 31 (60.8) | 46.1–74.2 | 5 (9.8) | 15 (29.4) | |
| Levofloxacin at 750 mg | 29 | 17 (58.6) | 38.9–76.5 | 4 (13.8) | 8 (27.6) | |
| ME | Plazomicin at 10 mg/kg | 7 | 6 (85.7) | 42.1–99.6 | 1 (14.3) | – |
| Plazomicin at 15 mg/kg | 35 | 31 (88.6) | 73.3–96.8 | 4 (11.4) | – | |
| Levofloxacin at 750 mg | 21 | 17 (81.0) | 58.1–94.6 | 4 (19.0) | – |
The difference in microbiological eradication rates between the group receiving plazomicin at 15 mg/kg and the group receiving levofloxacin was 2.2% (95% CI, −22.9 to 27.2%) for the MITT population and 7.6% (95% CI, −16.0 to 31.3%) for the ME population. The 95% CI for the difference was based on a normal approximation with a continuity correction.
–, patients with missing or indeterminate outcome data were excluded from the ME population.
The microbiological eradication rates by primary diagnosis (AP or cUTIs) and baseline pathogen were generally similar in each treatment group (Table 3). In patients with E. coli at the baseline, 88.0% who received plazomicin at 15 mg/kg and 75.0% who received levofloxacin had a favorable microbiological response at TOC. Although the numbers are small, the data suggest a trend toward lower microbiological eradication rates among patients with baseline pathogens with higher MICs for both plazomicin and levofloxacin (Table 3). Microbiological eradication in the group receiving plazomicin at 15 mg/kg was achieved in 93.1% (27/29) of the patients whose baseline urinary pathogens had a plazomicin MIC of ≤4 μg/ml, including two Enterobacteriaceae isolates with an MIC of 4 μg/ml, and in 66.7% (2/3) of the patients whose pathogens had a plazomicin MIC of >4 μg/ml. Microbiological eradication in the group receiving levofloxacin was achieved in 93.8% (15/16) of patients with levofloxacin-susceptible baseline urinary pathogens (MIC ≤ 4 μg/ml) and 33% (1/3) of patients with levofloxacin-resistant baseline urinary pathogens (MIC > 4 μg/ml). Both patients whose isolates were nonsusceptible to amikacin per EUCAST criteria (one in the plazomicin group, one in the levofloxacin group) had microbiological eradication at TOC.
TABLE 3.
Microbiological eradication at TOC according to primary diagnosis and baseline pathogen (ME population)
| Subgroup | Values for patients receiving: |
||
|---|---|---|---|
| Plazomicin at 10 mg/kg (n = 7) | Plazomicin at 15 mg/kg (n = 35) | Levofloxacin at 750 mg (n = 21) | |
| No. of patients with eradication at TOC/no. of patients with the following primary diagnosis (%): | |||
| AP | 2/2 (100) | 16/18 (88.9) | 12/15 (80.0) |
| 95% CI | 15.8–100 | 65.3–98.6 | 51.9–95.7 |
| cUTI | 4/5 (80.0) | 15/17 (88.2) | 5/6 (83.3) |
| 95% CI | 28.4–99.5 | 63.6–98.5 | 35.9–99.6 |
| No. of patients with eradication at TOC/no. of patients infected with the following pathogen at baseline (%): | |||
| Gram-positive bacteriaa | 1/1 (100.0) | 3/3 (100.0) | 1/1 (100.0) |
| Gram-negative bacteria | 5/6 (83.3) | 28/32 (87.5) | 16/20 (80.0) |
| E. coli | 3/4 (75.0) | 22/25 (88.0) | 12/16 (75.0) |
| K. pneumoniae | 1/1 (100.0) | 2/2 (100.0) | 1/1 (100.0) |
| Other Enterobacteriaceaeb | 1/1 (100.0) | 4/4 (100.0) | 3/3 (100.0) |
| P. aeruginosa | 0/0 (0.0) | 1/2 (50.0) | 0/0 (0.0) |
| No. of patients with eradication at TOC/no. of patients whose pathogen had the following MIC at baseline (%): | |||
| Plazomicin MIC ≤ 4 μg/ml | 5/6 (83.3) | 27/29 (93.1) | 16/19 (84.2) |
| Plazomicin MIC > 4 μg/ml | 0/0 (0.0) | 2/3 (66.7) | 1/1 (100.0) |
| Levofloxacin MIC ≤ 4 μg/ml | 4/4 (100.0) | 23/24 (95.8) | 15/16 (93.8) |
| Levofloxacin MIC > 4 μg/ml | 1/2 (50.0) | 5/7 (71.4) | 1/3 (33.3) |
Gram-positive bacteria included methicillin-susceptible Staphylococcus aureus (n = 1), Enterococcus faecalis (n = 3), and Staphylococcus saprophyticus (n = 1).
Other Enterobacteriaceae included Citrobacter freundii (n = 1), Enterobacter aerogenes (n = 1), M. morganii (n = 2), and P. mirabilis (n = 4).
Clinical outcomes.
Most patients in both treatment groups achieved clinical cure at TOC (Table 4). The median time to clinical cure was 5 days for patients in all three treatment groups (interquartile ranges, 5 to 14 days for the group receiving plazomicin at 10 mg/kg, 5 to 6 days for the group receiving plazomicin at 15 mg/kg, and 5 to 12 days for the group receiving levofloxacin). For patients in the ME population, clinical relapse at LFU occurred in 14.3% (4/28) and 6.3% (1/16) of patients in the groups receiving plazomicin at 15 mg/kg and levofloxacin, respectively.
TABLE 4.
Clinical outcome at TOC
| Population | Treatment | No. of patients | No. (%) of patients cured | 95% CI for cure (%) | No. (%) of patients with treatment failure | No. (%) of patients with indeterminate outcomea |
|---|---|---|---|---|---|---|
| MITT | Plazomicin at 10 mg/kg | 12 | 8 (66.7) | 34.9–90.1 | 3 (25.0) | 1 (8.3) |
| Plazomicin at 15 mg/kg | 51 | 36 (70.6) | 56.2–82.5 | 9 (17.6) | 6 (11.8) | |
| Levofloxacin at 750 mg | 29 | 19 (65.5) | 45.7–82.1 | 7 (24.1) | 3 (10.3) | |
| ME | Plazomicin at 10 mg/kg | 7 | 4 (57.1) | 18.4–90.1 | 3 (42.9) | – |
| Plazomicin at 15 mg/kg | 35 | 28 (80.0) | 63.1–91.6 | 7 (20.0) | – | |
| Levofloxacin at 750 mg | 21 | 16 (76.2) | 52.8–91.8 | 5 (23.8) | – |
–, patients with missing or indeterminate outcome data were excluded from the ME population.
The clinical cure rates for patients with antibiotic-resistant Enterobacteriaceae at the baseline are shown in Table 5. Of the 19 patients in the MITT population with levofloxacin-resistant Enterobacteriaceae, clinical cure was achieved in 75.0% (3/4), 77.8% (7/9), and 66.7% (4/6) in the groups receiving plazomicin at 10 mg/kg and 15 mg/kg and levofloxacin, respectively. Favorable cure rates were also achieved with plazomicin for patients with Enterobacteriaceae that were nonsusceptible to amikacin or resistant to ceftazidime, gentamicin, or trimethoprim-sulfamethoxazole at the baseline (Table 5).
TABLE 5.
Clinical cure at TOC for patients with antibiotic-resistant Enterobacteriaceae at baseline (MITT population)a
| Enterobacteriaceae susceptibility | No. of patients with cure at TOC/no. of patients with the specified pathogen at baseline (%) |
||
|---|---|---|---|
| Plazomicin at 10 mg/kg | Plazomicin at 15 mg/kg | Levofloxacin at 750 mg | |
| Levofloxacin resistant | 3/4 (75.0) | 7/9 (77.8) | 4/6 (66.7) |
| Ceftazidime resistant | 3/4 (75.0) | 2/3 (66.7) | 4/5 (80.0) |
| Gentamicin resistant | 3/3 (100) | 3/3 (100) | 4/4 (100) |
| Amikacin nonsusceptible | 0 | 1/1 (100) | 1/1 (100) |
| TMP-SMX resistant | 3/4 (75.0) | 13/16 (81.3) | 5/7 (71.4) |
Resistance and nonsusceptibility were defined according to CLSI and EUCAST breakpoints as follows: for levofloxacin, MIC > 4 μg/ml; for ceftazidime, CLSI MIC ≥ 16 μg/ml and EUCAST MIC > 4 μg/ml; for gentamicin, CLSI MIC ≥ 16 μg/ml; for amikacin, CLSI MIC ≥ 64 μg/ml and EUCAST MIC ≥ 16 μg/ml; and for trimethoprim-sulfamethoxazole, CLSI MIC ≥ 4 μg/ml and EUCAST MIC > 4 μg/ml (42, 43). TMP-SMX, trimethoprim-sulfamethoxazole.
Safety.
AEs were reported in 31.8% (7/22), 35.1% (26/74), and 47.7% (21/44) of the patients in the groups receiving plazomicin at 10 mg/kg and 15 mg/kg and levofloxacin, respectively (Table 6). The most frequent AEs reported in the plazomicin treatment groups included headache, nausea, vomiting, diarrhea, and dizziness, and most were graded by the investigator to be mild or moderate in severity.
TABLE 6.
Safety analysis (safety population)
| Eventa | Values for patients receiving: |
||
|---|---|---|---|
| Plazomicin at 10 mg/kg (n = 22) | Plazomicin at 15 mg/kg (n = 74) | Levofloxacin at 750 mg (n = 44) | |
| No. (%) of patients with any AE | 7 (31.8) | 26 (35.1) | 21 (47.7) |
| No. (%) of patients with the following AEs reported in ≥5% of patients in any treatment group: | |||
| Headache | 2 (9.1) | 6 (8.1) | 3 (6.8) |
| Diarrhea | 0 (0.0) | 4 (5.4) | 2 (4.5) |
| Vomiting | 0 (0.0) | 4 (5.4) | 1 (2.3) |
| Nausea | 0 (0.0) | 4 (5.4) | 0 (0.0) |
| Dizziness | 0 (0.0) | 4 (5.4) | 0 (0.0) |
| No. (%) of patients with: | |||
| AE related to renal functionb | 0 (0.0) | 2 (2.7) | 0 (0.0) |
| AE related to vestibular or cochlear functionc | 0 (0.0) | 2 (2.7) | 1 (2.3) |
| AE related to study drug | 2 (9.1) | 15 (20.3) | 12 (27.3) |
| AE leading to study drug discontinuation | 0 (0.0) | 4 (5.4) | 1 (2.3) |
| Any serious AE | 0 (0.0) | 1 (1.4) | 2 (4.5) |
| No. of patients with a ≥0.5-mg/dl increase in serum creatinine concn/total no. of patients tested (%): | |||
| At any time during the study | 1/22 (4.5) | 4/72 (5.6) | 1/41 (2.4) |
| While on i.v. study drug | 0/22 (0.0) | 3/72 (4.2) | 0/41 (0.0) |
AEs were coded according to the preferred terms in version 12.1 of MedDRA.
Events include preferred terms of azotemia and acute renal failure.
Events include preferred terms of tinnitus and vertigo and worsening of audiometry.
Three patients experienced a serious AE during the study. One was a patient in the group receiving plazomicin at 15 mg/kg, who had a negative pregnancy test at enrollment but experienced a spontaneous abortion on day 103 that was not considered related to study drug. The other two were patients in the levofloxacin group: one with a convulsion considered related to study drug and one with recurrent AP resulting in nephrectomy considered unrelated to study drug. There were no deaths during the study.
Five patients (four in the group receiving plazomicin at 15 mg/kg and one in the group receiving levofloxacin) had AEs that led to discontinuation of study drug. A 24-year-old woman experienced severe hypotension (systolic/diastolic blood pressure, 80/50 mm Hg) along with moderate dizziness on day 3, 30 min after receiving both the 30-min plazomicin infusion and the 90-min placebo infusion. The hypotension resolved at 2 h 15 min after onset, at which time the patient's blood pressure was 100/70 mm Hg. Both AEs (hypotension and dizziness) were considered related to study drug and resolved without sequelae. A 55-year-old woman discontinued plazomicin due to a worsening of preexisting diabetes mellitus (not related to study drug) and mild azotemia on day 3 of dosing (considered related to study drug), when her serum creatinine level increased from a baseline value of 1.6 mg/dl to 2.1 mg/dl on the day of the AE. At LFU, the patient's serum creatinine level was 1.8 mg/dl. Two patients, one with moderate dizziness and one with mild transient vertigo, discontinued plazomicin after two doses; both were considered related to study drug and resolved without sequelae. One patient discontinued levofloxacin due to a serious AE of convulsion that was considered related to study drug.
AEs associated with renal function occurred in two patients in the group receiving plazomicin at 15 mg/kg (Table 6): the previously described patient with mild azotemia that led to drug discontinuation and one patient with mild acute renal insufficiency whose serum creatinine level increased from 0.9 mg/dl at the baseline to 1.6 mg/dl at TOC and returned to near the baseline level (1.1 mg/dl) at LFU. For most patients, serum creatinine levels remained stable throughout the study. Five plazomicin-treated patients and one levofloxacin-treated patient had a ≥0.5-mg/dl increase in the serum creatinine level from that at the baseline during the study, including three patients in the group receiving plazomicin at 15 mg who had an increase while on intravenous (i.v.) therapy. The levels returned to near baseline by LFU in all but one plazomicin-treated patient, whose serum creatinine level increased from a baseline value of 1.2 mg/dl (normal range, 0.7 to 1.3 mg/dl) to 2.0 mg/dl on day 5 at the end of treatment and remained elevated (1.8 mg/dl) at LFU.
No patient met Hy's law criteria for liver injury (45).
AEs possibly associated with vestibular and cochlear function occurred in two patients in the group receiving plazomicin at 15 mg/kg and one patient in the group receiving levofloxacin (Table 6). As described above, one patient experienced mild transient vertigo after receiving the second dose of plazomicin. Her vertigo resolved without sequelae, and modified Romberg and audiogram test results were normal. Another patient, a 47-year-old woman with normal audiogram results, experienced mild unilateral tinnitus 14 days after the end of plazomicin therapy. Repeat audiograms at LFU and 3 months after the end of treatment showed normal hearing levels; however, mild unilateral tinnitus persisted at 3 months. One patient in the levofloxacin group experienced mild worsening of audiometry results at TOC; a small but consistent drop in hearing levels was detected, ranging from 10 to 40 dB in the frequencies tested (250 to 8,000 Hz), but the results did not meet the predefined sponsor criteria for sensorineural abnormalities. Modified Romberg testing generally showed no clinically important changes in vestibular function over the study; of the 93 patients who completed this testing, 89 showed normal results both at the baseline and at study days 10 to 14, and 2 showed a mild decline from the results at the baseline (1 in the plazomicin treatment group and 1 in the levofloxacin treatment group). No plazomicin-treated patients with evaluable audiometry data (0/75) showed postbaseline sensorineural, conductive, or mixed hearing loss.
There were no clinically important trends in laboratory parameters, vital signs, or electrocardiograms for patients who received plazomicin. AEs associated with vital signs were reported for four patients: the previously mentioned patient with severe hypotension that led to discontinuation of plazomicin and three patients with mild or moderate hypertension considered unrelated to study drug (one in the group receiving plazomicin at 10 mg/kg and two in the group receiving levofloxacin).
DISCUSSION
The results of this small phase 2 study demonstrate that the administration of plazomicin (10 or 15 mg/kg) once daily for 5 days was an effective treatment in adult patients with cUTI, including AP. Microbiological eradication was achieved in over 85% of plazomicin-treated patients in the ME population, and 80% of patients who received the 15-mg/kg dose of plazomicin were assessed by the investigator to be clinically cured, with complete resolution of baseline signs and symptoms of infection. Additionally, a lower rate of microbiological recurrence at LFU, which occurred ∼1 month after the last dose of study drug, was observed in the 15-mg/kg plazomicin treatment group than in the levofloxacin treatment group (6.5% versus 23.5%).
There are few large randomized, controlled trials that have evaluated the efficacy of aminoglycosides dosed once daily for cUTI or AP. Despite the small sample size of the current study and challenges in comparing data across studies with methodological differences, the high microbiological eradication rate observed with once-daily plazomicin therapy in the ME population was similar to that reported to be achieved with other aminoglycosides when dosed once daily (46, 47). Specifically, once-daily isepamicin therapy (8 or 15 mg/kg, depending on the severity of infection) was associated with microbiological eradication in 91% (92/101) of evaluable patients 4 to 15 days after the end of treatment (46), and once-daily amikacin therapy (15 mg/kg/day) was associated with microbiological eradication in 94% (32/34) of patients 7 to 10 days after the end of treatment (47). These numbers are comparable to the 89% (31/35) microbiological eradication rate at TOC for plazomicin (15 mg/kg) seen in the ME population in our study.
The clinical cure rates achieved with plazomicin were generally favorable in the small number of patients with antibiotic-resistant Enterobacteriaceae at the baseline, including aminoglycoside-resistant isolates (3/3 patients with gentamicin-resistant isolates and 1/1 patient with amikacin-resistant isolates in the 15-mg/kg plazomicin group). Although investigations in a larger group of patients with cUTIs due to resistant pathogens are needed to more precisely quantify the response rates to these pathogens, the cure rates associated with plazomicin in this study are promising, particularly in the current clinical environment in which options for the treatment of MDR pathogens are limited.
The causative uropathogens isolated in this study are typical and similar to those isolated in recent clinical trials of cUTIs (48, 49), although in our study relatively few P. mirabilis and P. aeruginosa isolates were recovered, perhaps due to the small number of patients enrolled. Levofloxacin resistance was detected in 28% of Enterobacteriaceae isolates recovered at the baseline in this study, which completed enrollment in 2012. Similarly high rates of levofloxacin resistance have been detected in recent registrational trials for this indication (48, 49), potentially introducing bias into noninferiority trials of cUTI and leading several experts to suggest that levofloxacin is no longer an appropriate choice of comparator for a noninferiority study or for empirical therapy of cUTI in patients with risk factors for antibiotic resistance (50, 51). In the context of this study, relatively few levofloxacin-resistant (MIC > 4 μg/ml) uropathogens were included in the primary analysis populations of the levofloxacin treatment group (6/25 and 3/19 in the MITT and ME populations, respectively), suggesting that study results may not have been overly impacted by fluoroquinolone resistance.
In this study, we closely monitored patients for toxicities associated with aminoglycosides, including nephrotoxicity and ototoxicity, although once-daily dosing has been associated with lower rates of nephrotoxicity than traditional dosing (23, 26, 29). Plazomicin administered at 10 or 15 mg/kg once daily as a 30-min infusion for 5 days was generally well tolerated by patients with cUTI or AP. The incidence of AEs was similar between the levofloxacin and plazomicin treatment groups, and most of the AEs were mild to moderate in intensity and rapidly resolved. In most patients, serum creatinine levels remained stable over the study. Postbaseline serum creatinine level increases of ≥0.5 mg/dl, which are considered to be a clinically meaningful measure of new-onset renal dysfunction (52), were observed in five plazomicin-treated patients and one levofloxacin-treated patient. These events resolved in all but one plazomicin-treated patient. These safety results are consistent with the known mechanism of aminoglycoside toxicity and phase 1 study data for healthy adults who received a once-daily plazomicin regimen of 15 mg/kg administered for 5 days (53). Additionally, no treatment-related changes in audiometry were observed.
Our study is limited by the small sample size, the limited number of patients with infections due to resistant pathogens, and the relatively large percentage of patients with an indeterminate outcome in the MITT population in each treatment group. Indeterminate outcomes at TOC were due to urine culture samples that were either contaminated, not obtained, or obtained outside the permitted window of 5 to 12 days after the end of treatment. Another limitation was the underrepresentation of patients from Europe, as patients from that continent were not enrolled. However, plazomicin activity and exposures are not expected to be impacted by race or ethnicity.
In summary, the results of this small phase 2 study suggest that plazomicin dosed at 15 mg/kg once daily for 5 days is effective in the treatment of adults with cUTIs, including patients with antibiotic-resistant Enterobacteriaceae. This dose and duration of plazomicin were well tolerated overall, with mild and generally reversible increases in serum creatinine levels being noted in a small number of patients. Although our findings suggest a potential dose-response effect of plazomicin on serum creatinine levels, the numbers are too small to draw conclusions and larger studies will be required to define exposure-response relationships for nephrotoxicity. Plazomicin has the potential to address an unmet medical need for patients with cUTIs or AP caused by MDR Enterobacteriaceae, and further studies using once-daily 15-mg/kg dosing are warranted.
MATERIALS AND METHODS
Study design.
This multicenter, double-blind, randomized, comparator-controlled, phase 2 study was conducted from 13 July 2010 to 3 April 2012 at 27 study sites in the United States, India, Colombia, and Chile. This study is registered at ClinicalTrials.gov under the identifier NCT01096849 and was performed in accordance with the Declaration of Helsinki, the International Conference on Harmonisation/Good Clinical Practice guidelines, and applicable regulatory requirements. An independent ethics committee or institutional review board at each site approved the protocol, and patients were required to provide written informed consent.
Inclusion and exclusion criteria.
Inpatients and outpatients were eligible for study participation. Patients participating on an outpatient basis were to receive study drug infusions and all other study activities by home health care or have the ability to return to the study center. Eligibility criteria included the following: age, 18 to 85 years; body weight, ≤100 kg, and a documented or suspected cUTI or AP with protocol-specified clinical signs and symptoms. A cUTI was defined by the presence of pyuria (≥5 white blood cells [WBC] per high-power field in urine sediment and/or a positive leukocyte esterase test on urinalysis) and at least one of the following signs or symptoms: fever (oral temperature, ≥38.5°C or ≥101.3°F), elevated WBC count (≥10,000/mm3 or a left shift of ≥15% immature polymorphonuclear leukocytes), dysuria, increased urinary frequency, urgency, or lower abdominal pain. At least one of the following complicating factors was also required in the definition of a cUTI: an indwelling catheter (to be removed or replaced by ≤12 h after randomization), urine residual volume of ≥100 ml, neurogenic bladder, or urinary retention in men due to previously diagnosed benign prostatic hypertrophy. AP was defined as the presence of signs or symptoms of an ascending tract infection, including fever or an elevated WBC count, lower back/flank pain, pyuria, and at least one of the following: costovertebral angle tenderness, nausea, chills, dysuria, increased urinary frequency, urgency, or vomiting. All patients were required to have a creatinine clearance (CLCR) of ≥60 ml/min using the Cockcroft-Gault formula (54).
Patients were excluded from the study if they had acute bacterial prostatitis, orchitis, epididymitis, chronic bacterial prostatitis, gross hematuria requiring intervention other than study drug, urinary tract surgery within 7 days before randomization or planned during the study period, a known nonrenal source of infection diagnosed within 7 days of randomization, a QTc interval of >440 ms, a history of hearing loss before the age of 40 years, sensorineural hearing loss, or a family history of hearing loss. Pregnant or breast-feeding women were also excluded. Patients were not permitted to receive any systemic antibacterial (oral, i.v., intramuscular) therapy or antibacterial bladder irrigation for the treatment of a bacterial cUTI or AP in the 48 h preceding randomization or during the study. Full inclusion and exclusion criteria are provided in the supplemental material.
Randomization and treatment.
Patients were enrolled and randomized (1:1:1) by a central interactive voice response system to receive i.v. plazomicin (10 or 15 mg/kg) or i.v. levofloxacin (750 mg) once daily for 5 days. Enrollment in the 10-mg/kg treatment group was stopped during the study to allow preferential enrollment in the higher-dose group (15 mg/kg). Patients were subsequently randomized in a 2:1 ratio to receive i.v. plazomicin (15 mg/kg) or i.v. levofloxacin (750 mg). Randomization was stratified by type of infection (AP, cUTI with an indwelling catheter, or cUTI without an indwelling catheter). Patients and investigators were blind to the treatment assignment. To maintain the blind, patients received two i.v. infusions on each of the 5 treatment days: a 30-min plazomicin infusion followed by a 90-min placebo infusion or a 30-min placebo infusion followed by a 90-min levofloxacin infusion. The plazomicin regimens were selected on the basis of the pharmacokinetic profile and the safety data observed in two previously completed phase 1 studies (53). The high-dose, short-course levofloxacin regimen used in this study is widely considered the standard of care for cUTI (3, 24, 55) and is approved as a 5-day regimen.
Analysis populations.
The intent-to-treat population included all randomized patients. The safety population included all randomized patients who received any amount of study drug. The MITT population included all randomized patients with at least one isolated causative bacterial pathogen at ≥105 CFU/ml from an appropriately collected pretreatment urine specimen. Patients with isolates present at ≥104 CFU/ml and <105 CFU/ml were evaluated for inclusion in conjunction with urinalysis and clinical signs and symptoms data. Patients were considered clinically evaluable if they had a protocol-defined cUTI or AP, received study medication as randomized, were blind to treatment assignment (except in the event of a treatment-emergent AE or serious AE), and received ≥80% of study drug for clinical success or 40% for clinical failure and the clinical response was assessed at TOC. The ME population included all clinically evaluable patients with a causative pathogen isolated at the baseline and results obtained from a noncontaminated urine culture collected at TOC within a specified window (5 to 12 days after the end of treatment, except in cases of early failure, in which the TOC visit was performed early). Patients with missing or indeterminate outcome data were excluded from the ME population.
Efficacy endpoints and assessments.
The coprimary efficacy endpoints were microbiological eradication in the MITT and the ME populations at TOC, 5 to 12 days after the last treatment. Secondary endpoints included microbiological eradication at TOC by primary diagnosis (cUTI or AP) and baseline uropathogen, the investigator's assessment of clinical cure at TOC, the time to clinical cure, and microbiological recurrence and clinical relapse at LFU, 33 to 47 days after the last treatment. Clinical outcomes in patients with antibiotic-resistant pathogens at the baseline were also assessed.
Microbiological outcomes were defined as eradication (TOC urine culture with <104 CFU/ml of the baseline pathogen), noneradication (TOC urine culture with ≥104 CFU/ml of the baseline pathogen), and indeterminate (any uropathogen that could not be classified as eradicated or persistent [including missing data]). Microbiological recurrence was defined as an LFU urine culture with >105 CFU/ml of regrowth of a baseline pathogen that was eradicated at TOC. Clinical outcomes were defined as cure (complete resolution of all baseline signs and symptoms at TOC without the use of additional antibiotic therapy or an AE leading to premature discontinuation of the study drug), failure (persistence, incomplete resolution, or worsening of baseline clinical signs and symptoms or development of new clinical signs and symptoms of cUTI or AP requiring additional antimicrobial therapy at any time through the TOC visit), and indeterminate (an outcome other than cure or failure, including loss to follow-up before the TOC visit). Clinical relapse at LFU was defined as the return of clinical signs and symptoms requiring antibiotic therapy in patients who were clinically cured at TOC.
Baseline pathogens were sent to a central laboratory (Eurofins Global Central Laboratory, Chantilly, VA) for identification and susceptibility testing. Results were interpreted according to CLSI (42) and EUCAST (43) criteria. The MICs of plazomicin, levofloxacin, and other comparator antibiotics, including amikacin, gentamicin, ceftazidime, and trimethoprim-sulfamethoxazole, were determined using broth microdilution. Enterobacteriaceae species were classified as having an MDR phenotype if they were nonsusceptible to at least one agent in three or more antimicrobial categories (44).
Safety assessments.
Safety investigations included physical examinations, measurement of vital signs, electrocardiograms, collection of reports of AEs, laboratory tests, cochlear tests (pure tone audiometry [PTA] with bone conduction at frequencies of up to 20,000 Hz with a minimum upper limit of 8,000 Hz), and vestibular function (modified Romberg) tests. AE reports, vital sign measurements, and blood and urine for safety analyses were collected at screening, daily while on treatment, and at TOC and LFU. Laboratory safety tests (hematology, serum chemistry, and urinalysis) were performed centrally at the ACM Medical Laboratory (Rochester, NY). An independent licensed audiologist reviewed the PTA with bone conduction results to assess changes from the baseline according to predefined criteria. Sensorineural hearing loss was defined as abnormal pure tone air and bone conduction of ≥25 dB and an air-bone gap of ≤10 dB, conductive hearing loss was defined as a normal bone conduction (<25 dB) and air conduction higher (worse) than bone conduction by >10 dB, and mixed hearing loss was defined as abnormal pure tone air and bone conduction (≥25 dB). Modified Romberg testing evaluated neurological function (including proprioception), vestibular function, and vision across four test conditions; a classification of “normal” required passing all four test conditions with no symptoms of disequilibrium or vertigo (56).
Statistical analyses.
This study was not powered for inferential statistics. With assumed microbiological eradication rates of 85% and 88% in the plazomicin treatment group in the MITT and ME populations, respectively, the final overall sample size of 145 patients (98 in the plazomicin treatment group) provided for 95% CIs of 73 to 94% and 74 to 95%, respectively.
Two-sided 95% CIs were calculated for the point estimates of microbiological eradication within each treatment group (Clopper-Pearson method) and for the difference in eradication rates between the plazomicin and levofloxacin treatment groups (based on the normal approximation with a continuity correction). Secondary efficacy endpoints were analyzed using descriptive statistics and CIs, where appropriate. Time-to-event analyses were conducted using Kaplan-Meier methods. Safety data were summarized descriptively.
Supplementary Material
ACKNOWLEDGMENTS
Editorial support was provided by Jean Turner of PAREXEL and funded by Achaogen, Inc.
This study was partially funded by the Wellcome Trust, a global charitable foundation, through grant no. 086065 (Seeding Drug Discovery 2008) and grant no. 090640 (Strategic Translation Award 2010).
L.E.C. is an employee of and stockholder in Achaogen, Inc., V.R. is a paid contractor to Achaogen, Inc., D.C. is an employee of and stockholder in Achaogen, Inc., E.S.A. is a former employee of and current stockholder in Achaogen, Inc., and L.G.M. has served as a consultant for Achaogen, Inc., and Tetraphase Pharmaceuticals and has received research grants from Achaogen, Gilead Sciences, Merck, Abbott, and Cepheid.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01989-17.
[This article was published on 27 March 2018 with a standard copyright line (“© 2018 American Society for Microbiology. All Rights Reserved.”). The authors elected to pay for open access for the article after publication, necessitating replacement of the original copyright line with the one above, and this change was made on 13 June 2018.]
REFERENCES
- 1.Zimlichman E, Henderson D, Tamir O, Franz C, Song P, Yamin CK, Keohane C, Denham CR, Bates DW. 2013. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med 173:2039–2046. doi: 10.1001/jamainternmed.2013.9763. [DOI] [PubMed] [Google Scholar]
- 2.Foxman B. 2014. Urinary tract infection syndromes: occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect Dis Clin North Am 28:1–13. doi: 10.1016/j.idc.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Nicolle LE. 2005. Complicated urinary tract infection in adults. Can J Infect Dis Med Microbiol 16:349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cek M, Tandogdu Z, Wagenlehner F, Tenke P, Naber K, Bjerklund-Johansen TE. 2014. Healthcare-associated urinary tract infections in hospitalized urological patients—a global perspective: results from the GPIU studies 2003-2010. World J Urol 32:1587–1594. doi: 10.1007/s00345-013-1218-9. [DOI] [PubMed] [Google Scholar]
- 5.Zowawi HM, Harris PN, Roberts MJ, Tambyah PA, Schembri MA, Pezzani MD, Williamson DA, Paterson DL. 2015. The emerging threat of multidrug-resistant Gram-negative bacteria in urology. Nat Rev Urol 12:570–584. doi: 10.1038/nrurol.2015.199. [DOI] [PubMed] [Google Scholar]
- 6.Bouchillon S, Hoban DJ, Badal R, Hawser S. 2012. Fluoroquinolone resistance among Gram-negative urinary tract pathogens: global SMART program results, 2009-2010. Open Microbiol J 6:74–78. doi: 10.2174/1874285801206010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoban DJ, Lascols C, Nicolle LE, Badal R, Bouchillon S, Hackel M, Hawser S. 2012. Antimicrobial susceptibility of Enterobacteriaceae, including molecular characterization of extended-spectrum beta-lactamase-producing species, in urinary tract isolates from hospitalized patients in North America and Europe: results from the SMART study 2009-2010. Diagn Microbiol Infect Dis 74:62–67. doi: 10.1016/j.diagmicrobio.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 8.Lob SH, Nicolle LE, Hoban DJ, Kazmierczak KM, Badal RE, Sahm DF. 2016. Susceptibility patterns and ESBL rates of Escherichia coli from urinary tract infections in Canada and the United States, SMART 2010–2014. Diagn Microbiol Infect Dis 85:459–465. doi: 10.1016/j.diagmicrobio.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 9.Tandogdu Z, Cek M, Wagenlehner F, Naber K, Tenke P, van Ostrum E, Johansen TB. 2014. Resistance patterns of nosocomial urinary tract infections in urology departments: 8-year results of the global prevalence of infections in urology study. World J Urol 32:791–801. doi: 10.1007/s00345-013-1154-8. [DOI] [PubMed] [Google Scholar]
- 10.Doi Y, Park YS, Rivera JI, Adams-Haduch JM, Hingwe A, Sordillo EM, Lewis JS, Howard WJ, Johnson LE, Polsky B, Jorgensen JH, Richter SS, Shutt KA, Paterson DL. 2013. Community-associated extended-spectrum beta-lactamase-producing Escherichia coli infection in the United States. Clin Infect Dis 56:641–648. doi: 10.1093/cid/cis942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guh AY, Bulens SN, Mu Y, Jacob JT, Reno J, Scott J, Wilson LE, Vaeth E, Lynfield R, Shaw KM, Vagnone PM, Bamberg WM, Janelle SJ, Dumyati G, Concannon C, Beldavs Z, Cunningham M, Cassidy PM, Phipps EC, Kenslow N, Travis T, Lonsway D, Rasheed JK, Limbago BM, Kallen AJ. 2015. Epidemiology of carbapenem-resistant Enterobacteriaceae in 7 US communities, 2012-2013. JAMA 314:1479–1487. doi: 10.1001/jama.2015.12480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Talan DA, Takhar SS, Krishnadasan A, Abrahamian FM, Mower WR, Moran GJ. 2016. Fluoroquinolone-resistant and extended-spectrum β-lactamase-producing Escherichia coli infections in patients with pyelonephritis, United States. Emerg Infect Dis 22:160148. doi: 10.3201/eid2209.160148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiner LM, Fridkin SK, Aponte-Torres Z, Avery L, Coffin N, Dudeck MA, Edwards JR, Jernigan JA, Konnor R, Soe MM, Peterson K, Clifford McDonald L. 2016. Vital signs: preventing antibiotic-resistant infections in hospitals—United States, 2014. Am J Transplant 16:2224–2230. doi: 10.1111/ajt.13893. [DOI] [PubMed] [Google Scholar]
- 14.Bartsch SM, McKinnell JA, Mueller LE, Miller LG, Gohil SK, Huang SS, Lee BY. 2017. Potential economic burden of carbapenem-resistant Enterobacteriaceae (CRE) in the United States. Clin Microbiol Infect 23:48.e49–48.e16. doi: 10.1016/j.cmi.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacVane SH, Tuttle LO, Nicolau DP. 2014. Impact of extended-spectrum beta-lactamase-producing organisms on clinical and economic outcomes in patients with urinary tract infection. J Hosp Med 9:232–238. doi: 10.1002/jhm.2157. [DOI] [PubMed] [Google Scholar]
- 16.Zilberberg MD, Nathanson BH, Sulham K, Fan W, Shorr AF. 2017. Carbapenem resistance, inappropriate empiric treatment and outcomes among patients hospitalized with Enterobacteriaceae urinary tract infection, pneumonia and sepsis. BMC Infect Dis 17:279. doi: 10.1186/s12879-017-2383-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mauldin PD, Salgado CD, Hansen IS, Durup DT, Bosso JA. 2010. Attributable hospital cost and length of stay associated with health care-associated infections caused by antibiotic-resistant Gram-negative bacteria. Antimicrob Agents Chemother 54:109–115. doi: 10.1128/AAC.01041-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwaber MJ, Carmeli Y. 2007. Mortality and delay in effective therapy associated with extended-spectrum beta-lactamase production in Enterobacteriaceae bacteraemia: a systematic review and meta-analysis. J Antimicrob Chemother 60:913–920. doi: 10.1093/jac/dkm318. [DOI] [PubMed] [Google Scholar]
- 19.Vardakas KZ, Rafailidis PI, Konstantelias AA, Falagas ME. 2013. Predictors of mortality in patients with infections due to multi-drug resistant Gram negative bacteria: the study, the patient, the bug or the drug? J Infect 66:401–414. doi: 10.1016/j.jinf.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 20.U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. 2013. Antibiotic resistance threats in the United States, 2013. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 21.World Health Organization. 2014. Antimicrobial resistance: global report on surveillance. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 22.Leibovici L, Vidal L, Paul M. 2009. Aminoglycoside drugs in clinical practice: an evidence-based approach. J Antimicrob Chemother 63:246–251. doi: 10.1093/jac/dkn469. [DOI] [PubMed] [Google Scholar]
- 23.Avent ML, Rogers BA, Cheng AC, Paterson DL. 2011. Current use of aminoglycosides: indications, pharmacokinetics and monitoring for toxicity. Intern Med J 41:441–449. doi: 10.1111/j.1445-5994.2011.02452.x. [DOI] [PubMed] [Google Scholar]
- 24.Grabe M, Bartoletti R, Bjerklund-Johansen TE, Cai T, Cek M, Koves B, Naber K, Pickard RS, Tenke P, Wagenlehner C, Wullt B. Guidelines on urological infections. European Association of Urology, Arnhem, The Netherlands: http://uroweb.org/wp-content/uploads/19-Urological-infections_LR2.pdf Accessed 29 September 2016. [Google Scholar]
- 25.Pallett A, Hand K. 2010. Complicated urinary tract infections: practical solutions for the treatment of multiresistant Gram-negative bacteria. J Antimicrob Chemother 65(Suppl 3):iii25–iii33. doi: 10.1093/jac/dkq298. [DOI] [PubMed] [Google Scholar]
- 26.Drusano GL, Ambrose PG, Bhavnani SM, Bertino JS, Nafziger AN, Louie A. 2007. Back to the future: using aminoglycosides again and how to dose them optimally. Clin Infect Dis 45:753–760. doi: 10.1086/520991. [DOI] [PubMed] [Google Scholar]
- 27.ter Braak EW, de Vries PJ, Bouter KP, van der Vegt SG, Dorrestein GC, Nortier JW, van Dijk A, Verkooyen RP, Verbrugh HA. 1990. Once-daily dosing regimen for aminoglycoside plus beta-lactam combination therapy of serious bacterial infections: comparative trial with netilmicin plus ceftriaxone. Am J Med 89:58–66. doi: 10.1016/0002-9343(90)90099-Y. [DOI] [PubMed] [Google Scholar]
- 28.Barza M, Ioannidis JP, Cappelleri JC, Lau J. 1996. Single or multiple daily doses of aminoglycosides: a meta-analysis. BMJ 312:338–345. doi: 10.1136/bmj.312.7027.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rybak MJ, Abate BJ, Kang SL, Ruffing MJ, Lerner SA, Drusano GL. 1999. Prospective evaluation of the effect of an aminoglycoside dosing regimen on rates of observed nephrotoxicity and ototoxicity. Antimicrob Agents Chemother 43:1549–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vidal L, Gafter-Gvili A, Borok S, Fraser A, Leibovici L, Paul M. 2007. Efficacy and safety of aminoglycoside monotherapy: systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother 60:247–257. doi: 10.1093/jac/dkm193. [DOI] [PubMed] [Google Scholar]
- 31.Aggen JB, Armstrong ES, Goldblum AA, Dozzo P, Linsell MS, Gliedt MJ, Hildebrandt DJ, Feeney LA, Kubo A, Matias RD, Lopez S, Gomez M, Wlasichuk KB, Diokno R, Miller GH, Moser HE. 2010. Synthesis and spectrum of the neoglycoside ACHN-490. Antimicrob Agents Chemother 54:4636–4642. doi: 10.1128/AAC.00572-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhanel GG, Lawson CD, Zelenitsky S, Findlay B, Schweizer F, Adam H, Walkty A, Rubinstein E, Gin AS, Hoban DJ, Lynch JP, Karlowsky JA. 2012. Comparison of the next-generation aminoglycoside plazomicin to gentamicin, tobramycin and amikacin. Expert Rev Anti Infect Ther 10:459–473. doi: 10.1586/eri.12.25. [DOI] [PubMed] [Google Scholar]
- 33.Landman D, Babu E, Shah N, Kelly P, Backer M, Bratu S, Quale J. 2010. Activity of a novel aminoglycoside, ACHN-490, against clinical isolates of Escherichia coli and Klebsiella pneumoniae from New York City. J Antimicrob Chemother 65:2123–2127. doi: 10.1093/jac/dkq278. [DOI] [PubMed] [Google Scholar]
- 34.Haidar G, Alkroud A, Cheng S, Churilla TM, Churilla BM, Shields RK, Doi Y, Clancy CJ, Nguyen MH. 2016. Association between presence of aminoglycoside modifying enzymes and in vitro activity of gentamicin, tobramycin, amikacin and plazomicin against KPC and ESBL-producing Enterobacter spp. Antimicrob Agents Chemother 60:5208–5214. doi: 10.1128/AAC.00869-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lopez-Diaz MD, Culebras E, Rodriguez-Avial I, Rios E, Vinuela-Prieto JM, Picazo JJ, Rodriguez-Avial C. 2017. Plazomicin activity against 346 extended-spectrum-beta-lactamase/AmpC-producing Escherichia coli urinary isolates, related to aminoglycoside-modifying enzymes characterized. Antimicrob Agents Chemother 61:e02454-16. doi: 10.1128/AAC.02454-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walkty A, Adam H, Baxter M, Denisuik A, Lagace-Wiens P, Karlowsky JA, Hoban DJ, Zhanel GG. 2014. In vitro activity of plazomicin against 5,015 Gram-negative and Gram-positive clinical isolates obtained from patients in Canadian hospitals as part of the CANWARD study, 2011-2012. Antimicrob Agents Chemother 58:2554–2563. doi: 10.1128/AAC.02744-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galani I, Souli M, Daikos GL, Chrysouli Z, Poulakou G, Psichogiou M, Panagea T, Argyropoulou A, Stefanou I, Plakias G, Giamarellou H, Petrikkos G. 2012. Activity of plazomicin (ACHN-490) against MDR clinical isolates of Klebsiella pneumoniae, Escherichia coli, and Enterobacter spp. from Athens, Greece. J Chemother 24:191–194. doi: 10.1179/1973947812Y.0000000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Almaghrabi R, Clancy CJ, Doi Y, Hao B, Chen L, Shields RK, Press EG, Iovine NM, Townsend BM, Wagener MM, Kreiswirth B, Nguyen MH. 2014. Carbapenem-resistant Klebsiella pneumoniae strains exhibit diversity in aminoglycoside-modifying enzymes, which exert differing effects on plazomicin and other agents. Antimicrob Agents Chemother 58:4443–4451. doi: 10.1128/AAC.00099-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Endimiani A, Hujer KM, Hujer AM, Armstrong ES, Choudhary Y, Aggen JB, Bonomo RA. 2009. ACHN-490, a neoglycoside with potent in vitro activity against multidrug-resistant Klebsiella pneumoniae isolates. Antimicrob Agents Chemother 53:4504–4507. doi: 10.1128/AAC.00641-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Livermore DM, Mushtaq S, Warner M, Zhang JC, Maharjan S, Doumith M, Woodford N. 2011. Activity of aminoglycosides, including ACHN-490, against carbapenem-resistant Enterobacteriaceae isolates. J Antimicrob Chemother 66:48–53. doi: 10.1093/jac/dkq408. [DOI] [PubMed] [Google Scholar]
- 41.Riddle VD, Cebrik DS, Armstrong ES, Cass RT, Clobes TC, Hillan KJ. 2012. Plazomicin safety and efficacy in patients with complicated urinary tract infection (cUTI) or acute pyelonephritis (AP). Abstr 52nd Intersci Conf Antimicrob Agents Chemother, San Francisco, CA, abstr L2-2118a. [Google Scholar]
- 42.Clinical and Laboratory Standards Institute. 2012. Performance standards for antimicrobial susceptibility testing; 22nd informational supplement. Document M100-S22. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 43.European Committee on Antimicrobial Susceptibility Testing. 2012. Breakpoint tables for interpretation of MICs and zone diameter, version 2.0. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/Breakpoint_table_v_2.0_120221.pdf Accessed 25 October 2017.
- 44.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. 2012. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 45.Robles-Diaz M, Lucena MI, Kaplowitz N, Stephens C, Medina-Caliz I, Gonzalez-Jimenez A, Ulzurrun E, Gonzalez AF, Fernandez MC, Romero-Gomez M, Jimenez-Perez M, Bruguera M, Prieto M, Bessone F, Hernandez N, Arrese M, Andrade RJ. 2014. Use of Hy's law and a new composite algorithm to predict acute liver failure in patients with drug-induced liver injury. Gastroenterology 147:109–118. doi: 10.1053/j.gastro.2014.03.050. [DOI] [PubMed] [Google Scholar]
- 46.Sturm W. 1995. Isepamicin versus amikacin in the treatment of urinary tract infection. J Chemother 7(Suppl 2):S149–S154. [PubMed] [Google Scholar]
- 47.Ipekci T, Seyman D, Berk H, Celik O. 2014. Clinical and bacteriological efficacy of amikacin in the treatment of lower urinary tract infection caused by extended-spectrum beta-lactamase-producing Escherichia coli or Klebsiella pneumoniae. J Infect Chemother 20:762–767. doi: 10.1016/j.jiac.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 48.Wagenlehner FM, Sobel JD, Newell P, Armstrong J, Huang X, Stone GG, Yates K, Gasink LB. 2016. Ceftazidime-avibactam versus doripenem for the treatment of complicated urinary tract infections, including acute pyelonephritis: RECAPTURE, a phase 3 randomized trial program. Clin Infect Dis 63:754–762. doi: 10.1093/cid/ciw378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wagenlehner FM, Umeh O, Steenbergen J, Yuan G, Darouiche RO. 2015. Ceftolozane-tazobactam compared with levofloxacin in the treatment of complicated urinary-tract infections, including pyelonephritis: a randomised, double-blind, phase 3 trial (ASPECT-cUTI). Lancet 385:1949–1956. doi: 10.1016/S0140-6736(14)62220-0. [DOI] [PubMed] [Google Scholar]
- 50.Fasugba O, Gardner A, Mitchell BG, Mnatzaganian G. 2015. Ciprofloxacin resistance in community- and hospital-acquired Escherichia coli urinary tract infections: a systematic review and meta-analysis of observational studies. BMC Infect Dis 15:545. doi: 10.1186/s12879-015-1282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bader MS, Loeb M, Brooks AA. 2017. An update on the management of urinary tract infections in the era of antimicrobial resistance. Postgrad Med 129:242–258. doi: 10.1080/00325481.2017.1246055. [DOI] [PubMed] [Google Scholar]
- 52.French MA, Cerra FB, Plaut ME, Schentag JJ. 1981. Amikacin and gentamicin accumulation pharmacokinetics and nephrotoxicity in critically ill patients. Antimicrob Agents Chemother 19:147–152. doi: 10.1128/AAC.19.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cass RT, Brooks CD, Havrilla NA, Tack KJ, Borin MT, Young D, Bruss JB. 2011. Pharmacokinetics and safety of single and multiple doses of ACHN-490 injection administered intravenously in healthy subjects. Antimicrob Agents Chemother 55:5874–5880. doi: 10.1128/AAC.00624-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cockcroft DW, Gault MH. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 55.Hooton TM, Bradley SF, Cardenas DD, Colgan R, Geerlings SE, Rice JC, Saint S, Schaeffer AJ, Tambayh PA, Tenke P, Nicolle LE. 2010. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 international clinical practice guidelines from the Infectious Diseases Society of America. Clin Infect Dis 50:625–663. doi: 10.1086/650482. [DOI] [PubMed] [Google Scholar]
- 56.Notermans NC, van Dijk GW, van der Graaf Y, van Gijn J, Wokke JH. 1994. Measuring ataxia: quantification based on the standard neurological examination. J Neurol Neurosurg Psychiatry 57:22–26. doi: 10.1136/jnnp.57.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.